Abstract
Autoantibodies associated with autoimmune limbic encephalitis (ALE) have been well-characterized, with intracellular neuronal antibodies being less responsive to immunotherapy than antibodies to cell surface antigens. Adenylate kinase 5 (AK5) is a nucleoside monophosphate kinase vital for neuronal-specific metabolism and is located intracellularly in the cytosol and expressed exclusively in the brain. Antibodies to AK5 had been previously identified but were not known to be associated with human disease prior to the report of two patients with AK5-related ALE (Tuzun et al., 2007). We present the complete clinical picture for one of these patients and the first reported neuropathology for AK5 ALE.
Keywords: Adenylate kinase 5, Autoimmunity, Limbic encephalitis, Neuronal antibodies, Rapidly progressive dementia
1. Introduction
Autoimmune-mediated limbic encephalitis (ALE) is a syndrome of rapidly progressive cognitive decline associated with psychiatric disturbances, memory deficits, and possibly seizures due to antibodies against central nervous system (CNS) targets. These disorders have been classified into two main groups: Group I with intracellular antigen targets, and Group II with cell surface targets (Graus et al., 2010). Most of the ALE syndromes with intracellular targets have been associated with paraneoplastic conditions (Gultekin et al., 2000), but there are an increasing number of patients in whom extensive investigation and follow-up exclude an underlying neoplasm (Graus et al., 2010). A few years ago, approximately 20% of patients with clinical and laboratory findings compatible with ALE test negative for all known autoantibodies (Bataller et al., 2007), although since then novel antibodies and associated antigens have been discovered, including anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antibodies (anti-AMPAR) (Lai et al., 2009), and anti-GABA(B) antibodies (Lancaster et al., 2010). Nevertheless, novel antibody/antigen syndromes are still being identified. Two patients were previously reported with ALE who were negative for all known neuronal antibodies at the time, but found in a research laboratory to have adenylate kinase 5 antibodies (Tuzun et al., 2007). Both had no evidence of any underlying cancer and remained refractory to aggressive immunomodulatory treatment resulting in progression to frank dementia. We now present one of these cases (Patient 1 in Tuzun et al., 2007) in detail with the first reported neuropathology for AK5 ALE, showing predominantly T-lymphocytic infiltrates of mainly CD8 subtype, confirming the inflammation as cytotoxic/CD8+ rather than an antibody-mediated/B-cell process, consistent with ALE associated with antibodies against intracellular antigens. Given that AK5 is intracellular, these findings are supportive of this concept.
2. Case report
A right-handed 71 year-old gentleman with a history of attention deficit disorder, depression, alcohol abuse and ischemic heart disease, was otherwise living independently till early August 2005 when he started to be forgetful, missing appointments, and misplacing items. This progressed to being mildly disoriented by the end of the month, with an acute deterioration a few weeks later with symptoms of apathy and behavioral change. He was admitted to hospital where brain MRI revealed FLAIR hyperintensity in the right temporal region (Fig. 1A). Standard dementia laboratory investigations were unremarkable. Body CT without contrast was reportedly normal. Cerebrospinal fluid (CSF) was negative/normal for herpes simplex, cell count, protein and glucose levels, but with mildly elevated IgG index (0.7; normal 0.28–0.66) and positive for oligoclonal bands. Family history was significant for his father dying in his forties from unknown cancer; his mother died in her nineties from a stroke. He has two healthy daughters. His identical twin suffered from hypertension, depression, and alcoholism.
