Abstract
The peptide alpha-helix represents one of Nature’s most featured protein shapes and is employed in a diversity of protein architectures, spanning the very cytoskeletal infrastructure of the cell to the most intimate contact points between crucial signaling proteins. By installing an all-hydrocarbon crosslink into native sequences, we recapitulate the shape and biological activity of natural peptide alpha-helices, yielding a chemical toolbox to both interrogate the protein interactome and modulate interaction networks for potential therapeutic benefit. Here, we describe our latest approach to synthesizing Stabilized Alpha-Helices (SAH) corresponding to key protein interaction domains. We emphasize a stepwise approach to the production of crosslinking non-natural amino acids, their incorporation into peptide templates, and the application of ruthenium-catalyzed ring closing metathesis to generate hydrocarbon-stapled peptides. Through facile derivatization and functionalization steps, SAHs can be tailored for a broad range of applications in biochemical, structural, proteomic, cellular and in vivo studies.
Keywords: α-helix, peptide, hydrocarbon stapling, olefin metathesis, photoreactive, protein interaction, targeting
Introduction
Why staple a peptide?
The simplest answer is: to restore and stabilize the natural alpha-helical structure of a peptide that otherwise unfolds when taken out of context from the full-length protein. By installing a non-natural all-hydrocarbon restraint within the peptide at one or more locations, we have consistently observed by circular dichroism that unfolded helical peptides can be zipped back into shape(Bird et al., 2010; Walensky et al., 2004; Walensky et al., 2006) (Fig. 1A). This staple-induced structural transformation turns out to have several beneficial side-effects:
First, the proteolytic resistance of the peptide is dramatically enhanced(Bird et al., 2010; Walensky et al., 2004) (Fig. 1B). Whereas proteases efficiently hydrolyze linear amide bonds, amide bonds engaged in the hydrogen-bonding network of a structured peptide helix are poor enzymatic substrates. In fact, we recently learned that there are two tiers of staple-induced protease resistance, including (1) slowing the kinetics of proteolysis through helical induction distal to the staple and (2) complete blockade of proteolysis in the constrained region immediately adjacent to and within the boundaries of the staple itself(Bird et al., 2010).
Second, stapled peptides gain entry into cells through a constitutive, ATP-dependent, vesicular important pathway called pinocytosis(Walensky et al., 2004). Confocal imaging of live cells treated with fluorescently-labeled stapled peptides enables tracking of peptide from the pinosomal import stage (e.g. colocalization with dextran) to ultimate release into the cytosol for distribution to the site(s) of target engagement (e.g. mitochondria) (Fig. 1C).
Third, target binding affinity is enhanced due to prefolding and structural reinforcement, which respectively establish and maintain the proper orientation of interacting residues. We have observed (1) leftward shifts in binding isotherms of stapled peptides compared to the corresponding unmodified templates (Fig. 1D, left) and (2) sequence-specific binding activity of stapled peptide helices but no detectable binding for the corresponding unstructured peptides (Fig. 1D, right)(Bernal et al., 2010; Bird et al., 2010; Walensky et al., 2004; Walensky et al., 2006) (Fig. 1D). In addition, we recently observed that an appropriately positioned staple can itself enhance binding activity even further and without compromising selectivity, by engaging hydrophobic contacts at the perimeter of an interaction site(Stewart et al., 2010).
Fig. 1.
Characteristic features of hydrocarbon-stapled peptides. (A) Circular dichroism demonstrates that hydrocarbon stapling can transform an unfolded peptide into a sturdy α-helix(Bird et al., 2010). (B) Protease resistance of hydrocarbon-stapled peptides. In this example, unmodified, singly-, and doubly-stapled peptides were exposed to chymotrypsin in vitro and the persistence of full-length peptide monitored over time by LC/MS(Bird et al., 2010). (C) Fluorescently-labeled stapled peptides are taken up by cells via the pinosomal pathway and gradually released into the cytosol for distribution to target binding sites, as shown here for a BCL-2 family α-helix (BID SAHB) that tracks to the mitochondria(Walensky et al., 2004). (D) Stapled helices can exhibit enhanced binding affinity for their target protein compared to the corresponding unmodified peptide. In this example, a BCL-2 family α-helix (BIM SAHB) demonstrated improved binding affinity for an anti-apoptotic target (left) and a previously undetected binding interaction with a pro-apoptotic target (right)(Walensky et al., 2006).
Thus, hydrocarbon stapling can produce a structurally-stabilized alpha-helical peptide with the capacity to resist proteolysis and gain cellular entry, overcoming key shortcomings of peptides as research tools and prototype therapeutics.
What can you do with stapled peptides?
Since we began applying hydrocarbon stapling to generate bioactive helices ten years ago, we have experimented with a variety of applications for their use in target binding analyses, structure determination, proteomic discovery, signal transduction research, cellular analyses, imaging, and in vivo bioactivity studies. We have explored protein targeting at the plasma membrane, within the cytosol and nucleus, and at specific organelles. We believe that the track record of stapled peptides in uncovering new protein interactions and targeting known interactions for therapeutic benefit in preclinical models speaks to their dual capacity to serve as effective research tools and promising drug prototypes. As with all new technologies, we continue to learn the rules, make improvements, and discover new applications. Below we present our most current methods for synthesizing and derivatizing stapled peptides for protein interaction research and therapeutic targeting.
Strategic Planning
The first step when preparing to generate stapled peptides is stockpiling the non-natural amino acids used for olefin metathesis-based crosslinking, whether they be (S)-2-(((9H–fluoren-9-yl)methoxy)carbonylamino)-2-methyl-hept-6-enoic acid (“S5”) used for i, i+4 staples or the additional (R)-2-(((9H–fluoren-9-yl)methoxy)carbonylamino)-2-methyl-dec-9-enoic acid (“R8”) paired with S5 to produce i, i+7 staples. We have tested and validated two methods for the production of these crosslinking amino acids. The first approach is based on the method developed by Williams and colleagues(Williams et al., 1988; Williams and Im, 1991; Williams et al., 2003), applied by Verdine and colleagues(Schafmeister et al., 2000; Walensky et al., 2004), and used extensively by our group, as described(Bird et al., 2008). As no significant changes have been made in this synthetic route since our last report(Bird et al., 2008), we will instead describe herein an alternative, facile approach that we have recently tested and adapted for generating the non-natural amino acids based on the methods of Hruby, Ryzhov, and colleagues(Belokon et al., 1998; Qiu et al., 2000).
With stapling amino acids in hand, the next step is to generate the designs for your first panel of peptides. The more structural data available for your template peptide and its interactor(s) the better, since the initial goal is to place the hydrocarbon staple(s) at the non-interacting face of the helix to avoid disruption of key helix-target interactions. If there is no structural data available but secondary structure prediction software suggests the peptide template is likely an alpha-helix, a panel of constructs with differentially localized staples can be generated at the outset to determine optimal staple placement. Once an effective binder is identified, subsequent panels can be geared toward generating mutant controls, alanine and/or staple scans, and any further iterations to optimize binding activity, protease resistance, cellular penetrance, and/or biological activity (see below). Depending on the experimental application, the N-terminus, for example, can be capped with acetyl, FITC, biotin, spin label, radiolabel, or other functionalities, so it is best to consider during the peptide design stage which derivatizations will be best suited for your work.
