Navitoclax is a selective small-molecule B-cell lymphoma 2 (BCL-2)/BCL-XL inhibitor that represents both the fruition of nearly three decades of BCL-2 protein research and the promise of a new generation of targeted therapeutics for reactivating apoptosis in cancer. In Journal of Clinical Oncology, Roberts et al1 report the promising results of their phase I study of navitoclax in patients with life-threatening chronic lymphocytic leukemia (CLL). Notwithstanding the dose-limiting adverse effect of thrombocytopenia, a type of hematologic toxicity commonly seen with chemotherapeutic agents and routinely managed by oncologists, navitoclax singlehandedly reduced pathologic lymphocytosis, lymphadenopathy, and splenomegaly in treatment-experienced patients. With a suitable dose and dosing schedule defined by this phase I study, navitoclax takes another giant step forward through the obstacle course of clinical translation.
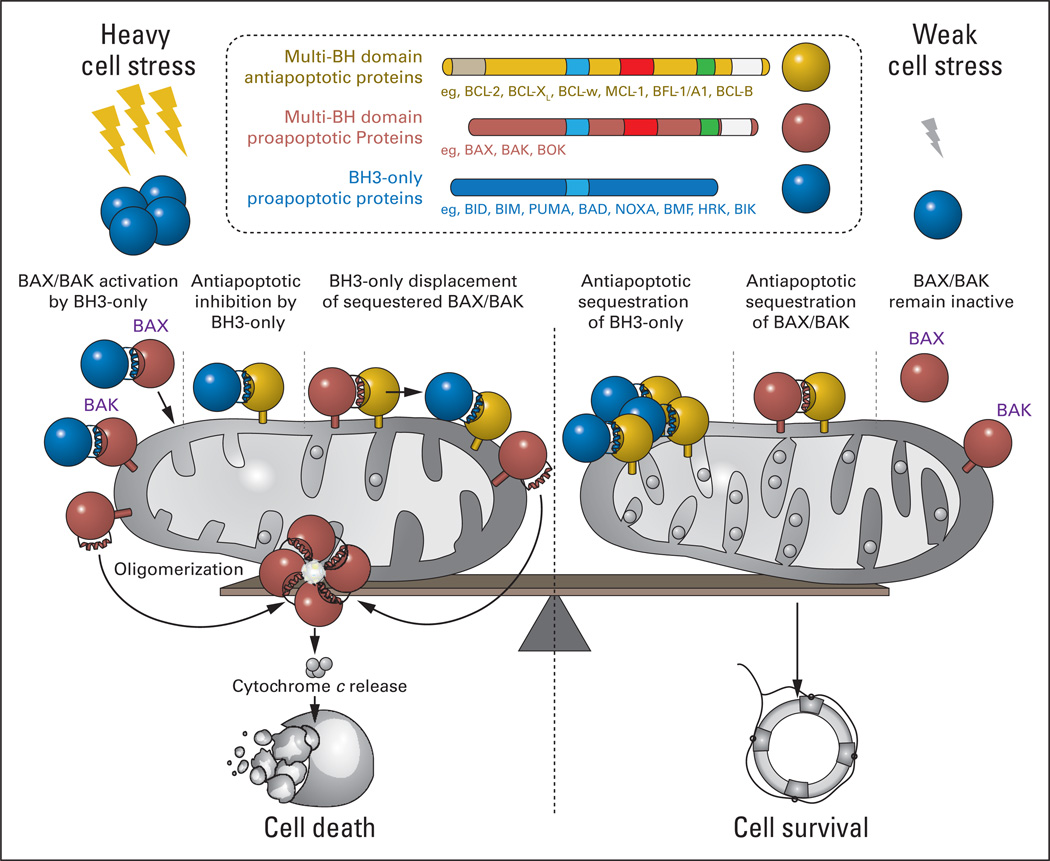
First discovered at the t(14:18) chromosomal translocation of malignant B cells,2,3 BCL-2 is the founding member of a large and influential family of proteins that render life or death decisions for cells during development and homeostasis, and when subjected to external or internal stress (Fig 1). On one side of the family, there are the survival proteins, represented by BCL-2 and its close functional homologues, BCL-XL, BCL-w, myeloid cell leukemia 1 (MCL-1), BFL-1/A1, and BCL-B. These antiapoptotic BCL-2 proteins are composed of up to four BCL-2 homology (BH) domains and suppress the programmed cell death pathway by binding and blocking the proapoptotic family members. The killer proteins are subdivided into two groups: multidomain members such as BAX and BAK, which contain three BH domains (BH1–3), and a heterogenous group of proteins sharing only a single conserved BH3 motif. The BH3-only proteins are situated throughout the cell and specialize in sensing stress signals, such as hypoxia, nutrient deprivation, DNA damage, unfolded proteins, and other intracellular and extracellular insults. Propelled into action by post-translational modification (eg, dephosphorylation, proteolytic cleavage) or transcriptional upregulation, BH3-only proteins such as BID, BIM, PUMA, BAD, and NOXA deliver their death message to the multidomain anti- and proapoptotic BCL-2 family proteins, a critical control point for signal integration and life-and-death decision making. The mitochondrion serves as center stage for this duel between the pro-survival and pro-death factions of the BCL-2 family. If survival proteins like BCL-2 cannot fend off the complement of BH3-only proteins and the activated forms of multidomain proapoptotic proteins at the mitochondria, then death signaling prevails. What ensues is a biochemical transformation of BAX and BAK monomers into homo-oligomeric pores, which pierce the mitochondrial outer membrane, releasing apoptogenic factors such as cytochrome c and smac/diablo that in turn activate caspases, which irreversibly execute the death program (Fig 1). Thus, BCL-2 and its antiapoptotic counterparts serve as mitochondrial guardians, protecting the power plant of the cell from destruction by the activated forms of BAX and BAK.
