Abstract
Objective
Endoluminal vascular interventions such as angioplasty initiate a sterile inflammatory response resulting from local tissue damage. This response drives the development of intimal hyperplasia (IH) that, in turn, can lead to arterial occlusion. We hypothesized that the ubiquitous nuclear protein and damage-associated molecular pattern molecule, high-mobility group box 1 (HMGB1), is one of the endogenous mediators that activates processes leading to IH after endoluminal injury to the arterial wall. The aim of this study is to investigate whether approaches that reduce the levels of HMGB1 or inhibit its activity suppresses IH after arterial injury.
Approach and Results
Here, we show that HMGB1 regulates IH in a mouse carotid wire injury model. Induced genetic deletion or neutralization of HMGB1 prevents IH, monocyte recruitment, and smooth muscle cell growth factor production after endoluminal carotid artery injury. A specific inhibitor of HMGB1 myeloid differentiation factor 2–toll-like receptor 4 (TLR4) interaction, P5779, also significantly inhibits IH. HMGB1 deletion is mimicked in this model by global deletion of TLR4 and partially replicated by myeloid-specific deletion of TLR4 but not TLR2 or receptor for advanced glycation endproducts deletion. The specific HMGB1 isoform known to activate TLR4 signaling (disulfide HMGB1) stimulates smooth muscle cell to migrate and produce monocyte chemotactic protein 1/CCL2) via TLR4. Macrophages produce smooth muscle cell mitogens in response to disulfide HMGB1 also in a TLR4/myeloid differentiation primary response gene (88)/Trif-dependent manner.
Conclusions
These findings place HMGB1 and its receptor, TLR4 as critical regulators of the events that drive the inflammation leading to IH after endoluminal arterial injury and identify this pathway as a possible therapeutic target to limit IH to attenuate damage-associated molecular pattern molecule–mediated vascular inflammatory responses.
Keywords: angioplasty, carotid artery injuries, HMGBI protein, hyperplasia, macrophages
Vascular interventions, such as surgical bypass procedures, endarterectomy,1 peripheral artery brachytherapy,2,3 angioplasty, and stent placement for arterial occlusive disease, can fail because of restenosis.4,5 These interventional procedures result in endothelial denudation with intimal and medial damage, which induces substantial local inflammation. This inflammation is manifested by monocyte infiltration as well as inflammatory mediator and growth factor production.6 This, in turn, stimulates vascular smooth muscle cell (SMC) accumulation and extracellular matrix deposition resulting in intimal hyperplasia (IH) and vessel or stent occlusion.7
The molecular processes that initiate inflammation in the arterial wall after mechanical injury are not fully understood. Because endoluminal vascular interventional procedures cause stretching of the vessel wall and cell necrosis,8 endogenous molecules released during cell death and stress, termed damage-associated molecular patterns (DAMP), could activate pattern recognition receptors leading to sterile inflammation. The nuclear protein, high-mobility group box 1 (HMGB1), functions as a DAMP when passively released during cell injury and necrosis or actively secreted during immune or parenchymal cell activation and cell stress.9,10 HMGB1 is upregulated in multiple cell types in atherosclerotic plaques11,12 and hyperplasia lesions in blood vessels,10,13 including endothelial cells, SMC, foam cells, macrophages, and activated platelets. In vitro, extracellular HMGB1 induces the release of proinflammatory cytokines and chemokines from macrophages and monocytes,14 upregulates chemokine, cytokine, plasminogen activator inhibitor 1, and tissue-type plasminogen activator expression in endothelial cell,14,15 and induces SMC proliferation and migration.12,16 Despite these observations, a role for DAMP, including HMGB1, in the remodeling response after endoluminal vascular injury has not been established.
Several toll-like receptors (TLRs) recognize DAMP and can drive sterile inflammation.17 Among these, TLR4 has been shown to be activated by the widest array of endogenous molecules, including HMGB1.18 Previous studies suggest that TLR4 is involved in a variety of vasculopathy processes, such as atherosclerosis, vein graft neointimal remodeling, and lipopolysaccharide-induced arterial IH.13,19,20 Human studies reveal that the common Asp299Gly TLR4 polymorphism, which attenuates receptor signaling, is associated with low levels of circulating inflammatory mediators and a decreased risk of atherosclerosis and cardiovascular events.21 TLR4 is expressed on several cell types involved in the vascular injury response, including monocytes, endothelial cell, platelets, and SMC; however, specific roles for TLR4 on a relevant cell type has not been established.
There is now strong support for a role for HMGB1–myeloid differentiation factor 2(MD2)/TLR4 interactions in driving both acute and chronic inflammatory responses to tissue trauma and hypoxia.22 Recently, a specific redox isoform of HMGB1, referred to as disulfide HMGB1, was shown to bind to MD2 in the TLR4 receptor complex and account for the cytokine-like or TLR4-stimulating activity of extracellular HMGB1.23 HMGB1 contains 3 conserved redox-sensitive cysteines (C23, C45, and C106); modification of these cysteines determines the biological activity of extracellular HMGB1. Cytokine-stimulating or disulfide HMGB1 has C23 and C45 in a disulfide linkage and C106 in its reduced form as a thiol.24,25
In this article, we tested the hypothesis that IH induced by endoluminal arterial injury is mediated through an HMGB1-and TLR4-driven mechanism. We found that both HMGB1 and TLR4 drive monocyte recruitment, inflammation, and IH after wire injury to the carotid artery. Both IH and monocyte recruitment after arterial injury involve TLR4 expression specifically on myeloid cells. Disulfide HMGB1 induced macrophage cytokine, chemokine, and SMC growth factor production as well as vascular SMC migration and monocyte chemotactic protein 1 (MCP1)/CCL2-CCR2 upregulation through TLR4 in vivo, whereas a specific inhibitor of HMGB1–MD2/TLR4 suppressed IH in vivo. These data provide evidence for a major role for the HMGB1-TLR4 axis and specifically the disulfide isoform of HMGB1 in the endoluminal arterial injury response that leads to IH.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Statistical Analysis
All data are expressed as mean±SEM. One-way analysis of variance after post hoc Student–Newman–Keuls test was used to determine the differences among multiple groups. The Mann–Whitney U test was applied on small-size comparisons with non-normal distributions between groups. The t test was applied only on experiments with normal distributions between the comparison groups. A P value <0.05 was considered statistically significant.
Results
HMGB1 and TLR4 are Essential for Acute Injury–Induced Inflammation and IH
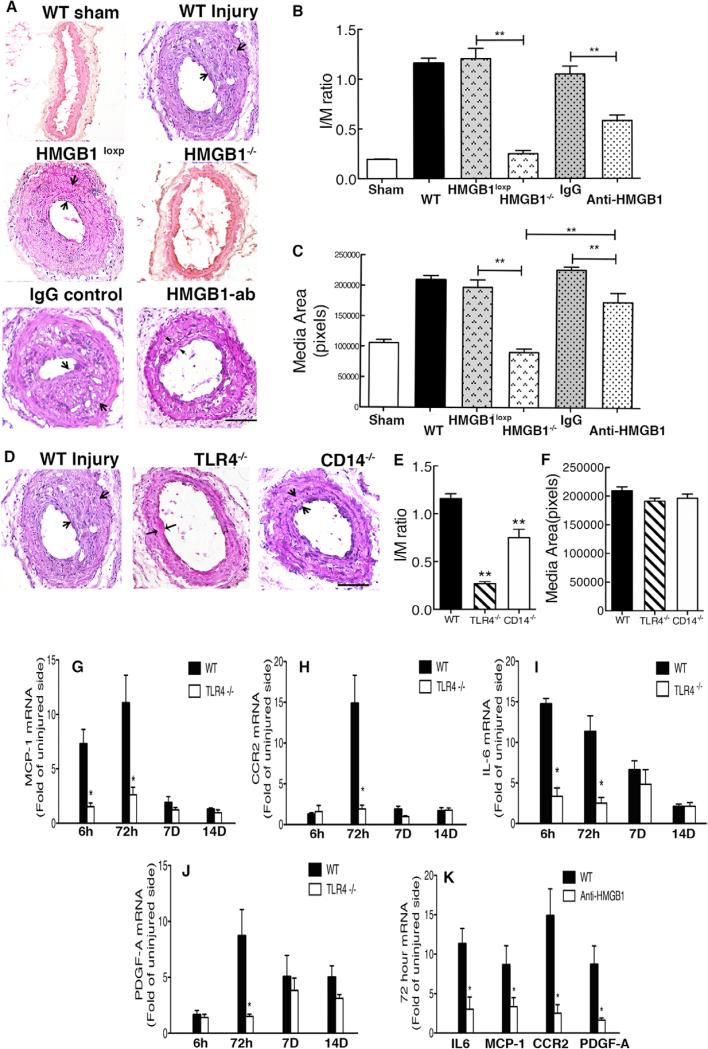
We tested the hypothesis that HMGB1 contributes to injury-induced IH and vascular remodeling in a carotid artery wire injury model in mice. Embryonic deletion of HMGB1 is lethal26; therefore, we generated an inducible HMGB1−/− mouse strain where the HMGB1 gene was globally deleted after tamoxifen treatment (Figure IA–IC in the online-only Data Supplement). As shown in Figure ID and IE in the online-only Data Supplement, tamoxifen treatment lead to a near complete and sustained loss of HMGB1 expression in the carotid artery, aorta, and heart (measured 35 days after tamoxifen treatment). A striking prevention of inward IH and vessel remodeling was observed in injured carotid arteries from inducible HMGB1−/− mice (P<0.01; Figure 1 A–1C) compared with the vessels from HMGB1loxp (no tamoxifen) control mice. Tamoxifen pretreatment had no impact on IH in WT mice.
