Abstract
Acute kidney injury is strongly associated with increased mortality and other adverse outcomes. Medical researchers have intensively investigated novel biomarkers to predict short- and long-term outcomes of acute kidney injury in many patient care settings, such as cardiac surgery, intensive care units, heart failure, and transplant. Future research should focus on leveraging this relationship to improve enrollment for clinical trials of acute kidney injury.
Keywords: acute kidney injury, AKI, biomarkers, chronic kidney disease, CKD
Introduction
Acute kidney injury (AKI) is a common complication, occurring in about 5% of hospitalizations, and is often associated with various short- and long-term complications 1. AKI has been associated with short-term outcomes, such as increased length of hospital stay, length of ventilation, and in-hospital mortality 2. A meta-analysis of observational studies demonstrated that survivors of AKI had a long-term mortality rate of more than twice that of patients without AKI 3. Furthermore, increasing duration of AKI was positively associated with long-term mortality 4, 5. Although both the magnitude and length of AKI are significantly associated with reduced long-term survival, no clear causal relationship between AKI and mortality has been established yet. However, mechanisms are currently being elucidated, and there is increasing evidence that AKI can cause distant organ injury, including lung and cardiac injury 6. Despite the relationship between AKI and adverse clinical outcomes, clinical trials investigating novel treatments for AKI have not demonstrated any benefit 7– 11.
While increased mortality is a well-documented complication of AKI, evidence from basic science and clinical literature suggests that there may be other consequences, including increased risk for development of hypertension, increased risk of progression to chronic kidney disease (CKD), and increased risk of cardiovascular events. Survivors of AKI are about 20 percent more likely to develop elevated blood pressure after an AKI event 12. Rat models have shown that there is rarefaction of peritubular capillaries after an episode of AKI, which leads to salt-sensitive hypertension 13, 14. A meta-analysis of observational data demonstrates that AKI increases the risk of CKD by almost eightfold 15. Cell cycle arrest in the gap (G)2/mitotic (M) phase checkpoint during proximal tubule injury activates fibrotic cellular signals, such as transforming growth factor-β and connective tissue growth factor, which may contribute to the increased risk of CKD 3, 15– 18. Epidemiologic data show that AKI may increase the risk of subsequent cardiovascular events by 1.5- to 2-fold 3. While the definite mechanism is not clear, there is evidence that ischemia-reperfusion increases apoptosis in the heart, macrophage infiltrate, and angiotensin-converting enzyme expression 19– 21. There is also evidence that ischemia-reperfusion injury alters the affinity of the calcium receptor in the heart for inotropic agents 22.
Potential uses of AKI biomarkers
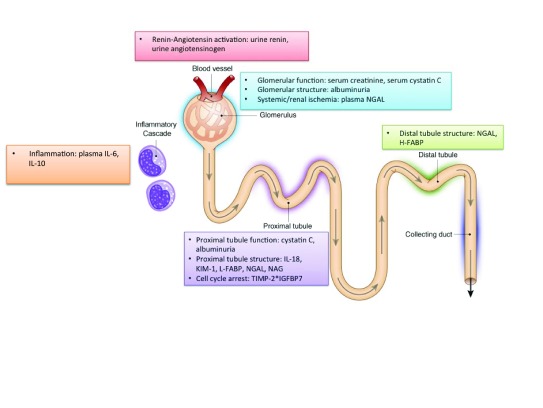
Novel biomarkers of AKI are recent developments and typically reflect a specific component of AKI pathophysiology, including tubular injury, cell cycle arrest, systemic inflammatory pathways, and glomerular filtration ( Figure 1). Biomarkers of AKI will hopefully have several uses in clinical care, including early diagnosis of AKI, prediction of clinical outcomes, and prediction of response to therapy. Initial investigations of biomarkers of AKI sought to determine if they could diagnose AKI earlier than serum creatinine, as it often takes 2 to 3 days before serum creatinine is elevated after a renal insult. Early diagnosis would be beneficial, as it can identify a window where clinicians could potentially intervene, such as by withdrawing nephrotoxic agents or providing treatment while injury is ongoing. If effective treatments for AKI are developed, clinicians could also potentially serially follow biomarkers of injury to determine if patients are responding to treatment. Evidence has also accumulated that biomarkers of AKI are associated with various patient outcomes, which is the focus of this review.
Figure 1. Physiology of biomarkers of AKI.
Adapted from 73. AKI, acute kidney injury; H-FABP, heart fatty acid binding protein; IGFBP-7, insulin-like growth factor binding protein 7; IL-6, interleukin-6; IL-10, interleukin-10; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid binding protein; NAG, N-acetyl-β-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; TIMP-2, tissue inhibitor metalloproteinase-2.
Biomarkers of AKI and prognosis
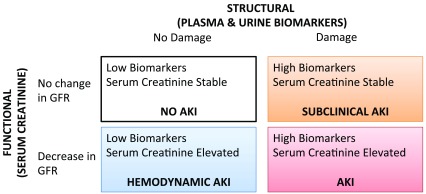
Many of the novel biomarkers of AKI are intricately involved in its pathogenesis, so it logically follows that they could be associated with adverse outcomes after AKI 23– 26. Biomarkers of AKI have been related to short- and long-term adverse outcomes in various patient care settings. The most commonly studied short-term outcomes are in-hospital mortality, need for renal replacement therapy (RRT), and length of stay. While there is evidence that biomarkers of AKI are related to long-term mortality, data are lacking to suggest that biomarkers of AKI are related to other important long-term patient outcomes, such as cardiovascular events and CKD, although prospective studies are ongoing 27. Since AKI independently increases the risk of subsequent cardiovascular events and CKD, it follows that diagnostic biomarkers of AKI will also be associated with these poor outcomes. However, some AKI biomarkers may yield additional prognostic information beyond that provided by the AKI event itself. Long-term follow-up studies have identified a subgroup of patients who suffer from “subclinical AKI”. While these patients do not have AKI as defined by serum creatinine, they have elevated biomarkers of tubular injury and fare worse than patients without elevated biomarkers of AKI 28, 29. This suggests that biomarkers of AKI may provide additional prognostic information beyond that offered by serum creatinine. This observation has generated a new proposed framework for organizing AKI: structural versus functional AKI ( Figure 2). This framework highlights two groups: the aforementioned patients with subclinical AKI and those with AKI as defined by serum creatinine but without any structural damage as indicated by low tubular injury markers, or “hemodynamic” AKI. This could prove meaningful, as it suggests that clinical trials investigating treatments for conditions such as acute tubular necrosis are enrolling patients unlikely to experience benefit (those with hemodynamic AKI) and failing to enroll patients who may benefit (those with subclinical AKI).
