Abstract
Peroxisomes contain numerous enzymatic activities that are important for mammalian physiology. Patients lacking either all peroxisomal functions or a single enzyme or transporter function typically develop severe neurological deficits, which originate from aberrant development of the brain, demyelination and loss of axonal integrity, neuroinflammation or other neurodegenerative processes. Whilst correlating peroxisomal properties with a compilation of pathologies observed in human patients and mouse models lacking all or individual peroxisomal functions, we discuss the importance of peroxisomal metabolites and tissue- and cell type-specific contributions to the observed brain pathologies. This enables us to deconstruct the local and systemic contribution of individual metabolic pathways to specific brain functions. We also review the recently discovered variability of pathological symptoms in cases with unexpectedly mild presentation of peroxisome biogenesis disorders. Finally, we explore the emerging evidence linking peroxisomes to more common neurological disorders such as Alzheimer’s disease, autism and amyotrophic lateral sclerosis. This article is part of a Special Issue entitled: Peroxisomes edited by Ralf Erdmann.
Keywords: Lipid metabolism, Plasmalogen, Zellweger spectrum disorder, D-bifunctional protein deficiency, X-linked adrenoleukodystrophy, Rhizomelic chondrodysplasia punctata
1. Introduction
Peroxisomes are single membrane-bound organelles, which harbor a variety of biochemical reactions and metabolic pathways that contribute to different physiological functions in eukaryotic organisms. Peroxisomes are found ubiquitously, but their number, shape and enzymatic content appear variable and differ between organisms and tissues and even upon changes in the environment [1]. In this review, we restrict the discussion to peroxisomal functions in the mammalian nervous system, with a specific focus on human physiology and pathophysiology supplemented by observations made in various mouse models. In mammals, peroxisomes contain around 50 different proteins [2], which exert a variety of catabolic and anabolic reactions as, for example, the degradation of very long-chain fatty acids (VLCFA)1, dicarboxylic acids, branched-chain fatty acids, or parts of the biosynthesis of ether phospholipids or specific polyunsaturated fatty acids [3].
The importance of peroxisomes for mammalian physiology is highlighted by the existence of a variety of severe inherited human diseases caused by the complete or partial loss of peroxisomal functions. These diseases have been subdivided into peroxisome biogenesis disorders (PBD), in which the formation of functional peroxisomes is disturbed, and single enzyme and transporter deficiencies lacking individual enzymatic activities that are performed by peroxisomes. Patients suffering from PBD show a broad spectrum of symptoms summarized as Zellweger spectrum disorders and rhizomelic chondrodysplasia punctata (RCDP) type 1. The genetic basis for each PBD is a mutation in one of 14 PEX genes, which encode proteins termed peroxins (PEX proteins or peroxisome biogenesis factors), which are involved in the biogenesis of the organelle (Table 1). All peroxisomal enzymes and membrane proteins contain a targeting signal, which is necessary and sufficient to mediate the interaction of the encoding protein with a receptor protein that translocates its cargo to peroxisomes and initiates the import. These processes are carried out by the PEX proteins (Fig. 1), which are either involved in the import of matrix proteins (PEX1, 2, 5, 6, 7, 10, 12, 13, 14, 26) or of membrane proteins (PEX3, 16 and 19) [4]. Soluble proteins harbor such peroxisome targeting signal (PTS) sequences either at their extreme C-terminus (type 1, PTS1) or close to their N-terminus (type 2, PTS2), whereas membrane proteins contain targeting signals for membrane proteins (mPTS). PTS1 is required for the interaction with the cytoplasmic receptor PEX5, PTS2 for the interaction with PEX7 and the mPTS for the interaction with PEX19. This is the reason why in Zellweger spectrum patients, on the cellular level, peroxisomes are either absent or empty (ghosts).
Table 1.