Finally, in preparation for peptide synthesis, it is best to identify a research group or core facility with longstanding experience in automated peptide synthesis, derivatization, purification, and quantitation. Importantly, the group must be willing to modify the standard Fmoc conditions used for generic peptide synthesis to accommodate the extended coupling times and other necessary protocol adjustments for optimal stapled peptide synthesis, as delineated below. To date, stapled peptides have been successfully generated in high yield and purity on the following automated synthesizers: ABI 433A (Applied Biosystems), Apex 396 (AAPTec), Tetras (CreoSalus), and Liberty-12 (CEM). Finally, once the stapled peptides are made and purified, we recommend quantitation by amino acid analysis (AAA), which will require identification of a core facility that offers reliable AAA services.
BASIC PROTOCOL 1
ASYMMETRIC SYNTHESIS OF THE STAPLING AMINO ACIDS VIA BENZYLPROLYLAMINOBENZOPHENONE
The following method for generating α,α-disubstituted non-natural amino acids bearing olefin tethers was adapted from Belokon et al, 1998 and Qiu et al, 2000 and employs a benzylprolylaminobenzophenone (BPB)-based chiral auxiliary(Belokon et al., 1998; Qiu et al., 2000). The advantages of this synthetic approach include its simplicity, alkylation efficiency, high stereochemical purity of the product, and the ability to recycle the chiral auxiliary.
Materials
Nitrogen source
Potassium hydroxide pellets (KOH)
Isopropanol, HPLC grade
d-proline
Benzyl chloride, anhydrous
Hydrochloric acid (HCl)
Chloroform, HPLC grade (CHCl3)
Magnesium sulfate powder, anhydrous (MgSO4)
Dichloromethane, reagent grade and anhydrous (DCM)
Thionyl chloride, anhydrous 2 M in DCM (SOCl2)
Racemic alanine
Nickel (II) nitrate hexahydrate (Ni(NO3)2•6H2O)
o-Aminobenzophenone
Solution of brine
Saturated sodium carbonate solution
Ethyl acetate, reagent grade (EtOAc)
Hexanes, reagent grade
Methanol, reagent grade (MeOH)
Acetic acid (AcOH)
Sodium iodide (NaI)
Acetone, HPLC grade
8-Bromo-1-octene
Dimethylformamide, anhydrous (DMF)
Acetonitrile (ACN)
Trifluoroacetic acid (TFA)
9-Fluorenylmethoxycarbonyl-N-hydroxysuccinimide (Fmoc-OSu)
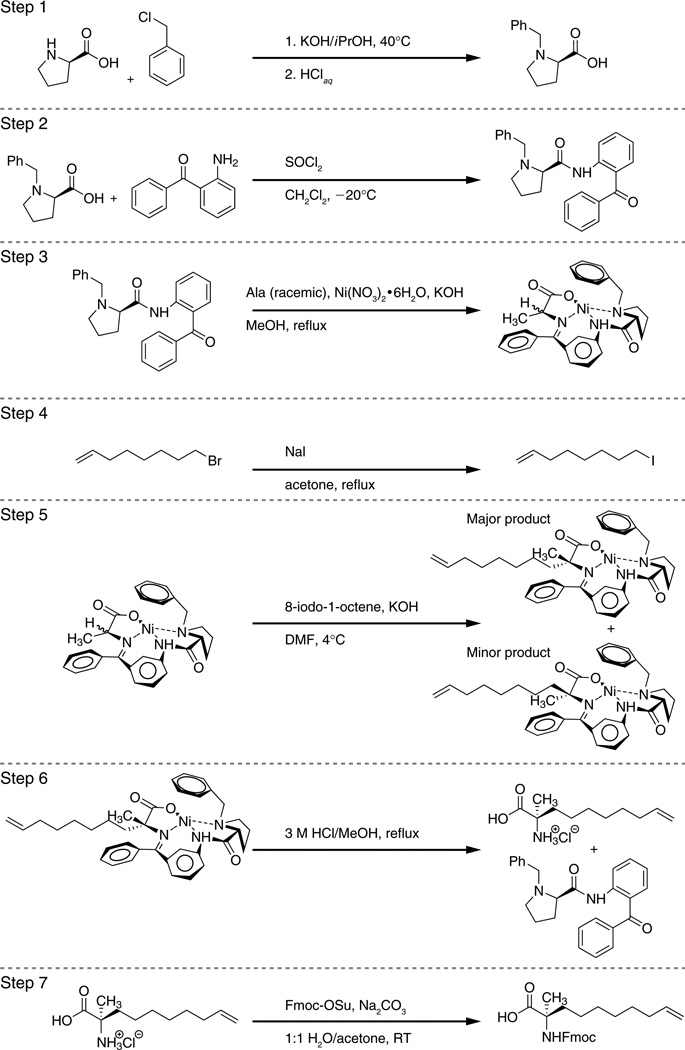
Synthetic Steps (Fig. 2)
Fig. 2.
Synthetic steps for generating α,α-disubstituted non-natural amino acids bearing olefin tethers using a BPB-based chiral auxiliary.
1. Synthesis of (R)-N-benzylproline (BP)
In a three-neck 500 mL round-bottom flask fitted with a glass stopper, addition funnel, and nitrogen line, dissolve freshly ground KOH (3.8 equiv) in isopropanol (110 mL). Add D-proline (15 g, 1 equiv), stirring the solution at 40°C until transparent, and then add benzyl chloride (1.5 equiv) via addition funnel at 40°C, stirring the cloudy solution at 40°C for 6 h. Quench the reaction by adding HCl until pH 5–6 is achieved. To purify the product, add chloroform (45 mL) and allow the solution to sit overnight. Filter the resulting precipitate and wash with three 20 mL portions of CHCl3. Combine the CHCl3 filtrates, dry over MgSO4, and concentrate in vacuo to afford a wet-appearing orange solid. Treat the solid with acetone to dissolve impurities and filter the resulting white solid, (R)-N-benzylproline (15.21 g, 74.1 mmol, 57% isolated yield).
