Abstract
Apical sodium-dependent bile acid transporter (ASBT) represents a highly efficient conservation mechanism of bile acids via mediation of their active transport across the luminal membrane of terminal ileum. To gain insight into the cellular regulation of ASBT, we investigated the association of ASBT with cholesterol and sphingolipid-enriched specialized plasma membrane microdomains known as lipid rafts and examined the role of membrane cholesterol in maintaining ASBT function. Human embryonic kidney (HEK)-293 cells stably transfected with human ASBT, human ileal brush-border membrane vesicles, and human intestinal epithelial Caco-2 cells were utilized for these studies. Floatation experiments on Optiprep density gradients demonstrated the association of ASBT protein with lipid rafts. Disruption of lipid rafts by depletion of membrane cholesterol with methyl-β-cyclodextrin (MβCD) significantly reduced the association of ASBT with lipid rafts, which was paralleled by a decrease in ASBT activity in Caco-2 and HEK-293 cells treated with MβCD. The inhibition in ASBT activity by MβCD was blocked in the cells treated with MβCD-cholesterol complexes. Kinetic analysis revealed that MβCD treatment decreased the Vmax of the transporter, which was not associated with alteration in the plasma membrane expression of ASBT. Our study illustrates that cholesterol content of lipid rafts is essential for the optimal activity of ASBT and support the association of ASBT with lipid rafts. These findings suggest a novel mechanism by which ASBT activity may be rapidly modulated by alterations in cholesterol content of plasma membrane and thus have important implications in processes related to maintenance of bile acid and cholesterol homeostasis.
Keywords: detergent-insoluble microdomains, floatation on Optiprep density gradient, bile acid absorption
the apical sodium-dependent bile acid transporter (ASBT) is an integral membrane glycoprotein responsible for active bile acid transport across plasma membrane of epithelial cells (12, 17, 24, 47). ASBT is expressed in distal ileum, renal proximal tubules, and bile ducts, where its polypeptide is localized to the apical membrane of polarized epithelial cells (3, 17, 27). The primary function of ASBT is the absorption of secreted bile acids to prevent their loss from the kidney and the intestine as a critical part of a salvage mechanism involved in their homeostasis (17, 24).
In the distal ileum, ASBT represents the major pathway for absorption of luminal bile acids and plays an important role in maintenance of cholesterol homeostasis (24). Also, disturbances in ASBT activity are implicated in diarrheal disorders secondary to an increase in intestinal luminal bile acid concentration and to an induction of electrolyte and water secretion (4, 9, 22, 35, 46). Because luminal bile acid content constantly varies between meals, it is expected that ileal ASBT undergoes rapid adaptive changes in response to the varying intestinal milieu. In this regard, previous studies have demonstrated the involvement of various signal transduction molecules and membrane trafficking events in the acute modulation of ASBT function and membrane expression. For example, a role of cAMP-dependent pathways and MAPKs has been demonstrated in the alteration of active bile acid absorption in both rat ileum and renal proximal tubular cells (36, 39). Also, secretin has been shown to stimulate the activity of ASBT by shuttling ASBT from subapical endosomes to the apical membrane and thus increasing its surface membrane expression in rat cholangiocytes (2).
Recent evidence suggested that optimal function of membrane transporters regulated by vesicular trafficking is dependent on their targeting to specific plasma membrane domains and is influenced by lipid and cholesterol composition of these microdomains (28, 32). This packing of membrane transporters with various kinases and signal transduction molecules within a tight structure of plasma membrane, such as lipid rafts, dictates their specific regulation (6, 16, 29). Lipid rafts of plasma membranes are microdomains enriched with sphingolipids and cholesterol and are resistant to solubilization by detergents such as Triton X-100 (6, 8, 16). Cholesterol plays an important role in maintaining the structure of these microdomain-condensing lipid and protein molecules (6, 16, 41). Cholesterol removal from plasma membrane leads to disassociation of raft proteins from lipids (41). The role of lipid rafts in regulation of several transport processes of the intestinal epithelial cells has been previously demonstrated (28, 32). Despite emerging evidence of the regulation of ASBT by vesicular trafficking (2, 42, 43), it is not yet known whether ASBT is associated with lipid rafts of plasma membrane and whether cholesterol content and physical state of plasma membrane affect the activity of ileal ASBT.
In the present study, we aimed to investigate the distribution of ASBT between various domains of plasma membrane and to examine the effect of lipid raft disruption and plasma membrane cholesterol depletion on ASBT activity. Our findings demonstrated the presence of ASBT polypeptide in insoluble fractions of plasma membrane. The pool of ASBT associated with the detergent-insoluble (DI) fraction of plasma membrane was shown to be associated with lipid raft microdomains whose disruption by cholesterol depletion significantly decreased the activity of ASBT.
MATERIALS AND METHODS
Cell culture
Caco-2 and human embryonic kidney (HEK)-293 cells were obtained from American Type Culture Collection and were grown routinely in T-75 plastic flasks at 37°C in a 5% CO2-95% air environment. The cells were cultured in MEM supplemented with FBS (20% for Caco-2 and 10% for HEK-293 cells). For uptake experiments, Caco-2 cells were plated at a density of 2 × 104 cell/cm2 in 24-well Falcon plates (treated by vacuum gas plasma; Becton Dickinson, Franklin Lakes, NJ). Confluent monolayers were then used for the transport experiments at day 14 postplating. Methyl-β-cyclodextrin (MβCD) and water-soluble cholesterol (49 mg cholesterol balanced by 561 mg of MβCD per each gram) were obtained from Sigma (St. Louis, MO). Solutions containing 1.25 mM cholesterol were supplemented with additional MβCD to reach a final concentration of 10 mM of the latter.
Plasmid construction
Full-length cDNA of human ASBT (hASBT) was amplified from total RNA extracted from Caco-2 cells by the methods of Chomczynski and Sacchi (10) utilizing RNazol solution (Tel-Test, Friendswood, TX) and essentially using the manufacturer's protocol. Total RNA (2 μg) was used for reverse transcription with random primers with the use of the SuperScript II reverse transcriptase kit (Invitrogen). The full-length cDNA of ASBT was then amplified by PCR, utilizing gene-specific primers and the proofreading Elongase enzyme mix (Invitrogen) according to the manufacturer's instructions. The primer sequences (designed based on GenBank accession number U10417) are as follows: 5′ primer is CTATCAACAAGTTTGTACAAAAAAGCAGGCTTGAAGGAGATAGAACCATGGCCAAT (Kozak sequence is underlined) and 3′ primer is GGGGACCACTTTGTACAAGAAAGCTGGGTCCTTTTCGTCAGGTTGAAA.
The PCR reaction conditions were as follows: 94°C for 5 min and then 40 cycles of amplification with two steps at 94°C for 30 s (denaturing) and at 68°C for 10 min (extension), followed by final extension at 68°C for 10 min. PCR products were excised from 1% agarose gel and purified utilizing Sephaglas BandPrep kit (Amersham Pharmacia Biotech, Piscataway, NJ). The amplified fragment was cut with BsrG1 restriction enzyme and subsequently cloned into pcDNA2.3/V5-DEST expression mammalian vector (Invitrogen) in frame with the V5 tagged. The orientation and the sequence of the insert were confirmed by sequencing, and the expression of hASBT-V5 fusion protein was examined by Western blotting with the use of anti-V5 antibodies (Invitrogen).