Figure 1.
High-mobility group box 1 (HMGB1) and toll-like receptor 4 (TLR4) contribute to wire injury-induced inflammation and intimal hyperplasia. A, Common carotid arteries were injured by a 0.4-mm guidewire plus external carotid artery ligation and harvested 28 days after surgery. Frozen tissues were sectioned. Representative hematoxylin & eosin (H&E)–stained sections of carotid arteries from wild-type (WT) mice (n=10), HMGB1loxP mice (n=9), inducible HMGB1−/− mice (n=8), IgG2b (control antibody)-treated mice (n=5), and anti-HMGB1 mAb-treated mice (n=8) are shown. B–C, The ratios of intima and media area (I/M) and vessel medial size were quantified by planimetry at 28 days. D, Representative H&E-stained sections of carotid arteries from WT mice (n=10), TLR4−/− mice (n=10), and CD14−/− mice (n=6) are shown. E–F, The ratio of I/M area and vessel medial size were quantified by planimetry at 28 days. G–K, Injured artery mRNA levels for monocyte chemotactic protein 1 (MCP-1)/CCL2, CCR2, interleukin 6 (IL-6), and platelet-derived growth factor-A (PDGF-A) were measured by reverse transcription polymerase chain reaction at 6 hours, 72 hours, 7 days, and 14 days in WT mice and TLR4−/− mice, and at 72 hours in IgG2b-treated mice and anti-HMGB1 mAb-treated mice. Gene expression was normalized to 18S transcript levels. Relative mRNA levels compared with the uninjured carotid artery are displayed. Data represent the mean±SEM. *P<0.05, **P<0.01 vs the indicated groups. Scale bar, 100 μm.
HMGB1 has compartment-specific roles inside and outside the cell.14,27 Extracellular HMGB1 can trigger inflammatory signaling through the activation of pattern recognition receptors. To establish the importance of extracellular HMGB1 on the IH process, C57Bl/6 wild-type (WT) mice were treated with a neutralizing anti-HMGB1 monoclonal antibody (mAb) daily for 28 days. Compared with the mice receiving the IgC2b isotype control mAb, anti-HMGB1 mAb treatment conferred a 50% reduction in IH measured at 28 days after wire injury (Figure 1A–1C).
Extracellular HMGB1 can trigger signaling through several receptors, including TLR4, TLR2, and receptor for advanced glycation endproducts (RAGE).28 To determine the extent to which carotid artery IH involves these receptors, TLR4−/−, TLR2−/−, and RAGE−/− mice were subjected to carotid artery wire injury. TLR4−/− mice were associated with the greatest reduction in IH (76% decrease compared with WT mice; Figure 1D and 1E; Figure II in the online-only Data Supplement). TLR4 deletion also led to an accelerated rate of re-endothelization by 7 days after wire injury (Figure V in the online-only Data Supplement). CD14 has been shown to participate in disulfide HMGB1–induced TLR4 activation.23 CD14−/− mice exhibited a significant reduction in carotid artery IH after wire injury (Figure 1D and 1E). Interestingly, no reduction in vascular media thickening after injury was observed in TLR4−/−, TLR2−/−, RAGE−/−, or CD14−/− mice (Figure 1D–1F; Figure II in the online-only Data Supplement).
We next investigated whether TLR4 and HMGB1 contributed to the expression of interleukin (IL)-6, platelet-derived growth factor-A (PDGF-A), or the monocyte chemoattractant MCP1/CCL2 and its receptor, CCR2,29 which are known to be involved in the vascular injury response. Quantitative reverse transcription polymerase chain reaction revealed that MCP1/CCL2 and IL-6 mRNA levels were increased by 6 hours and then approached basal levels by 7 days. CCR2 and PDGF-A mRNA peaked at 3 days after vascular injury, whereas CCR2 was attenuated by day 7, PDGF-A remained upregulated to 14 days in injured vessels of WT mice. The increases in MCP1/CCL2, CCR2, IL-6, and PDGF-A expression were attenuated in injured carotid arteries from TLR4−/− mice (Figure 1G–1J) and anti-HMGB1 mAb–treated mice at 3 days (Figure 1K).
HMGB1 Is Upregulated and Interacts With TLR4 Within the Injured Vessel
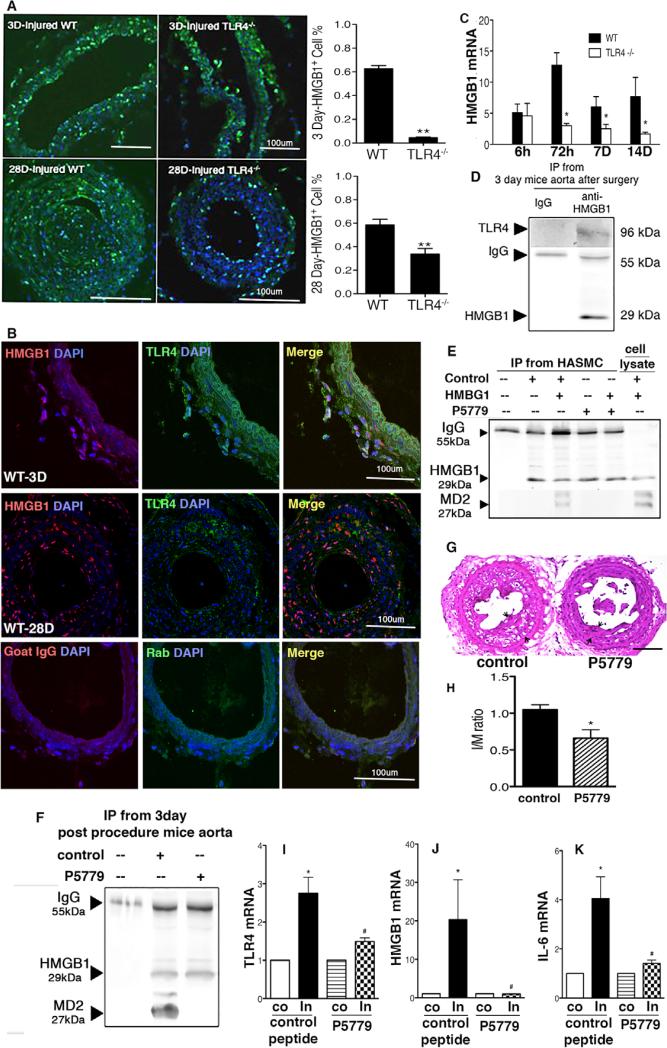
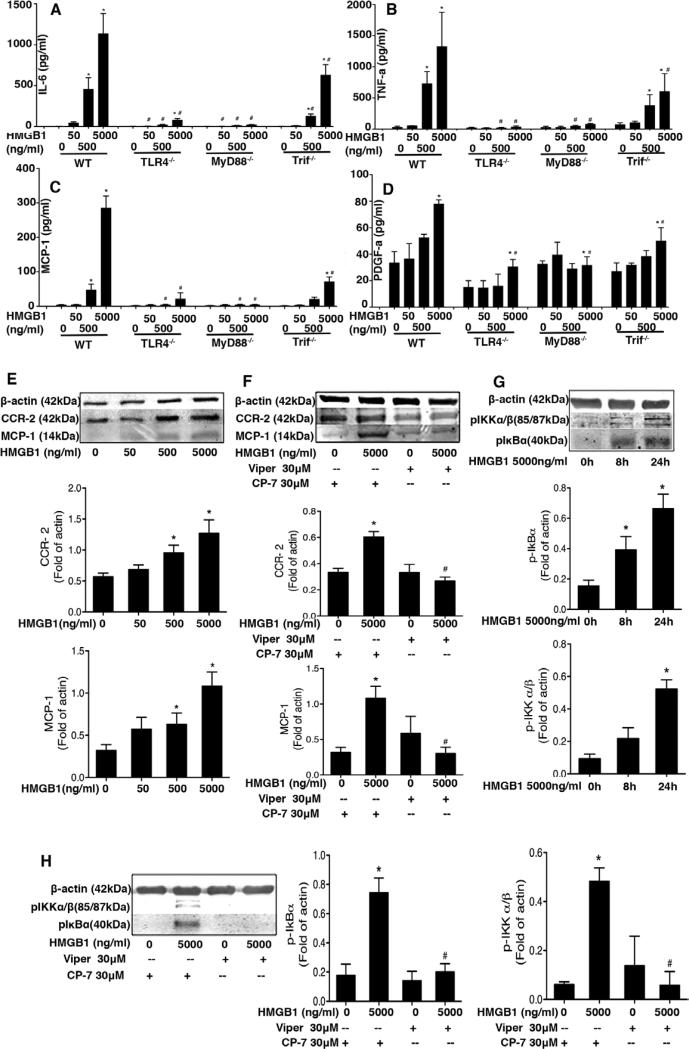
HMGB1 normally resides in the nucleus but can be upregulated and mobilized to the cytoplasm and actively secreted in the setting of cell stress and inflammation.9,28 The percentage of cells expressing HMGB1, as detected by immunofluorescence, was increased in the neointima at 3 and 28 days after wire injury in WT but not in TLR4−/− mice (Figure 2A). A corresponding increase in HMGB1 mRNA levels was also observed in WT and TLR4−/− mice by 6 hours but became significantly greater in the carotid arteries of the WT mice compared with the vessels from the TLR4−/− mice at 3, 7, and 14 days (Figure 2C). HMGB1 and TLR4 colocalized in cells in the adventitia on day 3, but not on day 28 (Figure 2B). Immunoprecipitation also revealed that HMGB1 and MD2/TLR4 interact on day 3 in the aorta (Figure 2D). HMGB1 immunoprecipitated with MD2 in lysates from disulfide HMGB1–treated human aortic SMC (HASMC) and the aorta of the mice at 3 days after wire injury. This interaction was blocked when WT mice were treated with a specific tetrapeptide inhibitor of disulfide HMGB1–MD2/TLR4 interaction, P5779, but not a control peptide (Figure 2E and 2F). This tetrapeptide (FSSE, P5779) has been shown to block disulfide HMGB1 binding to MD2 and prevent macrophage cytokine production in response to HMGB1.30 P5779 also partially prevented injury-induced IH (Figure 2G and 2H) and the upregulation of TLR4, HMGB1, and IL-6 mRNA at day 3 (Figure 2I–2K).
