INTRODUCTION
The dream of transforming thought into action is becoming possible, as research in brain–machine interfaces (BMIs) progresses. In fact, the last decade experienced a dramatic increase in BMI research with impressive demonstrations of rodents, nonhuman primates, and humans controlling robots, wheelchairs, and graphical cursors in real time through signals collected from the brain. BMIs aim to improve the quality of life for large numbers of neurological patients. In particular, the long-term goal of cortical BMIs is to create brain-controlled prostheses capable of reproducing the wide range of motor and sensory functions carried out by humans, so that patients can enact their voluntary motor intentions simply by thought. In particular, this novel technology is meant to play a major role in the near future as a serious contribution to spinal cord rehabilitation. The BMI paradigm contends that a user can perceive sensory information and enact voluntary motor actions through a direct interface between the brain and an artificial actuator in virtually the same way that we see, walk, or grab an object with our own natural limbs.
A primary goal over the last few years has been to develop algorithms capable of “translating” thoughts into a language that the interface computer could understand. Such understanding would trigger the desired output: movement, choice of letters or words, etc. There are multiple tools capable of providing information on a subject or animal’s brain status. To some extent these tools could be classified as invasive or noninvasive. Techniques such as magnetoencephalography (MEG) or electroencephalography (EEG) provide the possibility of recording activity from different brain regions with exquisite temporal resolution and moderate spatial resolution in intact behaving humans. Because of low signal to noise ratio (SNR), most of the published BMI works using EEG are offline studies in which numerous trials must be averaged for the SNR to be sufficient for use in a BMI system. Few groups are succeeding at online control studies with single trials, such as the wheelchair control work of Millan and colleagues (Galan et al., 2008). Two classes of EEG-based BMIs can be distinguished: asynchronous and synchronous BMIs. Asynchronous systems will look for changes in amplitude, frequency, or phase of natural rhythmic signals of the brain, linked to the volitional activity of the subject. For example, the amplitude of μ-rhythm (8–12 Hz) is modified not only when a subject performs a movement, but also when he or she imagines it. After proper training, subjects can learn how to control this rhythm to perform a specific action; a recent study (Galan et al., 2008) showed an impressive demonstration of a brain-actuated wheelchair. Synchronous systems aim at detecting specific events in the EEG signals, which are caused by stimuli perceived by the subject (ERPs: event-related potentials). Specifically, P300-type ERPs (meaning that the measured EEG potential occurs 300 ms after the stimulus) caused by visual, tactile, or auditory stimuli have been used to control computer-based tasks. One advantage of synchronous systems is that they do not require any training period from the user, since ERPs are an innate feature of the brain. However, several repetitions of the stimulus are needed in order to extract the information from noisy signals. Improvements may come from source localization methods, which allow better discrimination among events using spatial information.
Other techniques, such as transcranial magnetic (TMS) and direct current stimulation (tDCS), allow non-invasively the purposeful modulation of excitability in relatively focal cortical areas (Hallett, 2000; Nitsche et al., 2003; Siebner and Rothwell, 2003; Gandiga et al., 2006; Reis et al., 2008). While MEG and EEG are being used to measure brain activity with the purpose of evaluating cortical reorganization (Buch et al., 2008) or controlling BMIs (Wolpaw et al., 2002; Wolpaw and McFarland, 2004; Birbaumer and Cohen, 2007), TMS and tDCS have different uses. With particular stimulating parameters, TMS and tDCS can up- or downregulate activity in the stimulated cortical areas. This particular application has been used to evaluate the behavioral consequences of transient disruption of activity in specific cortical areas. If such disruption induces disruption in behavior, it has been inferred that there is a cause–effect link between activity in that area and control of the disrupted behavior. With different parameters of stimulation, TMS and tDCS could be used to facilitate excitability in superficial cortical areas. This particular application has been utilized in the setting of patients with brain lesions and to facilitate motor learning, for example, in healthy human subjects (Antal et al., 2004; Reis et al., 2009), and to enhance the beneficial effects of rehabilitative treatments after brain lesions such as stroke (Hummel and Cohen, 2005; Hummel et al., 2005). Taken together, these noninvasive brain stimulation techniques provide an exciting opportunity to purposefully increase or decrease cortical excitability in target cortical areas in order noninvasively to modulate behavior and improve training effects in neurorehabilitation. In addition to these noninvasive brain stimulation techniques, it has been proposed that direct cortical stimulation through invasive procedures could have comparable facilitatory effects, as, for example, tested in patients with stroke using epidural stimulation (Brown et al., 2006).
One advantage of the use of noninvasive brain stimulation techniques such as TMS and tDCS lies in the possibility of applying them to a wider variety of patients in neurorehabilitative settings – for example, immediately preceding or simultaneously with rehabilitative treatments after brain lesions. Once our understanding of the optimal parameters of stimulation and optimal timing of application to facilitate Hebbian learning improves, they are likely to become part of the armamentarium of neurologists, physiatrists, and therapists (Birbaumer and Cohen, 2007). At this time, though, all uses of these devices are carried out under specific research protocols.