Transfection experiments
For transfection studies, HEK-293 cells were seeded into 24-well plates (105 cells/well) and immediately transfected with mammalian expression vector for ASBT-V5 fusion protein using FuGENE 6 reagent (Roche). A total of 0.5 μg DNA/well and 1 μl of FuGENE 6 reagent/well were used for each transfection. After 48 h, cells were then incubated with medium containing 0.6 mg/ml of G418. Resistant clones of cells were then trypsinized, pooled, and maintained in medium containing the same concentration of G418 and designated as 2BT cells. The expression of ASBT-V5 in 2BT cells was confirmed by Western blotting using anti-V5 antibodies. Caco-2 cells were transfected with the Amaxa Nucleofector System according to the manufacturer's instructions. Briefly, ~2 × 106 cells were harvested and then electroporated in 100 μl of solution T (supplied by Amaxa) along with 10 μg of vector containing ASBT-V5 fusion protein. The cells were then transferred to full medium and plated on 6 wells of 24-well plates.
Labeling of lipid rafts and cholesterol depletion of plasma membrane
2BT cells were incubated with 3 μg/ml of biotin-conjugated subunit B of cholera toxin (CTxB) for 10 min at room temperature to label lipid rafts by binding GM1 gangliosides. The cells were then incubated for 30 min at 37°C in the presence or absence of 10 mM MβCD to deplete plasma cholesterol. The cells were then washed three times with PBS buffer and lysed in TNE buffer [50 mM Tris •HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA] supplemented with 1× complete protease inhibitor cocktail (Roche).
Cell surface biotinylation
Cell surface biotinylation was performed with sulfo-NHS-SS-biotin (0.5 mg/ml; Pierce, Rockford, IL) in borate buffer (in mM: 154 NaCl, 7.2 KCl, 1.8 CaCl2, and 10 H3BO3, pH 9.0), as previously described, with labeling for 60 min at 4°C to stop endocytosis and internalization of antigens (1). After immunoprecipitation of biotinylated antigens with streptavidin agarose, biotinylated proteins were released by incubation in 50 mM DTT, reconstituted in Laemmli buffer. The immunoprecipitates were subjected to SDS-PAGE, and blots were then probed with anti-V5 antibodies.
Floatation on a discontinuous Optiprep density gradient
Lipid rafts were isolated by floatation on Optiprep density gradient as previously described (19). Briefly, membrane preparations were centrifuged at 100,000 g for 30 min at 4°C and then resuspended and incubated for 30 min at 4°C in TNE buffer containing 25 mM Tris (pH 7.4), 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100 supplemented with 1× Complete protease inhibitor cocktail. The membranes were then adjusted to 40% final concentration of Optiprep (Nycomed, Oslo, Norway) and layered at the bottom of density gradient with steps of final concentrations of 35%, 30%, 25%, and 20% of Optiprep in TNE buffer. TNE buffer was laid on the top of the gradient, which was then centrifuged at 215,000 g for 4 h at 4°C. Fractions were collected from the top of the gradient and then analyzed by Western blotting. The specific activity of alkaline phosphatase was measured in each fraction of gradient. Alkaline phosphatase has been previously shown as a marker of lipid rafts (28). Protein concentrations in each fraction were assessed by the method of Bradford (7).
Isolation of human ileal brush-border membrane vesicles
Small intestine from healthy adult organ donors were obtained immediately after harvest of transplantation organs (Gift of Hope). The intestine was divided into three equal parts of which the middle one was discarded; the first third was designated as proximal small intestine (jejunum) and the third part was designated as distal small intestine (ileum). The intestine was then cleaned, and the mucosa was scraped from the seromuscular layer of these segments and stored at −80°C. Purified brush-border membrane vesicles (BBMVs) were prepared from mucosa as previously described (14, 15, 20). In the final step of the preparation, the vesicles were resuspended in PBS supplemented with 1× Complete protease inhibitor cocktail (Roche) and were then quick frozen and stored at −80°C for further use. The membrane protein was assessed by Bradford technique, using bovine plasma globulin as standard (7). The purity of membrane vesicles and the degree of contamination with intracellular organelles were assessed by measuring the activity of alkaline phosphatase as a marker for the intestinal apical membrane. The prepared ileal BBMVs exhibited 15- to 20-fold purity over the crude homogenate, as assessed by the enrichment of alkaline phosphatase activity.
Isolation of detergent-soluble and detergent-insoluble fractions from human ileal BBMVs
Detergent-soluble (DS) and insoluble (DI) fractions of ileal BBMVs were prepared essentially as previously described (28). Briefly, 3 mg of BBMVs were incubated, or left untreated, for 1 h at 37°C with 10 mM MβCD in 1× PBS and then were centrifuged for 30 min at 100,000 g at 4°C and resuspended in MES buffer containing 50 mM MES (pH 6.5), 60 mM NaCl, 3 mM EGTA, 5 mM MgCl2, 1% Triton X-100, and 1× Complete protease inhibitor cocktail. Membrane vesicles were then incubated with MES buffer on a rotary shaker for 30 min at 4°C. At the end of the incubation, BBMVs were centrifuged at 100,000 g at 4°C for 30 min, and supernatant was designated as DS fraction. The pellet was resuspended in buffer containing 15 mM HEPES (pH 7.4), 150 mM NaCl, 10 mM EDTA, 1 mM DTT, 1% Triton X-100, 0.1% SDS, and 1× Complete protease inhibitor cocktail and was designated as DI fraction. Both DS and DI fractions were frozen at −80°C until further analysis by Western blotting.
Western blotting
Equal amounts of protein from the DS and DI fractions of BBMVs (80 μg) or equal volumes from each fraction of the Optiprep gradient were solubilized in Laemmli sample buffer (2% SDS, 10% glycerol 100 mM DTT, 60 mM Tris, pH 6.8, 0.01% bromphenol blue) and separated on 10% Tris-glycine SDS polyacrylamide gel. Separated proteins were then electrotransferred onto nitrocellulose membranes, and Western blotting was performed by washing the nitrocellulose membranes three times and then blocking them overnight in blocking buffer containing 5% nonfat dry milk in PBS. The blots were then incubated with the primary antibodies diluted in the blocking solution for 1 h at room temperature and washed extensively after that with PBS containing 0.1% Tween 20. The blots were then incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (diluted 1:2,000) in the blocking buffer and were again washed extensively with PBS-0.1% Tween 20. The bands were visualized by enhanced chemiluminescence according to the manufacturer's instructions (Amersham, Arlington Heights, IL). hASBT was detected with the use of rabbit polyclonal anti-peptide antibodies that were raised against 16-amino acid peptide representing amino acid residues 314–329 (CHGKNKAEIPESKENG) of the COOH-terminal end of hASBT (Orbigen, San Diego, CA). Blots were also probed with anti-actin antibodies obtained from Sigma.