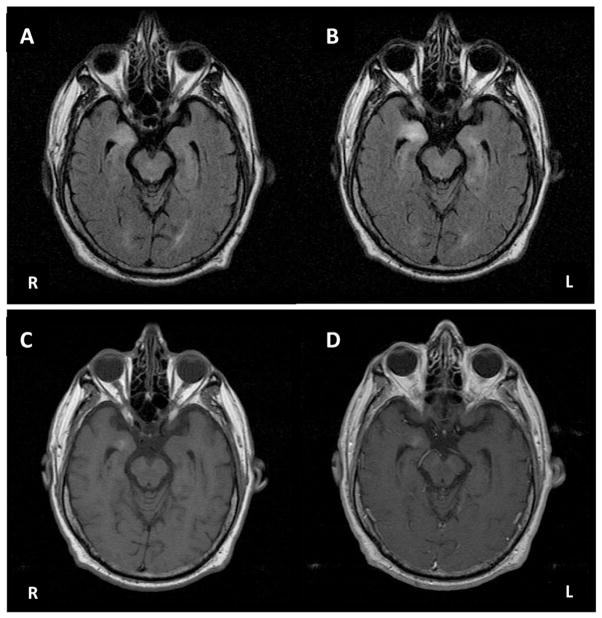
Fig. 1.
A. Axial FLAIR brain MRI at 2 months after onset showing right temporal hyperintensity. B. Axial FLAIR brain MRI 3 months after onset showing increase in right temporal hyperintensity and new left temporal hyperintensity. C. Axial T1-weighted brain MRI at 3 months after onset pre-contrast. D. Axial T1-weighted brain MRI at 3 months after onset post-contrast showing equivocal enhancement.
Repeat MRI a few weeks later showed increasing signal in the right temporal lobe on T2/FLAIR with equivocal enhancement on T1, and possibly new increased signal in the left temporal lobe (Fig. 1B–D). Persistent cognitive deficits prompted a referral to our center, three months after onset. He had worsening short-term memory and behavioral changes, with apathy, some episodes of mild disinhibition (walking around his apartment naked), and required assistance with most activities of daily living from a caregiver, but still remained able to use a microwave and watch TV. Neurological examination revealed mild bilateral postural tremor with mildly impaired tandem gait and mild postural instability on retropulsion testing. On neuropsychological testing, his Mini-Mental Status Exam score was 22/30, with deficits in memory and orientation. He performed significantly below average for his age and education (Master's degree) on measures of verbal and non-verbal memory, working memory, attention, processing speed, executive function and visuospatial skills (Table 1), with relative sparing of language. Further laboratory work-up including HIV, thyroid antibodies, ALE antibody screen (anti-Hu, anti-Yo, anti-Ri, anti-CV2, anti-Ma/Ta, voltage-gated potassium channel complex (anti-VGKC) antibodies) were negative. An electroencephalogram and repeat body PET/CT with contrast were normal. As his clinical syndrome and MRI findings were highly suggestive for ALE, serum and CSF were sent for novel antibody testing (Laboratory of J. Dalmau). While awaiting results of for this testing, he was started on immunotherapy 5 months after symptom onset. He received a five-day course of intravenous immunoglobulin (IVIG; 2 g/kg) and IV methylprednisolone (1 g/day), which resulted in short-lived improvement of only two days. He was moved to an assisted living facility as a result of his continued decline into a delirious state in which he was unable to feed himself and had fluctuating episodes of delusions, hallucinations and euphoria. Cancer surveillance during follow-up remained negative for neoplasm. Novel antibody testing in his serum and CSF showed intense neuronal reactivity in a pattern resembling CV2/CRMP5. Subsequent immunohistochemistry confirmed specific reactivity of both serum and CSF antibodies to AK5 (Tuzun et al., 2007). He was maintained on daily oral prednisone during this period, and subsequently received a course (5 cycles) of plasma exchange therapy (PLEX) which did not result in any clinical improvement (he had one generalized seizure after one cycle). He progressed further, requiring admission into a nursing home.
Table 1.