Figure 2.
High-mobility group box 1 (HMGB1) is upregulated after arterial wire injury. Inhibition of HMGB1 and myeloid differentiation factor 2 (MD2)/toll-like receptor 4 (TLR4) interaction by P5779 alleviated intimal hyperplasia. A, Representative photomicrographs of HMGB1 (green) immunofluorescent staining of arteries from wild-type (WT) and TLR4−/− mice at 3 and 28 days after vascular injury are shown. Nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). HMGB1-positive cell cross-sections were counted at 3 days (n=5) and 28 days (n=5). The number of HMGB1-positive cells was defined as the percentage of the total cells within a given area that stained positive for HMGB1. B, HMGB1 (red) and TLR4 (green) double immunofluorescence staining show colocalization of HMGB1 with TLR4 in the intimal hyperplasia section at 3 days, but not at 28 days. C, Injured artery HMGB1 mRNA levels were measured by reverse transcription polymerase chain reaction (RT-PCR) and normalized to 18S transcript levels at 6 hours, 72 hours, 7 days, and 14 days after carotid artery injury. D, Tissue lysates from aortic arteries (1 mg) were prepared from P5779 and control peptide-treated mice at 3 days after surgery. E, Human aortic smooth muscle cell (HASMC) in culture plates were stimulated with HMGB1 (1 μg/mL) plus P5779 or scrambled control peptide at 50 μg/mL for 6 hours and lysates subjected to Western blot analysis. F, Aortic arteries were harvested from P5779 and control peptide (500 μg/mouse twice daily) treated mice at 3 days after surgery. All were incubated with protein A/G PLUS-agarose beads and anti-HMGB1 antibody. Proteins bound to the beads were revealed by Western blot with anti-TLR4(D) or anti-MD2(E and F) antibody. Data shown is representative of 3 independent experiments. G, Administration of P5779 ameliorates intimal hyperplasia in WT mice. P5779 (or vehicle control) was administered intraperitoneally at 500 μg/mouse twice daily after surgery. Representative hematoxylin & eosin (H&E)–stained sections of carotid arteries from control mice (n=7) and P5779-treated mice (n=6) are shown. H, The ratio of intima and media area (I/M) and vessel medial size was quantified by planimetry at 28 days. I–K, Arterial TLR4, HMGB1, and interleukin 6 (IL-6) mRNA levels were measured by RT-PCR and normalized to 18S transcript levels at 72 hours (n=5) after carotid artery injury. Relative mRNA abundance in the injured artery compared with the uninjured carotid artery is displayed. Data represent the mean±SEM. *P<0.05 versus the respective control groups. Scale bar, 100 μm.
Both MyD88- and Trif-Dependent Signaling Contribute to IH Formation
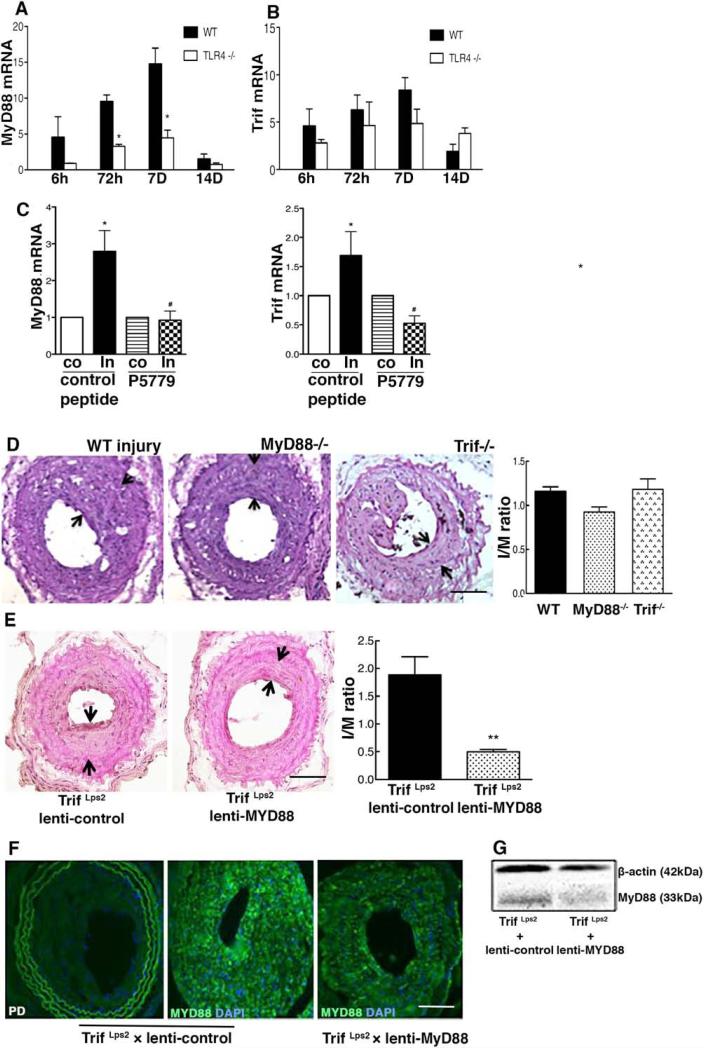
TLR4 activation leads to cell signaling through both myeloid differentiation primary response gene 88 (MyD88)- and Trif-dependent pathways. Carotid artery MyD88 and Trif mRNA levels, measured by quantitative reverse transcription polymerase chain reaction were upregulated by 6 hours after the injury compared with contralateral uninjured arteries in the WT mice. MyD88 mRNA levels were significantly lower in TLR4−/− mice (Figure 3A) and WT mice treated with P5779 than WT controls (Figure 3C); however, Trif mRNA levels were not influenced by TLR4 inhibition (Figure 3B). MyD88−/− mice exhibited a significant reduction by 20% in carotid artery IH after wire injury when compared with WT mice. There was no significant inhibition of IH in Trif−/− mice compared with the controls (Figure 3D).
Figure 3.
