Summary
The results of organ and cell allotransplantation continue to improve, but the field remains limited by a lack of deceased donor organs. Xenotransplantation, e.g., between pig and human, offers unlimited organs and cells for clinical transplantation. The immune barriers include a strong innate immune response in addition to the adaptive T cell response. The innate response has largely been overcome by the transplantation of organs from pigs with genetic modifications that protect their tissues from this response. T cell-mediated rejection can be controlled by immunosuppressive agents that inhibit costimulation. Coagulation dysfunction between the pig and primate remains problematic but is being overcome by the transplantation of organs from pigs that express human coagulation-regulatory proteins. The remaining barriers will be resolved by the introduction of novel genetically-engineered pigs. Limited clinical trials of pig islet and corneal transplantation are already underway.
Keywords: Immunobiology; Pig, genetically-engineered; Pig, islets; Pig, organs; Xenotransplantation
Introduction
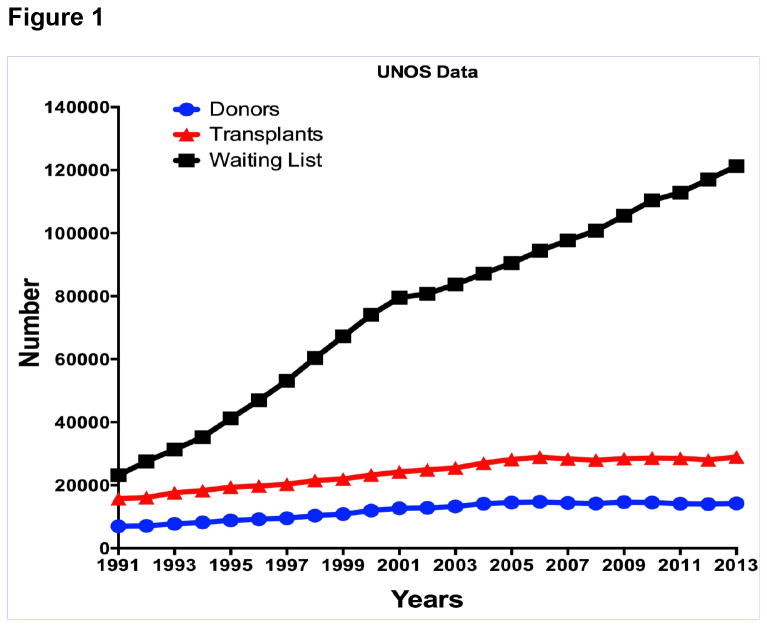
Organ allotransplantation is one of the great success stories of the latter part of the 20th century. The results continue to improve, but the field remains strictly limited by the number of deceased donor organs that become available each year (Figure 1). In the relatively new field of pancreatic islet allotransplantation, the results are improving significantly, but again the major limitation will be access to pancreases from deceased human donors, particularly as two or more pancreases are often required to provide sufficient islets to maintain normoglycemia in the recipient. Although there are sufficient numbers of deceased donor corneas available for the purpose of corneal transplantation in the U.S. and in some other western countries, worldwide there is a major shortage of corneas for the treatment of patients with corneal blindness [1,2].
Figure 1.
UNOS data showing the number of patients on the waiting list for organ transplantation, number of transplants carried out, and number of deceased human donors who become available each year (for the years 1991-2013).
Xenotransplantation, i.e., cross-species transplantation, e.g., between pig and human, offers the prospect of unlimited organs and cells for clinical transplantation [3-5]. The history of this experimental field has been reviewed elsewhere and will not be reviewed again except to state that kidney transplants from nonhuman primates (NHPs) into humans in the 1960s demonstrated that the immune response was greater than to an allograft [6,7]. Nevertheless, relatively prolonged survival (weeks or occasionally months) could be achieved in occasional patients with the primitive immunosuppressive therapy available at that time [8].
For a number of reasons, NHPs are no longer being considered as potential organ donors for humans (Table 1). Attention is being directed toward the pig as a potential source of organs and cells, and considerable progress has been made during the past 30 years [9,10].
Table 1. The advantages and disadvantages of the pig as a potential source of organs and cells for humans, in contrast with those of the baboon in this role.
| Pig | Baboon | |
|---|---|---|
| Availability | Unlimited | Limited |
| Breeding potential | Good | Poor |
| Period to reproductive maturity | 4-8 months | 3-5 years |
| Length of pregnancy | 114 ± 2 days | 173-193 days |
| Number of offspring | 5-12 | 1-2 |
| Growth | Rapid (adult human size within 6 months)** | Slow (9 years to reach maximum size) |
| Size of adult organs | Adequate | Inadequate* |
| Cost of maintenance | Significantly lower | High |
| Anatomical similarity to humans | Moderately close | Close |
| Physiological similarity to humans | Moderately close | Close |
| Relationship of immune system to humans | Distant | Close |
| Knowledge of tissue typing | Considerable (in selected herds) | Limited |
| Necessity for blood type compatibility with humans | Probably unimportant | Important |
| Experience with genetic engineering | Considerable | None |
| Risk of transfer of infection (xenozoonosis) | Low | High |
| Availability of specific pathogen-free animals | Yes | No |
| Public opinion | More in favor | Mixed |
The size of certain organs, e.g., the heart, would be inadequate for transplantation into adult humans.
Breeds of miniature swine are approximately 50% of the weight of domestic pigs at birth and sexual maturity, and reach a maximum weight of approximately 30% of standard breeds.
Pig-to-primate organ transplantation
Immune barriers and their resolution
The innate immune response
The immune barriers are much greater than when a NHP organ is transplanted or in the case of allotransplantation. These barriers include the almost immediate destruction of the graft by antibody-mediated complement activation (hyperacute rejection), although this has largely been overcome by the development of pigs with genetic modifications that protect their tissues from the human immune response. In this respect, two key modifications have been made.
One is the deletion of the gene for the enzyme, α1,3-galactosyltransferase, which led to the generation of α1,3-galactosyltransferase gene knock-out pigs [11]. This enzyme is responsible for adding galactose-α1,3-galactose (Gal) epitopes (that are the major target for primate anti-pig antibodies) to pig cells [12]. When these antibodies bind to the Gal antigens, they activate complement. This results in rapid graft failure through hyperacute rejection. This and a delayed form of antibody-mediated rejection, known variously as acute humoral xenograft rejection, delayed xenograft rejection, or acute vascular rejection, have largely been overcome by the transplantation of organs from pigs that do not express Gal (GTKO pigs) [13,14].
The second is the transgenic expression of one or more human complement-regulatory proteins, e.g., CD46, CD55, and CD59. Human complement is rarely damaging to a subject's own tissues as these tissues express species-specific (e.g., human) complement-regulatory proteins. Pig complement-regulatory proteins protect pig tissues from pig complement, but are relatively ineffective against human complement. Therefore, the expression of a human complement-regulatory protein in a pig largely protects against the effect of primate complement (whether this is human or NHP).
