Abstract
The endocannabinoid system (ECS) is abundantly expressed in the brain. This system regulates a plethora of physiological functions and is composed of cannabinoid receptors, their endogenous ligands (endocannabinoids), and the enzymes involved in the metabolism of endocannabinoids. In this review, we highlight the new advances in cannabinoid signaling, focusing on a key component of the ECS, the type-1 cannabinoid receptor (CB 1). In recent years, the development of new imaging and molecular tools has demonstrated that this receptor can be distributed in many cell types (e.g., neuronal or glial cells) and intracellular compartments (e.g., mitochondria). Interestingly, cellular and molecular effects are differentially mediated by CB 1 receptors according to their specific localization (e.g., glutamatergic or GABAergic neurons). Moreover, this receptor is expressed in the periphery, where it can modulate periphery-brain connections. Finally, the better understanding of the CB 1 receptor structure led researchers to propose interesting and new allosteric modulators. Thus, the advances and the new directions of the CB 1 receptor field will provide new insights and better approaches to profit from its interesting therapeutic profile.
Keywords: Endocannabinoid system, allosteric modulator, molecular pharmacology, cannabinoid ligands, CB1 receptor signaling
Introduction
The endocannabinoid system (ECS) is composed of G protein-coupled cannabinoid receptors, namely cannabinoid receptor-1 (CB 1) and cannabinoid receptor-2 (CB 2) 1, 2; the endogenous cannabinoids called endocannabinoids, such as the lipids anandamide and 2-arachidonoylglycerol 3, 4; and the enzymes involved in their synthesis and inactivation 5. The family of endocannabinoids has recently grown to include a group of peptide ligands (so-called pepcans) and other lipid molecules, such as lipoxin and pregnenolone, interestingly acting as allosteric enhancers or signal-specific inhibitors (SSIs) of CB 1 receptors 6.
One of the main characteristics of the ECS is its broad distribution throughout the body. In this review, we will specifically focus our attention on the CB 1 receptor-dependent functions in the nervous system (particularly the brain). The CB 1 receptor is considered the most abundant metabotropic receptor in the brain 7. It was cloned in 1990 1 and its distribution has been well characterized in both rodents 8, 9 and humans 10. These receptors are particularly rich in the central nervous system 11, 12, where they control a wide spectrum of physiological and pathological conditions, including brain development, learning and memory, motor behavior, regulation of appetite, body temperature, pain perception, inflammation, and they are involved in various psychiatric, neurological, and neurodevelopmental disorders 13– 17.
This review highlights recent findings that challenge or extend accepted “dogmas” of CB 1 receptor signaling. Thus, it discusses where CB 1 receptors are localized, the importance of CB 1 receptors outside the brain, and new strategies to pharmacologically act on these receptors. Importantly, the understanding of where, which, and how CB 1 receptor function is mandatory to improve the pharmacological strategies to act on this promising therapeutic target.
Localization of CB 1 receptors in different neuronal types
CB 1 receptor localization has been widely studied during the last few decades 18. Thus, early studies provided strong evidence for a presynaptic localization of CB 1 receptors, from where they can control the neurotransmitter release 7, 19. However, the somatodendritic localization of CB 1 receptors cannot be discarded, as processes of self-inhibition through these receptors have been demonstrated in the cortex 20– 23. According to this, recent work describes that somatodendritic CB 1 receptors control a specific postsynaptic signaling cascade important for the cognitive impairment induced by cannabinoids 24. Therefore, more studies are needed to clarify the relative involvement of pre- or post-synaptic CB 1 receptors in brain functions and how this can affect our general view of how the ECS controls synaptic transmission.
Interestingly, new experimental approaches (e.g., imaging tools) have shown the expression of CB 1 receptors in different neuronal types, including GABAergic, glutamatergic, and serotonergic neurons, among others 8, 25– 28. Moreover, although the anatomical presence of CB 1 receptors in cholinergic, noradrenergic, or dopaminergic neurons has not been fully characterized, cannabinoids are known to control acetylcholine and dopamine release 29, 30. For example, it has been recently shown that CB 1 receptors can specifically control cholinergic over glutamatergic transmission at single synapses that co-release both neurotransmitters 31.
Importantly, the expression levels of CB 1 receptors can drastically differ among different cell types and can diverge between different brain regions 12, 25, 32, 33. This widely distributed and differential expression in the brain reflects the complexity, and can explain the variety of functions, of the ECS. For instance, this specific distribution can explain some of the bimodal effects of cannabinoid drugs 34, 35. Thus, recent studies demonstrated how CB 1 receptors localized in GABAergic neurons can control food intake 34, running related behaviors 36, 37, drug addiction 38, 39, and learning and memory processes 40, 41, among other behaviors, whereas CB 1 receptors localized in glutamatergic neurons control neuroprotection 42, olfactory processes 25, fear memories 43, social behaviors 44, and anxiety 35, among others. Moreover, CB 1 receptors present in serotonergic neurons can modulate emotional responses 45.
Localization of CB 1 receptors in other cell types or intracellular organelles
The biased neuron-centric view in the ECS field changed when CB 1 receptors were found in another type of brain cells, the glial cells 46– 49. Moreover, recent studies have demonstrated how the astroglial CB 1 receptor can modulate important physiological functions in behavior and synaptic plasticity such as learning and memory and long-term depression in the hippocampus 50– 52. Therefore, this receptor can shape synaptic transmission via astroglial signaling 53. By doing this, it modulates the effects of exogenous cannabinoids on working memory 46 and, notably, can also determine the selective activity of specific circuits in the striatum 54. Thus, the improvement of the current tools will consolidate this knowledge to better elucidate the role of CB 1 receptors and astrocytes on brain functioning 55. Interestingly, recent findings have shown how CB 1 receptors can modulate microglia activation, suggesting its presence in this cell type 49.
