Abstract
Background and Purpose
Hemiparesis resulting in functional limitation of an upper extremity is common among stroke survivors. Although existing evidence suggests that increasing intensity of stroke rehabilitation therapy results in better motor recovery, limited evidence is available on the efficacy of virtual reality for stroke rehabilitation.
Methods
In this pilot, randomized, single-blinded clinical trial with 2 parallel groups involving stroke patients within 2 months, we compared the feasibility, safety, and efficacy of virtual reality using the Nintendo Wii gaming system (VRWii) versus recreational therapy (playing cards, bingo, or “Jenga”) among those receiving standard rehabilitation to evaluate arm motor improvement. The primary feasibility outcome was the total time receiving the intervention. The primary safety outcome was the proportion of patients experiencing intervention-related adverse events during the study period. Efficacy, a secondary outcome measure, was evaluated with the Wolf Motor Function Test, Box and Block Test, and Stroke Impact Scale at 4 weeks after intervention.
Results
Overall, 22 of 110 (20%) of screened patients were randomized. The mean age (range) was 61.3 (41 to 83) years. Two participants dropped out after a training session. The interventions were successfully delivered in 9 of 10 participants in the VRWii and 8 of 10 in the recreational therapy arm. The mean total session time was 388 minutes in the recreational therapy group compared with 364 minutes in the VRWii group (P=0.75). There were no serious adverse events in any group. Relative to the recreational therapy group, participants in the VRWii arm had a significant improvement in mean motor function of 7 seconds (Wolf Motor Function Test, 7.4 seconds; 95% CI, −14.5, −0.2) after adjustment for age, baseline functional status (Wolf Motor Function Test), and stroke severity.
Conclusions
VRWii gaming technology represents a safe, feasible, and potentially effective alternative to facilitate rehabilitation therapy and promote motor recovery after stroke.
Keywords: stroke, virtual reality, rehabilitation, Wolf Motor Function Test, outcome research, safety, feasibility, randomized clinical trial, Wii gaming system
Stroke is a devastating disease for patients and their families and a leading cause of adult disability. Up to 85% of stroke patients experience hemiparesis immediately after stroke, and between 55% and 75% of survivors continue to experience motor deficits associated with diminished quality of life.1 Traditional rehabilitative therapies can help regain motor function and ameliorate disability.2 Recent evidence suggests that repetitive, task-oriented training of the paretic extremity is beneficial.3,4 However, implementation of these techniques is tedious, resource-intensive, and costly, often requiring transportation of patients to specialized facilities.5,6 Moreover, there is a gap in knowledge of how these interventions influence patients within the first 3 months after stroke (subacute period). Recovery of function, both spontaneous and secondary to intense rehabilitative treatments, is sustained by plasticity and rewiring in the injured brain in adults.2,7,8 Neurons in the adult human brain increase their firing rates when a subject observes movements performed by other persons. Activation of this mirror–neuron system, including areas of the frontal, parietal, and temporal lobes, can induce cortical reorganization and possibly contribute to functional recovery because critical nodes in the system are also active when subjects actually perform movements.9–11
Virtual reality (VR) gaming systems are novel and potentially useful technologies that allow users to interact in 3 dimensions with a computer-generated scenario (a virtual world), engaging the mirror–neuron system. The gaming industry has developed a variety of VR systems for home use, making this technology both affordable and accessible with potential application in community settings (ie, patients’ homes). In particular, these technologies allow for interactive observation of avatar movements captured on the screen and combine features of increasing rehabilitation intensity required for induction of neuroplasticity.9,11,12
However, there has been limited research involving the incorporation of VR gaming systems into neurorehabilitation programs, particularly in the subacute period after stroke. There is an identified need for rigorous randomized controlled trials to establish the safety, feasibility, and efficacy of VR systems as therapeutic options in stroke rehabilitation.13,14 The objectives of this study were to examine the feasibility and safety of the VR Nintendo Wii gaming system (VRWii) compared with recreational therapy (RT) in facilitating motor function of the upper extremity required for activities of daily living among patients with subacute stroke receiving standard rehabilitation.
Methods
Study Design
The Effectiveness of Virtual Reality Exercises in Stroke Rehabilitation (EVREST) is a pilot, randomized, single-blind, parallel group trial to systematically compare the feasibility and safety of VRWii to RT in patients with a first stroke within 6 months before enrollment to determine whether VRWii enhances motor recovery after stroke.
Participants
Participants 18 to 85 years of age having a first-time ischemic or hemorrhagic stroke were eligible for the study. Although the protocol allowed the inclusion of patients up to 6 months after stroke, early recruitment was favored. This time window was chosen to maximize the opportunity for enhancing motor recovery. All participants had a clinically defined acute stroke confirmed by neuroimaging (CT or MRI) and neurological assessment and met a level of function of the upper extremity derived from the Chedoke–McMaster scale15 >3 either in the arm or hand (ie, shrug their shoulders, touch chin with the affected arm) at time of enrollment.