Fig 1.
Mitochondrial apoptosis is regulated by the protein interaction network of the B-cell lymphoma 2 (BCL-2) family, which is composed of dueling pro-survival and pro-death members. In response to stress stimuli, BCL-2 homology 3 (BH3) – only proteins promote activation of BAX and BAK through direct and indirect mechanisms, leading to the transformation of monomeric BAX/BAK into oligomeric pores that pierce the mitochondrial outer membrane and release apoptogenic factors. BH3-only proteins can activate BAX/BAK through direct binding interactions and/or by targeting the BH3-binding pocket of antiapoptotic proteins, releasing BH3-only proteins and conformationally active forms of BAX/BAK sequestered in heterodimeric complex (left). Conversely, antiapoptotic proteins prevent BAX/BAK-mediated mitochondrial apoptosis by impounding the BH3 domains of BH3-only proteins and BAX/BAK in a surface groove, effectively suppressing proapoptotic signaling (right). The life-or-death decision for the cell is ultimately dictated by the relative abundance and functional activity of pro- and antiapoptotic BCL-2 family proteins.
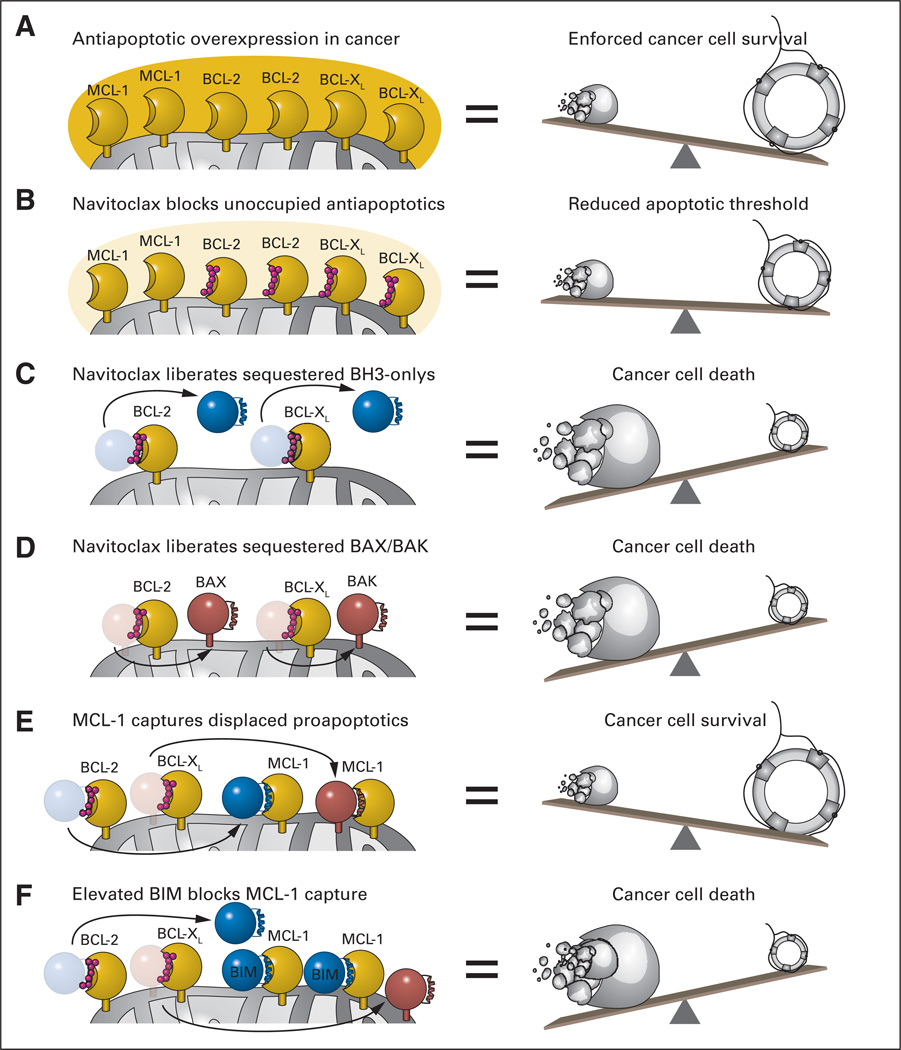
The crosstalk among BCL-2 family proteins is mediated by discrete binding interfaces. Antiapoptotic members contain a surface hydrophobic groove that binds and traps the α-helical BH3 domain of BH3-only and multidomain proapoptotic proteins. Because the BH3 helix is a critical effector domain of the death proteins, as demonstrated by loss-of-function BH3 mutagenesis,4 this sequestration event enforces cell survival. Cancer cells hijack this natural cell survival pathway by massively overexpressing individual or subsets of antiapoptotic proteins (Fig 2A). Indeed, the translocation of BCL-2 into the immunoglobulin locus results in ectopic overexpression of BCL-2 in B cells that constitutively express immunoglobulin. In 1997, Sattler et al5 reported the first structure of an antiapoptotic protein in complex with a BH3 death helix, providing the blueprint for a new pharmacologic paradigm to reactive apoptosis by inhibiting the inhibitors of cell death. Applying a powerful structure-activity relationship by nuclear magnetic resonance (SAR by NMR) methodology,6 Fesik, Rosenberg, and their Abbott Laboratory colleagues