Abstract
Objective
National estimates of HIV trends in generalised epidemics rely on HIV prevalence data from antenatal clinic (ANC) surveillance. We investigate whether HIV prevalence trends in ANC data reflect trends in men and women in the general population during the scale-up of anti-retroviral treatment (ART) in Manicaland, Zimbabwe.
Methods
Trends in HIV prevalence in local ANC attendees and adults aged 15-49yrs in towns, agricultural estates, and villages were compared using five rounds of parallel ANC (N≈1,200) and general-population surveys (N≈10,000) and multi-variable log-linear regression. Changes in the age-pattern of HIV prevalence and the age-distribution of ANC attendees were compared with those in the general population. Age-specific pregnancy prevalence rates were compared by HIV infection and ART status.
Results
Cumulatively, from 1998-2000 to 2009-2011, HIV prevalence fell by 60.0% (95% CI, 51.1%-67.3%) in ANC surveillance data and by 34.3% (30.8%-37.7%) in the general population. Most of the difference arose following the introduction of ART (2006-2011). The estates and villages reflected this overall pattern but HIV prevalence in the towns was lower at local ANCs than in the general population, largely due to attendance by pregnant women from outlying (lower prevalence) areas. The ageing of people living with HIV in the general population (52.4% aged >35yrs, 2009-2011) was under-represented in the ANC data (12.6%) due to lower fertility in older and HIV-infected women.
Conclusion
After the introduction of ART in Manicaland, HIV prevalence declined more steeply in ANC surveillance data than in the general population. Models used for HIV estimates must reflect this change in bias.
Keywords: HIV surveillance, ANC bias, HIV trends, prevalence, Zimbabwe
Introduction
Reliable estimates of trends in HIV prevalence continue to be of great importance for HIV control in sub-Saharan African populations. These estimates provide key information on healthcare needs in the population, and form the principal inputs to mathematical models used by UNAIDS and others to estimate levels and trends in the incidence of new infections, programme coverage, AIDS mortality, and orphanhood [1].
Since the late 1980s, data from pregnant women attending for routine check-ups at antenatal clinics (ANC) have been used to estimate levels and trends in HIV prevalence in generalised epidemics [2]. Early comparisons with general population data from local community studies suggested that HIV prevalence in pregnant women provided a reasonable approximation to prevalence in men and women combined [3, 4]. However, subsequent comparisons with national population sero-surveys showed that ANC surveillance data can over-estimate HIV prevalence in adults [5, 6], largely because ANC sites included in countries’ surveillance systems are more urban than the areas they are taken to represent [7]. Based on these findings, in 2007, UNAIDS recommended that ANC data for both urban and rural areas should be adjusted downwards by 20% when used in national estimates of HIV prevalence [6].
Today, most countries in sub-Saharan Africa have at least one national sero-survey that can be used to calibrate the level of HIV prevalence in the general population but data from pregnant women – increasingly in the form of data from routine PMTCT programme records – continue to be the main source of information on temporal trends in HIV prevalence [6]. The latter is a subject of growing concern since data from pregnant women attending antenatal clinics may over-state declines in HIV prevalence in the general population [8, 9] as populations of people living with HIV (PLHIV) age [10, 11], due to falling incidence of new infections and increasing survival on antiretroviral therapy (ART) [12]. Discrepancies between trends in pregnant women and in the general population may arise as more HIV-positive women experience the lower fertility associated with older age and if ART alters their fertility [13, 14] through reduced widowhood, reductions in the biological infertility associated with untreated HIV infection [15], and changes in fertility intentions and behaviour following increases in knowledge of infection status [16, 17].
In this paper, we compare trends in HIV prevalence in local ANC attendees with trends in men and women in the general population in a sub-Saharan African population over a period spanning the scale-up of ART services (1998-2011), and investigate reasons for observed discrepancies.
Methods
Data
The data for the study were collected in a longitudinal general-population survey and parallel ANC surveillance conducted for research purposes in east Zimbabwe [13]. The study areas for both surveys comprised 12 geographically-distinct sites in Manicaland Province (four subsistence farming areas, two roadside trading settlements, four large-scale commercial farming estates, and two small towns) which were enumerated in a set sequence. In each case, a baseline survey was carried out between July 1998 and January 2000, followed by four surveys, conducted at approximately two- or three-year intervals (July 2001 to March 2003, July 2003 to August 2005, July 2006 to September 2008, and September 2009 to July 2011).
In each round of the general-population survey, all households and residents were enumerated, and eligible adults were invited to join the study. In the first two rounds, one eligible adult (a male aged 17-54 years or a female aged 15-44 years who was a regular member of the household and had stayed in the household for at least 4 nights in the last month) per marital relationship was selected at random to avoid non-independence in the sample. From the third round, all adults aged 15-54 years (including visitors) were eligible; however, in the fourth and fifth rounds, adults were only interviewed in a random selection of two-thirds of households, owing to funding constraints. Study participants undertook a face-to-face interview and provided dried blood spots for anonymous HIV testing. In the interview, the data collected included information on socio-demographic characteristics, pregnancies in the last three years, and uptake of HIV treatment services.
