Summary
To lend insight into the overwintering strategy of the Alaska blackfish (Dallia pectoralis), we acclimated fish to 15°C or 5°C and then utilized whole-cell patch-clamp to characterize the effects of thermal acclimation and acute temperature change on the density and kinetics of ventricular L-type Ca2+ current (ICa). Peak ICa density at 5°C (−1.1± 0.1 pA pF−1) was 1/8th that at 15°C (−8.8 ± 0.6 pA pF−1). However, alterations of the Ca2+- and voltage-dependent inactivation properties of L-type Ca2+ channels partially compensated against the decrease. The time constant tau (τ) for the kinetics of inactivation of ICa was ~4.5-times greater at 5°C than at 15°C, and the voltage for half-maximal inactivation was shifted from −23.3 ± 1.0 mV at 15°C to - 19.8 ± 1.2 mV at 5°C. These modifications increase the open probability of the channel and culminated in an approximate doubling of the L-type Ca2+ window current, which contributed to approximately 15% of the maximal Ca2+ conductance at 5°C. Consequently, the charge density of ICa (QCa) and the total Ca2+ transferred through the L-type Ca channels (Δ[Ca2+]) were not as severely reduced at 5°C as compared to peak ICa density. In combination, the results suggest that while the Alaska blackfish substantially down-regulates ICa with acclimation to low temperature, there is sufficient compensation in the kinetics of the L-type Ca2+ channel to support the level of cardiac performance required for the fish to remain active throughout the winter.
Keywords: electrophysiology, ICa, heart, thermal acclimation
Introduction
The Alaska blackfish (Dallia pectoralis) is the only air-breathing fish to inhabit Arctic regions. The fish is native to the Yukon and Kuskokwim river basins of Western mainland Alaska, the Bering Sea Islands and Eastern Siberia, and has recently been introduced to the waters of Southcentral Alaska (Campbell and Lopéz 2014). The fish presumably utilizes air-breathing in the summer when water temperatures are high (12-15°C) to support reproduction and migration to waters that are dense in vegetation, have little mixing and are hypoxic (Lefevre et al. 2014; Ostdiek and Nardone 1959). However, unlike the majority of air-breathing fish that reside in the tropics and have year-round access to air (Graham and Wegner 2010), the Alaska blackfish is forcibly submerged beneath ice during the winter months, which precludes air-breathing. In addition, ice cover during the winter months prevents diffusion of atmospheric oxygen into the water column, restricting photosynthesis and leading to hypoxic conditions. Indeed, Alaska blackfish have been captured from ice-covered waters with a temperature of 4°C and oxygen levels ranging from 0.8 kPa at depth to 3.6 kPa in the water column (Lefevre et al. 2014). However, the fish appears incapable of surviving prolonged periods of severe oxygen deprivation (i.e., below their critical oxygen tension of 5.0 kPa) at low temperature if restricted from air-breathing (Lefevre et al. 2014; Scholander et al. 1953).
Interestingly, the constraints and demands placed upon the physiology of cold-acclimatized Alaska blackfish appear to be paradoxical. On the one hand, survival of hypoxic conditions when air-breathing is not possible would seemingly be facilitated by a reduction of cellular energy demands via a down-regulation of physiological and biochemical processes. However, on the other hand, the Alaska blackfish remains active at low temperature and has an equally impressive factorial aerobic scope (5- to 8-fold) at high (15°C) and low (5°C) acclimation temperatures (Lefevre et al. 2014; Ostdiek and Nardone 1959). Therefore, compensatory physiological and biochemical adjustments may be necessary to offset the passive depressive effect of decreased temperature on biological processes and permit the Alaska blackfish to remain active at low temperature.
It is well established that for fish of north-temperate climates that experience seasonal changes in temperature, physiological plasticity of cardiac physiology reflects overwintering strategy (Vornanen et al. 2002). Fish that overwinter in oxygen depleted waters, such as the crucian carp (Carassius carassius), down-regulate metabolic and cardiac activity upon exposure to low temperature (Vornanen 1996b). By comparison, cold-active species, such as rainbow trout (Oncorhynchus mykiss) and burbot (Lota lota), compensate against the depressive effects of low temperature (Vornanen et al. 2002; Aho and Vornanen 1999). With regard to cardiac excitation-contraction coupling, L-type Ca2+ current (ICa) is a predominant source of the Ca2+ required for the activation of the contractile filaments in the fish heart and is a robust proxy for cardiac contractility (Vornanen et al. 2002). While cold-dormant species down-regulate ICa with exposure to low temperature, cold-active species maintain cardiac contractility by up-regulating L-type Ca2+ channel density to maintain peak ICa density and also speed the rate of L-type Ca2+ channel inactivation to allow cardiomyocytes to re-activate more quickly (Klaiman et al. 2011; Shiels et al. 2000).
The objective of the present study was to lend insight into the overwintering strategy of the Alaska blackfish by examining the effect of temperature on its cardiac physiology, namely the effects of thermal acclimation and acute temperature change on the density and kinetics of the ventricular ICa. Using whole-cell patch-clamp, we characterized ICa of ventricular cardiomyocytes from Alaska blackfish acclimated to high (15°C) and low temperature (5°C). In addition, to distinguish acclimation effects from the passive effects of temperature change, ICa was also assessed after acute exposure of the cardiomyocytes to 10°C. We hypothesized that if energy conservation was the paramount determinant of cardiac physiology at low temperature, ICa would be down-regulated beyond the passive effects of temperature with acclimation to 5°C, as occurs in cardiac tissue of anoxia-tolerant and cold-dormant vertebrates (Stecyk et al. 2008). By contrast, we hypothesized that if compensation against low temperature was the predominant determinant of cardiac physiology at low temperature, compensatory changes in ICa would be apparent after acclimation to 5°C, as occurs in cold-active and cold stenothermic fish (Shiels et al. 2000; Shiels et al. 2006).
Materials and Methods
Experimental Animals
Animals were collected under appropriate Alaska Department of Fish and Game permits (SF-2013-010 and SF-2014-062) and the University of Alaska Anchorage (UAA) Institutional Animal Care and Use Committee approved all procedures (406888, 421896, and 421899). Seventeen Alaska blackfish (Dallia pectoralis) with a mean body mass of 33.8 ± 11.2 g and length of 15.3 ± 1.4 cm (means ± S.D.) were utilized (Table 1). Fish of both sexes were collected using minnow traps from local waters (Little Campbell Lake, Anchorage, AK; Duck Hunter's Training Pond, Palmer, AK) and were maintained indoors under a 12h:12h L:D photoperiod in 300-l fiberglass aquaria containing recirculating, dechlorinated and aerated water. Fish were initially held at their habitat temperature, which ranged between 10°C and 16°C, and then randomly divided into four aquaria for acclimation to 15°C or 5°C (2 aquaria per acclimation temperature). Acclimation to 15°C or 5°C occurred by gradually increasing (0.5°C per day) or decreasing (1°C per day) water temperature. The temperature of each aquarium was regulated with a cooler/heater system (Teco-TR20, Senkor Group, Inc., Terrell, TX, USA) and fish were held at their acclimation temperature for a minimum of 5 weeks prior to experimentation. Throughout, fish were fed ad libitum every 2 d with bloodworms (Brine Shrimp Direct, Ogden, UT, USA).
Table 1.
Length, mass and relative ventricular mass (RVM) of Alaska blackfish acclimated to 5°C or 15°C
| Length (cm) | Mass (g) | RVM (%) | N | |
|---|---|---|---|---|
| 5°C-acclimated | 16.1±0.3 | 40.5±2.7 | 0.075±0.001 | 10 |
| 15°C-acclimated | 14.1±0.4* | 24.1±2.4* | 0.066±0.002 | 7 |
An asterisk (*) indicates a significant difference (P<0.05) between 15°C- and 5°C-acclimated fish (unpaired t-tests)
Values are represented as means ± S.D for length and mass and means ± S.E.M for RVM.
Cardiomyocyte Isolation
Ventricular cardiomyocytes were enzymatically isolated using an established protocol for teleost fish (Shiels et al. 2000; Vornanen 1998). Fish were stunned by cranial concussion and pithed. The heart was immediately excised and a cannula was inserted through the bulbus arteriosus into the ventricle. The heart was retrograde perfused at room temperature (21°C) from a height of 50 cm, first with a nominally Ca2+-free, low-Na+ isolation saline solution (containing, in mmol l−1: 100 NaCl, 10 KCl, 4 MgSO4, 1.2 KH2PO4, 50 taurine, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 20 glucose; adjusted to pH 6.9 with KOH) for 10 minutes and then with fresh isolation solution supplemented with the proteolytic enzymes collagenase (1.5 mg ml−1; Type IA) and trypsin (1.0 mg ml−1; Type I), as well as bovine fatty acid serum albumin (1.5 mg ml−1), for an additional 10 minutes. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Solutions were constantly bubbled with 100% O2. The ventricle was then dissected from the bulbus arteriosus and atrium, weighed, minced with scissors in fresh isolation solution and triturated through the opening of a Pasteur pipette for 2-3 minutes. The solution was left to settle and the isolated cardiomyocytes were resuspended in fresh isolation solution. Cardiomyocytes were stored at 4°C for up to 8 h until use, as previously conducted for patch-clamp investigations on cardiomyocytes from crucian carp, rainbow trout, burbot, zebrafish (Danio rerio) and red-eared slider turtle (Trachemys scripta) (Hove-Madsen and Tort 1998; Shiels et al. 2006; Stecyk et al. 2007; Vornanen 1997; Zhang et al. 2011).