Genetic basis of peroxisomal disorders.
| Gene | Protein | Disease | Phenotype MIM | Reference |
|---|---|---|---|---|
| Peroxisome biogenesis disorders | Zellweger syndrome spectrum disorder | |||
| PEX1 | Peroxin 1 (PEX1) | Zellweger syndrome, | 214100 | [316] |
| neonatal adrenoleukodystrophy, infantile Refsum disease | 601539 | |||
| PEX2 | Peroxin 2 (PEX2) | Zellweger syndrome, | 614866 | [46] |
| infantile Refsum disease | 614867 | [317] | ||
| PEX3 | Peroxin 3 (PEX3) | Zellweger syndrome | 614882 | [318] |
| PEX5 | Peroxin 5 (PEX5) | Zellweger syndrome, | 214110 | [319] |
| neonatal adrenoleukodystrophy | 202370 | |||
| PEX6 | Peroxin 6 (PEX6) | Zellweger syndrome, | 614862 | [320] |
| neonatal adrenoleukodystrophy, infantile Refsum disease | 614863 | [321] | ||
| PEX10 | Peroxin 10 (PEX10) | Zellweger syndrome, | 614870 | [322] |
| neonatal adrenoleukodystrophy | 614871 | |||
| PEX12 | Peroxin 12 (PEX12) | Zellweger syndrome, | 614859 | [323] |
| neonatal adrenoleukodystrophy, infantile Refsum disease | 266510 | [324] | ||
| PEX13 | Peroxin 13 (PEX13) | Zellweger syndrome, | 614883 | [325] |
| neonatal adrenoleukodystrophy | 614885 | [326] | ||
| PEX14 | Peroxin 14 (PEX14) | Zellweger syndrome | 614887 | [327] |
| PEX16 | Peroxin 16 (PEX16) | Zellweger syndrome | 614876 | [328] |
| Mild Zellweger syndrome spectrum disorder | 614877 | [58] | ||
| PEX19 | Peroxin 19 (PEX19) | Zellweger syndrome | 614886 | [329] |
| PEX26 | Peroxin 26 (PEX26) | Zellweger syndrome, | 614872 | [330] |
| neonatal adrenoleukodystrophy, infantile Refsum disease | 614873 | |||
| PEX11β | Peroxin 11β (PEX11β) | Mild Zellweger syndrome spectrum disorder | 614920 | [331,332] |
| PEX7 | Peroxin 7 (PEX7) | Rhizomelic chondrodysplasia punctata type 1 | 215100 | [176–178] |
| 614879 | [190] | |||
| Single peroxisomal enzyme and transporter deficiencies | ||||
| Fatty acid β-oxidation | ||||
| ACOX1 | Acyl-CoA oxidase 1 (ACOX1) | Acyl-CoA oxidase deficiency | 264470 | [333] |
| HSD17B4 | D-Bifunctional proteina | D-Bifunctional protein deficiency | 261515 | [334] |
| Perrault syndrome 1 | 233400 | [85] | ||