Although CB 1 receptors are localized primarily at the plasma membrane, more and more evidence suggests the presence of functional intracellular CB 1 receptors 56, 57. For instance, a portion of these receptors is functionally present in cell mitochondria 58. In the past, previous data showed that cannabinoids can alter mitochondrial functions, but these effects were fully ascribed to unspecific membrane disturbance induced by these lipid molecules 59, 60. However, recent results challenge this idea, indicating that CB 1 receptors are also present in mitochondrial membranes in the periphery, such as in spermatozoa 61 or skeletal muscles 62, and in the brain, where they directly regulate mitochondrial oxidative phosphorylation (OXPHOS) activity 58, 63, 64 or can impact feeding behavior 65. However, further studies and more direct, specific, and powerful tools are needed to investigate the role of mitochondrial or other intracellular CB 1 receptors on synaptic transmission, brain functions, and behavior. Interestingly, brain mitochondrial functions have been recently causally associated to anxiety-related responses in the nucleus accumbens 66, demonstrating how brain energetics can impact behavior.
Localization of CB 1 receptors in the periphery
In the last two decades, CB 1 receptors have been described in a number of peripheral tissues, including fat tissue 67, gastrointestinal tract 68, mouth and oral cavity 69, eye 70, cardiovascular system 71, liver 72, pancreas 73, immune system 74, bone 75, skin 76, and skeletal muscle 77. Indeed, it seems that the ECS is present in a large majority of tissues and its specific functions have recently been investigated 78.
The complex interactions between peripheral organs and the central nervous system raised a particular interest within the neuroscience field. In this sense, it is worth discussing how the peripheral processes modulated by the CB 1 receptors are affecting the central nervous system functions. A recent study demonstrated that the peripheral sympathetic activity controlled by CB 1 receptors is necessary for central functions, such as hypophagia and anxiety-like effects 79. Other potential examples of the roles of CB 1 receptors in the periphery-brain connection are the control of the release of stress hormones from the adrenal glands 80 or the modulation of gut functions impacting on behavioral responses. Indeed, a close interaction between adipose tissue, gut bacteria, and the endocannabinoid system has been proposed in the context of obesity 81, 82.
New advances in the CB 1 receptor pharmacology
Several orthosteric ligands of CB 1 receptors have been described in the last few decades, including natural or synthetic CB 1 receptor agonists (e.g., Δ 9-tetrahydrocannabinol [THC], CP-55,940), antagonists (e.g., rimonabant), and orthosteric endocannabinoids 6, 83. Moreover, endocannabinoids seem also to target non-cannabinoid receptors (e.g., G protein-coupled receptor 55 receptors) 84, 85 and ion channels (e.g., serotonergic, nicotinic acetylcholine receptors, or vanilloid receptors) 86, particularly at concentrations at which they have been found to interact with CB 1 or CB 2 receptors 6, 87. Notably, the orthosteric action of CB 1 receptor agonists and antagonists induces important side effects 88, 89. For example, rimonabant, known as a partial antagonist/inverse agonist, showed different side effects in humans 88. In this sense, different strategies have been shown to improve the safety profile and overcome the side effects induced by CB 1 antagonists, such as the neutral CB 1 antagonists 90.
Interestingly, the pharmacology of CB 1 receptors is nowadays also focused in the recent developments on putative allosteric binding sites of these receptors and how this can be translated into new therapeutic approaches. As cannabinoid ligands present an interesting therapeutic profile 91, the development of new and safer drugs such as CB 1 receptor allosteric modulators is needed. Indeed, this strategy has become a hot topic in the G protein-coupled receptors field and there are different positive and negative allosteric modulators described (PAMs and NAMs, respectively) 92, 93. Consequently, different compounds have been developed as exogenous CB 1 allosteric modulators, including the indole derivatives (e.g., the NAM “ORG” compounds) 94, urea derivatives (e.g., the NAM PSNCBAM-1) 95, and other small molecules that also display a PAM profile, such as RTI-371 96. Importantly, recent work also identified natural PAMs and NAMs of CB 1 receptors, such as the lipoxin A4, the hemopressin pepcan-12, and pregnenolone 97, 98, which might represent model chemical structures for the development of new drugs. Although numerous studies have fully characterized the chemical and signaling properties of these new synthetic or natural compounds 97, 98, the in vivo effects of all these drugs modulating physiological or pathological conditions constitutes an emerging area in the cannabinoid field. In this context, the neurosteroid pregnenolone exerts peculiar effects on CB 1 receptor signaling. Indeed, pregnenolone, by binding to a specific identified site on CB 1 receptors, displays an interesting SSI profile: whereas CB 1-dependent modulation of cytoplasmic cyclic AMP signaling is unaltered by pregnenolone, the neurosteroid fully blocks the activation of extracellularly regulated kinases (ERKs) and the inhibition of mitochondrial activity by cannabinoids 63. By these mechanisms, the SSI pregnenolone blocks different central effects of THC, including memory impairment, hypolocomotion, and cannabinoid self-administration in rodents 63. Other compounds have been shown to alter CB 1 receptor-dependent effects. For instance, the synthetic PAM ZCZ011 reduces neuropathic pain 99, whereas the PAM lipoxin A4 shows anti-inflammatory effects 100. Interestingly, it was recently shown that cannabidiol, which has been previously reported as a CB 1 receptor antagonist, behaves also as a non-competitive NAM of CB 1 receptors, despite its low affinity to these receptors 101.
