Abstract
To study how the expression of the D1A dopamine receptor gene is regulated, a human genomic clone was isolated by using a rat cDNA as probe. A 2.3-kilobase genomic fragment spanning -2571 through -236 relative to the adenosine of the first methionine codon was sequenced. The gene has an intron of 116 base pairs in the 5' noncoding region, nucleotides -599 through -484 as determined by S1 mapping and reverse transcription-PCR. It has multiple transcription initiation sites located between -1061 and -1040. The promoter region lacks a TATA box and a CAAT box, is rich in G+C content, and has multiple putative binding sites for transcription factor Sp1. Thus, the promoter region of the human D1A gene has features of "housekeeping" genes. However, it also has consensus sequences for AP1 and AP2 binding sites and a putative cAMP response element. The ability of four deletion mutants of the 2.3-kilobase fragment to modulate transcription of the heterologous chloramphenicol acetyltransferase gene in the promoterless plasmid pCAT-Basic was determined. All mutants demonstrated substantial transcriptional activity in the murine neuroblastoma cell line NS20Y, which expresses the D1A gene endogenously. Transient expression assays suggested the presence of a positive modulator between nucleotides -1340 and -1102, and a negative modulator between -1730 and -1341. The four genomic fragments had no or very low transcriptional activity in NB41A3, C6, and Hep G2 cells, which are not known to express this gene. Thus, the human D1A gene belongs to the category of tissue-specific, regulated genes that have housekeeping-type promoters.
Full text
PDF

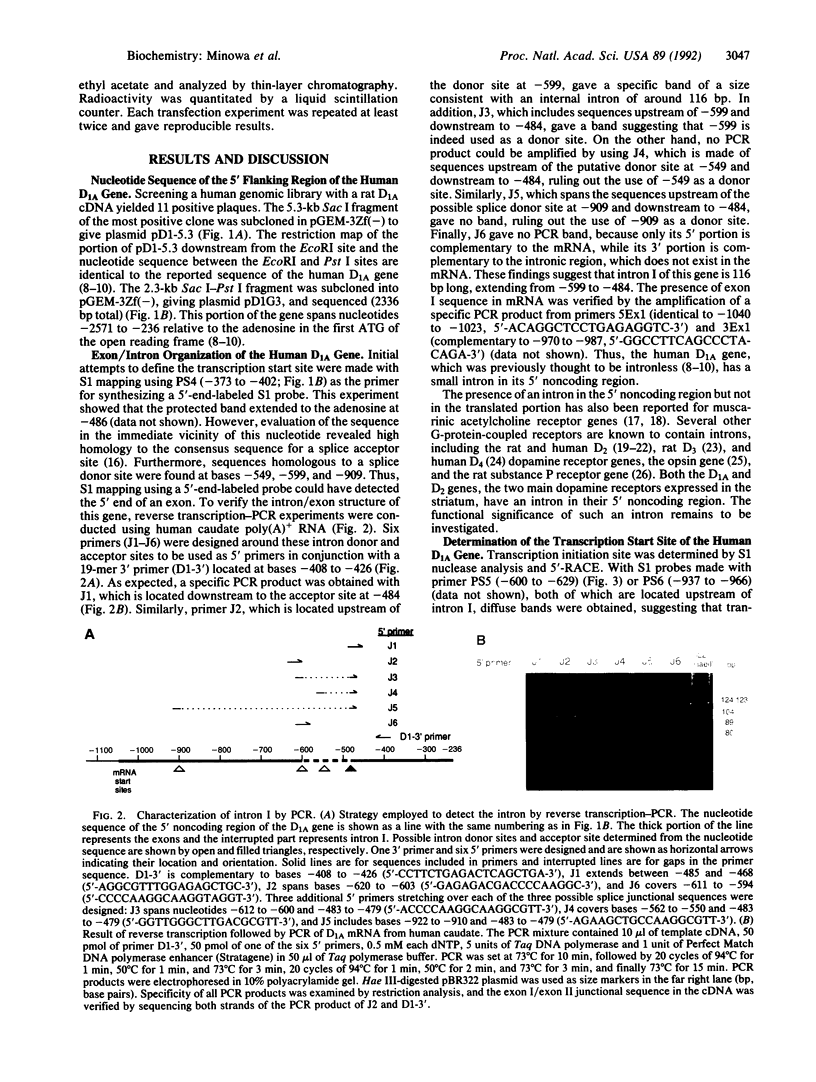


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Dal Toso R., Sommer B., Ewert M., Herb A., Pritchett D. B., Bach A., Shivers B. D., Seeburg P. H. The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J. 1989 Dec 20;8(13):4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearry A., Gingrich J. A., Falardeau P., Fremeau R. T., Jr, Bates M. D., Caron M. G. Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature. 1990 Sep 6;347(6288):72–76. doi: 10.1038/347072a0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Freissmuth M., Casey P. J., Gilman A. G. G proteins control diverse pathways of transmembrane signaling. FASEB J. 1989 Aug;3(10):2125–2131. [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandelman K. Y., Harmon S., Todd R. D., O'Malley K. L. Analysis of the structure and expression of the human dopamine D2A receptor gene. J Neurochem. 1991 Mar;56(3):1024–1029. doi: 10.1111/j.1471-4159.1991.tb02024.x. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grandy D. K., Marchionni M. A., Makam H., Stofko R. E., Alfano M., Frothingham L., Fischer J. B., Burke-Howie K. J., Bunzow J. R., Server A. C. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9762–9766. