Abstract
Interactions between motivation and cognition are implicated in producing functional impairments and poor quality of life in psychiatric patients. This interaction, however, is not well understood at either the behavioral or neural level. We developed a procedure for mice in which a cognitive measure, sustained attention, is modulated by a motivationally-relevant signal which predicts reward probability on a trial-by-trial basis. Using this paradigm, we tested the interaction between motivation and cognition in mice which model the increased striatal D2 receptor activity observed in schizophrenia patients (D2R-OE mice). In control mice, attention was modulated by signaled-reward probability. In D2R-OE mice, however, attention was not modulated by reward-related cues. This impairment was not due to any global deficits in attention or maintenance of the trial-specific information in working memory. Turning off the transgene in D2R-OE mice rescued the motivational modulation of attention. These results indicate that deficits in motivation impair the ability to use reward-related cues to recruit attention, and that improving motivation improves functional cognitive performance. These results further suggest that addressing motivational impairments in patients is critical to achieving substantive cognitive and functional gains.
Keywords: Dopamine D2 expression, motivation, attention, schizophrenia, cognition motivation interactions, mouse
Introduction
Impairments of motivation (the energizing of behavior in pursuit of a desired goal) and cognition are symptomatic of psychiatric diseases such as schizophrenia (Barch and Dowd, 2010; Gold, 2004; Heinrichs and Zakzanis, 1998; Kerns et al., 2008; Medalia and Brekke, 2010), and severity of this impairment is a significant predictor of functional outcomes (Bowie and Harvey, 2006; Green, 1996, 2006; Green and Nuechterlein, 2004; Ho et al., 1998). Furthermore, it is suggested that motivational impairments could interact with, or exacerbate, cognitive and functional impairments (Barch and Dowd, 2010; Barch, 2005; Gard et al., 2007; Nakagami et al., 2008). Thus, studying the neurobiology of the interaction between motivation and cognition is critical for understanding functional impairments in psychiatric disease and in the search for treatments.
Although the neurobiology of both motivation and cognition have long been topics of research interest, the specific functional neurocircuitry by which motivation impacts cognition has not been widely studied and remains poorly understood. The goal of the present study was to employ an experimental paradigm which explicitly assesses the interaction between motivation and cognition. We focused on the interaction between motivation and attention because attention is a fundamental cognitive process that modulates many other cognitive processes and is commonly impaired in patients (Heinrichs and Zakzanis, 1998). To do this, we modified a prefrontal-dependent visual-discrimination task that assesses sustained attention (Kahn et al., 2012) to signal the probability of reward for accurate cue detection on a trial-by-trial basis. Conceptually similar studies have been used in other species. For example, Burton et al. (2013) recently showed that signaling the size of potential rewards increases accuracy in a two-choice odor-discrimination paradigm in rats. Being able to assay the impact of motivation by signaling the probability of reward on a cognitive measure, sustained attention, in mice will allow us, and others, to take advantage of the methodological manipulations that have been optimized for use in mice to further investigate the relationship between these psychological processes.
Using this task as a methodological basis, we tested the interaction between motivation and attention in an animal model of the negative and cognitive symptoms of schizophrenia. We had earlier found a deficit in anticipatory motivation similar to that described in patients in a transgenic mouse model created to investigate the consequences of increased striatal D2 receptor activity (D2R-OE mice; Drew et al., 2007; Simpson et al., 2011; Ward et al., 2012), which is thought to be an important aspect of the pathophysiology of schizophrenia (Abi-Dargham et al., 2000). Specifically, we found that D2R-OE mice are impaired in their ability to anticipate the value of future rewards, and that this deficit is associated with poor cognitive performance (Ward et al., 2012). In addition, we found that the deficit in anticipatory motivation is reversible as it is rescued when the transgene is turned off. We therefore applied our behavioral task to test the following hypotheses: 1) this deficit in anticipatory motivation would compromise the ability of motivationally-significant cues to modulate attention in D2R-OE mice, and 2) modulation of attention by motivationally-significant cues would be restored after D2 expression is normalized.
Methods
Mice
Adult male mice in all experiments were F1 hybrids of the C57BL/6J and 129Svev (Tac) background strain. D2R-OE mice overexpress dopamine D2 receptors selectively in the post-synaptic medium spiny neurons of the striatum. Mice were bred and maintained as previously described (Drew et al., 2007; Simpson et al., 2011; Ward et al., 2012; Ward et al., 2009; Kellendonk et al., 2006; see Supplemental Information). All housing, breeding, and testing was compliant with the local IACUC guidelines.