The allosteric modulators of CB 1 receptors are not the only therapeutic agents recently proposed. Indeed, the effects of several phytocannabinoids in preclinical models of central nervous system diseases and, where available, clinical trials have been investigated, suggesting a promising phytocannabinoid-based medicine 102. Another factor that can change the CB 1 receptor pharmacology is heteromerization with other receptors. Heteromers of CB 1 receptors and other proteins recently emerged as an important target of the in vivo effects of cannabinoids 103– 105. Notably, these heterocomplexes could be potentially modulated 104 and this implies another pharmacological tool to act on CB 1 receptor signaling. Moreover, present evidence points to the membrane environment as another critical regulator of CB 1 receptor signaling, and this can be potentially exploited for the development of novel therapeutic compounds 106. Finally, a G protein-coupled receptor such as the CB 1 receptor may also have a constitutive, ligand-free mode of signaling, as has been shown in hippocampal GABAergic synapses 107. All of these new ideas demonstrate that the research community may dedicate more effort to tackle CB 1 receptors.
Conclusions
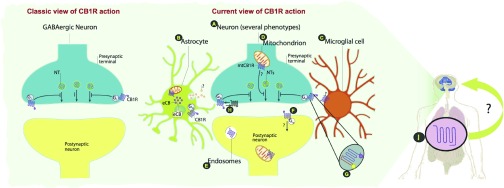
This short review focused on the new findings in CB 1 receptor research. However, the ECS comprises other components such as CB 2 receptors, the endocannabinoids, and the enzymes responsible for their synthesis and degradation. In this sense, recent advances have demonstrated the importance of CB 2 receptors in the brain 108– 110, the presence of other endocannabinoid-like molecules 111, 112, other potential receptors that can be activated by endocannabinoids 87, and interesting findings regarding the localization and pharmacology of the enzymes involved in the metabolism of these endocannabinoids 113, 114. In brief, the actual picture of how the endocannabinoid system works is quite complicated and more efforts are needed to try to merge the old and the new ideas in this field ( Figure 1).
Figure 1. Schematic comparison between the classic and the current view of the CB 1 receptor functional expression.
On the left panel, the classic view of the CB 1 receptor is represented. The CB 1 receptor was thought to be exclusively localized in GABAergic neurons, where it was demonstrated to inhibit neurotransmitter release. On the right panel, the current view of the CB 1 receptor is illustrated. Different advances have completely changed this picture: ( A) The CB 1 receptor is present in different neuronal types and in glial cells, both in astrocytes ( B) and potentially in microglia ( C). Furthermore, it is found intracellularly in the mitochondria ( D) and endosomes ( E). The view of a canonical retrograde system changed after the CB 1 receptor localization in postsynaptic somatodentritic neurons was demonstrated ( F). Nowadays, we know that CB 1 receptor presents allosteric binding sites ( G) and that it could form heteromers ( H). Beyond the brain, the CB 1 receptor is widely expressed in the periphery ( I), where it can modulate the periphery-brain connection. All of this new knowledge reflects the complexity of the central nervous system and the advance in neuroscience, positing the CB 1 receptor as an ideal tool for studying brain functions. CB 1, cannabinoid receptor-1; CB 2, cannabinoid receptor-2; eCB, endocannabinoid; NT, neurotransmitter.
An open question in the cannabinoid field is whether the cellular diversity of CB 1 functions could improve the therapeutic exploitation of cannabinoid-based drugs. One can speculate whether different CB 1 ligands can mediate different signaling pathways by selectively controlling different CB 1 receptors present in different cellular populations. Likewise, it is possible that specific drugs could target exclusively mitochondrial CB 1 (mtCB 1) receptors or could avoid activation of intracellular pools of CB 1. More studies will be needed to answer these questions, but there is already some evidence demonstrating a different pharmacological profile between CB 1 receptors expressed in GABAergic and glutamatergic cells. Thus, “glutamatergic” CB 1 receptors are more sensitive to low doses of agonists and are endowed with stronger intracellular coupling, whereas “GABAergic” pools of the receptor are activated by higher doses of agonists and produce lower activation of G proteins 34, 35, 43, 115. Therefore, one could speculate that specific compounds able to selectively activate different cellular subpopulations of CB 1 receptors could be developed. Moreover, combinations of drugs able to modulate glutamatergic or GABAergic neurotransmission with cannabinoid agonists have been shown to promote specific effects of CB 1 receptors and inhibit others 116. It is also interesting to note that both perisomatic and dendritic GABAergic synapses use phasic endocannabinoid signaling, but the tonic form of cannabinoid signaling is present only in perisomatic cells 107. Moreover, a recent study 80 shows that the peptide endocannabinoids, known as pepcans, act as endogenous allosteric modulators of CB 1 activity exclusively on noradrenergic neurons , demonstrating a cell type-specific regulatory role on endocannabinoid signaling. All of these new and exciting findings suggest that the better we understand cannabinoid signaling, the closer we are to developing specific and local pharmacological drugs that may have importance in brain disorders.