For the ANC surveillance, in each round, the principal clinics in each study area were visited at the same time that the general-population survey was conducted in that area. Up to 100 (120 from round three) pregnant women per site were enrolled for the ANC survey. A short face-to-face interview was conducted and dried blood spots were collected for anonymous HIV testing. The interview data included information on socio-demographic characteristics and uptake of HIV treatment services. HIV surveillance testing (for both surveys) was conducted at the Biomedical Research and Training Institute laboratory in Harare following a previously-described algorithm [18].
In both surveys, written informed consent was sought as a condition of enrolment and, at each round, a parallel free voluntary counselling and testing service was provided for study participants. In the two most recent rounds, clinical staging was undertaken in accordance with World Health Organisation guidelines and referrals were made for CD4 count testing and initiation on ART where appropriate. Prior ethical approval for the study was obtained from the Medical Research Council of Zimbabwe (MRCZ/A/681) and the Imperial College Research Ethics Committee (ICREC_9_3_13).
Household participation rates in the general population survey ranged from 93.7% (11865/12668) in round four (2006-2008) to 98.2% (8233/8386) in round one (1998-2000). Participation rates in the general-population survey, amongst members of enumerated households eligible for the current study, were 80.3% (9368/11661), 86.7% (6483/7480), 82.4% (14309/17371), 79.7% (10463/13136) and 77.6% (12357/15914) in the five survey rounds. Uptake of the free VCT service was low (<5%) in all rounds.
A total of 43 clinics were included in the ANC surveillance, of which 30 were visited in all five rounds of the survey. Clinics not visited in all rounds were mainly either small clinics or clinics that were not open at the time of the survey. 90.7% (5786/6380) of pregnant women interviewed in the ANC surveillance were interviewed in clinics that were visited in all rounds. 94% (1215/1289) of women attending ANCs in the study areas participated in the first round of ANC surveillance; no refusals were recorded in subsequent rounds.
Data analysis
The analysis was restricted to 15-49 year-olds interviewed in each survey. To improve comparability over time and the generalizability of the results, men and women interviewed in the general-population survey who were not regular members of the study households were excluded from the analysis.
HIV prevalence rates (with 95% exact confidence intervals) for each survey round for men and women aged 15-49 years (men aged 17-49 years and women aged 15-44 years in the first two rounds) in the general-population survey and for pregnant women aged 15-49 years participating in the ANC surveillance (including and excluding clinics not visited in all rounds) were calculated and plotted. Log-linear regression was used to calculate sex- and age-adjusted risk ratios for reductions in HIV prevalence relative to prevalence at baseline at each subsequent survey round. Differences in the extent and timing of declines in HIV prevalence between the two data sources were assessed by comparing the risk ratios and their associated 95% confidence intervals. HIV prevalence rates in the general-population survey were disaggregated by sex, recent pregnancy status (defined as currently pregnant or having completed a pregnancy in the last six months), and ANC attendance to investigate whether these factors contributed to the differences in the levels and trends over time observed between adults in the general population and pregnant women attending for check-ups at the local ANCs.
Levels and trends in HIV prevalence estimated using the ANC surveillance data were also compared with those for the general population after disaggregating by study location (towns, estates and villages) to assess whether differences between the data sources differed by location. The roadside settlement and subsistence farming sites were combined for this purpose because they both primarily comprised rural villages. Standardisation was used to explore whether spatial patterns of ANC attendance could account for differences in HIV prevalence observed between pregnant women living in the towns and pregnant women attending for antenatal check-ups at clinics in the towns.
To investigate the possibility that ageing of PLHIV contributed to the faster declines in HIV prevalence observed in ANC attendees than in the general population, age-patterns of HIV prevalence were plotted and compared between the two data sources for three time points – at the peak of the epidemic (1998-2000), at the introduction of ART services in Manicaland (2006-2008), and shortly after the scale-up of ART services (2009-2011). The age-distribution of PLHIV was also compared for each of these time-points and with the age-distributions of ANC attendees, pregnant women, and men and women in the population as a whole. Patterns of age- and HIV-associated sub-fertility were assessed by calculating age-specific pregnancy prevalence ratios (2009-2011) for HIV-infected women (with and without ART) relative to uninfected women using log-linear regression.
All data analyses were conducted using Stata version 12 (800-STATA-PC, College Station, Texas, USA; copyright 1985-2011).