Apparatus
Operant chambers (Med-Associates, St. Albans, VT; model ENV-307w) were used in all behavioral testing and have been described previously (Kahn et al., 2012; Ward et al., 2012; details in Supplemental Information).
Experimental procedures
A table showing all procedures and conditions completed by each cohort of mice is available in the Supplemental Information. Mice were first trained to consume the liquid reward from the dipper and to lever press as described previously (Ward et al., 2012). Discrimination training then occurred in several phases as described in Kahn et al. (2012; see Supplemental Information). A schematic of the final version of the sustained-attention task is shown in Figure 1. In this task, mice earn rewards for responding on one of two levers that has been cued at the beginning of the trial.
Figure 1.
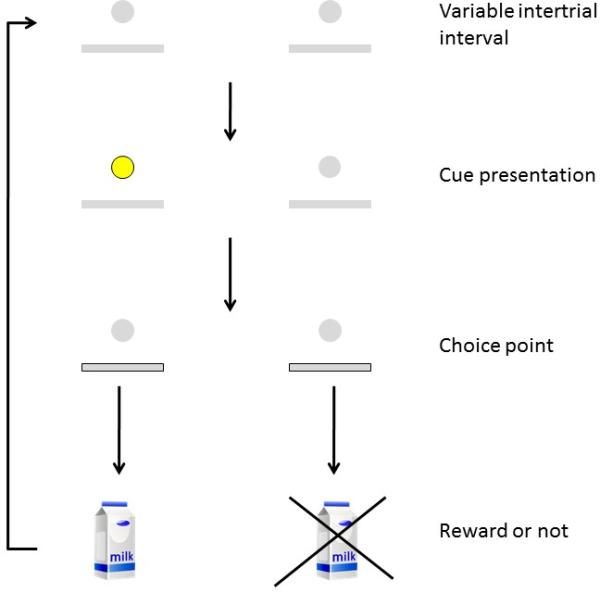
Schematic of the sustained-attention task. Trials began with both response levers retracted and a variable-duration inter-trial interval (mean = 45 s). Following the ITI, a cue light was lit above the position of either the left or right lever (p=0.5). Following cue presentation, choice levers were inserted, and a response on the lever that had been cued at the beginning of the trial resulted in a presentation of a drop of condensed milk via the dipper. Incorrect responses had no programmed consequences.
Signaled-probability condition with varying cue durations
A cohort of seven control mice performed the sustained-attention task (Figure 1), but the probability of reward for a correct choice response (either 1.0 or 0.1) on each upcoming trial was signaled on a trial-by-trial basis by turning the houselight on or off during the ITI (counterbalanced across mice such that houselight on signaled high reward probability and houselight off signaled low reward probability and vice versa). Mice received an equal number of high and low reward-probability trials per session (34 each). High and low reward-probability trials were presented pseudorandomly with the constraint that no more than four consecutive trial types of the same reward probability could be presented in a row. Only one cue duration was presented on all trials within each session.
Sustained-attention task (across session)
A separate cohort of 10 control and 10 D2R-OE mice performed the sustained-attention task with varying cue-light durations, presented in decreasing order (10 s, 5 s, 2 s, 1 s, 0.5 s, 0.1 s), 3 consecutive sessions for each duration.
Working-memory maintenance task
After completing the sustained-attention task, this same cohort of mice were retrained to a stable and high level of baseline performance. Mice were then tested with increasing delay intervals (2 s, 4 s, 8 s, 16 s, 24 s, 32 s, 40 s) between the cue offset and the lever presentation. Each delay duration was in place for 3 consecutive sessions.
Sustained-attention task (within session)
A separate cohort of 11 control and 10 D2R-OE mice were tested in the sustained-attention task, but the duration of the cue light was varied within instead of across session. Cue durations of 10 and 2 s were randomly chosen each trial with the constraint that each trial type appeared equally often.
Signaled-probability condition with 2-s cue duration in D2R-OE and control mice
A separate cohort of mice that consisted of 15 D2R-OE and 17 control littermates were tested in the signaled-probability paradigm for 14 sessions with a cue duration of 2 s. Data presented below are the average of the last two sessions.
Doxycycline rescue condition
Following the final signaled-probability condition, this same cohort of mice were treated with doxycycline for 2 weeks, after which they were retested in the signaled-probability condition for an additional 13 sessions. The data presented represent the average of the last two sessions.
Data analysis
The main data of interest was the proportion of correct responses. We also analyzed latency to make a choice response and latency to retrieve rewards during the signaled-probability conditions. For statistical comparison, repeated-measures analyses of variance with appropriate factors were conducted, followed by Bonferroni post-hoc tests where appropriate. Individual means were compared using paired samples t-tests. Latency to retrieve rewards was compared using a between subjects t-test.