Potential participants were excluded if they were unable to follow instructions, had a prestroke modified Rankin score of ≥2, were medically unstable or had uncontrolled hypertension according to the treating physician, were experiencing a severe illness with a life expectancy <3 months, experienced unstable angina, or had recent myocardial infarction (within 3 months), had a history of seizures or epilepsy (except for febrile seizures of childhood), were participating in another clinical trial involving an investigational drug or physical therapy, or had any condition that might put the patient at risk (ie, known shoulder subluxation or fracture) at study entry. (For a summary of inclusion and exclusion criteria, see Supplemental Table I, available online at http://stroke.ahajournals.org.)
Baseline Measures
Baseline characteristics were collected, including demographics (age and gender), handedness, comorbid conditions, stroke characteristics including location, type, and baseline disability based on the modified Rankin scale, and Barthel index for activities of daily living. Stroke severity was assessed using the Canadian Neurological Scale, a simple, reliable, and validated scale (in which lower scores indicate greater stroke severity) for estimating the neurological status, especially when the National Institutes of Health Stroke Scale is not available.16,17 Baseline motor function was assessed using the Wolf Motor Function Test18 and the Box and Block Test.19 Baseline quality of life was assessed using the Stroke Impact Scale.20 The blinded assessor was trained in the use of these scales.
All baseline, post-treatment, and 4-week follow-up assessments were performed by a trained outcome assessor, blinded to patient randomization, who was not involved in administration of study interventions.
Study Interventions
Description of Wii Gaming Technology
Nintendo introduced a new style of VR (2006) by using a wireless controller that interacts with the player through a motion detection system and avatar (computer user’s representation of himself or herself or alter ego) technology. The controllers use embedded acceleration sensors responsive to changes in direction, speed, and acceleration that enable participants to interact with the games while performing wrist, arm, and hand movements. A 2-point infrared light sensor, mounted on top of a television, captures and reproduces on the screen the movement from the controller as performed by participants. Because Wii is computer assisted, big sweeping movements in the games are not necessary. The feedback provided by the TV screen as well as the opportunity to observe their own movements in real time, generates positive reinforcement, thus facilitating training and task improvement. (Additional details are described online at http://www.nintendo.com/wii/what.)
As described, several distinctive features favored the selection VRWii over other VR systems, including novel and widely available 3D technology using gaming simulations, affordability, clinical applicability using simple graphics with real-time feedback with the possibility to reduce speed, making it usable for patients with cognitive impairments after stroke, and provision of direct multimodal sensory feedback (vision, touch, and auditory) with the avatar, thus allowing adjustments while performing and self-observing the execution of diverse tasks.
The software used in EVREST was the publicly available sports (ie, Wii Sports) and Cooking Mamma packages, accounting for 30 minutes each in the VRWii group.
Description of RT
RT sessions included leisure activities such as playing cards, stamping a seal while playing bingo, or playing Jenga. Adherence to standard rehabilitation and to the study tasks were monitored with a timer. RT was used as a control group to allow a fair comparison between the time spent in rehabilitation activities between groups and a lack of evidence that Wii gaming system is standard rehabilitation therapy. Additional details of the protocol have been published previously.21
Study Procedures
Randomization
Participants admitted to the Toronto Rehabilitation Institute were randomly allocated in a 1:1 ratio to the 2 study groups. The randomization schedule was computer generated using a basic random number generator.
Allocation
All participants received standard rehabilitation therapy for stroke, which accounts for an average of 1 hour of physiotherapy and another hour of occupational therapy per day on tolerance.
Sessions
Patients received an intensive program consisting of 8 interventional sessions (VRWii or RT) of 60 minutes each over a 14-day period. These 8 sessions were scheduled in a flexible manner as long as all 8 sessions were completed within the 2-week period with sessions separated by ≥5 hours. The arm movements involved in the use of the Wii included shoulder flexion and extension (bowling and tennis), shoulder rotation (tennis), elbow extension and flexion (Cooking Mama), wrist supination and pronation (tennis and Cooking Mama), and different degrees of wrist flexion and extension as well as thumb flexion involved in all activities. The recreational activities engaged similar movements. Patients were instructed to remain in a sitting position and primarily use their more affected arm/hand in these activities. Participants randomized to one arm were not exposed to the other intervention.
Video games may be associated with a risk of photosensitive-induced seizures (≈1:4000) and repetitive motion injuries.22,23 To reduce the likelihood of seizures, the lights were kept on during the VRWii gaming sessions, and patients were sitting ≥6 feet away from the television screen. The study coordinator remained in the room during the sessions and monitored the patient for symptoms suggestive of seizures or shoulder, arm, or hand pain. If the patient felt unwell at any time, the coordinator was instructed to stop the session.
Blinding of Caregivers and Outcomes Measures Assessment
The study coordinator and patients participating in this study were not blinded to the intervention group. To limit knowledge of the Wii gaming technology, and to ensure other caregivers and support staff were not aware of subject allocation, all study interventions were conducted by dedicated trial staff out of sight of ward staff. Trial staff and subjects were instructed not to divulge the intervention allocation to caregivers or other ward staff. Interventions were not recorded in the medical record. All postintervention and 4-week follow-up assessments were performed by a trained outcome assessor who was not involved in administration of study interventions and was blinded to patient randomization.
