Plasticity is a general feature of all nervous systems, essential for the success and survival of organisms, allowing them to respond and adapt to their environment through the processes of learning and memory. Even relatively simple forms of learning, such as habituation, the process by which animals reduce their responsiveness to stimuli that have no immediate consequences, or sensitization, an increase in overall responsiveness following an arousing stimulus, involve changes in neural gene transcription, protein translation and the modification of synapses and networks (1). In a recent paper published in Current Biology (2), Frost and colleagues examine the mechanisms that underlie the sensitization of a swimming response in the marine mollusk, Tritonia, and discover that certain populations of neurons in the pedal ganglion network are recruited to participate in the sensitized swim response, presumably to enhance the responsiveness of the animal to other potential threatening stimuli in the environment.
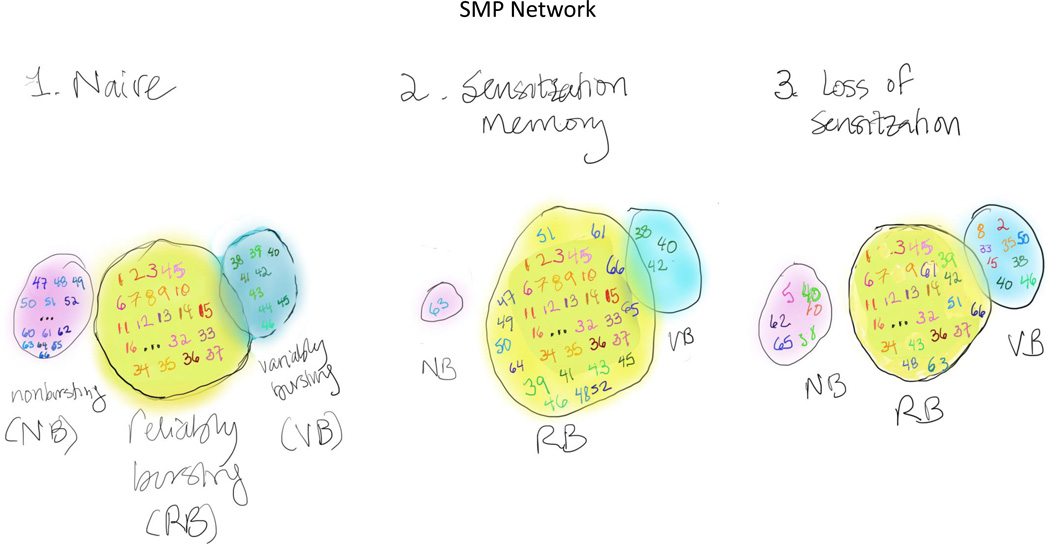
To identify the neurons and determine the network mechanisms that contribute to sensitization in Tritonia, Hill et al. made use of a voltage-sensitive dye that allowed them to monitor, simultaneously, the activity of up to ~65 neurons in the pedal ganglion (2,3). For many rhythmic motor behaviors like swimming, the neural activity that drives and sustains these behaviors involve central pattern generating circuits and “bursting”, clusters of action potentials elicited with a relatively high frequency, that serve to entrain and drive downstream neurons in the network. Previous studies have shown that following a sensitizing stimulus (shock to the pedal nerve), the onset latency for the Tritonia SMP is reduced, indicating that the system exhibits sensitization (4,5). In the current study, Hill et al. set out to examine the contribution of individual neurons to the SMP before, during and after sensitization. To do so, they built upon their earlier identification of neurons within the pedal ganglion that contribute to the swim motor program (SMP) with different propensities to burst, classified as reliable bursters, variable bursters and non-bursters (3). By monitoring the activity of each class of neuron, they observed that following sensitization, the number of neurons that exhibited reliable bursting behavior was significantly enhanced. This increase in the number of reliable bursters was due to the conversion of some neurons from variable or non-bursting to reliable bursting phenotypes. Consistent with sensitization arising from an expanded SMP network, dissipation of sensitization was accompanied by a return to the original network size. Remarkably, however, the constituent neurons in the network following loss of sensitization was distinct from that in the naïve network, indicating that the SMP is encoded by a dynamic network rather than by a fixed network of specific neurons.
To identify the cellular mechanisms that drive the reorganization observed during sensitization of the SMP, Hill et al. (2) focused on a class of serotonergic neurons previously identified to be a part of the swim central pattern generator (6). Not only did they find that stimulation of these neurons decreased the SMP latency, consistent with sensitization, but they also showed that direct application of the serotonin to the pedal ganglion decreased SMP latency and increased the number of reliable burster neurons in the SMP network. As such, activation of a small number of serotonergic neurons was sufficient to implant a “false sensitization memory ” in the system.
The findings of Hill et al. (2) add to a rich history of discoveries about the mechanisms of learning and memory in invertebrate “simple systems.” Although these simple systems contain a relatively small number of neurons, they undergo multiple and robust forms of learning. Two features contribute to the experimental tractability of these simple systems. First, the neurons are often identifiable, recognizable from animal to animal. Second, dissected preparations undergo forms of plasticity that mirror learning in the animal. These features facilitate the delineation of circuits underlying behavioral modification, and become even more powerful when combined, as by Hill et al. (2), with the use of voltage -sensitive dyes to monitor, simultaneously, the activity of many neurons in a circuit.