Results
Signaling reward probability impacts discrimination accuracy
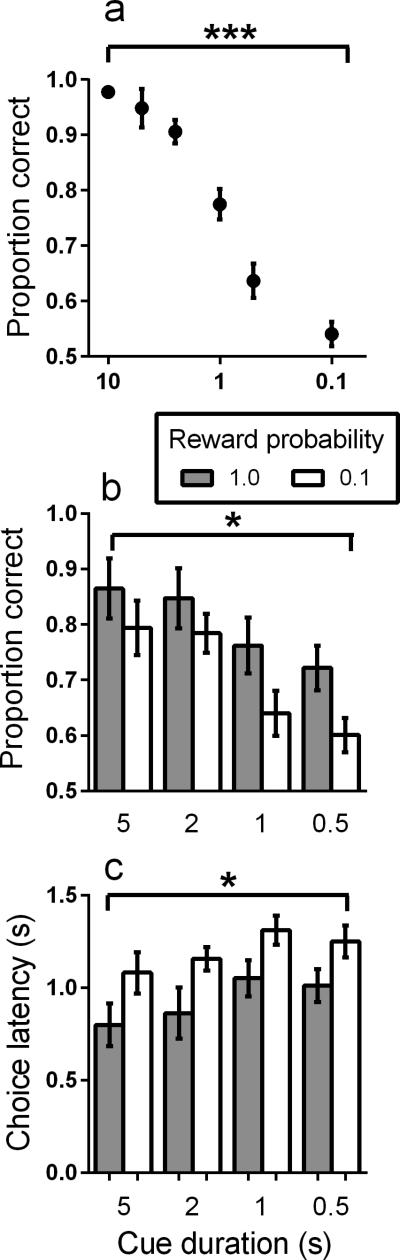
We have previously reported on the acquisition of the sustained-attention task in control mice (Kahn et al., 2012). As in that previous study, by the end of training, discrimination accuracy of the mice in this experiment was high (>.94 proportion correct). Decreasing cue duration decreased discrimination accuracy (Figure 2a) until mice performed at near-chance levels at the shortest cue duration (effect of cue duration; F(5,30) = 56.76, p<0.0001). These results confirm that this task is sensitive to the increased attentional demand of shorter cues.
Figure 2.
A. Proportion correct during the sustained attention task as a function of decreasing cue duration. Cue duration was decreased across sessions. X-axis is on a logarithmic scale. B. Proportion correct as a function of decreasing cue duration and signaled-reward probability. C. Latency to make a choice response as a function of decreasing cue duration and signaled-reward probability. (N=7). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
In the signaled-probability condition, as in Figure 2a, overall, the accuracy of discrimination declined with decreasing cue duration (Figure 2b) (effect of cue duration; F(3,24) = 12.17, p<0.0001). In addition, accuracy was greater on high reward-probability trials than on low reward-probability trials (effect of reward probability; F(1,24) = 6.06, p=0.02), and there was no interaction between cue duration and reward probability (F(3,24) = 0.17, p=0.91)). These data demonstrate that signaling an increase in reward probability improves attention.
To further examine the effects of signaled-reward probability, we calculated choice-response latencies. Figure 2c shows that, across all cue durations tested, latency to respond was shorter on high reward-probability trials than on low-probability trials (F(1,24) = 11.71, p=0.002), perhaps directly reflecting increased motivation to respond when the probability of reward is high. Latencies were longer with decreasing cue duration (F(3,24) = 3.15, p=0.04) and there was no interaction between cue duration and reward probability (F(3,24) = 0.02, p=0.99)).