The innate response, however, includes a cellular component, e.g., natural killer cells [15] and macrophages [16,17]. Several groups have demonstrated that transgenic expression of human leukocyte antigen (HLA)-E and/or G in the ‘donor’ pig organ inhibits the NK cell response [18]. More recently, studies by Miyagawa's group indicate that expression of these transgenes may also inhibit macrophage activity, which is likely to be important after organ, islet, and cell transplantation [19,20].
The adaptive immune response
The combination, therefore, of a graft from a pig that does not express the important Gal epitopes, but does express one or more human complement-regulatory proteins provides considerable protection against the primate innate immune response [21,22]. If, in addition, an effective immunosuppressive regimen is administered that prevents a T cell response to the graft, then prolonged graft survival has been obtained [23-29]. Inadequate immunosuppressive therapy, however, can result in the development of elicited anti-pig antibodies that result in graft failure.
The T cell response to an allograft or xenograft consists of a response to the antigens in the graft, but also requires a second response, known as costimulation, that is essential if the T cells are to respond adequately. Prevention of costimulation results in inhibition of the T cell response. The immunosuppressive regimens that have been tested in xenotransplantation models have demonstrated that the relatively new agents that are directed towards costimulation blockade are more effective than conventional immunosuppressive therapies (e.g., based on tacrolimus or cyclosporine) [30,31].
Genetic engineering of the donor pig can also contribute to reducing the T cell response [30]. For example, the absence of Gal expression reduces the T cell response [32], as does expression of CTLA4-Ig [33] or of a mutant MHC class II transactivator [34,35].
NonGal antigenic targets
Even when the innate and adaptive responses have been controlled by a combination of genetic engineering and immunosuppressive therapy, there remains a low-grade, but continuing, activation of the vascular endothelial cells of the graft related to the binding of other primate anti-pig (i.e., anti-nonGal) antibodies, complement deposition, and the activity of innate immune cells (e.g., neutrophils, monocytes). The targets for these anti-nonGal antibodies are generally unknown although two definitive antigen targets have been identified.
The first, N-glycolylneuraminic acid (NeuGc), has been known for some years and is present on pig and NHP tissues, but not on human tissues, and therefore humans develop anti-NeuGc antibodies [36,37]. NeuGc will therefore be a target when clinical xenotransplantation is undertaken, but is not relevant in pig-to-NHP models. Pigs that express neither Gal nor NeuGc have recently been produced [38], and initial in vitro testing is underway [39,40].
The second potential target for primate anti-nonGal antibodies is porcine β1,4 N-acetylgalactosaminyltransferase [41]. Its importance remains uncertain, but preliminary data suggest that, if it is depleted from the organ-source pig, primate antibody binding is reduced.
Coagulation dysregulation
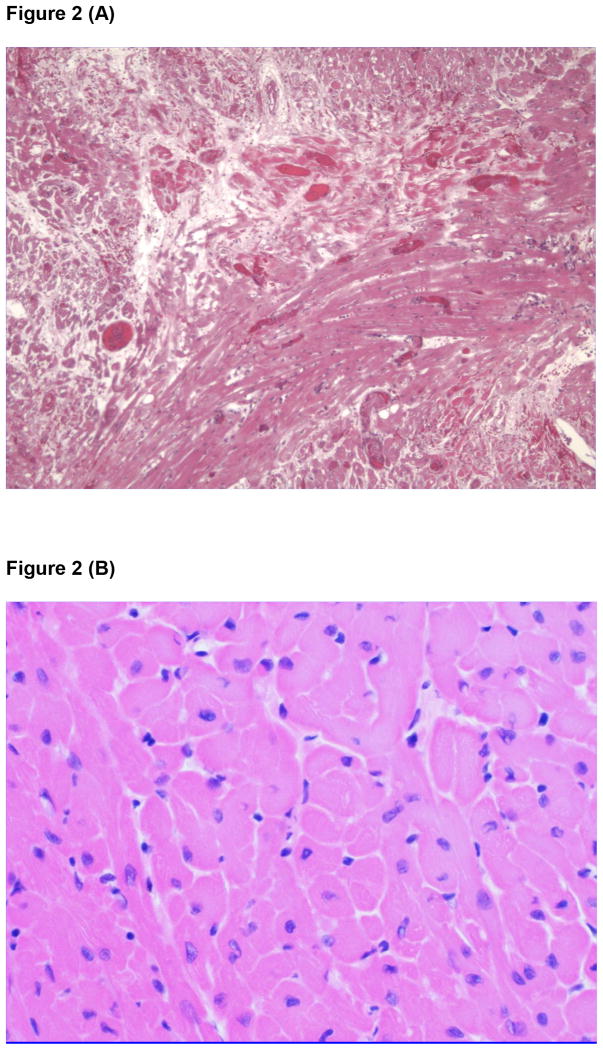
The activation of the vascular endothelium changes its normal anticoagulant state to procoagulant, and this process is exaggerated by the presence of several coagulation discrepancies between pig and primate [42]. For example, pig tissue factor pathway inhibitor is inadequate in preventing primate tissue factor activation. The normal anticoagulant state in the blood vessels of the pig organ graft is lost, and a procoagulant state develops. In heart transplant models this results in the development of a thrombotic microangiopathy in the graft, demonstrated by platelet aggregation and fibrin deposition, which ultimately leads to widespread myocardial ischemic injury (Figure 2A) [43,44]. When this is advanced and coagulation factors are depleted, a consumptive coagulopathy can develop in the recipient that can be fatal [45]. Rapid excision of the graft can inhibit further consumption of coagulation factors and result in survival of the recipient, indicating that the initiating problem for the development of consumptive coagulopathy is in the graft.
Figure 2.
(A) Microscopic appearance of the myocardium of a GTKO pig heart transplanted heterotopically into an immunosuppressed baboon almost 6 months previously, showing advanced features of thrombotic microangiopathy, with numerous vessels occluded by thrombus with surrounding areas of ischemic fibrosis. Reproduced from The Lancet, Vol .379, Ekser et al, Clinical xenotransplantation: the next medical revolution?, pp672-83, Copyright 2012, with permission from Elsevier [3].
(B) Microscopic appearance of the myocardium of a GTKO/CD46/TBM pig heart transplanted heterotopically into an immunosuppressed baboon one year previously. No significant histopathological changes are seen. (Courtesy Muhammad Mohiuddin MD, NHLBI, NIH, Bethesda, MD, USA.)
This problem is being resolved by the development of pigs that express one or more human coagulation-regulatory proteins, e.g., thrombomodulin (TBM), endothelial protein C-receptor, CD39, or tissue factor pathway inhibitor, thus balancing procoagulation and anticoagulation in the graft [46,47].
Inflammatory response
Increasing attention is being paid to an inflammatory response that develops after the transplantation of a pig organ into a NHP [48-50]. The inflammatory response can augment the immune response and the coagulation discrepancies [51]. The administration of anti-inflammatory agents, therefore, may further prolong graft survival or allow less intensive immunosuppressive therapy. In particular, interleukin (IL)-6 is being targeted as a potential proinflammatory cytokine [50].