Contamination
At the time of final follow-up measurements, the blinded assessor was asked to select (forced choice) to which group they thought the patient had been allocated. This process allowed us to assess the effectiveness of blinding the assessor to group allocation.
Follow-Up Visit
Follow-up data were collected 4 weeks (±3 days) after the final study intervention session.
Outcome Measures
Primary Outcome Measures
The coprimary end points of the present study relate to feasibility and safety. Time tolerance and adaptation to playing Wii have not been formally tested in stroke patients. Therefore, the primary feasibility outcome was defined as the total time receiving the VRWii intervention.
The primary safety outcome was defined as the proportion of patients experiencing intervention-related adverse events or any serious adverse event during the study period. A serious adverse event was defined as any untoward medical occurrence, whether or not considered to be causally related to the study intervention, that resulted in death, life-threatening illness or injury, required inpatient hospitalization or prolonged inpatient hospitalization, or resulted in persistent disability or incapacity. The Borg perceived exertion scale was used to determine fatigability or level of effort required to perform tasks at the end of the sessions.24 The scale ranges from 6 (no exertion at all) to 20 (maximal exertion). Excessive fatigue is considered any score >13. For a healthy person, this represents slowly walking at his or her own pace and finding an exercise somewhat difficult but not severe enough to discontinue the task. Patients were instructed to rate their perception of exertion reflecting how heavy and strenuous the exercise felt to them, combining all sensations and feelings of physical stress, effort, and fatigue.
Secondary Outcome Measures
Because EVREST was designed as a feasibility study, efficacy was a secondary outcome assessed at 4 weeks after intervention. Efficacy was measured as an improvement in motor function determined by the total time elapsed to complete a shorter version of the Wolf Motor Function Test (WMFT).18 This test contains 15 timed and 2 strength tasks (lifting the weighted limb and grip strength), ordered from simple to complex, administered sequentially to each upper extremity and controlling for patient positioning and distance the extremity segment must traverse. Tasks specifically measured in the study included forearm to box (side), hand to box (front), lift a can, lift a paper clip, flip cards, and fold a towel. The 2 strength tasks included forward flexion of the shoulder in a seated position to the top of a box placed on the table using weights ≤20 pounds strapped to the forearm, as well as dynamometer grip strength for 3 seconds with the elbow bent to 90°.18 Other efficacy end points included a 4-block improvement on the Box and Block Test,19,25 a performance-based measure assessment of gross manual dexterity. The respondent was instructed to move as many blocks as possible, one at a time, from one compartment to the other for a period of 60 seconds. Quality of life was measured by the Stroke Impact Scale.20 This is a simple, validated, stroke-specific, health status measure. Version 2.0 was composted of 64 items in 8 domains (strength, hand function, activities of daily living/instrumental activities of daily living, mobility, communication, emotion, memory and thinking, and participation). Hand function and a composite score of the physical domains (strength, hand function, mobility, and activities of daily living/instrumental activities of daily living) were considered a priori as the more clinically relevant Stroke Impact Scale outcomes.
Study Organization and Data Management
The study was completed at Toronto Rehabilitation Institute, which receives referrals for inpatient stroke rehabilitation from 4 acute care facilities in Toronto.
Administrative activities, data management, research coordination, and statistical analyses were conducted at the Applied Health Research Centre at St. Michael’s Hospital, University of Toronto. Operational procedures, guidelines for the implementation of both arms of the study, and informed consent forms were approved by the ethics review boards at St. Michael’s Hospital and the Toronto Rehabilitation Institute. Written informed consent was obtained at the Toronto Rehabilitation Institute before enrollment.
Analysis
Given the pilot nature of this study, primarily descriptive statistics were calculated. The average total therapy time, the average therapy time per session, and differences 4 weeks after intervention from baseline in clinical outcomes were computed for each group, along with 95% CIs. The Welch t test was used to rule out large differences in therapy times. For relevant clinical outcomes, descriptive statistics (mean, SD, median, and quartiles) were computed for each assessment. Adjusted treatment analysis was planned a priori assuming differences in baseline characteristics as expected in a small sample study. Adjustment treatment effects were obtained from the parameter estimate pertaining to treatment group from multiple linear regression, for which age, Canadian Neurological Scale, and the baseline score for the particular outcome being analyzed were adjusted. An examination of the residual plots did not suggest concerns regarding the normality of the errors. A 2-sided P value of <0.05 was considered statistically significant. All statistical analysis was performed in the R language for statistical computing (R Foundation for Statistical Computing; Vienna, Austria).