| SCP2 | Sterol carrier protein 2 (SCP2)b | Sterol-carrier-protein X deficiency | 613724 | [102] |
| AMACR | α-Methylacyl-CoA racemase | α-Methylacyl-CoA racemase deficiency | 614307 | [93] |
| Congenital bile acid synthesis defect 4 | 214950 | |||
| ABCD1 | ATP-binding cassette transporter, subfamily D, member 1 (ABCD1) | X-linked adrenoleukodystrophy | 300100 | [108] |
| ABCD3 | ATP-binding cassette transporter, subfamily D, member 3 (ABCD3) | ATP-binding cassette transporter, subfamily D, member 3 deficiency | 616278 | [335] |
| Fatty acid α-oxidation | ||||
| PHYH/PAHX | Phytanoyl-CoA hydroxylase (PHYH, PAHX) | Refsum disease | 266500 | [170,336] |
| Ether phospholipid biosynthesis | ||||
| GNPAT | Dihydroxyacetone phosphate acyltransferase (DHAPAT) | Rhizomelic chondrodysplasia punctata type 2 | 222765 | [179] |
| AGPS | Alkyl-dihydroxyacetone phosphate synthase (ADHAPS) | Rhizomelic chondrodysplasia punctata type 3 | 600121 | [180] |
| FAR1 | Fatty acyl-CoA reductase 1 (FAR1) | Rhizomelic chondrodysplasia punctata type 4/peroxisomal fatty acyl-CoA reductase 1 deficiency | 616154 | [183] |
| PEX5 | Peroxin 5 long isoform (PEX5L) | Rhizomelic chondrodysplasia punctata type 5 | - | [185] |
| Bile acid maturation | ||||
| BAAT | Bile acid CoA:amino acid N-acyl-transferase (BAAT) | Familiar hypercholanemia/bile acid-CoA: amino acid N-acyltransferase deficiency | 607748 | [337] |
| Glyoxylate metabolism | ||||
| AGXT | Alanine-glyoxylate aminotransferase (AGXT, AGT) | Primary hyperoxaluria type I | 259900 | [338] |
| Hydrogen peroxide metabolism | ||||
| CAT | Catalase | Acatalasemia | 614097 | [339] |
| Others | ||||
| ALDH3A2 | Fatty aldehyde dehydrogenase (FALDH)c | Sjögren–Larsson syndrome | 270200 | [340] |
| DAO | D-Amino acid oxidase (DAO, DAAO) | Amyotrophic lateral sclerosis | 105400 | [254] |
Alternative names: 17-β-hydroxysteroid dehydrogenase IV (HSD17B4)/multifunctional protein 2 (MFP2).
Alternative name: sterol carrier protein X (SCPX).
Two isoforms are known residing in peroxisomes and the ER, which precludes attribution of the disease to a particular variant.
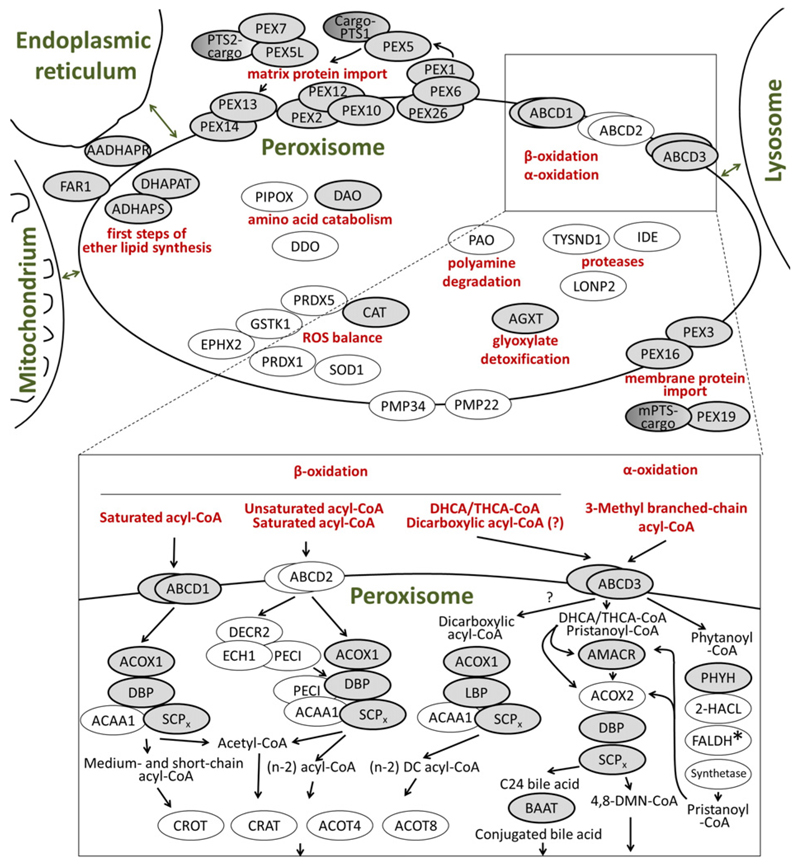
Fig.1.