Figure 2. Structural versus functional classification of AKI.
Adapted from 74. AKI, acute kidney injury; GFR, glomerular filtration rate.
Utility of novel biomarkers in various patient settings
While novel biomarkers of AKI reflect the same pathophysiology regardless of the patient care setting, challenges for interpreting biomarkers of AKI differ between patient care settings, including cardiac surgery, critically ill, cardiorenal syndrome, transplant, and hospitalized patients.
Cardiac surgery
Cardiac surgery is one of the most popular settings for the evaluation of biomarkers of AKI, partially because there is a clear point of injury. AKI after cardiac surgery has been commonly ascribed to an inflammatory cascade, although thus far interventions to quell the inflammatory cascade have been unsuccessful 10. Biomarkers of AKI could help identify patients at higher risk of adverse outcomes.
Short-term outcomes. One of the most carefully studied cohorts for biomarkers, Translational Research Investigating Biomarker Endpoints of AKI (TRIBE-AKI), featured over 1200 patients who underwent cardiac surgery. Levels of post-operative tubular injury biomarkers, such as urinary interleukin (IL)-18, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and liver fatty acid binding protein (L-FABP), were associated with increased risk of in-hospital death, increased length of stay, and need for RRT ( Table 1) 30, 31. Patients with elevated urinary cystatin C, another tubular injury biomarker, additionally had increased risk of requiring RRT 32.
Table 1. Biomarkers of AKI and outcomes in cardiac surgery.
| Biomarkers | OUTCOMES | ||||||
|---|---|---|---|---|---|---|---|
| Short-term (1 Month) outcomes | Three year mortality | ||||||
| LOS - ICU | LOS - Hospital | AKI recovery | Mortality or RRT | AKI | No AKI | Overall | |
|
Urine IL-18
TRIBE-AKI 28, 30 |
Q1: 2.8 d (SD 6)* Q5: 5.3 d (SD 15.3) |
Q1: 6.7 d (SD 6.7)* Q5: 12.2 d (SD 18) |
Q1: 0.4%* Q5: 5.8% |
HR 3.16 (1.53, 6.53) |
HR 1.23 (1.02, 1.48) |
||
|
Urine NGAL
TRIBE-AKI 28, 30 |
Q1: 2.7 d (SD 2.6)* Q5: 5.4 d (SD 15.9) |
Q1: 6.4 d (SD 3.1)* Q5: 12.2 d (SD 18) |
Q1: 0.4%* Q5: 5.4% |
HR 2.52 (1.86, 3.42) |
HR 0.9 (0.5, 1.63) |
||
|
Urine L-FABP
TRIBE-AKI 30, 31 |
Q1: 2 d (IQR: 1, 3)* Q5 2.0 d (IQR: 1, 4) |
Q1: 6 d (IQR: 5,8)* Q5: 7 d (IQR: 6, 10) |
Q1: 1.7%§ Q5: 5.0% |
HR 2.35 (1.64, 3.37) |
HR 0.65 (0.45, 0.92) |
||
|
Urine KIM-1
TRIBE-AKI 30, 31 |
Q1: 2 d (IQR: 1, 3) § Q5: 2 d (IQR: 1, 3) |
Q1: 6 d (IQR: 5, 7)* Q5: 7 d (IQR: 5, 9) |
Q1: 1.3%* Q5: 5.4% |
HR 2.10 (1.31, 3.10) |
HR 1.83 (1.44, 3.33) |
||
|
Blood IL-6
TRIBE-AKI 36 |
HR 1.4 (0.65, 2.90) |
||||||
|
Blood IL-10
TRIBE-AKI 36 |
HR 0.72 (0.56, 0.93) ↑NGAL: 0.51 (0.36, 0.73) |
||||||
|
IL-18 and KIM-1
Arthur 2014 33 |
AUC 0.93(0.80, 0.98) |
||||||
|
Urine angiotensinogen
Alge 2013 34 |
AUC 0.75(0.65, 0.85) |
||||||
|
Urine Renin
Alge 2013 34 |
AUC 0.70(0.57, 0.83) |
||||||
|
Urine TIMP-2*IGFBP7
Meersch 2014 35 |
AUC 0.79(0.65, 0.92) |
||||||
*p-value for trend significant, §p-value for trend not significant.
All values in ( ) are 95% confidence intervals, unless otherwise noted.
AKI, acute kidney injury; AUC, area under the curve; HR, hazard ratio (3 rd tertile compared to 1 st tertile); IGFBP7, insulin like growth factor binding protein 7; IL-6, interleukin-6; IL-10, interleukin-10; IL-18, interleukin-18; IQR, interquartile range; KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid binding protein; LOS, length of stay; NGAL, neutrophil gelatinase-associated lipocalin; Q1, quintile 1; Q5, quintile 5; RRT, renal replacement therapy; SD, standard deviation; TIMP-2, tissue inhibitor of metalloproteinases-2; d, days.