D2R-OE mice have no deficit in sustained attention or working-memory maintenance
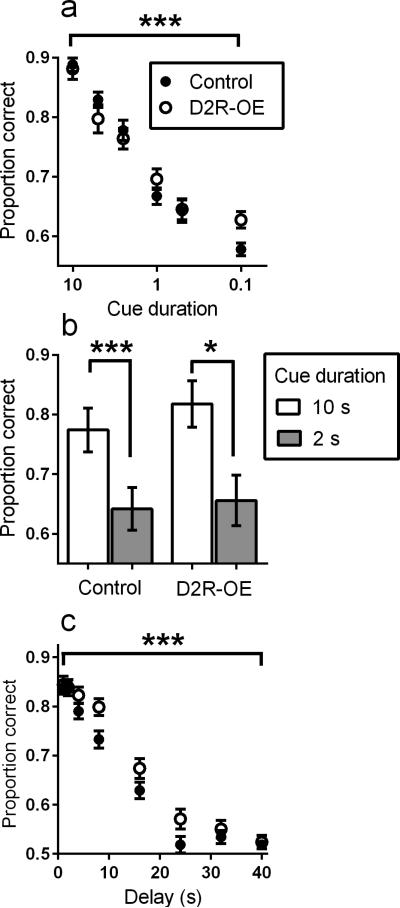
Before exploring whether motivationally-significant cues modulate attention in D2R-OE mice, we first determined if there was a baseline deficit in sustained attention in D2R-OE mice. As shown in Figure 3a, sustained attention of both control and D2R-OE mice declined as cue duration decreased across sessions (F(5,18)=59.76, p<0.0001), but there was no difference in performance between genotypes (F(1,18)=0.06, p=0.86), with no interaction (F(5,18)=1.14, p=0.35)). Similar results were obtained when two cue durations were manipulated within session (Figure 3b), There was a significant effect of cue duration (F(1,19)=37.91, p<0.0001), but not of genotype (F(1,19)=0.35, p=0.56), with no interaction (F(1,19)=0.38, p=0.55)). Thus, sustained attention does not differ between D2R-OE and control mice.
Figure 3.
A. Proportion correct during the sustained-attention task as a function of decreasing cue duration for control and D2R-OE mice. Cue duration was decreased across sessions. X-axis is on a logarithmic scale. Control N=10, D2R-OE N=10. B. Proportion correct as a function of decreasing cue duration (within session) for control and D2R-OE mice. Control N=11, D2R-OE N=10. C. Proportion correct as a function of increasing delay between cue presentation and presentation of the choice levers for control and D2R-OE mice. Delay duration was increased across sessions. Control N=10, D2R-OE N=10. Other details as in Figure 2.
To earn rewards in this task, mice must maintain a representation of the cue that signals the correct lever during the choice period. A deficit in this ability would complicate the interpretation of results from the signaled-probability paradigm. We therefore tested working-memory maintenance in D2R-OE mice. Discrimination accuracy declined in both control and D2R-OE mice with increasing retention interval (Figure 3c; F(7,18)=132.10, p<0.0001) reaching a floor at around 24 s, but there was no significant difference between genotypes (F(1,18)=3.22, p=0.09) and no interaction (F(7,18)=1.07, p=0.39)). These results indicate that D2R-OE mice are not impaired in maintaining the specific cue information in working memory that is required in the signaled-probability task.
Attention of D2R-OE mice is not modulated by reward signals
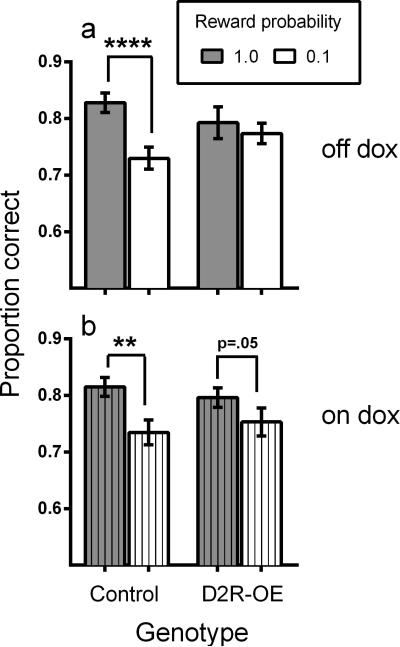
Figure 4a shows that, as we saw in the previous cohort of mice (Figure 2b), signaling the probability of reward modulated accuracy in control mice, with higher accuracy on high reward-probability trials than on low reward-probability trials. In contrast, signaling the probability of reward had little impact on performance of D2R-OE mice (Figure 4a). An ANOVA found a significant effect of reward probability (F(1,30)=11.26, p=0.002), but not of genotype (F(1,30)=0.03, p=0.86), with a significant interaction (F(1,30)=5.10, p=0.03). Post-hoc comparisons showed a significant effect of reward probability on accuracy for control (t(16)=2.59, p=0.02) but not D2R-OE mice (t(14)=0.60, p=0.56).
Figure 4.
A. Proportion correct as a function of signaled-reward probability for control and D2R-OE mice. B. Proportion correct as a function of signaled-reward probability for control and D2R-OE mice following doxycycline treatment (see text for details). Control N=17, D2R-OE N=15. Other details as in Figure 2.