Pig organ graft survival in nonhuman primates
The transplantation of hearts from GTKO/CD46/TBM pigs into baboons has resulted in survival of heterotopic (non-life supporting) grafts for periods >1 year, with normal function (demonstrated by echocardiography) and histology (Figure 2B) recorded at that time [23-26]. Indeed in one case, graft survival extended for >2 years. These encouraging results were obtained using an immunosuppressive regimen based on costimulation blockade with an anti-CD40 monoclonal antibody (mAb).
Using similar pigs as sources of kidneys and a similar immunosuppressive regimen, kidney graft survival has extended to almost five months in one baboon [29]. Even in the absence of a human coagulation-regulatory protein, if recipient baboons are selected that have extremely low levels of anti-pig antibody (therefore reducing the activation of the vascular endothelium of the graft), graft survival has extended to >6 months [27].
Transplantation of pig lungs or livers has been associated with significantly greater problems, largely from problems related to coagulation dysfunction and platelet consumption. To date, graft (or recipient) survival in NHPs has extended to only a few days [52-55]. More extensive genetic manipulation of the source-pigs will be required [56].
Pig-to-primate cell transplantation
Pancreatic islet transplantation
In the field of cell xenotransplantation, e.g., of pancreatic islets, similar approaches are leading to improving results.
For many compelling reasons, the pig would be a suitable donor for islets intended for transplantation as a treatment of patients with diabetes. One of the most important advantages is the structural similarity between pig and human insulin, which differs at only one amino acid. Porcine insulin was used clinically for several decades before recombinant human insulin became available.
The ideal ‘donor’ pig age is still under debate [57]. Adult (>2 years of age) pigs provide higher islet yields and improved functional performance in terms of insulin release, but neonatal islets (from pigs <1 month old) are less expensive and easier to isolate, more resistant to ischemic injury, and proliferate in vivo after transplantation. There is therefore a strong interest in the use of neonatal pigs as islet donors [57].
The genetic modifications that have been found valuable in pig organ xenotransplantation have also proven helpful in pig-to-NHP islet transplantation, especially GTKO (which is particularly important for neonatal islets where Gal is more highly expressed), and expression of human complement and coagulation modulators. Transgenes can be ubiquitously expressed in all tissues, but specific expression in select cells (e.g., β cells, with expression driven under an insulin promoter) is possible [58].
One major barrier to islet xenotransplantation is the early loss of a large number of islets after their infusion into the blood of the portal vein of the recipient (the standard current approach in allotransplantation), a condition known as the instant blood-mediated inflammatory reaction (IBMIR) [59]. This was initially believed to be a nonspecific response involving complement and coagulation activation related to the direct exposure of the islets to blood. More recent evidence suggests that an immune-mediated response (antibody, complement, and innate immune cells) may be playing a much greater role than originally anticipated, and that indeed it may be a form of hyperacute rejection [60]. Overcoming this barrier is proving difficult, although genetic modification of the pigs may prove helpful [61]. An alternative would be the transplantation of the islets in sites other than the liver, such as the gastric submucosal space, where the islets are not immediately exposed directly to blood, but still receive a good supply of oxygen and nutrients [62,63]. An ideal site should feature portal delivery of insulin, but be accessible by a minimally-invasive procedure associated with minimal complications. While other sites are being investigated, the liver, despite its limitation, remains the primary transplantation site.
The T cell response plays an important role in islet rejection, but can be efficiently controlled by costimulation blockade-directed therapy [61,64-69].
Pig islet graft survival in nonhuman primates
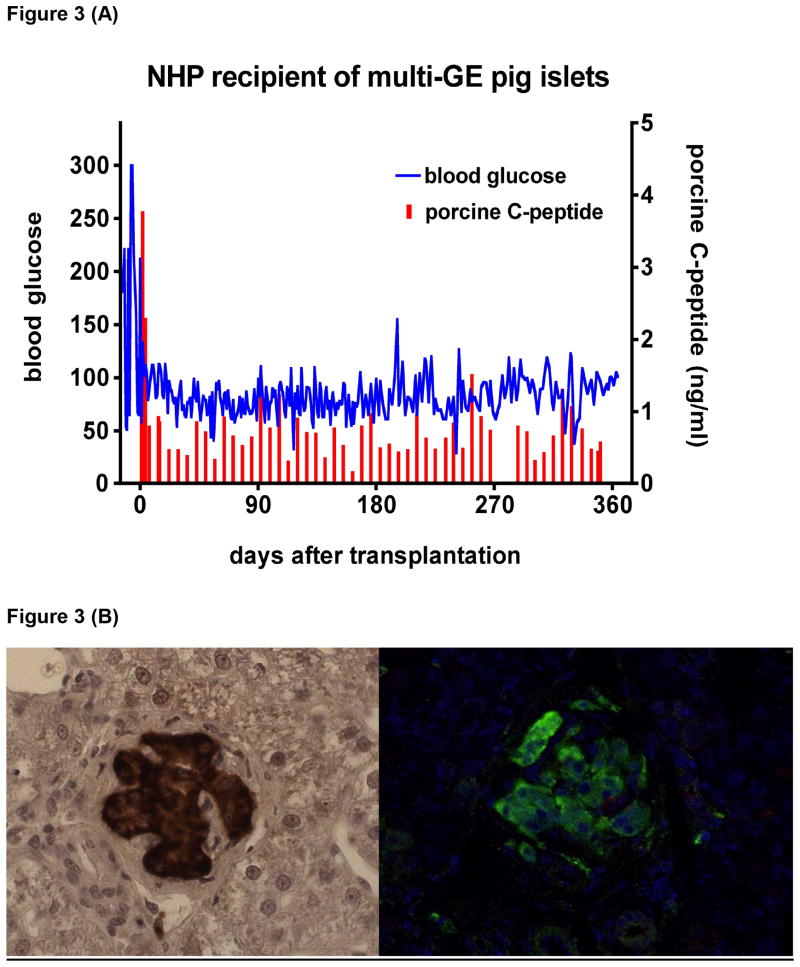
Several groups have independently demonstrated maintenance of normoglycemia by pig islet transplantation in diabetic NHPs for >6 months in the absence of exogenous insulin. These studies have included islets from various types and ages of pigs and various immunosuppressive regimens. Normalization of blood glucose has been reported after the transplantation of encapsulated adult pig islets [70], but free (non-encapsulated) islets infused intraportally have been associated with greater success. Genetically-unmodified pig islets from adult donors [66,71,72], fetal cells [73], neonatal islet-like cell clusters [65,69], genetically-engineered pig islets (both adult [67] and neonatal [68]) and, finally, adult pig islets with multiple genetic modifications [61] have all achieved successful outcomes. Islets from genetically-engineered pigs have functioned in diabetic monkeys for >1 year under an anti-CD154mAb-based immunosuppressive therapy (Figure 3) [58,61,67].
Figure 3.
(A) Blood glucose (blue) in a streptozotocin-induced diabetic cynomolgus monkey that remained insulin-independent and normoglycemic for more than one year after adult porcine islet xenotransplantation (using a GTKO pig transgenic for the human complement-regulatory protein CD46, the human coagulation-regulatory protein tissue factor pathway inhibitor, and the immunosuppressive agent CTLA4-Ig) at the University of Pittsburgh. NHP= nonhuman primate; GE= genetically-engineered.
