DEFINITION
The idea that the cerebral cortex is dynamically organized was proposed in 1912, when Brown and Sherrington stimulated the motor cortex of chimpanzees and found that “a point which began by yielding primary extension may come to yield primary flexion in the latter part of the stimulation series” (Brown and Sherrington, 1912). In many investigations since then these phenomena have been referred to as neural plasticity. Neural plasticity can be defined as the ability of the central nervous system (CNS) to adapt in response to changes in the environment or lesions. This property of the CNS may involve modifications in overall cognitive strategies to successfully cope with new challenges (i.e., attention, behavioral compensation) (Bury and Jones, 2002), recruitment of new/different neural networks (Johansen-Berg et al., 2002; Fridman et al., 2004; Lotze et al., 2006; Heuninckx et al., 2008), or changes in strength of such connections or specific brain areas in charge of carrying out a particular task (i.e., movement, language, vision, hearing) (Cohen et al., 1997; Grefkes et al., 2008). At the cellular level, changes in membrane excitability, synaptic plasticity, as well as structural changes in dendritic and axonal anatomy as measured in vivo and in vitro may be demonstrated in animals and humans (Clarkson et al., 2010; Li et al., 2010). The study of neuroplasticity engages scientists from many different disciplines because of the profound implications it has for understanding the functional underpinnings of action and cognition in the healthy and lesioned brain (Dimyan and Cohen, 2010). Mechanistic understanding of neuroplastic changes in the process of functional recovery following brain lesions, one of the focuses of this volume, is already starting to lead to the development of more rational strategies to facilitate neurorehabilitation (Taub et al., 2002; Cheeran et al., 2009).
At a cellular level, neuronal circuits consist of synaptic connections between axons and dendrites. As these circuits extend over the brain there is the potential for a large number of possible interactive combinations allowing for great flexibility. Modification of sensory input may induce rapid changes in cortical representations through various mechanisms including unmasking of connections that are silent in the native state (Calford and Tweedale, 1991a, b). For example, blocking inhibition pharmacologically within a small region of the primary motor cortex (M1) immediately unveils new representational patterns (Jacobs and Donoghue, 1991), through unmasking horizontal excitatory connections previously hidden by inhibitory neurons. The strength of these horizontal connections and the balance of excitation and inhibition appear to shape cortical representations. Corticofugal connections make extensive long-range (±1 mm) links with other pyramidal tract neurons, and with local inhibitory interneurons (Landry et al., 1990; McGuire et al., 1991). It is now known that long-term potentiation (LTP) can be induced in these horizontal connections of adult M1, contributing to long-lasting associations among neurons within a motor cortical area (Hess and Donoghue, 1994). Moreover, vertical synaptic pathways in M1 can experience short-term depression, short-term facilitation, long-term depression and, under conditions of disinhibition, also LTP (Castro-Alamancos et al., 1995). In addition, slower, progressive plastic changes can be driven by learning (Robertson and Irvine, 1989; Chino et al., 1997), competition with other inputs (Merzenich et al., 1983), and use (Nudo et al., 1996b).
Basic science investigations have substantially advanced our understanding of the mechanisms of plasticity and metaplasticity, important in multiple areas of human cognition such as learning and memory, and in functional recovery from lesions in the CNS, as in stroke (Buonomano and Merzenich, 1998; Floel and Cohen, 2006). The term “metaplasticity” is often, but incorrectly, used interchangeably with “homeostatic” plasticity (see below) (Abraham and Bear, 1996; Fischer et al., 1997; Gentner et al., 2008; Jung and Ziemann, 2009). In the past few years it has become evident that these findings have direct implications for the way in which human disease is treated, and new efforts have been invested in research that translates these advances in the basic science domain to the formulation of new, rational strategies for promoting recovery of function in humans. To accomplish this goal, it is important to demonstrate that similar principles to those described in animal models apply to the human cerebral cortex in relevant behavioral settings.
SITESOF PLASTICITY
In most cases, the cerebral cortex has been the target of studies of human plasticity (Wolpaw and Tennissen, 2001). However, reorganization requires fine-tuning of activity at cortical as well as subcortical sites. In the motor domain, for example, spinal processes play a role in modulating locomotor learning (Bizzi et al., 2000) and plasticity after amputations and nerve transections (Wu and Kaas, 1999). Plastic changes following deafferentation can be identified at cortical (Kaas et al., 1983) and subcortical (Devor and Wall, 1981) sites. The extent to which plastic changes detected at cortical levels reflect reorganization in subcortical structures is incompletely understood and still underinvestigated (Wu and Kaas, 2000). Therefore, it is important to keep in mind that the neural substrates of recovery of function are likely distributed over multiple sites at different levels of the neuroaxis and not restricted to one specific location. It still represents a challenge to understand how these different levels interact with one another to accomplish a particular behavioral goal.