2. Synthesis of (R)-2-[N-(N’-benzylprolyl)amino]benzophenone (BPB)
To a three-neck 500 mL flask equipped with an addition funnel, nitrogen line, and glass stopper, dissolve BP (15.21 g, 1 equiv) in 34 mL anhydrous DCM and stir at −20°C for 20 minutes. Slowly add thionyl chloride (1.25 equiv, 2 M in DCM) via syringe at −20°C and stir the solution at −15°C for 20 min. Add a solution of o-aminobenzophenone (1 equiv) in DCM (60 mL) dropwise via addition funnel at −30°C. Slowly raise the temperature of the reaction to RT and then stir overnight. If a precipitate forms, dissolve the solid by quenching the reaction with a saturated sodium carbonate solution (2 equiv in 60 mL H2O). Transfer the solution to a 500 mL separatory funnel and extract the aqueous solution with three 50 mL portions of DCM. Combine the organic layers and wash with 100 mL brine. Dry the organic layer over MgSO4 and concentrate in vacuo to yield a brown-orange solid, BPB, which is used without further purification. If desired, a short silica column using an EtOAc/hexanes gradient (0 to 20%, Rf=0.29) can be used to purify the product.
3. Synthesis of the chiral auxiliary, BPB-Ni(II)-Ala
Combine BPB (9.16 g, 1 equiv), racemic alanine (2 equiv), and Ni(NO3)2•6H2O (2 equiv) under nitrogen in a 250 mL two-neck round bottom flask equipped with a glass stopper and condenser. Add methanol (85 mL) and stir the solution for 10 min at 60°C, and then add a solution of KOH (7 equiv) in MeOH (36 mL). The reaction mixture will turn color from green to brick red. Stir the reaction at reflux for 2 h and then cool to RT with stirring. Quench the reaction by the addition of 9.6 mL AcOH (7 equiv) and transfer to a 500 mL erlenmeyer flask and dilute with 268 mL H2O. Allow the flask to sit undisturbed overnight to crystallize BPB-Ni(II)-Ala, which is then filtered and washed with H2O to yield 9.5 g of pure chiral auxiliary (18.5 mmol, 80%; Rf =0.31 in 5% MeOH/DCM).
4. Synthesis of 8-iodo-1-octene by Finkelstein reaction
Dissolve NaI (35.7 g, 2 equiv) in acetone (180 mL) in a 250 mL two-neck round bottom flask equipped with a rubber septum and condenser, and then add 8-bromo-1-octene (20 mL, 1 equiv) via syringe (the solution will become cloudy). Reflux the reaction with stirring for 2 h. Cool the reaction to RT and then transfer to a 250 mL separatory funnel. Extract the organic mixture with three 50 mL portions of hexanes and then wash with three 20 mL portions of water. Dry the organic layer over MgSO4 and concentrate in vacuo to afford clear liquid 8-iodo-1-octene, obtained in near quantitative yield. Depending on their reactivity, alkenyl bromides may be used directly in the subsequent alkylation step, obviating the need for halide exchange.
5. Alkylation to form (R)-BPB-Ni(II)-2-methyldec-9-enoate
Dissolve BPB-Ni(II)-Ala (9.5 g, 1 equiv) in 95 mL DMF under nitrogen in a 250 mL round bottom flask submerged in an ice bath. Add freshly ground KOH (10 equiv) and stir for 20 min at 4°C. Subsequently, add 8-iodo-1-octene (1.2 equiv) as a solution in DMF (8.4 mL) via syringe at 4°C. Stir the reaction, allowing it to warm to RT, and monitor for generation of the reaction product (Rf =0.41, 5% MeOH/DCM), which migrates just above the starting material upon TLC. Quench after 1.5 h by adding the reaction mixture to 422 mL of chilled 5% AcOH in a 1 L Erlenmeyer flask. Transfer the solution to a 1 L separatory funnel and extract the organic layer with three 100 mL portions of DCM and wash with three 50 mL portions of water. Dry the organic layer over MgSO4 and concentrate in vacuo to afford a deep-red oil. Adsorb the crude material onto celite, dry load a silica column, and elute with a 0% to 70 % EtOAc/hexanes gradient. Combine only the exquisitely clean fractions of product to avoid trace contamination by the unwanted diastereomer, which elutes after the desired product. (R)-BPB-Ni(II)-2-methyldec-9-enoate was obtained in 77% yield (8.7 g, 14.0 mmol).
6. Decomposition to yield unprotected (R)-2-amino-2-methyldec-9-enoic acid
Dissolve (R)-BPB-Ni(II)-2-methyldec-9-enoate (3.5 g, 1 equiv) in 23 mL of MeOH and then add the solution to a 1:1 mixture of 3M HCl/MeOH (38 mL, 10 equiv HCl) in a 250 mL two-neck round bottom flask equipped with a condenser and rubber septum. Reflux the reaction for 30 min and then cool to RT. Quench the sea green-colored reaction by concentrating under high vacuum. Precipitate BPB as a white solid by adding approximately 20 mL H2O. Filter and wash the solid with water to yield 2.15 g BPB (5.61 mmol, 100%), which can be stored for reuse. Transfer the remaining aqueous filtrate to a 50 mL separatory funnel and wash with five 10 mL portions of DCM to remove any residual BPB. Concentrate the aqueous layer in vacuo to yield crude (R)-2-amino-2-methyldec-9-enoic acid, which is dried further by lyophilization. To purify the amino acid, dissolve the crude material in a minimum amount of 50:50 H2O/ACN and load onto a 43 g reverse phase (C18) MPLC column. Elute with a 5% to 95% ACN/H2O (+0.1 % TFA) gradient to yield 1.11 g of pure (R)-2-amino-2-methyldec-9-enoic acid (5.57 mmol, 99% yield). Of note, the blue-green nickel complex will elute first in approximately 4 × 25 mL fractions, followed by the desired product.
7. Fmoc protection to form (R)-2-(((9H–fluoren-9-yl)methoxy)carbonylamino)-2-methyl-dec-9-enoic acid
Dissolve (R)-2-amino-2-methyldec-9-enoic acid (2.56 g, 1 equiv) in a 1:1 H2O/acetone solution (150 mL) in a 250 mL round bottom flask equipped with a septum and nitrogen line. Add Fmoc-OSu (4.52 g, 1.10 equiv), followed by Na2CO3 (5.43 g, 4 equiv). After stirring overnight at RT, add 150 mL of 2:1 H2O/hexanes, transfer the mixture to a 500 mL separatory funnel, and pour off the organic layer. Wash the aqueous layer with an additional 50 mL hexanes and then back extract twice with 30 mL of 2:1 hexanes/EtOAc. Adjust the aqueous layer to pH 2–3 with 6 M HCl and extract the organic layer with 3 × 30 mL portions of DCM. Combine and dry the DCM layers over MgSO4, and then concentrate in vacuo to yield a pale yellow oil. For purification, load the crude material in a minimum amount of DCM onto a 43 g MPLC column, and elute with a 0% to 10% MeOH/CHCl3 gradient to isolate 3.06 g of (R)-2-(((9H–fluoren-9-yl)methoxy)carbonylamino)-2-methyl-dec-9-enoic acid (7.3 mmol, 57% yield).