Subject's neuropsychological testing results, with accompanying Z-scores and University of California, San Francisco (UCSF) normative means and standard deviations for his age and education. Neuropsychological batteries in bold denote ones in which the subject scored 2 or more standard deviations below the mean for his age and education.
| Neuropsychological tests | Subject's score | Z-scores | UCSF normative means (SD) for his age & education |
|---|---|---|---|
| Global cognition | |||
| Mini Mental Status Examination (MMSE) | 22 out of 30 missed points for date, month, day of the week, name, floor of clinic; delayed recall 0 out of 3 | ||
| Memory | |||
| California Verbal Learning Test-San Francisco (CVLT-SF) | Learning T1, T2, T3, and T4: 2, 4, 5, and 5 words | LT4: Z = −2.5 | LT4: 7.8 (1.1) |
| 2 words after 30 s | 30 s: Z = −3.5 | 30 s: 7.2 (1.5) | |
| 0 words after 10 min | 10 min: Z = −3.0 | 10 min: 6.4 (2.1) | |
| Recognized 4 words correctly with 5 false positives | Recognition: Z = −3.8 | Recognition: 8.2 (1.1) | |
| Modified Rey–Osterrieth figure | Recall 0 out of 17; unable to recognize on multiple choice | Recall: Z = −4.1 | Recall: 11.5 (2.8) |
| Working memory, attention, speed & executive function | |||
| Verbal fluency (per min) | Lexical fluency: 16 D-words with 6 repetitions and 1 rule violation | D-words: Z = −0.1 | D-words: 16.3 (4.5) |
| Animals: Z = −3.5 | Animals: 23.5 (5.0) | ||
| Semantic fluency: 6 animals with 1 repetition | |||
| Design fluency | 4 with 1 repetition | Z = −2.1 | 11.3 (3.4) |
| Digit span backward/working memory | 3 digits backwards | Z = −2.3 | 5.8 (1.2) |
| Able to spell WORLD backwards but with hesitancy | |||
| Modified trails | 3 out of 14 with 2 errors, timed out at 120 s | Trail time: 27.4 (10.4) s | |
| Stroop color naming | 75 correct | Z = −1.2 | 91.3 (13.4) |
| Stroop interference | 34 words/min with no errors | Z = −1.7 | 51.9 (10.6) |
| Abstract reasoning (proverbs + similarities) | Interpreted 1 out of 3 proverbs | Z = −1.3 | Total: 5.2 (0.9) |
| Able to identify conceptual similarities between 3 word pairs | |||
| Language | |||
| Abbreviated Boston Naming Test (BNT) | 14 out of 15 items | Z = −0.4 | 14.3 (0.8) |
| Repetition | 4 out of 5 sentences | Z = −1.0 | 4.6 (0.6) |
| Reading | 10 out of 10 words | Within normal limits | |
| PPVT-R | 16 out of 16 items | Z = 0.5 | 15.6 (0.8) |
| Visuospatial skills | |||
| Pentagons | Copied intersecting pentagons without error | ||
| Visual-Object Space Perception Battery (VOSP) | 5 out of 10 items | Z = −3.3 | 9.0 (1.2) |
| Modified Rey–Osterrieth figure | Able to copy Fig. 17 out of 17 components | Copy: Z = 1.4 | Copy: 15.5 (1.1) |
| Miscellaneous | |||
| Calculations | 3 out of 5 calculations | Z = −4.5 | 4.8 (0.4) |
| Geriatric Depression Scale | 6 out of 30 items | Minimal | N/A |
Although his behavioral symptoms and delusions responded slightly to risperidone, he showed no response to immunotherapy and continued to deteriorate cognitively. He was unable to name his grandchildren and occasionally his daughter, and introduced a male caretaker as his “husband”. Motorically, he had relatively intact power but required assistance to stand and a parkinsonian gait. He unfortunately continued to remain in a demented state and died two years after symptom onset from an acute myocardial injury. Due to a holiday weekend, total body autopsy was performed 4 days after death.
