ABSTRACT
Cutaneous wound repair in adult mammals typically does not regenerate original dermal architecture. Skin that has undergone repair following injury is not identical to intact uninjured skin. This disparity may be caused by differences in the mechanisms that regulate postnatal cutaneous wound repair compared to embryonic skin development and thus we seek a deeper understanding of the role that Wnt signaling plays in the mechanisms of skin repair in both fetal and adult wounds. The influence of secreted Wnt signaling proteins in tissue homeostasis has galvanized efforts to identify small molecules that target Wnt-mediated cellular responses. Wnt signaling is activated by wounding and participates in every subsequent stage of the healing process from the control of inflammation and programmed cell death, to the mobilization of stem cell reservoirs within the wound site. Endogenous Wnt signaling augmentation represents an attractive option to aid in the restoration of cutaneous wounds, as the complex mechanisms of the Wnt pathway have been increasingly investigated over the years. In this review, we summarize recent data elucidating the roles that Wnt signaling plays in cutaneous wound healing process.
Keywords: ß-catenin, regeneration, repair, stem cells, skin, Wnt
Introduction
Regeneration is the process of restoration, renewal, and growth that fosters the ability for genomes, cells, and organs to be resilient to the natural fluctuations and events that cause damage. It is important to distinguish between repair, healing via formation of scar tissue, and regeneration, which is restoration to the pre-injury state. Full-thickness skin loss in adult mammals typically results in a reparative rather than regenerative response, leading to the formation of scar tissue.1 Deposition of a collagen-rich matrix in the neo-dermis makes it prone to contracture, decreases elasticity and tensile strength, and promotes hypertrophic scar formation.2 Epithelialization without the development of an epidermal appendage over a large surface area leads to alopecia and thermal imbalance.3
Processes involved in the healing of a skin wound parallel embryonic skin development in many ways. Both processes involve the differentiation, migration, proliferation, and apoptosis of various cell types to create the multilayered tissue that constitutes the skin. Many of the same key signaling pathways that are activated during embryonic skin development are also activated during postnatal wound healing; e.g. Wnt/ß-catenin, Notch, and Hedgehog pathways.4
Maintenance of epidermal homeostasis is achieved by separate populations of stem cells in the skin which includes stem cells from the bulb region of the hair follicles, the interfollicular epidermis, and the sebaceous glands.5 While both epidermal and bulb stem cells have demonstrated the potential to regenerate epidermis,6,7 an effective cell-based approach utilizing these population to promote “scarless” wound healing remains elusive. There is increasing evidence that Wnt proteins are necessary for normal skin development.8,9,10 Recent data demonstrate that the epidermis of wounded adult mice can regenerate new hair follicles during healing and that the origins of cells that develop new hair follicles are Wnt-responsive interfollicular stem cells, not stem cells from the existing hair follicle bulb.11
In the following sections we present a summation of data which provides strong evidence that augmenting the endogenous Wnt pathway improves cutaneous regeneration after injury through activation of tissue-resident stem cells.4,12-14
The three different Wnt signaling pathways
The Wnt signaling pathway is an evolutionarily conserved pathway that regulates crucial aspects of cell fate determination, cell polarity, cell migration, neural patterning and organogenesis during embryonic development. Intracellular Wnt signaling diversifies into 3 main branches: 1. the β-catenin pathway (canonical Wnt pathway), which activates target genes in the nucleus; 2. the planar cell polarity (PCP) pathway, which involves jun N-terminal kinase (JNK) and cytoskeletal rearrangements; and 3. the Wnt/Ca2+ pathway. In humans, there are currently 19 different known Wnt proteins and 10 different Frizzled receptors. This review will focus on canonical/ß-catenin dependent Wnt signaling, which has been implicated in tissue regeneration and repair.
The hallmark of canonical Wnt signaling is the accumulation and translocation of the adherens junction-associated protein, β-catenin, into the nucleus.15 Without Wnt signaling, cytoplasmic β-catenin is degraded by a β-catenin destruction complex, which includes Axin, adenomatosis polyposis coli (APC), glycogen synthase kinase 3 (GSK3), protein phosphatase 2A (PP2A), and casein kinase 1α (CK1α). Phosphorylation of β-catenin within this complex by Casein Kinase and GSK3 targets it for ubiquitination and subsequent proteolytic destruction by the proteosomal complex. Binding of Wnt to its receptor complex composed of the Fz (frizzled) and the LRP5/6 triggers a series of events that disrupt the APC/Axin/GSK3 complex that is required for the targeted destruction of β-catenin. The binding of Wnt to the Fz/LRP5/6 complex induces the membrane translocation of a key negative regulator of signaling. Binding of Axin has been proposed as the mechanism for reduction of the inhibitory activity of Axin on canonical Wnt signaling.53 This leads to activation of the phosphoprotein Dishevelled (Dsh), which inhibits the activity of the GSK3 enzyme, preventing the degradation of β-catenin, allowing consequent stabilization and accumulation in the cytoplasm. Stabilized β-catenin translocates into the nucleus, exerting its effect on gene transcription by functioning as a transcriptional co-activator. A large number of binding partners for β-catenin in the nucleus has been uncovered and perhaps the best characterized are the members of the LEF/TCF DNA-binding transcription factors. This complex binds to the promoter of target genes (e.g., Cyclin D1, MT1-MMP (membrane-type 1-matrix metalloproteinase), MMP-7 or Dkk-1 (Dickkopf) and these genes are required for organizer formation during embryogenesis.16 (Fig. 1).