WINDOWOFOPPORTUNITY
Neural plasticity occurs throughout the life span (Elias and Wagster, 2007). During normal human development the CNS must continue to optimize performance and learn and adapt in the presence of changes in anatomical constraints (such as, for example, changes in limb length or muscle mass or strength) and experience (Gaillard et al., 2000). Additionally, neuroplastic changes identified following CNS abnormalities during development have been particularly impressive given their ability to reestablish almost normal behavior (Chen et al., 2002). One such example is the substantial recovery of motor function or language in children posthemispherectomy, implemented to ameliorate intractable seizures (Vargha-Khadem et al., 1997). The potential of neuroplastic changes to influence behavior and recovery of function was first widely accepted in relation to the developing brain. Only more recently was it understood that neuroplastic changes of substantial clinical relevance could occur in the adult CNS and in the elderly (Merzenich et al., 1996). It has now been proposed, for example, that recruitment of wider brain networks in the elderly and after stroke may play a beneficial role in maintaining the ability of individuals to carry out specific tasks or even in facilitating relearning (Heuninckx et al., 2008; Hummel et al., 2010).
FUNCTIONAL RELEVANCE
Plasticity of cortical representations within and across different brain regions is thought to represent the neural basis underlying sensory substitution, for example in blind and deaf humans (Rauschecker, 1995), as well as in the recovery of motor function after cortical lesions like stroke (Nudo et al., 1996a). Although neuroplasticity, as defined above, is a ubiquitous phenomenon (our brain is constantly changing), it may have different impact on different behaviors. It may be beneficial (often referred to as adaptive plasticity, the most common forms of plasticity studied; Cohen et al., 1997; Lee, 2009), have no influence (representing only epiphenomena of the modified behavior), or even result in deleterious consequences (i.e., maladaptive; Flor et al., 2006) on performance of particular tasks or sensory experiences. This concept has been referred to as functional relevance of neuroplasticity. Conceptually, it would not be surprising that plastic changes in, for example neuronal networks, may have beneficial implications on a particular behavior but at a cost to other behavior (Chklovskii et al., 2004). This concept of cost of neuroplastic changes, which has been to some extent overlooked, is starting to receive attention. Understanding these changes and how they can be influenced is pivotal in developing better treatments and therapies for patients (Hodics et al., 2006; Hummel and Cohen, 2006; Cramer, 2008).
PLASTICITY, METAPLASTICITY, ANDHOMEOSTATIC PLASTICITY
Plasticity likely depends on multiple mechanisms evolving on different temporal scales – minutes to months, even years. Rapid onset-mechanisms, which may operate over a limited period of time, are believed to represent initial steps of more slowly evolving processes of reorganization through which functional gains (or losses) may be sustained (Classen et al., 1998; Kleim and Jones, 2008). At the level of neuronal synapses, multiple transformations may occur from relatively short-lasting LTP, which appears to be largely independent of protein synthesis, to long-lasting LTP, which may persist along the life span. These synaptic changes are complemented by changes in neuronal excitability and structural changes, with the latter ones being detectable using light microscopy, for example. From a behavioural perspective, consolidation refers to a process that results in enduring performance improvements (Cohen et al., 2005; Krakauer, 2009) that is underway during training or learning but typically occurs after. Through consolidation, newly acquired skills become more robust in the face of disruptive experiences or may even improve further, a process termed off-line learning. Through reconsolidation, stored memories may be purposefully modified in order to strengthen or weaken them (Censor et al., 2010).
Metaplasticity refers to the influence that baseline neural activity immediately preceding presentation of a plasticity-inducing protocol (for example in vitro theta burst stimulation) can substantially influence the ability of neuronal elements to exhibit plasticity. The functional significance of metaplasticity may include, but is not limited to, controlling homeostasis of neural network excitability, for example, by virtue of modifying synaptic efficacy in an operational range. Metaplasticity may, therefore, protect against potentially noxious excitability increases (Abraham and Bear, 1996). It should be kept in mind that the specific characteristics of the baseline activity preceding application of the same plasticity-inducing protocol can then result in opposite effects on synaptic efficacy (Seol et al., 2007). More attention is now paid to these phenomena in humans because it is thought that they can substantially impact information coding and cortical reorganization (Gentner et al., 2008; Jung and Ziemann, 2009).