Macroscopically, brain autopsy revealed severe softening of the anterior halves of the temporal lobes with increased prominence and flattening of sulci and a slight decrease of the left superior temporal gyrus. Microscopically, there was extensive loss of neurons, microgliosis and astrogliosis, involving the temporal cortex, hippocampi, insular cortex and claustrum. Infiltration of lymphocytes, in perivascular spaces and brain parenchyma of the temporal lobe, insular cortex, and mid brain as well as demyelination of white matter bordering the right lateral ventricle was noted. This predominance for the temporal lobes seen microscopically was consistent with his MRI findings of bilateral temporal FLAIR hyperintensity. The rest of the brainstem, cerebellum, basal ganglia and cerebral cortices did not show significant pathological abnormality. To characterize the nature of the disease process, immunohistochemical (IHC) staining for CD3, CD4, CD8, CD20, TIA-1, glial fibrillary acidic protein (GFAP), and CD68 was performed. The CD68 stain confirmed the presence of extensive microgliosis and the GFAP stain demonstrated reactive astrogliosis. The lymphocytic inflammation present in cortical sections was predominantly composed of CD3-positive T-lymphocytes (Fig. 2). Importantly, IHC showed prominent CD8 and TIA-1 staining (Fig. 3), confirming the presence of a cytotoxic/CD8+ process rather than an antibody-mediated B-cell reaction. These histopathologic features are consistent with those of ALE, which is reactive against intracellular antigens, and in which cytotoxic T-cell mechanisms appear to play a dominant role in pathogenicity. Lymphocyte and macrophage markers (CD3, CD20 and CD68) were mostly negative within the frontal lobe, with only a few perivascular macrophages and T cells, reinforcing the predominance of the disease for the temporal region. Staining for hyperphosphorylated tau showed only a few neuro-fibrillary tangles in the hippocampus, and some scattered glia and threads throughout the frontal lobe/temporal lobe. Staining for amyloid beta was completely negative, clarifying the point that there was no Alzheimer's pathology detected. No cancer was found on whole body autopsy.
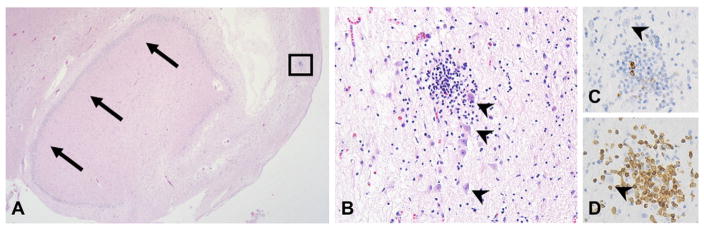
Fig. 2.
Microscopic images from the hippocampus, showing T-cell predominant inflammation among cortical neurons (arrowheads indicate neurons in all images). A. Coronal section of the hippocampus (large arrows indicate dentate gyrus) showing extensive rarefaction and tissue damage (hematoxylin and eosin, 2×). B. A focus of cortical lymphocytic inflammation (hematoxylin and eosin, 40×). C. Only rare lymphocytes show immunostaining for CD20 (40×). D. CD3 immunohistochemistry shows the infiltrate to be T-cell predominant (40×).
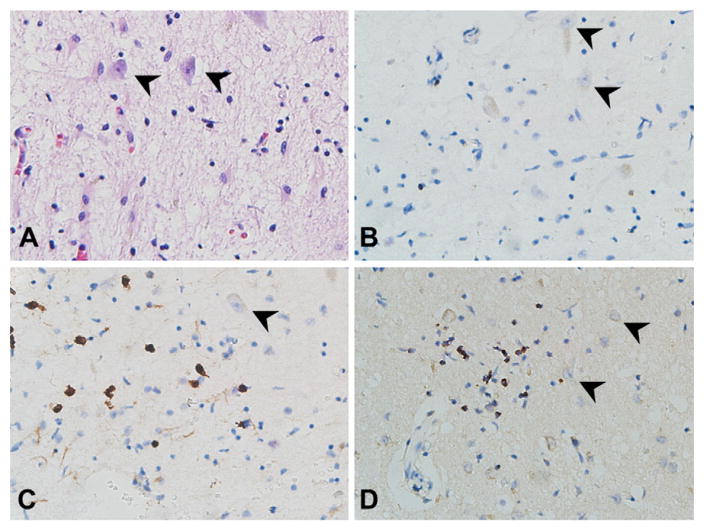
Fig. 3.