Overall, the new and exciting findings suggesting different and specific localizations of the ECS components and the new strategies proposed to tackle their activity of this receptor open the door to new questions ( Table 1). Indeed, the endocannabinoid system has been related to many physiological and pathological functions 13, 18, 117, and the better understanding of these new evidences will bring more light to exploit the therapeutically beneficial properties of this widely spread neuromodulator system in the brain and in the body.
Table 1. Open questions in the cannabinoid receptor-1 (CB 1) receptor field.
| Open questions in the endocannabinoid field. |
|---|
| Is the cell type-specific CB
1 receptor signaling an open door to
develop new therapeutic tools? |
| Is the endocannabinoid system exclusively a retrograde
neuromodulator system? |
| How is the subcellular CB
1 receptor distributed in the different
cell types? |
| How can CB 1 receptors control neurotransmitter co-release? |
| Which physiological and pathological functions are modulated
by intracellular CB 1 receptors? |
| Is there specific or differential CB
2 receptor expression in
different cell types? |
| Is the allosteric modulation of CB
1 receptors a good therapeutic
approach for pathological conditions? |
| Will it be possible to create compounds that target CB
1 receptors
in specific cell types or subcellular localizations? |
CB 1, cannabinoid receptor-1; CB 2, cannabinoid receptor-2.
Abbreviations
CB 1, cannabinoid receptor-1; CB 2, cannabinoid receptor-2; ECS, endocannabinoid system; NAM, negative allosteric modulator; PAM, positive allosteric modulator; SSI, signal-specific inhibitor; THC, Δ9-tetrahydrocannabinol.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Carsten T Wotjak, Department of Stress Neurobiology and Neurogenetics, Max Planck Institute of Psychiatry, Munich, Germany
Istvan Katona, Momentum Laboratory of Molecular Neurobiology, Institute of Experimental Medicine, Hungarian Academy of Sciences, Budapest, Hungary
Alberto Bacci, Institut du Cerveau et de la Moelle épinière (ICM), INSERM U1127, CNRS UMR 7225, Sorbonne Universités, UPMC-P6 UMR S 1127, Paris, France
Funding Statement
We thank all the members of the GM lab for useful discussions. This work was supported by INSERM (to GM), EU-FP7 (PAINCAGE, HEALTH-603191 to GM and FP7-PEOPLE-2013-IEF-623638 to AB-G), European Research Council (Endofood, ERC-2010-StG-260515; CannaPreg, ERC-2014-PoC-640923, to GM), Fondation pour la Recherche Medicale (DRM20101220445, to GM and LB), Human Frontiers Science Program (to GM), Region Aquitaine (to GM), French State/Agence Nationale de la Recherche/LabEx BRAIN (ANR-10-LABX-0043 to GM), Fyssen Foundation (to ES-G), CONACyT (to ES-G), French State/Agence Nationale de la Recherche/IdEx (ANR-10-IDEX-03-02 to AB-G), and French State/Agence Nationale de la Recherche/Blanc (NeuroNutriSens ANR-13-BSV4-0006-02 to GM).
[version 1; referees: 3 approved]
References
- 1. Matsuda LA, Lolait SJ, Brownstein MJ, et al. : Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–4. 10.1038/346561a0 [DOI] [PubMed] [Google Scholar]
- 2. Howlett AC, Barth F, Bonner TI, et al. : International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202. [DOI] [PubMed] [Google Scholar]
- 3. Little PJ, Compton DR, Johnson MR, et al. : Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther. 1988;247(3):1046–51. [PubMed] [Google Scholar]
- 4. Devane WA, Hanus L, Breuer A, et al. : Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–9. 10.1126/science.1470919 [DOI] [PubMed] [Google Scholar]
- 5. Pacher P, Bátkai S, Kunos G: The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. 10.1124/pr.58.3.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pertwee RG: Endocannabinoids and Their Pharmacological Actions. Handb Exp Pharmacol. 2015;231:1–37. 10.1007/978-3-319-20825-1_1 [DOI] [PubMed] [Google Scholar]
- 7. Kano M, Ohno-Shosaku T, Hashimotodani Y, et al. : Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89(1):309–80. 10.1152/physrev.00019.2008 [DOI] [PubMed] [Google Scholar]
- 8. Herkenham M, Lynn AB, Johnson MR, et al. : Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11(2):563–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsou K, Brown S, Sañudo-Peña MC, et al. : Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. 10.1016/S0306-4522(97)00436-3 [DOI] [PubMed] [Google Scholar]
- 10. Westlake TM, Howlett AC, Bonner TI, et al. : Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer's brains. Neuroscience. 1994;63(3):637–52. 10.1016/0306-4522(94)90511-8 [DOI] [PubMed] [Google Scholar]
- 11. Hu SS, Mackie K: Distribution of the Endocannabinoid System in the Central Nervous System. Handb Exp Pharmacol. 2015;231:59–93. 10.1007/978-3-319-20825-1_3 [DOI] [PubMed] [Google Scholar]
- 12. Freund TF, Katona I, Piomelli D: Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83(3):1017–66. 10.1152/physrev.00004.2003 [DOI] [PubMed] [Google Scholar]
- 13. Katona I, Freund TF: Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012;35:529–58. 10.1146/annurev-neuro-062111-150420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heifets BD, Castillo PE: Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. 10.1146/annurev.physiol.010908.163149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mechoulam R, Parker LA: The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. 10.1146/annurev-psych-113011-143739 [DOI] [PubMed] [Google Scholar]