Consistent with our previous experiment (Figure 2c), we found that choice latency was significantly decreased in trials with a higher probability of reward (F(1,30)=9.16, p=0.005). Choice latency was not affected by genotype (F(1,30)=0.89, p=0.35), and there was no interaction between genotype and reward probability (F(1,30)=3.65, p=0.07). We also analyzed the percentage of trials in which mice failed to make a choice response (omissions). Overall, mice completed the vast majority of trials (>95%), but completed significantly fewer low reward-probability trials (effect of reward probability; (F(1,30)=24.45, p=0.000)), indicating decreased motivation to participate on these trials. D2R-OE mice completed significantly fewer trials overall than controls (effect of genotype; (F(1,30)=4.79, p=0.036)), consistent with our previous reports of decreased motivation in these mice. The interaction between reward probability and genotype was not significant (F(1,30)=2.51, p=0.12)).
Finally, we found no statistically significant difference between control and D2R-OE mice in the latency to retrieve the dipper reward (t(30)=0.67, p=0.51), suggesting that D2R-OE and control mice were equally interested in consuming the milk reward.
Turning off transgene expression rescues modulation of attention by reward signals in D2R-OE mice
To determine if the deficit in modulation of attention by signaled-reward probability in D2R-OE mice was caused by D2R overexpression, we turned off the transgene by treating the same cohort of mice with doxycycline for two weeks and then retested them in the signaled-probability assay. Figure 4b shows that, as in Figures 2b and 4a, signaling the probability of reward resulted in differential discrimination accuracy in control mice. The modulation of attention by signaled-reward probability was restored in D2R-OE mice treated with doxycycline (effect of reward probability; (F(1,30)=14.04, p=0.001)), resulting in no effect of genotype (F(1,30)=0.00, p=0.997)) and no genotype-probability interaction (F(1,30)=1.27, p=0.27)). These results were further confirmed by a repeated-measures ANOVA comparing the discrimination accuracy in the sessions prior to doxycycline treatment and the discrimination accuracy during the doxycycline treatment (when the transgene was switched off). This ANOVA found a significant doxycycline-genotype interaction F(1,30)=5.25, p=0.029)).
Normalization of D2R levels also rescued motivation as indexed by the percentage of trials omitted. As before, mice omitted significantly more low-reward probability trials (F(1,30)=32.28, p=0.000), but there was no difference in the percentage of trials omitted between genotypes (F(1,30)=1.51, p=0.229), with no interaction (F(1,30)=0.10, p=0.76)).
Taken together, the data in Figure 4 indicate that modulation of attention by signaled-reward probability is impaired in D2R-OE mice, and is rescued when D2R levels (and motivation) are normalized following doxycycline treatment.
Discussion
In the present study we developed a method to assess the interaction between motivation and cognition in mice. We found that performance of a sustained-attention task could be modulated by reward-associated cues. This effect of reward probability in the current study is similar to that of other manipulations of reward “value” (size of reward or immediacy of reward delivery) as described for other species including monkeys (Bendiksby and Platt, 2006), pigeons (Brown and White, 2005), and rats (Burton et al., 2013). Therefore, our paradigm can be used in the mouse, a species for which many new neurobiological methods have been developed, to explore factors such as circuitry, molecular cell types and neurotransmitter functions involved in the interaction between anticipation and cognitive systems.
We found no impairment of attention in D2R-OE mice. While impairments in attention (broadly defined) have been widely reported in schizophrenia (Heinrichs and Zakzanis, 1998; Demeter et al., 2013; Nuechterlein et al., 2004), detailed analysis of the specific nature of attentional deficits in schizophrenia shows unimpaired selection of highly-salient inputs for attentional processing (Luck and Gold, 2008; Gold et al., 2006), as in our sustained-attention task. Instead, patients with schizophrenia are impaired in the executive control of attention, such as guided searching and resistance to distraction (Luck and Gold, 2008). In addition, we found no impairment in the ability of D2R-OE mice to maintain information in working memory. We previously published that D2R-OE mice display a deficit in performing working memory tasks that involve updating or manipulating information in working memory, a “win-shift” radial maze task and a “delayed-non-match-to place” T-maze task (Kellendonk et al., 2006). These results are consistent with the finding that the working-memory deficit observed in patients with schizophrenia is not due to an inability to retain information during delay intervals. Instead, working-memory impairments in patients are due to deficits in manipulation, capacity, or resistance to distraction (Anticevic et al., 2012; Gold et al., 2010; Lee et al., 2005). Future work would be needed to determine what other specific aspects of working memory, such as working memory capacity, might be disrupted in D2R-OE mice. In the case of both attention deficits as well as working-memory deficits, both are present in medicated patients (Anticevic et al., 2012; Gold et al., 2010; Gold et al., 2006), illustrating the importance of developing new medications to alleviate these symptoms.