On the invasive side, two main recording technologies dominate the BMI spectrum: electrocorticography (ECoG), and cortically implanted microelectrode arrays. The nature of the signals recorded via ECoG is similar to EEG, since it measures electrical potentials resulting from the spatial average of a large area of the brain, and hence utilizes a large group of neurons. However, the fact that the recording electrodes are placed under the dura leads to higher spatial resolution than EEG (i.e., tenths of millimeters versus centimeters), broader bandwidth (i.e., 0–500 Hz versus 0–50 Hz), higher characteristic amplitude (i.e., 50–100 μV versus 10–20 μV), and far less vulnerability to artifacts such as EMG or ambient noise. The main drawback with respect to EEG is the invasiveness of the procedure as it requires opening the skull, and in the case of subdural implants, also opening the dura mater. Also similar to EEG, ECoG can be used either in an asynchronous or a synchronous manner. Schalk and colleagues showed recently an application of asynchronous ECoG to 2D cursor control with good success rates. In this study, ECoG features, such as spectrum amplitude at specific frequencies and electrode locations, were used to drive the cursor in one or the other direction (Schalk et al., 2008).
Microelectrode arrays are a much more invasive physical interface, but they allow for the collection of spiking data from neural ensembles. To date, this is the only recording technique with the ability to reconstruct the intended movements of the subject with high accuracy. Microelectrode arrays are chronically implanted in frontoparietal areas of the brain, such as the primary motor cortex (M1), the dorsal premotor cortex (PMd), or the posterior parietal cortex (PPC). Single unit and multiunit activity are recorded on each electrode. The activity of each recording site must be sorted in real time for use in a BMI paradigm; units with a clearly distinguishable waveform are isolated. An “instantaneous” spiking frequency, or spike count for a given time bin, is derived for each cell, and then used as input by the decoding algorithm. Of great concern with implanted microelectrode arrays is their stability, both in short and long periods of time. In the short term, one must confirm, from one day to another, that the same cells are used by the decoder. This is not taken for granted, since microelectrodes can move into the brain, leading to changes in the observed waveforms, or to a below-threshold SNR. In the long term, the brain tends to protect itself by creating a layer of scar tissue around the electrodes, leading to a slow decrease of the SNR. Thus far, studies have reported high quality recordings up to 18 months after surgery (Nicolelis et al., 2003).
The rest of this chapter is structured as follows: in the second section we focus on cortical BMIs that use microelectrode arrays to sample the activity of neural ensembles in awake animals. The third section reviews MEG and its applications to noninvasive BMI, and the fourth section covers the use of TMS. Finally, we discuss the field with respect to future implications for spinal injury in humans.
CORTICAL BRAIN^MACHINE INTERFACES
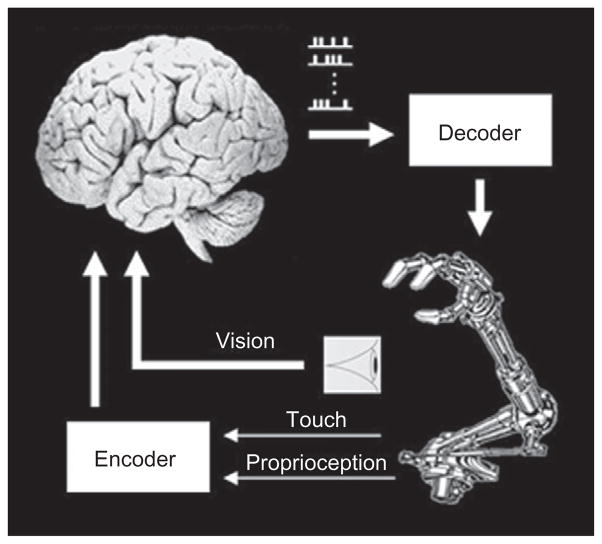
Current technology for interfacing the brain consists of microwire arrays that are implanted chronically in sensory and motor-related areas of the brain. The neuronal signals recorded from these microelectrodes are fed into a mathematical algorithm (i.e., the decoder) that translates these signals into a motor plan (i.e., the subject’s intention of movement), which is then streamed to an artificial actuator, the prosthetic arm. A closed control loop is established by providing the subject with visual feedback of the prosthetic arm (Nicolelis, 2001, 2003). Current directions in BMI research explore ways of increasing the feedback by also delivering tactile and proprioceptive feedback from the prosthetic device. Figure 27.1 illustrates the concept of a sensorimotor BMI.
Fig. 27.1.
Schematic description of a BMI. Components include chronically implanted microelectrodes, data processing and telemetry, decoding algorithm, control signal, and a robotic arm. The loop is closed through visual and sensory feedback from the prosthesis.
Estimation and control
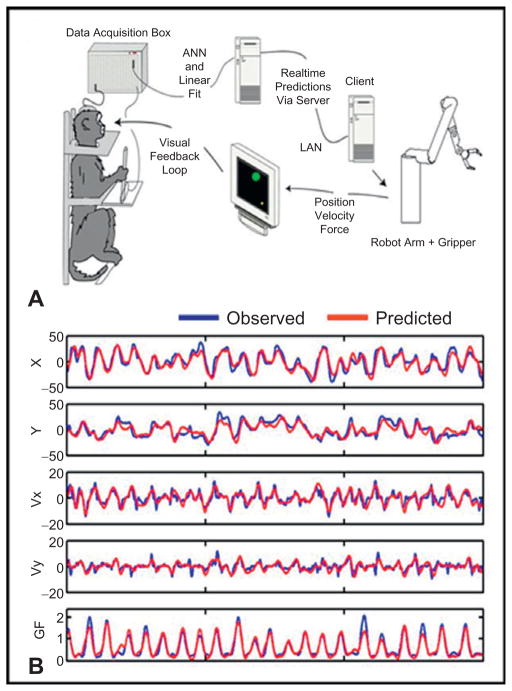
The role of the decoding algorithm is to transform the neural activity into a control signal for the prosthetic device. The usual procedure to build a decoder is to first have the subject perform the task manually while spike activity and motor parameters are being recorded (also referred to as “manual control”), followed by training of the decoder with the chosen algorithm. The trained model is then used to generate predictions of motor parameters from neural data recorded online (also referred to as ‘brain control’). Figure 27.2A depicts the experimental setup for a reach and grasp BMI study in nonhuman primates (Carmena et al., 2003).