An example in humans is that the magnitude of the response to noninvasive brain stimulation protocols applied to the primary motor cortex critically depends on the previous history of neural activity (Ziemann et al., 1998; Iyer et al., 2003; Gentner et al., 2008). As long as the modulation is confined to the magnitude, but not the sign, of responses (increases vs. decreases in excitability, for example), most of these findings may be interpreted within the framework of homeostatic plasticity as proposed in the Bienenstock–Cooper–Munro (BCM) theory of synaptic plasticity (Bienenstock et al., 1982; Abraham and Bear, 1996). Fundamental to the BCM theory is a time-variable induction threshold. For example, prolonged low levels of postsynaptic activity decrease the induction threshold, thereby increasing the probability for LTP. Alternatively, a history of enhanced postsynaptic activity would increase the threshold for LTP and therefore increase the likelihood for long-term depression induction (Ragert et al., 2009). Based on this combination of basic science and human neurophysiological evidence, it is attractive to speculate that the response to motor training protocols could depend on the history of activity at the time training is imparted. In other words, activities carried out in the period of time preceding the actual rehabilitative treatment (sleep, caffeine intake, reading, feeding, etc.) could have substantial influence, so far not well characterized, on outcomes and perhaps to some extent contribute to well-described interindividual variability in treatment response. Human studies have indeed provided experimental support for a homeostatic model of plasticity (Jung and Ziemann, 2009). On the other hand, experimental or therapeutic manipulations applied after the treatment may also provide an opportunity for modulating the ultimate behavioral response (Reis et al., 2009).
GENETICINFLUENCES
Genetic factors can influence the human brain’s ability to experience neuroplastic changes. For example, a genetic polymorphism (Val66Met) in brain-derived neurotrophic factor (BDNF) reduces electrophysiological measurements of training-dependent plasticity of the primary motor cortex (Kleim et al., 2006; Cheeran et al., 2008). Although the implications for motor learning and recovery of function need to be firmly established, these factors could partially explain interindividual variability in functional recovery or response to pharmacological or training-based interventions. BDNF is almost certainly one of the first of many possible genetic polymorphisms that affect training-dependent plasticity and also the ability to learn (Fritsch et al., 2010). Other mechanisms such as polymorphisms in the gene coding for catechol-O-methyltransferase, serotonin transporter, and other proteins involved in modulating or regulating neurotransmission are being explored and will likely lead to more individually tailored rehabilitative protocols after brain lesions (Pearson-Fuhrhop et al., 2009).
NONINVASIVETECHNIQUESCAPABLE OF EVALUATINGNEUROPLASTICITY IN HUMANS
One important factor that contributed to substantial advances in the understanding of neuroplasticity at a systems level in the human brain has been the development of techniques that allowed the noninvasive measurement of these changes. Techniques like positron emission tomography, magnetic resonance imaging (MRI), both functional (fMRI) and structural (particularly diffusion tensor imaging, DTI), magneto (MEG) and electro (EEG) encephalography, and transcranial magnetic (TMS) and direct current stimulation have all played important roles in the noninvasive evaluation of neuroplastic processes associated with recovery of function after CNS lesions. These techniques provide information on the possible relation between anatomical connectivity (DTI) or functional activity in specific brain areas as well as interactions between neural networks (fMRI) and a particular behavior, recovery process, or response to treatment. fMRI has excellent spatial resolution but less sharp temporal resolution and alone does not allow firm conclusions on cause—effect links between these associations (Cohen et al., 1997).
Dramatic increases in readily available computational power have led to the development of novel analytical approaches to functional imaging. Some of these apply economic theories, such as structural equation modeling (Simon, 1953) and Granger’s causality (Granger, 1969), to model the interactions between (sub)cortical regions (Deshpande et al., 2009; Kim and Horwitz, 2009). Dynamic causal modeling also explores regional interactions but does so within a Bayesian framework (Penny et al., 2004). These approaches explore interactions that are overlooked by conventional activation analysis (Rowe et al., 2002).
Model “free” analysis using multivariate statistical approaches (such as independent component analysis and principal component analysis) have established the existence of resting state networks (De Luca et al., 2006). The study of these networks represents a fertile area of research given their ability to experience neuroplastic changes, for example in relation to learning (Albert et al., 2009; Mantini et al., 2009). Changes in resting networks may be lost in classical fMRI study designs that involve contrasting one condition (task) with a control condition (most commonly rest). Another development that takes advantage of the increasing sophistication in these analytical tools is real-time fMRI (deCharms, 2008). The excitement of this approach is that it potentially can demonstrate the ability of training in human and nonhuman subjects to recruit specific brain regions or neural networks.
Although a lot of work has focused on the evaluation of dynamic changes in neural networks, there is mounting evidence that motor training can induce structural changes as well. These include changes in gray matter density (measured using voxel-based morphometry) (Smith et al., 2007; Wrigley et al., 2009) and in white matter (measured using DTI) (Johansen-Berg, 2007; Ciccarelli et al., 2008; Johansen-Berg and Rushworth, 2009; Scholz et al., 2009). Structural changes in gray and white matter have also been described in the elderly (Boyke et al., 2008). A word of caution: the cellular changes that underpin these changes are not yet clear. Nevertheless, in healthy volunteers, learning to juggle produces changes in gray matter density as well as in white matter (Scholz et al., 2009) in biologically plausible regions. Although there are numerous technical difficulties that have prevented the application of these techniques to the lesioned brain, some interesting studies are starting to appear in patient populations. In a recent study it was shown that constraint-induced therapy, a treatment proposed to improve motor function after stroke (Wolf et al., 2006), induced increases in matter density in the affected hemisphere, which is in keeping with functional MRI data (Gauthier et al., 2008). There were also increases in gray matter density in the nonaffected hemisphere, which are difficult to predict in our current framework of understanding. In some cases, identification of these changes allows the formulation of predicting algorithms after stroke (Stinear, 2010).