- 16. Lu H, Mackie K: An Introduction to the Endogenous Cannabinoid System. Biol Psychiatry. 2016;79(7):516–25. 10.1016/j.biopsych.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lutz B, Marsicano G, Maldonado R, et al. : The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci. 2015;16(12):705–18. 10.1038/nrn4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Busquets-Garcia A, Desprez T, Metna-Laurent M, et al. : Dissecting the cannabinergic control of behavior: The where matters. Bioessays. 2015;37(11):1215–25. 10.1002/bies.201500046 [DOI] [PubMed] [Google Scholar]
- 19. Ohno-Shosaku T, Tanimura A, Hashimotodani Y, et al. : Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist. 2012;18(2):119–32. 10.1177/1073858410397377 [DOI] [PubMed] [Google Scholar]
- 20. Bacci A, Huguenard JR, Prince DA: Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431(7006):312–6. 10.1038/nature02913 [DOI] [PubMed] [Google Scholar]
- 21. Min R, Testa-Silva G, Heistek TS, et al. : Diacylglycerol lipase is not involved in depolarization-induced suppression of inhibition at unitary inhibitory connections in mouse hippocampus. J Neurosci. 2010;30(7):2710–5. 10.1523/JNEUROSCI.BC-3622-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marinelli S, Pacioni S, Bisogno T, et al. : The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. J Neurosci. 2008;28(50):13532–41. 10.1523/JNEUROSCI.0847-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marinelli S, Pacioni S, Cannich A, et al. : Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat Neurosci. 2009;12(12):1488–90. 10.1038/nn.2430 [DOI] [PubMed] [Google Scholar]
- 24. Maroso M, Szabo GG, Kim HK, et al. : Cannabinoid Control of Learning and Memory through HCN Channels. Neuron. 2016;89(5):1059–73. 10.1016/j.neuron.2016.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soria-Gómez E, Bellocchio L, Reguero L, et al. : The endocannabinoid system controls food intake via olfactory processes. Nat Neurosci. 2014;17(3):407–15. 10.1038/nn.3647 [DOI] [PubMed] [Google Scholar]
- 26. Marsicano G, Lutz B: Neuromodulatory functions of the endocannabinoid system. J Endocrinol Invest. 2006;29(3 Suppl):27–46. [PubMed] [Google Scholar]
- 27. Kawamura Y, Fukaya M, Maejima T, et al. : The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26(11):2991–3001. 10.1523/JNEUROSCI.4872-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Häring M, Enk V, Aparisi Rey A, et al. : Cannabinoid type-1 receptor signaling in central serotonergic neurons regulates anxiety-like behavior and sociability. Front Behav Neurosci. 2015;9:235. 10.3389/fnbeh.2015.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hernandez G, Cheer JF: To Act or Not to Act: Endocannabinoid/Dopamine Interactions in Decision-Making. Front Behav Neurosci. 2015;9:336. 10.3389/fnbeh.2015.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin HG, Bernabeu A, Lassalle O, et al. : Endocannabinoids Mediate Muscarinic Acetylcholine Receptor-Dependent Long-Term Depression in the Adult Medial Prefrontal Cortex. Front Cell Neurosci. 2015;9:457. 10.3389/fncel.2015.00457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soria-Gómez E, Busquets-Garcia A, Hu F, et al. : Habenular CB 1 Receptors Control the Expression of Aversive Memories. Neuron. 2015;88(2):306–13. 10.1016/j.neuron.2015.08.035 [DOI] [PubMed] [Google Scholar]
- 32. Mackie K: Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005; (168):299–325. 10.1007/3-540-26573-2_10 [DOI] [PubMed] [Google Scholar]
- 33. Katona I: Cannabis and Endocannabinoid Signaling in Epilepsy. Handb Exp Pharmacol. 2015;231:285–316. 10.1007/978-3-319-20825-1_10 [DOI] [PubMed] [Google Scholar]
- 34. Bellocchio L, Lafenêtre P, Cannich A, et al. : Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci. 2010;13(3):281–3. 10.1038/nn.2494 [DOI] [PubMed] [Google Scholar]
- 35. Rey AA, Purrio M, Viveros MP, et al. : Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA B receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37(12):2624–34. 10.1038/npp.2012.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dubreucq S, Durand A, Matias I, et al. : Ventral tegmental area cannabinoid type-1 receptors control voluntary exercise performance. Biol Psychiatry. 2013;73(9):895–903. 10.1016/j.biopsych.2012.10.025 [DOI] [PubMed] [Google Scholar]
- 37. Fuss J, Steinle J, Bindila L, et al. : A runner's high depends on cannabinoid receptors in mice. Proc Natl Acad Sci U S A. 2015;112(42):13105–8. 10.1073/pnas.1514996112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martín-García E, Bourgoin L, Cathala A, et al. : Differential Control of Cocaine Self-Administration by GABAergic and Glutamatergic CB1 Cannabinoid Receptors. Neuropsychopharmacology. 2015. 10.1038/npp.2015.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Talani G, Lovinger DM: Interactions between ethanol and the endocannabinoid system at GABAergic synapses on basolateral amygdala principal neurons. Alcohol. 2015;49(8):781–94. 10.1016/j.alcohol.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Albayram Ö, Passlick S, Bilkei-Gorzo A, et al. : Physiological impact of CB 1 receptor expression by hippocampal GABAergic interneurons. Pflugers Arch. 2016;468(4):727–37. 10.1007/s00424-015-1782-5 [DOI] [PubMed] [Google Scholar]
- 41. Puighermanal E, Marsicano G, Busquets-Garcia A, et al. : Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12(9):1152–8. 10.1038/nn.2369 [DOI] [PubMed] [Google Scholar]