BASIC PROTOCOL 2
SYNTHESIS AND DERIVATIZATION OF HYDROCARBON-STAPLED PEPTIDES
Automated solid phase peptide synthesis using Fmoc chemistry is an efficient and reliable method for generating hydrocarbon-stapled peptides. We employ a standard Fmoc protocol that is adjusted to lengthen and/or repeat deprotection and coupling reactions as needed to optimize incorporation of both the stapling amino acids and those natural residues that immediately follow a non-natural amino acid. With these minor modifications, as outlined below, the yields of singly- and doubly-stapled peptides can readily match those of the corresponding unmodified peptides. If the peptide has natural α-helical propensity, olefin metathesis proceeds smoothly at room temperature within 2 hours. Facile derivatization of peptides affords a variety of functionalized stapled peptides tailored for diverse experimental applications.
Materials
Automated solid phase peptide synthesizer
High performance liquid chromatography/mass spectrometer (LC/MS)
Fmoc-protected amino acids
N-Methylpyrrolidinone (NMP, Aldrich; Anhydrous, 99.5% for reagent solutions; ReagentPlus, 99%, for washing)
N,N-Dimethylformamide (DMF, Aldrich; HPLC grade for washing)
Methanol (MeOH, Reagent grade, 99%)
Diisopropylethylamine (DIEA)
2-(6-Chloro-1H–benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate (HCTU, Peptides International)
Piperidine
Rink Amide AM resin (200–400 mesh, EMD Biosciences)
Trifluoroacetic acid (TFA)
Triisopropylsilane (TIS)
Ethanedithiol
Grubbs Catalyst, 1st generation
Acetic anhydride (Ac2O)
d-Biotin
Fmoc-4-benzoyl-L-phenylalanine (Bpa, Advanced ChemTech)
Fluorescein isothiocyanate isomer I (FITC, Sigma, ≥90%)
Synthetic steps (Fig. 3)
Fig. 3.
Synthetic steps for the automated production of hydrocarbon-stapled peptides using Fmoc chemistry.
1. Design the stapled peptide(s)
When the structure of the helical binding interface is available, locate hydrocarbon staples on the non-interacting face of the helix to avoid disruption of target binding. To properly space the non-natural amino acids, place S5 residues at i and i+4 positions for a staple that spans one helical turn, and locate R8 at i and S5 at i+7 positions, respectively, for a staple that spans two helical turns.
2. Replace amino acids sparingly
One of the goals of peptide stapling is to maximally preserve the natural bioactive peptide sequence. However, we typically replace methionine, whose thioether can decrease the efficiency of ruthenium catalysis, with an isosteric amino acid such as norleucine to preserve chain length and aliphatic character. An important advantage of stapled peptides for protein interaction research is the ability to carefully vet specificity of action through mutagenesis that intentionally disrupts bioactivity. Whether by alanine scanning to sequentially substitute each natural residue with ala or by staple scanning to iteratively sample all staple positions along the helical surfaces, the development of negative control mutants is a valuable feature of employing stapled peptides for biological and therapeutic targeting studies. In addition, we have recently employed Fmoc-Bpa to install a photoreactive benzophenone moiety at a sampling of locations throughout the peptide (including, in particular, at the binding interface and its perimeter), in order to crosslink stapled peptide helices to their protein interactors for target discovery and binding site identification using proteomic methods(Braun et al., 2010).
3. Create the peptide synthesis methods file
Modify the synthesizer’s standard Fmoc settings to define all non-natural amino acids using the available letters B, J, O, U, X, and Z. For example, we choose B for norleucine, U for Bpa, X for S5, and Z for R8. Adjust the method file for incorporation of the stapling non-natural amino acids. In α,α-disubstituted amino acids, the amine is linked to a quaternary carbon center, and therefore, to achieve optimal reaction yields, the Fmoc removal and acylation steps of the standard Fmoc protocol require modification. For example, whereas 2 × 10 min Fmoc deprotections are standard for natural Fmoc amino acids, we use 4 × 10 min deprotections for the stapling amino acids. For acylation, coupling frequency and incubation times are typically 2 × 30 min for standard residues, 2 × 45 min for the stapling amino acids, and 3 × 45 min for the residue following a stapling amino acid.
4. Prepare the peptide synthesis reagents and solutions
For a 50 µmol scale peptide synthesis, weigh out ∼70 mg of Rink amide AM resin (loading levels of 0.4–0.6 mmol/g resin) per reaction chamber and dissolve the calculated amount of each amino acid in anhydrous NMP. Although more expensive than DMF, we employ NMP because of its greater stability at room temperature in the context of synthetic runs that can last approximately one week for a typical 24-member library of ∼30 residues per peptide. Prepare the deprotection solution, which is comprised of 20% (v/v) piperidine in NMP, and stock sufficient NMP for the repeated washes. Deprotection and wash solutions can alternatively employ DMF to mitigate costs. Prepare a 0.5 M solution of the coupling reagent 2-(6-chloro-1H–benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate (HCTU). We choose HCTU over other aminium-based coupling reagents, such as HBTU or HATU, because HCTU is more active than HBTU and more stable than HATU over a 1 week period. Typically, 4 equivalents of Fmoc-amino acid, 3.9 equivalents of coupling reagent, and 8 equivalents of N,N-diisopropylethylamine (DIEA) are used, and double coupling is performed. It is essential to monitor the consumption of reagents and solutions throughout the synthesis to avoid depletion of ingredients during the run.
Execute the peptide synthesis
A summary of the reaction steps for Fmoc-based, solid phase, stapled peptide synthesis is presented in Fig. 3.
Gauge the success of peptide synthesis
Once the synthesis is complete, perform a test cleave to determine if full-length peptide was generated as the predominant reaction product. Touch a glass pipette to the resin and transfer approximately 1/8 inch-worth of sample to a 0.5 mL solution of TFA:TIS:water (95:2.5:2.5) and stir for 1.5 hours. After removing the resin by filtration, precipitate the peptide by adding the solution dropwise to 1.5 mL of ether:hexanes (1:1). Incubate on ice for 10 min and then tabletop centrifuge at maximum speed for 2 min to pellet the precipitated peptide. Dissolve the sample in 150 µL of water:acetonitrile (1:1) and inject 30 µL for LC/MS analysis. Monitor for the desired mass and analyze truncated products for missing residues, which could reflect difficult couplings (e.g. residues following the stapling amino acid). A common M+44 impurity derives from incomplete hydrolysis of the tryptophan carbamic acid, which ultimately hydrolyzes to Trp upon exposure to aqueous solution for >3 hours in the autosampler vial.
Apportion resin for the desired functionalizations
The steps taken to finalize stapled peptide synthesis depend on the selected N-terminal or other functionalization. If no derivatization is desired, the N-terminal Fmoc is removed and the peptide is capped with an acetyl group prior to metathesis. Having a free N-terminus will otherwise result in a sluggish metathesis reaction. For functionalizations that contain sulfur, such as FITC or biotin, the N-terminal Fmoc of an appended βAla is retained and metathesis performed prior to N-terminal capping to avoid interference with ruthenium catalysis.