Results
Comparison of HIV prevalence declines in ANC surveillance and the general population
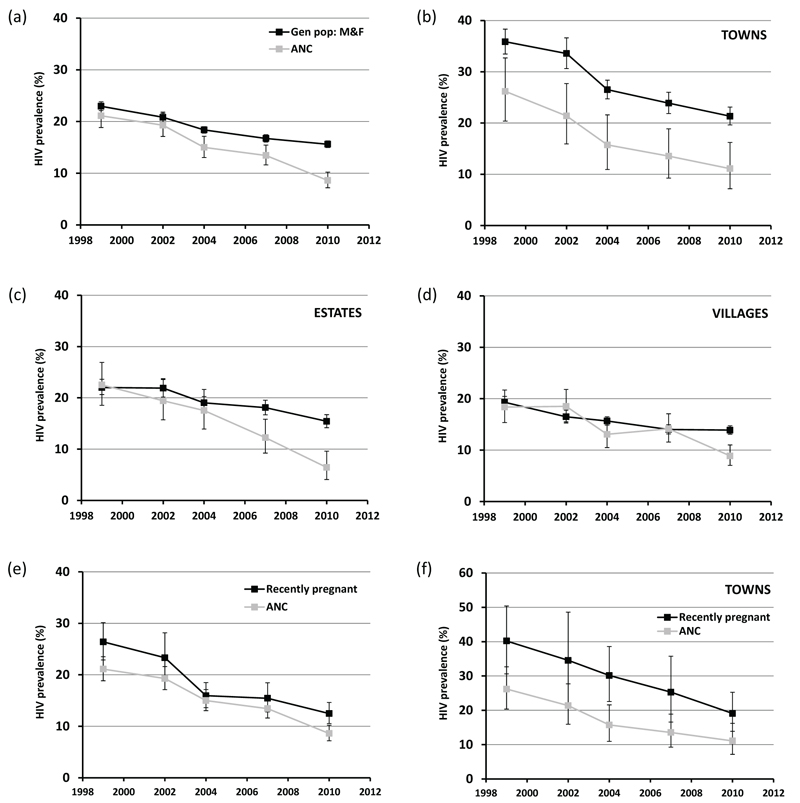
The first two rounds of the general-population survey, covering the late 1990s and early 2000s, were restricted to men aged 17-49 years and women aged 15-44 years. Since HIV prevalence, generally, is low in young men and in older women compared to intermediate ages, the survey data may yield slight over-estimates of HIV prevalence in 15-49 year-olds. Taking this into consideration, the ANC surveillance data provided reasonable estimates of HIV prevalence in men and women in the general population (Figure 1a). Both data sources indicated a decline in HIV prevalence over this period although the decline in the ANC data was not statistically significant (p=0.2). In subsequent periods – and especially after the scale-up of ART (2006-2011) – HIV prevalence declined more rapidly in the ANC surveillance data than in the general population (Figure 1a). Cumulatively, between 1998-2000 and 2009-2011, HIV prevalence fell by 60.0% (95% CI, 51.1%-67.3%) in the ANC data but by only 34.3% (30.8%-37.7%) in the general population (Table 1). Between 2003-2005 (the last survey before introduction of ART services) and 2009-2011, HIV prevalence fell by 46.7% (33.9%-57.0%) in the ANC data compared to 19.5% (15.3%-23.5%) in men and women in the general population.
Figure 1. Comparison of HIV prevalence trends in ANC surveillance with trends in the general population, Manicaland, Zimbabwe, 1998 to 2011.
HIV prevalence (whiskers show 95% confidence intervals) in pregnant women attending local ANCs versus all males and females aged 15-49 years in the general population, for all sites combined (graph (a)), towns (b), agricultural estates (c) and rural villages (d). Recently pregnant women in the general population survey versus pregnant women attending local ANCs for all sites combined (e) and towns (f).
Table 1. Comparison of HIV prevalence trends in ANC surveillance versus the general population, Manicaland, Zimbabwe, 1998 to 2011.
| 1998-2000a | 2001-2003a | 2003-2005 | 2006-2008 | 2009-2011 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % HIV+ | N | % HIV+ | N | aRR | (95% CI) | % HIV+ | N | aRR | (95% CI) | % HIV+ | N | aRR | (95% CI) | % HIV+ | N | aRR | (95% CI) | |
| General population survey | ||||||||||||||||||
| Men and women | 23.0 | 9368 | 20.8 | 6483 | 0.898 | (0.848-0.950) | 18.4 | 14309 | 0.817 | (0.780-0.857) | 16.7 | 10463 | 0.724 | (0.686-0.763) | 15.6 | 12357 | 0.657 | (0.623-0.692) |
| Men | 19.1 | 4160 | 18.0 | 2621 | 0.905 | (0.825-0.993) | 14.8 | 5703 | 0.792 | (0.731-0.858) | 12.8 | 4296 | 0.649 | (0.592-0.712) | 12.1 | 4946 | 0.575 | (0.525-0.630) |
| Women | 26.0 | 5208 | 22.7 | 3862 | 0.900 | (0.839-0.966) | 20.7 | 8606 | 0.824 | (0.777-0.873) | 19.5 | 6167 | 0.750 | (0.703-0.801) | 18.0 | 7411 | 0.680 | (0.638-0.725) |
| Pregnant women | 26.4 | 595 | 23.3 | 343 | 0.862 | (0.685-1.084) | 16.0 | 909 | 0.615 | (0.504-0.749) | 15.5 | 660 | 0.577 | (0.463-0.719) | 12.5 | 1057 | 0.447 | (0.364-0.549) |
| Pregnant women attending ANCs | 27.4 | 471 | 23.0 | 270 | 0.829 | (0.642-1.071) | 16.0 | 720 | 0.601 | (0.483-0.749) | 14.3 | 469 | 0.522 | (0.402-0.678) | 12.5 | 790 | 0.432 | (0.343-0.545) |
| Pregnant women attending local ANCs | 27.5 | 375 | 24.0 | 229 | 0.871 | (0.663-1.146) | 15.9 | 604 | 0.601 | (0.472-0.765) | 13.4 | 417 | 0.496 | (0.372-0.662) | 13.0 | 676 | 0.453 | (0.353-0.582) |
| Antenatal clinic survey | ||||||||||||||||||
| All clinics visited | 21.1 | 1218 | 19.3 | 1229 | 0.918 | (0.787-1.071) | 15.0 | 1200 | 0.739 | (0.624-0.876) | 13.4 | 1272 | 0.665 | (0.559-0.792) | 8.6 | 1381 | 0.400 | (0.327-0.489) |
| Clinics visited in all rounds | 21.0 | 1170 | 18.8 | 1082 | 0.901 | (0.765-1.061) | 15.3 | 1087 | 0.759 | (0.637-0.905) | 13.0 | 1124 | 0.641 | (0.533-0.772) | 8.9 | 1243 | 0.413 | (0.336-0.509) |
aRR, Adjusted risk ratio compared to round 1 (1998-2000), adjusted for age and sex; CI, confidence interval
In the first two rounds of the general population survey, eligibility was limited to men aged 17-49 years and women aged 15-44 years.