Another cohort of cardiac surgery patients, Southern Acute Kidney Injury Network (SAKInet), were evaluated to determine whether combinations of biomarkers could predict if patients with stage I AKI (AKIN) after cardiac surgery progressed to stage III AKI or death. Urinary IL-18 combined with percentage change in serum creatinine or urinary KIM-1 had the best discriminative ability to identify patients at high risk for progressing to higher stages of AKI or death within 30 days 33. The SAKInet cohort study additionally evaluated urinary angiotensinogen and renin, biomarkers of intra-renal renin-angiotensin system activity, and found that they were associated with severe AKI or death within 30 days 34.
Changes in urinary biomarkers have been associated with improved patient outcomes. One study examined biomarkers that induce cell cycle arrest in cardiac surgery by examining the product of tissue inhibitor metalloproteinase 2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP-7). Differences in the decline of these biomarkers within 24 hours after surgery discriminated between patients who recovered and those who failed to recover from AKI 35.
Long-term outcomes. In the TRIBE-AKI study, patients with AKI as defined by serum creatinine and elevated peak post-operative tubular injury markers, including urinary IL-18, NGAL, KIM-1, and L-FABP, had an increased risk of long-term mortality 28. Patients without AKI as defined by serum creatinine, elevated urinary IL-18, and KIM-1 also had an increase in long-term mortality of about 1.5-fold. These patients may have experienced subclinical AKI that was not detected by serum creatinine, leading to increased mortality, or it may be that urinary IL-18 or KIM-1 is capturing a systematic inflammatory process which increases the risk of death independently of AKI.
Biomarkers that probe inflammatory pathways involved in AKI have additionally been investigated in TRIBE-AKI. Elevated first post-operative serum IL-10, which is known to attenuate the inflammatory cascade, was found to decrease the risk of long-term mortality 36. Interestingly, this effect was isolated to patients with elevated urinary markers of tubular injury, which suggests that IL-10 protected those patients who were experiencing proximal tubular cell damage.
Critical illness
AKI is a common complication in critical illness and is consistently associated with high mortality, although this is a challenging setting in which to study biomarkers of AKI 1. There may be multiple renal insults, and the timing of renal insults is not always clear. Regardless, biomarker data have identified subphenotypes for complex intensive care unit (ICU) syndromes, such as acute respiratory distress syndrome (ARDS), which benefit from different treatment strategies 37. The incorporation of prognostic information from AKI biomarkers could eventually lead to similar benefit. Multiple urinary biomarkers have been studied in critically ill patients and are associated with adverse outcomes, although the data are strongest for short-term outcomes ( Table 2).
Table 2. Biomarkers of AKI and outcomes in critically ill patients.
| Biomarkers | SHORT-TERM (IN-HOSPITAL) OUTCOMES | |||
|---|---|---|---|---|
| Recovery of AKI | RRT | RRT or mortality | Mortality | |
|
Urine IL-18
VALID 38 Doi 2011 42 ARDS Network 41 |
OR 1.05 (0.56, 1.98) |
OR 1.76 (1.19, 2.59) |
OR 2.02 (1.41, 2.89) AUC 0.83 (0.68, 0.91) OR 2.32 (1.2, 4.4) § |
|
|
Urine NGAL
VALID 39, 40 Doi 2011 42 ATN Trial 44 |
AUC 0.70 (0.55, 0.84) |
OR 2.60 (1.55, 4.35) |
HR 1.38 (0.93, 1.96) |
OR 1.44 (1.00, 2.07)
AUC 0.83 (0.69, 0.91) |
|
Urine L-FABP
Doi 2011 42 VALID 39 |
HR 1.15 (0.82, 1.61) | AUC 0.90 (0.84, 0.94) | ||
|
Urine NAG
Doi 2011 42 |
AUC 0.66 (0.50, 0.80) | |||
|
Urine Cystatin C
Nejat 2010 43 |
OR 1.60 (1.16, 2.21) † | |||
|
Urine HGF
ATN Trial 44 |
AUC 0.74 (0.53, 0.94) | |||
|
Urine TIMP2xIGFB7
Sapphire 45 |
HR 2.16 (1.32, 3.53)* § | |||
|
Urine Albumin
Doi 2011 42 |
AUC 0.72 (0.61, 0.86) | |||
All values in ( ) are 95% confidence intervals, unless otherwise noted.
*Outcomes at 9 months in patients with AKI § greater than cut-off versus less than cut-off † for 10-fold increase.
AKI, acute kidney injury; AUC, area under the curve; HGF, hepatocyte growth factor; HR, hazard ratio (continuous unless otherwise noted); IL-18, interleukin-18; IGFBP7, insulin like growth factor binding protein 7; L-FABP, liver fatty acid binding protein; NAG, N-acetyl-beta-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; OR, odds ratio; TIMP-2, tissue inhibitor of metalloproteinases-2.
Short-term outcomes. One ICU cohort study, Validation of Biomarkers for Acute Lung Injury Diagnosis (VALID), comprising critically ill patients in both medical and surgical ICU settings, collected urinary biomarker specimens on admission and 48 hours after admission in over 400 patients. In VALID, admission levels of urinary IL-18 were independently associated with the composite outcome of 28-day mortality or dialysis even after adjustment for APACHE-II score, serum creatinine, and sepsis criteria 38. Despite the relationship between urinary IL-18 and the composite outcome, other tubular injury markers, such as urinary NGAL and L-FABP, were associated only with the initiation of dialysis at 28 days and were not associated with the composite outcome 39, 40. This persisted despite the authors excluding patients with unidentified CKD or AKI upon enrollment into the study 39. Similar results for urinary IL-18 and NGAL have been found in other cohorts of critically ill patients. A post hoc case-control study of 132 patients in the ARDS Network additionally found that increasing levels of urinary IL-18 on admission were associated with increased 1-month mortality 41. Another prospective cohort of 339 patients in a mixed ICU setting showed that admission urinary L-FABP, IL-18, and NGAL could discriminate between survivors and non-survivors, and a combination of urinary NGAL and L-FABP was best able to discriminate between survivors and non-survivors 42. Additionally, increasing levels of urinary cystatin C, a biomarker of glomerular filtration, in a group of 444 ICU patients were associated with increased risk of 1-month mortality 43.