Perform olefin metathesis to generate the staple(s)
Staple the olefin-containing peptide using Grubbs generation 1 catalyst (Benzylidene-bis(tricyclohexylphosphine) dichlororuthenium, bis(tricyclohexylphosphine) benzylidine ruthenium(IV) dichloride). For a 50 µmol scale reaction, dissolve 8 mg catalyst in 2 mL 1,2-dichloroethane (DCE) and stir for 1.5–3 hours. Then, remove the reaction solution, wash the resin with DCE, and repeat the reaction procedure once or twice more using freshly prepared catalyst. A freshly prepared solution of catalyst should be purple and the color will gradually turn brown during metathesis. Of note, we have observed that the reaction does not strictly require an inert atmosphere of nitrogen or argon. More importantly, because olefin metathesis is an equilibrium reaction, allowing for the gaseous ethylene byproduct to escape will drive the reaction to completion. It is desirable to use DCE because of its higher vapor pressure compared to dichloromethane (DCM), though DCM can be substituted (and replenished during the reaction) if DCE is not available. Because separating the stapled from unstapled peptide by HPLC can be challenging, it is important to confirm by LC/MS that metathesis has gone to completion. If necessary, repeat the stapling reaction for longer intervals (even overnight) and/or with a higher reaction temperature. In general, peptides with a sequence-based predisposition to assume an α-helical configuration undergo facile metathesis; therefore, if the structure of the peptide is unknown and the metathesis reaction is unsuccessful or incomplete, this may be an indication that the peptide is not naturally shaped as an α-helix.
9. Acylate with sulfur-containing moieties post-metathesis
Remove the Fmoc group by shaking in 2 mL 20% piperidine/DMF for 30 min. For FITC derivatization, a preceding non-α-amino acid such as β-alanine or 6-aminohexanoic acid is required to avoid an Edman-type elimination reaction. Then, capping with FITC is accomplished by stirring the resin overnight in a 2 mL solution of 0.1 M fluorescein isothiocyanate (isomer I)/0.2 M DIEA. For biotinylation, a spacer may also be desired and we typically use β-alanine or a short PEG linker (e.g. Fmoc-NH-(PEG)n-COOH; n = 1–5). The biotinylation reaction employs a 2 mL solution of 0.1 M biotin-OSu/0.4 M DIEA, stirring overnight. At this stage, a test cleave is again performed to ensure that the desired reaction has gone to completion.
10. Perform a full-scale cleavage reaction to liberate the unprotected peptide
Vigorously stir the resin in 2 mL of 95:2.5:2.5 TFA:TIS:water for 2 h. If the peptide sequence contains cysteine, include ethanedithiol (2%) in the cleavage mixture to prevent oxidation. Filter off the resin and apply the cleavage solution dropwise into 35 mL of ether:hexanes, mix by inversion, release the pressure, and allow to incubate on dry ice for 20 min. Isolate the precipitate by centrifugation at 1500 x g for 20 min at 4°C, remove the reaction solution, and dry the peptide pellet at RT overnight.
11. Purify the desired stapled peptide product by LC/MS
Dissolve the peptide precipitate in 1.5 mL of water:acenonitrile, 3% formic acid and centrifuge to remove insoluble material. Purify the desired product by HPLC (typically using a C18 column) or, if available, by LC/MS with mass-triggered fraction collection. Reinject the fractions containing the desired full-length peptide, pool all of the pure fractions, and lyophilize, making sure that the acetonitrile concentration is <40% so that the solution remains frozen. Resuspend the pure peptide powder in a known volume of 30% acetonitrile:water, take 1:10 and 1:20 dilutions for amino acid analysis (AAA), and then aliquot and lyophilize for storage of the dried powder at −20°C. Apply the AAA results to calculate the amount of peptide in each aliquot.
12. Perform off-resin derivatizations
For certain applications, off-resin derivatizations of the stapled peptide may be desirable. For example, we have generated paramagnetically labeled stapled peptides for paramagnetic relaxation enhancement (PRE) NMR by derivatization with MTSL (S-(2,2,5,5-tetramethyl-2,5-dihydro-1H–pyrrol-3-yl)methyl methanesulfonothioate). Dissolve MTSL in 10 mM DMSO and the purified stapled peptide (containing an installed cysteine) in 1 mM anhydrous DMF. Mix the two solutions together (1:1), monitor the reaction for completion by LC/MS, and purify the desired product as described in step 9.
COMMENTARY
Background Information
Current drug therapy is based on two major classes of pharmaceuticals that recognize protein targets in and on the cell: small molecules penetrate intact cells to bind focal, greasy cavities within target proteins/enzymes, and antibodies specialize in binding to large and complex cell surface protein topographies. Along with these major strengths, each class has its accompanying weakness: (1) molecules are often too small to blanket the large, flat, and complex intracellular protein interaction landscape required for disrupting pathologic protein engagement and (2) antibodies, ideally suited for binding to complex and extended protein surfaces, cannot access intracellular targets. To fill the drug gap, a class of “compromise” compounds are envisioned that are sufficiently small to maintain cell penetrance but large enough to achieve a greater breadth of binding terrain to modulate intracellular protein interactions for therapeutic benefit. In the last decade, there has been an explosion of renewed interest in peptides, among other natural motifs (such as RNA), to populate this middle ground of drug development. Because protein interactions are typically mediated by protein subdomains with defined structure, chemists, chemical biologists, and drug developers have explored a battery of new approaches to emulate Nature’s solution to protein targeting and modulation. Important goals of these efforts include recapitulating and stabilizing native bioactive structure, overcoming vulnerability to proteolysis, and achieving efficient cellular uptake.