Fig. 27.2.
(A) Behavioral setup consisting of the data acquisition system, the computer running multiple linear models in real time, the robot arm equipped with a gripper, and the visual display. (B) Performance of linear model predicting multiple parameters of arm movement and gripping force.
Various types of modeling approaches have been tested in previous studies. Among them, three different kinds of linear models have been widely used to predict movement based on spike activity: the population vector algorithm, multidimensional linear regression methods such as the Wiener filter, and dynamic filters such as the Kalman filter.
The population vector algorithm used in several BMI studies (Taylor et al., 2002; Velliste et al., 2008) was proposed by Georgopoulos and colleagues in the 1980s (Georgopoulos et al., 1986). They originally observed that M1 neurons were broadly tuned to hand movement direction, noting that each neuron had a preferred direction for which it exhibited maximal firing rate. In this algorithm, the preferred directions of individual neurons are independently determined during a training phase. Then, during the online phase, the preferred directions of individual neurons are weighted by their instantaneous firing rate. The sum of the weighted preferred directions defines the population vector which estimates the current movement direction. The movement speed can be inferred from the magnitude of the population vector. Regression models use behavioral variables, such as hand position or velocity, as a weighted linear combination of neuronal activity (Wessberg et al., 2000; Serruya et al., 2002; Carmena et al., 2003; Hochberg et al., 2006). The filter weights are determined using a multidimensional linear regression. The spike count for each neuron is typically binned and regressed at several time lags, that is, for a movement prediction at time t, neural data from time t, t-1, … t-l will be used, where l is the number of time lags. This allows the model to capture time-delayed dependencies between neural activity and movement that exist across different cortical areas. Similar approaches using nonlinear elements, such as artificial neural networks, have also proved to be efficient. The models described thus far can be viewed as attempts to directly approximate the mapping from neural firing rates to movement. In contrast, most models of neural encoding express the neural activity as a function of a stimulus. This motivated other groups (Mulliken et al., 2008) to use dynamic filtering models, where an explicit generative model of neural firing rate is used. In the Kalman filter framework, one can model the hand behavior as the system state, and the firing rate as the observation (measurement). The observation is a linear function of the state at time t, assumed to be a linear function of state a time t-1, plus Gaussian noise. The linear relationships between consecutive states and between state and observation are estimated from training data using least squares estimation, thus building the Kalman model. The Kalman filter is derived from the model, allowing online estimation of the state (behavior) based on the observation (neural data). Recently, more sophisticated decoding schemes have been proposed based on hidden Markov models and Bayesian inference approaches. Data acquired during the manual task is generally divided into two sets: a training set, and a test set. The test set is used for evaluating the prediction performance of the decoding algorithms. The metric commonly used to evaluate the performance of a decoder is the squared correlation coefficient between the predicted movement and the actual movement. This performance is often referred to as the “offline performance” of the decoding algorithm because it does not imply any real-time prediction of the movement, nor any feedback to the user. Figure 27.2B shows records of observed (blue traces) movement parameters (position X, position Y, velocity X, velocity Y, and gripping force) and model predictions (red traces) from a macaque monkey performing a reaching and grasping task.
Once the motor parameters are estimated, the next step in the BMI loop is to control the prosthetic device. Research in cortical BMIs has flourished in the last decade with impressive demonstrations of control of robots and computer cursors in real time through single unit, multiunit, and field potential signals collected from the brain. These demonstrations can be divided largely into two categories: either continuous control of position or velocity (Serruya et al., 2002; Taylor et al., 2002; Carmena et al., 2003; Hochberg et al., 2006; Mortiz et al., 2008; Velliste et al., 2008), or discrete control of more abstract information such as intended targets, intended actions, and the onset of movements (Musallam et al., 2004; Santhanam et al., 2006). The former are typically referred to as motor prosthetics whereas the latter are referred to as communication prosthetics.
In BMI systems that use the continuous control strategy, much of the work has treated the primate as a pure motion source. This makes intuitive sense considering that numerous studies show that most cortical activity is broadly tuned for higher-level features of hand movement, such as position (Georgopoulos et al., 1983) and velocity (Schwartz, 1994; Moran and Schwartz, 1999). Using this approach, neural recordings have been used to predict hand trajectory with reasonable accuracy (Wessberg et al., 2000; Serruya et al., 2002; Taylor et al., 2002; Carmena et al., 2003; Hochberg et al., 2006; Velliste et al., 2008). The continuous control strategy has proven to be a successful approach for confirming the potential of BMIs to decode movement features from cortical areas in order to drive neuroprostheses for the impaired.