Results
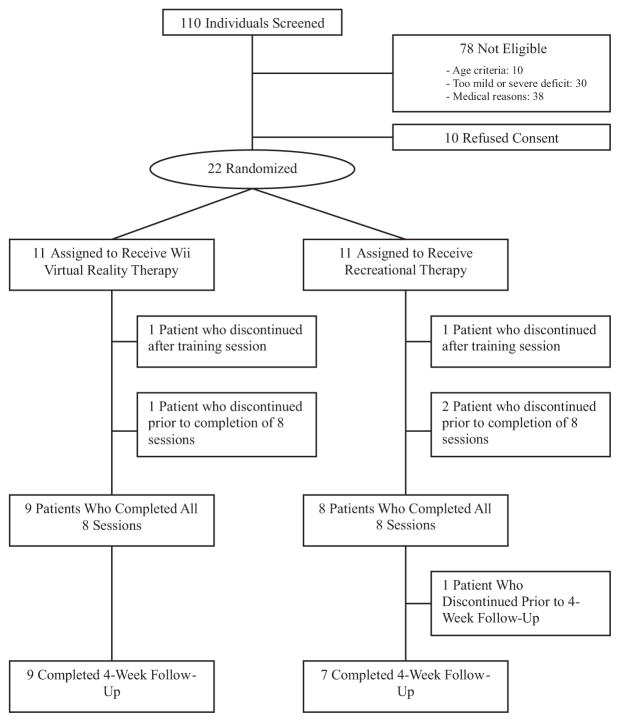
From November 2008 to October 2009, 110 potential candidates were screened to participate in EVREST (Figure). A total of 88 patients were excluded. Ten patients refused consent, 10 were >85 years of age, 30 had a very mild or severe deficit, and 38 had an underlying medical condition (angina, recent myocardial infarction, language barrier, or global aphasia) that affected their ability to participate in the study. A total of 22 (20%) participants were enrolled in EVREST. Although 11 patients were randomized to each group, one patient in each arm dropped out after the first training session, leaving 20 patients randomized (10 to each intervention arm). Eight participants randomized to RT and 9 randomized to VRWii completed all sessions (Figure).
Figure.
Flow of patients through the trial.
The mean age was 61 years (range 41 to 83 years), and the mean time from stroke onset to enrollment was 25 days (range 10 to 56 days). Baseline characteristics are presented in Table 1. Relative to patients randomized to the RT groups, patients randomized to VRWii were younger (mean age 55 years versus 67 years), had more severe strokes (median Canadian Neurological Scale 9.0 versus 10.0), and had lower arm function (median Chedoke–McMaster scale 4.0 versus 4.5) at baseline. There were no significant imbalances between groups with respect to gender, comorbidities, affected side, handedness, stroke type.
Table 1.
Study Participant Characteristics
| Characteristics | RT (n=11) | VRWii (n=11) |
|---|---|---|
| Mean age (range), y | 67.3 (46 – 83) | 55.3 (41–72) |
| Sex, male | 7 (64) | 7 (64) |
| Handedness, right | 10 (91) | 10 (91) |
| Comorbidities | ||
| Hypertension | 5 (45) | 9 (82) |
| Dyslipidemia | 2 (18) | 1 (9) |
| Diabetes mellitus | 4 (36) | 2 (18) |
| Atrial fibrillation | 0 (0) | 1 (9) |
| Stroke type, hemorrhagic | 2 (18) | 2 (18) |
| Affected side, right | 6 (55) | 6 (55) |
| Baseline functional status at time of randomization | ||
| Chedocke–McMaster, median [IQR] | 4.5 [4.0–5.0] | 4.0 [3.5–4.0] |
| Stroke severity, median CNS [IQR] | 10.0 [8.1–10.4] | 9.0 [7.8–9.5] |
| Barthel index, median [IQR] | 65 [60–88] | 65 [55–72.5] |
| Modified Rankin scale | ||
| 1 | 1 (9) | 0 (0) |
| 2 | 3 (27) | 2 (18) |
| 3 | 5 (45) | 5 (45) |
| 4 | 2 (18) | 4 (36) |
| Mean [SD] days from onset to randomization | 22.7 [8.6] | 26.7 [16.4] |
Numbers between brackets represent percentages unless otherwise specified.
IQR indicates interquartile range; CNS, Canadian Neurological Scale.
Both groups received similar durations of standard rehabilitation (physiotherapy and occupational therapy) during the study period: ≈20 hours during the 2-week intervention period (mean time of physiotherapy and occupational therapy 100 minutes on weekdays and 81 minutes on weekends).
In assessing the success of blinding, we found no evidence of failure of concealment at the end of the study. The blinded assessor was correct 9 times out of 16 decisions, or 56% of the time, which is similar to the 50% expected by chance.
Primary End Points: Safety and Feasibility
None of the participants in either group experienced a serious adverse event during the study period. Table 2 summarizes the primary outcomes. There was no difference in the proportion of patients reporting any symptom during the interventions (difference VRWii-RT 0.2 (95% CI, −0.229, 0.629]). Two participants in the RT and 3 participants in the VRWii reported exertion fatigue (defined as a Borg >13 during therapy). The difference in the proportion of patients ever reporting fatigue was not significant.
Table 2.