As in cardiac surgery, a decline in tubular injury biomarkers is also associated with improved patient outcomes. A post hoc analysis of 76 patients from the Acute Renal Failure Trial Network (ATN) trial, which evaluated the effect of dialysis dose on critically ill patients, investigated whether urinary biomarkers were associated with recovery from AKI and found that decreasing levels of urinary NGAL and hepatocyte growth factor (HGF) over a 2-week period predicted an increased chance of recovery of renal function 60 days after ICU admission 44.
Long-term outcomes. While biomarkers of tubular injury are studied most carefully, another cohort comprising 744 ICU patients (Sapphire) found that urinary biomarkers of cell cycle arrest (TIMP-2*IGFBP-7) were associated with a twofold increased risk of RRT and 9-month mortality, although this relationship was found only in patients with AKI as defined by serum creatinine 45.
Cardiorenal syndrome
Heart failure patients commonly suffer from AKI, which is strongly associated with increased mortality 46. Cardiorenal syndrome, or the co-existence of AKI and heart failure, is often ascribed to hemodynamic perturbations or venous congestion. This is likely an over-simplification, as patients with cardiorenal syndrome respond differently to standard heart failure therapies, such as diuresis and inotropic support. Biomarker research has attempted to untangle the numerous phenotypes that exist within cardiorenal syndrome 47.
Long-term outcomes. There is extensive literature surrounding long-term prognosis and biomarkers of tubular injury in heart failure patients. Tubular injury biomarkers, including urinary KIM-1, NGAL, and N-acetyl-β-D-glucosaminidase (NAG), were associated with long-term mortality and recurrent heart failure hospitalization even after accounting for glomerular filtration rate (GFR) in multiple case-control studies and cohorts ( Table 3) 48– 50. KIM-1 and NAG were significantly correlated with New York Heart Association class, suggesting that tubular injury may contribute to the pathophysiology of cardiorenal syndrome 50. While urinary biomarkers of tubular injury have been consistently associated with mortality and other adverse outcomes, plasma NGAL has not demonstrated a consistent relationship, particularly after adjustment for renal function 51– 53. This has also been found in cardiac surgery and is likely because plasma NGAL is renally excreted, effectively rendering it another marker of GFR 54.
Table 3. Biomarkers of AKI and outcomes in cardiorenal syndrome.
| Biomarkers | LONG-TERM OUTCOMES (>1 YEAR FOLLOW-UP) | ||
|---|---|---|---|
| MACE | Mortality | Hospitalization | |
|
Urine NGAL
Damman 2011 48 |
HR 1.23 (1.07, 1.41) | HR 1.01 (0.90, 1.13) | |
|
Urine NAG
Damman 2010 49 Damman 2011 48 |
HR 1.43 (1.10, 1.84) † HR 1.22 (1.10, 1.36) |
HR 1.30(1.11–1.51) | HR 1.17(1.02, 1.33) |
|
Urine KIM-1
Damman 2010 49 Damman 2011 48 |
HR 1.13 (1.00, 1.28) HR 1.22 (1.03, 1.45) |
HR 1.11 (0.98–1.26) | HR 1.11(0.98, 1.26) |
|
Plasma NGAL
GALLANT 51 COACH 53 Shrestha 2011 52 |
HR 29.83 (5.43, 163.96)* § HR 1.06 (0.66, 1.84) |
HR 1.44(1.22, 1.69) § |
|
All values in ( ) are 95% confidence intervals, unless otherwise noted.
*unadjusted and 30 day follow-up, § greater than cut-off versus less than cut-off, † per 5U/gram Creatinine increase.
AKI, acute kidney injury; HR, hazard ratio (per standard deviation increase unless otherwise noted); KIM-1, kidney injury molecule-1; MACE, major adverse cardiac events; NAG, N-acetyl-beta-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin.
Kidney transplantation
Novel urinary biomarkers have also been investigated for prognostic utility in the kidney transplant setting and can have unique applications in the transplant field. Given the persistent shortage of organs and the increasing number of patients on the transplant list, biomarkers of AKI can potentially be used to decide if a deceased donor organ is acceptable for transplant as well as improve prediction for adverse recipient outcomes, such as delayed graft function (DGF) and graft failure ( Table 4).
Table 4. Biomarkers of AKI and outcomes in kidney transplantation.
| Urine biomarkers | OUTCOMES | ||
|---|---|---|---|
| Short-term (1 week) | Long-term (>1 year follow-up) | ||
| Delayed graft function | Graft failure at 1 year | Graft failure + death | |
|
Recipient IL-18
Hall 2012 58 Hall 2010 55 |
OR 5.1 (1.14, 2.28)* |
HR 1.26 (0.74, 2.14) |
|
|
Recipient NGAL
Nauta 2011 59 Parikh 2006 56 Hall 2010 55 Hall 2012 58 |
OR 6.8 (1.42, 32.2)* |
HR 1.61 (1.04, 2.50) |
HR 1.5 (1.2, 1.8) HR 1.2 (1.0, 1.5) † |
|
Recipient NAG
Nauta 2011 59 |
HR 1.4 (1.2, 1.7) | ||
|
Recipient KIM-1
Nauta 2011 59 |
HR 1.3 (1.1, 1.7) | ||
|
Recipient H-FABP
Nauta 2011 59 |
HR 1.5 (1.2, 1.8) | ||
All values in ( ) are 95% confidence intervals, unless otherwise noted.