In the general-population survey, the HIV prevalence decline was greater in men (42.5%; 95% CI, 37.0%-47.5%) than in women (32.0%; 27.5%-36.2%) (Table 1). However, the reduction in pregnant women (55.3%; 45.1%-63.6%) was much greater than the reductions in women overall (32.0%) and in both sexes combined (34.3%). Whilst the levels and decline in HIV prevalence in pregnant women were similar to those in women overall in the first two rounds of the survey, the subsequent decline was considerably steeper. By 2009-2011, HIV prevalence in pregnant women (12.5%, 10.6%-14.6%) was almost a third lower than prevalence in women overall (18.0%, 17.1%-18.9%).
ANC attendance amongst pregnant women was high – 76.3% in all survey participants; 90.4% in women who had delivered – and differences in HIV prevalence between pregnant women who had and who had not attended for ANC check-ups (locally or elsewhere) were small (Table 1).
The extent and pattern of HIV prevalence decline in pregnant women in the general-population survey were similar to those measured in the surveillance data from local antenatal clinics (Figure 1e). However, except in 2003-2005, the level of HIV prevalence was consistently lower in the ANC surveillance data than in pregnant women in the general population. This difference was particularly noticeable in the towns (Figure 1f).
Geographical participation bias in the ANC surveillance data
The pattern of HIV prevalence decline over time observed in the commercial farming estates reflected the overall pattern (Figure 1c) whilst, in the rural villages, the ANC surveillance estimates matched those for the general population until 2006-2008 but declined rapidly following the scale-up of ART when HIV prevalence in the general population was stable (Figure 1d). In the towns, the ANC surveillance data showed remarkably similar trends to those in the general population (Figure 1b). However, the ANC estimates were considerably lower than the direct estimates for HIV prevalence in both sexes combined in the general population throughout the survey.
The discrepancies in HIV prevalence between local ANC attendees and men and women as a whole (and pregnant women in particular) living in the general population in the towns were largely accounted for by use of urban antenatal clinics by pregnant women from the surrounding rural areas where HIV prevalence is lower. For example, in 2001-2003, 60% of attendees at the urban ANCs reported living in outlying villages, and HIV prevalence was considerably lower in these women (16.7%) than in women attending the same clinics who lived in the town (33.9%) (Table 2). The proportion of urban ANC attendees who were resident in outlying villages increased over time but the bias was little affected because the proportion resident in surrounding farming estates (where HIV prevalence was also lower) fell over the same period. This form of participation bias accounted for between 74.5% and 94.6% of the discrepancy between HIV prevalence in urban ANC attendees and pregnant women across the last four rounds of the survey.
Table 2. Effect of spatial patterns of ANC attendance on ANC surveillance estimates for HIV prevalence in small towns in Manicaland, Zimbabwe.
| 2001-2003 |
2003-2005 |
2006-2008 |
2009-2011 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANC attendees |
Pregnant women |
ANC attendees |
Pregnant women |
ANC attendees |
Pregnant women |
ANC attendees |
Pregnant women |
|||||||||
| Place of residence | % HIV+ | n | n/N | % HIV+ | % HIV+ | n | n/N | % HIV+ | % HIV+ | n | n/N | % HIV+ | % HIV+ | n | n/N | % HIV+ |
| Towns | 33.9 | 59 | 0.29 | 34.6 | 27.5 | 40 | 0.20 | 30.2 | 17.9 | 39 | 0.18 | 25.3 | 7.7 | 39 | 0.19 | 19.1 |
| Estates | 13.6 | 22 | 0.11 | 22.4 | 26.1 | 23 | 0.12 | 14.6 | 0.0 | 5 | 0.02 | 12.1 | 28.6 | 7 | 0.03 | 11.6 |
| Villages | 16.7 | 120 | 0.60 | 20.4 | 10.4 | 134 | 0.68 | 12.8 | 12.9 | 170 | 0.79 | 15.0 | 11.2 | 161 | 0.78 | 10.7 |
| Alla | 21.4 | 201 | 1.00 | 24.7 | 15.7 | 197 | 1.00 | 16.5 | 13.6 | 214 | 1.00 | 16.8 | 11.1 | 207 | 1.00 | 12.3 |
| Participation biasb | 74.5% | 94.6% | 72.5% | 85.3% | ||||||||||||
The figures in italics indicate HIV prevalence rates in pregnant women standardised by place of residence, based on the residence distribution of ANC attendees.