Both myeloid differentiation primary response gene 88 (MyD88) and Trif contribute to intimal hyperplasia formation after wire injury. A–C, Arterial mRNA levels for MyD88 and Trif were measured by reverse transcription polymerase chain reaction and normalized to 18S transcript levels at indicated time points after wire injury. Relative mRNA levels compared with the uninjured carotid artery are displayed (n=4–6). D, Carotid arteries from wild-type (WT) mice (n=10), MyD88−/− mice (n=16), and Trif Lps2 mice (n=7) were analyzed at day 28 after arterial injury. E, Arterial intimal hyperplasia after local MyD88 silencing in TrifLps2 mice (Trif Lps2 +lenti-control, n=8; Trif Lps2+lenti-shMyD88, n=7) is shown. Intima and media area (I/M) was reduced in the lenti-shMyD88 group compared with the lenti-control. F, Focal MyD88 expression is shown in carotid arteries locally treated with lenti-control or lenti-shMyD88. Representative hematoxylin & eosin–stained and fluorescent-stained sections are shown. The I/M ratios were quantified by planimetry at 28 days. G, Tissue lysates from aortic arteries (30 μg) were prepared from lenti-shMyD88 and lenti-control at 28 days after application of lentivirus and subjected to Western blot with anti–high-mobility group box 1 and β-actin antibodies. Data represent the mean±SEM. *P<0.05, **P<0.01 vs controls, respectively. Scale bar, 100 μm.
Because signaling downstream of TLR4 involves both MyD88 and Trif, we then blocked signaling through both MyD88 and Trif by local application of lenti-shMyD88 to the carotid artery in Trif−/− mice. Detection of MyD88 expression by immunohistochemistry and Western blot verified that treatment with lenti-shMyD88 reduced MyD88 content in the injured artery (Figure 3F and 3G). Suppression of MyD88 expression in Trif−/− led to a 73.6% reduction of intima and media area ratio in the injured artery compared with lenti-control treated Trif−/− mice (Figure 3E), which is even greater than the reduction in IH observed in the MyD88−/− mice. These data show that both TLR4 adapters pathways are involved in IH after arterial injury that is revealed only when the expression of both receptors is suppressed.
HMGB1-TLR4 Signaling Drive Monocyte Infiltration
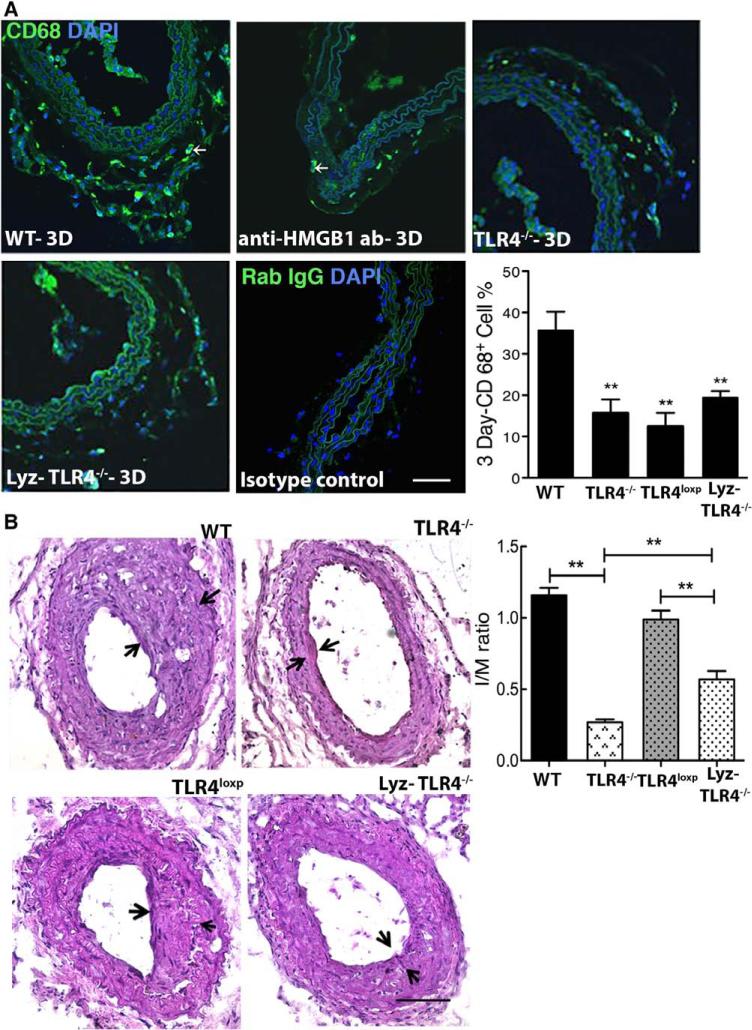
Macrophage infiltration plays a crucial role in the pathophysiological mechanisms, leading to vascular restenosis.31 Immunofluorescence staining for the macrophage marker CD68 demonstrated adventitial infiltration of CD68+ cells at day 3 in WT mice after wire injury (Figure 4A). However, a marked reduction in macrophage accumulation was observed in injured arteries from anti–HMGB1-treated WT mice and TLR4−/− mice compared with arteries from untreated WT mice (Figure 4A) or WT mice that received control isotype antibody (data not shown). The increase in adventitial CD68+ cells in WT mice was not associated with an increase costaining for Ki67 but was associated with an increase in tunel-positive CD68+ cells, which was not seen in injured TLR4−/− carotids (Figure IV in the online-only Data Supplement).
Figure 4.
High-mobility group box 1 (HMGB1)-toll-like receptor 4 (TLR4) signaling drive monocyte infiltration. A, Mouse carotid arteries at 3 days after injury that were immunostained for macrophage markers (CD68-positive cells). The number of CD68-positive cells was defined as the percentage of the total cells within a given area that stained positive for CD68. B, Carotid arteries from WT mice (n=10), TLR4−/− mice (n=10), TLR4loxP mice (n=10), and Lyz-TLR4−/− mice (n=7) were analyzed after arterial injury. Representative hematoxylin & eosin (H&E)–stained sections are shown. The ratio of neointimal to medial areas (I/M) was quantified by planimetry at 28 days. Representative H&E-stained and immunofluorescent-stained sections are shown. Data represent the mean±SEM. *P<0.05, **P<0.01 vs the WT groups. Scale bar, 100 μm.
To determine if TLR4 expression on macrophages plays an essential role in IH, we selectively depleted the TLR4 gene from the myeloid cell population by crossing TLR4loxp/loxp mice with LyzM-cre (Lyz-TLR4−/− mice). We have previously confirmed the functional, selective deletion of TLR4 from myeloid cells in the Lyz-TLR4−/− mice.32 This strategy significantly suppressed IH by 57.5% at day 28 (Figure 5B) as well as CD68+ cell infiltration by 42.3% at day 3 after carotid arterial injury (Figure 4A). Thus, TLR4 expression on myeloid cells is involved in the infiltration of monocytes and the progression of IH through a process that does not seem to involve local proliferation.
Figure 5.
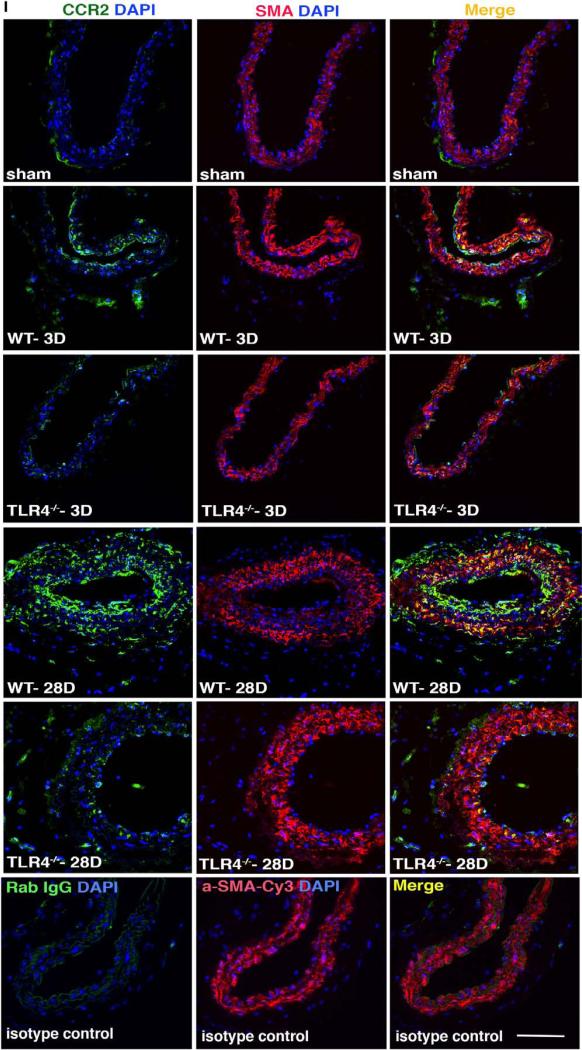
High-mobility group box 1 (HMGB1) induces cytokine and chemokine release via toll-like receptor 4 (TLR4) in macrophages and smooth muscle cells. A–D, Thioglycollate-elicited murine peritoneal macrophages from wild-type (WT), TLR4−/−, myeloid differentiation primary response gene 88 (MyD88)−/−, and Trif Lps2 mice treated with 0, 50, 500, and 5000 ng/mL HMGB1. Eight hours later, media was collected and tumor necrosis factors-α (TNF-α; A), interleukin 6 (IL-6; B), monocyte chemotactic protein 1 (MCP-1)/CCL2 (C), and platelet-derived growth factor-A (PDGF-A; D) were measured by ELISA. Data are presented as mean±SEM (n=4). *P<0.05 compared with control. #P<0.05 vs HMGB1-treated group. E, Western blot analysis showing that HMGB1 dose-dependently increased MCP-1/CCL2 and CCR2 expression in human aortic SMC (HASMC) lysates at 24 hours after HMGB1 treatment. F, HASMC were pretreated with TLR4 inhibitory peptide for 1 hour. The cells were then treated with HMGB1 for 24 hours. Upregulation of MCP-1/CCL2 and CCR2 were blocked by Viper at 30 μmol/L. G, HMGB1 5000 ng/mL increased IκBα and IKK phosphorylation in a time-dependent manner in HASMC. H, HASMC were pretreated with TLR4 inhibitory peptide for 1 hour. The cells were then treated with HMGB1 for 8 hours. Upregulation of pIκBα and pIKKα/β were blocked by Viper at 30 μmol/L. β-actin was used as loading control. MCP-1/CCL2, CCR2, pIκBα, and pIKKα/β were detected by Western blotting. These parameters effect is blocked by Viper at 30 μmol/L. Graphic data show quantification of 4 to 5 independent experiments. Data are shown as mean±SEM. *P<0.05 compared with control. #P<0.05 versus HMGB-treated group. I, Double immunofluorescence staining was performed to detect CCR2-positive cells (green) and smooth muscle actin (SMA)–positive cells (red) in WT and TLR4−/− mice at 3 and 28 days. Nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Representative fluorescent-stained sections are shown. Scale bar, 100 μm.