Experimental Procedures and Data Analysis
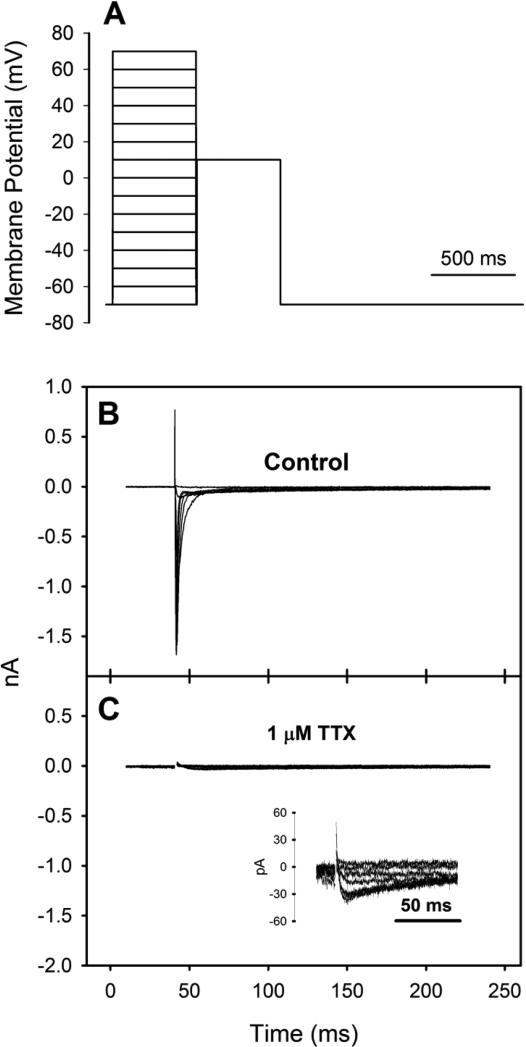
Electrophysiological measurements and the analysis of ICa were achieved using established methods and solutions for teleost fish (Shiels et al. 2000; Shiels et al. 2006; Vornanen 1997, 1998). Whole-cell voltage-clamp recordings were made using an Axopatch 200B amplifier, a CV-203BU headstage and ClampEx 10.2 software (Axon Instruments, Foster City, CA, USA). An aliquot of dissociated cardiomyocytes was placed into a recording chamber (RC-22C; Warner Instruments, Hamden, CT, USA) mounted on the stage of an inverted microscope (Nikon Ti-S, Tokyo, Japan) and allowed to settle. The cardiomyocytes were then superfused at a rate of 1-2 ml min−1 with an extracellular saline solution (containing, in mmol l−1: 150 NaCl, 5.4 CsCl, 1.2 MgSO4, 0.4 NaH2PO4, 1.8 CaCl2, 10 HEPES and 10 glucose adjusted to pH 7.6 with CsOH). Additionally, 1 μmol l−1 tetrodotoxin (TTX) was added to the perfusate to completely block fast Na+ channels (Fig. 1; Shiels et al. 2000; Vornanen 1997; Zhang et al. 2011). Perfusate temperature was regulated using a SC-20 dual in-line solution heater/cooler and a CL-100 bipolar temperature controller (Warner Instruments). A thermocouple positioned no less than 5 mm from the cardiomyocyte under investigation was used to continuously monitor temperature.
Fig. 1.
(A) Square pulse stimulation protocol used for all ICa measurements of Alaska blackfish ventricular cardiomyocytes and representative recordings of ICa from a cardiomyocyte isolated from a 15°C-acclimated Alaska blackfish (B) in the absence of and (C) in the presence of 1 μM TTX. The inset in panel B shows ICa at a greater resolution.
Patch pipettes were pulled from borosilicate glass (GC150T-7.5; Harvard Apparatus, St. Laurent, QC, Canada) with a Sutter Flaming/Brown P-1000 puller (Sutter Instruments, Novato, CA, USA) and fire-polished with a MF-200 micro-forge (WPI, Sarasota, FL, USA). Pipettes had a resistance of 1.5-2.5 MΩ when filled with pipette solution (containing, in mmol l−1: 130 CsCl, 1 MgCl2, 5 MgATP, 0.03 Na2-GTP, 5 ethylene glycol tetraacetic acid (EGTA), 15 tetraethylammonium chloride (TEA-Cl, to block K+ currents; Hove-Madsen and Tort 1998) and 10 HEPES adjusted to pH 7.2 with CsOH). Offset potentials were zeroed just before the formation of the gigaohm seal and pipette capacitance was compensated after seal formation. The patch was ruptured by delivering a short voltage pulse (zap) to the cardiomyocyte and capacitive transients were eliminated by iterative adjustments of the series resistance and cell capacitance circuits. Mean cell capacitance was 26.0 ± 1.6 pF (mean ± S.E.M.; N=24) and mean series resistance was 12.1 ± 1.6 MΩ (mean ± S.E.M.; N=24). Compensation of series resistance was not performed. However, given the size of the currents recorded, voltage errors would be negligible (i.e., less than 3 mV). ICa was elicited from the holding potential of −70 mV by 500 ms depolarized square pulses to voltages between −70 and +70 mV in 10 mV steps (Fig. 1). Each voltage step was followed by a square test pulse to +10 mV for 500 ms to assess the steady-state activation and inactivation of ICa. The two pulses were separated by a 5 ms return to the holding potential. Sampling rate was 10 kHz and signals were low-pass filtered at 2 kHz on-line with the Axopatch amplifier. Signals were analyzed off-line with Clampfit 10.2 software (Axon Instruments).
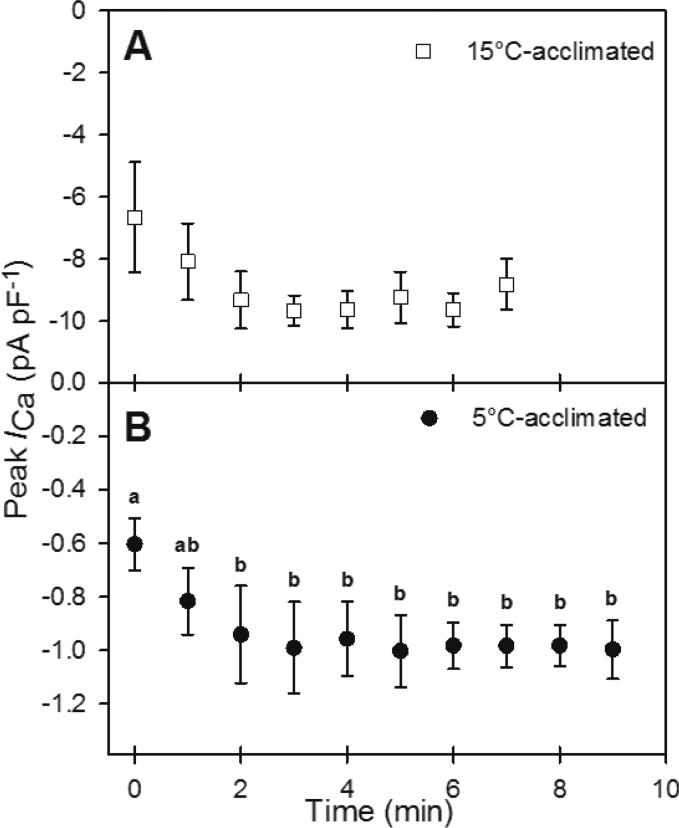
The experimental protocol consisted of recording from a cardiomyocyte first at the acclimation temperature of the animal (i.e., at 15°C or 5°C) and then after an acute exposure to 10°C. The recordings at the common test temperature of 10°C were conducted to distinguish acclimation effects from direct temperature effects. To ensure that any observed difference in ICa after acute temperature change was not due to rundown of ICa, which can occur over time when ICa is measured in the whole-cell configuration, the magnitude of ICa rundown for 15°C- and 5°C-acclimated cardiomyocytes was assessed by recording at 1 min intervals for up to 7 min at 15°C and 9 min at 5°C (ICa became unstable after 7 min at 15°C; Fig. 2). The peak ICa density of 15°C-acclimated cardiomyocytes did not exhibit any statistically significant change over the 7-min period (Fig. 2A). However, the peak current tended to increase by approximately 40% between 0 min and 3 min. For 5°C-acclimated cardiomyocytes, peak ICa density increased by 55% by 2 min, but then stabilized (Fig. 2B). Therefore, at both 15°C and 5°C, the experimental recordings did not commence until 3 min after membrane rupture. The acute temperature change to 10°C occurred immediately after the initial recording of ICa at acclimation temperature and was accomplished within 2-3 min. The recording of ICa at 10°C was therefore completed within the time period that ICa remained stable for both temperature acclimation groups.
Fig. 2.