[3H]taurocholic acid uptake
Sodium-dependent taurocholic acid (TC) transport in transfected HEK-293 or Caco-2 cells was assessed as previously described by our group (5). Briefly, medium was aspirated off, and the cells were incubated for 15 min at 25°C with buffer containing (in mM) 110 NaCl (with sodium) or choline chloride (without sodium), 4 KCl, 1 MgSO4, 1 CaCl2, 50 mannitol, and 10 HEPES (pH 7.4). Cells were then incubated with the same buffer containing the indicated concentration of TC along with 1 μCi/ml of [3H]TC (Perkin Elmer, Boston, MA) for the designated period of time. The transport process was terminated by washing the cells twice with ice-cold PBS. Cells were then solubilized with 0.5 N NaOH for at least 4 h. The protein concentration was measured by the method of Bradford (7), and the radioactivity was counted by Packard liquid scintillation analyzer Tri-CARB 1600-TR (Packard Instrument, Downers Grove, IL). The uptake was measured at 5 min (and expressed as pmol•mg protein−1 •5 min−1). For the kinetic experiments, the uptake values were analyzed for simple Michaelis-Menten kinetics utilizing a nonlinear regression data analysis from a computerized model (GraphPad, PRISM, San Diego, CA).
Statistical analysis
Results are expressed as means ± SE. Student's t-test was utilized in statistical analysis. P < 0.05 was considered statistically significant.
RESULTS
ASBT association with lipid rafts
Previous studies utilizing several in vitro models have demonstrated that optimal function of certain plasma membrane transporters is dependent on their orderly distribution among various microdomains of plasma membrane such as lipid rafts (28, 32). Human embryonic kidney HEK-293 cell line has been shown to be a suitable in vitro model to assess the role of lipid rafts and membrane cholesterol in sustaining the function of membrane transporters (23, 32). We generated HEK-293 cells stably transfected with ASBT-V5 fusion protein (designated as 2BT cells) to investigate the association of ASBT with lipid rafts. As depicted in Fig. 1A, Western blot analysis utilizing anti-V5 antibodies showed the expected size band (~40 kDa) in 2BT cells but not in wild-type HEK-293 cells. It should be noted that ASBT-V5 fusion protein is detected as a pair of bands indicating a glycosylated and unglycosylated polypeptide of ASBT as previously reported (47). Functional studies also demonstrated the presence of high-sodium-dependent [3H]TC activity in 2BT cells compared with wild-type HEK-293 cells in which the sodium-dependent [3H]TC uptake was insignificant (data not shown). These data indicate that the exogenously expressed ASBT tagged to V5 epitope is fully functional in 2BT cells.
Fig. 1.
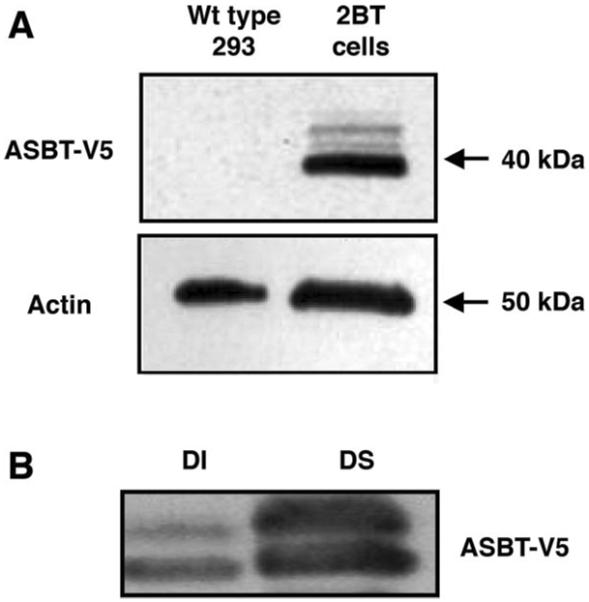
Expression of apical sodium-dependent bile acid transporter (ASBT)-V5 fusion protein in transfected human embryonic kidney (HEK)-293 cells. A: HEK-293 fibroblasts were transfected with pcDNA 2.3/V5 vector engineered to stably express ASBT protein fused to V5 tagged at the COOH terminus. Western blot analysis with anti-V5 antibodies demonstrates expression of ASBT-V5 fusion protein in HEK-293 cells stably transfected with ASBT-V5 (designated as 2BT cells) compared with wild-type (WT) cells. Actin expression from the same samples is shown in both WT and transfected cells. B: total membrane preparations were made from 2BT cells and lysed with 1% Triton X-100 at 4°C, as described in materials and methods. Detergent-soluble (DS) and detergent-insoluble (DI) fractions were isolated, and level ASBT-V5 fusion protein associated with these fractions was assessed by Western blot analyses utilizing anti-V5 antibodies.
We next investigated the association of ASBT with lipid rafts of plasma membrane. Because of their enrichment with sphingolipids and cholesterol, lipid rafts are characterized by their resistance to solubilization with Triton X-100 at 4°C and by high buoyancy and floatation on a discontinuous density gradient (28, 32, 44). We first investigated the association of ASBT-V5 protein with the DS and DI fractions of total membrane preparations of 2BT cells. As shown in Fig. 1B, ASBT-V5 was detected in both DS and DI fraction of the membrane. These results suggest that a significant pool of ASBT-V5 protein is associated with lipid rafts or could possibly indicate that ASBT-V5 is precipitated in the DI fraction via its link to cytoskeleton elements such as actin. Therefore, we further examined the association of ASBT-V5 fusion protein with the floating pools of membrane preparations of 2BT cells on Optiprep density gradient.
Lipid rafts were first labeled in 2BT cells by incubation with the nontoxic CTxB, which attaches to the cellular surface by binding to ganglioside GM1 of plasma membrane, the widely used marker for lipid raft microdomains (13, 34). The cells were then lysed in the presence of 1% Triton X-100 at 4°C, and total membrane was overlaid with a discontinuous Optiprep gradient. The Western blot in Fig. 2A shows the presence of ASBT-V5 fusion protein in the floating fractions on the top of Optiprep density gradient, indicating its presence in membrane lipid rafts. Because the integrity of lipid rafts depends on their content of cholesterol (41), we exposed 2BT cells to the oligosaccharide MβCD, which selectively removes the majority of cholesterol from the membrane (11). Cells were then lysed, and their membrane extracts were subjected to centrifugation on a density gradient. As shown in Fig. 2A, the presence of ASBT-V5 fusion protein in the top floating fractions was significantly reduced with cholesterol depletion of plasma membrane. To confirm our observations, we examined the floatation profile of CTxB as a marker of lipid rafts in the membrane preparations from 2BT cells. The distribution profile of CTxB on Optiprep density gradient is depicted in Fig. 2B. The presence of GM1 is evident in all fractions, including the floating fractions containing lipid rafts.
Fig. 2.