In contrast to MRI techniques, MEG and EEG allow a millisecond by millisecond analysis of the activity in functional networks in relation to behavior (Birbaumer and Cohen, 2007; Pantev et al., 2009). As such, they can provide information on the timing of neuroplastic changes or serial processing in a way that imaging techniques alone still cannot. Additionally, MEG and EEG do provide important information on activity in neural networks with a very accurate temporal resolution (Fujioka et al., 2006). Of note is that activity originating in specific brain networks as measured with MEG has been successfully used to control a hand orthosis that controls movements of a completely paralyzed hand after stroke (Buch et al., 2008). Similar results in terms of output control using EEG in completely paralyzed patients have been reported previously (Birbaumer et al., 1999; Wolpaw and McFarland, 2004).
Noninvasive brain stimulation techniques have contributed in different ways to the evaluation of systems’ neuroplasticity in the healthy and lesioned brain. In particular, TMS allows the evaluation of the behavioral consequences of disruption of activity (virtual lesion) in relatively focal brain regions, for example those shown to be active during a particular behavior in fMRI studies (Pascual-Leone et al., 2000). Disruption of a specific behavior as a consequence of TMS-induced disruption of a particular brain region has been interpreted as indicative of a cause—effect link between the two (Cohen et al., 1997). In this sense, neuroimaging and TMS virtual lesion experiments are complementary. An example of the way in which these two techniques operate to address fundamental hypotheses in motor control has been the evaluation of the role of the supplementary motor area or primary motor cortex in motor learning (Muellbacher et al., 2002; Perez et al., 2007; Censor et al., 2010). In another example of how these tools can be creatively utilized, TMS has been applied in the fMRI environment to examine fundamental questions of interregional connectivity within neural networks, otherwise impossible to address experimentally (Bestmann et al., 2005). This armamentarium has created an important momentum in human systems neuroscience, making possible the experimental evaluation of hypotheses until recently beyond the scope of investigation.
MODULATIONOF NEUROPLASTICITY
Given its proposed influence on learning processes and recovery of function, one goal of present investigations has been to develop strategies to modulate neuroplasticity: to facilitate it when it plays an adaptive function and downregulate it when it is maladaptive (see above). Different approaches have been tried in animal and human settings. Use of pharmacological agents like amphetamine, L-dopa, or selective serotonin reuptake inhibitors (SSRIs) (for review see Floel and Cohen, 2010) in association with motor training protocols may result in behavioral gains accompanied by cortical reorganization in humans (Tardy et al., 2006). Clearly, the development of better training protocols that take into account advances in basic science is an important area of research (Luft et al., 2004; Wolf et al., 2006; Ramachandran and Altschuler, 2009). Examples of proposed new rehabilitative paradigms include the combination of customarily used training protocols with action observation (Stefan et al., 2005), motor imagery (Sharma et al., 2006; Page et al., 2009; Sharma et al., 2009), and focused attention (Stefan et al., 2004) thought to ameliorate function by facilitating the mirror neuron system (Nelissen et al., 2005).
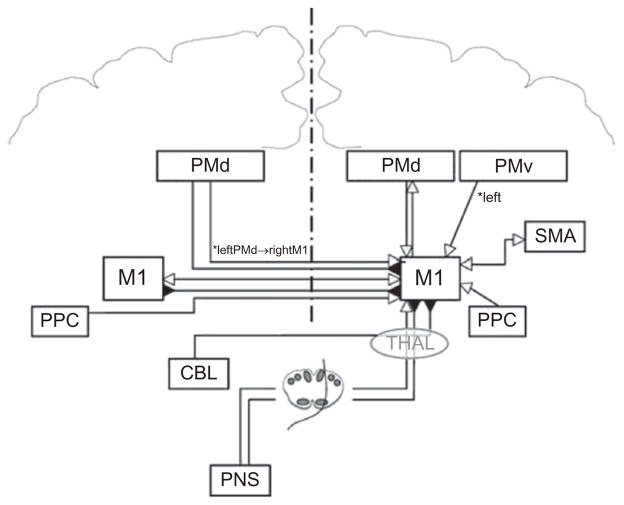
In addition to these approaches, recent years have seen the formulation of interventions based on better understood principles of intracortical interactive functions (see Fig. 1.1 for a summary of the interregional influences on M1). For example, the identification of persistent interhemispheric inhibitory interactions between the primary motor cortices after stroke (Murase et al., 2004) led to the hypothesis that facilitating excitability in the ipsilesional M1 or downregulating excitability in the contralesional M1 could enhance either static excitability or plastic processes leading to improved function. Both strategies have been tried in different laboratories leading to promising proof of principle behavioral and physiological results (for review see Fregni and Pascual-Leone, 2006; Hummel and Cohen, 2006; Talelli and Rothwell, 2006). Studies in healthy subjects pointed to the importance of synchronous application of cortical stimulation to M1 and motor training protocols (Reis et al., 2008a). Possible advantages of combining different stimulating modalities have been suggested as well. For example, a combination of facilitatory stimulation of the ipsilesional and inhibitory stimulation of the contralesional primary motor cortices (Vines et al., 2008) or combination of peripheral nerve stimulation applied to the paretic hand and facilitatory stimulation of the ipsilesional M1 (Celnik et al., 2009) in association with training have shown potential benefits in patients with chronic stroke. Invasive cortical stimulation through epidural electrodes over the primary motor cortex may have similar effects and has been proposed after stroke in humans and in animal models (Brown et al., 2008; Plow et al., 2009). While most reports seem to point to benefits of these techniques, it should be kept in mind that negative results are often underreported and that results from well-controlled, multicenter clinical trials under way are still not available.