The approach to estimation and control of motor intention in BMIs depends on a deep-rooted argument in neuroscience about how arm movement is encoded in the motor cortex. Does the motor cortex only specify high-level movement features and leave the conversion of this relatively abstract representation to muscle commands for neurons downstream, or, in conjunction with other cortical areas, does it calculate the inverse dynamics and kinematics of the limb in order to send muscle activity information directly? In support of the “abstract motion” view, Georgopoulous and colleagues originally observed that M1 neurons were broadly tuned to hand movement direction, noting that each neuron had a preferred direction for which it exhibited maximal firing rate (Georgopoulos et al., 1982). Yet there is persuasive evidence supporting that M1 does not purely encode motion in hand space. Kalaska et al. (1989) and Sergio and Kalaska (1998) observed that the same cells showing directional tuning for movement also encode force on an object during movement in an isometric task. Moreover, several studies have found systematic differences in neural activity depending on joint configuration for identical movement directions (Kalaska et al., 1989; Kakei et al., 1999). Thus, which of the two views, direction of movement or muscle activity, better describes the function of the motor cortex remains uncertain. The most likely answer is a combination of both, as suggested by recent studies (Li et al., 2001; Morrow et al., 2007).
As seen above, current BMI demonstrations have shown the potential for controlling a neuroprosthesis under pure motion control, that is, predicting end effector kinematics from neural ensemble activity with reasonable accuracy using linear decoders. However, these demonstrations failed to show a precise control of limb posture, lacking the ability to slow or stop motion with the same neural signals that were driving the device. In most of these studies, the behavioral tasks did not require holding at a given position for a long time, and trials initiated at the center of the workspace. In fact, for real world tasks, pure motion control lacks the information required for versatile manipulation in which the dynamic interactions of forces and torques between the musculoskeletal system and the environment play a crucial role. In the human body, the neuromuscular system naturally modulates mechanical impedance (i.e., intended inertia, damping and stiffness). Numerous studies show that this modulation is essential for versatile interaction with the environment (Hogan, 1985; Burdet et al., 2001; Hogan, 2002; Selen et al., 2006). For example, when using a screwdriver, stabilization of the hand by stiffness modulation is required to prevent slipping off the screw head. To date, modulation of mechanical impedance has not been utilized as a control signal in BMIs. Hence, neuroprosthetic devices that incorporate modulation of upper limb stiffness will provide more reliable performance in real world scenarios.
Prosthetic feedback
Finally, one of the new avenues towards bringing this technology to the clinical realm is the delivery of sensory feedback from the prosthetic device to the subject’s brain via intracortical electrical microstimulation (ICMS). While visual feedback has proven to be enough to close the loop and prove the concept of brain control, it is probably insufficient for more complex motor tasks that require dexterous manipulation of objects or precise information of the limb in space. Successful encoding of proprioceptive and tactile feedback from the prosthetic device should make control of a prosthetic device more natural and therefore increase performance and reliability.
Electrical stimulation of brain structures has been used for over 100 years to uncover how the brain mediates various psychological processes as well as for clinical applications. Previous ICMS work has sought to deliver artificial sensory stimuli to the somatosensory, visual, and auditory cortices. In fact, cortical microstimulation is known to evoke motor and sensory effects that mimic the functional contribution of the stimulated area (Salzman et al., 1990; Graziano et al., 2002; Cohen and Newsome, 2004; Tehovnik and Slocum, 2007). Recent advances in cortically controlled motor prostheses have sparked interest in encoding somatosensory feedback using cortical microstimulation. In the somatosensory system, it has been shown that animals can detect microstimulation (Otto et al., 2005), discriminate between stimulation in different cortical regions (Talwar et al., 2002), and discriminate different spatiotemporal patterns of stimulation (Fitzsimmons et al., 2007). Furthermore, pioneering work in the monkey somatosensory system (Romo et al., 1998, 2000) has shown that microstimulation of quickly adapting cells can mimic the perception of flutter stimulus on fingers and can be memorized and compared to other stimuli. However, there are both technological and conceptual barriers to overcome before the full potential of ICMS for therapeutic technology may be realized. Specifically, current hardware makes it difficult to study the early (< 10 ms) neural response simultaneously with behavior, leaving experimenters to infer rather than observe the full effects of ICMS. Furthermore, little work has addressed how ICMS alters local neural activity over time, as well as how behavioral use structures this neural response.
Also, in order to achieve long-term chronic microstimulation, as will be required in a neuroprosthetic application, it is essential to ensure that no long-term damage is caused to either the neighboring cells or the recording/stimulating electrodes. Of primary importance is that the net charge in each stimulation waveform is kept zero. If charge balance is not maintained, the net charge accumulation on the electrode over time may increase the electrode potential to the point where electrolysis occurs. Even while using charge-balanced waveforms, two safety limits should be considered. Neural damage limits are dependent on the ability of biological tissue to withstand the electric current without degrading. Electrochemical limits are based on the ability of the electrode to store or dissipate electrical charge without exceeding the water window, which is the potential window outside of which significant bubble formation is evident at the interface. Iridium oxide is the material of choice in present-day stimulating systems.
Towards shared control
Here we have shown how an artificial device (cursor/robot) can be controlled directly by the brain. This was achieved by volitionally modulating neural ensemble activity through a decoding algorithm that performs a linear mapping between the neuronal activation in several areas of the primate’s brain and its behavioral output. However, in order fully to restore control of upper limb movements, a neuroprosthetic device may need to incorporate general physiological principles of how motor signals underlying these movements are encoded in the brain. In other words, the decoding algorithms may need to incorporate physiological knowledge. Still, this may not be enough to reach the performance level an injured patient would desire. In fact, dexterous manipulation in humans is one of the most impressive examples of motor control, and requires a significant amount of skill. The relatively low bandwidth in current BMI works (~10 Hz) and the lack of sensory feedback makes the task of restoring hand dexterity using an artificial limb (or robotic actuator) extremely challenging, and perhaps not feasible with current technology.