Figure 1.
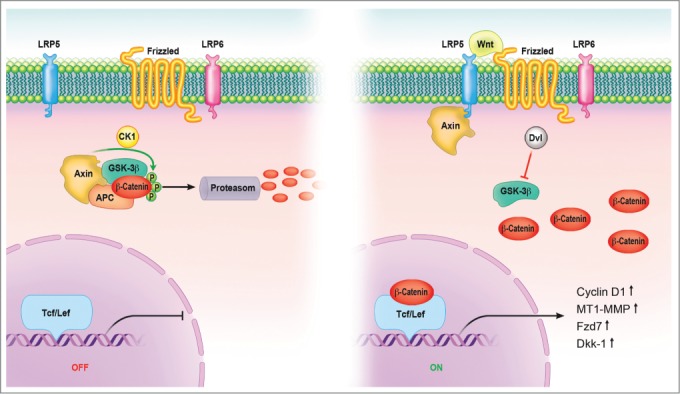
Canonical Wnt signaling pathway. In the absence of signal, action of the destruction complex (CKIα, GSK-3β, APC, Axin) creates a hyperphosphorylated β-catenin, which is a target for ubiqitination and degradation by the proteosome. Binding of Wnt ligand to a Frizzled/LRP-5/6 receptor complex leads to stabilization of hypophosphorylated β-catenin, which interacts with TCF/LEF proteins in the nucleus to activate transcription. In a canonical pathway, CKIα, GSK-3β, APC, and Axin act as negative regulators and all other components act positively. Abbreviations: APC = adenomatous polyposis coli, CK = casein kinase, GSK = glycogen synthase kinase, Fzd = Frizzled-Rezeptor, LRP = low-density-lipoprotein receptor related protein, Tcf/Lef = T cell-specific transcription factor/lymphoid enhancer-binding factor.
The role of Wnt signaling in tissue regeneration/repair
The Wnt pathway regulates cell proliferation in the adult epidermis, which directly impacts the rate and extent of skin wound healing. Using the Axin2-Cre lineage reporter mice, one study was able to show that the majority of the basal epidermal layer requires Wnt/β-catenin signaling to proliferate and these same cells contribute robustly to wound healing, with no requirement for a quiescent stem cell subpopulation.13 Wnt proteins also serve as niche signals for at least 2 types of skin stem cells that contribute to skin wound healing; those in the bulge region of the hair follicle,(17) and those in the basal layer of the interfollicular epidermis.18 Several lines of other evidence indirectly supports a role for Wnt signaling in cutaneous repair.12,19,20
The concept that wound healing recapitulates embryonic development is illustrated by the interesting finding that the source of new follicles is not cells within the hair follicle stem cell niche.21 Recent studies showed that fibroblast growth factor (FGF) signaling plays multiple inductive roles during development of vertebrates.22 FGF-9 modulates hair follicle regeneration after skin injury in adult mice and FGF-9 triggers Wnt expression with subsequent Wnt activation in wound fibroblasts.23 Through a unique feedback mechanism, activated fibroblasts then express FGF-9, thus amplifying Wnt activity throughout the wound dermis during a crucial phase of skin regeneration (Fig. 2). Skin wounds express various Wnt proteins during the early phases of healing, with transcripts of Wnts 1, 3, 4, 5a, and 10b being present in murine full-thickness cutaneous wounds up to 7 d after injury.24 In the epithelium, Wnt 10b protein can be detected in migrating epithelial cells up to 3 d after wounding, while Wnt 4, 5a, and 10b localize to hair follicles.25 Wnt 2a and 4 are expressed in the dermis, although reports vary with respect to the time-course of their expression (range: 30 h – 7 d after wounding). It appears that Wnt signaling, through its ability to activate stem cells with induction of their self-renewal and proliferation, serves as a positive stimulus for wound repair.