Sections from the hippocampus/entorhinal cortex showing T-lymphocytic inflammation around neurons (arrowheads, 40×). A. Hematoxylin and eosin staining. B. CD4 immunopositivity was present in only a few cells. C. CD8 and D. TIA-1 (a granule-associated protein of cytotoxic T-cells and NK cells) immunostaining was prevalent among the T-lymphocytes, supporting a cytotoxic subtype.
3. Discussion
Adenylate kinases (AKs) catalyze the reversible transfer of the γ-phosphate group from a phosphate donor (normally ATP) to AMP, releasing two molecules of ADP. They play a vital role in homeostasis of adenine nucleotide metabolism and are involved in cellular energetics through complex phospho-transfer networks regulating intracellular ATP-producing processes (Dzeja et al., 2002). Among the members of the AK family, AK5 is specifically expressed in brain (Van Rompay et al., 1999), and has critical neuronal-specific metabolic functions, including energy transfer and synthesis of RNA and DNA (Ren et al., 2005). Increased CSF levels of AK may also occur in patients with extensive neuronal damage caused by stroke and neoplastic processes (Buttner et al., 1986; Ronquist et al., 1977) To examine whether the antibodies to AK5 could have been triggered after neuronal damage by encephalitis or as a remote effect of an occult breast cancer, Tuzun et al. included fifty control patients with paraneoplastic encephalitis or cerebellar degeneration, and nineteen patients with breast cancer. None of the patients had antibodies to AK5 in serum or CSF, suggesting that the antibodies detected in our patient were likely related to the underlying immune disorder (Tuzun et al., 2007). Of note, antibodies to other protein kinases have also been found in paraneoplastic and non-paraneoplastic neurological conditions (Sabater et al., 2006), with lung cancer being the most frequently-associated neoplasm. Tuzun et al. searched for antibodies to AK5 in the sera of 74 patients with lung cancer and found all to be negative, providing further evidence that AK5 autoimmunity is more likely to be a non-paraneoplastic entity (Tuzun et al., 2007). Graus et al. proposed a classification of neuronal antibodies, with AK5 classified under group 1c–group 1 being antibodies that target intracellular antigens and are probably not pathogenic; with subdivision into 1c being determined by its association with a non-paraneoplastic neurological syndrome (Graus et al., 2010). The authors however, mention that the inclusion of such novel antibodies in their classification must be viewed with caution until more cases are described.
It is well-known that ALE associated with antibodies to intracellular antigens (i.e., Hu, Ma2, CV2/CRMP5) respond much more poorly to immunotherapy than ALE associated with antibodies to cell surface neuronal antigens (i.e., VGKC, NMDAR) as a result of cytotoxic T-cell mechanisms (Bataller et al., 2007; Gultekin et al., 2000; Titulaer et al., 2013; Vincent et al., 2004). This was reflected in our patient with AK5-autoimmunity who had histopathological confirmation of a predominantly T-cell mediated process of neuronal destruction in regions of the brain frequently targeted in limbic encephalitis. And like many patients with ALE associated with intracellular antigens, he remained minimally responsive to immunotherapy, and continued to progress. As mentioned earlier, there has also been a growing body of evidence that many cases of immune-mediated non-infectious related limbic encephalitis may not have a paraneoplastic etiology. These patients have clinical and radiological evidence of ALE but remain negative for cancer even on follow-up (Bien, 2007). Also, although profiling of auto-antigens associated with immune-mediated ALE has been prolific, allowing identification of the target autoantigen in roughly 80% of ALE patients, nearly 20% have novel auto-antigens remaining to be identified (Bataller et al., 2007). Many of these cases remain undiagnosed, or excluded from studies that include specific antibodies.