Time-dependent changes in the peak amplitude of ICa of (A) 15°C- and (B) 5°C-acclimated Alaska blackfish ventricular cardiomyocytes. Values are means ± S.E.M. Dissimilar letters indicate a statistically significant difference (P<0.05) between time points (repeated measures ANOVA with Student-Newman-Keuls post hoc analysis). N=4 cells from 3 fish at 15°C, N=5 cells from 5 fish at 5°C.
The peak amplitude of ICa was calculated as the difference between peak inward current and the current at the end of the initial depolarizing pulses. The charge density of ICa (QCa) at 0 mV was determined by integrating the inactivating portion of the Ca2+ current for the 500 ms initial depolarizing pulse and was normalized to cell capacitance (normalized QCa). The total Ca2+ that was transferred through the L-type Ca2+ channels (Δ[Ca2+]) was calculated from normalized QCa and cell volume and was expressed as a function of an assumed non-mitochondrial myoplasmic volume (i.e., myofibrillar volume) of 55% (Vornanen 1997, 1998). Cell volume was calculated from measured cell capacitance and the experimentally determined surface-to-volume ratio of the cardiomyocytes. Surface area was calculated from length and width measurements of cardiomyocytes imaged with an Olympus FSX100 microscope (Olympus America, Center Valley, PA, USA; Table 2). The cardiomyocytes were considered to be flat elliptical cylinders with a 1:2 axis ratio for the elliptical cross-section (Vornanen 1997). Membrane capacitance was converted to surface area using a value of 61.28 μm2 pF−1 (specific membrane capacitance of 1.63 μF cm−2), which was determined by dividing the mean surface area by the mean capacitance of the cardiomyocytes.
Table 2.
Alaska blackfish ventricular cardiomyocyte morphology
| Length (μm) | Width (μm) | Area (μm2) | Volume (μm3) | Surface-to-volume ratio (μm−1) | N |
|---|---|---|---|---|---|
| 93.8±5.8 | 6.6±0.4 | 1595±156 | 1730±267 | 1.03±0.07 | 14 (1 fish) |
Values are means ± S.E.M.
The kinetics of inactivation of ICa were assessed by fitting the decaying portion of ICa with a single exponential function using the Chebyshev transformation in the Clampfit 10.2 software to derive a time constant, tau (τ) (Shiels et al. 2006). A single-order exponential function fit the decaying portion of ICa better than a double-order exponential function, as evidenced by less variable τ values among cardiomyocytes recorded at the same temperature.
The voltage-dependence of steady-state activation and inactivation were determined using previously established calculations (Vornanen 1998). The voltage-dependence of steady-state activation was determined as the normalized Ca2+ conductance (gCa/gmax), where gmax is the maximum value for Ca2+ conductance. The voltage-dependence of the peak conductance of the L-type Ca2+ channels was calculated using the following equation: gCa = ICa/(V-Vrev), where gCa is the membrane Ca2+ conductance, ICa is the peak current amplitude elicited at a given voltage (V) and Vrev is the apparent reversal potential elicited by extrapolating the ascending portion of the current-voltage (I-V) relationship to zero current. Inactivation voltage-dependence was calculated by dividing the amplitude of the test current by the maximal current elicited. The steady-state activation and inactivation parameters, namely the half-activating potential (Vh,act), the half-inactivating potential (Vh,inact), the slope factor of activation (kact) and the slope factor of inactivation (kinact), were determined for each recording from an individual cell by plotting peak conductance against membrane potential and the fitting the data to the Boltzmann equation (MacFarlane and Sontheimer 1997). Curve fitting was conducted for individual recordings such that the effect of acute temperature change on the steady-state activation and inactivation parameters could be analyzed by repeated measures statistical analysis (see Statistical Analysis below). The window current was calculated using the product of the steady-state activation and inactivation curves.
Statistical Analysis
Results are reported as means ± S.E.M. or means ± S.D., as indicated. For whole-animal measurements, N represents individual animals, whereas for patch-clamp data, N represents individual cardiomyocytes. A one-way repeated-measures analysis of variance (ANOVA) with Student-Newman-Keuls post hoc analysis was utilized to assess the rundown of peak ICa density over time. Unpaired t-tests were utilized to determine statistically significant differences between acclimation temperatures, as well between 15°C- and 5°C-acclimated cardiomyocytes measured at 10°C. Paired t-tests were used to determine statistically significant effects of acute exposure to 10°C from an acclimation temperature. Differences were considered statistically significant when P<0.05.
Results
Relative Ventricular Mass
RVM was unchanged with acclimation temperature. RVM was 0.066 ± 0.002% at 15°C and 0.075 ± 0.001% at 5°C (Table 1).
Cardiomyocyte Morphology
Alaska blackfish ventricular cardiomyocytes were spindle-shaped (Fig. 3) and had a mean length and width of 93.8 ± 5.8 μm and 6.6 ± 0.4 μm, respectively (Table 2). Assuming a 1:2 axis ratio for the elliptical cross-section of the cardiomyocyte (Vornanen 1997), cell surface area was 1595 ± 156 μm2 and cell volume was 1730 ± 267 μm3 (1.73 ± 0.27 pl). Accordingly, the mean surface-to-volume ratio was 1.03 ± 0.07 μm−1 (21.16 ± 4.3 pF pl−1). There was no difference in cell capacitance between acclimation groups (Table 3).
Fig. 3.
Phase contrast light microscopy image of a live cardiomyocyte enzymatically isolated from the Alaska blackfish ventricle. Scale bar is 20 μM.
Table 3.
Peak ICa density and charge transfer (QCa), cell capacitance and the change in total cellular [Ca2+] (Δ[Ca2+]) expressed as a function of non-mitochondrial volume of Alaska blackfish ventricular cardiomyocytes at low (5°C) and high (15°C) acclimation temperatures and following acute exposure to 10°C.
| Treatment | Peak ICa density (pA pF−1) | QCa (pC) | Cell capacitance (pF) | Normalized QCa (pC pF−1) | Δ[Ca2+] (μmol l−1) | N |
|---|---|---|---|---|---|---|
| 5°C-acclimated | −1.1±0.1a | −3.8±0.6a | 26.2±2.3 | −0.142±0.01a | 27.4±2.0a | 13 (9 fish) |
| 10°C acute from 5°C | −2.3±0.4* | −7.0±1.0* | 25.0±1.5 | −0.279±0.03* | 53.9±5.9* | 7 (5 fish) |
| 10°C acute from 15°C | −4.1±0.5* † | −7.3±1.3* | 21.0±2.6 | −0.343±0.04* | 66.1±7.3* | 6 (3 fish) |
| 15°C-acclimated | −8.8±0.6b | −15.7±2.3b | 26.1±2.9 | −0.585±0.06b | 112.9±12.5b | 10 (6 fish) |
Dissimilar letters indicate a significant difference (P<0.05) between 15°C and 5°C acclimation groups (unpaired t-tests).
An asterisk (*) indicates a statistically significant effect (P<0.05) of acute exposure to 10°C from an acclimation temperature (paired t-tests).
A dagger (†) indicates a significant difference (P<0.05) between 15°C and 5°C acclimation groups after acute exposure to and recording at 10°C (unpaired t-tests).
Values are means ± S.E.M.
ICa Current-Voltage Relationship
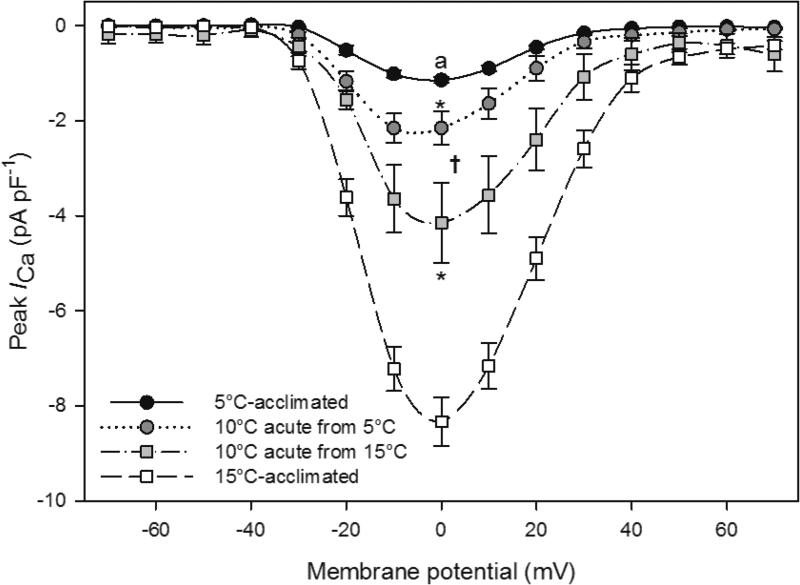
At nearly all test temperatures, ICa activated at approximately −30 mV and peaked at 0 mV (Fig. 4). Exceptions included the 5°C-acclimated cardiomyocytes measured at 5°C, where ICa did not activate until voltages positive of −30 mV, and the 5°C-acclimated cardiomyocytes acutely warmed to 10°C, where peak ICa density appeared to shift to a slightly more negative membrane potential. The peak ICa density at 15°C was −8.8 ± 0.6 pA pF−1 (Table 3). By comparison, the peak ICa density at 5°C was −1.1 ± 0.1 pA pF−1 (Table 3). The 8.0-fold difference in ICa density between acclimation temperatures corresponds to a Q10 of 8.0. When 15°C-acclimated cardiomyocytes were acutely cooled to 10°C, peak ICa density decreased to −4.1 ± 0.6 pA pF−1, corresponding to a Q10 of 4.6. When 5°C-acclimated cardiomyocytes were acutely warmed to 10°C, peak ICa density increased to −2.4 ± 0.4 pA pF−1, corresponding to a Q10 of 4.5. Despite the large sensitivity of peak ICa density to acute temperature change, the peak ICa density of 5°C-acclimated cardiomyocytes was less than that of the 15°C-acclimated cardiomyocytes when measured at the common test temperature of 10°C.