*greater than cut-off versus less than cut-off, † per 100 ng/mg increase.
AKI, acute kidney injury; H-FABP, heart fatty acid binding protein; HR, hazard ratio (continuous unless otherwise noted); IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NAG, N-acetyl-beta-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; OR, odds ratio.
Short-term outcomes. In the perioperative period, the presence of urinary IL-18 and NGAL immediately after transplant was associated with an increased risk of DGF 55, 56. While the relationship between recipient kidney injury biomarkers and outcomes is relatively clear, the relationship between donor kidney injury biomarkers and recipient outcomes is more complex. One large multi-center cohort found that elevated donor urinary NGAL had a modest association with DGF in the recipient, although other tubular injury biomarkers were not significantly associated after multivariable adjustment 57.
Long-term outcomes. Single-center studies of transplant recipients found that elevated urinary tubular injury biomarkers, such as urinary IL-18, NGAL, NAG, and KIM-1, are associated with an increased risk of graft failure or death ( Table 4) 58, 59. However, urinary donor biomarkers, including urinary IL-18, NGAL, and L-FABP, modified the relationship between DGF and 6-month recipient GFR. For recipients with DGF, higher levels of tubular injury biomarkers were associated with higher 6-month GFR, while for recipients who did not experience DGF, higher urinary donor biomarkers were associated with lower 6-month GFR 57.
Heterogeneous populations: hospitalized patients and the emergency department
While the most extensive evidence for the association between biomarkers of AKI and prognosis is in specific patient populations, biomarkers of AKI also have prognostic utility for short-term outcomes in heterogeneous populations. Multiple single-center cohorts of patients requiring nephrology consultation found that biomarkers of tubular injury were associated with an increased risk of in-hospital RRT or death ( Table 5) 60– 63. Alternatively, elevated plasma NGAL, a marker of glomerular filtration, in patients hospitalized with pneumonia and severe AKI meant they were twice as likely to fail to recover renal function by discharge in adjusted analysis 64. In another cohort of hospitalized patients requiring nephrology consultation owing to presumed acute tubular necrosis, elevated urinary epidermal growth factor levels allowed the authors to distinguish between patients who recovered renal function within 1 week of renal consult and those who did not. Additionally, elevated inflammatory cytokines identified patients who failed to recover renal function within 1 week of renal consult 65. Urinary NGAL and KIM-1 measured at the time of emergency room visit were both independently associated with in-hospital RRT initiation or death after accounting for clinical parameters 66.
Table 5. Biomarkers of AKI and outcomes in heterogeneous populations.
| Biomarkers | IN-HOSPITAL OUTCOMES | |
|---|---|---|
| RRT or death | Failure to recover from AKI | |
|
Urine IL-18
Hall 2011 60 |
OR 2.7 (1.4, 5.0)* | |
|
Urine NGAL
Hall 2011 60 Singer 2011 63 Nickolas 2011 66 |
OR 2.6 (1.6, 4.3)* OR 4.2 (1.9, 9.0) § OR 2.43 (1.42, 4.16) § |
|
|
Urine KIM-1
Liangos 2007 61 Hall 2011 60 Nickolas 2011 66 |
AUC 0.61 (0.53, 0.69) OR 2.8 (1.5, 5.3)* OR 2.54 (1.50, 4.30) § |
|
|
Urine NAG
Liangos 2007 61 |
AUC 0.71 (0.63, 0.78) | |
|
Plasma NGAL
Srisawat 2011 64 |
OR 2.02 (1.03, 3.31) † | |
|
Urine EGF
Kwon 2010 65 |
AUC 0.83 (0.70, 0.96) | |
All values in ( ) are 95% confidence intervals, unless otherwise noted.
*4 th quartile versus 1 st–3 rd quartile, § greater than cut-off versus less than cut-off, † per 300 ng/ml increase.
AKI, acute kidney injury; AUC, area under the curve; EGF, epidermal growth factor; HR, hazard ratio; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; NAG, N-acetyl-beta-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; OR, odds ratio; RRT, renal replacement therapy.
Future directions
Next-generation biomarkers
The bioinformatics revolution has improved discovery techniques for the identification of next-generation biomarkers. Biomarker research has primarily focused on biomarkers that are considered part of the proteome. Recent studies have begun examining other layers of cellular data, including microRNA and mitochondrial DNA 67– 70. While these biomarkers are in the early stages of investigation, they hold great promise, as they provide opportunities to probe other components of cellular pathways.
Limitations for clinical application and future directions
Several barriers remain for the clinical application of biomarkers despite the extensive evidence that they are associated with prognosis. Biomarkers of AKI often provide modest improvement in discriminative ability compared to traditional clinical models and have different trajectories and time scales depending upon the patient population and biomarker being studied. This will pose a challenge for busy clinicians attempting to incorporate a cornucopia of biomarkers into clinical practice. Moreover, data are lacking to support whether biomarkers are associated with organ-specific long-term outcomes, such as CKD or cardiovascular disease. Technical challenges also exist, as developing, standardizing, and validating biomarker assays is a laborious process 71. Most importantly, researchers have yet to identify therapeutic changes to institute once patients are identified as being at high risk for adverse outcomes based on biomarkers of AKI.
Given the heterogeneity and dynamic nature of AKI, one biomarker will not accurately predict all adverse outcomes across several settings, nor will it accurately identify the various stages in the natural history of AKI. One important task is to definitively identify the natural stages of AKI as demarcated by biomarkers: injury, maintenance, and repair. This could help create a more balanced picture of prognostic outcomes for patients. For example, patients may have different outcomes depending on the magnitude of “injury” biomarkers balanced by the level of “repair” biomarkers.