(B) Histopathology with insulin immunostaining of the liver 12 months after porcine islet xenotransplantation into the portal vein in the same cynomolgus monkey as in (A). Left panel shows insulin immuno-staining in paraffin-embedded liver tissue. Right panel shows green fluorescent insulin immuno-reactive cells in paraformaldehyde-fixed liver tissue. Islet morphology is well preserved. No islets were detectable in the monkey's native pancreas.
It remains to be determined which approach will provide the greatest benefit. When encapsulation of islets in alginate has been utilized, no immunosuppressive therapy has been administered, clearly a major advantage. However, encapsulation techniques are limited by the biocompatibility of the membrane, the duration of capsule integrity, volume and size of the capsules, and difficulties in establishing long-term pig insulin release.
In our opinion, it is unlikely that any form of encapsulation will lead to greatly prolonged graft survival. It is essential that the capsules allow insulin produced by the islets to escape, but this will also enable cytokines and chemokines, as well as possibly antibodies and complement to enter the capsules to injure the islets. Furthermore, even if this does not occur, it is uncertain that encapsulation allows oxygen and other nutrients to continue to reach the islets, which may result in graft failure.
Free (non-encapsulated) islets require immunosuppressive therapy. Protocols including costimulation blockade with anti-CD154mAb have been associated with the best results, with prolongation of islet graft survival from both wild-type as well as genetically-modified pig donors. Although in these studies anti-CD154mAb did not lead to secondary complications, such as thrombosis, this drug is unlikely to be clinically applicable due to conflicting reports on its thromboembolic effects. It is expected that the anti-CD40mAb therapy that has proved effective after pig organ transplantation will prove equally effective in islet xenotransplantation.
Transplantation of pig neuronal cells and corneas
Although less emphasis has been directed to the transplantation of pig neuronal cells in monkeys with a Parkinson-like state, encouraging results have been reported [74]. Similar encouraging results have been reported following pig corneal transplantation in monkeys [75,76].
Clinical trials of xenotransplantation
There have been no scientific clinical trials of pig organ transplantation even though there have been historic efforts that all resulted in early graft failure [6,7]. The risks of cell transplantation are clearly less than the risks of whole organ transplantation, largely because consumptive coagulopathy does not develop. Therefore, there have already been a number of small trials of pig islet and corneal transplantation. To date, all of the islet xenotransplantation trials (with the exception of the original trial by Groth et al [77], have involved encapsulation of the islets using various techniques aimed to protect them from the human immune response. The results have not always been fully reported, but it would appear that they have not been entirely satisfactory in protecting the islets [78,79].
Pig corneal transplants are being assessed in clinical trials in China [80], where there are an estimated several million people with corneal blindness who might benefit from corneal transplantation [2]. There are limited published data, but these have been moderately encouraging.
Potential impact on clinical medicine
If the immune barriers to clinical pig organ transplantation can be completely overcome, the impact that xenotransplantation will have on clinical medicine will be immense [3-5]. There are a number of advantages of pig organ transplantation over allotransplantation (Table 2). When it is considered that there is a significant mortality of patients waiting for human organs, the potential impact of xenotransplantation is obvious. Furthermore, because of the limited number of pancreases from deceased human donors, allotransplantation (of the pancreas or islets) will never offer therapy for the estimated 30 million or more patients (in the USA alone) with Type I or Type II diabetes. In the pig, the absence of brain death [81,82], with its associated metabolic derangements will provide an advantage over human islets. Because of differences in amino acid composition between human and pig islet amyloid polypeptide, pig islets do not accumulate amyloid, a feature typically associated with β cell dysfunction [83].
Table 2. Major advantages of xenotransplantation over allotransplantation.
|
Potential alternatives to xenotransplantation
The potential of xenotransplantation has surprisingly not been fully realized by a majority of those involved in medical research or clinical medicine. Other potential approaches have perhaps received more attention and more funding even though progress in most of these fields still lags well behind that in xenotransplantation.
These alternative approaches include mechanical devices that support the failing heart, e.g., ventricular assist devices, which are now relatively successful over periods of months or even years [84]. Even so, ventricular assist devices are still problematic in respect of thromboembolic and infectious complications (largely related to the necessity of having a power source external to the body with a driveline connecting the device percutaneously with the power source). Furthermore, ventricular assist devices can result in sensitization to human leukocyte antigens (HLA), which might preclude or reduce the possibility of a subsequent heart allotransplant. (Current limited evidence is that even a failed pig organ graft does not sensitize to HLA antigens, and therefore would not place the patient at risk of not being able to obtain an allograft [85].) Nevertheless, these devices are playing a significant role in bridging patients to cardiac allotransplantation and in supporting patients who are not candidates for allotransplantation. There are, however, currently no implantable mechanical devices that will realistically support patients with renal, hepatic, or pulmonary failure.
A second potential alternative to xenotransplantation is based on tissue engineering and regenerative medicine [86]. Regenerative medicine techniques include, in particular, using a decellularized organ, e.g., a lung (obtained from a deceased human or a pig), and recellularizing the remaining matrix with pluripotent progenitor (stem) cells from a potential human recipient. Progress in this field is far behind that of xenotransplantation, and to our knowledge there is no experience in the transplantation of such an organ into a NHP.
The development of a whole organ from pluripotent progenitor cells is also very much in its infancy, although progenitor cell-derived β islet cells may possibly resolve the problem of diabetes [87]. However, there remains concern with regard to possible development of teratomas from embryonic stem cells, and there is also the risk that, in patients with autoimmune diabetes, autoantibodies may damage the transplanted β cells. Furthermore, it remains uncertain whether the transplantation of pancreatic β cells alone (in the absence of the other cells found in islets) will be sufficient to maintain normoglycemia.
A final potential alternative to xenotransplantation lies with blastocyst complementation, though it is also in its relative infancy [88-91]. In this highly-innovative approach, the genes for an organ, e.g., the lung, are deleted in the pig, which then in embryonic life receives human pluripotent cells from which a human lung is generated. If these cells are taken from the specific human who will receive the lung transplant, there will be only a weak immune response against the graft. However, the organ would have to be generated on a case-by-case basis and so fulfilling the needs of those thousands or millions who need organ or cell transplants would be a time-consuming and very costly process.
Expert commentary
Pig organ xenotransplantation in NHPs has progressed far more than any alternative approach (with the exception of cardiac mechanical devices), and could well resolve the major problem of lack of suitable deceased human organs for purposes of transplantation. Nevertheless, further progress needs to be made before a clinical trial can be undertaken.
In view of the success of ventricular assist devices in supporting patients with heart failure, it is likely that the first clinical trials of whole organ xenotransplantation will be in patients with kidney failure. These patients might be selected on the basis of a high degree of HLA sensitization, precluding them from readily obtaining an allograft, but also may include those who no longer have any suitable access sites for continuing dialysis. In such patients, an attempt at pig kidney xenotransplantation may be ethically acceptable and potentially life-saving.