Fig. 1.1.
The currently described influences of other brain areas on the output of the primary motor cortex (M1) are shown. Open arrows denote facilitation, while filled arrows denote inhibition. In many cases the influence shown represents a net effect of several specific interactions, whose details are discussed in the relevant section of the text and are shown in subsequent figures. These influences include projections from motor areas in the ipsi- and contralateral hemispheres and the effects of afferent sensory input. PMd = dorsal premotor cortex; PMv = ventral premotor cortex; SMA = supplementary motor area; PPC = posterior parietal cortex; CBL = cerebellum; THAL = thalamus; PNS = peripheral nervous system. (Figure reproduced with permission from Reis et al., 2008b.)
Manipulation of somatosensory input elicits clear effects on somatosensory as well as motor function. In healthy humans, somatosensory stimulation of median, ulnar, and/or radial nerves at the wrist induces clear increases in fMRI activation and cortical excitability in the stimulated hand motor cortical representations (Ridding et al., 2001; Kaelin-Lang et al., 2002; Wu et al., 2005; Conforto et al., 2010) while hand anesthesia induces increases in excitability and improved tactile discrimination in the non-anesthesized hand (Werhahn et al., 2002a, b). In stroke patients, somatosensory stimulation of the paretic limb (Conforto et al., 2002; Sheffler et al., 2006; Celnik et al., 2007) and anesthesia of the non-paretic hand (Floel et al., 2004) show comparable short-lasting behavioral and electrophysiological beneficial effects on paretic hand function, consistent with the documented correction of abnormalities in interhemispheric interactions between the primary motor cortices (Murase et al., 2004; Floel et al., 2008). Results from studies using transcutaneous electrical stimulation are consistent with those carried out using peripheral nerve stimulation and should therefore be considered in neurorehabilitation.
More information is becoming available on the neural mechanisms underlying recovery of motor function and neuroplasticity after stroke (see for example Prabhakaran et al., 2008; Swayne et al., 2008; Marshall et al., 2009). An emerging body of evidence is providing new insight into the interregional interactions between the premotor and parietal areas and primary motor cortex in healthy individuals, as well as strategies to modulate the strength of these interactions (Koch et al., 2006; Koch and Rothwell, 2009). It is only reasonable to expect the development of newer interventional proposals based on the emerging understanding of these mechanisms.
CONCLUSIONS
The last decade saw impressive improvements in our understanding of the ability of the CNS to reorganize in response to changes in the environment and lesions. This understanding resulted in parallel gains in our insight into mechanisms of both action and cognition in health and disease. Understanding of these neuroplastic principles is evolving into the development of more rational, hypothesis-driven strategies to promote recovery of function and will likely result in improvements in patient care along the bench to bedside translational pipeline (Cheeran et al., 2009).