HMGB1 Induces Cytokine and Chemokine Release Via TLR4 in Macrophages and SMCs
Infiltrating macrophages promote IH progression and vessel remodeling by releasing inflammatory mediators and growth factors. To confirm that disulfide HMGB1 induced macrophage activation via TLR4, peritoneal macrophages from WT, TLR4−/−, MyD88−/−, or TrifLps2 mice were treated with recombinant disulfide HMGB1 for 8 hours. Disulfide HMGB1 induced a concentration-dependent increase in the release of MCP1/CCL2, IL-6, PDGF-A, and tumor necrosis factor (TNF) into the supernatents of WT macrophages (Figure 5A–5D). The production of MCP1/CCL2, IL-6, and TNF was almost completely TLR4-dependent, whereas the production of PDGF-A was partially TLR4 dependent. A similar reduction in MCP1/CCL2, IL-6, TNF, and PDGF-A release in HMGB1-treated MyD88−/− macrophages to that seen in the TLR4−/− cells confirmed that production of these mediators requires MyD88. Production of MCP1/CCL2, TNF, and PDGF-A also seemed to be at least partially Trif dependent.
In addition to directing macrophage infiltration, the chemokine–chemokine receptor pair MCP-1/CCL2-CCR2 is known to promote intimal expansion.33–35 We next determined if HMGB1 could induce MCP1/CCL2 and CCR2 expression in cultured HASMCs. Exposure to disulfide HMGB1 5000 ng/mL for 24 hours induced concentration-dependent increases in MCP-1/CCL2 and CCR2 protein expression in cultured HASMC (Figure 5E). Disulfide HMGB1 also induced time-dependent increases in pIKKα/β and pIκBα protein expression between 0 and 24 hours of exposure (Figure 5G), without impacting total IKKα/β and IκBα protein levels (Figure VI in the online-only Data Supplement), which indicates nuclear factor κB activation in HMGB1-elicited HASMC. The role of TLR4 in this response was confirmed by pretreating the HASMC with a TLR4 inhibitory or control peptide, Viper versus CP-7 (30 μmol/L for each), respectively, for 1 hour (Figure 5F and 5H). Viper blocked HMGB1-induced MCP1/CCL2, pIKKα/β and pIκBα expression by HASMC whereas CP-7 did not.
Analyses by immunofluorescence demonstrated that CCR2 expression was induced in both the intima and adventitia of injured carotid arteries (Figure 5I). CCR2 started to colocalize with smooth muscle actin-α+ cells at 3 days and because expression continued to increase by 28 days, CCR2 became colocalized with smooth muscle actin-α. The expression of CCR2 was lower in the injured arteries from TLR4−/− mice compared with injured carotids from WT mice at both 3 and 28 days. These results suggest that MCP1/CCL2-CCR2 is involved in both early and late events in injury-induced vascular inflammation and that TLR4 is required for upregulation of both MCP1/CCL2 and CCR2.
HMGB1-Induced HASMC Migration, But Not Cell Viability, Is Mediated Via a TLR4-Dependent Mechanism
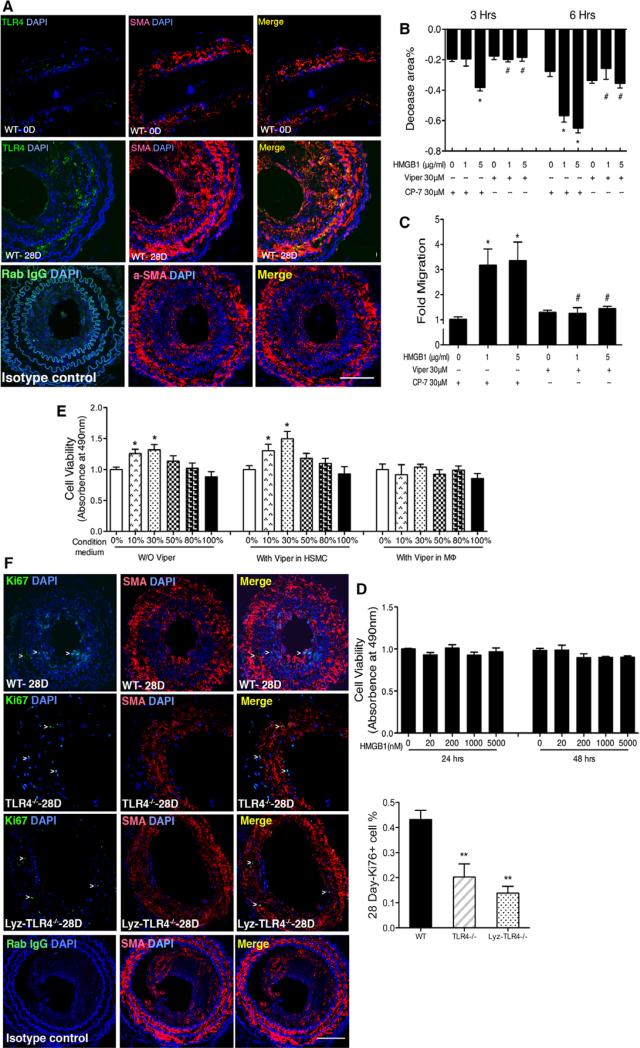
We next sought evidence for TLR4 expression in SMC in vivo after wire injury. As shown in Figure 7A, TLR4 expression was colocalized with smooth muscle actin-α at both baseline and at 28 days after injury. Two of the vascular smooth muscle responses critical for IH are migration and proliferation. Using a wound healing system, we found that disulfide HMGB1–induced migration of HASMC in a time and concentration-dependent manner (Figure 6B and 6C). This effect was blocked by the TLR4 antagonist (Viper) but not the control peptide CP-7. In contrast, disulfide HMGB1 had no effect on HASMC cell viability measured at 24 hours (Figure 6D).
Figure 7.
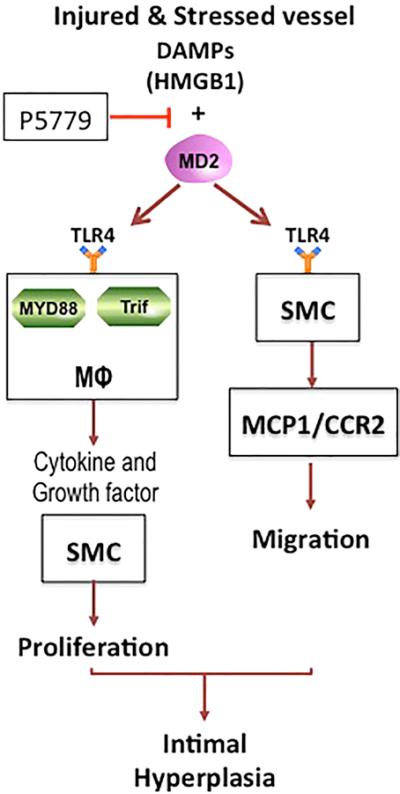
An illustration of potential mechanisms in injury-induced vascular intimal hyperplasia is depicted. We propose that intimal hyperplasia is driven by toll-like receptor 4 (TLR4)–myeloid differentiation factor 2 (MD2) on macrophages responding to high-mobility group box 1 (HMGB1) released by injured and stressed cells in the vessel wall. Blockade of HMGB1–MD2 interaction could be a therapeutic target. For detailed discussion of the pathways, please see the text. DAMP indicates damage-associated molecular pattern molecule; and SMC, smooth muscle cell.