Progress with liver and lung xenotransplantation has been limited to date, and clinical trials may therefore be some way in the future. However, as there is no effective method of supporting a patient with acute liver failure who is a candidate for a liver allotransplant, if a pig liver could function satisfactorily in a NHP for a period of 1-2 weeks (in the absence of thrombocytopenia and other complications), then pig liver transplantation (or ex vivo liver perfusion) as a bridge to allotransplantation may be ethically justified and successful.
As the numbers of deceased human pancreases that become available are so limited, a case could be made for the transplantation of pig islets into severely diabetic patients, particularly those who experience life-threatening hypoglycemic attacks. The risk to the patient would be small because graft failure would be unlikely to be associated with life-threatening complications. If islet allotransplantation into the gastric submucosal space, for example, proves effective and therapeutic, then a trial could be considered of introducing pig islets into this site (although the immunosuppressive regimen would probably need to be different from that used in allotransplantation).
One topic that has attracted much interest is whether pig organ or cell transplantation will be safe from the perspective of infectious complications [92]. In particular, will a porcine infectious microorganism be transferred to the patient with the graft and, of greater importance, could that microorganism be transferred to the human contacts of the recipient, e.g., medical and nursing staff, family, etc., and thus become a health hazard to the community. Most attention has been directed to the risk of transfer of porcine endogenous retroviruses that are present in the genome of every pig cell. Current opinion is that the risks are very small, and that their inevitable transfer will not preclude clinical xenotransplantation from proceeding [93]. Nevertheless, the regulatory authorities will insist on long-term monitoring of the patient for any signs of disease transmission.
When all of the pathobiological barriers have been overcome, it will be much easier to investigate whether there are physiological differences that limit the efficacy of xenotransplantation [94,95]. For example, even though some of the products of the pig liver seem to function adequately in NHPs [96], it is unlikely that the multiple products of the pig liver will fulfill all of the metabolic needs of the human recipient. Here again, in selected instances where it is essential to correct a deficiency, genetic manipulation of the source-pig may enable any discrepancies to be overcome. For example, if porcine albumin proves insufficient in a human recipient, the pig could be genetically-engineered to produce human albumin.
If xenotransplantation could be made fully successful, the implications for clinical medicine would be truly immense. The numbers of patients undergoing organ transplantation would increase significantly, particularly in countries where deceased organ donation is not embraced culturally (Table 2). Cures for diabetes, certain neurodegenerative diseases, e.g., Parkinson's disease, and corneal blindness would revolutionize the management of patients with these conditions.
Five-year view
The remaining barriers are likely to be resolved within the next 5 years by the introduction of novel genetically-engineered pigs, particularly as the speed and ease by which these modifications can be made have been greatly facilitated by new techniques that have been introduced recently, e.g., TALENS (transcription activator-like effector nucleases) [97], and CRISPR/Cas9 (clustered regularly interspaced short palindromic repeat-associated system) [98-101]. There are now an estimated 40 different genetically-engineered pigs worldwide (Table 3), with some expressing 5 or 6 manipulations [29,58,61,102]. Furthermore, the number and efficiency of novel immunosuppressive and anti-inflammatory agents are also rapidly increasing.
Table 3. Selected genetically-modified pigs currently available for xenotransplantation research.
| Complement regulation by human complement-regulatory gene expression |
| CD46 (membrane cofactor protein) |
| CD55 (decay-accelerating factor) |
| CD59 (protectin or membrane inhibitor of reactive lysis) |
| Gal or nonGal antigen ‘masking’ or deletion |
| Human H-transferase gene expression (expression of blood type O antigen) |
| Endo-beta-galactosidase C (reduction of Gal antigen expression) |
| α1,3-galactosyltransferase gene-knockout (GTKO) |
| Cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) gene-knockout (NeuGcKO) |
| β4GalNT2 (β1,4 N-acetylgalactosaminyltransferase) gene-knockout (β4GalNT2KO) |
| Human GnT-III (N-acetylglucosaminyltransferase III) gene |
| Human tumor necrosis factor-α receptor 1 |
| Suppression of cellular immune response by gene expression or downregulation |
| CIITA-DN (MHC class II transactivator knockdown, resulting in swine leukocyte antigen class II knockdown) |
| Class I MHC-knockout (MHC-IKO) |
| HLA-E/human β2-microglobulin (inhibits human natural killer cell and macrophage cytotoxicity) |
| Human FAS ligand (CD95L) |
| Porcine CTLA4-Ig (Cytotoxic T-Lymphocyte Antigen 4 or CD152) |
| Human TRAIL (tumor necrosis factor-alpha-related apoptosis-inducing ligand) |
| Anticoagulation and anti-inflammatory gene expression or deletion |
| von Willebrand factor (vWF)-deficient (natural mutant) |
| Human tissue factor pathway inhibitor (TFPI) |
| Human thrombomodulin |
| Human endothelial protein C receptor (EPCR) |
| Human CD39 (ectonucleoside triphosphate diphosphohydrolase-1) |
| Anticoagulation, anti-inflammatory, and anti-apoptotic gene expression |
| Human A20 (tumor necrosis factor-alpha-induced protein 3) |
| Human heme oxygenase-1 (HO-1) |
| Human CD47 (species-specific interaction with SIRP-α inhibits phagocytosis) |
| Porcine asialoglycoprotein receptor 1 gene-knockout (ASGR1-KO) (decreases platelet phagocytosis) |
| Human signal regulatory protein α (SIRPα) (decreases platelet phagocytosis by ‘self’ recognition) |
| Prevention of porcine endogenous retrovirus (PERV) activation |
| PERV siRNA |
Based on a table published in J Pathol (Cooper DKC, et al, 2015, in press), courtesy of Burcin Ekser MD, PhD.
There is a relative lack of enthusiasm for clinical islet xenotransplantation unless a method can be introduced that allows the patient to avoid replacement of insulin with immunosuppressive agents. We are not enthusiastic that encapsulation techniques will allow successful islet xenotransplantation in the absence of exogenous immunosuppression, and tolerance to pig cells is likely to take longer than 5 years to achieve. However, the ability to express an immunosuppressive agent in the pig islets, e.g., one that inhibits costimulation, may allow islet transplantation to be carried out without the need for intensive systemic immunosuppressive therapy.
Similarly, many physicians would be reluctant to recommend corneal xenotransplantation because of the long-term potentially detrimental effects of immunosuppressive therapy, even though blindness is such major disability that we believe many of those afflicted would be prepared to take some long-term risks in exchange for being able to see again. Here again, the ability to provide local immunosuppression may allow pig corneal transplantation with minimal or no systemic therapy. The ability to genetically engineer pigs opens possibilities that those in transplantation have never considered previously.
As with many other fields of medical research, the major factor inhibiting progress is lack of sufficient funding from governmental agencies, medical charitable foundations, and industry (discussed in [5]). Perhaps, as the field progresses towards clinical trials of solid organs, pharmaceutical and biotechnology companies will begin to take a serious interest again. Nevertheless, we believe there will be major progress towards clinical trials within 5 years.
Key issues
It will be necessary to demonstrate consistent 6-month recipient survival (with evidence that some recipients survive for 12 months) after life-supporting organ (heart or kidney) transplantation in 5-10 consecutive pig-to-NHP experiments before a clinical trial could be justified.