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J. M., Larin Z., Taylor I. C., Prentice H., Gwinn K. A., Kingston R. E. Multiple basal elements of a human hsp70 promoter function differently in human and rodent cell lines. Mol Cell Biol. 1987 Oct;7(10):3646–3655. doi: 10.1128/mcb.7.10.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey A. D., Dykema P. E., Krause J. E. Organization, structure, and expression of the gene encoding the rat substance P receptor. J Biol Chem. 1991 Mar 5;266(7):4366–4374. [PubMed] [Google Scholar]
- Lee T., Seeman P., Rajput A., Farley I. J., Hornykiewicz O. Receptor basis for dopaminergic supersensitivity in Parkinson's disease. Nature. 1978 May 4;273(5657):59–61. doi: 10.1038/273059a0. [DOI] [PubMed] [Google Scholar]
- Lee T., Seeman P., Tourtellotte W. W., Farley I. J., Hornykeiwicz O. Binding of 3H-neuroleptics and 3H-apomorphine in schizophrenic brains. Nature. 1978 Aug 31;274(5674):897–900. doi: 10.1038/274897a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lovenberg T. W., Roth R. H., Nichols D. E., Mailman R. B. D1 dopamine receptors of NS20Y neuroblastoma cells are functionally similar to rat striatal D1 receptors. J Neurochem. 1991 Nov;57(5):1563–1569. doi: 10.1111/j.1471-4159.1991.tb06352.x. [DOI] [PubMed] [Google Scholar]
- Meeker T. C., Loeb J., Ayres M., Sellers W. The human Pim-1 gene is selectively transcribed in different hemato-lymphoid cell lines in spite of a G + C-rich housekeeping promoter. Mol Cell Biol. 1990 Apr;10(4):1680–1688. doi: 10.1128/mcb.10.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma F. J., Jr, Brassard D. L., Sibley D. R. Identification and characterization of D1 and D2 dopamine receptors in cultured neuroblastoma and retinoblastoma clonal cell lines. Brain Res. 1989 Jul 17;492(1-2):314–324. doi: 10.1016/0006-8993(89)90915-3. [DOI] [PubMed] [Google Scholar]
- Monsma F. J., Jr, Mahan L. C., McVittie L. D., Gerfen C. R., Sibley D. R. Molecular cloning and expression of a D1 dopamine receptor linked to adenylyl cyclase activation. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6723–6727. doi: 10.1073/pnas.87.17.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouradian M. M., Heuser I. J., Baronti F., Chase T. N. Modification of central dopaminergic mechanisms by continuous levodopa therapy for advanced Parkinson's disease. Ann Neurol. 1990 Jan;27(1):18–23. doi: 10.1002/ana.410270105. [DOI] [PubMed] [Google Scholar]
- Mouradian M. M., Juncos J. L., Fabbrini G., Schlegel J., Bartko J. J., Chase T. N. Motor fluctuations in Parkinson's disease: central pathophysiological mechanisms, Part II. Ann Neurol. 1988 Sep;24(3):372–378. doi: 10.1002/ana.410240304. [DOI] [PubMed] [Google Scholar]
- Muller P., Seeman P. Dopaminergic supersensitivity after neuroleptics: time-course and specificity. Psychopharmacology (Berl) 1978 Dec 15;60(1):1–11. doi: 10.1007/BF00429171. [DOI] [PubMed] [Google Scholar]