Contribution of ANC attendees resident in surrounding rural areas to under-statement of HIV prevalence in urban pregnant women.
Effects of the ageing of the HIV epidemic and low fertility in older and HIV-infected women
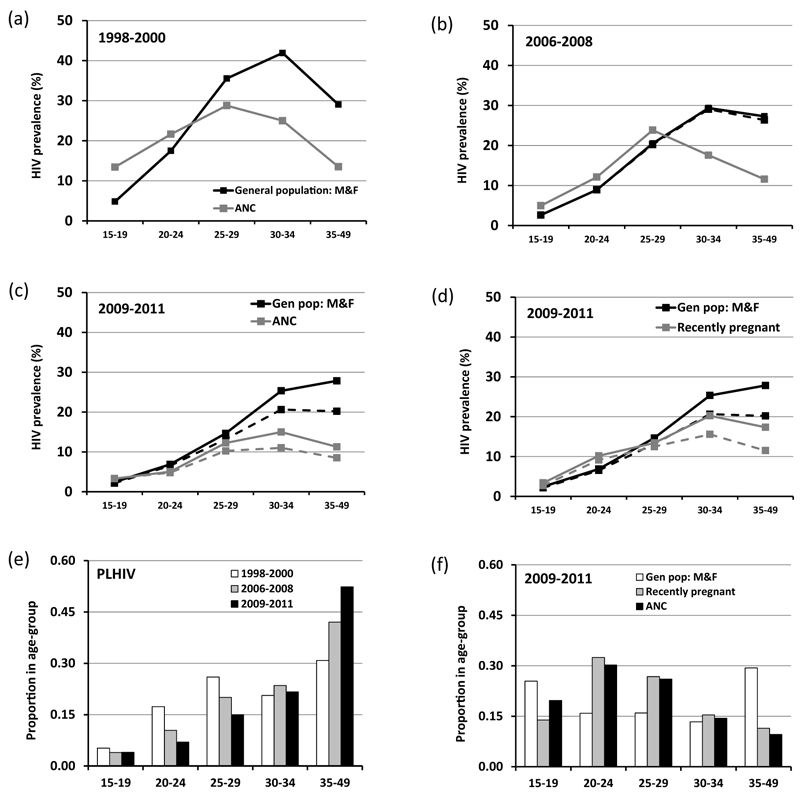
Figures 2a-c compare the age-patterns of HIV prevalence in the ANC surveillance data with those in the general population. Large declines in prevalence over time can be seen at younger ages, most likely reflecting reductions in HIV incidence [19]. These declines are somewhat greater in the ANC surveillance data, such that the initial over-estimation of prevalence at younger ages in this data source disappears by 2009-2011. At older ages, HIV prevalence is relatively stable, particularly between 2006 and 2011 (the period of ART scale-up), in both data sources, and the substantial under-estimation of general population prevalence evident in the ANC surveillance data persists. A similar picture is seen when comparing recently pregnant women with all adults in the general population (Figure 2d), although HIV prevalence at older ages is somewhat closer to that in the general population as a whole. ART coverage in all HIV-infected individuals aged 30 years and above increased from 3.5% (40/1148) in 2006-2008 to 31.2% (445/1428) in 2009-2011 (Figures 2b-d). Whilst the population of PLHIV overall aged considerably over time with more than half (52.4%) now being 35-49 years old (Figure 2e), ANC attendees remain primarily women under 30 years of age (Figure 2f).
Figure 2. Changes in the age-pattern of HIV prevalence over time and differences between the age-distributions of pregnant women attending local ANCs and the general population (aged 15-49 years), Manicaland, Zimbabwe, 1998 to 2011.
Age-specific HIV prevalence rates in local ANC attendees and males and females in the general population in 1998-2000 (graph (a)), 2006-2008 (b) and 2009-2011 (c). Age-specific HIV prevalence rates in local ANC attendees and locally-resident pregnant women in 2009-2011 (d). Dashed lines in graphs (b)-(d) show HIV prevalence in people not on anti-retroviral therapy (before the pregnancy for ANC attendees). Changes over time in the age-distribution of HIV-positive adults in the general population (e); and comparison of the age-distributions of local ANC attendees and all adults and locally-resident pregnant women in the general population, 2009-2011 (f).
Biological sub-fertility associated with HIV is particularly severe after longer periods of infection [20] and therefore affects older women disproportionately. In the most recent round of the general population survey (2009-2011), pregnancy rates in uninfected women peaked at ages 25-29 years and then declined steadily at older ages (Table 3). In HIV-infected women, pregnancy rates were highest in 15-24 year-olds and fell sharply thereafter. Pregnancy rates in 30-34 year-old and 35-49 year-old HIV-infected women – the age-groups in which women with HIV are now concentrated (Figure 2e) – were 40% and 50% lower, respectively, than those in uninfected women of the same age. Whilst the numbers of younger women on ART are small, this pattern was observed for HIV-infected women irrespective of ART status.