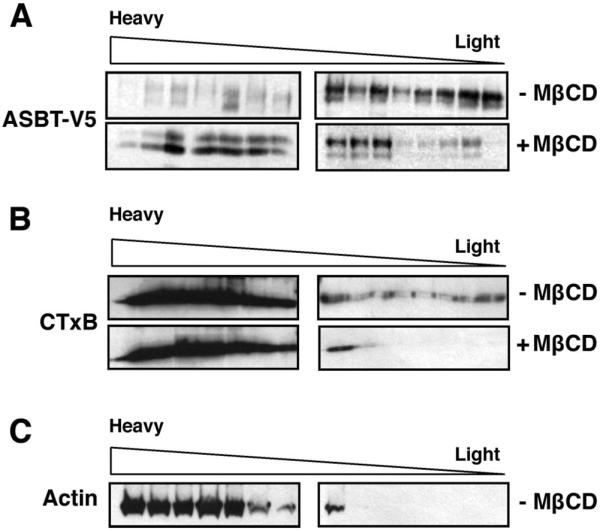
Association of ASBT-V5 fusion protein with lipid rafts in 2BT cells. 2BT cells were incubated with biotin-conjugated cholera toxin subunit B (CTxB) to label lipid rafts of plasma membrane. Labeled cells were incubated with methyl-β-cyclodextrin (MβCD) to remove cholesterol from plasma membrane or with vehicle alone. Cells were then lysed with 1% Triton X-100, and total membrane extracts were prepared as described in materials and methods. Samples were subjected to floatation on Optiprep density gradients, and fractions were collected from top of the gradients. Fractions were separated by electrophoresis and analyzed by Western blotting. A: Western blot results using anti-V5 antibodies. B: blots were probed with avidin peroxidase antibodies to label the biotinylated CTxB. C: blots were probed with actin antibodies.
Similar to the shift in ASBT-V5 fusion protein distribution, GM1 distribution profile on Optiprep gradient was also shifted to the heavier fractions on cholesterol depletion of plasma membrane by MβCD. On the other hand, actin of the cytoskeleton that is insoluble by detergent but is not associated with lipid rafts was recovered as expected only in the heavy fractions of the density gradient (Fig. 2C). Together, these findings suggest the association of ASBT with lipid rafts of cellular membranes.
Depletion of plasma membrane cholesterol decreases hASBT activity
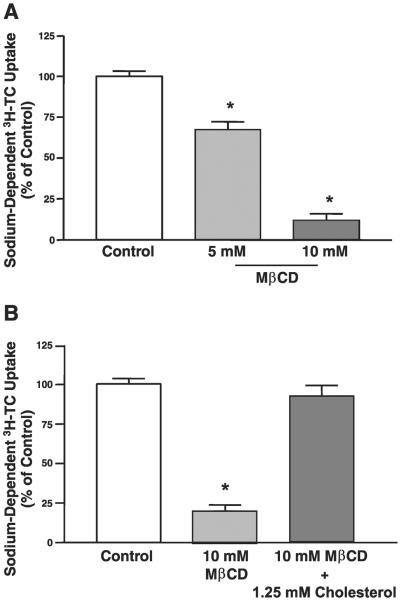
To evaluate the significance of ASBT localization in lipid rafts, we examined the effect of lipid raft disruption by cholesterol depletion on ASBT activity. 2BT cells were incubated with different concentrations of MβCD for 30 min at 37°C, and ASBT activity was assessed by measuring sodium-dependent [3H]TC uptake. As shown in Fig. 3A, sodium-dependent [3H]TC uptake in 2BT cells was significantly reduced in a dose-dependent manner with a maximal inhibition occurring at 10 mM (incubation with 20 mM resulted in the same degree of reduction; data not shown).
Fig. 3.
MβCD decreased ASBT function in 2BT cells. A: 2BT cells were treated with different concentrations of MβCD for 30 min at 37°C. Cells were washed and then incubated at 25°C with HEPES-buffered uptake solution (pH 7.4) containing 110 mM of NaCl or choline chloride along with 10 μM [3H]taurocholic acid (TC) for 5 min, and sodium-dependent [3H]TC uptake was determined. Results are presented as % of control and are means ± SE obtained from at least 3 separate experiments. *P < 0.05 compared with control. B: 2BT cells were treated with 10 mM MβCD alone or with 10 mM of MβCD + 1.25 mM of cholesterol for 30 min at 37°C, and Na+-dependent [3H]TC uptake was assessed. Data are expressed as % of control and are means ± SE obtained from at least 3 separate experiments. *P < 0.05 compared with control.
To determine whether the observed reduction in ASBT activity was a result of cholesterol depletion, 2BT cells were treated with 10 mM MβCD alone or along with 1.25 mM cholesterol. As depicted in Fig. 3B, the presence of cholesterol prevented MβCD-induced inhibition of ASBT, indicating that the observed reduction in ASBT function is indeed due to cholesterol depletion of the plasma membrane.
ASBT association with lipid rafts of brush-border membrane of intestinal epithelial cells
To further examine whether ASBT is associated with lipid microdomains of plasma membranes of human intestinal epithelial cells, we first investigated the partitioning of ASBT between DS and DI fractions of human ileal BBMVs prepared from organ donors. Human ileal BBMVs were treated with Triton X-100 at 4°C, and the DI pool was collected by high-speed sedimentation; the supernatant contained the DS fraction of plasma membrane. As depicted in Fig. 4A, Western blot analysis demonstrated the presence of ASBT polypeptide predominantly in the DI fractions of human ileal BBMVs. Actin cytoskeleton was also predominantly detected in the DI fractions. We next investigated the effect of cholesterol depletion from human ileal BBMVs on ASBT solubility with Triton X-100. Human ileal BBMVs were exposed to the oligosaccharide MβCD and then solubilized with Triton X-100. As shown in Fig. 4A, the level of ASBT but not actin in the DI fraction was remarkably decreased on depletion of cholesterol from plasma membrane. Also, MβCD resulted in a parallel increase in the level of ASBT in the DS fraction. However, in the presence of cholesterol, the redistribution of ASBT from DI to DS fractions by MβCD was blocked. Treatment with MβCD led to an ~50% (Fig. 4B) decrease in the level of ASBT associated with DI fraction, whereas the level of actin remained unaltered. These findings strongly suggest the presence of ASBT in lipid rafts of human ileal BBMVs.
Fig. 4.
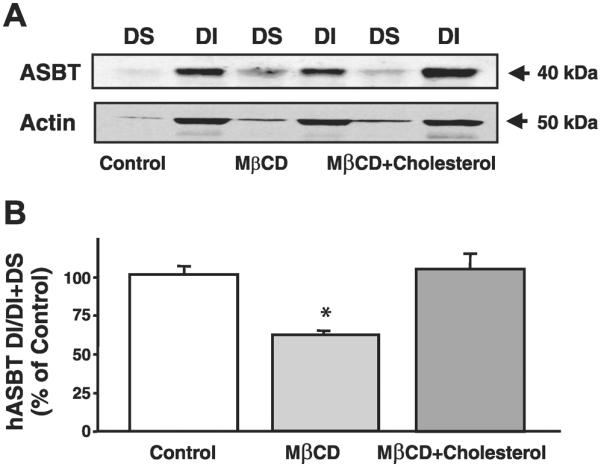
Human ASBT (hASBT) is associated with DI fractions of human ileal brush-border membrane vesicles (BBMVs). A: human ileal BBMVs were treated with MβCD in the presence or the absence of 1.25 mM cholesterol or with vehicle alone and solubilized with Triton X-100, and then DS and DI fractions were isolated as described in materials and methods. Equal amounts of proteins (~80 μg) from DI and DS fractions were separated by electrophoresis on 10% polyacrylamide gel and then analyzed by Western blotting for ASBT and actin expression. B: blots were scanned, and the densities of the bands were determined by densitometric analysis. y-Axis indicates the density of DI fractions from the Optiprep gradient. Data are expressed as arbitrary units with control set as 100% and are means ± SE of 4 separate experiments. *P < 0.05 compared with control.