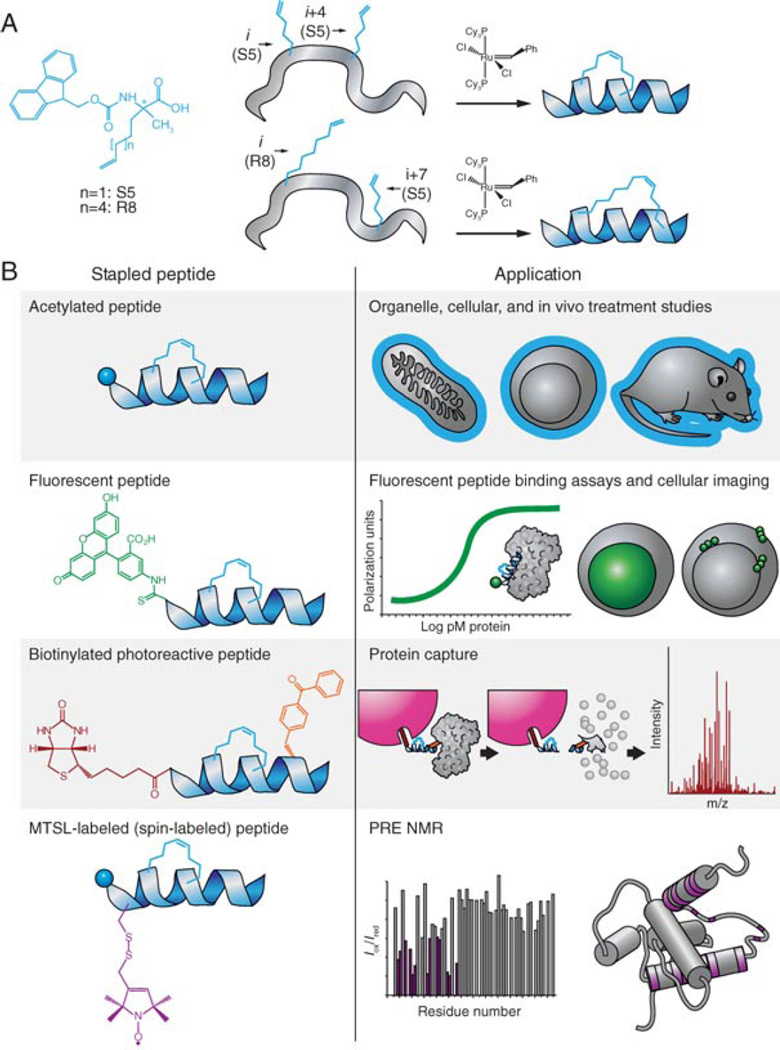
Within the realm of α-helical peptide mimicry, a variety of exciting new strategies have emerged, including peptide stabilization chemistries and alternative non-natural scaffolds for presenting key amino acid moieties(Henchey et al., 2008). An important byproduct of these approaches is a wealth of new reagents for fundamental biological investigation, which in turn can lead to the discovery of novel targets and protein interactions that can serve as the substrates for next-generation therapeutics. Here, we describe the production of hydrocarbon-stapled peptides and their derivatives, based on the incorporation of olefinic non-natural amino acids that when crosslinked by ruthenium-catalyzed olefin metathesis, yield sturdy, protease-resistant, and cell-permeable α-helices for protein interaction research and therapeutic targeting (Fig. 4). By deploying our stapled peptide approach, we have developed a new therapeutic strategy for targeting intracellular protein interactions(Walensky et al., 2004), uncovered an unanticipated function for a death protein in metabolism(Danial et al., 2008), structurally defined the elusive activation site on an essential executioner protein of the cell death pathway(Gavathiotis et al., 2008), identified a natural alpha-helical peptide that can function as an exclusive inhibitor of a formidable anti-apoptotic protein linked to cancer(Stewart et al., 2010), remedied the proteolytic instability of a lengthy peptide therapeutic(Bird et al., 2010), defined the key conformational changes that transform an inactive death protein into a toxic mitochondrial oligomer(Gavathiotis et al., 2010), and recapitulated the essential features of a key transcription factor motif to restore the tumor suppressor pathway in cancer cells(Bernal et al., 2010). In describing our latest synthetic approaches, we hope to further expand accessibility to stapled peptides and broaden their utility in advancing protein interaction research and novel therapeutic strategies.
Fig. 4.
Production and derivatization of hydrocarbon-stapled peptide α-helices for a diversity of experimental and therapeutic applications.
Critical Parameters
Chemical synthesis of the stapling amino acids
The most challenging synthetic steps include isolating (1) the correct diastereomer in step 5 and (2) the free amino acid following cleavage from the chiral auxiliary in step 6. To purify the correct diastereomer, be certain to first resolve the product by TLC, co-spotting against BPB-Ni(II)-Ala using a 5% MeOH/DCM solvent system. For example, the desired R8 product (Rf=0.41) travels just ahead of the starting material, whereas the undesired diastereomer migrates just below the starting material. Determining the TLC migration profile of the desired product will ensure pooling of the correct, clean fractions following silica purification.
Following acid-catalyzed cleavage of (R)-BPB-Ni(II)-2-methyldec-9-enoate, the free amino acid must be isolated from the aqueous solvent by vigorously drying the material using strong vacuum techniques, including lyophilization. Once dry, the amino acid can be purified using a C18 reverse phase silica column. Because the product has no UV activity, analyzing the fractions by LC/MS is recommended to delineate the pure amino acid-containing fractions. Typically, we find that the sea-green fractions containing free nickel elute quickly, with (R)-2-amino-2-methyldec-9-enoic acid eluting in the subsequent four to six 25 mL fractions. Once the fractions containing pure amino acid are pooled, the material is again concentrated in vacuo and thoroughly dried by lyophilization.
Stapled peptide synthesis
A variety of automated peptide synthesizers and Fmoc protocols have been applied to successfully generate stapled peptides. If you are performing peptide synthesis for the first time, optimize the equipment and method using the unmodified template peptide first to ensure that the standard protocol is generating the desired product in high yield and purity before advancing to stapled peptide synthesis. If an automated synthesizer is not available, the entire synthesis can be performed manually. Although laborious, we routinely made stapled peptides in this fashion before adapting the procedure for automation.
Once your first panel of stapled peptides is designed and synthesized, a series of critical characterization studies must be performed prior to application of the reagents to biological investigation:
-
(1)
What is the solubility profile of the stapled peptide? It is essential to determine the optimal approach to solubilizing your material. Some stapled peptides are highly soluble in water alone and others need to be dissolved in 100% DMSO prior to dilution into aqueous buffers. The HPLC elution profile is an excellent guide, since late-eluting peptides are more hydrophobic and potentially more challenging to solubilize. We suggest that you experiment with dissolving your peptide in a variety of aqueous buffers, varying the pH and salt concentration. Whether you are dissolving your peptide powder in 100% aqueous or diluting it from a DMSO stock into aqueous buffers, always be sure to verify that the peptide is actually in solution by performing a tabletop spin at maximum speed and then checking for the presence of a pellet, which indicates incomplete solubility. If inadequately dissolved, rigorous evaluation of stapled peptide activity will be compromised regardless of the assay employed. Iterative dilution of the DMSO stock into increasingly larger volumes of aqueous buffer until the goal concentration is reached can be an effective strategy for achieving solubilization. In the extreme circumstance that you are unable to identify conditions to solubilize your construct, redesign your peptide to incorporate hydrophilic or charged residues to decrease overall hydrophobicity.
-
(2)
What is the structure of the stapled peptide in solution? To perform a rapid assessment of α-helical structural stabilization, perform circular dichroism spectroscopy (Bird et al., 2008) to compare the unmodified peptide to your stapled construct, ensuring that both peptides are completely solubilized. By varying staple location, you may identify particular constructs with optimal α-helicity.