developed the small molecule ABT-7377 and then the orally bioavailable navitoclax (ABT-263),8 both of which successfully mimic a key portion of a BH3 α-helix that selectively targets BCL-2/BCL-XL.9,10 Targeted inhibition of BCL-2/BCL-XL contributes to apoptosis induction in three ways: (1) blocking unoccupied BCL-2/BCL-XL pockets reduces the threshold for apoptosis—a sensitizing feature (Fig 2B); (2) liberating sequestered BH3-only proteins enables them to occupy other antiapoptotic pockets and/or directly activate BAX/BAK (Fig 2C); and (3) displacing the trapped forms of BAX/BAK frees their BH3 death helices to propel the homo-oligomerization process and consequent mitochondrial outer membrane permeabilization (Fig 2D). Indeed, Roberts et al1 document that navitoclax-induced reductions in pathologic lymphocytosis correlated with the biochemical and morphologic hallmarks of apoptosis in circulating CLL cells.
Fig 2.
(A) A common mechanism employed by cancer cells to withstand mitochondrial assault by proapoptotics and thereby ensure pathologic survival is to overload mitochondria with antiapoptotic BCL-2 family proteins. By simulating the natural α-helical BH3 domain that selectively engages the surface groove of antiapoptotic BCL-2 and BCL-XL, navitoclax inhibits the inhibitors of mitochondrial apoptosis. Among its repertoire of proapoptotic activities, navitoclax can (B) lower the threshold for apoptosis by blocking the BH3-binding pockets of unoccupied BCL-2/BCL-XL proteins, (C) displace BCL-2/BCL-XL–sequestered BH3-only proteins to activate BAX/BAK and/or inhibit antiapoptotic proteins not blocked by navitoclax, and (D) competitively release activated forms of BAX/BAK from the heterodimeric lock hold of BCL-2/BCL-XL. (E) Whereas the capacity of navitoclax to potently inhibit BCL-XL leads to unwanted platelet apoptosis, its inability to block a broader spectrum of antiapoptotic proteins, such as myeloid cell leukemia 1 (MCL-1), can account for drug resistance. (F) Such resistance is averted by elevated levels of endogenous BIM, which can mitigate the antiapoptotic activity of MCL-1. Thus, a delicate (yet feasible) pharmacologic balancing act is required to achieve a therapeutic window for navitoclax-induced apoptosis in chronic lymphocytic leukemia.