Figure 2.
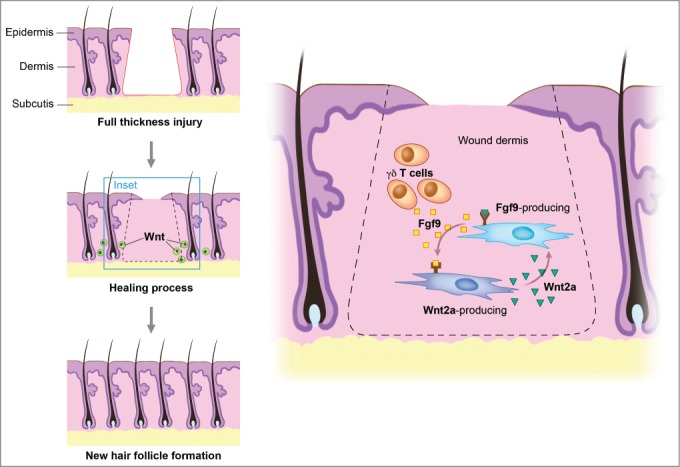
Wnt signaling maintains the hair-inducing activity in skin repair. Fibroblast growth factor (Fgf) 9 is a secreted signaling molecule that is expressed in epithelium. Mesenchymal Fgf signaling interacts with β-catenin-mediated Wnt signaling in a feed-forward loop that functions to sustain mesenchymal Fgf responsiveness and mesenchymal Wnt/β-catenin signaling. Wnt2a is a canonical Wnt ligand that activates mesenchymal Wnt/β-catenin signaling, whereas Fgf9 is the only known ligand that signals to mesenchymal Fgf receptors (FGFRs). Mesothelial Fgf9 and mesenchymal Wnt2a are principally responsible for maintaining mesenchymal Fgf-Wnt/β-catenin signaling, whereas epithelial Fgf9 primarily affects epithelial branching. In summary, Fgf signaling is primarily responsible for regulating mesenchymal proliferation, whereas β-catenin signaling is a required permissive factor for mesenchymal Fgf signaling. Abbreviations: Fgf = Fibroblast growth factor.
Stages of Skin Regeneration
Hemostasis and inflammation are dependent on Wnt signaling
Wound healing is classically described as a process involving 3 overlapping phases (Fig. 3). The resolution of injury begins with hemostasis. Vasoconstriction and clot formation lead to cessation of bleeding. Hemostasis is achieved through the activation of platelets and the coagulation cascade.26 Recent data suggests that Wnt signaling is essential for development of megakaryocytes and for stimulating proplatelet function in vitro.27 Interestingly, canonical Wnt has been shown to inhibit platelet aggregation.28 whereas non-Canonical Wnt-5A, stimulates platelet aggregation.29
Figure 3.
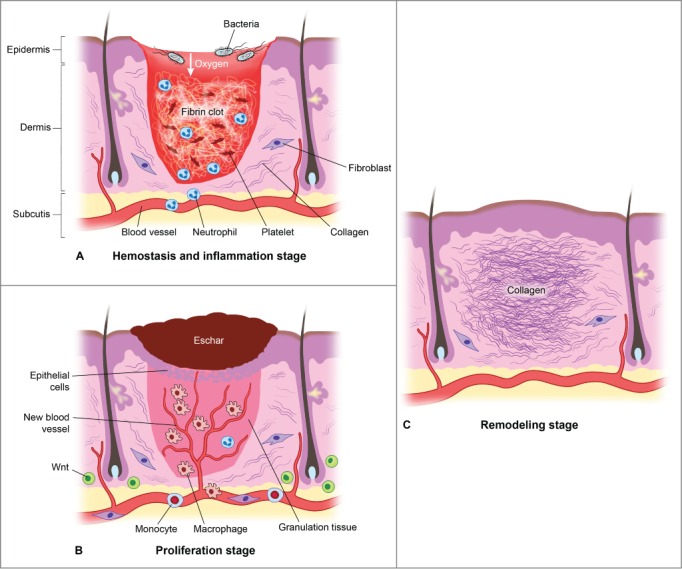
There are 3 classic stages of wound repair: inflammation (a), proliferation (b) and remodeling (c). (a) Inflammation. This stage lasts until about 48 h after injury and depicted is a skin wound at about 24–48 h after injury. The wound is characterized by a hypoxic (ischemic) environment in which a fibrin clot has formed and platelets aggregate. Platelets adhere to the injured endothelium and release chemokines, thereby attracting the cellular components of the inflammatory stage. The inflammatory stage of wound healing is characterized by the presence of neutrophils, macrophages, lymphocytes and local Wnt signaling begins to increase. The inflammatory cells then serve to release proinflammatory cytokines, growth factors and vascular endothelial growth factor, ingest foreign materials, increase vascular permeability, and promote fibroblast activity. (b) Proliferation. This stage occurs about 2–10 d after injury and depicted is a skin wound at about 5–10 d after injury. This stage includes an increased local Wnt response, capillary growth and granulation tissue formation occur and an eschar has formed on the surface of the wound. (c) Remodeling. This stage lasts for a year or longer and depicted is a skin wound about 1–12 months after repair. The final stage of wound healing is a long process of tissue remodeling and increasing wound strength. During this stage, type I collagen synthesis and turnover continues, and fibroblasts differentiate into myofibroblasts, allowing further wound contraction.