Effect of VRWii on Primary End Points Between Groups
| Outcomes | RT (n=10) | VRWii (n=10) | Difference VRWii-RT (95% CI) |
|---|---|---|---|
| Primary end points | |||
| Primary feasibility outcomes | |||
| No. (%) of patients completing all 8 sessions | 8 (80) | 9 (90) | 0.1 (−0.21, 0.41)* |
| Mean (SD) total session time, in minutes | 388 (171) | 364 (148) | −23.3 (−173.7, 127.1)† |
| Mean (SD) session time, in minutes | 56.2 (6.8) | 46.5 (16.0) | −9.7 (−21.7, 2.2)‡ |
| Primary safety outcomes | |||
| Serious adverse events | 0 (0) | 0 (0) | NA |
| Dizziness or nausea | 1 (10) | 0 (0) | NA |
| Mean Borg exertion scale at the end of the interventions (SD) | 9.38 (4.7) | 9.0 (2.8) | −0.4 (−4.1, 3.4) |
| Borg exertion scale (fatigability) >13 in any session | 2 (20) | 3 (33) | 0.13 (−0.26, 0.53)§ |
| No. of patients reporting any symptom during any session | 4 (40) | 6 (60) | 0.2 (−0.23, 0.63)¶ |
NA indicates not applicable.
Difference in the proportion completing all 8 sessions between groups;
difference (95% CI) in the total session time of the delivered intervention between groups;
difference (95% CI) in the average total session length between groups;
difference (95% CI) in the proportion of patients ever reporting fatigue (Borg exertion scale >13) during the intervention;
difference (95% CI) in the proportion of patients reporting any symptom during the intervention.
Ten participants in each group started the intervention after the training session. The interventions were successfully delivered in 9 of 9 participants in the VRWii and 8 of 10 in the RT arm (Figure).
The primary end point for time of delivered interventions showed no difference between groups. The mean time for the delivered intervention was 388±171 minutes in the RT and 364±148 minutes in the VRWii group (P=0.75; Table 2). The total delivery time comprised 80% (RT) and 76% (VRWii) of the scheduled time.
Secondary End-Points: Clinically Relevant Outcomes
Table 3 summarizes the unadjusted changes in clinical outcomes at baseline through 4 weeks after the interventions within the VRWii and RT groups. Participants in the VRWii group had a significant improvement in WMFT (represented as a shorter time in completing the tasks) and grip strength from baseline, whereas both groups had an improvement in Box and Block Test (Table 3).
Table 3.
Effect of Interventions on Secondary Outcomes at Baseline Through 4-Week Follow-Up and Change From Baseline Within Wii and RT Groups
| Secondary Efficacy Outcomes | Virtual Reality using Wii (VRWii)
|
Recreational Therapy (RT)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n=9) | Postintervention (n=9) | Four Weeks After Intervention (n=9) | Change From Baseline (95% CI)* | Baseline (n=10) | Postintervention (n=9) | Four Weeks After Intervention (n=7) | Change From Baseline (95% CI)* | |
| WMFT, mean time in seconds | 29.5 | 19.8 | 19.0 | −10.5 (−19.3, −1.8) | 39.7 | 27.4 | 25.7 | −14 (−32.1, 4.1) |
| BBT, mean No. of blocks | 33.4 | 40.1 | 42.0 | 8.6 (0.9, 16.2) | 24.4 | 34.1 | 36.4 | 12 (3.8, 20.2) |
| SIS hand function, % | 70.6 | 71.1 | 85.8 | 15.3 (−1.2, 31.8) | 63.4 | 70.0 | 74.6 | 11.2 (−21.3, 43.8) |
| SIS composite function, % | 74.4 | 74.2 | 82.7 | 8.3 (−3.3, 19.9) | 67.0 | 74.3 | 80.3 | 13.3 (0.9, 25.8) |
| SIS perception of recovery, % | 62.1 | 76.7 | 76.1 | 14 (−2.7, 12.6) | 61.4 | 66.4 | 77.1 | 15.7 (−9.1, 40.5) |
| Grip strength, mean in kg | 22.4 | 24.6 | 28.8 | 6.4 (3.8, 9.0) | 18.7 | 21.5 | 21.5 | 2.8 (−1.6, 7.1) |
SIS indicates Stroke Impact Scale; BBT, Box and Block Test.
SIS composite includes the following physical domains: strength, hand function, mobility, and activities of daily living.
Difference and 95% CI in secondary efficacy outcomes between baseline and 4-week postintervention measures in each group;
one patient was unable to complete some baseline tasks for the WMFT.
Multivariable analyses using linear regression 4 weeks after intervention were performed using baseline measures as covariates to evaluate the efficacy of VRWii versus RT. After adjustment for age, baseline functional status (WMFT), and stroke severity, participants in the VRWii arm performed, on average, significantly better on the WMFT than the RT group (−7.4 seconds; 95% CI, −14.5, −0.2). Similarly, participants randomized to VRWii achieved a nonsignificant improvement in grip strengths when compared with RT after adjusting for age, baseline grip strength, and stroke severity (1.9 kg; 95% CI, −2.5, 6.2). No significant differences were observed in the adjusted analysis for the Stroke Impact Scale (hand or composite) and Box and Block Test.