Fig. 4.
Mean current-voltage relationship for Alaska blackfish ventricular cardiomyocytes recorded at acclimation temperature (15°C or 5°C) and then acutely exposed to and recorded at 10°C. Values are means ± S.E.M. Dissimilar letters indicate a significant difference (P<0.05) between 15°C- and 5°C-acclimated cells at 0 mV (unpaired t-tests). An asterisk (*) indicates a statistically significant effect of acute exposure to 10°C from an acclimation temperature (P<0.05) at 0 mV (paired t-tests). A dagger (†) indicates a significant difference (P<0.05) at 0 mV between 15°C- and 5°C-acclimated cells acutely exposed and recorded at 10°C (unpaired t-tests). N=13 cells from 9 fish at 5°C, N=7 cells from 5 fish at 10°C (from 5°C), N=6 cells from 3 fish at 10°C (from 15°C), and N=10 cells from 6 fish at 15°C.
Ca2+ Influx through L-type Ca2+ Channels
Similar to peak ICa density, QCa, normalized Q and Δ[Ca2+] were greatest in 15°C-acclimated cardiomyocytes and least in 5°C-acclimated cardiomyocytes (Table 3). There was a 4.1-fold difference in Δ[Ca2+] between acclimation temperatures. When 15°C-acclimated cardiomyocytes were acutely cooled to 10°C, normalized QCa and Δ[Ca2+] decreased by 44%. When 5°C-acclimated cardiomyocytes were acutely warmed to 10°C, normalized QCa and Δ[Ca2+] nearly doubled. Subsequently, normalized QCa and Δ[Ca2+] were similar for 15°C- and 5°C-acclimated ventricular cardiomyocytes when measured at the common test temperature of 10°C.
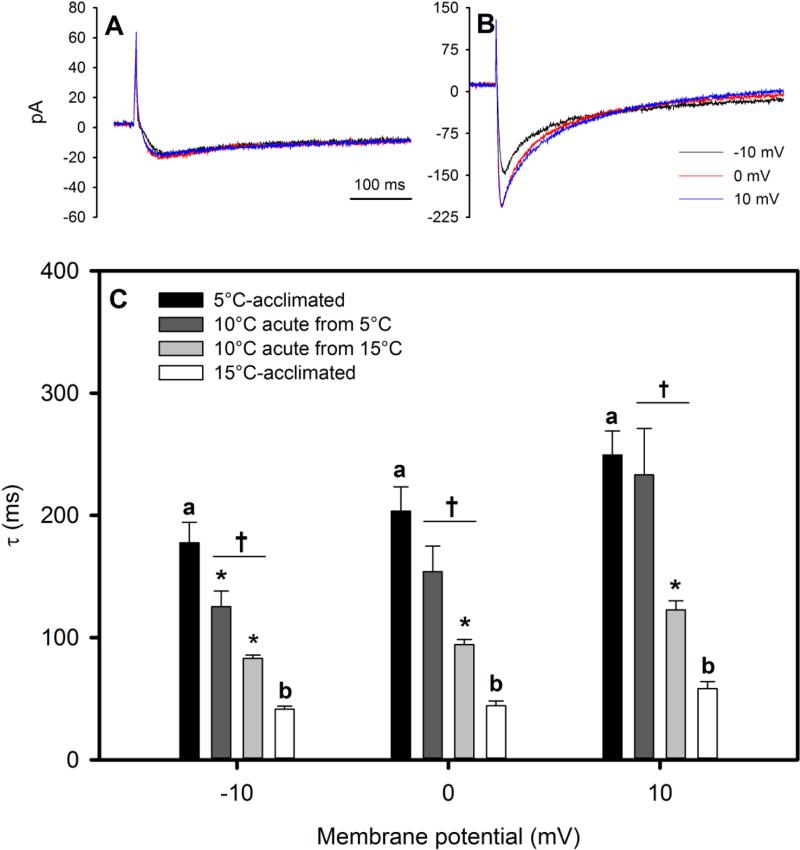
ICa Kinetics of Inactivation
τ was statistically significantly greater in 5°C-acclimated cardiomyocytes than 15°C-acclimated cardiomyocytes regardless of the membrane potential at which it was calculated (i.e., −10, 0 or +10 mV; Fig. 5). Specifically, ICa inactivation was between 4.3- and 4.6-times slower at 5°C than 15°C. When 15°C-acclimated cardiomyocytes were acutely cooled to 10°C, τ approximately doubled, corresponding to Q10 values between 4.0 and 4.5. By contrast, when 5°C-acclimated cardiomyocytes were acutely warmed to 10°C, τ did not consistently decrease (a statistically significant decrease of 29% was only observed at −10 mV, corresponding to a Q10 of 2.0). Consequently, τ of 5°C-acclimated cardiomyocytes measured at 10°C was greater than the τ of 15°C-acclimated cardiomyocytes measured at 10°C across the range of membrane potentials investigated.
Fig. 5.
Representative recordings of peak ICa and inactivating portion of ICa at −10, 0 and +10 mV in (A) 5°C- and (B) 15°C-acclimated cardiomyocytes, and (C) the time constant tau (τ) for the kinetics of inactivation of ICa from Alaska blackfish ventricular cardiomyocytes. τ was derived by fitting a single exponential function to the inactivating portion of ICa at −10, 0, and +10mV. Values are means ± S.E.M. Dissimilar letters indicate a significant difference (P<0.05) between 15°C and 5°C cells at a given voltage (unpaired t-tests). An asterisk (*) indicates a statistically significant effect of acute exposure to 10°C from an acclimation temperature (P<0.05) at a given voltage (paired t-tests). A dagger (†) indicates a significant difference (P<0.05) at a given voltage between 15°C- and 5°C-acclimated cells acutely exposed and recorded at 10°C (unpaired t-tests) at a given voltage. N=12 cells from 9 fish at 5°C, N=7 cells from 5 fish at 10°C (from 5°C), N=5 cells from 2 fish at 10°C (from 15°C), and N=10 cells from 5 fish at 15°C.
Steady-State Activation and Inactivation of ICa
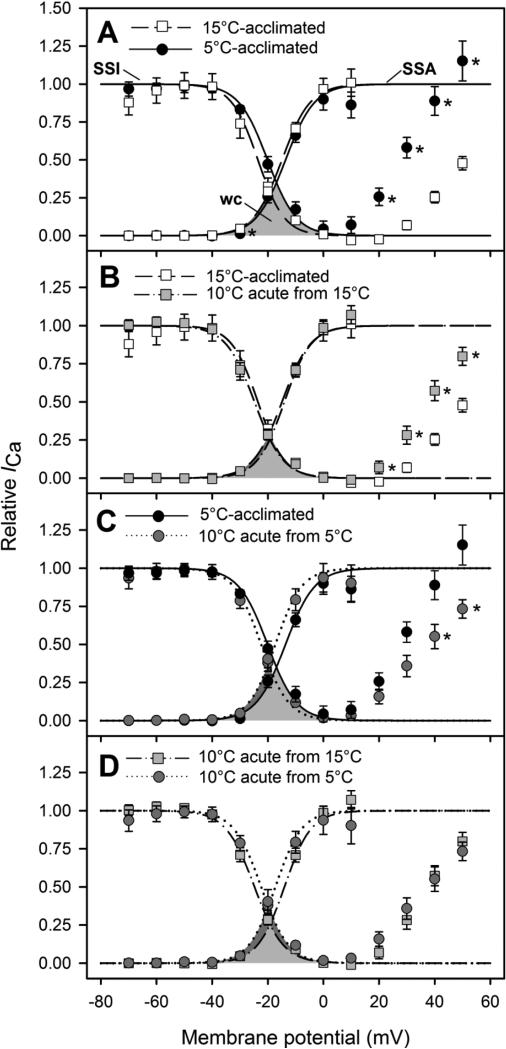
The rates of transition between active and inactive states (i.e., kact and kinact) were not affected by acclimation temperature or acute temperature change (Table 4). Likewise, the voltage for half-maximal activation (Vh,act) did not differ between acclimation temperatures, nor was it affected by acute temperature change. By contrast, steady-state inactivation was affected by acclimation temperature and acute temperature change. Acclimation to 5°C shifted the voltage for half-maximal inactivation (Vh,inact) to a more depolarized voltage (from −23.3 ± 1.0 mV to −19.8 ± 1.2 mV), whereas acute cooling of 15°C-acclimated cardiomyocytes to 10°C shifted Vh,inact to a more polarized voltage (i.e., in the opposite direction to −25.0 ± 0.4 mV). In addition, between +20 and +50 mV, the attenuation of inactivation was significantly greater at 5°C than at 15°C (Fig. 6A). Similarly, acute cooling of 15°C-acclimated cardiomyocytes resulted in a greater attenuation of inactivation between +20 and +50 mV (Fig. 6C), whereas acute warming of 5°C-acclimated cardiomyocytes resulted in a decreased attenuation of inactivation between +40 and +50 mV (Fig. 6B). Consequently, the attenuation of inactivation between +20 and +50 mV was similar for 15°C- and 5°C-acclimated cardiomyocytes when measured at 10°C (Fig. 6D).