Following hemostasis is the start of the inflammatory phase which is characterized by the presence of erythema (rubor), warmth (calor), edema (tumor), and pain (dolor).30 At a cellular level, inflammation involves blood vessel dilation, increased vascular permeability, and leukocyte recruitment to the site of injury. Two leukocyte populations sequentially dominate the inflammatory events of wound healing: neutrophils and macrophages.31 Both provide the critical function of wound debridement, whereas the latter population is critical in orchestrating the subsequent steps in wound healing. More recently, Wnt signaling has been shown to be involved in the regulation of inflammatory processes. Wnt5a is induced in human macrophages in response to mycobacteria and conserved bacterial structures, contributing to the regulation of pro-inflammatory cytokines via its receptor Frizzled (Fzd) 5.32 Wnt5a is also induced in other infectious and inflammatory diseases such as tuberculosis, sepsis, psoriasis, rheumatoid arthritis and atherosclerosis.32 ß-Catenin-dependent Wnt signaling, therefore, robustly enhances the inflammatory response.33
Proliferation
Wound debridement is critical in enhancing the inflammatory process by reducing wound bacterial counts and removing necrotic tissue which impedes the healing process.34 Once debrided, the wound enters the proliferative phase which takes place around post-injury days 4 through 12. During this time period, fibroblasts, smooth muscle cells, and endothelial cells infiltrate the wound as epithelial cells begin to cover the site of injury.35 In concert, these cells reestablish tissue continuity through matrix deposition, angiogenesis, and epithelialization. ß-Catenin is an important regulator of fibroblast behavior during the proliferative phase of dermal wound repair.36 ß-Catenin protein levels and transcriptional activity are elevated in dermal fibroblasts during the proliferative phase of healing in murine cutaneous wounds and return to baseline during the remodeling phase. Human wounds similarly show increased expression of ß-catenin and its target genes, such as fibronectin and MMP7, during the proliferative phase.37 While increased ß-catenin activity during the proliferative phase is crucial for successful wound repair, prolonged or aberrant ß-catenin activity beyond the normal parameters of healing contributes to excessive fibrosis and scar formation. Indeed, human hypertrophic scars and keloids exhibit elevated ß-catenin levels.38 Interestingly, while Wnt ligands may participate in stimulating dermal ß-catenin during wound repair, Wnt signaling is not crucial for maintaining elevated ß-catenin levels during the proliferative phase of cutaneous healing. This has been demonstrated in mice treated with an adenovirus expressing the Wnt signaling inhibitor Dickkopf (DKK1, which binds LRP6/Arrow), without a significant decline in ß-catenin protein levels during the proliferative phase of skin wound healing, in contrast to the situation in bone repair.39 This suggests that other factors play a role in regulating ß-catenin levels during the proliferative phase of healing. Indeed, ß-catenin levels in fibroblasts can be stimulated by growth factors, such as TGF-ß1, which are released during the early stages of wound repair. Furthermore, ß-catenin activity in dermal fibroblasts is regulated by extracellular matrix (ECM) components, such as fibronectin, which activates ß-catenin through a GSK3ß-dependent, ß1 integrin mediated pathway.40 Hypertrophic scars and keloids represent a dysregulated response to cutaneous wounding, resulting in an excessive deposition of ECM, especially collagen. TGF-β is believed to be responsible for excessive ECM deposition in hypertrophic scars, keloids and other fibrotic conditions.41 Since β-catenin is known to accumulate during fibroproliferation,42 we speculate that it could play a role in the mechanisms that lead to hypertrophic/keloid scarring. ß-Catenin and Wnt signaling are intrinsically involved in the formation of the dermis and of epidermal structures, both during wound repair and during skin development. It will be interesting to elucidate whether non-Wnt activators of ß-catenin, such as ECM proteins and growth factors, modulate ß-catenin during skin development as they do during the response to injury.