To our knowledge, only two cases of AK5 autoimmune ALE have been reported in the literature (Tuzun et al., 2007), and this is the only case of the two to provide pathological evidence for an immune-mediated process that correlated with his clinical course and MRI findings. The fact that no evidence for cancer was found even on autopsy contributes to our current understanding of this devastating disease as being primarily related to an underlying immune disorder rather than a paraneoplastic syndrome. With further recognition of this novel auto-immune neurological disorder, more studies can be done to further our understanding of this devastating disease, and allow for targeted therapeutic approaches. Meanwhile, as AK5 autoimmunity in a patient with ALE may herald a refractory or poorly-responsive course, physicians should be aware of this syndrome to allow aggressive immunotherapy to be instituted at the earliest possible.
Acknowledgments
A.S.L. Ng was supported by the SingHealth Health Manpower Development Plan. MDG was supported by NIH NIA K23 AG021989, R01 AG031189, and AG21601, and the Michael J. Homer Family Fund.
References
- Bataller L, Kleopa KA, Wu GF, Rossi JE, Rosenfeld MR, Dalmau J. Autoimmune limbic encephalitis in 39 patients: immunophenotypes and outcomes. J Neurol Neurosurg Psychiatry. 2007;78:381–385. doi: 10.1136/jnnp.2006.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien CG. Limbic encephalitis: extension of the diagnostic armamentarium. J Neurol Neurosurg Psychiatry. 2007;78:332–333. doi: 10.1136/jnnp.2006.106757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner T, Hornig CR, Busse O, Dorndorf W. CSF cyclic AMP and CSF adenylate kinase in cerebral ischaemic infarction. J Neurol. 1986;233:297–303. doi: 10.1007/BF00314162. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Bortolon R, Perez-Terzic C, Holmuhamedov EL, Terzic A. Energetic communication between mitochondria and nucleus directed by catalyzed phosphotransfer. Proc Natl Acad Sci U S A. 2002;99:10156–10161. doi: 10.1073/pnas.152259999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. 2010;257:509–517. doi: 10.1007/s00415-009-5431-9. [DOI] [PubMed] [Google Scholar]
- Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481–1494. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, Shu H, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65(4):424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Wang L, Bennett M, Liang Y, Zheng X, Lu F, et al. The crystal structure of human adenylate kinase 6: an adenylate kinase localized to the cell nucleus. Proc Natl Acad Sci U S A. 2005;102:303–308. doi: 10.1073/pnas.0407459102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist G, Ericsson P, Frithz G, Hugosson R. Malignant brain tumours associated with adenylate kinase in cerebrospinal fluid. Lancet. 1977;1:1284–1286. doi: 10.1016/s0140-6736(77)91320-4. [DOI] [PubMed] [Google Scholar]
- Sabater L, Bataller L, Carpentier AF, Aguirre-Cruz ML, Saiz A, Benyahia B, et al. Protein kinase Cgamma autoimmunity in paraneoplastic cerebellar degeneration and non-small-cell lung cancer. J Neurol Neurosurg Psychiatry. 2006;77:1359–1362. doi: 10.1136/jnnp.2006.097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzun E, Rossi JE, Karner SF, Centurion AF, Dalmau J. Adenylate kinase 5 autoimmunity in treatment refractory limbic encephalitis. J Neuroimmunol. 2007;186:177–180. doi: 10.1016/j.jneuroim.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rompay AR, Johansson M, Karlsson A. Identification of a novel human adenylate kinase. cDNA cloning, expression analysis, chromosome localization and characterization of the recombinant protein. Eur J Biochem. 1999;261:509–517. doi: 10.1046/j.1432-1327.1999.00294.x. [DOI] [PubMed] [Google Scholar]
- Vincent A, Buckley C, Schott JM, Baker I, Dewar BK, Detert N, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–712. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]