While challenges remain, biomarkers can offer several potential benefits to the diagnosis, management, and treatment of AKI in the foreseeable future. Perhaps the most immediate application is to identify patients who are experiencing renal injury from nephrotoxic agents, prolonged surgeries, cardiorenal syndrome, or hepatorenal syndrome. Patients with elevated injury or fibrosis markers could be considered for alternative therapy instead of waiting for these patients to develop AKI or CKD as defined by serum creatinine. However, for biomarkers to become the “standard of care” for the management of these patients, prospective trials should be conducted to identify cut-points for biomarker levels which will identify patients at a sufficiently high risk of renal complications to warrant providing a therapeutic intervention or adjusting surgical management.
Biomarkers of AKI can have two potential applications in clinical trials of putative drug treatments for AKI. First, they will allow us to identify patients who have ongoing injury so that therapies may be provided while the injury process is occurring. Second, the greatest potential for biomarkers is if they can identify patients at high risk of adverse outcomes. In such a scenario, treatment can then be personalized for high-risk subgroups of patients. Trials in AKI have been hindered by low event rates and are likely enrolling patients with multiple phenotypes of AKI. Given the wealth of information showing that biomarkers are related to important patient outcomes, such as RRT and death, future clinical trials for AKI should enroll patients with elevated biomarkers of AKI in order to increase the likelihood that patients would actually benefit from the putative treatment. The TOPCAT trial, which examined the benefit of spironolactone in heart failure patients with preserved ejection fraction, found that treatment benefit was isolated to patients with elevated natriuretic peptide levels 72. Future AKI trials should employ similar enrollment strategies, as treatment benefit may be isolated to particular AKI phenotypes. The Assessment, Serial Evaluation and Subsequent Sequelae of Acute Kidney Injury study is ongoing to determine if patients with AKI experience increased risk of long-term complications, such as cardiovascular events and CKD 27. This study should help ascertain if it is worthwhile enrolling patients with AKI as defined by biomarkers in lieu of serum creatinine in future clinical trials.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Brian Reeves, Division of Nephrology, Department of Medicine, Penn State Hershey Medical Center and College of Medicine, Hershey, PA, USA
Charuhas Thakar, Director, Division of Nephrology, Kidney CARE Program, University of Cincinnati College of Medicine, Cincinnati, OH, USA
Funding Statement
Chirag R. Parikh is supported by the NIH grants K24DK090203, P30 DK079310 O'Brien Center Grant and R01HL085757. Jennifer A. Schaub is supported by T32DK007276-36.
[version 1; referees: 2 approved]
References
- 1. Uchino S, Kellum JA, Bellomo R, et al. : Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8. 10.1001/jama.294.7.813 [DOI] [PubMed] [Google Scholar]
- 2. Bagshaw SM, Uchino S, Bellomo R, et al. : Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2(3):431–9. 10.2215/CJN.03681106 [DOI] [PubMed] [Google Scholar]
- 3. Coca SG, Yusuf B, Shlipak MG, et al. : Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–73. 10.1053/j.ajkd.2008.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coca SG, King JT, Jr, Rosenthal RA, et al. : The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78(9):926–33. 10.1038/ki.2010.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uchino S, Bellomo R, Bagshaw SM, et al. : Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25(6):1833–9. 10.1093/ndt/gfp624 [DOI] [PubMed] [Google Scholar]
- 6. Grams ME, Rabb H: The distant organ effects of acute kidney injury. Kidney Int. 2012;81(10):942–8. 10.1038/ki.2011.241 [DOI] [PubMed] [Google Scholar]
- 7. Chen HH, Anstrom KJ, Givertz MM, et al. : Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310(23):2533–43. 10.1001/jama.2013.282190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg AX, Kurz A, Sessler DI, et al. : Perioperative aspirin and clonidine and risk of acute kidney injury: a randomized clinical trial. JAMA. 2014;312(21):2254–64. 10.1001/jama.2014.15284 [DOI] [PubMed] [Google Scholar]
- 9. Hausenloy DJ, Candilio L, Evans R, et al. : Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med. 2015;373(15):1408–17. 10.1056/NEJMoa1413534 [DOI] [PubMed] [Google Scholar]
- 10. Whitlock RP, Devereaux PJ, Teoh KH, et al. : Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10000):1243–53. 10.1016/S0140-6736(15)00273-1 [DOI] [PubMed] [Google Scholar]
- 11. Chen HH, AbouEzzeddine OF, Anstrom KJ, et al. : Targeting the kidney in acute heart failure: can old drugs provide new benefit? Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE AHF) trial. Circ Heart Fail. 2013;6(5):1087–94. 10.1161/CIRCHEARTFAILURE.113.000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu CY, Hsu RK, Yang J, et al. : Elevated BP after AKI. J Am Soc Nephrol. 2016;27(3):914–23. 10.1681/ASN.2014111114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pechman KR, De Miguel C, Lund H, et al. : Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1358–63. 10.1152/ajpregu.91022.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spurgeon-Pechman KR, Donohoe DL, Mattson DL, et al. : Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol. 2007;293(1):F269–78. 10.1152/ajprenal.00279.2006 [DOI] [PubMed] [Google Scholar]