Wound remodeling
In the skin epithelium, remodeling consists of deposition of matrix and subsequent changes in its organization and composition over time. This final phase of wound healing occurs throughout the entire wound repair process and continues for up to 1 y after injury.43 Fibrin clot formed in the early inflammatory phase is replaced by granulation tissue that is rich in type III collagen and blood vessels during the proliferative phase and subsequently replaced by a collagenous scar predominantly of type I collagen.44 Wnt is responsible for the differentiation of myofibroblasts of mesenchymal stem cell;45 myofibroblasts cause wound contracture, decreasing the surface of the developing scar.46 Wnt has also been shown as critical in the process of angiogenesis and endogenous enhancement of Wnt can correct vascular defects.47 As angiogenic processes diminish, wound blood flow declines, and acute wound metabolic activity slows, eventually stopping.
Differences between Fetal Wound Healing and Adult Wound Healing
Although it has been almost 3 decades since the discovery of scarless fetal healing, the key to scarless repair remains elusive.48 Investigators have now begun applying more comprehensive transcriptomic techniques to the study of scarless wound healing. In particular, there has been a focus on the time during fetal gestation when regenerative healing changes to adult wound healing with scar formation in order to understand the phenomena occurring immediately before and after this transition. In rats, wounds made on the 16th day of gestation (gestation period: 21 days) histologically regenerate, but wounds made on the 18th day of gestation are associated with scarring.49,50 In wounds made during the regenerative phase, the skin, including appendages and panniculus carnosus muscle, is completely regenerated, whereas the scarring reparative response does not include regeneration of the hair bulb and skin texture.51 The major objective of skin wounding research is restoration of the extracellular matrix architecture, and a subsequent return of strength and function to the injured skin. It therefore must overcome the fibrotic nature of post-natal wound healing. It is clear from studies conducted in mammals that normal skin development absolutely depends on a tight regulation of the activities of secreted signaling molecules that display potent organizing properties in the embryo.52 These signaling molecules include members of the Hedgehog (Hh), transforming growth factor-β (TGF-β), and Wnt families of secreted factors (Table 1). Based on the studies mentioned above, it is clear that fetal wound healing eventually changes over to adult wound healing. Here, we focus on a particular mode of regulation of the activity of Wnt proteins to aid in the regeneration of skin architecture after wounding.
Table 1.
Summary of developmental signaling pathways in mammalian skin development and repair
| Signaling pathway | Skin section | Skin development | Skin repair |
|---|---|---|---|
| Wnt | Wnt | Development and morphogenesis of hair follicles | Regeneration of hair follicles in large wounds |
| Dermis | Development of the dermis | Reconstitution of the dermis: fibroblast numbers and behavior, matrix production | |
| Sonic hedgehog | Epidermis | Development and morphogenesis of hair follicles | Present in regenerated hair follicles |
| Dermis | Role previously unknown | Involved in dermal reconstitution: effects on matrix, cellularity and vascularity | |
| TGF-β | Epidermis | No significant role in hair follicle development | Inhibitory role in re-epithelialization |
| Dermis | Role previously unknown Expressed in developing dermis | Reconstitution of the dermis: fibroblast proliferation and behavior, myofibroblast formation, matrix production, wound contraction | |
| Notch | Epidermis | Epidermal differentiation | Role previously unknown |
| Dermis | Role previously unknown | Involved in dermal reconstitution:effects on macrophage behavior,angiogenesis |
Conclusions and Clinical Aspects of Skin Regeneration/Repair
Our understanding of skin wound healing mechanisms has progressed considerably in recent years. The wound epithelium in adult mammals is just as capable of responding to morphogenic signals from the dermis as it does in the embryo during hair placode formation. Adult stem cells have tremendous therapeutic potential, and the skin epithelium represents an enormous source of accessible stem cells that might be a starting point for generating cells to replace diseased tissue. Skin stem cells have already been used to replace skin lost to burns; whether it will be possible to use skin stem cell plasticity to engineer treatments for other disorders remains to be determined. Although increasing evidence supports a role for Wnt signaling in skin epithelial stem cell maintenance and/or determination, deregulated Wnt signaling activation has long been implicated in human cancers. Wnt signaling is essential at multiple steps during the complex organogenesis of the skin and its appendages. It is required to induce the formation of the dorsal dermis and regulates the size of the different skin appendage tracts. Later, Wnt signaling is required for the very early stages of skin appendage formation. Skin appendage distribution and pigmentation are regulated, in part by Wnt signaling. Disruption of the pathway can lead to the formation of skin appendage tumors. Any strategy that attempts to target the Wnt pathway to augment tissue regeneration will have to take into consideration the need to selectively and locally activate signaling in the tissue or area of interest, while simultaneously restricting Wnt signaling in other parts of the body. The intricate and dynamic nature of the wound environment suggests that successful therapies for treating wound healing disorders will not rely upon a single all-encompassing agent, but will likely require a multitude of factors for a finely tuned attenuation of endogenous Wnt signaling during the wound healing process. Identifying the relationships between developmental signaling pathways in adult wound repair and fetal skin development and/or regeneration will certainly propel the research community closer to this goal, and is a fruitful area of future investigation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Funding for this research have been provided by the Hagey Family Endowed Found in Stem Cell Research and Regenerative Medicine, The Oak Foundation, and the National Library of Medicine (#LM0077033 to MJ).