Table 4.
Steady-state activation and inactivation parameters of Alaska blackfish ventricular cardiomyocytes at low (5°C) and high (15°C) acclimation temperatures and following acute exposure to 10°C.
| Treatment | Vh,act (mV) | kact (mV) | Vh,inact (mV) | kinact (mV) | N |
|---|---|---|---|---|---|
| 5°C-acclimated | −13.7±1.2 | 3.9±1.5 | −19.8±1.2b | 5.4±0.4 | 11 (8 fish) |
| 10°C acute from 5°C | −13.8±1.1 | 5.7±0.9 | −20.7±1.1 | 5.4±0.5 | 5 (4 fish) |
| 10°C acute from 15°C | −14.2±0.8 | 4.9±0.2 | −25.0±0.4*† | 4.9±0.1 | 5 (2 fish) |
| 15°C-acclimated | −14.1±0.8 | 5.8±0.7 | −23.3±1.0a | 4.6±0.1 | 9 (5 fish) |
Vh,act: half-activating potential; Vh,inact: half-inactivating potential; kact: the slope factor of activation; kinact: the slope factor of inactivation.
Dissimilar letters indicate a significant difference (P<0.05) between 15°C and 5°C acclimation groups (unpaired t-tests).
An asterisk (*) indicates a statistically significant effect (P<0.05) of acute exposure to 10°C from an acclimation temperature (paired t-tests).
A dagger (†) indicates a significant difference (P<0.05) between 15°C and 5°C acclimation groups after acute exposure to and recording at 10°C (unpaired t-tests).
Values are means ± S.E.M.
Fig. 6.
Steady-state activation and inactivation of ICa in Alaska blackfish ventricular cardiomyocytes at acclimation temperature (5°C or 15°C) and after acute exposure to 10°C. Steady-state activation and inactivation are compared between (A) 5°C- and 15°C-acclimated cardiomyocytes, (B) 5°C-acclimated cardiomyocytes recorded at 5°C and then acutely exposed to 10°C, (C) 15°C-acclimated cardiomyocytes recorded at 15°C and then acutely exposed to 10°C and (D) 15°C- and 5°C-acclimated cells acutely exposed to 10°C. The data from individual recordings were fit to the Boltzmann equation and the mean coordinates of the fitted curves are plotted (see Materials and Methods). Steady-state inactivation (SSI) curves decrease sigmoidally from 1, while steady-state activation (SSA) curves increase sigmoidally from 0. The shaded areas below the two curves represents the window current (WC) of ICa. Values are means ± S.E.M. Asterisks (*) indicate a statistically significant difference (P<0.05) at a specific voltage (unpaired t-tests for panels A and D; paired t-tests for panels B and C). N values are detailed in Table 4.
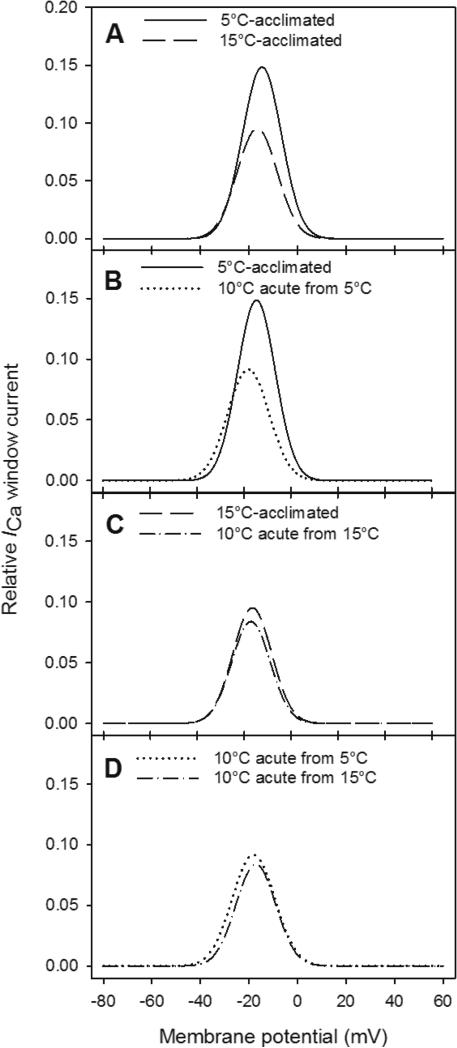
As evidenced by the overlap of the steady-state activation and inactivation curves, a window current was present in all conditions (Fig. 7). The window current of 15°C-acclimated cardiomyocytes was maximal at −18 mV, where it contributed to approximately 9% of the maximal conductance (Fig. 7A). With acclimation to 5°C, the peak of the window current was shifted to a more depolarized voltage (−15 mV) and its contribution to maximal conductance was almost doubled (to approximately 15%; Fig. 7A). By contrast, acute cooling of 15°C-acclimated cardiomyocytes to 10°C did not result in a shift of the window current peak to a more depolarized membrane potential or an increase in the contribution of the window current to maximal conductance (Fig. 7C). However, when 5°C-acclimated cardiomyocytes were acutely warmed to 10°C, the effects of acclimation to low temperature were reversed. The peak of the window current was shifted to a more polarized voltage that was similar to that observed for 15°C cells and its contribution to maximal conductance was decreased (from 15% to 9%; Fig. 7B). Consequently, the window currents of 15°C- and 5°C-acclimated cardiomyocytes when measured at 10°C were nearly identical (Fig. 7D).
Fig. 7.
Window current of ICa from Alaska blackfish ventricular cardiomyocytes. Window currents are compared between (A) 5°C- and 15°C-acclimated cardiomyocytes, (B) 5°C-acclimated cardiomyocytes recorded at 5°C and then acutely exposed to 10°C, (C) 15°C-acclimated cardiomyocytes recorded at 15°C and then acutely exposed to 10°C and (D) 15°C-and 5°C-acclimated cells acutely exposed to 10°C. N values are detailed in Table 4.
Discussion
Effects of Temperature on ICa
Temperature is perhaps the most influential abiotic factor influencing the cardiac physiology of ectothermic vertebrates. For teleosts of northern temperate climates, low seasonal water temperatures during the winter months present a physiological challenge, especially for the heart. Cardiac muscle must accommodate the temperature-driven effects on muscle contractility, blood viscosity and vascular resistance and continue to provide the force necessary to perfuse tissues (Young and Egginton 2011). In response, fish utilize one of two primary overwintering strategies. Some fish down-regulate metabolic and cardiac activity and consequently spend the winter in a dormant state (Vornanen et al. 2002). The crucian carp, which overwinters in anoxic ponds, exemplifies this strategy. At low temperature, the crucian carp suppresses heart rate and ventricular contraction velocity (Matikainen and Vornanen 1992). Correspondingly, peak ICa density is decreased to 1/6th of that displayed at high temperatures, with no resulting changes in L-type Ca2+ channel kinetics (Vornanen 1998). By contrast, other fish compensate against the depressive effects of low temperature in order to remain active. The rainbow trout exemplifies this strategy. With cold exposure, the rainbow trout increases RVM to overcome the negative inotropic effects of low temperature and increased blood viscosity, up-regulates L-type Ca2+ channel density to maintain peak ICa density and speeds the rate of L-type Ca2+ channel inactivation so that cardiomyocytes can re-activate more quickly (Klaiman et al. 2011; Shiels et al. 2000). The compensatory modifications result in an increased rate of contraction in both atrial and ventricular tissue with low temperature acclimation (Aho and Vornanen 1999). Similar compensatory physiological adaptations are exhibited by cold stenothermal burbot (Shiels et al. 2006).
The present findings reveal that the cardiophysiological responses of Alaska blackfish to low temperature acclimation include a mix of cold-compensatory and down-regulatory modifications. At the tissue level, the absence of ventricular hypertrophy with cold acclimation reflects an absence of cold-compensation In comparison, the RVM of 5°C-acclimated Alaska blackfish was approximately half that of cold-acclimated rainbow trout and the cold-stenothermic burbot (Klaiman et al. 2011; Tiitu and Vornanen 2002). The finding suggests that cardiac power output (i.e., cardiac ATP demand) and stroke volume are likely reduced at low temperature. Nevertheless, the RVM of Alaska blackfish falls within the average range for teleost fish (Wilber et al. 1961), including other Alaskan fish species that overwinter in similar habitats (Thymallus arcticus: 0.09%; Catostomus catostomus: 0.08%; Cameron 1975).