The failure of reward signals to modulate attention in D2R-OE mice suggests that compromised DA signaling affected recruitment of attention in response to signaled-reward probability. Indeed several studies have demonstrated an involvement of dopamine signaling in the modulation of behavioral performance by signaled-reward probability (Day et al., 2010; Gan et al., 2010; Fiorillo et al., 2003). Therefore, perturbation of dopamine signaling could lead to the lack of motivational modulation of attention in D2R-OE mice and perhaps in patients who evidence this type of deficit.
There are several potential ways that dopamine transmission could be disrupted or dampened in D2R-OE mice. First, it has been suggested that phasic dopamine signaling is important in modulation of behavior by cues that signal a high-reward probability (Day et al., 2010; Gan et al., 2010). This phasic signal may not have sufficient impact because of the increase in the basal level of postsynaptic D2 receptor activity in the striatum in D2R-OE mice. Second, increased postsynaptic D2 receptor expression in striatum could impact presynaptic dopamine function. In fact, in vivo electrophysiology experiments have determined that the firing patterns of midbrain dopamine neurons are altered in D2R-OE mice. Specifically, the firing frequency is reduced and there is decreased burst activity in the VTA. (Krabbe et al. 2015). This significant reduction in activity of VTA dopamine will result in less release of dopamine at terminal sites, thereby reducing dopamine transmission. Third, Gold and colleagues recently suggested that failure of performance to be modulated by increased reward probability could be due to areas of the prefrontal cortex known to participate in value computation (mPFC, OFC) being compromised in patients (Gold et al., 2012; 2013). In D2R-OE mice, abnormalities in PFC function, including reduced dopamine turnover and altered sensitivity to D1 and D2 agonists in the PFC (Kellendonk et al., 2006; Li et al., 2011) may also be foundational to the deficit. Further work is needed to localize specific prefrontal dysfunction in the motivational control of attention.
We found that modulation of attention by reward anticipation was rescued after D2R levels were normalized in D2R-OE mice (a manipulation that also restores anticipatory motivation; Ward et al., 2012). There are at least two potential explanations for this behavioral rescue. It may be that D2R-OE mice are incapable of utilizing the signaled probability to modulate performance, and this incapacity was resolved by switching off the transgene. Alternatively, because we tested mice on doxycycline after the initial testing period, it may be that D2R-OE mice are slower to learn to utilize the signaled probability to modulate performance, and this is resolved by extended experience with the task. This interpretation is less likely, however, because an analysis of the data from the doxycycline condition showed that attention was differentially modulated in D2R-OE mice on the first day of testing after doxycycline treatment.
The fact that turning off the transgene, and thereby normalizing D2R levels rescued the motivational modulation of attention here suggests the possibility that both the motivational and cognitive deficits in this model are caused by D2R overexpression. To the extent that D2R-OE mice model the conditions found in schizophrenia, this result suggests the hopeful possibility that normalizing D2R function in patients will improve both motivation and cognition. Unfortunately, treatment with antipsychotic agents, which predominantly target D2R activity, has no appreciable positive impact on either motivation or cognition in schizophrenia (Stahl & Buckley, 2007; Bilder et al., 1992; Nielsen et al., 2015) and may even worsen symptoms (Bilder et al., 1992; Hill et al., 2010). This difference may be explained by the fact that the D2R-OE model includes the overexpression of D2Rs selectively in postsynaptic medium spiny neurons of the striatum. Antipsychotic medications are administered systemically, and they therefore antagonize not only the postsynaptic D2Rs in striatum, but also the presynaptic D2Rs in the midbrain. Also, doses of antipsychotic agents that are effective for preventing positive symptoms in patients have been shown to block 60-80% of D2Rs in rodents (Kapur et al., 2003; Naiker et al., 2006), while turning off the transgene removes only the approximately 15% excess D2Rs present in our model. Given that acute blockade of D2Rs produces profound impairments in motivation in rats (see Salamone et al., 2007 for review), the lack of therapeutic efficacy of D2R blockade in patients is likely due to the difficulty in targeting only the excess D2R activity. Indeed, we failed to find any improvement of motivation (instead, the deficit was worsened) in D2R-OE mice after chronic treatment with haloperidol at a dose that was analogous to clinically efficacious doses used in patients (Simpson et al., 2011).