Acknowledgments
This work was supported by the Intramural Research Program of the NINDS, NIH.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;9:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, et al. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage. 2005;28:22–29. doi: 10.1016/j.neuroimage.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Cohen LG. Brain-computer interfaces: communication and restoration of movement in paralysis. J Physiol. 2007;579:621–636. doi: 10.1113/jphysiol.2006.125633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Ghanayim N, Hinterberger T, et al. A spelling device for the paralysed. Nature. 1999;398:297–298. doi: 10.1038/18581. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Tresch MC, Saltiel P, et al. New perspectives on spinal motor systems. Nat Rev Neurosci. 2000;1:101–108. doi: 10.1038/35039000. [DOI] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, et al. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Lutsep HL, Weinand M, et al. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2008;62:853–862. doi: 10.1227/01.neu.0000316287.37618.78. [DOI] [PubMed] [Google Scholar]
- Brown TG, Sherrington CS. On the instability of a cortical point. Proc R Soc Lond (Biol) 1912;85:250–277. [Google Scholar]
- Buch E, Weber C, Cohen LG, et al. Think to move: a neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke. 2008;39:910–917. doi: 10.1161/STROKEAHA.107.505313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Ann Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Acute changes in cutaneous receptive fields in primary somatosensory cortex after digit denervation in adult flying fox. J Neurophysiol. 1991a;65:178–187. doi: 10.1152/jn.1991.65.2.178. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate expansion of receptive fields of neurons in area 3b of macaque monkeys after digit denervation. Somatosens Mot Res. 1991b;8:249–260. doi: 10.3109/08990229109144748. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Donoghue JP, Connors BW. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 1995;15:5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celnik P, Hummel F, Harris-Love M, et al. Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Arch Phys Med Rehabil. 2007;88:1369–1376. doi: 10.1016/j.apmr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Celnik P, Paik NJ, Vandermeeren Y, et al. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke. 2009;40:1764–1771. doi: 10.1161/STROKEAHA.108.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Dimyan MA, Cohen LG. Modification of existing human motor memories is enabled by primary cortical processing during memory reactivation. Curr Biol. 2010;20:1545–1549. doi: 10.1016/j.cub.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran B, Cohen L, Dobkin B, et al. The future of restorative neurosciences in stroke: driving the translational research pipeline from basic science to rehabilitation of people after stroke. Neurorehabil Neural Repair. 2009;23:97–107. doi: 10.1177/1545968308326636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Chino YM, Smith EL, Hatta S, et al. Postnatal development of binocular disparity sensitivity in neurons of the primate visual cortex. J Neurosci. 1997;17:296–307. doi: 10.1523/JNEUROSCI.17-01-00296.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Catani M, Johansen-Berg H, et al. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008;7:715–727. doi: 10.1016/S1474-4422(08)70163-7. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, et al. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, et al. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Cohen DA, Pascual-Leone A, Press DZ, et al. Off-line learning of motor skill memory: a double dissociation of goal and movement. Proc Natl Acad Sci U S A. 2005;102:18237–18241. doi: 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG, Celnik P, Pascual-Leone A, et al. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389:180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Kaelin-Lang A, Cohen LG. Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol. 2002;51:122–125. doi: 10.1002/ana.10070. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Ferreiro KN, Tomasi C, et al. Effects of somatosensory stimulation on motor function after subacute stroke. Neurorehabil Neural Repair. 2010;24:263–272. doi: 10.1177/1545968309349946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol. 2008;63:549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- deCharms RC. Applications of real-time fMRI. Nat Rev Neurosci. 2008;9:720–729. doi: 10.1038/nrn2414. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, et al. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Deshpande G, LaConte S, James GA, et al. Multivariate Granger causality analysis of fMRI data. Hum Brain Mapp. 2009;30:1361–1373. doi: 10.1002/hbm.20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M, Wall P. Effect of peripheral nerve injury on receptive fields of cells in the cat spinal cord. J Comp Neurol. 1981;199:277–291. doi: 10.1002/cne.901990209. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of functional recovery mechanisms after stroke. Neurorehabil Neural Repair. 2010;24:125–135. doi: 10.1177/1545968309345270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JW, Wagster MV. Developing context and background underlying cognitive intervention/training studies in older populations. J Gerontol B Psychol Sci Soc Sci. 2007;62:5–10. doi: 10.1093/geronb/62.special_issue_1.5. [DOI] [PubMed] [Google Scholar]
- Fischer TM, Blazis DE, Priver NA, et al. Metaplasticity at identified inhibitory synapses in Aplysia [see comments] Nature. 1997;389:860–865. doi: 10.1038/39892. [DOI] [PubMed] [Google Scholar]
- Floel A, Cohen LG. Translational studies in neurorehabilitation: from bench to bedside. Cogn Behav Neurol. 2006;19:1–10. doi: 10.1097/00146965-200603000-00001. [DOI] [PubMed] [Google Scholar]
- Floel A, Cohen LG. Recovery of function in humans: cortical stimulation and pharmacological treatments after stroke. Neurobiol Dis. 2010;37:243–251. doi: 10.1016/j.nbd.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, Nagorsen U, Werhahn KJ, et al. Influence of somatosensory input on motor function in patients with chronic stroke. Ann Neurol. 2004;56:206–212. doi: 10.1002/ana.20170. [DOI] [PubMed] [Google Scholar]