Figure 6.
High-mobility group box 1 (HMGB1)–induced human aortic smooth muscle cell (HASMC) migration, but not cell viability, is mediated via a toll-like receptor 4 (TLR4)–dependent mechanism. A, Double immunofluorescence staining was performed to detect TLR4 expression (green) colocalized with SMA (red) in carotid arteries from wild-type (WT) mice at 3 and 28 days. Nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Representative fluorescent–stained sections are shown. B–C, TLR4 inhibitory peptide reduced HMGB1-induced HASMC migration in vitro. For in vitro migration experiments, HASMC were cultured in 6-well plates and cultured in serum-free medium in the absence or the presence of disulfide HMGB1. Images of the scratched areas under each condition were photographed and quantified at 3 and 6 hours. Quantitative analysis of HASMC migration toward medium with HMGB1 after 22 hours of incubation is shown. Data are presented as relative fold compared with medium without HMGB1. D, HMGB1 had no significant effect on HASMC proliferation. HASMC seeded in 96-well plates were treated with various doses of disulfide HMGB1 for 24 or 48 hours and the cell viability was determined at the end of experiment. E, Conditioned media from HMGB1-treated macrophages produced a dose-dependent effect on HASMC proliferation at 24 hours, which was diminished by blocking TLR4 on macrophages, not by blocking TLR4 on HASMC. The data are the mean±SEM of 3 independent experiments. F, Intimal Ki67+ positive cells (green) were reduced in TLR4−/− and Lyz-TLR4−/− mice compared with WT mice at 28 days postarterial injury. Nuclei are stained with DAPI (blue). Representative fluorescent–stained sections are shown. Data are presented as mean±SEM (n=3–4). Ki67-positive cell cross-sections were counted at 3 days (n=5) and 28 days (n=5). The number of K-positive cells was defined as the percentage of the total cells within a given area that stained positive for Ki67. *P<0.05 compared with control. #P<0.05 versus HMGB1-treated group. Scale bar, 100 μm
TNF-α,36 MCP-1/CCL2,29,35 IL-6,37 and PDGF-A38 are known to induce HASMC proliferation. As shown above, disulfide HMGB1 induces the production of these mediators from macrophages (Figure 5A–5D). We further determined whether conditioned media from disulfide HMGB1–treated macrophages promoted an increase in viable HASMC numbers by measuring cell viability. Conditioned media was collected from THP-1 macrophages after 8 hours incubation with 5000 ng/mL disulfide HMGB1 and transferred to HASMC. The number of viable HASMC was determined after 24 hours of conditioned media exposure at concentrations ranging from 10% to 50%. Conditioned media induced a concentration-dependent increase in HASMC viability (Figure 6E).
To investigate whether disulfide HMGB1 could enhance HASMC viability through a paracrine mechanism via TLR4 on macrophages, HASMC were cotreated with THP-1 cell-conditioned media and Viper 30 μmol/L or its control (CP-7 30 μmol/L). As shown in Figure 6E, Viper did not prevent the increase in HASMC viability induced by condition media. However, conditioned media from Viper-pretreated THP-1 macrophages failed to increase HASMC viability.
To seek in vivo evidence that signaling through TLR4 modulates cell proliferation, Ki67 staining analyses was performed to determine the number of proliferating cells in carotid arteries from WT, myeloid TLR4−/− (Lyz-TLR4−/−), and global TLR4−/− mice. Significantly, fewer Ki67 positive–stained cells were observed in the injured arteries of TLR4−/− mice and Lyz-TLR4−/− mice than vessels from WT mice at 28 days (Figure 6F). Thus, TLR4 signaling specifically on myeloid cells regulates cell proliferation within the vessel wall after arterial injury.
Discussion
This study was undertaken to determine if HMGB1 and one of its key receptors, TLR4, play central roles in vascular inflammation after endoluminal arterial injury. We show that HMGB1 expression is upregulated after wire injury to the carotid artery and that HMGB1 promotes IH and monocyte recruitment. TLR4 deletion also markedly suppresses IH, monocyte recruitment, HMGB1 upregulation, and inflammation after arterial injury, effects that are partially replicated by deletion of TLR4 on specifically on myeloid cells. A specific inhibitor of HMGB1–MD2/TLR4 interaction (P5779) suppressed IH after wire injury. Our mechanistic studies show that the disulfide isoform of HMGB1 stimulates vascular SMC migration and production of MCP1/CCL2, as well as the production of SMC growth factors by macrophages via TLR4. The key role of TLR4 on myeloid cells in the development of IH was confirmed in vivo using myeloid-specific TLR4−/− mice. Combined, these findings identify HMGB1 and TLR4 as regulators of the arterial response to endoluminal injury by regulating cell migration, monocyte trafficking, and inflammatory mediator and growth factor production. The findings also highlight the possibility of developing novel therapeutic strategies to attenuate DAMP-TLR4–mediated vascular inflammatory responses.
HMGB1 has emerged as an important proximal regulator of sterile inflammatory responses resulting from tissue trauma22 and ischemia.39 Most cells, including vascular SMC, endothelial cell, and monocytes, express HMGB1 in the nucleus at baseline.14 It can be readily mobilized to the cytoplasm under conditions of cellular stress (ie, hypoxia) where it regulates autophagy and nucleic acid sensing.10,28 HMGB1 reaches the extracellular space by passive mechanisms when cells die by necrosis or pyroptosis; or can be actively secreted in response to hypoxia or exposure to proinflammatory signals, including HMGB1 itself.28 HMGB1 exhibits proinflammatory cytokine-like activity, promotes chemotaxis, and stimulates cellular migration and growth.14 HMGB1 has been shown to trigger signaling through several plasma membrane and endosomal receptors which accounts, in part, for the diversity of its activities. Receptor specificity is only partially understood but is known to involve the redox status of HMGB1 as well as extraceullar- and intracellular-binding partners.40 For example, when all 3 cysteines, C23, C45, and C106, within HMGB1 are in the thiol configuration extracellular HMGB1 avidly interacts with C–X–C motif chemokine (CXCL)12 and activates signaling through CXCR4.24 Mild oxidation of this form leads to formation of a disulfide bond between C23 and C45 and it is this isoform that binds to MD2 to trigger TLR4 signaling.10,18 Further oxidation of any of the thiols inactivates extracellular HMGB1. We studied the role of 3 well-established HMGB1 receptors, including TLR4, TLR2, and RAGE, in the wire injury model. TLR4 and its coreceptor CD14 contributed most significantly to IH and inflammation in the mouse carotid wire injury model. Disulfide HMGB1 recognition by TLR4 in macrophages requires CD14.23 Therefore, our findings that both HMGB1 and TLR4/CD14 contribute to monocyte recruitment and IH and the finding that 5779 blocks this response are consistent with a role for the HMGB1-TLR4 axis and specifically disulfide HMGB1 in driving inflammation in acute arterial injury.
Others have implicated TLR4 in arterial remodeling by showing that lipopolysaccharide administration promotes IH induced by femoral artery periadventitial cuff placement in WT but not TLR4 mutant mice.19 We show that even in the absence of exogenous lipopolysaccharide, TLR4 is a major driver of the arterial response to endoluminal wire injury and provide evidence that TLR4 on myeloid cells is critical to the response. Myeloid-specific deletion of TLR4 only partially replicated the prevention of IH seen in the global TLR4−/− mice. Therefore, it is likely that TLR4 on other cell types also contributes to the injury response. This concept is further supported by our results showing that TLR4 and HMGB1 are both upregulated and colocalize in vascular SMC in the neointima. We also show that disulfide HMGB1 triggers both MCP1/CCL2-CCR2 upregulation and vascular SMC migration via TLR4 in vitro. However, the contribution of TLR4 on nonmyeloid cells, such as SMC, to IH after arterial injury remains to be determined.