At the cellular level, a down-regulatory overwintering strategy is most clearly construed by the marked suppression of peak ICa density. The Q10 of 8.0 for peak ICa density between 15°C and 5°C is much greater than the Q10 of 2-3 that would be expected to occur solely from the passive effects of low temperature. The large magnitude of suppression signifies an active down-regulation of L-type Ca2+ channel density with cold acclimation and indicates that the Alaska blackfish employs channel arrest and inverse thermal compensation to prepare for low oxygen conditions during the winter. Similarly large suppressions of peak ICa density with cold temperature exposure have been reported for the truly anoxia-tolerant vertebrate species (Q10 values ranging between 3.6 and 5.0; Stecyk et al. 2007; Vornanen and Paajanen 2004). By comparison, the cold-active rainbow trout displayed a Q10 value of 1.1 for ventricular peak ICa density following acclimation to 4°C from 17°C (Vornanen 1998). The active down-regulation of L-type Ca2+ channel density with cold acclimation is further demonstrated by the fact that peak ICa density of 5°C-acclimated cardiomyocytes remained less than that of 15°C-acclimated cardiomyocytes when measured at the common test temperature of 10°C.
The slowed kinetics of L-type Ca2+ channel inactivation (i.e., greater τ) at 5°C, which directly contrasts with the response of cold-acclimated rainbow trout, is also reflective of an active down-regulatory response. However, compensatory alterations of the inactivation properties of L-type Ca2+ channels with cold acclimation also play an important role in intracellular Ca2+ management. Namely, they serve to counteract against the large decrease of peak ICa density by increasing sarcolemmal Ca2+ influx. L-type Ca2+ channels inactivate due to Ca2+-dependent and voltage-dependent mechanisms (Morotti et al. 2012; Peterson et al. 1999). Accordingly, a decreased peak ICa density should correspond to a slower rate of channel inactivation because less Ca2+ is available to bind to the calmodulin-tethered channel complexes that trigger channel inactivation. Indeed, the greater attenuation of steady-state inactivation observed with acute cooling and chronic exposure to 5°C (i.e., as evidenced by the statistically significant differences between steady-state inactivation curves at membrane potentials greater than 20 mV; Fig. 6) is reflective of a reduction of Ca2+-induced inactivation of the L-type Ca2+ channels. Nevertheless, the fact that the delayed time course of L-type Ca2+ channel inactivation at 5°C persisted after acute warming to 10°C, when peak ICa density simultaneously more than doubled, indicates that acclimation to 5°C resulted in an attenuation of Ca2+-dependent inactivation, independent of changes in peak ICa density. One possible mechanism worthy of future investigation is a decreased sarcoplasmic reticulum (SR) Ca2+ load and/or decreased SR Ca2+ release after acclimation to 5°C (Cros et al. 2014). Likewise, cold acclimation induced a change in the coupling mechanism between the voltage sensor and inactivation gate of the L-type Ca2+ channel. The polarizing shift of Vh,inact that occurred with cold acclimation decreases the probability of the channel inactivation gate to close. In contrast, the polarizing shift of Vh,inact that occurred with acute cooling increases the probability of the channel inactivation gate to close.
Ultimately, the attenuation of Ca2+- and voltage-dependent inactivation of the L-type Ca2+ channel with cold acclimation, in combination with the unaltered activation kinetics, culminated in a pronounced window current at 5°C. The physiological significance of the window current is that at this range of voltages, which overlap with the plateau of the ventricular action potential, L-type Ca2+ channels can recover from inactivation and re-open, thereby providing a consistent influx of Ca2+ into the cardiomyocyte (Hirano et al. 1992). This mechanism can partially offset the extremely pronounced decrease of peak ICa density at 5°C. Indeed, the magnitude of reduction in the charge density of ICa and Δ[Ca2+] with acclimation to 5°C from 15°C (4.1-fold difference) was substantially less than that for peak ICa density (8.0-fold difference). In nature, a large window current at 5°C may allow sufficient influx of Ca2+ through L-type Ca2+ channels to meet the demands of the myofilaments.
Comparison of Alaska blackfish Cardiomyocyte Morphology and ICa to other Species
To the best of our knowledge, the present study is the first to examine the cardiac electrophysiology of an air-breathing teleost. A number of in vivo and in vitro studies have examined the effects of temperature and oxygen deprivation on the cardiovascular status and cardiac physiology of air-breathing fishes (Costa et al. 2005; Costa et al. 2009; Iversen et al. 2013; McKenzie et al. 2007), but none have investigated cardiac excitation-contraction coupling at the level of the isolated cardiomyocyte. Our measurements of cardiomyocyte morphology revealed that ventricular cardiomyocytes of Alaska blackfish are long and thin, much like those of other non-mammalian species (Galli et al. 2006; Galli et al. 2009; Vornanen et al. 2002). However, cell size, capacitance and surface area were generally 15-50% less than reported for other ectothermic species (Galli et al. 2006; Galli et al. 2009; Tiitu and Vornanen 2002; Vornanen 1997, 1998). Overall, the surface-to-volume ratio (1.03 μm−1) was less than reported for rainbow trout, burbot and crucian carp (1.15-1.2 μm−1; Tiitu and Vornanen 2002; Vornanen 1997, 1998).
The force and duration of cardiac contraction is determined by changes in the membrane potential (i.e., electrical activity) of the sarcolemmal membrane of a cardiomyocyte and the attendant transient rise in intracellular free Ca2+ concentration. For cardiomyocytes of ectothermic vertebrates, a large surface-to-volume ratio is thought to increase the efficiency of trans-sarcolemmal Ca2+ influx (Vornanen 1997). In this regard, the smaller surface-to-volume ratio of Alaska blackfish ventricular cardiomyocytes may equate to a lower efficiency of transsarcolemmal Ca2+ influx. However, the small size of the cardiomyocytes should result in smaller diffusion distances between the sarcolemmal membrane and the myofilaments. Moreover, the large peak ICa density of Alaska blackfish ventricular cardiomyocytes should offset the potential negative effects of a smaller surface-to-volume ratio on trans-sarcolemmal Ca2+ influx. At 15°C, peak ICa density was 1.2- to 2.8-times greater than peak ICa density reported for ventricular cardiomyocytes of most other ectothermic species examined to date (Table 5). Likewise, at 5°C, peak ICa density in Alaska blackfish ventricular cardiomyocytes was 1.4- to 2.8-times greater than observed for other species acclimated to and recorded at low temperature (Table 5). Only the European river lamprey (Lampetra fluviatilis), a cold-active species known to have a high metabolic rate at low temperature has a greater ventricular peak ICa density than the 5°C-acclimated Alaska blackfish (Hardisty et al. 2011).
Table 5.
Comparison of ventricular cell capacitance, peak ICa density, charge transfer (QCa), and the change in total cellular [Ca2+] (Δ[Ca2+]) between 15°C and 5°C-acclimated Alaska blackfish and select ectothermic species.
| Species | Acclimation Temperature (°C) | Cell Capacitance (pF) | Peak ICa Density (pA pF−1) | Normalized QCa (pC pF−1) | Δ[Ca2+] (μmol l−1) |
|---|---|---|---|---|---|
| Squalus acanthias | 22-25a | 55.1a | −10.6a | ND | ND |
| Danio rerio | 20-23b | 26.0b | −9.6b | 0.490c | 71.0c |
| Dallia pectoralis | 15 | 26.1 | −8.8 | 0.585 | 112.9 |
| Carassius carassius | 18d | 20.6d | −6.6d | 0.325e | 39.3e |
| Trachemys scripta | 21f | 50.2f | −5.7f | ND | ND |
| Scomber japonicas | 20g | 41.8g | −5.6g | ND | ND |
| Oncorynchus mykiss | 24e | 46.0e | −4.5e | 0.270e | 63.0e |
| Thunnus orientalis | 20g | 43.1g | −4.5g | ND | 45h |
| Trachemys scripta scripta | 20-21i | 42.4i | −3.2i | 0.270i | 64.1i |
| Varanus exanthematicus | 25j | 41.2j | −3.1j | ND | 64.0j |
| Acipenser baerii | 18k | 37.7k | −2.3k | ND | ND |
| Lampetra fluviatilis | 5l | ND | −3.0l | ND | ND |
| Dallia pectoralis | 5 | 26.2 | −1.1 | 0.142 | 27.4 |
| Carassius carassius | 4d | 21.3d | −1.1d | ND | ND |
| Lota lota | 4m | 23.7m | −0.8m | 0.250m | ND |
| Perca fluviatilis | 4-5l | ND | −0.8l | ND | ND |
| Trachemys scripta | 5f | 50.2f | −0.4f | ND | ND |
Species are arranged in descending order of peak ICa density within high (18-25°C) and low (4-5°C) acclimation temperatures. All peak ICa measurements are from patch-clamp experiments on isolated ventricular cardiomyocytes recorded at acclimation temperature (unless otherwise indicated below). Results obtained in the present study are bolded for emphasis. Results are means ± SEM. ND, Not determined. Species are: Squalus acanthias, spiny dogfish; Danio rerio, zebrafish; Dallia pectoralis, Alaska blackfish; Carassius carassius, crucian carp; Trachemys scripta, red-eared slider turtle; Scomber japonicas, Pacific mackerel; Oncorynchus mykiss, rainbow trout; Thunnus orientalis, Pacific bluefin tuna; Trachemys scripta scripta, yellow-bellied turtle; Varanus exanthematicus, Varanid lizard; Acipenser baerii, Siberian sturgeon; Lampetra fluviatilis, European lamprey; Lota lota, burbot; Perca fluviatilis, European perch.