- Floel A, Hummel F, Duque J, et al. Influence of somatosensory input on interhemispheric interactions in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22:477–485. doi: 10.1177/1545968308316388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873–881. doi: 10.1038/nrn1991. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Hand motor recovery after stroke: tuning the orchestra to improve hand motor function. Cogn Behav Neurol. 2006;19:21–33. doi: 10.1097/00146965-200603000-00003. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, et al. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka T, Ross B, Kakigi R, et al. One year of musical training affects development of auditory cortical-evoked fields in young children. Brain. 2006;129:2593–2608. doi: 10.1093/brain/awl247. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, et al. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gauthier LV, Taub E, Perkins C, et al. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, et al. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 2008;18:2046–2053. doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- Granger C. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37:424–438. [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63:236–246. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol. 1994;71:2543–2547. doi: 10.1152/jn.1994.71.6.2543. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28:91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodics T, Cohen LG, Cramer SC. Functional imaging of intervention effects in stroke motor rehabilitation. Arch Phys Med Rehabil. 2006;87:S36–S42. doi: 10.1016/j.apmr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Heise K, Celnik P, et al. Facilitating skilled right hand motor function in older subjects by anodal polarization over the left primary motor cortex. Neurobiol Aging. 2010;31:2160–2168. doi: 10.1016/j.neurobiolaging.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:10867–10872. doi: 10.1523/JNEUROSCI.23-34-10867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H. Structural plasticity: rewiring the brain. Curr Biol. 2007;17:R141–R144. doi: 10.1016/j.cub.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF. Using diffusion imaging to study human connectional anatomy. Annu Rev Neurosci. 2009;32:75–94. doi: 10.1146/annurev.neuro.051508.135735. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, et al. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung P, Ziemann U. Homeostatic and nonhomeostatic modulation of learning in human motor cortex. J Neurosci. 2009;29:5597–5604. doi: 10.1523/JNEUROSCI.0222-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Merzenich MM, Killackey HP. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- Kaelin-Lang A, Luft AR, Sawaki L, et al. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540:623–633. doi: 10.1113/jphysiol.2001.012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Horwitz B. How well does structural equation modeling reveal abnormal brain anatomical connections? An fMRI simulation study. Neuroimage. 2009;45:1190–1198. doi: 10.1016/j.neuroimage.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Koch G, Rothwell JC. TMS investigations into the task-dependent functional interplay between human posterior parietal and motor cortex. Behav Brain Res. 2009;202:147–152. doi: 10.1016/j.bbr.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, et al. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006;26:7452–7459. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol. 2009;629:405–421. doi: 10.1007/978-0-387-77064-2_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry P, Labelle A, Deschenes M. Intracortical distribution of axonal collaterals of pyramidal tract cells in the cat motor cortex. Brain Res. 1990;191:327–336. doi: 10.1016/0006-8993(80)91284-6. [DOI] [PubMed] [Google Scholar]
- Lee JL. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Overman JJ, Katsman D, et al. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci. 2010;13:1496–1504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, et al. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26:6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft AR, McCombe-Waller S, Whitall J, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Perrucci MG, et al. Large-scale brain networks account for sustained and transient activity during target detection. Neuroimage. 2009;44:265–274. doi: 10.1016/j.neuroimage.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RS, Zarahn E, Alon L, et al. Early imaging correlates of subsequent motor recovery after stroke. Ann Neurol. 2009;65:596–602. doi: 10.1002/ana.21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire BA, Gilbert CD, Rivlin PK, et al. Targets of horizontal connections in macaque primary visual cortex. J Comp Neurol. 1991;305:370–392. doi: 10.1002/cne.903050303. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall JT, et al. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Merzenich M, Wright B, Jenkins W, et al. Cortical plasticity underlying perceptual, motor, and cognitive skill development: implications for neurorehabilitation. Cold Spring Harb Symp Quant Biol. 1996;61:1–8. [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, et al. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, et al. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Luppino G, Vanduffel W, et al. Observing others: multiple action representation in the frontal lobe. Science. 2005;310:332–336. doi: 10.1126/science.1115593. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes FS, et al. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996a;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, et al. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996b;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SJ, Szaflarski JP, Eliassen JC, et al. Cortical plasticity following motor skill learning during mental practice in stroke. Neurorehabil Neural Repair. 2009;23:382–388. doi: 10.1177/1545968308326427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantev C, Lappe C, Herholz SC, et al. Auditory-somatosensory integration and cortical plasticity in musical training. Ann N Y Acad Sci. 2009;1169:143–150. doi: 10.1111/j.1749-6632.2009.04588.x. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience – virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10:232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Pearson-Fuhrhop KM, Kleim JA, Cramer SC. Brain plasticity and genetic factors. Top Stroke Rehabil. 2009;16:282–299. doi: 10.1310/tsr1604-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, et al. Modelling functional integration: a comparison of structural equation and dynamic causal models. Neuroimage. 2004;23:S264–S274. doi: 10.1016/j.neuroimage.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, et al. Neural substrates of intermanual transfer of a newly acquired motor skill. Curr Biol. 2007;17:1896–1902. doi: 10.1016/j.cub.2007.09.058. [DOI] [PubMed] [Google Scholar]