In a recent study, acute viral overexpression of D2R in the striatum enhanced motivation in adult mice (Trifilieff et al., 2013), which is in apparent contradiction to our other studies (including this one) showing that genetic overexpression of D2R results in decreased motivation (Drew et al., 2007; Simpson et al., 2011; Ward et al., 2012). These seemingly-opposing findings likely reflect the several differences between an acute virally mediated system and a chronic developmental genetic upregulation. In addition to the temporal difference in D2R overexpression, the cell-type and regional expression pattern within striatum differ between these models, likely also contributing to the different phenotypes. Specifically, it has recently been shown that developmental overexpression of D2Rs results in increased excitability of indirect pathway neurons (Cazorla et al., 2012), as well as structural changes in the circuitry connecting striatum with substantia nigra (Cazorla et al., 2014). Perhaps of most direct relevance, is the recently identified reduction in DA transmission in D2R-OE mice described above (Krabbe et al., 2015). The fact that developmental D2R overexpression results in a reduction in DA transmission in adulthood may explain why D2R-OE mice show deficits in motivation, similar to mice treated with D2R antagonists, whereas acute overexpression in adulthood results in a hyperdopaminergic phenotype.
In conclusion, these results highlight the need for further study of the interaction between cognitive and motivational symptoms in schizophrenia and other psychiatric diseases (Barch and Dowd, 2010). Here, we have demonstrated that rescuing a motivational deficit restores the interaction between motivation and cognition, critically implicating motivation in adaptive cognitive function. Therefore, failure to treat motivational impairments may contribute to the relative lack of functional improvements observed in patients, in spite of gains in specific domains of cognitive function following cognitive-remediation therapy (Hogarty et al., 2004; Medalia and Choi, 2009; Velligan et al., 2006). The present results suggest the encouraging possibility that by treating motivational impairments, already debilitating in their own right, we may also be able to meliorate the functional impairment that results from cognitive dysfunction in patients.
Supplementary Material
Acknowledgments
We thank Iram Haq and Tamara Azayeva for maintaining the mouse colony. This work was supported by the National Institutes of Health grants 1K99MH095835-01 (R.D.W.), 1P50MH086404 (E.R.K and E.H.S.), and 5R01MH068073-10 (P.D.B.). This work was also supported by the Howard Hughes Medical Institute (E.R.K) and a grant from the Lieber Institute for Brain Development (E.R.K, E.H.S, R.D.W).
Footnotes
The authors declare no biomedical financial interests or potential conflicts of interest.
References
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proceeding of the National Academy of Sciences USA. 2000;97:8104–109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Krystal JH, Barch DM. A broken filter: prefrontal functional connectivity abnormalities in schizophrenia during working memory interference. Schizophrenia Research. 2012;141:8–14. doi: 10.1016/j.schres.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: How much and how little we know. Schizophrenia Bulletin. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophrenia Bulletin. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendiksby MS, Platt ML. Neural correlates of reward and attention in macaque area LIP. Neuropsychologia. 2006;44:2411–2420. doi: 10.1016/j.neuropsychologia.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Turkel E, Lipshutz-Broch L, Lieberman JA. Antipsychotic medication effects on neuropsychological functions. Psychopharmacology Bulletin. 1992;28:353–366. [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatric Disease & Treatment. 2006;2:531–536. doi: 10.2147/nedt.2006.2.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GS, White KG. On the effects of signaling reinforcer probability and magnitude in delayed matching to sample. Journal of the Experimental Analysis of Behavior. 2005;83:119–128. doi: 10.1901/jeab.2005.94-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla M, de Carvalho FD, Chohan MO, Shegda M, Chuhma N, Rayport S, Ahmari SE, Moore H, Kellendonk C. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81:153–164. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla M, Shegda M, Ramesh B, Harrison NL, Kellendonk C. Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. Journal of Neuroscience. 2012;32:2398–2409. doi: 10.1523/JNEUROSCI.6056-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM. Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biological Psychiatry. 2010;68:306–309. doi: 10.1016/j.biopsych.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter E, Guthrie SK, Taylor SF, Sarter M, Lustig C. Increased distractor vulnerability but preserved vigilance in patients with schizophrenia: evidence from a translational sustained attention task. Schizophrenia Research. 2013;144:136–41. doi: 10.1016/j.schres.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. Journal of Neuroscience. 2007;27:7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Gan JO, Walton ME, Phillip PE. Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nature Neuroscience. 2010;13:25–27. doi: 10.1038/nn.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, et al. Negative symptoms and the failure to represent the expected reward value of actions: Behavioral and computational modeling evidence. Archives of General Psychiatry. 