- Nathans J. Molecular biology of visual pigments. Annu Rev Neurosci. 1987;10:163–194. doi: 10.1146/annurev.ne.10.030187.001115. [DOI] [PubMed] [Google Scholar]
- O'Malley K. L., Mack K. J., Gandelman K. Y., Todd R. D. Organization and expression of the rat D2A receptor gene: identification of alternative transcripts and a variant donor splice site. Biochemistry. 1990 Feb 13;29(6):1367–1371. doi: 10.1021/bi00458a003. [DOI] [PubMed] [Google Scholar]
- Peralta E. G., Winslow J. W., Peterson G. L., Smith D. H., Ashkenazi A., Ramachandran J., Schimerlik M. I., Capon D. J. Primary structure and biochemical properties of an M2 muscarinic receptor. Science. 1987 May 1;236(4801):600–605. doi: 10.1126/science.3107123. [DOI] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerwald A., Hoesche C., Oschwald R., Kilimann M. W. The 5'-flanking region of the synapsin I gene. A G+C-rich, TATA- and CAAT-less, phylogenetically conserved sequence with cell type-specific promoter function. J Biol Chem. 1990 Sep 5;265(25):14932–14937. [PubMed] [Google Scholar]
- Sehgal A., Patil N., Chao M. A constitutive promoter directs expression of the nerve growth factor receptor gene. Mol Cell Biol. 1988 Aug;8(8):3160–3167. doi: 10.1128/mcb.8.8.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P., Giros B., Martres M. P., Bouthenet M. L., Schwartz J. C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990 Sep 13;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sunahara R. K., Niznik H. B., Weiner D. M., Stormann T. M., Brann M. R., Kennedy J. L., Gelernter J. E., Rozmahel R., Yang Y. L., Israel Y. Human dopamine D1 receptor encoded by an intronless gene on chromosome 5. Nature. 1990 Sep 6;347(6288):80–83. doi: 10.1038/347080a0. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T., Toyama R., Itoh H., Kozasa T., Matsuoka M., Kaziro Y. Structure of the human gene and two rat cDNAs encoding the alpha chain of GTP-binding regulatory protein Go: two different mRNAs are generated by alternative splicing. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2974–2978. doi: 10.1073/pnas.88.8.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Bunzow J. R., Guan H. C., Sunahara R. K., Seeman P., Niznik H. B., Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991 Apr 18;350(6319):610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Werner H., Stannard B., Bach M. A., LeRoith D., Roberts C. T., Jr Cloning and characterization of the proximal promoter region of the rat insulin-like growth factor I (IGF-I) receptor gene. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1021–1027. doi: 10.1016/0006-291x(90)91996-6. [DOI] [PubMed] [Google Scholar]
- Zhou Q. Y., Grandy D. K., Thambi L., Kushner J. A., Van Tol H. H., Cone R., Pribnow D., Salon J., Bunzow J. R., Civelli O. Cloning and expression of human and rat D1 dopamine receptors. Nature. 1990 Sep 6;347(6288):76–80. doi: 10.1038/347076a0. [DOI] [PubMed] [Google Scholar]