REFERENCES
- 1.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci September 15 2009; 122(Pt 18):3209-13; PMID:19726630; http://dx.doi.org/ 10.1242/jcs.031187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song C. Hypertrophic scars and keloids in surgery: current concepts. Ann Plast Surg September 2014; 73 Suppl 1:S108-118; PMID:25115371 [DOI] [PubMed] [Google Scholar]
- 3.Martin P. Wound healing–aiming for perfect skin regeneration. Sci April 4 1997; 276(5309):75-81; PMID:9082989; http://dx.doi.org/ 10.1126/science.276.5309.75 [DOI] [PubMed] [Google Scholar]
- 4.Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci June 2013; 70(12):2059-81; PMID:23052205; http://dx.doi.org/ 10.1007/s00018-012-1152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lough D, Dai H, Yang M, Reichensperger J, Cox L, Harrison C, Neumeister MW. Stimulation of the follicular bulge LGR5+ and LGR6+ stem cells with the gut-derived human α defensin 5 results in decreased bacterial presence, enhanced wound healing, and hair growth from tissues devoid of adnexal structures. Plast Reconst Surg November 2013; 132(5):1159-71; PMID:24165598; http://dx.doi.org/ 10.1097/PRS.0b013e3182a48af6 [DOI] [PubMed] [Google Scholar]
- 6.Morasso MI, Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell March 2005; 97(3):173-83; PMID:15715523; http://dx.doi.org/ 10.1042/BC20040098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med December 2005; 11(12):1351-4; PMID:16288281; http://dx.doi.org/ 10.1038/nm1328 [DOI] [PubMed] [Google Scholar]
- 8.Fuchs E. Scratching the surface of skin development. Nat February 22 2007; 445(7130):834-42; PMID:17314969; http://dx.doi.org/ 10.1038/nature05659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, Niswander L, Conlon RA. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol August 1 2006; 296(1):164-76; http://dx.doi.org/ 10.1016/j.ydbio.2006.04.449 [DOI] [PubMed] [Google Scholar]
- 10.Lim X, Nusse R. Wnt signaling in skin development, homeostasis, and disease. Cold Spring Harb Perspect Biol February 2013; 5(2):a008029; http://dx.doi.org/ 10.1101/cshperspect.a008029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature May 17 2007; 447(7142):316-20; PMID:17507982; http://dx.doi.org/ 10.1038/nature05766 [DOI] [PubMed] [Google Scholar]
- 12.Fathke C, Wilson L, Shah K, Kim B, Hocking A, Moon R, Isik F. Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Biol 2006; 7:4; PMID:16426441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, van Amerongen R, Klein AM, Nusse R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science December 6 2013; 342(6163):1226-30; PMID:24311688; http://dx.doi.org/ 10.1126/science.1239730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whyte JL, Smith AA, Liu B, Manzano WR, Evans ND, Dhamdhere GR, Fang MY, Chang HY, Oro AE, Helms JA. Augmenting endogenous Wnt signaling improves skin wound healing. PLoS One 2013; 8(10):e76883; PMID:24204695; http://dx.doi.org/ 10.1371/journal.pone.0076883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis April 2008; 4(2):68-75; PMID:19279717; http://dx.doi.org/ 10.4161/org.4.2.5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schambony A, Kunz M, Gradl D. Cross-regulation of Wnt signaling and cell adhesion. Differentiation September 2004; 72(7):307-18; http://dx.doi.org/ 10.1111/j.1432-0436.2004.07207002.x [DOI] [PubMed] [Google Scholar]
- 17.Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Investigative Dermatol January 2013; 133(1):31-41; http://dx.doi.org/ 10.1038/jid.2012.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev May 15 2003; 17(10):1189-200; PMID:12756224; http://dx.doi.org/ 10.1101/gad.1086903 [DOI] [PubMed] [Google Scholar]
- 19.Lam AP, Gottardi CJ. β-catenin signaling: a novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol November 2011; 23(6):562-7; http://dx.doi.org/ 10.1097/BOR.0b013e32834b3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carre AL, James AW, MacLeod L, Kong W, Kawai K, Longaker MT, Lorenz HP. Interaction of wingless protein (Wnt), transforming growth factor-beta1, and hyaluronan production in fetal and postnatal fibroblasts. Plast Reconstr Surg January 2010; 125(1):74-88; PMID:20048602; http://dx.doi.org/ 10.1097/PRS.0b013e3181c495d1 [DOI] [PubMed] [Google Scholar]