On the other hand, there is the availability of the robotic domain, namely exploiting the fields of control theory and artificial intelligence, among others, and creating a hybrid BMI that will incorporate both real (neuronal) and artificial signals in a way that would allow a patient to accomplish tasks more accurately than when using neuronal signals only. For example, one could think of a BMI that will decode the intention of movement directly from neuronal signals, and leave the path planning execution, obstacle avoidance, and final refinement on grasping to a control module incorporated into the robot. This control module would have inputs from neuronal signals as well as readings from sensors embedded in the robot. At this point, the following question arises: what ratio of neuronal versus artificial signal is needed for optimal control of a BMI? In response, Kim et al. (2006) introduced the concept of continuous shared control (CSC) in BMIs. As the authors indicate:
the control is continuous because the interaction is immediate and does not have the “wait and see” characteristics of a planner-based approach or the switching characteristic of a traded-control. The control is shared because it always reflects input of both brain and sensor, as distinguished from traded control where control switches discreetly from direct operator control to the autonomy of the robot depending on task and situation.
Kim et al. (2006) tested this idea on real data from a previous study in which macaque monkeys were reaching and grasping virtual objects using only brain-derived signals. However, to reach and squeeze a real object with the required force at the right location is an extremely difficult task. A three degrees of freedom (3 DOF) robot with a pneumatic gripper that incorporated optical sensors was used to replicate the task using CSC. This gripper produced reflex-like reactions to augment the brain-controlled trajectories, providing obstacle avoidance and stabilized grasping. Different levels of sensor-based reflex effort were tested, and the ratio of 70% brain command and 30% sensor command was the optimal level, resulting in a seven-fold increase in task performance. This significant improvement in performance suggests the use of CSC to be critical in the future development of BMI systems.
MAGNETOENCEPHALOGRAPHY
As seen so far, in the last few years, an increasing number of BMI systems have been proposed (Nicolelis, 2001, 2003; Birbaumer and Cohen, 2007). All of them record, decode, and translate measurable neurophysiological signals into action of a mechanical device. BMI systems used invasive microelectrode arrays to gain information from single-unit spiking activity (Carmena et al., 2003; Hochberg et al., 2006) and/or local field potentials, and subdural electrodes to record ECoG (Leuthardt et al., 2006; Schalk et al., 2008). Noninvasive approaches have utilized EEG (Wolpaw et al., 2002; Wolpaw and McFarland, 2004), MEG (Mellinger, 2007), blood oxygen level-dependent functional magnetic resonance imaging (BOLD fMRI) (Weiskopf et al., 2003), and near infrared spectroscopy (NIRS) (Sitaram et al., 2007). Relatively few attempts have been made to apply these technologies to patient groups, focusing primarily on patients with amyotrophic lateral sclerosis (ALS) or tetraplegia (Wolpaw et al., 2002; Birbaumer and Cohen, 2007). More recently, an MEG-based BMI system has been proposed for patients with chronic stroke and substantial hand motor paralysis (Buch et al., 2008). It was shown that most patients with chronic stroke and complete hand paralysis can learn to modulate μ rhythm amplitude to achieve binary control of an orthosis that manipulates the grasping posture of the plegic hand. What was more interesting about these findings was that such control was achieved using MEG signals recorded over the ipsilesional hemisphere. Therefore, it is possible using such MEG-based approach to “train” ipsilesional brain areas after stroke that control movement, facilitating perhaps cortical reorganization. One advantage of MEG is that it provides exquisite temporal resolution and reasonable spatial resolution. One problem is that, while very useful to “train” the brain in a laboratory environment, it cannot be used when the patient leaves the hospital. Therefore, it has been proposed that after learning to control a hand pros-thesis in the MEG environment using up to 256 sensors that provide optimal noninvasive spatial resolution, patients could use what they learn to control an EEG-based BMI in real life neurorehabilitative settings and daily living situations. General purposes of the use of MEG in neurorehabilitative settings include: characterization of cortical reorganization associated with brain lesions or with training effects (as in the example of BMI in stroke). Additionally, MEG could potentially be used to “train” activation of particular brain networks in a way similar to what has been achieved using fMRI recently (Weiskopf et al., 2007).
TRANSCRANIAL MAGNETIC STIMULATION
Neural plasticity, defined as any enduring change in cortical properties either morphological or functional in response to environmental changes or lesions, contributes to motor learning in health and disease. Plastic processes are more prominent in the maturing nervous system, but they are also present in the adult brain (Nudo, 2003; Celnik and Cohen, 2004). The cortex, with its extensive network of synaptic connections, provides the ideal neural setting for plasticity. Training represents the central tenet of neurorehabilitative treatments. However, recovery of function after brain lesions such as stroke remains incomplete in most patients and therefore, there is a need for the development of adjuvant strategies to enhance the beneficial effects of training-based rehabilitative treatments. Noninvasive brain stimulation techniques have been recently evaluated as possible adjuvants to facilitate cortical plasticity and training effects. Noninvasive cortical stimulation can be delivered using TMS (Hallett, 2000; Reis et al., 2008) and transcranial direct current stimulation (tDCS) (Nitsche et al., 2005). Repetitive TMS is a non-invasive, relatively painless strategy to increase or decrease excitability and may influence motor, sensory, and cognitive functions. In TMS a brief high electrical current is passed through an insulated coil of copper wire placed over the scalp, which generates a transient magnetic field perpendicular to the coil. This magnetic field passes into the brain decreasing as a cubic function of the distance between the coil and the target tissues, where it induces electric currents that flow at right angles to the magnetic field. The induced electrical currents in a cortical region depend on coil shape and size, magnetic field strength (intensity), and frequency and duration of magnetic pulse trains, as well as on the anatomy of the particular anatomical structures, CSF, and the presence or absence of brain lesions. rTMS is used to temporarily disrupt cortical function in specific cortical sites to investigate the behavioral consequences of such disruption (Siebner and Rothwell, 2003; Reis et al., 2008). In general, “high-frequency stimulation” refers to frequencies of about 5 Hz and above, while “low frequency stimulation” refers to frequencies of about 1 Hz (Chen et al., 1997). Low-frequency rTMS usually results in inhibitory effects that depend on the duration of stimulation. A 15 minute train of 0.9 Hz applied over the primary motor cortex decreased corticospinal excitability for at least 15 minutes after stimulation (Chen et al., 1997; Muellbacher et al., 2000; Perez et al., 2007). Long-term depression-like mechanisms have been proposed as operating mechanisms in this modulatory effect (Iyer et al., 2003). Recently, it has been shown that transcranial DC current application can induce an intracerebral current flow sufficiently large to achieve changes in cortical excitability.