We next examined the distribution of ASBT of human ileal BBMVs on Optiprep density gradient. In Fig. 5A, the Western blot shows the presence of ASBT protein in the floating fractions on the top of Optiprep density gradient, further indicating its presence in membrane lipid rafts. To confirm the collection of lipid rafts in the top floating fractions of the Optiprep gradient, we measured the enzymatic activity of alkaline phosphatase, a well-known marker for these microdomains (28). The specific activity of alkaline phosphatase throughout the density gradient is expressed as nanomoles per milligram of protein per minute. As shown in Fig. 5B, the specific activity of alkaline phosphatase was the highest in the top floating fractions, indicating that these fractions contain lipid rafts. Together, these data provide strong evidence showing that the majority of ASBT pool in the plasma membrane intestinal epithelial cells is associated with the DI lipid raft microdomains.
Fig. 5.
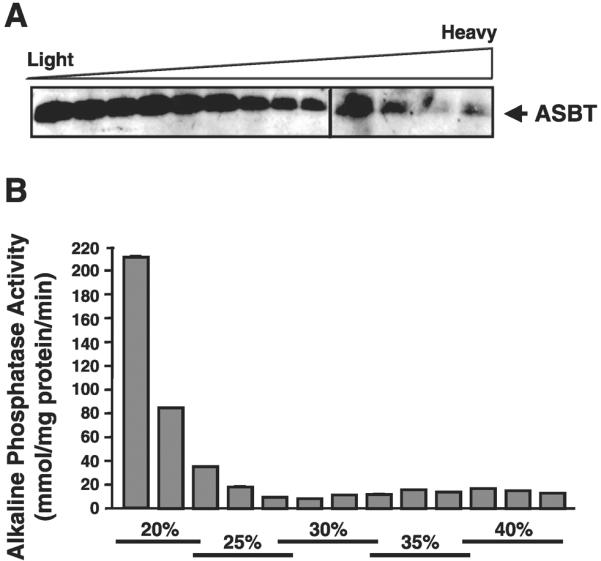
ASBT is detected in the floating fractions of human ileal BBMVs treated with Triton X-100 on Optiprep density gradient. A: Human ileal BBMVs were treated with 1% Triton X-100 for 30 min at 4°C and then were layered at the bottom of a discontinuous density gradient of Optiprep as described in materials and methods. After high-speed centrifugation, fractions were collected from the top to the bottom of the gradient, and their proteins were separated on 2 separate SDS-PAGE gels and analyzed simultaneously by Western blotting utilizing ASBT antibodies. The blots shown are a representative of 3 separate experiments. B: specific activity of alkaline phosphatase (a marker of lipid rafts) was assessed in each fraction of the Optiprep floatation gradient of human ileal BBMVs. Alkaline phosphatase activity is expressed as nmol•mg protein−1 •min−1, and data are means ± SE of 3 separate measurements from different occasions.
Effect of MβCD on ASBT activity in intestinal epithelial cells
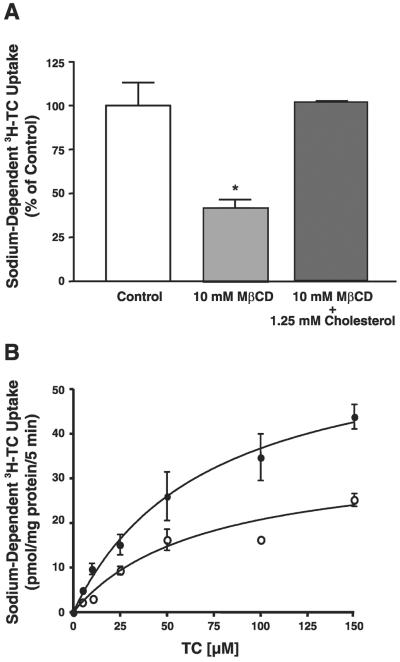
To further explore the significance of ASBT association with lipid rafts in intestinal epithelial cells, we investigated the effect of MβCD on ASBT function in the human intestinal Caco-2 cell line. Similar to the decrease in the function of ASBT in 2BT fibroblasts, incubation with MβCD for 1 h at 37°C also led to a significant reduction in the activity of the endogenously expressed ASBT in intestinal epithelial Caco-2 cells (Fig. 6A). Furthermore, Fig. 6A shows that the inhibition of ASBT function appears to be secondary to cholesterol depletion of plasma membrane as the presence of cholesterol blocked the inhibitory effect of MβCD on ASBT function. To better understand the mechanism by which incubation with MβCD decreases TC uptake, we evaluated its effect on the kinetic parameters of Na+-dependent TC uptake in Caco-2 cells. As shown in Fig. 6B, 1-h incubation with 10 mM MβCD significantly decreased the Vmax (63 ± 8 vs. 35 ± 4 pmol•mg protein−1 •5 min−1) of [3H]TC uptake compared with that shown for control, with no significant changes in the Km for TC. These observations indicate that the effect of MβCD may have occurred by reducing the number of active hASBT molecules rather than changing the affinity of the transporter for its substrate, TC. Furthermore, the data clearly indicate the critical role of plasma membrane cholesterol for optimal activity of ASBT in intestinal epithelial cells.
Fig. 6.
MβCD inhibits ASBT activity in human intestinal Caco-2 cells. A: postconfluent Caco-2 cells were incubated with either 10 mM MβCD alone or with 10 mM MβCD + 1.25 mM cholesterol for 1 h at 37°C. Na+-dependent [3H]TC uptake (10 μM) was then determined for 5 min. Results are presented as % of control and are means ± SE obtained from 5 separate experiments. *P < 0.05 compared with control. B: postconfluent Caco-2 cells were incubated with vehicle alone (●) or with 10 mM MβCD (◯) for 1 h at 37°C, and Na+-dependent TC uptake was performed in the presence of increasing concentrations of the substrate TC. Sodium-dependent TC uptake was expressed as pmol•mg protein−1 •5 min−1. Experiments were performed 4 times in triplicate in separate groups of cells. Michaelis-Menten plot of a representative experiment is shown. Results indicate a decrease in the Vmax of the transport process from 63 ± 8 (control) to 35 ± 4 pmol•mg protein−1 •5 min−1 in MβCD-treated cells.
To determine whether the reduction in ASBT activity is a result of cholesterol depletion rather than reduction in level of ASBT at the plasma membrane, we transiently transfected Caco-2 cells with ASBT-V5 fusion protein and then labeled surface proteins of apical membrane with biotin in the presence or the absence of MβCD. It should be emphasized that, although ASBT is endogenously expressed in Caco-2 cells, transfection with ASBT-V5 was utilized to obtain better membrane detection with anti-V5 antibodies. Biotinylated proteins were then precipitated with avidin, and the level of biotinylated (surface) ASBT-V5 fusion protein was assessed by Western blotting. Figure 7 also shows a representative densitometric analysis of three experiments demonstrating that the relative abundance of plasma membrane hASBT-V5 fusion protein normalized to its total expression (surface + intracellular expression of hASBT-V5) remains unchanged by cholesterol depletion of plasma membrane with MβCD. These findings indicate that the decrease in ASBT activity is likely to be a result of cholesterol depletion but not a consequence of a decrease in its level of expression on plasma membrane.
Fig. 7.