- 21.Stenn KS, Cotsarelis G. Bioengineering the hair follicle: fringe benefits of stem cell technology. Curr Opin Biotechnol October 2005; 16(5):493-7; http://dx.doi.org/ 10.1016/j.copbio.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 22.Dorey K, Amaya E. FGF signalling: diverse roles during early vertebrate embryogenesis. Development November 2010; 137(22):3731-42; PMID:20978071; http://dx.doi.org/ 10.1242/dev.037689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, Ito M, Yang Z, Treffeisen E, Kim CD, et al.. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nat Med July 2013; 19(7):916-23; PMID:23727932; http://dx.doi.org/ 10.1038/nm.3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R, Whetstone H, Guha A, Alman BA. Beta-catenin regulates wound size and mediates the effect of TGF-β in cutaneous healing. FASEB J April 2006; 20(6):692-701; PMID:16581977; http://dx.doi.org/ 10.1096/fj.05-4759com [DOI] [PubMed] [Google Scholar]
- 25.Widelitz RB. Wnt signaling in skin organogenesis. Organogenesis April 2008; 4(2):123-33; PMID:19279724; http://dx.doi.org/ 10.4161/org.4.2.5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Browder T, Folkman J, Pirie-Shepherd S. The hemostatic system as a regulator of angiogenesis. J Biol Chemistry January 21 2000; 275(3):1521-4; http://dx.doi.org/ 10.1074/jbc.275.3.1521 [DOI] [PubMed] [Google Scholar]
- 27.Macaulay IC, Thon JN, Tijssen MR, Steele BM, MacDonald BT, Meade G, Burns P, Rendon A, Salunkhe V, Murphy RP, et al.. Canonical Wnt signaling in megakaryocytes regulates proplatelet formation. Blood January 3 2013; 121(1):188-96; PMID:23160460; http://dx.doi.org/ 10.1182/blood-2012-03-416875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steele BM, Harper MT, Macaulay IC, Morrell CN, Perez-Tamayo A, Foy M, Habas R, Poole AW, Fitzgerald DJ, Maguire PB. Canonical Wnt signaling negatively regulates platelet function. Proc Natl Acad Sci U S A November 24 2009; 106(47):19836-41; PMID:19901330; http://dx.doi.org/ 10.1073/pnas.0906268106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SY, Kim S, Yun-Choi HS, Jho EH. Wnt5a potentiates U46619-induced platelet aggregation via the PI3K/Akt pathway. Mol Cells October 2011; 32(4):333-6; PMID:21870110; http://dx.doi.org/ 10.1007/s10059-011-0134-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alessandri AL, Sousa LP, Lucas CD, Rossi AG, Pinho V, Teixeira MM. Resolution of inflammation: mechanisms and opportunity for drug development. Pharmacol Ther August 2013; 139(2):189-212; PMID:23583354; http://dx.doi.org/ 10.1016/j.pharmthera.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 31.Greaves NS, Ashcroft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J Dermatol Sci December 2013; 72(3):206-17 [DOI] [PubMed] [Google Scholar]
- 32.Schaale K, Neumann J, Schneider D, Ehlers S, Reiling N. Wnt signaling in macrophages: augmenting and inhibiting mycobacteria-induced inflammatory responses. European J Cell Biol Jun-Jul 2011; 90(6-7):553-9 [DOI] [PubMed] [Google Scholar]
- 33.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol August 2008; 8(8):581-93; PMID:18617885 [DOI] [PubMed] [Google Scholar]
- 34.Nazarko L. Advances in wound debridement techniques. British J Community Nursing June 2015; 20 Suppl 6:S6-8 [DOI] [PubMed] [Google Scholar]
- 35.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clinics Dermatol Jan-Feb 2007; 25(1):9-18; PMID:17276196 [DOI] [PubMed] [Google Scholar]
- 36.Poon R, Nik SA, Ahn J, Slade L, Alman BA. Beta-catenin and transforming growth factor β have distinct roles regulating fibroblast cell motility and the induction of collagen lattice contraction. BMC Cell Biol 2009; 10:38; PMID:19432963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheon S, Poon R, Yu C, Khoury M, Shenker R, Fish J, Alman BA. Prolonged β-catenin stabilization and tcf-dependent transcriptional activation in hyperplastic cutaneous wounds. Lab Invest March 2005; 85(3):416-25 [DOI] [PubMed] [Google Scholar]