- 15. Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–8. 10.1038/ki.2011.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chawla LS, Amdur RL, Shaw AD, et al. : Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014;9(3):448–56. 10.2215/CJN.02440213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Canaud G, Bonventre JV: Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol Dial Transplant. 2015;30(4):575–83. 10.1093/ndt/gfu230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang L, Besschetnova TY, Brooks CR, et al. : Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–43, 1p following 143. 10.1038/nm.2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burchill L, Velkoska E, Dean RG, et al. : Acute kidney injury in the rat causes cardiac remodelling and increases angiotensin-converting enzyme 2 expression. Exp Physiol. 2008;93(5):622–30. 10.1113/expphysiol.2007.040386 [DOI] [PubMed] [Google Scholar]
- 20. Kelly KJ: Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14(6):1549–58. 10.1097/01.ASN.0000064946.94590.46 [DOI] [PubMed] [Google Scholar]
- 21. Tokuyama H, Kelly DJ, Zhang Y, et al. : Macrophage infiltration and cellular proliferation in the non-ischemic kidney and heart following prolonged unilateral renal ischemia. Nephron Physiol. 2007;106(3):p54–62. 10.1159/000103910 [DOI] [PubMed] [Google Scholar]
- 22. Robinson SC, Bowmer CJ, Yates MS: Cardiac function in rats with acute renal failure. J Pharm Pharmacol. 1992;44(12):1007–14. 10.1111/j.2042-7158.1992.tb07083.x [DOI] [PubMed] [Google Scholar]
- 23. Han WK, Bailly V, Abichandani R, et al. : Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–44. 10.1046/j.1523-1755.2002.00433.x [DOI] [PubMed] [Google Scholar]
- 24. Melnikov VY, Ecder T, Fantuzzi G, et al. : Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107(9):1145–52. 10.1172/JCI12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Melnikov VY, Faubel S, Siegmund B, et al. : Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest. 2002;110(8):1083–91. 10.1172/JCI15623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mishra J, Ma Q, Prada A, et al. : Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–43. 10.1097/01.ASN.0000088027.54400.C6 [DOI] [PubMed] [Google Scholar]
- 27. Go AS, Parikh CR, Ikizler TA, et al. : The assessment, serial evaluation, and subsequent sequelae of acute kidney injury (ASSESS-AKI) study: design and methods. BMC Nephrol. 2010;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coca SG, Garg AX, Thiessen-Philbrook H, et al. : Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol. 2014;25(5):1063–71. 10.1681/ASN.2013070742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haase M, Devarajan P, Haase-Fielitz A, et al. : The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57(17):1752–61. 10.1016/j.jacc.2010.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parikh CR, Coca SG, Thiessen-Philbrook H, et al. : Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22(9):1748–57. 10.1681/ASN.2010121302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parikh CR, Thiessen-Philbrook H, Garg AX, et al. : Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8(7):1079–88. 10.2215/CJN.10971012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koyner JL, Garg AX, Shlipak MG, et al. : Urinary cystatin C and acute kidney injury after cardiac surgery. Am J Kidney Dis. 2013;61(5):730–8. 10.1053/j.ajkd.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arthur JM, Hill EG, Alge JL, et al. : Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 2014;85(2):431–8. 10.1038/ki.2013.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alge JL, Karakala N, Neely BA, et al. : Association of elevated urinary concentration of renin-angiotensin system components and severe AKI. Clin J Am Soc Nephrol. 2013;8(12):2043–52. 10.2215/CJN.03510413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meersch M, Schmidt C, van Aken H, et al. : Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9(3):e93460. 10.1371/journal.pone.0093460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang WR, Garg AX, Coca SG, et al. : Plasma IL-6 and IL-10 Concentrations Predict AKI and Long-Term Mortality in Adults after Cardiac Surgery. J Am Soc Nephrol. 2015;26(12):3123–32. 10.1681/ASN.2014080764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Calfee CS, Delucchi K, Parsons PE, et al. : Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–20. 10.1016/S2213-2600(14)70097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siew ED, Ikizler TA, Gebretsadik T, et al. : Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol. 2010;5(8):1497–505. 10.2215/CJN.09061209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siew ED, Ware LB, Bian A, et al. : Distinct injury markers for the early detection and prognosis of incident acute kidney injury in critically ill adults with preserved kidney function. Kidney Int. 2013;84(4):786–94. 10.1038/ki.2013.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siew ED, Ware LB, Gebretsadik T, et al. : Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20(8):1823–32. 10.1681/ASN.2008070673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parikh CR, Abraham E, Ancukiewicz M, et al. : Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16(10):3046–52. 10.1681/ASN.2005030236 [DOI] [PubMed] [Google Scholar]
- 42. Doi K, Negishi K, Ishizu T, et al. : Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med. 2011;39(11):2464–9. 10.1097/CCM.0b013e318225761a [DOI] [PubMed] [Google Scholar]
- 43. Nejat M, Pickering JW, Walker RJ, et al. : Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit Care. 2010;14(3):R85. 10.1186/cc9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Srisawat N, Wen X, Lee M, et al. : Urinary biomarkers and renal recovery in critically ill patients with renal support. Clin J Am Soc Nephrol. 2011;6(8):1815–23. 10.2215/CJN.11261210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koyner JL, Shaw AD, Chawla LS, et al. : Tissue Inhibitor Metalloproteinase-2 (TIMP-2)·IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Long-Term Outcomes in Patients with AKI. J Am Soc Nephrol. 2015;26(7):1747–54. 10.1681/ASN.2014060556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith GL, Lichtman JH, Bracken MB, et al. : Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47(10):1987–96. 10.1016/j.jacc.2005.11.084 [DOI] [PubMed] [Google Scholar]