The clinical impact of navitoclax can be influenced by BCL-2 family signaling dynamics in both the pathologic tissue and normal host cells. For example, the predominant dose-limiting toxicity of navitoclax treatment is on-target platelet apoptosis deriving from BCL-XL inhibition. Of note, the unexpected finding of thrombocytopenia in preclinical models8,11 led to the fundamental discovery that BCL-XL controls the biologic clock and function of platelets,12,13 an important example of bedside-to-bench research. Whereas dosing level and schedule can ameliorate the depth and duration of thrombocytopenia, the ultimate solution to avoiding this dose-limiting toxicity may be to tailor drug specificity even further to a BCL-2–only binding profile, a chemically viable objective.14 The expression of antiapoptotic proteins that lie outside the binding spectrum of navitoclax can also affect clinical response. For example, Roberts et al1 found that higher levels of MCL-1 in CLL cells before therapy correlated with decreased efficacy of navitoclax in reducing lymphocytosis, consistent with in vitro studies that first revealed MCL-1 to be a significant resistance factor for ABT-737 (Fig 2E).15,16 This resistance scenario was abetted in the setting of elevated BIM expression, as reflected by measurement of the ratio of BIM to MCL-1,1 suggesting that BIM can heighten the apoptotic response as a result of its broad BCL-2 family–targeting capacity (Fig 2F).17–20 In contrast to narrowing the antiapoptotic binding spectrum as a potential antidote for navitoclax-induced thrombocytopenia, drug resistance deriving from diverse antiapoptotic protein expression would require broadening the antiapoptotic binding spectrum or combining navitoclax with other agents21–23 or indirect strategies24 targeting those antiapoptotic proteins, such as MCL-1, not inhibited by navitoclax.
With three phase I studies documenting the safety, optimal dosing regimen, and preliminary efficacy of navitoclax in patients with relapsed and refractory lymphoid malignancies,25 small-cell lung cancer,26 and now CLL,1 this new modality for therapeutic activation of apoptosis through BCL-2 targeting advances to phase II testing as a single agent and in combination to combat cancer chemoresistance. Lessons learned from navitoclax will continue to inform its clinical translation and the development of next-generation agents designed to target BCL-2 family proteins and their interaction network. Although the biology is complex, and much remains to be learned about the roles of BCL-2 family proteins in death pathways, unlocking the tremendous therapeutic potential of modulating these arbiters of cellular life and death warrants our continued laser focus and unabashed persistence.
Footnotes
AUTHOR’S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Loren D. Walensky, Aileron Therapeutics (C) Stock Ownership: Loren D. Walensky, Aileron Therapeutics Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
REFERENCES
- 1.Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: Results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsujimoto Y, Finger LR, Yunis J, et al. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 3.Pegoraro L, Palumbo A, Erikson J, et al. A 14;18 and an 8;14 chromosome translocation in a cell line derived from an acute B-cell leukemia. Proc Natl Acad Sci U S A. 1984;81:7166–7170. doi: 10.1073/pnas.81.22.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K, Gross A, Waksman G, et al. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol Cell Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sattler M, Liang H, Nettesheim D, et al. Structure of Bcl-xL-Bak peptide complex: Recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 6.Shuker SB, Hajduk PJ, Meadows RP, et al. Discovering high-affinity ligands for proteins: SAR by NMR. Science. 1996;274:1531–1534. doi: 10.1126/science.274.5292.1531. [DOI] [PubMed] [Google Scholar]
- 7.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 8.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 9.Lee EF, Czabotar PE, Smith BJ, et al. Crystal structure of ABT-737 complexed with Bcl-xL: Implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 2007;14:1711–1713. doi: 10.1038/sj.cdd.4402178. [DOI] [PubMed] [Google Scholar]
- 10.Petros AM, Nettesheim DG, Wang Y, et al. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 2000;9:2528–2534. doi: 10.1110/ps.9.12.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Nimmer PM, Tahir SK, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 12.Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 13.Schoenwaelder SM, Jarman KE, Gardiner E, et al. Bcl-xL inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood. 2011;118:1663–1674. doi: 10.1182/blood-2011-04-347849. [DOI] [PubMed] [Google Scholar]
- 14.Petros AM, Huth JR, Oost T, et al. Discovery of a potent and selective Bcl-2 inhibitor using SAR by NMR. Bioorg Med Chem Lett. 2010;20:6587–6591. doi: 10.1016/j.bmcl.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 15.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 16.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merino D, Giam M, Hughes PD, et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol. 2009;186:355–362. doi: 10.1083/jcb.200905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Willis SN, Wei A, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Certo M, Del Gaizo Moore V, Nishino M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Walensky LD, Pitter K, Morash J, et al. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Stewart ML, Fire E, Keating AE, et al. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Biol. 2010;6:595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen M, Marcellus RC, Roulston A, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moretti L, Li B, Kim KW, et al. AT-101, a pan-Bcl-2 inhibitor, leads to radiosensitization of non-small cell lung cancer. J Thorac Oncol. 2010;5:680–687. doi: 10.1097/JTO.0b013e3181d6e08e. [DOI] [PubMed] [Google Scholar]
- 24.Lin X, Morgan-Lappe S, Huang X, et al. “Seed” analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007;26:3972–3979. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 25.Wilson WH, O’Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: A phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandhi L, Camidge DR, Ribeiro de Oliveira M, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]