This must be achieved in the absence of the development of graft atherosclerosis or other severe histopathological injury and without major complications related to the need for intensive systemic immunosuppressive therapy.
Perhaps less stringent milestones are necessary to warrant a clinical trial of pig islet transplantation.
A bridging clinical trial of pig liver transplantation might be justified if life-supporting pig livers could consistently support NHPs for periods of 10-14 days in the absence of major complications, e.g., severe thrombocytopenia, and with evidence of adequate pig hepatic function. Recipient and graft survival for 10-14 days would be sufficient to indicate that a patient in fulminant hepatic failure is likely to be supported by the pig liver long enough for a liver from a deceased human donor to be obtained.
Even though NHPs provide a very poor model for the study of transfer of porcine endogenous retroviruses, there must be no evidence from studies in humans or NHPs that a clinical trial would be associated with a definitive risk of any other porcine infectious agent spreading into the human community.
Acknowledgments
Financial and competing interests disclosure: We thank our many colleagues at the University of Pittsburgh, Allegheny-Singer Research Institute, and Revivicor Inc. (Blacksburg, VA) who have contributed to our own studies included in this review. Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants #U19 AI090959, #U01 AI068642, and # R21 A1074844, and # PO1 HL107152 by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Blacksburg, VA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Abbreviations
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- mAb
monoclonal antibody
- NeuGc
N-glycolylneuraminic acid
- NHP
nonhuman primate
- TBM
thrombomodulin
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Hara H, Cooper DK. The immunology of corneal xenotransplantation: a review of the literature. Xenotransplantation. 2010;17(5):338–49. doi: 10.1111/j.1399-3089.2010.00608.x. [DOI] [PubMed] [Google Scholar]
- 2.Hara H, Cooper DK. Xenotransplantation--the future of corneal transplantation? Cornea. 2011;30(4):371–8. doi: 10.1097/ICO.0b013e3181f237ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekser B, Ezzelarab M, Hara H, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379(9816):672–83. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 4.van der Windt DJ, Bottino R, Kumar G, et al. Clinical islet xenotransplantation: how close are we? Diabetes. 2012;61(12):3046–55. doi: 10.2337/db12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper DK. The case for xenotransplantation. Clin Transplant. 2015;29(4):288–93. doi: 10.1111/ctr.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taniguchi S, Cooper DK. Clinical xenotransplantation: past, present and future. Ann R Coll Surg Engl. 1997;79(1):13–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper DK. A brief history of cross-species organ transplantation. Proc (Bayl Univ Med Cent) 2012;25(1):49–57. doi: 10.1080/08998280.2012.11928783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reemtsma K, McCracken BH, Schlegel JU, et al. Renal heterotransplantation in man. Ann Surg. 1964;160:384–410. doi: 10.1097/00000658-196409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambrigts D, Sachs DH, Cooper DK. Discordant organ xenotransplantation in primates: world experience and current status. Transplantation. 1998;66(5):547–61. doi: 10.1097/00007890-199809150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Cooper DK, Satyananda V, Ekser B, et al. Progress in pig-to-nonhuman primate transplantation models (1998-2013): a comprehensive review of the literature. Xenotransplantation. 2014;21(5):397–419. doi: 10.1111/xen.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi T, Cooper DK. Anti-Gal, alpha-Gal epitopes, and xenotransplantation. Subcell Biochem. 1999;32:229–57. doi: 10.1007/978-1-4615-4771-6_10. [DOI] [PubMed] [Google Scholar]
- 13.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 14.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–4. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 15.Inverardi L, Clissi B, Stolzer AL, Bender JR, Sandrin MS, Pardi R. Human natural killer lymphocytes directly recognize evolutionarily conserved oligosaccharide ligands expressed by xenogeneic tissues. Transplantation. 1997;63(9):1318–30. doi: 10.1097/00007890-199705150-00021. [DOI] [PubMed] [Google Scholar]
- 16.Terpstra W, Leenen PJ, van den Bos C, et al. Facilitated engraftment of human hematopoietic cells in severe combined immunodeficient mice following a single injection of Cl2MDP liposomes. Leukemia. 1997;11(7):1049–54. doi: 10.1038/sj.leu.2400694. [DOI] [PubMed] [Google Scholar]
- 17.Basker M, Alwayn IP, Buhler L, et al. Clearance of mobilized porcine peripheral blood progenitor cells is delayed by depletion of the phagocytic reticuloendothelial system in baboons. Transplantation. 2001;72(7):1278–85. doi: 10.1097/00007890-200110150-00017. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki H, Xu XC, Smith DM, Howard T, Mohanakumar T. HLA-G expression protects porcine endothelial cells against natural killer cell-mediated xenogeneic cytotoxicity. Transplantation. 1999;67(1):31–7. doi: 10.1097/00007890-199901150-00005. [DOI] [PubMed] [Google Scholar]
- 19.Maeda A, Kawamura T, Ueno T, Usui N, Eguchi H, Miyagawa S. The suppression of inflammatory macrophage-mediated cytotoxicity and proinflammatory cytokine production by transgenic expression of HLA-E. Transpl Immunol. 2013;29(1-4):76–81. doi: 10.1016/j.trim.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Esquivel EL, Maeda A, Eguchi H, et al. Suppression of human macrophage-mediated cytotoxicity by transgenic swine endothelial cell expression of HLA-G. Transpl Immunol. 2015;32(2):109–15. doi: 10.1016/j.trim.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 21.McGregor CG, Ricci D, Miyagi N, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012;93(7):686–92. doi: 10.1097/TP.0b013e3182472850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azimzadeh A, Kelishadi S, Ezzelarab MB, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement-regulatory protein. Xenotransplantation. 2015 doi: 10.1111/xen.12176. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohiuddin MM, Corcoran PC, Singh AK, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12(3):763–71. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohiuddin MM, Singh AK, Corcoran PC, et al. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J Thorac Cardiovasc Surg. 2014;148(3):1106–13. doi: 10.1016/j.jtcvs.2014.06.002. discussion 13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohiuddin MM, Singh AK, Corcoran PC, et al. Role of anti-CD40 antibody-mediated costimulation blockade on non-Gal antibody production and heterotopic cardiac xenograft survival in a GTKO. hCD46Tg pig-to-baboon model Xenotransplantation. 2014;21(1):35–45. doi: 10.1111/xen.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohiuddin MM, Singh AK, Corcoran PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14(2):488–9. doi: 10.1111/ajt.12562. ** This and the other publications from this group report on non-life-supporting genetically-engineered pig heterotopic heart grafts functioning in baboons for more than a year, a major milestone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22:221–30. doi: 10.1111/xen.12166. * Extended life-supporting pig kidney graft survival in two monkeys with low anti-pig antibody levels beyond 125 days using anti-CD154mAb-based therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwase H, Ekser B, Satyananda V, et al. Pig-to-baboon heart transplantation - first experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22:211–20. doi: 10.1111/xen.