- 42. Chiarlone A, Bellocchio L, Blázquez C, et al. : A restricted population of CB 1 cannabinoid receptors with neuroprotective activity. Proc Natl Acad Sci U S A. 2014;111(22):8257–62. 10.1073/pnas.1400988111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Metna-Laurent M, Soria-Gómez E, Verrier D, et al. : Bimodal control of fear-coping strategies by CB 1 cannabinoid receptors. J Neurosci. 2012;32(21):7109–18. 10.1523/JNEUROSCI.1054-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Terzian AL, Micale V, Wotjak CT: Cannabinoid receptor type 1 receptors on GABAergic vs. glutamatergic neurons differentially gate sex-dependent social interest in mice. Eur J Neurosci. 2014;40(1):2293–8. 10.1111/ejn.12561 [DOI] [PubMed] [Google Scholar]
- 45. Dubreucq S, Matias I, Cardinal P, et al. : Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology. 2012;37(8):1885–900. 10.1038/npp.2012.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Han J, Kesner P, Metna-Laurent M, et al. : Acute cannabinoids impair working memory through astroglial CB 1 receptor modulation of hippocampal LTD. Cell. 2012;148(5):1039–50. 10.1016/j.cell.2012.01.037 [DOI] [PubMed] [Google Scholar]
- 47. Molina-Holgado E, Vela JM, Arévalo-Martín A, et al. : Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci. 2002;22(22):9742–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stella N: Endocannabinoid signaling in microglial cells. Neuropharmacology. 2009;56(Suppl 1):244–53. 10.1016/j.neuropharm.2008.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mecha M, Feliú A, Carrillo-Salinas FJ, et al. : Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun. 2015;49:233–45. 10.1016/j.bbi.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 50. Oliveira da Cruz JF, Robin LM, Drago F, et al. : Astroglial type-1 cannabinoid receptor (CB 1): A new player in the tripartite synapse. Neuroscience. 2016;323:35–42. 10.1016/j.neuroscience.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 51. Metna-Laurent M, Marsicano G: Rising stars: modulation of brain functions by astroglial type-1 cannabinoid receptors. Glia. 2015;63(3):353–64. 10.1002/glia.22773 [DOI] [PubMed] [Google Scholar]
- 52. Navarrete M, Díez A, Araque A: Astrocytes in endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2014;369(1654):20130599. 10.1098/rstb.2013.0599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pérez-Alvarez A, Araque A: Astrocyte-neuron interaction at tripartite synapses. Curr Drug Targets. 2013;14(11):1220–4. 10.2174/13894501113149990203 [DOI] [PubMed] [Google Scholar]
- 54. Martín R, Bajo-Grañeras R, Moratalla R, et al. : Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science. 2015;349(6249):730–4. 10.1126/science.aaa7945 [DOI] [PubMed] [Google Scholar]
- 55. Oliveira JF, Sardinha VM, Guerra-Gomes S, et al. : Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci. 2015;38(9):535–49. 10.1016/j.tins.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 56. Dudok B, Barna L, Ledri M, et al. : Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat Neurosci. 2015;18(1):75–86. 10.1038/nn.3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thibault K, Carrel D, Bonnard D, et al. : Activation-dependent subcellular distribution patterns of CB1 cannabinoid receptors in the rat forebrain. Cereb Cortex. 2013;23(11):2581–91. 10.1093/cercor/bhs240 [DOI] [PubMed] [Google Scholar]
- 58. Bénard G, Massa F, Puente N, et al. : Mitochondrial CB 1 receptors regulate neuronal energy metabolism. Nat Neurosci. 2012;15(4):558–64. 10.1038/nn.3053 [DOI] [PubMed] [Google Scholar]
- 59. Howlett AC: The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68–69:619–31. [DOI] [PubMed] [Google Scholar]
- 60. Bartova A, Birmingham MK: Effect of delta9-tetrahydrocannabinol on mitochondrial NADH-oxidase activity. J Biol Chem. 1976;251(16):5002–6. [PubMed] [Google Scholar]
- 61. Aquila S, Guido C, Santoro A, et al. : Rimonabant (SR141716) induces metabolism and acquisition of fertilizing ability in human sperm. Br J Pharmacol. 2010;159(4):831–41. 10.1111/j.1476-5381.2009.00570.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arrabal S, Lucena MA, Canduela MJ, et al. : Pharmacological Blockade of Cannabinoid CB 1 Receptors in Diet-Induced Obesity Regulates Mitochondrial Dihydrolipoamide Dehydrogenase in Muscle. PLoS One. 2015;10(12):e0145244. 10.1371/journal.pone.0145244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vallée M, Vitiello S, Bellocchio L, et al. : Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343(6166):94–8. 10.1126/science.1243985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hebert-Chatelain E, Reguero L, Puente N, et al. : Cannabinoid control of brain bioenergetics: Exploring the subcellular localization of the CB 1 receptor. Mol Metab. 2014;3(4):495–504. 10.1016/j.molmet.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Koch M, Varela L, Kim JG, et al. : Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519(7541):45–50. 10.1038/nature14260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hollis F, van der Kooij MA, Zanoletti O, et al. : Mitochondrial function in the brain links anxiety with social subordination. Proc Natl Acad Sci U S A. 2015;112(50):15486–91. 10.1073/pnas.1512653112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cota D, Marsicano G, Tschöp M, et al. : The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112(3):423–31. 10.1172/JCI200317725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Izzo AA, Sharkey KA: Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther. 2010;126(1):21–38. 10.1016/j.pharmthera.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 69. Jyotaki M, Shigemura N, Ninomiya Y: Modulation of sweet taste sensitivity by orexigenic and anorexigenic factors. Endocr J. 2010;57(6):467–75. 10.1507/endocrj.K10E-095 [DOI] [PubMed] [Google Scholar]