Discussion
The field of poststroke rehabilitation is evolving. The current paradigm of stroke rehabilitation strategies to improve motor function is focused on high-intensity, repetitive, and task-specific practice.2,26 Long-term potentiation, implicated in the acquisition of new and retrieval of learned motor patterns, develops from repeated stimulation or tasks. VR gaming is a technology that allows a user to interact with a computer-simulated environment and receive near real-time feedback on performance. As reported in recent studies,2,14 the extent to which VR systems can facilitate conventional therapy currently in use remains to be determined.
The EVREST trial represents the first randomized controlled study to systematically test an interactive 3D VR technology using the Wii gaming system as neurorehabilitation therapy among patients who experienced a first stroke within 2 months before enrollment. The effect of the VRWii gaming technology was compared with RT among patients receiving usual/standard rehabilitation. We found that VRWii use was feasible and safe when performed under the prespecified criteria. For the efficacy outcomes (secondary clinical end points), this feasibility study showed a significant improvement in motor function (WMFT) in the unadjusted (Table 3) and adjusted analysis for the VRWii group. When reporting outcomes, it is worth knowing whether an observed difference indicates a clinically significant effect.27 The observed 7-second benefit for the VRWii group in the adjusted analysis appears clinically meaningful given the 1.5-to 2-second change reported as a the minimal clinically important difference in previous studies.28
The stroke rehabilitation research landscape has been evolving.2 The results of the EXCITE (Extremity Constraint Induced Therapy Evaluation) trial provided new insight in stroke rehabilitation by showing that constraint-induced motor therapy can produce a clinically relevant improvement in arm function for patients within 6 months of a stroke.4 A study using functional MRI showed that repetitive bimanual stimulation produced bilateral activation of the motor cortices, one marker of brain plasticity.8 Interestingly, the comparison of Bobath, proprioceptive neuromuscular facilitation, or motor relearning conventional techniques has shown that no one approach improves functional outcomes over another.29–32 According to a recent systematic review, constraint-induced motor therapy represents, thus far, the most promising intervention to date for improving upper limb function.2
Limited evidence exists regarding the use of VR gaming systems in stroke rehabilitation. Two recent systematic reviews have summarized the available studies on VR in stroke rehabilitation.13 Among 11 identified studies, there were only 3 randomized clinical trials with diverse outcomes measures (memory retraining, walking, gait, and postural stability). Most of the reported studies focused on lower extremities, especially in gait training, including the use of a treadmill.13 Only 3 studies addressed upper limb rehabilitation, and none of them were randomized trials. None of the studies reported any significant adverse effects. Moreover, several studies compared an intervention plus conventional physical therapy versus conventional physical therapy alone, which, by necessity allowed for more rehabilitation time in the experimental group.33 This creates a bias in favor of the new intervention because the intensity and frequency of rehabilitation per se is known to directly and beneficially affect functional outcomes. A more recent systematic review on VR gaming and arm motor function found only 2 randomized clinical trials, with just one including patients in the acute phase of stroke.14 No significant differences were observed between virtual-environment training and conventional therapy groups in motor strength and functional scores. Finally, the authors of this systematic review highlighted the potential value and safety of VR gaming as a tool for stroke rehabilitation but concluded that VR gaming in stroke rehabilitation is an unproven treatment, and much more evidence is needed from well-designed randomized trials.13,14 Considering the paucity of well-designed randomized studies, the scarce funding for stroke rehabilitation research, and the limitations of conventional rehabilitation, VRWii technology is accessible for all segments of the population at a relatively low cost, without requiring special resources, assistance, or transportation to a specific facility.
The limitations of our study deserve comment. First, EVREST was a pilot study with a small sample size, limiting any definitive conclusions about the efficacy of VRWii. EVREST was designed as a feasibility study and therefore not powered to detect a difference between groups. However, these results will allow for an informed estimate of the sample size required for an adequately powered large-scale randomized clinical trial. Second, because this study was single-blinded, it was possibly subject to bias in that patients using the “new” technology may have been more motivated by the use of this treatment and also may have inadvertently disclosed their treatment allocation to the examiner. Nevertheless, this is a common issue in the design and implementation of randomized clinical trials in stroke rehabilitation, and not unique to EVREST. However, we had made an effort to determine whether concealed allocation was preserved by asking the blinded assessor to guess the allocation group for each participant. No difference was found. Third, because safety and feasibility were the primary outcome measures in EVREST, we have no information on the potential effects of bimanual training in this population. Fourth, the VRWii group was significantly younger than the RT group. Older subjects typically have slower reaction times, with an apparent poorer performance in the RT group. However, the results remained consistent after adjusting for age and other baseline differences. Finally, the short duration of the intervention (8 sessions within 2 weeks) may underestimate the effect of VRWii gaming technology. In addition, the Wii system only provides feedback on the movement itself such that persons might adopt a variety of movement strategies to successfully play the game. Some of these strategies (eg, significant shoulder/trunk motions) are not necessarily ideal adaptive strategies to reinforce.34 As a result, patient supervision may be an important component of subsequent trials, as might be the addition of additional sensors to better represent feedback of limb/arm position.