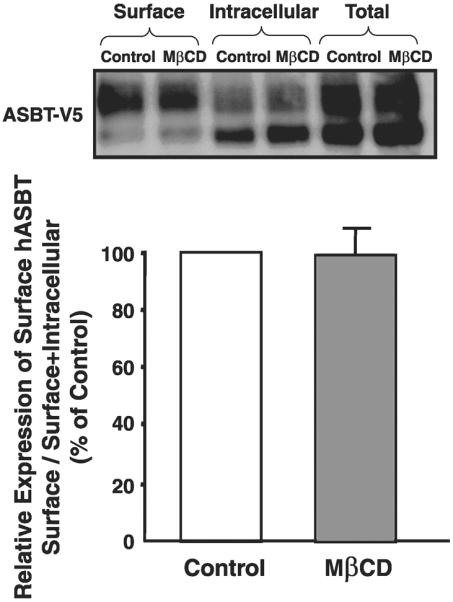
Plasma membrane expression of ASBT-V5 fusion protein in Caco-2 cells. Caco-2 cells were transfected with ASBT-V5 by electroporation utilizing Amaxa Nucleofector System as described in materials and methods; 24 h posttransfection, monolayers grown on plastic support were treated with 10 mM MβCD or vehicle alone for 1 h at 37°C and subjected to biotinylation at 4°C using sulfo-NHS-SS-biotin. Cells were then lysed, and surface biotinylated proteins were precipitated with streptavidin-agarose from equal amounts of total cellular protein. Precipitated proteins (surface) were separated on SDS-polyacrylamide gel electrophoresis and electroblotted to nitrocellulose blots. Western blotting analysis was performed with anti-V5. The relative abundance of ASBT-V5 fusion protein in the biotinylated fractions is shown and is expressed as the density of ASBT-V5 band normalized to the density of total hASBT-V5 (surface+intracellular). Data were obtained from 3 separate experiments.
MAPKs are not involved in the downregulation of hASBT function by cholesterol depletion
Recent studies demonstrated an increase in ERK1/2 phosphorylation by MβCD (45). Therefore, we investigated the possible role of ERK1/2 in the observed decrease in ASBT activity by cholesterol depletion. MβCD-induced inhibition of ASBT activity was unaltered in the presence of 30 μM of the ERK1/2 inhibitor PD-98059 (Na+-dependent [3H]TC uptake was 51 ± 13.7% and 59 ± 8.3% of control in response to 1-h incubation with 10 mM MβCD alone or 10 mM MβCD + 30 μM PD-98059, respectively). These findings rule out the involvement of ERK1/2-mediated signaling pathway in the MβCD-mediated reduction in ASBT function. Together, these data further support the notion that the reduction in ASBT function results from the depletion of plasma membrane cholesterol.
DISCUSSION
Lipid rafts are specialized microdomains of the plasma membrane that are essential for the normal functioning of various membrane transporters (6, 40, 41). These microdomains of the plasma membranes are widely viewed as platforms that selectively cluster signaling molecules with their target proteins (21, 29), permitting their regulation and modulation by membrane trafficking events (6, 37). The involvement of lipid rafts in hepatic bile secretion has recently been revealed (44); however, their role in intestinal bile acid absorption is yet to be elucidated. In the present study, we provide compelling novel evidence demonstrating the association of ASBT with lipid raft microdomains of luminal membranes of intestinal epithelial cells. Furthermore, our findings demonstrate the modulation of ASBT activity by cholesterol depletion of the membranes.
Utilizing Optiprep floatation and Triton X-100 insolubility, we have demonstrated that ASBT is associated with lipid rafts. First, we utilized HEK-293 cells as an in vitro model to establish the association of ASBT with lipid rafts. HEK-293 cells have been previously described as a suitable model to elucidate the influence of lipid rafts on function of membrane transporters (32, 38). Because HEK-293 cells express low levels of endogenous ASBT (23), we generated HEK-293 cells that stably overexpress ASBT-V5 fusion protein, designated as 2BT cells, and evaluated ASBT-V5 localization with lipid rafts. The presence of ASBT-V5 fusion protein in the high-buoyancy fractions of the density gradient provided strong evidence for the association of ASBT with lipid raft microdomains. Interestingly, the floatation of ASBT-V5 was reduced when lipid rafts were disrupted by cholesterol removal on treatment with MβCD. The fact that actin was not recovered in the floating fractions negates the possibility that ASBT floated as a result of insufficient detergent solubilization of 2BT membranes. These findings also suggest that floatation of ASBT-containing membrane microdomains in 2BT cells is dependent on its content of cholesterol.
We also examined the partitioning of ASBT in human ileal BBMVs extracted from native intestinal epithelium. Lipid raft-associated proteins are generally detected in DI fractions of plasma membrane (28, 30). Interestingly, hASBT polypeptide was predominantly recovered in Triton-X 100 DI fractions of human ileal BBMVs. Disruption of lipid rafts by cholesterol depletion from BBMVs utilizing MβCD exclusively reduced the level of ASBT (but not actin) in DI fraction. The association of ASBT with lipid rafts was also confirmed based on their high buoyancy on the Optiprep density gradient. ASBT protein was present in the fractions that were also enriched with alkaline phosphatase, a marker of lipid rafts in the intestine (28). It should be noted that the distribution of hASBT in the DI and DS fractions is different in BBMVs compared with that shown in 2BT cells (Fig. 4A compared with Fig. 1B). In fact, the partitioning of hASBT in DI and DS fractions of Caco-2 cells was similar to that observed in 2BT cells (data not shown). This difference in distribution of hASBT in DS and DI fractions in BBMVs and cell culture models might be attributed to the fact that DS and DI fractions were isolated from crude membrane preparations from total cell lysate containing plasma membrane and other cellular membranous structures. On the other hand, BBMVs represent only the purified brush-border membranes of intestinal epithelial cells.
Our findings demonstrating the presence of ASBT in lipid rafts of plasma membrane of 2BT cells and human ileal BBMVs might indicate that the basal activity of ASBT is influenced by lipid composition of these microdomains. In this regard, previous studies demonstrated a crucial role of plasma membrane cholesterol in sustaining the basal activity of several intestinal transporters such as the apical sodium/hydrogen exchanger NHE3 (33) and basolateral calcium-activated potassium (BK) channels (25). Various methods have been previously utilized to disrupt lipid raft domains by sequestering cellular and plasma membrane cholesterol to characterize the functional roles (18). MβCD treatment of living cells or membrane preparations has been the most widely employed method for rapid and highly efficient extraction of cellular and membrane cholesterol (11, 18, 26). We determined the effect of MβCD on ASBT activity in both 2BT cells and human intestinal Caco-2 cells. Interestingly, incubation of 2BT cells with MβCD profoundly reduced the activity of the exogenously expressed ASBT in a cholesterol-dependent manner, and the inhibition was reversed in the presence of cholesterol. Similarly, treatment of human intestinal Caco-2 cells with MβCD also led to a significant decrease in the endogenously expressed ASBT function.