Table 3. Pregnancy prevalence ratesa by HIV infection and ART status, Manicaland, Zimbabwe, 2009-2011.
| HIV- |
HIV+ |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All |
ART |
no ART |
||||||||||||
| Age-group | % | N | aIRR | (95% CI) | % | N | aIRR | (95% CI) | % | N | aIRR | (95% CI) | % | N |
| 15-24 | 17.2 | 2619 | 1.14 | (0.87-1.49) | 27.0 | 148 | - | - | 50.0 | 10 | 1.05 | (0.79-1.40) | 25.4 | 138 |
| 25-29 | 23.9 | 1027 | 0.71 | (0.52-0.97) | 17.0 | 224 | 0.42 | (0.14-1.24) | 10.0 | 30 | 0.75 | (0.55-1.04) | 18.0 | 194 |
| 30-34 | 18.1 | 720 | 0.60 | (0.42-0.86) | 10.9 | 302 | 0.57 | (0.30-1.08) | 10.3 | 87 | 0.62 | (0.41-0.93) | 11.2 | 215 |
| 35-49 | 5.8 | 1712 | 0.50 | (0.31-0.78) | 3.2 | 659 | 0.52 | (0.26-1.05) | 3.0 | 263 | 0.48 | (0.27-0.84) | 3.3 | 396 |
| 15-49 | 15.2 | 6078 | 0.75 | (0.63-0.90) | 9.9 | 1333 | 0.63 | (0.43-0.93) | 6.4 | 390 | 0.78 | (0.64-0.94) | 11.3 | 943 |
Prevalence of a current pregnancy or a pregnancy which ended in the last 6 months.
IRR, Risk ratio adjusted for 5-year age-group (or single year within 5- and 10-year age-groups) and place of residence (town, estate or village). CI, confidence interval.
Discussion
HIV prevalence fell substantially in eastern Zimbabwe from the late 1990s. Despite ART-associated reductions in AIDS mortality, this decline continued through to 2011 due to further reductions in prevalence at younger adult ages which, in turn, reflect earlier and sustained declines in HIV incidence [19, 21]. However, the results of this analysis show that local ANC surveillance data have exaggerated this decline since the mid-2000s by a factor of 1.8.
Our findings also suggest that this divergence in trends was caused largely by the ageing of the HIV epidemic due to falling HIV incidence and increased survival on ART. Several interwoven factors probably contributed. We found that the ageing of the population of PLHIV was only partially reflected in data on pregnant women mainly because HIV-infected women (≥25 years) continued to have lower fertility than uninfected women. Almost half (380/805) of these women were not on ART so could still be subject to the biological sub-fertility found previously in untreated infected women [20]. The degree and age-pattern of reduced fertility (compared to uninfected women) were similar to the pre-ART era [22], suggesting that substantial changes in fertility intentions and behaviour have not occurred in this group. HIV-infected women who were on ART also had lower fertility than uninfected women [15], although numbers of cases at young ages were small. An early study in Uganda also found that ART did not increase fertility in infected women despite finding an association between ART and greater fertility desire [17]. Reductions in widowhood might also increase fertility disproportionately in infected women including those on ART; therefore, any effect of ART in reversing or preventing biological sub-fertility may be small or slow to take effect.
An important further factor contributing to the divergence in HIV prevalence trends in ANC and general population data in the current study was the disappearance over time in the over-statement of HIV prevalence at young ages (<25 years) in the ANC data. Possible explanations include the rise in age at first sex noted in the study population [18, 23] and (more speculatively) reductions in the risks of early sexual activity due to fewer partners being infected and reduced transmission from (often older [24]) infected partners now on ART [25]. Finally, introduction of new and enhanced PMTCT/ART services could alter spatial patterns of ANC attendance and distort trends in surveillance data. However, we found little evidence in the current study that changes of this nature contributed to the divergence in HIV prevalence trends.
The results from this in-depth community study in eastern Zimbabwe are consistent with findings from a recent analysis of national survey data. In a comparison of changes in HIV prevalence between the pre- / early ART scale-up period (2003-2008) and the ART period (2008-2012) in 13 countries, Eaton and colleagues found that HIV prevalence fell by 19% in pregnant women but remained unchanged amongst all women. The results of this study also confirm findings from an earlier analysis of data from the baseline survey in Manicaland which showed evidence of lower HIV prevalence in ANC surveillance data than in pregnant women in the general population [26]. Here we found that this pattern has persisted over time and is strongest in small towns, where the majority of pregnant women attending the local ANCs live in outlying villages and estates where HIV prevalence is lower. These findings conflict with results from an analysis of five national surveys in which HIV prevalence in ANC sentinel sites close to survey clusters was similar to prevalence in adults living in or close to these clusters [27]. However, in a study in Zimbabwe, HIV prevalence was non-significantly lower at urban ANC surveillance sites (18.6%) than in recently pregnant women living in the clinic catchment areas (20.9%) [28]; a finding which may reflect similar spatial patterns of ANC attendance to those observed in the current study. Under-estimation of HIV prevalence in towns in ANC surveillance data could be important if these data are used in targeting resources to areas of high prevalence [29].