Previous reports show that arterialized vein grafts from TLR4 mutant mice exhibit less thickening than WT controls and that TLR4 is involved in the vascular lesions associated with hypoxia-induced pulmonary artery hypertension. These studies combined with our findings suggest a more generalized role for TLR4 in injury-induced vascular wall remodeling.13 Inhibitors of TLR7 and TLR9 also limit neointimal thickening and macrophage activation in the femoral artery cuff model.41 HMGB1 can promote the sensing of intracellular self-DNA through TLR7 and TLR9.27 Our data show that anti-HMGB1 mAb is less effective at reducing IH than genetic deletion of HMGB1 suggesting that intracellular actions of HMGB1 may also be important to the arterial injury response. Therefore, HMGB1 may contribute to DAMP signaling through more than a single receptor or mechanisms after acute arterial injury.
The MCP1/CCL2-CCR2 axis is known to contribute to IH through multiple mechanisms, including the recruitment of monocytes to the injury site,29 as well as through stimulation of SMC migration and proliferation.33,34 We found that MCP1/CCL2 and CCR2 were upregulated in the arterial wall, including in SMC early after injury in a TLR4-dependent manner. In vitro studies confirmed that disulfide HMGB1 could upregulate MCP1/CCL2 through TLR4 in both vascular SMC and macrophages. This suggests that endogenous activators of TLR4, such as disulfide HMGB1, could be 1 important mechanism for the upregulation of chemokines and their receptors as well as growth factor production by both SMC and macrophages attracted to the site of injury.
Others have shown that unspecified isoforms of HMGB1 can stimulate both the migration and the proliferation of vascular SMC in vitro.14,42 Interestingly, we found that the disulfide isoform of HMGB1 triggers only SMC migration through TLR4 and not proliferation. Our data suggest that HMGB1-TLR4 axis contributes indirectly to vascular SMC proliferation by stimulating macrophages to produce SMC growth factors, such as IL-6 and PDGF-A in addition to MCP1/CCL2. Therefore, the effects of HMGB1 could be isoform and receptor specific. We did not determine if other HMGB1 isoforms can directly regulate SMC proliferation. Furthermore, neither the genetic HMGB1 deletion nor the anti-HMGB1 antibody used in this study differentiates between the isoforms of HMGB1 or its receptor targets in vivo.
TLR4 is unique among the TLR receptors in that it signals through both MyD88- and Trif-dependent signaling pathways.43 A role for MyD88 in flow and cuff-mediated vascular remodeling has been established,44 whereas TLR4/Trif-dependent signaling has been implicated in vascular matrix metalloproteinase upregulation.45 We extend these observations to show that MyD88 mRNA is upregulated in the vessel wall in a TLR4-dependent manner after endoluminal arterial injury. We confirm that both MyD88 and Trif contribute to wire injury-induced IH. The slight but significant reduction in IH observed in MyD88−/− was enhanced even greater if both My88 and Trif expression was suppressed. Therefore, both MyD88- and Trif-dependent signaling pathways are likely to overlap in their roles after endoluminal arterial injury. Both pathways can lead to nuclear factor κB activation and the induction of cytokine and chemokine production.43 The greater impact on IH by MyD88 deletion reported in the periadventitial cuff model44 than we observed in the wire injury model may reflect differences in the mechanism of vascular injury in the 2 models. Our findings suggest that TLR4 is one of the pathways that may activate signaling through Trif and MyD88.
In conclusion, our results provide support for the proposed sequence depicted in Figure 7, where injury induces the release of DAMP, such as HMGB1, from several cell sources. This triggers TLR4 activation leading to chemokine and growth factor production, which attracts monocytes into the injury site. These inflammatory cells become further activated via disulfide HMGB1–TLR4 interactions to produce SMC growth factors and inflammatory mediators. We speculate that disulfide HMGB1 may promote vascular SMC migration directly via TLR4, although this is not proven in vivo. We propose that these mechanisms act synergistically in the development of IH. This scenario offers numerous potential therapeutic targets, including HMGB1, the interaction of disulfide HMGB1 with TLR4/MD2, the CD14/TLR4/MD2 receptor complex, postreceptor signaling and specific growth factors or inflammatory mediators.
Supplementary Material
Significance.
In this study, we show for the first time that a damage-associated molecular pattern molecule, high-mobility group box 1, drives intimal hyperplasia and vascular remodeling through toll-like receptor 4/myeloid differentiation factor 2-myeloid differentiation primary response gene (88)/Trif signaling. Furthermore, we provide evidence that a specific isoform of high-mobility group box 1 known to trigger toll-like receptor 4 signaling (disulfide high-mobility group box 1) is involved in this process. We specifically delineated toll-like receptor 4 on monocytes as one of the critical receptors driving inflammation after acute arterial injury. These observations and the detailed mechanistic insights identify several potential therapeutic targets to limit vascular restenosis not previously considered.
Acknowledgments
Sources of Funding
This work was supported, in part, by the National Basic Research Program (973 Program) of China 2014CB542400 and the National Science Foundation of China Projects 81130004, 81370359, and 913392019 (all to Dr Chen), Project 81200236 and 81570271 (to J.J. Cai), the China National Major Scientific and Technological Special Project for Significant New Drugs Development 2012ZX09303014-001 (to H. Yuan), and National Institutes of Health R01 GM50441 (to T.R. Billiar).
Nonstandard Abbreviations and Acronyms
- DAMPs
damage-associated molecular pattern molecules
- HASMC
human aortic smooth muscle cell
- HMGB1
high-mobility group box 1
- IH
intimal hyperplasia
- IL
interleukin
- MCP-1/CCL2
mchemotactic protein 1
- MD2
myeloid differentiation protein 2
- MYD88
myeloid differentiation primary response gene (88)
- PDGF-A
platelet-derived growth factor-A
- RAGE
receptor for advanced glycation endproducts
- TLR
toll-like receptor
- TNF-α
tumor necrosis factors-α
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.115.305789.
Disclosures
None.
References
- 1.Fokkema M, den Hartog AG, Bots ML, van der Tweel I, Moll FL, de Borst GJ. Stenting versus surgery in patients with carotid stenosis after previous cervical radiation therapy: systematic review and meta-analysis. Stroke. 2012;43:793–801. doi: 10.1161/STROKEAHA.111.633743. doi: 10.1161/STROKEAHA.111.633743. [DOI] [PubMed] [Google Scholar]
- 2.Andras A, Hansrani M, Stewart M, Stansby G. Intravascular brachytherapy for peripheral vascular disease. Cochrane Database Syst Rev. 2014;1:CD003504. doi: 10.1002/14651858.CD003504.pub2. doi: 10.1002/14651858.CD003504.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seedial SM, Ghosh S, Saunders RS, Suwanabol PA, Shi X, Liu B, Kent KC. Local drug delivery to prevent restenosis. J Vasc Surg. 2013;57:1403–1414. doi: 10.1016/j.jvs.2012.12.069. doi: 10.1016/j.jvs.2012.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rensing BJ, Hermans WR, Vos J, Beatt KJ, Bossuyt P, Rutsch W, Serruys PW. Angiographic risk factors of luminal narrowing after coronary balloon angioplasty using balloon measurements to reflect stretch and elastic recoil at the dilation site. The CARPORT Study Group. Am J Cardiol. 1992;69:584–591. doi: 10.1016/0002-9149(92)90146-p. [DOI] [PubMed] [Google Scholar]
- 5.Dangas G, Kuepper F. Cardiology patient page. Restenosis: repeat narrowing of a coronary artery: prevention and treatment. Circulation. 2002;105:2586–2587. doi: 10.1161/01.cir.0000019122.00032.df. [DOI] [PubMed] [Google Scholar]
- 6.Liu MW, Roubin GS, King SB III. Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989;79:1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- 7.Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–1776. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Gardner SE, Clarke MC. Cell death, damage-associated molecular patterns, and sterile inflammation in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31:2781–2786. doi: 10.1161/ATVBAHA.111.224907. doi: 10.1161/ATVBAHA.111.224907. [DOI] [PubMed] [Google Scholar]