- Plow EB, Carey JR, Nudo RJ, et al. Invasive cortical stimulation to promote recovery of function after stroke: a critical appraisal. Stroke. 2009;40:1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- Ragert P, Camus M, Vandermeeren Y, et al. Modulation of effects of intermittent theta burst stimulation applied over primary motor cortex (M1) by conditioning stimulation of the opposite M1. J Neurophysiol. 2009;102:766–773. doi: 10.1152/jn.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. 2009;132:1693–1710. doi: 10.1093/brain/awp135. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Compensatory plasticity and sensory substitution in the cerebralcortex. Trends Neurosci. 1995;18:36–43. doi: 10.1016/0166-2236(95)93948-w. [DOI] [PubMed] [Google Scholar]
- Reis J, Robertson E, Krakauer JW, et al. Consensus: “Can tDCS and TMS enhance motor learning and memory formation?”. Brain Stimul. 2008a;1(4):363–369. doi: 10.1016/j.brs.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008b;586(2):325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, McKay DR, Thompson PD, et al. Changes in corticomotor representations induced by prolonged peripheral nerve stimulation in humans. Clin Neurophysiol. 2001;112:1461–1469. doi: 10.1016/s1388-2457(01)00592-2. [DOI] [PubMed] [Google Scholar]
- Robertson D, Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- Rowe J, Stephan KE, Friston K, et al. Attention to action in Parkinson’s disease: impaired effective connectivity among frontal cortical regions. Brain. 2002;125:276–289. doi: 10.1093/brain/awf036. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, et al. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, et al. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Pomeroy VM, Baron JC. Motor imagery: a backdoor to the motor system after stroke? Stroke. 2006;37:1941–1952. doi: 10.1161/01.STR.0000226902.43357.fc. [DOI] [PubMed] [Google Scholar]
- Sharma N, Simmons LH, Jones PS, et al. Motor imagery after subcortical stroke: a functional magnetic resonance imaging study. Stroke. 2009;40:1315–1324. doi: 10.1161/STROKEAHA.108.525766. [DOI] [PubMed] [Google Scholar]
- Sheffler LR, Hennessey MT, Naples GG, et al. Peroneal nerve stimulation versus an ankle foot orthosis for correction of footdrop in stroke: impact on functional ambulation. Neurorehabil Neural Repair. 2006;20:355–360. doi: 10.1177/1545968306287925. [DOI] [PubMed] [Google Scholar]
- Simon HA. Causal ordering and identifiability. In: Hood WC, Koopmans TC, editors. Studies in Econometric Method. Wiley; New York: 1953. pp. 49–74. [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, et al. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28:1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, et al. Formation of a motor memory by action observation. J Neurosci. 2005;25:9339–9346. doi: 10.1523/JNEUROSCI.2282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9:1228–1232. doi: 10.1016/S1474-4422(10)70247-7. [DOI] [PubMed] [Google Scholar]
- Swayne OB, Rothwell JC, Ward NS, et al. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb Cortex. 2008;18:1909–1922. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P, Rothwell J. Does brain stimulation after stroke have a future? Curr Opin Neurol. 2006;19:543–550. doi: 10.1097/WCO.0b013e32801080d1. [DOI] [PubMed] [Google Scholar]
- Tardy J, Pariente J, Leger A, et al. Methylphenidate modulates cerebral post-stroke reorganization. Neuroimage. 2006;33:913–922. doi: 10.1016/j.neuroimage.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Elbert T. New treatments in neurorehabilitation founded on basic research. Nat Rev Neurosci. 2002;3:228–236. doi: 10.1038/nrn754. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Carr LJ, Isaacs E, et al. Onset of speech after left hemispherectomy in a nine-year-old boy. Brain. 1997;120:159–182. doi: 10.1093/brain/120.1.159. [DOI] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Kaelin-Lang A, et al. Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain. 2002a;125:1402–1413. doi: 10.1093/brain/awf140. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Van Boven RW, et al. Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci. 2002b;5:936–938. doi: 10.1038/nn917. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc Natl Acad Sci U S A. 2004;101:17849–17854. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci. 2001;24:807–843. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Gustin SM, Macey PM, et al. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. 2009;19:224–232. doi: 10.1093/cercor/bhn072. [DOI] [PubMed] [Google Scholar]
- Wu CW, Kaas JH. Reorganization in primary motor cortex of primates with long-standing therapeutic amputations. J Neurosci. 1999;19:7679–7697. doi: 10.1523/JNEUROSCI.19-17-07679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Kaas JH. Spinal cord atrophy and reorganization of motoneuron connections following long-standing limb loss in primates. Neuron. 2000;28:967–978. doi: 10.1016/s0896-6273(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Wu CW, van Gelderen P, Hanakawa T, et al. Enduring representational plasticity after somatosensory stimulation. Neuroimage. 2005;27:872–884. doi: 10.1016/j.neuroimage.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci. 1998;18:7000–7007. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]