Although the extraction of cholesterol could be toxic to the cells, however, previous studies in HEK-293 and Caco-2 cells have clearly shown that MβCD treatment similar to the one used in the present study did not affect cell viability (26, 32). Interestingly, it has been reported that Caco-2 cells are relatively resistant to the cholesterol-depleting effect of MβCD compared with other cell lines, such as Madin-Darby canine kidney and HEK-293 cells, which might be because of the presence of high levels of glycosphingolipids in the brush-border membrane of intestinal epithelial cells, including Caco-2 cells (26). This resistance is reflected in our present experiments by the fact that longer incubation with MβCD (1 h) was required to attain significant changes in detergent solubility of ASBT of human ileal BBMVs and to trigger significant alterations in the activity of ASBT in Caco-2 cells. On the other hand, 30-min incubation was sufficient to produce the same effects in HEK-293 cells. Also, treatment of human BBMVs with MβCD altered the floatation of hASBT on Optiprep density gradient (data not shown) but to a lower degree than that shown with 2BT cells. These observations further indicate that lipid raft microdomains in the brush-border membrane of intestinal epithelial cells are more resistant to the cholesterol-depleting effect of MβCD than HEK-293 fibroblasts.
It has been previously demonstrated that incubation of polarized Caco-2 cells with MβCD disrupts the barrier function of the epithelial monolayer (26). However, this effect is unlikely to confound the observed inhibition of ASBT function in response to MβCD because ASBT was exclusively localized to the apical membrane of Caco-2 cells. Thus, even in cases of permeability changes, ASBT activity determined as Na+-sensitive component of [3H]TC uptake into the cells will remain the same whether cells are exposed to [3H]TC from apical side or both apical and basolateral sides.
With respect to the mechanism of MβCD-induced inhibition of ASBT function, kinetic analysis suggested that cholesterol depletion caused a significant reduction in the Vmax of the transporter, whereas the affinity of ASBT for TC remained unchanged. These findings indicated a decrease in the number of active molecules of ASBT on the apical membranes of Caco-2 cells. Interestingly, membrane biotinylation studies of Caco-2 cells showed that reduction in ASBT activity was not accompanied by a parallel decrease in membrane expression of ASBT. Furthermore, cholesterol depletion resulted in a redistribution of ASBT protein between DI and DS fractions of luminal ileal BBMVs, and it is likely that MβCD also caused a redistribution of ASBT between different microdomains of the apical plasma membrane of Caco-2 cells. One possibility is that hASBT inhibition by MβCD could be a result of activation of intracellular signal transduction pathway. In this regard, recent studies have shown cholesterol depletion to activate the ERK1/2 pathway (45). Our results utilizing the ERK inhibitor, however, ruled out the involvement of ERK-dependent pathway in the MβCD-mediated effects on ASBT function. Altogether, these findings suggest that disruption of lipid rafts by cholesterol removal in response to MβCD redistributes ASBT to the DS fractions of plasma membranes, causing a reduction in its activity, rather than changes in overall surface expression of ASBT molecules and not as a consequence of activating intracellular signal-transduction pathway.
Recent studies have shown that lipid content of the brush-border membrane of intestinal epithelial cells is altered by type of diet. In this regard, Ma et al. (31) demonstrated a reduction in the level of cholesterol in murine colonic brush-border membrane in response to diet enriched with n-3 polyunsaturated fatty acids. In light of these previous findings, the observed reduction in the activity of ASBT by cholesterol depletion may represent a novel mechanism by which intestinal bile acid absorption via ASBT could be modulated by diet composition that alters lipid and cholesterol content of apical membrane of epithelial cells. This speculation may require further in vivo investigations to confirm that ASBT activity and association with lipid rafts is altered in response to n-3 polyunsaturated fatty acids.
In conclusion, our study demonstrated the distribution of ASBT in DS and DI fractions of apical plasma membranes of intestinal epithelial cells. The DI pool of ASBT appears to be associated with lipid raft microdomains. We further demonstrated that the disruption of these microdomains by cholesterol depletion leads to an inhibition of ASBT function. Our studies demonstrate a vital role of plasma membrane cholesterol for optimal function of ASBT and suggest a novel mechanism by which ASBT activity may be rapidly modulated in response to physiological postprandial changes between meals and in response to the content of the diet.
Acknowledgments
GRANTS These studies were supported by the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-71596 (W. A. Alrefai), DK-54016 and DK-68324 (P. K. Dudeja), DK-61931 and DK-68271 (J. R. Turner), and PO1 DK-067887 (P. K. Dudeja, J. R. Turner).
REFERENCES
- 1.Akhter S, Cavet ME, Tse CM, Donowitz M. C-terminal domains of Na+/H+ exchanger isoform 3 are involved in the basal and serum-stimulated membrane trafficking of the exchanger. Biochemistry. 2000;39:1990–2000. doi: 10.1021/bi991739s. [DOI] [PubMed] [Google Scholar]
- 2.Alpini G, Glaser S, Baiocchi L, Francis H, Xia X, Lesage G. Secretin activation of the apical Na+-dependent bile acid transporter is associated with cholehepatic shunting in rats. Hepatology. 2005;41:1037–1045. doi: 10.1002/hep.20653. [DOI] [PubMed] [Google Scholar]
- 3.Alpini G, Glaser SS, Rodgers R, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology. 1997;113:1734–1740. doi: 10.1053/gast.1997.v113.pm9352879. [DOI] [PubMed] [Google Scholar]
- 4.Alrefai WA, Saksena S, Tyagi S, Gill RK, Ramaswamy K, Dudeja PK. Taurodeoxycholate modulates apical Cl−/OH− exchange activity in Caco2 cells. Dig Dis Sci. 2007;52:1270–1278. doi: 10.1007/s10620-006-9090-8. [DOI] [PubMed] [Google Scholar]
- 5.Alrefai WA, Sarwar Z, Tyagi S, Saksena S, Dudeja PK, Gill RK. Cholesterol modulates human intestinal sodium-dependent bile acid transporter. Am J Physiol Gastrointest Liver Physiol. 2005;288:G978–G985. doi: 10.1152/ajpgi.00379.2004. [DOI] [PubMed] [Google Scholar]
- 6.Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 7.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 8.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 9.Chen F, Ma L, Sartor RB, Li F, Xiong H, Sun AQ, Shneider B. Inflammatory-mediated repression of the rat ileal sodium-dependent bile acid transporter by c-fos nuclear translocation. Gastroenterology. 2002;123:2005–2016. doi: 10.1053/gast.2002.37055. [DOI] [PubMed] [Google Scholar]
- 10.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- 12.Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH, Dawson PA. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol Gastrointest Liver Physiol. 1998;274:G157–G169. doi: 10.1152/ajpgi.1998.274.1.G157. [DOI] [PubMed] [Google Scholar]