Acclimation temperature 21°C (Zhang et al. 2011)
Carassius carassius: acclimation temperature 17°C; Both Carassius carassius and Oncorynchus mykiss recorded at 22°C (Vornanen 1998)
Acclimation temperature 14°C, recorded at 19°C (Shiels et al. 2014)
The charge density of ICa (QCa and normalized QCa) and the change of total cellular [Ca2+] (Δ[Ca2+]) at 15°C was also in general greater (1.2- to 2.6-times) than reported for other ectothermic species at high temperature (Table 5). In fact, a similar charge density has only been reported for 7°C rainbow trout atrial cardiomyocytes stimulated with 1μmol l−1 adrenaline and 21°C adult zebrafish ventricular cardiomyocytes (Shiels et al. 2003; Zhang et al. 2011). By contrast, at 5°C, normalized QCa and Δ[Ca2+] were approximately 45% less than reported for ventricular cardiomyocytes of the cold-acclimated burbot (Shiels et al. 2006).
Combined, the data suggest that trans-sarcolemmal Ca2+ influx via L-type Ca2+ channels is a predominant source of Ca2+ for excitation-contraction coupling in Alaska blackfish ventricular cardiomyocytes at both high and low acclimation temperatures. Indeed, the charge density of ICa for 15°C-acclimated Alaska blackfish ventricular cardiomyocytes is greater than that of newborn rat ventricular cardiomyocytes (0.43 pC pF−1), which have a poorly developed SR and rely primarily on sarcolemmal Ca2+ entry for excitation-contraction coupling (Vornanen 1996a). Nonetheless the comparatively low surface-to-volume ratio of Alaska blackfish ventricular cardiomyocytes compared to other teleost species suggests that SR Ca2+ release may also be physiologically important for excitation-contraction coupling. Future studies that assess the relative contributions of trans-sarcolemmal and SR Ca2+ to cardiac contraction at high and low acclimation temperatures and examine the ultrastructure of Alaska blackfish cardiomyocytes are required to resolve this possibility.
It is important to note that the methodologies employed to quantify ICa may have led to an overestimation of peak ICa density, charge density of ICa and Δ[Ca2+] compared to what occurs under natural physiological conditions. First, square pulse stimulation waveforms can overestimate current amplitude as compared to the use of physiological action potential waveforms (Shiels et al. 2000). In addition, the pipette solution contained 5 mmol l−1 of the Ca2+ chelator EGTA, which will serve to augment current amplitude (Bers and Perez-Reyes 1999). Nevertheless, similar stimulation protocols and pipette solutions were employed by the studies that assessed ICa in other fish and reptile species, making the interspecies comparisons valid.
Concluding Remarks and Perspectives
In combination, our findings are consistent with the apparent paradoxical demands and constraints placed upon overwintering Alaska blackfish in their natural environment. The results suggest that while the Alaska blackfish substantially down-regulates ICa with acclimation to low temperature, perhaps as an energy saving strategy to survive exposure to low oxygen levels via channel arrest (Lutz et al. 1986; Hochachka 1986), there may be sufficient compensation in the kinetics of L-type Ca2+ channel inactivation to support the level of cardiac performance required for the fish to remain active at low temperature when oxygen is available. Of course, other potential mechanisms may also contribute to modifying ventricular cardiomyocyte Ca2+ cycling at low temperature to allow cardiac performance to be matched to metabolic constraints and demands. L-type Ca2+ channels are phosphorylated and dephosphorylated by excitatory adrenergic and inhibitory cholinergic signaling, respectively (McDonald et al. 1994; Mery et al. 1997). Such control presents the possible opportunity for powerful and rapid control of cardiac contraction via modulation of ICa. In addition, Alaska blackfish could modulate action potential frequency (i.e., heart rate) and action potential duration to match metabolism. Cold-acclimated crucian carp exhibit action potential arrest (i.e., bradycardia), as well as prolonged action potential duration (Paajanen and Vornanen 2004). A slowed heart rate in 5°C-acclimated Alaska blackfish would conserve energy by reducing the frequency per unit time that Ca2+ channels would be recruited, whereas a prolonged action potential duration would serve to keep L-type Ca2+ channels open longer and further augment the large window current. Moreover, like the cold stenothermic burbot and river lamprey (Shiels et al. 2006; Vornanen and Haverinen 2013), Alaska blackfish could rely extensively on the sodium-calcium exchanger and/or SR Ca2+ stores to support cardiac contractility at low temperature. Future studies are required to examine each of these possibilities. Likewise, future investigation is required to elucidate the mechanism through which the coupling between the voltage sensor and L-type Ca2+ channel activity is modified for inactivation, but not activation.
Acknowledgements
This study was supported by Graduate Student Research Awards from LGL Limited Environmental Research Associates (to K.L.K.) and an Institutional Development Award (IDeA) from the US National Institute of General Medical Sciences of the National Institutes of Health (grant number P20GM103395 to J.A.W.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Dr. Matti Vornanen for providing assistance with data analysis calculations.
Reference List
- Aho E, Vornanen M. Contractile properties of atrial and ventricular myocardium of the heart of rainbow trout Oncorhynchus mykiss: Effects of thermal acclimation. J Exp Biol. 1999;202(19):2663–2677. doi: 10.1242/jeb.202.19.2663. [DOI] [PubMed] [Google Scholar]
- Bers DM, Perez-Reyes E. Ca channels in cardiac myocytes: Structure and function in Ca influx and intracellular Ca release. Cardiovasc Res. 1999;42:339–360. doi: 10.1016/s0008-6363(99)00038-3. [DOI] [PubMed] [Google Scholar]
- Brette F, Luxan G, Cros C, Dixey H, Wilson C, Shiels HA. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem Biophys Res Commun. 2008;374(1):143–146. doi: 10.1016/j.bbrc.2008.06.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JN. Morphometric and flow indicator studies of the teleost heart. Can J Zool. 1975;53:691–698. doi: 10.1139/z75-084. [DOI] [PubMed] [Google Scholar]
- Campbell MA, Lopéz JA. Mitochondrial phylogeography of a Beringian relict: the endemic freshwater genus of blackfish Dallia (Esociformes). J Fish Biol. 2014;84(2):523–538. doi: 10.1111/jfb.12314. [DOI] [PubMed] [Google Scholar]
- Costa MJ, Olle CD, Rantin FT, Kalinin AL. Influence of temperature on calcium sensitivity in the ventricular myocardium of the South American lungfish: Effects of extracellular calcium and adrenaline. J Therm Biol. 2005;30:259–266. [Google Scholar]
- Costa MJ, Rantin FT, Kalinin AL. Differences in Ca2+-management between the ventricle of two species of neotropical teleosts: the jeju, Hoplerythrinus unitaeniatus (Spix & Agassiz, 1829), and the acara, Geophagus brasiliensis (Quoy & Gaimard, 1824). Neotrop Ichthyol. 2009;7(3):471–478. [Google Scholar]
- Cros C, Salle L, Warren DE, Shiels HA, Brette F. The calcium stored in the sarcoplasmic reticulum acts as a safety mechanism in rainbow trout heart. AJP: Regulatory, Integrative and Comparative Physiology. 2014;307:R1493–R1501. doi: 10.1152/ajpregu.00127.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli GLJ, Taylor EW, Shiels HA. Calcium flux in turtle ventricular myocytes. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1781–R1789. doi: 10.1152/ajpregu.00421.2006. [DOI] [PubMed] [Google Scholar]
- Galli GLJ, Warren DE, Shiels HA. Ca2+ cycling in cardiomyocytes from a high- performance reptile, the varanid lizard (Varanus exanthematicus). Am J Physiol Regul Integr Comp Physiol. 2009;297(15):R1636–R1644. doi: 10.1152/ajpregu.00381.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JB, Wegner NC. In: Respiratory Physiology of Vertebrates: Life With and Without Oxygen. Cambridge University Press; 2010. Breathing air in water and in air: the air-breathing fishes. pp. 174–221. [Google Scholar]
- Hardisty MW, Potter IC, Hilliard RW. Physiological adaptations of the living agnathans. Trans R Soc Edinb Earth Sci. 2011;80(3-4):241–254. [Google Scholar]