- 70. Nadolska K, Goś R: [The role of endocannabinoid system in physiological and pathological processes in the eye]. Klin Oczna. 2008;110(10–12):392–6. [PubMed] [Google Scholar]
- 71. Mach F, Steffens S: The role of the endocannabinoid system in atherosclerosis. J Neuroendocrinol. 2008;20(Suppl 1):53–7. 10.1111/j.1365-2826.2008.01685.x [DOI] [PubMed] [Google Scholar]
- 72. Osei-Hyiaman D, DePetrillo M, Pacher P, et al. : Endocannabinoid activation at hepatic CB 1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115(5):1298–305. 10.1172/JCI200523057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cota D: CB1 receptors: emerging evidence for central and peripheral mechanisms that regulate energy balance, metabolism, and cardiovascular health. Diabetes Metab Res Rev. 2007;23(7):507–17. 10.1002/dmrr.764 [DOI] [PubMed] [Google Scholar]
- 74. Jean-Gilles L, Braitch M, Latif ML, et al. : Effects of pro-inflammatory cytokines on cannabinoid CB 1 and CB 2 receptors in immune cells. Acta Physiol (Oxf). 2015;214(1):63–74. 10.1111/apha.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Idris AI, van 't Hof RJ, Greig IR, et al. : Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med. 2005;11(7):774–9. 10.1038/nm1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bíró T, Tóth BI, Haskó G, et al. : The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30(8):411–20. 10.1016/j.tips.2009.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cavuoto P, McAinch AJ, Hatzinikolas G, et al. : The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochem Biophys Res Commun. 2007;364(1):105–10. 10.1016/j.bbrc.2007.09.099 [DOI] [PubMed] [Google Scholar]
- 78. Maccarrone M, Bab I, Bíró T, et al. : Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci. 2015;36(5):277–96. 10.1016/j.tips.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bellocchio L, Soria-Gómez E, Quarta C, et al. : Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB 1 receptor blockade. Proc Natl Acad Sci U S A. 2013;110(12):4786–91. 10.1073/pnas.1218573110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hofer SC, Ralvenius WT, Gachet MS, et al. : Localization and production of peptide endocannabinoids in the rodent CNS and adrenal medulla. Neuropharmacology. 2015;98:78–89. 10.1016/j.neuropharm.2015.03.021 [DOI] [PubMed] [Google Scholar]
- 81. Cani PD, Plovier H, Van Hul M, et al. : Endocannabinoids - at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol. 2016;12(3):133–43. 10.1038/nrendo.2015.211 [DOI] [PubMed] [Google Scholar]
- 82. Geurts L, Everard A, Van Hul M, et al. : Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat Commun. 2015;6: 6495. 10.1038/ncomms7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vemuri VK, Makriyannis A: Medicinal chemistry of cannabinoids. Clin Pharmacol Ther. 2015;97(6):553–8. 10.1002/cpt.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pertwee RG: GPR55: a new member of the cannabinoid receptor clan? Br J Pharmacol. 2007;152(7):984–6. 10.1038/sj.bjp.0707464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lauckner JE, Jensen JB, Chen HY, et al. : GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A. 2008;105(7):2699–704. 10.1073/pnas.0711278105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xiong W, Cui T, Cheng K, et al. : Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J Exp Med. 2012;209(6):1121–34. 10.1084/jem.20120242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Di Marzo V, De Petrocellis L: Why do cannabinoid receptors have more than one endogenous ligand? Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3216–28. 10.1098/rstb.2011.0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Christensen R, Kristensen PK, Bartels EM, et al. : Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370(9600):1706–13. 10.1016/S0140-6736(07)61721-8 [DOI] [PubMed] [Google Scholar]
- 89. Volkow ND, Baler RD, Compton WM, et al. : Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219–27. 10.1056/NEJMra1402309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Janero DR: Cannabinoid-1 receptor (CB1R) blockers as medicines: beyond obesity and cardiometabolic disorders to substance abuse/drug addiction with CB1R neutral antagonists. Expert Opin Emerg Drugs. 2012;17(1):17–29. 10.1517/14728214.2012.660916 [DOI] [PubMed] [Google Scholar]
- 91. Whiting PF, Wolff RF, Deshpande S, et al. : Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015;313(24):2456–73. 10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- 92. Gentry PR, Sexton PM, Christopoulos A: Novel Allosteric Modulators of G Protein-coupled Receptors. J Biol Chem. 2015;290(32):19478–88. 10.1074/jbc.R115.662759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. van der Westhuizen ET, Valant C, Sexton PM, et al. : Endogenous allosteric modulators of G protein-coupled receptors. J Pharmacol Exp Ther. 2015;353(2):246–60. 10.1124/jpet.114.221606 [DOI] [PubMed] [Google Scholar]