Despite these limitations, EVREST is the first randomized clinical trial showing that VRWii is a feasible, safe, and potentially effective intervention to enhance motor function recovery in patients with a recent stroke and represents a proof-of-concept trial.14 EVREST used a novel, simple, wireless, and widely available 3D VR technology, allowing the implementation of proven concepts in stroke rehabilitation to improve motor function. Repetitive intense training and the observation, practice, and representation on the screen of task-specific activities can facilitate brain plasticity mechanisms that engage the mirror neuron system or long-term potentiation effects.
In summary, the EVREST study constitutes the initial step in the understanding of potential benefits of a novel interactive approach in neurorehabilitation after stroke that can be easily implemented in routine clinical practice. Further, VR technology could be used as adjuvant therapy to other proven successful interventions (ie, constraint-induced therapy). As such, it provides hope for enhancing motor function and improving quality of life in stroke survivors.
Acknowledgments
The authors thank the support provided by the Ontario Stroke System (OSS), the Ministry of Health and Long Term Care of Ontario (MoHLTC), and Heart and Stroke Foundation Ontario (HSFO). The authors also thank Florencia E. Saposnik (9), source of inspiration for the design of EVREST. The authors thank the co-op students at Li Ka Shing Knowledge Institute for their assistance with database development and data entry. We also thank the Li Ka Shing Knowledge Institute at St Michael’s Hospital and Toronto Rehabilitation Institute for the efficient organization and implementation of the trial. We especially thank Jacqueline Willems, manager of the South East Toronto Stroke Network, and Dr Neville Bayer (deceased), former director of the Stroke Program at St. Michael’s Hospital, for their invaluable support. We also appreciate the support, comments, and suggestions from Drs Vladimir Hachinski and Sandra Black during conception of the study.
The EVREST Research Study Group comprised G.S. (principal investigator), M.B., M.M., J.H., D.C., W.M., K.E.T., Jacqueline Willems, Alexis Dishaw (Toronto, Canada), R.T., and L.G.C.
The EVREST team at Toronto Rehabilitation Institute was composed of M.B. (site principal investigator), Laura Langer, Jennifer Shaw, Hannah Cheung, and W.M.
The EVREST Steering Committee included G.S. (principal investigator of EVREST), M.B. (principal investigator at Toronto Rehabilitation), J.H. (research manager), and M.M. (M.M.) at the Applied Health Research Centre, who made all decisions concerning the implementation and conduction of the study.
Sources of Funding
This study was supported by a grant from the Ministry of Health and Long Term Care (MoHLTC) through the Ontario Stroke System (OSS), administered by Heart and Stroke Foundation of Ontario (HSFO). We are most grateful for the initial funding provided by South East Toronto Stroke Network, which helped with the early organization of the study design and coordination.
Footnotes
Disclosures
The authors declare no financial conflicts of interest. Specifically, we have not received support from Nintendo or other software development companies.
The sponsors were not involved in the design, execution, analysis, interpretation, or reporting of the results.
We declare that we have participated in the conception, design, analysis, interpretation of the results, drafting the manuscript, and made a critical revision of the manuscript.
K.T. (biostatistician), G.S., and J.H. all had full access to the data. G.S. was responsible for obtaining funds and is supported by the clinician-scientist award from Heart and Stroke Foundation Ontario.
References
- 1.Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke. 2005;36:1480–1484. doi: 10.1161/01.STR.0000170706.13595.4f. [DOI] [PubMed] [Google Scholar]
- 2.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 3.Barreca S, Wolf SL, Fasoli S, Bohannon R. Treatment interventions for the paretic upper limb of stroke survivors: a critical review. Neurorehabil Neural Repair. 2003;17:220–226. doi: 10.1177/0888439003259415. [DOI] [PubMed] [Google Scholar]
- 4.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. J Am Med Assoc. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 5.Jutai JW, Teasell RW. The necessity and limitations of evidence-based practice in stroke rehabilitation. Top Stroke Rehabil. 2003;10:71–78. doi: 10.1310/CRDA-PGFW-KHEL-20E1. [DOI] [PubMed] [Google Scholar]
- 6.Teasell R, Meyer MJ, McClure A, Pan C, Murie-Fernandez M, Foley N, Salter K. Stroke rehabilitation: an international perspective. Top Stroke Rehabil. 2009;16:44–56. doi: 10.1310/tsr1601-44. [DOI] [PubMed] [Google Scholar]
- 7.Kwakkel G, Wagenaar RC, Koelman TW, Lankhorst GJ, Koetsier JC. Effects of intensity of rehabilitation after stroke. A research synthesis. Stroke. 1997;28:1550–1556. doi: 10.1161/01.str.28.8.1550. [DOI] [PubMed] [Google Scholar]