tDCS can be applied in humans painlessly to induce focal, lasting, but reversible shifts of cortical excitability. The duration and direction of shifts of excitability depend on the stimulus duration, the strength, and the polarity. tDCS (1–2 mA) is delivered through surface electrodes (25–35 cm2). For primary motor cortex, for example, one electrode is positioned above the motor cortical representation of the target body part, the other electrode over the contralateral supraorbital region. Depending on the polarity, cortical excitability is upregulated (anodal tDCS) or downregulated (cathodal tDCS). In recent studies, tDCS has been delivered for up to 30 minutes, inducing effects outlasting the stimulation period by up to 90 minutes (Hummel and Cohen, 2005; Hummel et al., 2005). One advantage of this type of stimulation is that it can be shammed, facilitating blinding of patients and investigators (Gandiga et al., 2006). In summary, TMS and tDCS can facilitate the beneficial effects of motor training (Butefisch et al., 2004), as well as visuomotor coordination (Antal et al., 2004), implicit motor learning (Nitsche et al., 2003; Kincses et al., 2004), skilled finger movements (Kobayashi et al., 2004), working memory (Fregni et al., 2005) and sleep-dependent consolidation of declarative memories (Marshall et al., 2004) in healthy volunteers. Studies presently under way are evaluating the ability to facilitate control of robotic devices (Takahashi et al., 2008).
DISCUSSION
BMI research has implications for both neuroengineering and systems neuroscience. In the former, as we have seen in this chapter, neuroprosthetic systems will play a major role in restoring communication and sensorimotor function for patients suffering from spinal cord injuries and other neurological disorders. On the other hand, BMIs are also a powerful tool for studying sensorimotor learning and control, as well as cortical plasticity. In the BMI paradigm, the experimenter has full control of the motor transformation linking the neural activity to the behavior, or the sensory transformation linking a behavioral or external event to neural activity. For example, sensorimotor maps can be arbitrarily changed by the experimenter, allowing the neural adaptations to environmental changes to be studied in a controlled way.
When it comes to comparing decoder performance using different modeling approaches, it is important to realize the fundamental difference between open-loop and closed-loop BMIs. In an open-loop BMI, neural data and behavioral data are recorded, and the goal is to build a decoder that maximizes the prediction power (e.g., correlation between predicted and actual movement). The problem can be seen as a pure statistical learning issue. However, a good predictive power in an open-loop BMI does not guarantee good performance of the BMI system in closed loop. This is because the recorded neurons may behave differently when performing the BMI task as opposed to when performing the manual task. In fact, it has been shown that the directional tuning of neurons is subject to change when switching from the manual task to the BMI task (Taylor et al., 2002; Carmena et al., 2003). One possible explanation for these changes in the firing properties of the cells is learning; as the subject adapts to the BMI task, the behavior of the neurons involved in the BMI task also changes. Nevertheless, a good performance of the decoder in open loop is still important because it will provide a “first guess” during closed-loop operation, where performance increases with practice (Serruya et al., 2002; Taylor et al., 2002; Carmena et al., 2003; Hochberg et al., 2006; Mulliken et al., 2008). The fact that neurons behave differently during BMI control as compared to manual control suggests that there is a “BMI space” of motor control. During a closed-loop BMI experiment, the activity of the cells entirely determines the motor behavior. And conversely, to perform a given motor action, neurons must behave in a specific way. Thus, the subject must volitionally modulate the activity of the neurons to achieve the desired task. This fundamental principle for BMI was proposed by Eberhard Fetz in the 1970s (Fetz, 2007).
Future directions
Several questions concerning BMI research are currently being addressed by the scientific community. For instance, understanding the learning process during closed-loop BMI is crucial (Jarosiewicz et al., 2008). Building a computational model of this process could help determine which type of decoder would enhance the learning process: for instance, decreasing the duration of a subject’s adaptation to the BMI task, or increasing final performance of the BMI.