The Manicaland study is unique in having collected parallel HIV surveillance data from local ANCs and from the general population in the same areas over a 14-year period spanning the introduction and scale-up of ART services. Participation in the general population survey was close to 80% in all rounds but temporal changes in participation rates amongst, for example, high-risk groups such as commercial sex workers could still have distorted observed trends in HIV prevalence. Generally, in the ANC-based HIV surveillance, it is preferable to measure trends in a consistent set of clinics [30]. We found similar HIV prevalence in all clinics and in clinics visited in all rounds, possibly because changes largely reflected changes in the main clinics operational within the study sites. To maintain representativeness of the study sites and a reasonable sample size, we included all clinics in subsequent analyses. PMTCT services were introduced in ANCs in Zimbabwe in 2002 and the national HIV testing policy changed from opt-in to opt-out testing from 2007. These developments might have affected HIV prevalence trends measured using routine surveillance or programme data but we were unable to investigate effects of this nature because these data were not available for the study.
These limitations notwithstanding, the finding of faster HIV prevalence declines in pregnant women attending ANCs than in the general population is important because ANC surveillance data are relied upon extensively in models of trends in national and regional HIV estimates, which, in turn, are used in planning and evaluating services. For countries without national population surveys, our findings suggest that HIV prevalence in pregnant women is less likely than in the past to provide a reasonable estimate for prevalence in the general population, even at local level. Further comparisons of data from longitudinal general population surveys with information on recent pregnancies could be conducted to test the generalizability of these findings.
Acknowledgements
S.G. designed the study and analysed the data with input from K.D., M.P., N.S. and A.T. All authors contributed to interpretation of results and read and approved the final manuscript. We are grateful to Kimberley Marsh for revisions to the ANC survey questionnaire, to the Manicaland Study team for assistance with data collection and processing, and to the study participants for providing the necessary data.
Source of funding: Wellcome Trust programme grant (084401/Z/07/B).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Stover J, Andreev K, Slaymaker E, Gopalappa C, Sabin K, Velasquez C, et al. Updates to the Spectrum model to estimate key HIV indicators for adults and children. AIDS. 2014;28:s427–s434. doi: 10.1097/QAD.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin J. Public health surveillance of AIDS and HIV infections. Bulletin of the World Health Organization. 1990;68:529–536. [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. Report on the UNAIDS Epidemiology Reference Group Meeting; Rome. October 8-10, October 2000; Oxford University: Oxford; 2000. In. [Google Scholar]
- 4.Walker N, Stanecki KA, Brown T, Stover J, Lazzari S, Garcia-Calleja JM, et al. Methods and procedures for estimating HIV/AIDS and its impact: the UNAIDS/WHO estimates for the end of 2001. AIDS. 2003;17:2215–2225. doi: 10.1097/00002030-200310170-00010. [DOI] [PubMed] [Google Scholar]
- 5.Boerma JT, Ghys PD, Walker N. Estimates of HIV-1 prevalence from national population-based surveys as a new gold standard. The Lancet. 2003;362:1929–1931. doi: 10.1016/S0140-6736(03)14967-7. [DOI] [PubMed] [Google Scholar]
- 6.Gouws E, Mishra V, Fowler TB. Comparison of adult HIV prevalence from national population-based surveys and antenatal clinic surveillance in countries with generalised epidemics: implications for calibrating surveillance data. Sexually Transmitted Infections. 2008;84:i17–i23. doi: 10.1136/sti.2008.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghys PD, Walker N, McFarland W, Miller R, Garnett GP. Improved data, methods and tools for the 2007 HIV and AIDS estimates and projections. Sexually Transmitted Infections. 2008;84:i1–i4. doi: 10.1136/sti.2008.032573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaton JW, Rehle TM, Jooste S, Nkambule R, Kim AA, Mahy M, et al. Recent HIV prevalence trends among pregnant women and all women in sub-Saharan Africa: implications for HIV estimates. AIDS. 2014;28:s507–s514. doi: 10.1097/QAD.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh KA, Mahy M, Salomon JA, Hogan DR. Assessing and adjusting for differences between HIV prevalence estimates derived from national population-based surveys and antenatal care surveillance, with applications for Spectrum 2013. AIDS. 2014;28:s497–s505. doi: 10.1097/QAD.0000000000000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills EJ, Barninghausen T, Negin J. HIV and ageing - preparing for the challenges ahead. New England Journal of Medicine. 2012;366:1270–1273. doi: 10.1056/NEJMp1113643. [DOI] [PubMed] [Google Scholar]