-
(3)
What is the behavior of the stapled peptide in solution? Like many chemical compounds, peptides can aggregate depending upon the composition and concentration. For most applications, stapled peptides are employed in the nanomolar and low micromolar range, concentrations at which self-association is rarely observed. Nevertheless, the self-association propensity of the peptide can be rapidly assessed by evaluating samples at various concentrations using native gel electrophoresis and/or gel filtration chromatography. If aggregation is observed at a particular concentration, employ the stapled peptide below this concentration in biological studies, experiment with alternate solubilization buffers, or redesign your peptide to remedy its propensity to self-associate.
-
(4)
Is the stapled peptide cell permeable? We have observed cellular uptake of stapled peptides in a time-, temperature-, and ATP-dependent manner, consistent with a pinocytotic mechanism(Walensky et al., 2004). In the event that your stapled peptide binds to serum proteins such as albumin, initiate your analysis of cellular uptake using a serum-free medium (e.g. Opti-MEM) or by treating in the absence of serum for a 1–4 hour period followed by serum replacement. We evaluate cellular uptake of FITC-stapled peptides by live confocal microscopy, FACS analysis of treated cells, and fluorescence scan of electrophoresed lysates from treated cells. Prior to analysis, cells are washed to remove stapled peptide-containing media, and for the FACS and cell lysate evaluation, the cells are further treated with trypsin to digest surface protein and eliminate any non-specifically bound peptide. Based on our evaluation of many series of stapled peptides, we have observed that their propensity to be taken up by cells derives from a combination of factors, including charge, hydrophobicity, and α-helical structure, with negatively charged and less structured constructs typically requiring modification to achieve cell penetrance. Successful interventions include (1) substituting Gln for Glu and/or Asn for Asp, or appending native or non-native charged residues at the N- or C-termini to adjust the overall charge to 0 to +2, and (2) producing constructs with greater α-helical content through differential staple placement.
-
(5)
Does the stapled peptide exhibit on-target activity? A key benefit of working with peptides in biological systems is the ability to track binding activity and selectivity by use of negative control point mutants or scrambled peptide constructs. In addition, protein targeting in cells or cellular lysates can be carefully examined with stapled peptide pull-down assays that employ FITC-tagged, biotinylated, and/or photoreactive constructs, followed by protein detection by western blotting and/or proteomic analyses(Bernal et al., 2010; Braun et al., 2010; Walensky et al., 2006). It is important to be aware that certain peptides can disrupt membranes as a result of their amino acid composition (e.g. cationic antimicrobial peptides)(Bechinger, 1997). Therefore, if you are planning to treat cells or purified organelles with your stapled peptide, be sure to perform a maximally tolerated dose titration to screen for constructs that perturb membranes based on composition or dose range. Simple studies such as monitoring cells by light microscopy (e.g. trypan blue exclusion) immediately after treatment can flag disruptive peptides. Stapled peptides should be used at tolerated doses only, and if necessary, redesigned to eliminated unwanted biophysical properties so that on-target, sequence-dependent biological activity is achieved.
Anticipate that generating the optimal stapled peptide for your scientific application, whether for in vitro binding studies, cellular localization analyses, structure determination, cellular signal transduction studies, or in vivo activity analyses, is likely to require several rounds of synthetic iteration to achieve the desired solubility, structural stability, cell permeability, and biological activity. Based on our own work and numerous collaborative experiences, we have found that a commitment to taking a rigorous, step-wise, and iterative approach to stapled peptide production, optimization, and application is the best formula for successfully developing stapled peptides to advance your protein interaction and therapeutic targeting research.
Troubleshooting
Chemical synthesis of the stapling amino acids
The most frequent problems associated with the non-natural amino acid synthesis detailed above, and our suggested solutions, are outlined below:
Step 1: If you cannot isolate (R)-N-benzylproline from the reaction mixture, be sure that when quenching the reaction, you did not acidify below pH 5.
Step 3: If BPB-Ni(II)-Ala does not crystallize, scour the Erlenmeyer flask with a Pasteur pipette.
Step 4: If you do not obtain a clear liquid product from the Finkelstein reaction, you likely evaporated the alkenyl iodide due to its low vapor pressure. Use gentle vacuum to evaporate the organic solvent only.
Step 5: If you cannot resolve the reaction products, use a larger silica column and broader EtOAc gradient.
Step 6: If you cannot adequately dry the (R)-2-amino-2-methyldec-9-enoic acid reaction product by vacuum pump, flash freeze the product in liquid nitrogen and lyophilize.
Step 7: If Fmoc protection fails, make certain that the starting material, (R)-2-amino-2-methyldec-9-enoic acid, is free of nickel and other impurities.
Step 8: If you do not recover the reaction product, (R)-2-(((9H–fluoren-9-yl)methoxy)carbonylamino)-2-methyl-dec-9-enoic acid, from the DCM layer, TLC each wash and extraction, as the Fmoc-protected amino acid can enter the aqueous layer if the correct pH (2–3) is not achieved or maintained.
Stapled peptide synthesis
The most frequent complication of peptide synthesis is failure to generate the full-length construct due to difficult amino acid couplings. Common examples include coupling (1) β-branched amino acids (e.g. Thr, Ile, and Val), (2) Arg residues, (3) the non-natural amino acids, and (4) residues following the non-natural amino acids. To overcome difficult couplings, extend the deprotection and coupling times and perform multiple rounds of coupling using fresh reagent, as described above. Anticipating difficult sequences at the outset and adjusting the method accordingly will improve your success rate and yield.
Additional complications can include cross-reactions and progressive inaccessibility of the N-terminus due to on-resin aggregation. The dipeptide motif Asp-Gly is the most likely amino acid pair to undergo a reaction known as aspartimide formation, which occurs when the -NH- of Asp, upon repeated exposure to piperidine, attacks its ester-protected side chain, displacing t-BuOH to form a 5-membered ring. Subsequent attack and ring opening by water or piperidine, results in the production of a peptide containing racemized Asp or a piperamide, respectively. This side reaction can be completely suppressed by employing the commerically available side-chain protected dipeptide pair, Fmoc-Asp(OtBu)-(Dmb)Gly-OH (EMD Biosciences). Exposure of the reactive N-terminus can be hindered by aggregation of the growing peptide chains as β-sheets on the polymeric bead. Interventions that prevent aggregation include incorporation of the stapling amino acids themselves, which promote α-helical conformation, and the use of pseudoproline Ser and Thr dipeptides (EMD Biosciences), which can be substituted at X-Ser and X-Thr positions, producing a kink that disrupts β-sheet formation.
Anticipated Results
The overall synthetic yield for generating the non-natural amino acids using the above method is ∼20%. For optimized stapled peptide synthesis, one can expect to achieve the purity and yield of the corresponding unmodified peptide. A purity of 90% for the post-cleavage crude material is common and can be improved to >95% after HPLC, with overall yields of 30% routinely obtained. Absent any unanticipated coupling challenges, side reactions, or on-bead aggregation, the majority of stapled peptides can be generated successfully on the first attempt.
Time Considerations
An experienced chemist can anticipate completing the synthesis of the non-natural amino acid(s) used for all-hydrocarbon peptide stapling in 3–4 weeks.