- 38.Carothers AM, Rizvi H, Hasson RM, Heit YI, Davids JS, Bertagnolli MM, Cho NL. Mesenchymal stromal cell mutations and wound healing contribute to the etiology of desmoid tumors. Cancer Res January 1 2012; 72(1):346-55; PMID:22094874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bielefeld KA, Amini-Nik S, Whetstone H, Poon R, Youn A, Wang J, Alman BA. Fibronectin and β-catenin act in a regulatory loop in dermal fibroblasts to modulate cutaneous healing. J Biol Chem August 5 2011; 286(31):27687-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowley E, O'Gorman DB, Gan BS. Beta-catenin signaling in fibroproliferative disease. J Surgical Research March 2007; 138(1):141-50 [DOI] [PubMed] [Google Scholar]
- 41.Singer AJ, Clark RA. Cutaneous wound healing. New England J Medicine September 2 1999; 341(10):738-46. [DOI] [PubMed] [Google Scholar]
- 42.Cheon SS, Nadesan P, Poon R, Alman BA. Growth factors regulate β-catenin-mediated TCF-dependent transcriptional activation in fibroblasts during the proliferative phase of wound healing. Exp Cell Res February 15 2004; 293(2):267-74; PMID:14729464 [DOI] [PubMed] [Google Scholar]
- 43.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature May 15 2008; 453(7193):314-21; PMID:18480812 [DOI] [PubMed] [Google Scholar]
- 44.Yates CC, Bodnar R, Wells A. Matrix control of scarring. Cell Mol Life Sci June 2011; 68(11):1871-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Z, Wang C, Shi C, Xu X, Qian W, Nie S, Han X. Activated Wnt signaling induces myofibroblast differentiation of mesenchymal stem cells, contributing to pulmonary fibrosis. Int J Mol Med May 2014; 33(5):1097-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarrazy V, Billet F, Micallef L, Coulomb B, Desmouliere A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen September 2011; 19 Suppll 1:s10-15; PMID:21793960; http://dx.doi.org/ 10.1111/j.1524-475X.2011.00708.x [DOI] [PubMed] [Google Scholar]
- 47.Birdsey GM, Shah AV, Dufton N, Reynolds LE, Osuna Almagro L, Yang Y, Aspalter IM, Khan ST, Mason JC, Dejana E, et al.. The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/β-catenin signaling. Dev Cell January 12 2015; 32(1):82-96; PMID:25584796; http://dx.doi.org/ 10.1016/j.devcel.2014.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Adv Clin Chem 2009; 48:137-61; http://dx.doi.org/ 10.1016/S0065-2423(09)48006-5 [DOI] [PubMed] [Google Scholar]
- 49.Chen W, Fu X, Ge S, Sun T, Zhou G, Han B, Li H, Sheng Z. Profiling of genes differentially expressed in a rat of early and later gestational ages with high-density oligonucleotide DNA array. Wound Repair Regen Jan-Feb 2007; 15(1):147-55; PMID:17244330; http://dx.doi.org/ 10.1111/j.1524-475X.2006.00195.x [DOI] [PubMed] [Google Scholar]
- 50.Chen W, Fu XB, Ge SL, Sun TZ, Sheng ZY. Analysis of differentially expressed genes in fetal skin of scarless and scar-forming periods of gestational rats. Chin J Traumatol April 2006; 9(2):94-9; PMID:16533435 [PubMed] [Google Scholar]
- 51.Kishi K, Okabe K, Shimizu R, Kubota Y. Fetal skin possesses the ability to regenerate completely: complete regeneration of skin. Keio J Medicine 2012; 61(4):101-8; http://dx.doi.org/ 10.2302/kjm.2011-0002-IR [DOI] [PubMed] [Google Scholar]
- 52.Veraitch O, Kobayashi T, Imaizumi Y, Akamatsu W, Sasaki T, Yamanaka S, Amagai M, Okano H, Ohyama M. Human induced pluripotent stem cell-derived ectodermal precursor cells contribute to hair follicle morphogenesis in vivo. J Invest Dermatol June 2013; 133(6):1479-88; http://dx.doi.org/ 10.1038/jid.2013.7 [DOI] [PubMed] [Google Scholar]
- 53.Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3s phosphorylation of β-catenin. Proc Natl Acad Sci USA June 10 2008; 105(23):8032-7; PMID:18509060; http://dx.doi.org/ 10.1073/pnas.0803025105 [DOI] [PMC free article] [PubMed] [Google Scholar]