- 13.Del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 14.Dudeja PK, Baldwin ML, Harig JM, Cragoe EJ, Jr, Ramaswamy K, Brasitus TA. Mechanisms of Na+ transport in human distal colonic apical membrane vesicles. Biochim Biophys Acta. 1994;1193:67–76. doi: 10.1016/0005-2736(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 15.Gill RK, Saksena S, Alrefai WA, Sarwar Z, Goldstein JL, Carroll RE, Ramaswamy K, Dudeja PK. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol. 2005;289:C846–C852. doi: 10.1152/ajpcell.00112.2005. [DOI] [PubMed] [Google Scholar]
- 16.Guan JL. Cell biology. Integrins, rafts, Rac, and Rho. Science. 2004;303:773–774. doi: 10.1126/science.1094376. [DOI] [PubMed] [Google Scholar]
- 17.Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflügers Arch. 2004;447:566–570. doi: 10.1007/s00424-003-1130-z. [DOI] [PubMed] [Google Scholar]
- 18.Hansen GH, Immerdal L, Thorsen E, Niels-Christiansen LL, Nystrom BT, Demant EJ, Danielsen EM. Lipid rafts exist as stable cholesterol-independent microdomains in the brush border membrane of enterocytes. J Biol Chem. 2001;276:32338–32344. doi: 10.1074/jbc.M102667200. [DOI] [PubMed] [Google Scholar]
- 19.Hanwell D, Ishikawa T, Saleki R, Rotin D. Trafficking and cell surface stability of the epithelial Na+ channel expressed in epithelial Madin-Darby canine kidney cells. J Biol Chem. 2002;277:9772–9779. doi: 10.1074/jbc.M110904200. [DOI] [PubMed] [Google Scholar]
- 20.Harig JM, Dudeja PK, Knaup SM, Shoshara J, Ramaswamy K, Brasitus TA. Apical plasma membrane vesicles formed from organ donor colon demonstrate Na+ and H+ conductances and Na+/H+ exchange. Biochem Biophys Res Commun. 1990;167:438–443. doi: 10.1016/0006-291x(90)92042-x. [DOI] [PubMed] [Google Scholar]
- 21.Hoessli DC, Ilangumaran S, Soltermann A, Robinson PJ, Borisch B, Nasir-Ud D. Signaling through sphingolipid microdomains of the plasma membrane: the concept of signaling platform. Glycoconj J. 2000;17:191–197. doi: 10.1023/a:1026585006064. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 23.Jung D, Fried M, Kullak-Ublick GA. Human apical sodium-dependent bile salt transporter gene (SLC10A2) is regulated by the peroxisome proliferator-activated receptor alpha. J Biol Chem. 2002;277:30559–30566. doi: 10.1074/jbc.M203511200. [DOI] [PubMed] [Google Scholar]
- 24.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Lam RS, Shaw AR, Duszyk M. Membrane cholesterol content modulates activation of BK channels in colonic epithelia. Biochim Biophys Acta. 2004;1667:241–248. doi: 10.1016/j.bbamem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Lambert D, O'Neill CA, Padfield PJ. Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight-junction proteins. Biochem J. 2005;387:553–560. doi: 10.1042/BJ20041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazaridis KN, Pham L, Tietz P, Marinelli RA, deGroen PC, Levine S, Dawson PA, LaRusso NF. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest. 1997;100:2714–2721. doi: 10.1172/JCI119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Galli T, Leu S, Wade JB, Weinman EJ, Leung G, Cheong A, Louvard D, Donowitz M. Na+-H+ exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3. J Physiol. 2001;537:537–552. doi: 10.1111/j.1469-7793.2001.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Leu S, Cheong A, Zhang H, Baibakov B, Shih C, Birnbaum MJ, Donowitz M. Akt2, phosphatidylinositol 3-kinase, and PTEN are in lipid rafts of intestinal cells: role in absorption and differentiation. Gastroenterology. 2004;126:122–135. doi: 10.1053/j.gastro.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Zhang H, Cheong A, Leu S, Chen Y, Elowsky CG, Donowitz M. Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent. J Physiol. 2004;556:791–804. doi: 10.1113/jphysiol.2004.060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma DW, Seo J, Switzer KC, Fan YY, McMurray DN, Lupton JR, Chapkin RS. n-3 PUFA and membrane microdomains: a new frontier in bioactive lipid research. J Nutr Biochem. 2004;15:700–706. doi: 10.1016/j.jnutbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Magnani F, Tate CG, Wynne S, Williams C, Haase J. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. J Biol Chem. 2004;279:38770–38778. doi: 10.1074/jbc.M400831200. [DOI] [PubMed] [Google Scholar]
- 33.Murtazina R, Li X. Donowitz M. Brush border (BB) NHE3 activity is lipid rafts dependent (Abstract) Gastroenterology. 2004;126:A295. [Google Scholar]
- 34.Nichols BJ. GM1-containing lipid rafts are depleted within clathrin-coated pits. Curr Biol. 2003;13:686–690. doi: 10.1016/s0960-9822(03)00209-4. [DOI] [PubMed] [Google Scholar]
- 35.Potter GD. Bile acid diarrhea. Dig Dis. 1998;16:118–124. doi: 10.1159/000016855. [DOI] [PubMed] [Google Scholar]
- 36.Reymann A, Braun W, Drobik C, Woermann C. Stimulation of bile acid active transport related to increased mucosal cyclic AMP content in rat ileum in vitro. Biochim Biophys Acta. 1989;1011:158–164. doi: 10.1016/0167-4889(89)90203-6. [DOI] [PubMed] [Google Scholar]
- 37.Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40:10507–10513. doi: 10.1021/bi010730z. [DOI] [PubMed] [Google Scholar]
- 39.Schlattjan JH, Benger S, Herrler A, von Rango U, Greven J. Regulation of taurocholate transport in freshly isolated proximal tubular cells of the rat kidney by protein kinases. Nephron Physiol. 2005;99:35–42. doi: 10.1159/000082870. [DOI] [PubMed] [Google Scholar]
- 40.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 41.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 42.Sun AQ, Balasubramaniyan N, Liu CJ, Shahid M, Suchy FJ. Association of the 16-kDa subunit c of vacuolar proton pump with the ileal Na+-dependent bile acid transporter: protein-protein interaction and intracellular trafficking. J Biol Chem. 2004;279:16295–16300. doi: 10.1074/jbc.M312838200. [DOI] [PubMed] [Google Scholar]
- 43.Sun AQ, Salkar R, Sachchidanand, Xu S, Zeng L, Zhou MM, Suchy FJ. A 14-amino acid sequence with a beta-turn structure is required for apical membrane sorting of the rat ileal bile acid transporter. J Biol Chem. 2003;278:4000–4009. doi: 10.1074/jbc.M207163200. [DOI] [PubMed] [Google Scholar]
- 44.Tietz P, Jefferson J, Pagano R, Larusso NF. Membrane microdomains in hepatocytes: potential target areas for proteins involved in canalicular bile secretion. J Lipid Res. 2005;46:1426–1432. doi: 10.1194/jlr.M400412-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Wang PY, Weng J, Anderson RG. OSBP is a cholesterol-regulated scaffolding protein in control of ERK 1/2 activation. Science. 2005;307:1472–1476. doi: 10.1126/science.1107710. [DOI] [PubMed] [Google Scholar]
- 46.Wong MH, Oelkers P, Dawson PA. Identification of a mutation in the ileal sodium-dependent bile acid transporter gene that abolishes transport activity. J Biol Chem. 1995;270:27228–27234. doi: 10.1074/jbc.270.45.27228. [DOI] [PubMed] [Google Scholar]
- 47.Zhang EY, Phelps MA, Banerjee A, Khantwal CM, Chang C, Helsper F, Swaan PW. Topology scanning and putative three-dimensional structure of the extracellular binding domains of the apical sodium-dependent bile acid transporter (SLC10A2) Biochemistry. 2004;43:11380–11392. doi: 10.1021/bi049270a. [DOI] [PubMed] [Google Scholar]