Another challenge for BMI research will be to show the ability to finely control high-dimensional robotic actuators. Ultimately, and particularly when dealing with rehabilitation prostheses, BMI systems must provide a similar degree of control when interacting with the environment. To achieve this goal, decoding algorithms may need to include modules that account for the viscoelastic properties of muscles. For example, instead of directly predicting endpoint kinematics of the hand, the BMI could predict the muscle activity based on neural recordings, and then use these predictions to drive a musculoskeletal model of the arm. This will provide access to other types of variables concerning the movement, such as endpoint force or stiffness. Indeed, such biomimetic algorithms constitute an elegant way to solve the decoder problem; ideally, every component of the natural motor-control loop would be modeled and implemented in the BMI system. However, while the biomimetic approach is appealing, computational issues may arise: for example, the human arm is driven by more than 30 muscles, each of which exhibits complex, nonlinear behavior. Building such a large musculoskeletal model, estimating its parameters, and running it online is not a straightforward task. Software tools that specifically target such applications will be crucial for progress in both invasive and noninvasive BMI systems. Important areas of work are the human applications for which different patients could potentially choose in the future for the use of invasive or for noninvasive BMI approaches, each with its advantages and disadvantages. It is also conceivable that different patients might in the future receive more benefit from one or the other, based on disability type or lesion characteristics. Finally, serious attempts are under way to attempt facilitated control of BMI systems by focal brain stimulation.
References
- Antal A, Nitsche MA, Kincses TZ, et al. Facilitation of visuomotor learning by transcranial direct current stimulation of the motor and extrastriate visual areas in humans. Eur J Neurosci. 2004;19:2888–2892. doi: 10.1111/j.1460-9568.2004.03367.x. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Cohen LG. Brain–computer interfaces: communication and restoration of movement in paralysis. J Physiol. 2007;579:621–636. doi: 10.1113/jphysiol.2006.125633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Lutsep HL, Weinand M, et al. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2006;58:464–473. doi: 10.1227/01.NEU.0000197100.63931.04. [DOI] [PubMed] [Google Scholar]
- Buch E, Weber C, Cohen LG, et al. Think to move: a neuromagnetic brain–computer interface (BCI) system for chronic stroke. Stroke. 2008;39:910–917. doi: 10.1161/STROKEAHA.107.505313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdet E, Osu R, Franklin DW, et al. The CNS skillfully stabilizes unstable dynamics by learning optimal impedance. Nature. 2001;414:446–449. doi: 10.1038/35106566. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Khurana V, Kopylev L, et al. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91:2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- Carmena JM, Lebedev MA, Crist RE, et al. Learning to control brain–machine interface for reaching and grasping by primates. PLoS Biol. 2003;1:192–208. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celnik PA, Cohen LG. Modulation of motor function and cortical plasticity in health and disease. Restor Neurol Neurosci. 2004;22:261–268. [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Newsome WT. What electrical microstimulation has revealed about the neural basis of cognition. Curr Opin Neurobiol. 2004;14:169–177. doi: 10.1016/j.conb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Fetz EE. Volitional control of neural activity: implications for brain–computer interfaces. J Physiol. 2007;579:571–579. doi: 10.1113/jphysiol.2006.127142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons NA, Drake W, Hanson TL, et al. Primate reaching cued by multichannel spatiotemporal cortical microstimulation. J Neurosci. 2007;27:5593–5602. doi: 10.1523/JNEUROSCI.5297-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche M, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- Galan F, Nuttin M, Lew E, et al. A brain-actuated wheelchair: asynchronous and non-invasive brain–computer interfaces for continuous control of robots. Clin Neurophysiol. 2008;119:2159–2169. doi: 10.1016/j.clinph.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, et al. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Caminiti R, Kalaska JF, et al. Spatial coding of movement: a hypothesis concerning coding of movement direction by motor cortical population. Exp Brain Res. 1983;7:327–336. [Google Scholar]
- Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Hogan N. Impedance Control: an approach to manipulation: Parts I–III. ASME J Dyn Syst Meas Control. 1985;107:1–24. [Google Scholar]
- Hogan N. Skeletal muscle impedance in the control of motor actions. J Mech Med Biol. 2002;2:359–373. [Google Scholar]
- Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19:14–19. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:10867–10872. doi: 10.1523/JNEUROSCI.23-34-10867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosiewicz B, Chase SM, Fraser GW, et al. Functional network reorganization during learning in a brain–computer interface paradigm. Proc Natl Acad Sci U S A. 2008;105:19486–19491. doi: 10.1073/pnas.0808113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei S, Hoffman D, Strick PL. Muscle and movement representations in the primary motor cortex. Science. 1999;285:2136–2139. doi: 10.1126/science.285.5436.2136. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Cohen DAD, Hyde ML, et al. A comparison of movement direction-related versus load direction-related activity in primate motor cortex, using a two-dimensional reaching task. J Neurosci. 1989;9:2080–2102. doi: 10.1523/JNEUROSCI.09-06-02080.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Biggs SJ, Schloerb DW, et al. Continuous shared control stabilizes reach and grasping with brain–machine interfaces. IEEE Trans Biomed Eng. 2006;53:1164–1173. doi: 10.1109/TBME.2006.870235. [DOI] [PubMed] [Google Scholar]
- Kincses TZ, Antal A, Nitsche MA, et al. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia. 2004;42:113–117. doi: 10.1016/s0028-3932(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Theoret H, et al. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–98. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- Leuthardt EC, Miller KJ, Schalk G, et al. Electrocorticography-based brain computer interface – the Seattle experience. IEEE Trans Neural Syst Rehabil Eng. 2006;14:194–198. doi: 10.1109/TNSRE.2006.875536. [DOI] [PubMed] [Google Scholar]