For stapled peptide synthesis, a 2–3 week time frame is standard, but will depend on the type of synthesizer employed and variables such as the length and complexity of the peptide (i.e. need for multiple coupling reactions per residue) and the planned derivatizations. An automated XYZ probe-type synthesizer (e.g. APEX396 [AAPPTec]) excels at generating many peptides at once, with only marginal increases in time for expanding the size of the library. The duration of a synthetic run will vary depending on whether you choose to double couple with fewer equivalents of amino acid or single couple with larger amino acid quantities. We typically double couple at 4:1 equivalents of amino acid to resin for 40 min each, followed by 2 NMP washes, a 2–15 min 20% piperidine/NMP Fmoc-deprotection, and repeat methanol and NMP washes. Accounting for all robotic arm movements, reagent delivery, and probe washings, one complete cycle of amino acid incorporation for 24 peptides takes ∼3–5 h, depending on the variety of amino acids at each position. For 24 peptides of 22–30 residues in length, an automated synthesis run is completed in 1 week’s time, which nicely parallels the window of stability for key reagents, such as HCTU. By comparison, a microwave-based peptide synthesizer (Liberty, CEM) that generates one peptide at a time requires only ∼12 h to complete the synthesis of a 22-mer peptide.
N-terminal derivatization and olefin metathesis can take 1–3 days to complete. N-terminal acetylation with 5 mL of 4:1:0.1 (NMP:Ac2O:DIEA) is accomplished in ∼30 min, whereas Fmoc-β-Ala or -PEG can be coupled using HCTU:DIEA in ∼1 h. Olefin metathesis using Grubbs I catalyst is performed in 3 × 2 h, after which a small scale cleave is recommended to confirm that the reaction has gone to completion. Depending on the number of peptides being processed, small scale test cleavage and LC/MS analysis requires 3–15 h. With completion of metathesis confirmed, removal of the N-terminal Fmoc from β-Ala or PEG takes 30 min, followed by coupling of FITC (14 h) or biotin (4 h). A test cleave and LC/MS analysis is also recommended at this stage to confirm successful and complete derivatization.
To finish the production process, large scale cleavage takes 2 h, followed by drying the precipitated peptide in a hood overnight. HPLC or LC/MS purification can require 2 h per peptide and analysis of the fractions for the desired product can take hours to days depending on the number of peptides being generated and the number of fractions per peptide. Lyophilization of the purified and pooled peptide fractions can take 1–3 days, depending on the number and volume of the fractions. Finally, amino acid analysis of the peptide will take at least 2 days in order to conduct the acid hydrolysis, derivatization, and analysis steps.
Acknowledgments
We thank E. Smith for figure design and editorial assistance, and Walensky laboratory members past and present, including F. Bernal, C. Braun, E. Gavathiotis, M. Stewart, S. Katz, and J. LaBelle, for their scientific contributions to the advancement of stapled peptide research. We are tremendously grateful for the federal, foundation, and professional society support of our protein interaction and therapeutic targeting research using stapled peptides, including NIH grants 5R01CA50239, 1R01OD005851, 1R01AI084102, 5P01CA92625, a Leukemia and Lymphoma Society Specialized Center of Research Award, a Stand Up To Cancer Innovative Research Award, and a grant from the Wolpoff Family Foundation.
Literature Cited
- Bechinger B. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J Membr Biol. 1997;156:197–211. doi: 10.1007/s002329900201. [DOI] [PubMed] [Google Scholar]
- Belokon Y, Tararov V, Maleev V, Savel’eva T, Ryzhov M. Improved procedures for the synthesis of (S)-2-[N-(N′-benzylprolyl)amino]benzophenone (BPB) and Ni(II) complexes of Schiff's bases derived from BPB and amino acids. Tetrahedron: Asymmetry. 1998;9:4249–4252. [Google Scholar]
- Bernal F, Wade M, Godes M, Davis TN, Whitehead DG, Kung AL, Wahl GM, Walensky LD. A stapled p53 helix overcomes HDMX-mediated suppression of p53. Cancer Cell. 2010;18:411–422. doi: 10.1016/j.ccr.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird GH, Bernal F, Pitter K, Walensky LD. Synthesis and biophysical characterization of stabilized alpha-helices of BCL-2 domains. Methods Enzymol. 2008;446:369–386. doi: 10.1016/S0076-6879(08)01622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird GH, Madani N, Perry AF, Princiotto AM, Supko JG, He X, Gavathiotis E, Sodroski JG, Walensky LD. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proc Natl Acad Sci U S A. 2010;107:14093–14098. doi: 10.1073/pnas.1002713107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun CR, Mintseris J, Gavathiotis E, Bird GH, Gygi SP, Walensky LD. Photoreactive stapled BH3 peptides to dissect the BCL-2 family interactome. Chem Biol. 2010;17:1325–1333. doi: 10.1016/j.chembiol.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, Datta SR, Pitter KL, Bird GH, Wikstrom JD, Deeney JT, Robertson K, Morash J, Kulkarni A, Neschen S, Kim S, Greenberg ME, Corkey BE, Shirihai OS, Shulman GI, Lowell BB, Korsmeyer SJ. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–492. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchey LK, Jochim AL, Arora PS. Contemporary strategies for the stabilization of peptides in the alpha-helical conformation. Curr Opin Chem Biol. 2008;12:692–697. doi: 10.1016/j.cbpa.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Soloshonok V, Cai C, Tang X, Hruby V. Convenient, Large-Scale Asymmetric Synthesis of Enantiomerically Pure trans-Cinnamylglycine and -α-Alanine. Tetrahedron. 2000;56:2577–2582. [Google Scholar]
- Schafmeister C, Po J, Verdine G. An all-hydrocarbon cross-linking system for enhancing the helicity and metabolic stability of peptides. J Am Chem Soc. 2000;122:5891–5892. [Google Scholar]
- Stewart ML, Fire E, Keating AE, Walensky LD. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Biol. 2010;6:595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, Smith E, Verdine GL, Korsmeyer SJ. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Williams RM, Sinclair PJ, Zhai D, Chen D. Practical asymmetric syntheses of .alpha.-amino acids through carbon-carbon bond constructions on electrophilic glycine templates. J Am Chem Soc. 1988;110:1547–1557. [Google Scholar]
- Williams RM, Im MN. Asymmetric synthesis of monosubstituted and alpha, alpha-disubstituted amino acids via diastereoselective glycine enolate alkylations. J Am Chem Soc. 1991;113:9276–9286. [Google Scholar]
- Williams RM, Sinclair PJ, DeMong DE, Chen D, Zhai D. Asymmetric Synthesis of N-tert-butoxycarbonyl Alpha-Amino Acids: Synthesis of (5S, 6R)-4-tert-butoxycarbonyl-5,6-diphenylmorpholin-2-one. Organic Syntheses. 2003;80:18–30. [Google Scholar]