- Haverinen J, Egginton S, Vornanen M. Electrical Excitation of the Heart in a Basal Vertebrate, the European River Lamprey (Lampetra fluviatilis). Physiol Biochem Zool. 2014;87(6):817–828. doi: 10.1086/678954. [DOI] [PubMed] [Google Scholar]
- Haworth TE, Haverinen J, Shiels HA, Vornanen M. Electrical excitability of the heart in a Chondrostei fish, the Siberian sturgeon (Acipenser baerii). Am J Physiol Regul Integr Comp Physiol. 2014;307(9):R1157–R1166. doi: 10.1152/ajpregu.00253.2014. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Moscucci a, January CT. Direct measurement of L-type Ca2+ window current in heart cells. Circul Res. 1992;70(3):445–455. doi: 10.1161/01.res.70.3.445. [DOI] [PubMed] [Google Scholar]
- Hochachka PW. Defense strategies against hypoxia and hypothermia. Science. 1986;231(4735):234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L, Tort L. L-type Ca2+ current and excitation-contraction coupling in single atrial myocytes from rainbow trout. Am J Physiol Regul Integr Comp Physiol. 1998;275:R2061–R2069. doi: 10.1152/ajpregu.1998.275.6.R2061. [DOI] [PubMed] [Google Scholar]
- Iversen NK, Lauridsen H, Huong DTT, Van Cong N, Gesser H, Buchanan R, Bayley M, Pedersen M, Wang T. Cardiovascular anatomy and cardiac function in the air-breathing swamp eel (Monopterus albus). Comp Biochem Physiol, A: Mol Integr Physiol. 2013;164(1):171–180. doi: 10.1016/j.cbpa.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Klaiman JM, Fenna AJ, Shiels HA, Macri J, Gillis TE. Cardiac remodeling in fish: strategies to maintain heart function during temperature Change. PloS one. 2011;6(9):1–11. doi: 10.1371/journal.pone.0024464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre S, Damsgaard C, Pascale DR, Nilsson G, Stecyk JAW. Air breathing in the Arctic: influence of temperature, hypoxia, activity and restricted air access on respiratory physiology of the Alaska blackfish Dallia pectoralis. J Exp Biol. 2014;217(24):4387–4398. doi: 10.1242/jeb.105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PL, Rosenthal M, Sick TJ. Living without oxygen: turtle brain as a model of anaerobic metabolism. Mol Phys. 1986;8(3) [Google Scholar]
- MacFarlane SN, Sontheimer H. Electrophysiological changes that accompany reactive gliosis in vitro. J Neurosci. 1997;17(19):7316–7329. doi: 10.1523/JNEUROSCI.17-19-07316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen N, Vornanen M. Effect of season and temperature acclimation on the function of crucian carp (Carassius carassius) heart. J Exp Biol. 1992;167:203–220. [Google Scholar]
- Maylie J, Morad M. Evaluation of T- and L-type Ca2+ currents in shark ventricular myocytes. Am J Physiol Regul Integr Comp Physiol. 1995;269(5):H1695–H1703. doi: 10.1152/ajpheart.1995.269.5.H1695. [DOI] [PubMed] [Google Scholar]
- McKenzie DJ, Campbell Ha, Taylor EW, Micheli M, Rantin FT, Abe aS. The autonomic control and functional significance of the changes in heart rate associated with air breathing in the jeju, Hoplerythrinus unitaeniatus. J Exp Biol. 2007;210:4224–4232. doi: 10.1242/jeb.009266. [DOI] [PubMed] [Google Scholar]
- Morotti S, Grandi E, Summa A, Ginsburg KS, Bers DM. Theoretical study of L-type Ca(2+) current inactivation kinetics during action potential repolarization and early afterdepolarizations. J Physiol. 2012;590:4465–4481. doi: 10.1113/jphysiol.2012.231886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostdiek JL, Nardone RM. Studies on the Alaskan Blackfish Dallia pectoralis I. Habitat, Size and Stomach Analyses. Am Midl Nat. 1959;61(1):218–229. [Google Scholar]
- Paajanen V, Vornanen M. Regulation of action potential duration under acute heat stress by I(K,ATP) and I(K1) in fish cardiac myocytes. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R405–R415. doi: 10.1152/ajpregu.00500.2003. [DOI] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+ - dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- Scholander PF, Flagg W, Hock RJ, Irving L. Studies on the physiology of frozen plants and animals in the Arctic. J Cell Comp Physiol. 1953;42:1–56. doi: 10.1159/000054843. [DOI] [PubMed] [Google Scholar]
- Shiels HA, Blank JM, Farrell AP, Block BA. Electrophysiological properties of the L-type Ca(2+) current in cardiomyocytes from bluefin tuna and Pacific mackerel. Am J Physiol Regul Integr Comp Physiol. 2004;286:R659–R668. doi: 10.1152/ajpregu.00521.2003. [DOI] [PubMed] [Google Scholar]
- Shiels HA, Galli GLJ, Block BA. Cardiac function in an endothermic fish: Cellular mechanisms for overcoming acute thermal challenges during diving. Proc Biol Sci. 2014;282(1800):20141989–20141989. doi: 10.1098/rspb.2014.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels HA, Paajanen V, Vornanen M. Sarcolemmal ion currents and sarcoplasmic reticulum Ca2+ content in ventricular myocytes from the cold stenothermic fish, the burbot (Lota lota). J Exp Biol. 2006;209(16):3091–3100. doi: 10.1242/jeb.02321. [DOI] [PubMed] [Google Scholar]
- Shiels HA, Vornanen M, Farrell AP. Temperature-dependence of L-type Ca2+ channel current in atrial myocytes from rainbow trout. J Exp Biol. 2000;203(18):2771–2780. doi: 10.1242/jeb.203.18.2771. [DOI] [PubMed] [Google Scholar]
- Shiels HA, Vornanen M, Farrell AP. Acute Temperature Change Modulates the Response of ICa to Adrenergic Stimulation in Fish Cardiomyocytes. Physiol Biochem Zool. 2003;76(6):816–824. doi: 10.1086/378918. [DOI] [PubMed] [Google Scholar]
- Stecyk JAW, Galli GLJ, Shiels HA, Farrell AP. Cardiac survival in anoxia-tolerant vertebrates: An electrophysiological perspective. Comp Biochem Physiol, C: Toxicol Pharmacol. 2008;148(4):339–354. doi: 10.1016/j.cbpc.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Stecyk JAW, Paajanen V, Farrell AP, Vornanen M. Effect of temperature and prolonged anoxia exposure on electrophysiological properties of the turtle (Trachemys scripta) heart. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R421–437. doi: 10.1152/ajpregu.00096.2007. [DOI] [PubMed] [Google Scholar]
- Tiitu V, Vornanen M. Morphology and fine structure of the heart of the burbot, a cold stenothermal fish. J Fish Biol. 2002;61:106–121. [Google Scholar]
- Vornanen M. Contribution of sarcolemmal calcium current to total cellular calcium in postnatally developing rat heart. Cardiovasc Res. 1996a;32:400–410. doi: 10.1016/0008-6363(96)00083-1. [DOI] [PubMed] [Google Scholar]
- Vornanen M. Effect of extracellular calcium on the contractility of warm-and cold- acclimated crucian carp heart. Journal of Comparative Physiology B. 1996b;165(7):507–517. doi:10.1007/BF00387511. [Google Scholar]
- Vornanen M. Sarcolemmal Ca influx through L-type Ca channels in ventricular myocytes of a teleost fish. Am J Physiol. 1997;272(5):R1432–1440. doi: 10.1152/ajpregu.1997.272.5.R1432. [DOI] [PubMed] [Google Scholar]
- Vornanen M. L-type Ca2+ current in fish cardiac myocytes: effects of thermal acclimation and beta-adrenergic stimulation. J Exp Biol. 1998;201(4):533–547. doi: 10.1242/jeb.201.4.533. [DOI] [PubMed] [Google Scholar]
- Vornanen M, Haverinen J. A significant role of sarcoplasmic reticulum in cardiac contraction of a basal vertebrate, the river lamprey (Lampetra fluviatilis). Acta Physiol. 2013;207(2):269–279. doi: 10.1111/j.1748-1716.2012.02479.x. [DOI] [PubMed] [Google Scholar]
- Vornanen M, Paajanen V. Seasonality of dihydropyridine receptor binding in the heart of an anoxia-tolerant vertebrate, the crucian carp (Carassius carassius L.). Am J Physiol Regul Integr Comp Physiol. 2004;287:R1263–R1269. doi: 10.1152/ajpregu.00317.2004. [DOI] [PubMed] [Google Scholar]
- Vornanen M, Shiels HA, Farrell AP. Plasticity of excitation–contraction coupling in fish cardiac myocytes. Comp Biochem Physiol, A: Mol Integr Physiol. 2002;132(4):827–846. doi: 10.1016/s1095-6433(02)00051-x. [DOI] [PubMed] [Google Scholar]
- Wilber CG, Robinson PF, Hunn JB. Heart size and body size in fish. Anat Rec. 1961;140:285–287. doi: 10.1002/ar.1091400402. [DOI] [PubMed] [Google Scholar]
- Young S, Egginton S. Temperature acclimation of gross cardiovascular morphology in common carp (Cyprinus carpio). J Therm Biol. 2011;36(7):475–477. [Google Scholar]
- Zhang P-C, Llach A, Sheng XY, Hove-Madsen L, Tibbits GF. Calcium handling in zebrafish ventricular myocytes. Am J Physiol Regul Integr Comp Physiol. 2011;300(1):R56–66. doi: 10.1152/ajpregu.00377.2010. [DOI] [PubMed] [Google Scholar]