2012;69:129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biological Psychiatry. 2013;74:130–136. doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophrenia Research. 2004;72:21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson B, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory in schizophrenia. Journal of Abnormal Psychology. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- Gold JM, Hahn B, Zhang WW, Robinson BM, Kappenman ES, Beck VM, Luck SJ. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Archives of General Psychiatry. 2010;67:570–577. doi: 10.1001/archgenpsychiatry.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. Journal of Clinical Psychiatry. 2006;67:3–8. [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophrenia Research. 2004;72:1–3. doi: 10.1016/j.schres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hill SK, Bishop JR, Palumbo D, Sweeney JA. Effect of second-generation antipsychotics on cognitiona: current issues and future challenges. Expert Review of Neurotherapeutics. 2010;10:43–57. doi: 10.1586/ern.09.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Nopoulos P, Flaum M, Arndt S, Andreason NC. Two-year outcome in first episode schizophrenia: Predictive value of symptoms for quality of life. American Journal of Psychiatry. 1998;155:1196–1201. doi: 10.1176/ajp.155.9.1196. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, et al. Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Archives of General Psychiatry. 2004;61:866–876. doi: 10.1001/archpsyc.61.9.866. [DOI] [PubMed] [Google Scholar]
- Kahn JB, Ward RD, Kahn L, Balsam PD, Simpson EH. Medial prefrontal lesions in mice impair sustained attention but spare maintenance of information in working memory. Learning & Memory. 2012;19:513–517. doi: 10.1101/lm.026302.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. Journal of Pharmacology and Experimental Therapuetics. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biological Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Krabbe S, Duda J, Schiemann J, Poetschke C, Schneider G, Kandel ER, Liss B, Roeper J, Simpson EH. Increased dopamine D2 receptor activity in the striatum alters the firing pattern of dopamine neurons in the ventral tegmental area. Proceedings of the National Academy of Sciences U. S. A. doi: 10.1073/pnas.1500450112. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. Journal of Abnormal Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Li YC, Kellendonk C, Simpson E, Kandel ER, Gao WJ. D2 receptor overexpression in the striatum leads to a deficit in inhibitory transmission and dopamine sensitivity in mouse prefrontal cortex. Proceedings of the National Academy of Sciences USA. 2011;108:12107–12112. doi: 10.1073/pnas.1109718108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The construct of attention in schizophrenia. Biological Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Brekke J. In search of a theoretical structure for understanding motivation in schizophrenia. Schizophrenia Bulletin. 2010;36:912–918. doi: 10.1093/schbul/sbq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Choi J. Cognitive remediation in schizophrenia. Neuropsychological Review. 2009;19:353–364. doi: 10.1007/s11065-009-9097-y. [DOI] [PubMed] [Google Scholar]
- Naiker DV, Catts SV, Bedi KS, Bryan-Lluka LJ. Dose determination of haloperidol, risperidone and olanzapine using an in vivo dopamine D2-receptor occupancy method in the rat. European Journal of Pharmacology. 2006;540:87–90. doi: 10.1016/j.ejphar.2006.04.048. [DOI] [PubMed] [Google Scholar]
- Nakagami E, Xie B, Hoe M, Brekke JS. Intrinsic motivation, neurocognition, and psychosocial functioning in schizophrenia: testing mediator and moderator effects. Schizophrenia Research. 2008;105:95–104. doi: 10.1016/j.schres.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Nielsen RE, Levander S, Kjaersdam TG, Jensen SO, Ostergaard CT, Leucht S. Second-generation antipsychotic effect on cognition in patients with schizophrenia-a meta-analysis of randomized clinical trials. Acta Psychiatrica Scandinavica. 2015 doi: 10.1111/acps.12374. DOI: 10.1111/acps.12374. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophrenia Research. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S, et al. Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biological Psychiatry. 2011;69:928–935. doi: 10.1016/j.biopsych.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM, Buckley PF. Negative symptoms of schizophrenia: a problem that will not go away. Acta Psychiatrica Scandinavica. 2007;115:4–11. doi: 10.1111/j.1600-0447.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, et al. Increasing dopamine D2 receptor expression in adult nucleus accumbens enhances motivation. Molecular Psychiatry. 2013;18:1025–1033. doi: 10.1038/mp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Kern RS, Gold JM. Cognitive rehabilitation for schizophrenia and the putative role of motivation and expectancies. Schizophrenia Bulletin. 2006;32:474–485. doi: 10.1093/schbul/sbj071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Kellendonk C, Simpson EH, Lipatova O, Drew MR, Fairhurst S, et al. Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits. Behavioral Neuroscience. 2009;123:720–730. doi: 10.1037/a0016503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI, et al. Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology. 2012;37:1699–1707. doi: 10.1038/npp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.