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015 doi: 10.1111/xen.12174. (In press) * Extended life-supporting pig kidney graft survival in a baboon with a very high anti-pig antibody level to 136 days using anti-CD40mAb-based therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satyananda V, Hara H, Ezzelarab MB, Phelps C, Ayares D, Cooper DK. New concepts of immune modulation in xenotransplantation. Transplantation. 2013;96(11):937–45. doi: 10.1097/TP.0b013e31829bbcb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowan PJ, Cooper DK, d'Apice AJ. Kidney xenotransplantation. Kidney Int. 2014;85(2):265–75. doi: 10.1038/ki.2013.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilhite T, Ezzelarab C, Hara H, et al. The effect of Gal expression on pig cells on the human T-cell xenoresponse. Xenotransplantation. 2012;19(1):56–63. doi: 10.1111/j.1399-3089.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phelps CJ, Ball SF, Vaught TD, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16(6):477–85. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 34.Hara H, Witt W, Crossley T, et al. Human dominant-negative class II transactivator transgenic pigs - effect on the human anti-pig T-cell immune response and immune status. Immunology. 2013;140(1):39–46. doi: 10.1111/imm.12107. ** Provided in vitro evidence that cells from pigs transgenic for a mutant MHC class II transactivator generate a reduced T cell response from primates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwase H, Satyananda V, Zhou H, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transpl Immunol. 2015;32:99–108. doi: 10.1016/j.trim.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouhours D, Pourcel C, Bouhours JE. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Gal alpha 1-3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J. 1996;13(6):947–53. doi: 10.1007/BF01053190. [DOI] [PubMed] [Google Scholar]
- 37.Padler-Karavani V, Varki A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 2011;18(1):1–5. doi: 10.1111/j.1399-3089.2011.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutz AJ, Li P, Estrada JL, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20(1):27–35. doi: 10.1111/xen.12019. ** First report of production of a pig that does not express the important N-glycolylneuraminic acid antigen that is a known target for human anti-pig antibodies. [DOI] [PubMed] [Google Scholar]
- 39.Burlak C, Paris LL, Lutz AJ, et al. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am J Transplant. 2014;14(8):1895–900. doi: 10.1111/ajt.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015;22:194–202. doi: 10.1111/xen.12161. ** Demonstrated reduced human antibody binding to cells from pigs with deletion of three antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byrne GW, Du Z, Stalboerger P, Kogelberg H, McGregor CG. Cloning and expression of porcine beta1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 2014;21(6):543–54. doi: 10.1111/xen.12124. ** Identified a pig antigen that provides a new target for human anti-pig antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowan PJ, Robson SC, d'Apice AJ. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16(2):214–21. doi: 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houser SL, Kuwaki K, Knosalla C, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11(5):416–25. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 2008;172(6):1471–81. doi: 10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin CC, Ezzelarab M, Shapiro R, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10(7):1556–68. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miwa Y, Yamamoto K, Onishi A, et al. Potential value of human thrombomodulin and DAF expression for coagulation control in pig-to-human xenotransplantation. Xenotransplantation. 2010;17(1):26–37. doi: 10.1111/j.1399-3089.2009.00555.x. [DOI] [PubMed] [Google Scholar]
- 47.Yazaki S, Iwamoto M, Onishi A, et al. Production of cloned pigs expressing human thrombomodulin in endothelial cells. Xenotransplantation. 2012;19(2):82–91. doi: 10.1111/j.1399-3089.2012.00696.x. [DOI] [PubMed] [Google Scholar]
- 48.Burdorf L, Stoddard T, Zhang T, et al. Expression of human CD46 modulates inflammation associated with GalTKO lung xenograft injury. Am J Transplant. 2014;14(5):1084–95. doi: 10.1111/ajt.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ezzelarab MB, Ekser B, Azimzadeh A, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22(1):32–47. doi: 10.1111/xen.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwase H, Ekser B, Zhou H, et al. Further evidence for a sustained systemic inflammatory response in xenograft recipients (SIXR) Xenotransplantation. 2015 doi: 10.1111/xen.12182. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011;9(Suppl 1):182–8. doi: 10.1111/j.1538-7836.2011.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10(2):273–85. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 53.Ekser B, Burlak C, Waldman JP, et al. Immunobiology of liver xenotransplantation. Expert Rev Clin Immunol. 2012;8(7):621–34. doi: 10.1586/eci.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeh H, Machaidze Z, Wamala I, et al. Increased transfusion-free survival following auxiliary pig liver xenotransplantation. Xenotransplantation. 2014;21(5):454–64. doi: 10.1111/xen.12111. [DOI] [PubMed] [Google Scholar]
- 55.Harris DG, Quinn KJ, French BM, et al. Meta-analysis of the independent and cumulative effects of multiple genetic modifications on pig lung xenograft performance during ex vivo perfusion with human blood. Xenotransplantation. 2015;22(2):102–11. doi: 10.1111/xen.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper DK, Ekser B, Burlak C, et al. Clinical lung xenotransplantation--what donor genetic modifications may be necessary? Xenotransplantation. 2012;19(3):144–58. doi: 10.1111/j.1399-3089.2012.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagaraju S, Bottino R, Wijkstrom M, Trucco M, Cooper DK. Islet xenotransplantation: what is the optimal age of the islet-source pig? Xenotransplantation. 2015;22(1):7–19. doi: 10.1111/xen.12130. [DOI] [PubMed] [Google Scholar]
- 58.Wijkstrom M, Bottino R, Iwase H, et al. Glucose metabolism in pigs expressing human genes under an insulin promoter. Xenotransplantation. 2015;22(1):70–9. doi: 10.1111/xen.12145. * This study indicates that genetically-engineered pigs expressing transgenes in the pancreatic islets (directed by an insulin promotor) retain near-normal glucose metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation. 2007;14(4):288–97. doi: 10.1111/j.1399-3089.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 60.van der Windt DJ, Marigliano M, He J, et al. Early islet damage after direct exposure of pig islets to blood: has humoral immunity been underestimated? Cell Transplant. 2012;21(8):1791–802. doi: 10.3727/096368912X653011. [DOI] [PubMed] [Google Scholar]
- 61.Bottino R, Wijkstrom M, van der Windt DJ, et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant. 2014;14(10):2275–87. doi: 10.1111/ajt.12868. ** The first report of pig islet transplantation in which there was both multiple genetic modifications and islet-specific transgene expression achieved through use of an insulin promotor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Echeverri GJ, McGrath K, Bottino R, et al. Endoscopic gastric submucosal transplantation of islets (ENDO-STI): technique and initial results in diabetic pigs. Am J Transplant. 2009;9(11):2485–96. doi: 10.1111/j.1600-6143.2009.02815.x. [DOI] [PubMed] [Google Scholar]