- 8.Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, Schulz JB, Goldberg AP, Hanley DF. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. J Am Med Assoc. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buccino G, Solodkin A, Small SL. Functions of the mirror neuron system: implications for neurorehabilitation. Cogn Behav Neurol. 2006;19:55–63. doi: 10.1097/00146965-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Celnik P, Stefan K, Hummel F, Duque J, Classen J, Cohen LG. Encoding a motor memory in the older adult by action observation. Neuroimage. 2006;29:677–684. doi: 10.1016/j.neuroimage.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 11.Rizzolatti G, Fabbri-Destro M. The mirror system and its role in social cognition. Curr Opin Neurobiol. 2008;18:179–184. doi: 10.1016/j.conb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Celnik P, Webster B, Glasser DM, Cohen LG. Effects of action observation on physical training after stroke. Stroke. 2008;39:1814–1820. doi: 10.1161/STROKEAHA.107.508184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crosbie JH, Lennon S, Basford JR, McDonough SM. Virtual reality in stroke rehabilitation: still more virtual than real. Disabil Rehabil. 2007;29:1139–1146. doi: 10.1080/09638280600960909. discussion 1147–1152. [DOI] [PubMed] [Google Scholar]
- 14.Lucca LF. Virtual reality and motor rehabilitation of the upper limb after stroke: a generation of progress? J Rehabil Med. 2009;41:1003–1100. doi: 10.2340/16501977-0405. [DOI] [PubMed] [Google Scholar]
- 15.Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, Sanford J, Barreca S, Vanspall B, Plews N. Measuring physical impairment and disability with the chedoke-mcmaster stroke assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 16.Cote R, Battista RN, Wolfson C, Boucher J, Adam J, Hachinski V. The Canadian Neurological Scale: validation and reliability assessment. Neurology. 1989;39:638– 643. doi: 10.1212/wnl.39.5.638. [DOI] [PubMed] [Google Scholar]
- 17.Bushnell CD, Johnston DC, Goldstein LB. Retrospective assessment of initial stroke severity: comparison of the NIH stroke scale and the Canadian Neurological Scale. Stroke. 2001;32:656–660. doi: 10.1161/01.str.32.3.656. [DOI] [PubMed] [Google Scholar]
- 18.Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–132. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 19.Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K. Analysis of fmri and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- 20.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 21.Saposnik G, Mamdani M, Bayley M, Thorpe KE, Hall J, Cohen LG, Teasell R. Effectiveness of Virtual Reality Exercises in Stroke Rehabilitation (EVREST): rationale, design, protocol and baseline data of a pilot randomized clinical trial assessing the Wii gaming system. Int J Stroke. 2010;5:47–51. doi: 10.1111/j.1747-4949.2009.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher RS, Harding G, Erba G, Barkley GL, Wilkins A. Photic- and pattern-induced seizures: a review for the Epilepsy Foundation of America Working Group. Epilepsia. 2005;46:1426–1441. doi: 10.1111/j.1528-1167.2005.31405.x. [DOI] [PubMed] [Google Scholar]
- 23.Bonis J. Acute Wiiitis. N Engl J Med. 2007;356:2431–2432. doi: 10.1056/NEJMc070670. [DOI] [PubMed] [Google Scholar]
- 24.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 25.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39:386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 26.Dobkin BH. Training and exercise to drive poststroke recovery. Nat Clin Pract Neurol. 2008;4:76–85. doi: 10.1038/ncpneuro0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barak S, Duncan PW. Issues in selecting outcome measures to assess functional recovery after stroke. NeuroRx. 2006;3:505–524. doi: 10.1016/j.nurx.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin KC, Hsieh YW, Wu CY, Chen CL, Jang Y, Liu JS. Minimal detectable change and clinically important difference of the Wolf Motor Function Test in stroke patients. Neurorehabil Neural Repair. 2009;23:429–434. doi: 10.1177/1545968308331144. [DOI] [PubMed] [Google Scholar]
- 29.Langhammer B, Stanghelle JK. Bobath or motor relearning programme? A comparison of two different approaches of physiotherapy in stroke rehabilitation: a randomized controlled study. Clin Rehabil. 2000;14:361–369. doi: 10.1191/0269215500cr338oa. [DOI] [PubMed] [Google Scholar]
- 30.Logigian MK, Samuels MA, Falconer J, Zagar R. Clinical exercise trial for stroke patients. Arch Phys Med Rehabil. 1983;64:364–367. [PubMed] [Google Scholar]
- 31.Lord JP, Hall K. Neuromuscular reeducation versus traditional programs for stroke rehabilitation. Arch Phys Med Rehabil. 1986;67:88–91. doi: 10.1016/0003-9993(86)90108-5. [DOI] [PubMed] [Google Scholar]
- 32.van Vliet PM, Lincoln NB, Foxall A. Comparison of bobath based and movement science based treatment for stroke: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2005;76:503–508. doi: 10.1136/jnnp.2004.040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JH, Jang SH, Kim CS, Jung JH, You JH. Use of virtual reality to enhance balance and ambulation in chronic stroke: a double-blind, randomized controlled study. Am J Phys Med Rehabil. 2009;88:693–701. doi: 10.1097/PHM.0b013e3181b33350. [DOI] [PubMed] [Google Scholar]
- 34.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123:940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]