- 94. Price MR, Baillie GL, Thomas A, et al. : Allosteric modulation of the cannabinoid CB 1 receptor. Mol Pharmacol. 2005;68(5):1484–95. 10.1124/mol.105.016162 [DOI] [PubMed] [Google Scholar]
- 95. Horswill JG, Bali U, Shaaban S, et al. : PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB 1 receptors with hypophagic effects in rats. Br J Pharmacol. 2007;152(5):805–14. 10.1038/sj.bjp.0707347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Navarro HA, Howard JL, Pollard GT, et al. : Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br J Pharmacol. 2009;156(7):1178–84. 10.1111/j.1476-5381.2009.00124.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Abood ME: Allosteric Modulators: A Side Door. J Med Chem. 2016;59(1):42–3. 10.1021/acs.jmedchem.5b01824 [DOI] [PubMed] [Google Scholar]
- 98. Morales P, Goya P, Jagerovic N, et al. : Allosteric Modulators of the CB 1 Cannabinoid Receptor: A Structural Update Review. Cannabis and Cannabinoid Research. 2016;1(1):22–30. 10.1089/can.2015.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ignatowska-Jankowska BM, Baillie GL, Kinsey S, et al. : A Cannabinoid CB 1 Receptor-Positive Allosteric Modulator Reduces Neuropathic Pain in the Mouse with No Psychoactive Effects. Neuropsychopharmacology. 2015;40(13):2948–59. 10.1038/npp.2015.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pamplona FA, Ferreira J, Menezes de Lima O, Jr, et al. : Anti-inflammatory lipoxin A 4 is an endogenous allosteric enhancer of CB 1 cannabinoid receptor. Proc Natl Acad Sci U S A. 2012;109(51):21134–9. 10.1073/pnas.1202906109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Laprairie RB, Bagher AM, Kelly ME, et al. : Cannabidiol is a negative allosteric modulator of the cannabinoid CB 1 receptor. Br J Pharmacol. 2015;172(20):4790–805. 10.1111/bph.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hill AJ, Williams CM, Whalley BJ, et al. : Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther. 2012;133(1):79–97. 10.1016/j.pharmthera.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 103. Chiodi V, Ferrante A, Ferraro L, et al. : Striatal adenosine-cannabinoid receptor interactions in rats over-expressing adenosine A 2A receptors. J Neurochem. 2016;136(5):907–17. 10.1111/jnc.13421 [DOI] [PubMed] [Google Scholar]
- 104. Viñals X, Moreno E, Lanfumey L, et al. : Cognitive Impairment Induced by Delta9-tetrahydrocannabinol Occurs through Heteromers between Cannabinoid CB 1 and Serotonin 5-HT 2A Receptors. PLoS Biol. 2015;13(7):e1002194. 10.1371/journal.pbio.1002194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Callén L, Moreno E, Barroso-Chinea P, et al. : Cannabinoid receptors CB 1 and CB 2 form functional heteromers in brain. J Biol Chem. 2012;287(25):20851–65. 10.1074/jbc.M111.335273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Maccarrone M, Bernardi G, Agrò AF, et al. : Cannabinoid receptor signalling in neurodegenerative diseases: a potential role for membrane fluidity disturbance. Br J Pharmacol. 2011;163(7):1379–90. 10.1111/j.1476-5381.2011.01277.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lee SH, Ledri M, Tóth B, et al. : Multiple Forms of Endocannabinoid and Endovanilloid Signaling Regulate the Tonic Control of GABA Release. J Neurosci. 2015;35(27):10039–57. 10.1523/JNEUROSCI.4112-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang HY, Gao M, Shen H, et al. : Expression of functional cannabinoid CB 2 receptor in VTA dopamine neurons in rats. Addict Biol. 2016. 10.1111/adb.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li Y, Kim J: CB2 Cannabinoid Receptor Knockout in Mice Impairs Contextual Long-Term Memory and Enhances Spatial Working Memory. Neural Plast. 2016;2016: 9817089. 10.1155/2016/9817089 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 110. Li Y, Kim J: Deletion of CB2 cannabinoid receptors reduces synaptic transmission and long-term potentiation in the mouse hippocampus. Hippocampus. 2016;26(3):275–81. 10.1002/hipo.22558 [DOI] [PubMed] [Google Scholar]
- 111. Pistis M, Melis M: From surface to nuclear receptors: the endocannabinoid family extends its assets. Curr Med Chem. 2010;17(14):1450–67. 10.2174/092986710790980014 [DOI] [PubMed] [Google Scholar]
- 112. Fezza F, Bari M, Florio R, et al. : Endocannabinoids, related compounds and their metabolic routes. Molecules. 2014;19(11):17078–106. 10.3390/molecules191117078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Viader A, Blankman JL, Zhong P, et al. : Metabolic Interplay between Astrocytes and Neurons Regulates Endocannabinoid Action. Cell Rep. 2015;12(5):798–808. 10.1016/j.celrep.2015.06.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Viader A, Ogasawara D, Joslyn CM, et al. : A chemical proteomic atlas of brain serine hydrolases identifies cell type-specific pathways regulating neuroinflammation. eLife. 2016;5:e12345. 10.7554/eLife.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Steindel F, Lerner R, Häring M, et al. : Neuron-type specific cannabinoid-mediated G protein signalling in mouse hippocampus. J Neurochem. 2013;124(6):795–807. 10.1111/jnc.12137 [DOI] [PubMed] [Google Scholar]
- 116. Bellocchio: Patent: Compositions targeting cb1 receptor for controlling food intake. Application Number: EP09306163.8. European Patent Office.2010. [Google Scholar]
- 117. Di Marzo V, Stella N, Zimmer A: Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci. 2015;16(1):30–42. 10.1038/nrn3876 [DOI] [PMC free article] [PubMed] [Google Scholar]