- 63.Fujita M, McGrath KM, Bottino R, et al. Technique of endoscopic biopsy of islet allografts transplanted into the gastric submucosal space in pigs. Cell Transplant. 2013;22(12):2335–44. doi: 10.3727/096368912X662381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buhler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69(11):2296–304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 65.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–6. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 66.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–3. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 67.van der Windt DJ, Bottino R, Casu A, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9(12):2716–26. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 68.Thompson P, Badell IR, Lowe M, et al. Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. Am J Transplant. 2011;11(12):2593–602. doi: 10.1111/j.1600-6143.2011.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thompson P, Cardona K, Russell M, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011;11(5):947–57. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dufrane D, Goebbels RM, Gianello P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation. 2010;90(10):1054–62. doi: 10.1097/TP.0b013e3181f6e267. [DOI] [PubMed] [Google Scholar]
- 71.Cardona K, Milas Z, Strobert E, et al. Engraftment of adult porcine islet xenografts in diabetic nonhuman primates through targeting of costimulation pathways. Am J Transplant. 2007;7(10):2260–8. doi: 10.1111/j.1600-6143.2007.01933.x. [DOI] [PubMed] [Google Scholar]
- 72.Shin JS, Kim JM, Kim JS, et al. Long-Term Control of Diabetes in Immunosuppressed Nonhuman Primates (NHP) by the Transplantation of Adult Porcine Islets. Am J Transplant. 2015 doi: 10.1111/ajt.13345. [DOI] [PubMed] [Google Scholar]
- 73.Hecht G, Eventov-Friedman S, Rosen C, et al. Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proc Natl Acad Sci U S A. 2009;106(21):8659–64. doi: 10.1073/pnas.0812253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leveque X, Cozzi E, Naveilhan P, Neveu I. Intracerebral xenotransplantation: recent findings and perspectives for local immunosuppression. Curr Opin Organ Transplant. 2011;16(2):190–4. doi: 10.1097/MOT.0b013e32834494b5. [DOI] [PubMed] [Google Scholar]
- 75.Choi HJ, Kim MK, Lee HJ, et al. Efficacy of pig-to-rhesus lamellar corneal xenotransplantation. Invest Ophthalmol Vis Sci. 2011;52(9):6643–50. doi: 10.1167/iovs.11-7273. [DOI] [PubMed] [Google Scholar]
- 76.Choi HJ, Lee JJ, Kim MK, et al. Cross-reactivity between decellularized porcine corneal lamellae for corneal xenobridging and subsequent corneal allotransplants. Xenotransplantation. 2014;21(2):115–23. doi: 10.1111/xen.12075. [DOI] [PubMed] [Google Scholar]
- 77.Groth CG, Korsgren O, Tibell A, et al. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344(8934):1402–4. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 78.Matsumoto S, Tan P, Baker J, et al. Clinical porcine islet xenotransplantation under comprehensive regulation. Transplant Proc. 2014;46(6):1992–5. doi: 10.1016/j.transproceed.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 79.Wynyard S, Nathu D, Garkavenko O, Denner J, Elliott R. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation. 2014 May 7; doi: 10.1111/xen.12102. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 80.Zhang MC, Liu X, Jin Y, Jiang DL, Wei XS, Xie HT. Lamellar keratoplasty treatment of fungal corneal ulcers with acellular porcine corneal stroma. Am J Transplant. 2015;15(4):1068–75. doi: 10.1111/ajt.13096. [DOI] [PubMed] [Google Scholar]
- 81.Cooper DK, Novitzky D, Wicomb WN, Basker M, Rosendale JD, Myron Kauffman H. A review of studies relating to thyroid hormone therapy in brain-dead organ donors. Front Biosci (Landmark Ed) 2009;14:3750–70. doi: 10.2741/3486. [DOI] [PubMed] [Google Scholar]
- 82.Novitzky D, Mi Z, Sun Q, Collins JF, Cooper DK. Thyroid hormone therapy in the management of 63,593 brain-dead organ donors: a retrospective analysis. Transplantation. 2014;98(10):1119–27. doi: 10.1097/TP.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 83.Potter KJ, Abedini A, Marek P, et al. Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc Natl Acad Sci U S A. 2010;107(9):4305–10. doi: 10.1073/pnas.0909024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014;33(6):555–64. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 85.Cooper DK, Tseng YL, Saidman SL. Alloantibody and xenoantibody cross-reactivity in transplantation. Transplantation. 2004;77(1):1–5. doi: 10.1097/01.TP.0000105116.74032.63. [DOI] [PubMed] [Google Scholar]
- 86.Atala A, Murphy S. Regenerative medicine. JAMA. 2015;313(14):1413–4. doi: 10.1001/jama.2015.1492. [DOI] [PubMed] [Google Scholar]
- 87.Fox IJ, Daley GQ, Goldman SA, Huard J, Kamp TJ, Trucco M. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science. 2014;345(6199):1247391. doi: 10.1126/science.1247391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang L, Wang J, Zhang Y, Kou Z, Gao S. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell. 2009;5(2):135–8. doi: 10.1016/j.stem.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Kobayashi T, Yamaguchi T, Hamanaka S, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142(5):787–99. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 90.Matsunari H, Nagashima H, Watanabe M, et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci U S A. 2013;110(12):4557–62. doi: 10.1073/pnas.1222902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feng W, Dai Y, Mou L, Cooper DK, Shi D, Cai Z. The Potential of the combination of CRISPR/Cas9 and pluripotent stem cells to provide human organs from chimaeric pigs. Int J Mol Sci. 2015;16(3):6545–56. doi: 10.3390/ijms16036545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Onions D, Cooper DK, Alexander TJ, et al. An approach to the control of disease transmission in pig-to-human xenotransplantation. Xenotransplantation. 2000;7(2):143–55. doi: 10.1034/j.1399-3089.2000.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Denner J. Infectious risk in xenotransplantation--what post-transplant screening for the human recipient? Xenotransplantation. 2011;18(3):151–7. doi: 10.1111/j.1399-3089.2011.00636.x. [DOI] [PubMed] [Google Scholar]
- 94.Soin B, Smith KG, Zaidi A, et al. Physiological aspects of pig-to-primate renal xenotransplantation. Kidney Int. 2001;60(4):1592–7. doi: 10.1046/j.1523-1755.2001.00973.x. [DOI] [PubMed] [Google Scholar]
- 95.Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13(6):488–99. doi: 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 96.Ekser B, Echeverri GJ, Hassett AC, et al. Hepatic function after genetically engineered pig liver transplantation in baboons. Transplantation. 2010;90(5):483–93. doi: 10.1097/TP.0b013e3181e98d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tesson L, Usal C, Menoret S, et al. Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol. 2011;29(8):695–6. doi: 10.1038/nbt.1940. [DOI] [PubMed] [Google Scholar]
- 98.Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Methods. 2013;10(10):957–63. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–78. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096. doi: 10.1126/science.1258096. ** Introduced a new method for the genetic modification of pigs, which is likely to have several advantages over previous methods. [DOI] [PubMed] [Google Scholar]
- 101.Ni W, Qiao J, Hu S, et al. Efficient gene knockout in goats using CRISPR/Cas9 system. PLoS One. 2014;9(9):e106718. doi: 10.1371/journal.pone.0106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li P, Estrada JL, Burlak C, et al. Efficient generation of genetically distinct pigs in a single pregnancy using multiplexed single-guide RNA and carbohydrate selection. Xenotransplantation. 2015;22(1):20–31. doi: 10.1111/xen.12131. [DOI] [PubMed] [Google Scholar]