- 47. Brisco MA, Testani JM: Novel renal biomarkers to assess cardiorenal syndrome. Curr Heart Fail Rep. 2014;11(4):485–99. 10.1007/s11897-014-0226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Damman K, Masson S, Hillege HL, et al. : Clinical outcome of renal tubular damage in chronic heart failure. Eur Heart J. 2011;32(21):2705–12. 10.1093/eurheartj/ehr190 [DOI] [PubMed] [Google Scholar]
- 49. Damman K, van Veldhuisen DJ, Navis G, et al. : Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96(16):1297–302. 10.1136/hrt.2010.194878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jungbauer CG, Birner C, Jung B, et al. : Kidney injury molecule-1 and N-acetyl-β- D-glucosaminidase in chronic heart failure: possible biomarkers of cardiorenal syndrome. Eur J Heart Fail. 2011;13(10):1104–10. 10.1093/eurjhf/hfr102 [DOI] [PubMed] [Google Scholar]
- 51. Maisel AS, Mueller C, Fitzgerald R, et al. : Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: the NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail. 2011;13(8):846–51. 10.1093/eurjhf/hfr087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shrestha K, Borowski AG, Troughton RW, et al. : Renal dysfunction is a stronger determinant of systemic neutrophil gelatinase-associated lipocalin levels than myocardial dysfunction in systolic heart failure. J Card Fail. 2011;17(6):472–8. 10.1016/j.cardfail.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van Deursen VM, Damman K, Voors AA, et al. : Prognostic value of plasma neutrophil gelatinase-associated lipocalin for mortality in patients with heart failure. Circ Heart Fail. 2014;7(1):35–42. 10.1161/CIRCHEARTFAILURE.113.000242 [DOI] [PubMed] [Google Scholar]
- 54. Moledina DG, Parikh CR, Garg AX, et al. : Association of Perioperative Plasma Neutrophil Gelatinase-Associated Lipocalin Levels with 3-Year Mortality after Cardiac Surgery: A Prospective Observational Cohort Study. PLoS One. 2015;10(6):e0129619. 10.1371/journal.pone.0129619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hall IE, Yarlagadda SG, Coca SG, et al. : IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21(1):189–97. 10.1681/ASN.2009030264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Parikh CR, Jani A, Mishra J, et al. : Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6(7):1639–45. 10.1111/j.1600-6143.2006.01352.x [DOI] [PubMed] [Google Scholar]
- 57. Reese PP, Hall IE, Weng FL, et al. : Associations between Deceased-Donor Urine Injury Biomarkers and Kidney Transplant Outcomes. J Am Soc Nephrol. 2016;27(5):1534–43. 10.1681/ASN.2015040345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hall IE, Doshi MD, Reese PP, et al. : Association between peritransplant kidney injury biomarkers and 1-year allograft outcomes. Clin J Am Soc Nephrol. 2012;7(8):1224–33. 10.2215/CJN.00310112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nauta FL, Bakker SJ, van Oeveren W, et al. : Albuminuria, proteinuria, and novel urine biomarkers as predictors of long-term allograft outcomes in kidney transplant recipients. Am J Kidney Dis. 2011;57(5):733–43. 10.1053/j.ajkd.2010.12.022 [DOI] [PubMed] [Google Scholar]
- 60. Hall IE, Coca SG, Perazella MA, et al. : Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis. Clin J Am Soc Nephrol. 2011;6(12):2740–9. 10.2215/CJN.04960511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liangos O, Perianayagam MC, Vaidya VS, et al. : Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18(3):904–12. 10.1681/ASN.2006030221 [DOI] [PubMed] [Google Scholar]
- 62. Nejat M, Pickering JW, Devarajan P, et al. : Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int. 2012;81(12):1254–62. 10.1038/ki.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Singer E, Elger A, Elitok S, et al. : Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80(4):405–14. 10.1038/ki.2011.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Srisawat N, Murugan R, Lee M, et al. : Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int. 2011;80(5):545–52. 10.1038/ki.2011.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kwon O, Ahn K, Zhang B, et al. : Simultaneous monitoring of multiple urinary cytokines may predict renal and patient outcome in ischemic AKI. Ren Fail. 2010;32(6):699–708. 10.3109/0886022X.2010.486496 [DOI] [PubMed] [Google Scholar]
- 66. Nickolas TL, Schmidt-Ott KM, Canetta P, et al. : Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59(3):246–55. 10.1016/j.jacc.2011.10.854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee CG, Kim JG, Kim HJ, et al. : Discovery of an integrative network of microRNAs and transcriptomics changes for acute kidney injury. Kidney Int. 2014;86(5):943–53. 10.1038/ki.2014.117 [DOI] [PubMed] [Google Scholar]
- 68. Ramachandran K, Saikumar J, Bijol V, et al. : Human miRNome profiling identifies microRNAs differentially present in the urine after kidney injury. Clin Chem. 2013;59(12):1742–52. 10.1373/clinchem.2013.210245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Saikumar J, Hoffmann D, Kim TM, et al. : Expression, circulation, and excretion profile of microRNA-21, -155, and -18a following acute kidney injury. Toxicol Sci. 2012;129(2):256–67. 10.1093/toxsci/kfs210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Whitaker RM, Stallons LJ, Kneff JE, et al. : Urinary mitochondrial DNA is a biomarker of mitochondrial disruption and renal dysfunction in acute kidney injury. Kidney Int. 2015;88(6):1336–44. 10.1038/ki.2015.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pepe MS, Etzioni R, Feng Z, et al. : Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93(14):1054–61. 10.1093/jnci/93.14.1054 [DOI] [PubMed] [Google Scholar]
- 72. Pitt B, Pfeffer MA, Assmann SF, et al. : Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–92. 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 73. Koyner JL, Parikh CR: Clinical utility of biomarkers of AKI in cardiac surgery and critical illness. Clin J Am Soc Nephrol. 2013;8(6):1034–42. 10.2215/CJN.05150512 [DOI] [PubMed] [Google Scholar]
- 74. Huen SC, Parikh CR: Molecular phenotyping of clinical AKI with novel urinary biomarkers. Am J Physiol Renal Physiol. 2015;309(5):F406–13. 10.1152/ajprenal.00682.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]