- 9.Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011;14:1315–1335. doi: 10.1089/ars.2010.3356. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai J, Wen J, Bauer E, Zhong H, Yuan H, Chen A. The role HMGB1 in cardiovascular biology: danger signals [published online ahead of print June 12, 2015]. Antioxid Redox Signal. doi: 10.1089/ars.2015.6408. doi: 10.1089/ars.2015.6408. http://online.liebertpub.com/doi/10.1089/ars.2015.6408. [DOI] [PubMed]
- 11.Kalinina N, Agrotis A, Antropova Y, DiVitto G, Kanellakis P, Kostolias G, Ilyinskaya O, Tararak E, Bobik A. Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol. 2004;24:2320–2325. doi: 10.1161/01.ATV.0000145573.36113.8a. doi: 10.1161/01.ATV.0000145573.36113.8a. [DOI] [PubMed] [Google Scholar]
- 12.Inoue K, Kawahara K, Biswas KK, Ando K, Mitsudo K, Nobuyoshi M, Maruyama I. HMGB1 expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques. Cardiovasc Pathol. 2007;16:136–143. doi: 10.1016/j.carpath.2006.11.006. doi: 10.1016/j.carpath.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Karper JC, de Vries MR, van den Brand BT, Hoefer IE, Fischer JW, Jukema JW, Niessen HW, Quax PH. Toll-like receptor 4 is involved in human and mouse vein graft remodeling, and local gene silencing reduces vein graft disease in hypercholesterolemic APOE*3Leiden mice. Arterioscler Thromb Vasc Biol. 2011;31:1033–1040. doi: 10.1161/ATVBAHA.111.223271. doi: 10.1161/ATVBAHA.111.223271. [DOI] [PubMed] [Google Scholar]
- 14.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 16.Porto A, Palumbo R, Pieroni M, Aprigliano G, Chiesa R, Sanvito F, Maseri A, Bianchi ME. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J. 2006;20:2565–2566. doi: 10.1096/fj.06-5867fje. doi: 10.1096/fj.06-5867fje. [DOI] [PubMed] [Google Scholar]
- 17.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93:865–873. doi: 10.1189/jlb.1212662. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vink A, Schoneveld AH, van der Meer JJ, van Middelaar BJ, Sluijter JP, Smeets MB, Quax PH, Lim SK, Borst C, Pasterkamp G, de Kleijn DP. In vivo evidence for a role of toll-like receptor 4 in the development of intimal lesions. Circulation. 2002;106:1985–1990. doi: 10.1161/01.cir.0000032146.75113.ee. [DOI] [PubMed] [Google Scholar]
- 20.Hollestelle SC, De Vries MR, Van Keulen JK, Schoneveld AH, Vink A, Strijder CF, Van Middelaar BJ, Pasterkamp G, Quax PH, De Kleijn DP. Toll-like receptor 4 is involved in outward arterial remodeling. Circulation. 2004;109:393–398. doi: 10.1161/01.CIR.0000109140.51366.72. doi: 10.1161/01.CIR.0000109140.51366.72. [DOI] [PubMed] [Google Scholar]
- 21.Boekholdt SM, Agema WR, Peters RJ, Zwinderman AH, van der Wall EE, Reitsma PH, Kastelein JJ, Jukema JW. REgression GRowth Evaluation Statin Study Group. Variants of toll-like receptor 4 modify the efficacy of statin therapy and the risk of cardiovascular events. Circulation. 2003;107:2416–2421. doi: 10.1161/01.CIR.0000068311.40161.28. doi: 10.1161/01.CIR.0000068311.40161.28. [DOI] [PubMed] [Google Scholar]
- 22.Venereau E, Schiraldi M, Uguccioni M, Bianchi ME. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol. 2013;55:76–82. doi: 10.1016/j.molimm.2012.10.037. doi: 10.1016/j.molimm.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Kim SY, Pribis JP, Lotze M, Mollen KP, Shapiro R, Loughran P, Scott MJ, Billiar TR. Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med. 2013;19:88–98. doi: 10.2119/molmed.2012.00306. doi: 10.2119/molmed.2012.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venereau E, Casalgrandi M, Schiraldi M, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–1528. doi: 10.1084/jem.20120189. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Lundbäck P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, Al-Abed Y, Andersson U, Tracey KJ, Antoine DJ. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol Med. 2012;18:250–259. doi: 10.2119/molmed.2011.00389. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Calogero S, Grassi F, Aguzzi A, Voigtländer T, Ferrier P, Ferrari S, Bianchi ME. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet. 1999;22:276–280. doi: 10.1038/10338. doi: 10.1038/10338. [DOI] [PubMed] [Google Scholar]
- 27.Yanai H, Ban T, Wang Z, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 28.Tang D, Billiar TR, Lotze MT. A Janus tale of two active high mobility group box 1 (HMGB1) redox states. Mol Med. 2012;18:1360–1362. doi: 10.2119/molmed.2012.00314. doi: 10.2119/molmed.2012.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Wang H, Ju Z, et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J Exp Med. 2015;212:5–14. doi: 10.1084/jem.20141318. doi: 10.1084/jem.20141318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hui DY. Intimal hyperplasia in murine models. Curr Drug Targets. 2008;9:251–260. doi: 10.2174/138945008783755601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nace GW, Huang H, Klune JR, Eid RE, Rosborough BR, Korff S, Li S, Shapiro RA, Stolz DB, Sodhi CP, Hackam DJ, Geller DA, Billiar TR, Tsung A. Cellular-specific role of toll-like receptor 4 in hepatic ischemia-reperfusion injury in mice. Hepatology. 2013;58:374–387. doi: 10.1002/hep.26346. doi: 10.1002/hep.26346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schepers A, Eefting D, Bonta PI, Grimbergen JM, de Vries MR, van Weel V, de Vries CJ, Egashira K, van Bockel JH, Quax PH. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006;26:2063–2069. doi: 10.1161/01.ATV.0000235694.69719.e2. doi: 10.1161/01.ATV.0000235694.69719.e2. [DOI] [PubMed] [Google Scholar]
- 34.Kim WJ, Chereshnev I, Gazdoiu M, Fallon JT, Rollins BJ, Taubman MB. MCP-1 deficiency is associated with reduced intimal hyperplasia after arterial injury. Biochem Biophys Res Commun. 2003;310:936–942. doi: 10.1016/j.bbrc.2003.09.088. [DOI] [PubMed] [Google Scholar]
- 35.Ali ZA, Bursill CA, Douglas G, McNeill E, Papaspyridonos M, Tatham AL, Bendall JK, Akhtar AM, Alp NJ, Greaves DR, Channon KM. CCR2-mediated antiinflammatory effects of endothelial tetrahydrobiopterin inhibit vascular injury-induced accelerated atherosclerosis. Circulation. 2008;118(suppl 14):S71–S77. doi: 10.1161/CIRCULATIONAHA.107.753558. doi: 10.1161/CIRCULATIONAHA.107.753558. [DOI] [PubMed] [Google Scholar]
- 36.Heo SK, Yun HJ, Park WH, Park SD. Rhein inhibits TNF-alpha-induced human aortic smooth muscle cell proliferation via mitochondrial-dependent apoptosis. J Vasc Res. 2009;46:375–386. doi: 10.1159/000189798. doi: 10.1159/000189798. [DOI] [PubMed] [Google Scholar]
- 37.Savale L, Tu L, Rideau D, Izziki M, Maitre B, Adnot S, Eddahibi S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res. 2009;10:6. doi: 10.1186/1465-9921-10-6. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Räber L, Wohlwend L, Wigger M, et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation. 2011;123:2819–28. doi: 10.1161/CIRCULATIONAHA.110.004762. 6 p following 2828. doi: 10.1161/CIRCULATIONAHA.110.004762. [DOI] [PubMed] [Google Scholar]
- 39.Izuishi K, Tsung A, Jeyabalan G, Critchlow ND, Li J, Tracey KJ, Demarco RA, Lotze MT, Fink MP, Geller DA, Billiar TR. Cutting edge: high-mobility group box 1 preconditioning protects against liver ischemia-reperfusion injury. J Immunol. 2006;176:7154–7158. doi: 10.4049/jimmunol.176.12.7154. [DOI] [PubMed] [Google Scholar]
- 40.Antoine DJ, Harris HE, Andersson U, Tracey KJ, Bianchi ME. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol Med. 2014;20:135–137. doi: 10.2119/molmed.2014.00022. doi: 10.2119/molmed.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karper JC, Ewing MM, Habets KL, de Vries MR, Peters EA, van Oeveren-Rietdijk AM, de Boer HC, Hamming JF, Kuiper J, Kandimalla ER, La Monica N, Jukema JW, Quax PH. Blocking toll-like receptors 7 and 9 reduces postinterventional remodeling via reduced macrophage activation, foam cell formation, and migration. Arterioscler Thromb Vasc Biol. 2012;32:e72–e80. doi: 10.1161/ATVBAHA.112.249391. doi: 10.1161/ATVBAHA.112.249391. [DOI] [PubMed] [Google Scholar]
- 42.Degryse B, Bonaldi T, Scaffidi P, Müller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pålsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saxena A, Rauch U, Berg KE, Andersson L, Hollender L, Carlsson AM, Gomez MF, Hultgårdh-Nilsson A, Nilsson J, Björkbacka H. The vascular repair process after injury of the carotid artery is regulated by IL-1RI and MyD88 signalling. Cardiovasc Res. 2011;91:350–357. doi: 10.1093/cvr/cvr075. doi: 10.1093/cvr/cvr075. [DOI] [PubMed] [Google Scholar]
- 45.Monaco C, Gregan SM, Navin TJ, Foxwell BM, Davies AH, Feldmann M. Toll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation. 2009;120:2462–2469. doi: 10.1161/CIRCULATIONAHA.109.851881. doi: 10.1161/CIRCULATIONAHA.109.851881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.