The “simple” conclusion from Hill et al. (2) is that memories are stored as expansion in the number of neurons in networks underlying behavior. The idea is that neurons are predisposed to join a given network, and that learning, acting via neuromodulation, commits these predisposed neurons to the network. This “simple” idea is contrasted with what the authors consider the prevailing view that memories are stored as activity-dependent changes in synaptic strength and number, or synaptic plasticity. However, just as simple systems generate complex behaviors from a small number of neurons and circuits, they also have been shown to do so using multiple mechanisms. While studies in the marine mollusk Aplysia californica have emphasized the importance of changes in synaptic strength and number in mediating learning, including sensitization (7), other studies in Aplysia and the related mollusk Hermissenda, have identified “nonsynaptic” mechanisms, including changes in excitability that occur together with synaptic changes in both nonassociative and associative forms of learning (8,9). A remarkable set of studies on a central pattern generator in another invertebrate “simple system,” the lobster stomatogastric ganglion (STG), has revealed tremendous functional variability in neuronal networks, emerging from activity-dependent changes in synaptic strength and excitability (10). The findings of Frost and colleagues are indeed reminiscent of the STG work that established that neurons switch allegiance from one motor pattern to another under neuromodulatory control (11), indicating that the same circuit elements can be recombined in numerous ways, to generate behavioral flexibility.
As such, Hill et al’s (2) partisan framework of synaptic plasticity opposed to network expansion, of changes in synaptic strength opposed to alterations in neuronal excitability, is simply too simple. The authors do not explore the mechanism(s) by which the bursting profile of the individual neurons within the network are altered- changes in bursting behavior could be elicited by changes in intrinsic ionic conductances but also could be mediated by alterations in excitatory and/or inhibitory transmission in the network. In fact, the two mechanisms often co-occur (10). For example, the usual line-up of ion channels that alter excitability in neuronal processes can also change the properties of axon terminal depolarization and repolarization, resulting in changes in the kinetics and amount of transmitter released. Thus, our bet is that, in the end, the answer will be that it’s not one single mechanism as opposed to another, but rather that “it’s both,” a conclusion reached more often than not in arguments of mechanism in neurobiology.
Figure 1.
The swimming motor program (SMP) network in Tritonia consists of approximately 65 neurons. These can be divided into regularly bursting (RB, yellow circle, orange/red numbers), variably bursting (VB, blue circle, green numbers) and nonbursting (pink circle, blue numbers). During sensitization, both VB and NB neurons are converted to RB neurons, such that the number of RB neurons is increased. Following loss of sensitization, there is a return to the basal number of RB, VB and NB neurons, but the actual composition of neurons is different, with some previously RB neurons now in the VB or NB category, some VB neurons now in the RB or NB category, and some NB neurons now in the VB or RB category.
Contributor Information
K.C. Martin, Email: kcmartin@mednet.ucla.edu.
E.M. Schuman, Email: erin.schuman@brain.mpg.de.
References
- 1.Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Hill ES, Vasireddi SK, Wang J, Bruno AM, Frost WN WN. Memory formation in Tritonia via recruitment of variably committed neurons. Current Biology. 2015;25:2879–2888. doi: 10.1016/j.cub.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill ES, Vasireddi SK, Wang J, Bruno AM, Frost WN WN. Variable neuronal participation in stereotypic motor programs. PLoS One. 2012;7:e40579. doi: 10.1371/journal.pone.0040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown GD, Frost WN, Getting PA. Habituation and interative enhancement of multiple components of the Tritonia swim response. Behav Neurosci. 1996;110:478–485. doi: 10.1037//0735-7044.110.3.478. [DOI] [PubMed] [Google Scholar]
- 5.Frost WN, Brandon CL, Mongeluzi DL. Sensitization of the Tritonia escape swim. Neurobiol. Learn. Mem. 1998;69:126–135. doi: 10.1006/nlme.1997.3816. [DOI] [PubMed] [Google Scholar]
- 6.Getting PA, Lennard PR, Hume RI. Central pattern generator mediating swimming in Tritonia. I. Identification and synaptic interactions. J Neurophysiol. 1980;44:151–164. doi: 10.1152/jn.1980.44.1.151. [DOI] [PubMed] [Google Scholar]
- 7.Bailey CH, Kandel ER. Synaptic remodeling, synaptic growth and the storage of long-term memory in Aplysia. Prog. Brain Res. 2008;169:179–198. doi: 10.1016/S0079-6123(07)00010-6. [DOI] [PubMed] [Google Scholar]
- 8.Schuman EM, Clark GA. Synaptic facilitation at connections of Hermissenda type B photoreceptors. J Neurosci. 1994;14:1613–1622. doi: 10.1523/JNEUROSCI.14-03-01613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkins RD, Byrne JH. Associative learning in invertebrates. Cold Spring Harb Perspect Biol. 2015;7:pii: a021709. doi: 10.1101/cshperspect.a021709. doi: 10.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Destexhe A, Marder E. Plasticity in single neuron and circuit computations. Nature. 2004;431:789–795. doi: 10.1038/nature03011. [DOI] [PubMed] [Google Scholar]
- 11.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]