- Li CSR, Padoa-Schioppa C, Bizzi E. Neural correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron. 2001;30:593–607. doi: 10.1016/s0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- Marshall L, Molle M, Hallschmid M, et al. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci. 2004;24:9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellinger J, Schalk G, Braun C, et al. An MEG-based brain-computer interface (BCI) Neuroimage. 2007;36:581–593. doi: 10.1016/j.neuroimage.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran DW, Schwartz AB. Motor cortical representation of speed and direction during reaching. J Neurophysiol. 1999;82:2676–2692. doi: 10.1152/jn.1999.82.5.2676. [DOI] [PubMed] [Google Scholar]
- Morrow MM, Jordan LR, Miller LE. Direct comparison of the task-dependent discharge of M1 in hand space and muscle space. J Neurophysiol. 2007;97:1786–1798. doi: 10.1152/jn.00150.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortiz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456:639–642. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, et al. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol. 2000;111:1002–1007. doi: 10.1016/s1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Mulliken GH, Musallam M, Andersen RA. Decoding trajectories from posterior parietal cortex ensembles. J Neurosci. 2008;28:12913–12926. doi: 10.1523/JNEUROSCI.1463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musallam S, Corneil BD, Greger B, et al. Cognitive control signals for neural prosthetics. Science. 2004;305:258–262. doi: 10.1126/science.1097938. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA. Actions from thoughts. Nature. 2001;409:403–407. doi: 10.1038/35053191. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL. Brain–machine interfaces to restore motor function and probe neural circuits. Nat Rev Neurosci. 2003;4:417–422. doi: 10.1038/nrn1105. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Dimitrov D, Carmena JM, et al. Chronic, multi-site, multi-electrode recordings in macaque monkeys. Proc Natl Acad Sci U S A. 2003;100:11041–11046. doi: 10.1073/pnas.1934665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ. Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. J Rehabil Med. 2003;41:7–10. doi: 10.1080/16501960310010070. [DOI] [PubMed] [Google Scholar]
- Otto KJ, Rousche PJ, Kipke DR. Cortical microstimulation in auditory cortex of rat elicits best-frequency dependent behaviors. J Neural Eng. 2005;2:42–51. doi: 10.1088/1741-2560/2/2/005. [DOI] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, et al. Neural substrates of intermanual transfer of a newly acquired motor skill. Curr Biol. 2007;17:1896–1902. doi: 10.1016/j.cub.2007.09.058. [DOI] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, et al. Noninvasive cortical stimulation enhances skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Hernández A, Zainos A, et al. Somatosensory discrimination based on cortical microstimulation. Nature. 1998;392:387–390. doi: 10.1038/32891. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernández A, Zainos A, et al. Sensing without touching: psychophysical performance based on cortical microstimulation. Neuron. 2000;26:273–278. doi: 10.1016/s0896-6273(00)81156-3. [DOI] [PubMed] [Google Scholar]
- Santhanam G, Ryu SI, Yu BM, et al. A high-performance brain–computer interface. Nature. 2006;442:195–198. doi: 10.1038/nature04968. [DOI] [PubMed] [Google Scholar]
- Schalk G, Miller KJ, Anderson NR, et al. Two-dimensional movement control using electrocorticographic signals in humans. J Neural Eng. 2008;5:75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AB. Direct cortical representation of drawing. Science. 1994;265:540–542. doi: 10.1126/science.8036499. [DOI] [PubMed] [Google Scholar]
- Selen LPJ, Beek PJ, van Dieen JH. Impedance is modulated to meet accuracy demands during goal-directed arm movements. Exp Brain Res. 2006;172:129–138. doi: 10.1007/s00221-005-0320-7. [DOI] [PubMed] [Google Scholar]
- Sergio LE, Kalaska JF. Changes in temporal pattern of primary motor cortex activity in a directional isometric force versus limb movement task. J Neurophysiol. 1998;80:1557–1583. doi: 10.1152/jn.1998.80.3.1577. [DOI] [PubMed] [Google Scholar]
- Serruya MD, Hatsopoulos NG, Paninski L, et al. Instant neural control of a movement signal. Nature. 2002;416:141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Sitaram R, Zhang H, Guan C, et al. Temporal classification of multichannel near-infrared spectroscopy signals of motor imagery for developing a brain–computer interface. Neuroimage. 2007;34:1416–1427. doi: 10.1016/j.neuroimage.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Takahashi CD, Der-Yeghiaian L, Le V, et al. Robot-based hand motor therapy after stroke. Brain. 2008;131:425–437. doi: 10.1093/brain/awm311. [DOI] [PubMed] [Google Scholar]
- Talwar SK, Xu S, Hawley ES, et al. Rat navigation guided by remote control. Nature. 2002;417:37–38. doi: 10.1038/417037a. [DOI] [PubMed] [Google Scholar]
- Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296:1829–1832. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM. Phosphene induction by microstimulation of macaque V1. Brain Res Rev. 2007;53:337–343. doi: 10.1016/j.brainresrev.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velliste M, Perel S, Spalding MC, et al. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–1101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Veit R, Erb M, et al. Physiological self-regulation of regional brain activity using real-time functional magnetic resonance imaging (fMRI): methodology and exemplary data. Neuroimage. 2003;19:577–586. doi: 10.1016/s1053-8119(03)00145-9. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Sitaram R, Josephs O, et al. Real-time functional magnetic resonance imaging: methods and applications. Magn Reson Imaging. 2007;25:989–1003. doi: 10.1016/j.mri.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Wessberg J, Stambaugh C, Kralik J. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000;408:361–365. doi: 10.1038/35042582. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain–computer interface in humans. Proc Natl Acad Sci U S A. 2004;101:17849–17854. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Birbaumer N, McFarland DJ, et al. Brain–computer interfaces for communication and control. Clin Neurophysiol. 2002;113:767–791. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]