- 11.UNAIDS. HIV and Ageing: A Special Supplement to the UNAIDS report on the Global AIDS Epidemic 2013. Geneva: UNAIDS; 2013. p. 8. [Google Scholar]
- 12.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2012. Geneva: UNAIDS; 2012. [Google Scholar]
- 13.Kaida A, Andia I, Maier M, Strathdee SA, Bangsberg DR, Spiegel J, et al. The potential impact of ART on fertility in sub-Saharan Africa. Current HIV Reports. 2006;3:187–194. doi: 10.1007/s11904-006-0015-0. [DOI] [PubMed] [Google Scholar]
- 14.Zaba B, Gregson S. Measuring the impact of HIV on fertility in Africa. AIDS. 1998;12:S41–S50. [PubMed] [Google Scholar]
- 15.Myer L, Carter RJ, Katyal M, Toro P, El-Sadr WM, Abrams MJ. Impact of antiretroviral therapy on incidence of pregnancy among HIV-infected women in sub-Saharan Africa: a cohort study. Public Library of Science Medicine. 2010;7:e1000229. doi: 10.1371/journal.pmed.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeatman SE. The impact of HIV status and perceived status on fertility desires in rural Malawi. AIDS and Behaviour. 2009;13:S12–S19. doi: 10.1007/s10461-009-9534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maier M, Andia I, Emenyonu N, Guzman D, Kaida A, Pepper L, et al. ART is associated with increased fertility desire, but not pregnancy or live birth, among HIV+ women in an early HIV treatment program in rural Uganda. AIDS and Behaviour. 2009;13:28–37. doi: 10.1007/s10461-008-9371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregson S, Garnett GP, Nyamukapa CA, Hallett TB, Lewis JJC, Mason PR, et al. HIV decline associated with behaviour change in eastern Zimbabwe. Science. 2006;311:664–666. doi: 10.1126/science.1121054. [DOI] [PubMed] [Google Scholar]
- 19.Gregson S, Takavarasha F, Schumacher C, Mugurungi O, Nyamukapa CA, Garnett GP. Transmission dynamics underlying a decade of HIV prevalence decline in Manicaland, Zimbabwe, 1998-2008. XIX International AIDS Conference; Washington DC. 2012. [Google Scholar]
- 20.Ross A, Van der Paal L, Lubega R, Mayanja B, Shafer LA, Whitworth JAG. HIV-1 disease progression and fertility: the incidence of recognized pregnancy and pregnancy outcome in Uganda. AIDS. 2004;18:799–804. doi: 10.1097/00002030-200403260-00012. [DOI] [PubMed] [Google Scholar]
- 21.Halperin DT, Mugurungi O, Hallett TB, Muchini B, Campbell B, Magure T, et al. A surprising prevention success: Why did the HIV epidemic decline in Zimbabwe? Public Library of Science Medicine. 2011;8:e1000414. doi: 10.1371/journal.pmed.1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terceira N, Gregson S, Zaba B, Mason PR. The contribution of HIV to fertility decline in rural Zimbabwe. Population Studies. 2003;57:149–164. doi: 10.1080/0032472032000097074. [DOI] [PubMed] [Google Scholar]
- 23.Zaba B, Boerma T, White R. Monitoring the AIDS epidemic using HIV prevalence data among young women attending antenatal clinics: prospects and problems. AIDS. 2000;14:1633–1645. doi: 10.1097/00002030-200007280-00020. [DOI] [PubMed] [Google Scholar]
- 24.Gregson S, Nyamukapa C, Garnett GP, Mason PR, Zhuwau T, Careal M, et al. Sexual mixing patterns and sex-differentials in teenage exposure to HIV infection in rural Zimbabwe. The Lancet. 2002;359:1896–1903. doi: 10.1016/S0140-6736(02)08780-9. [DOI] [PubMed] [Google Scholar]
- 25.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregson S, Terceira N, Kakowa M, Mason PR, Anderson RM, Chandiwana SK, et al. Study of bias in antenatal clinic HIV-1 surveillance data in a high contraceptive prevalence population in sub-Saharan Africa. AIDS. 2002;16:643–652. doi: 10.1097/00002030-200203080-00017. [DOI] [PubMed] [Google Scholar]
- 27.Montana LS, Mishra V, Hong R. Comparison of HIV prevalence estimates from antenatal care surveillance and population-based surveys in sub-Saharan Africa. Sexually Transmitted Infections. 2008;84:i78–i84. doi: 10.1136/sti.2008.030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonese E, Dzangare J, Jonga N, Mugurungi O, Gregson S, Walkup R, et al. Zimbabwe Working Papers, No 1. Calverton, Maryland, USA: ICF Macro; 2010. Comparison of HIV Prevalence Estimates for Zimbabwe from National Antenatal Clinic Surveillance (2006) and the 2-005-06 Zimbabwe Demographic and Health Survey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson S-J, Cherutich P, Kilonzo N, Cremin I, Fecht D, Kimanga D, et al. Maximising the effect of combination HIV prevention through prioritisation of the people and places in greatest need: a modelling study. The Lancet. 2014;384:249–256. doi: 10.1016/S0140-6736(14)61053-9. [DOI] [PubMed] [Google Scholar]
- 30.Glynn JR, Buve A, Carael M, Kahindo M, Macauley IB, Musonda RM, et al. Decreased fertility among HIV-1-infected women attending antenatal clinics in three African cities. Journal of Acquired Immune Deficiency Syndromes. 2000;25:345–352. doi: 10.1097/00042560-200012010-00008. [DOI] [PubMed] [Google Scholar]