Schematic drawing linking peroxisomal disease-related proteins to individual metabolic pathways. Upper part: Proteins are grouped according to their function in biosynthetic or degradative metabolic pathways, ROS homeostasis, proteolytic activity, transport of metabolites across the peroxisomal membrane (ABCD and PMP proteins), and the import of matrix and membrane proteins (PEX proteins). Ovals represent proteins that are involved in peroxisomal functions (not complete); gray ovals, proteins for which mutations have been linked to a human disease (for full name see Table 1). The degradation of various fatty acids and bile acid precursors is symbolized by the frame depicting the homodimeric transporters (ABCD1–3) and the terms α- and β-oxidation, illustrated in more detail below. Lower part: Proteins are grouped into the degradation pathways for different activated fatty acids (fatty acyl-CoA: saturated, unsaturated, dicarboxylic, branched-chain) and the side chain shortening of di- and trihydroxycholestanoic acid (DHCA/THCA) during bile acid biosynthesis (all via β-oxidation) and the oxidative removal of one carbon unit from branched-chain fatty acids (α-oxidation). Several proteins are involved in the subsequent modification of the β-oxidation products, either by thiolytic cleavage (thioesterases, ACOT), substitution of CoA for carnitine (carnitine transferases, CRAT and CROT) or amidation of the CoA-activated side chain of bile acids (amino transferase, BAAT). FALDH*, two isoforms are known residing in peroxisomes and the ER, respectively, which precludes attribution of the linked disease, Sjögren–Larsson syndrome, to a particular variant. Synthetase, CoA-activation is essential for the link between α- and β-oxidation, but the exact enzyme has not yet been assigned. PEX, peroxin; cargo-PTS1 and PTS2-cargo, representative peroxisomal matrix proteins harboring a PTS1 or PTS2 motif, respectively; mPTS-cargo, representative peroxisomal membrane protein harboring a motif for targeting of peroxisomal membrane proteins (mPTS). 4,8-DMN-CoA, 4,8-dimethylnonanoyl-CoA. Proteins not included in Table 1: 2-HACL, 2-hydroxyacyl-CoA lyase; ABCD2, ATP-binding cassette transporter D2; ACAA1, acetyl-CoA acyltransferase 1; ACOT4, acyl-CoA thioesterase 4; ACOT8, acyl-CoA thioesterase 8; ACOX2, acyl-CoA oxidase 2; CRAT, carnitine O-acetyltransferase; CROT, carnitine O-octanoyltransferase; DDO, D-aspartate oxidase; DECR2, dienoyl-CoA reductase 2; ECH1, enoyl-CoA hydratase 1; EPHX2, epoxide hydroxylase 2; GSTK1, glutathione S-transferase kappa-1, IDE, insulin-degrading enzyme; LONP2, lon peptidase 2; PAO, polyamine oxidase; PIPOX, pipecolic acid oxidase; PECI, peroxisomal D3,D2-enoyl-CoA isomerase; PMP22, peroxisomal membrane protein of 22 kDa; PMP34, peroxisomal membrane protein of 34 kDa; PRDX1, peroxiredoxin 1; PRDX5, peroxiredoxin 5; SOD1, superoxide dismutase 1; TYSND1, trypsin domain-containing 1
The symptoms of patients with peroxisomal single enzyme and transporter deficiencies have a broad heterogeneity, related to differences in the physiological role of the affected metabolic pathway or reaction [5]. In this group of inherited diseases, mutations have been identified in 13 different genes encoding peroxisomal enzymes and in two genes encoding peroxisomal transporter proteins (Table 1; Fig. 1).
The brain is the most elaborate organ of the mammalian body and consists of a variety of tissue-specific cell types: neurons (with hundreds of different subtypes), oligodendrocytes, astrocytes and microglia. These differ in structure and function but cooperate tightly to perform all the tasks attributed to the brain. Moreover, the structural complexity of brain organization requires a precisely coordinated developmental process to accomplish its proper formation. The central nervous system (CNS; brain and spinal cord) and the peripheral nervous system (PNS) use the same mechanisms for communication between neurons, which transmit information by chemical synapses between cells. In addition, efficient propagation of the electrical signal (action potential) along the nerve fibers is facilitated by myelin ensheathment of the axons. The complexity of the nervous system and the tight interaction of the involved cell types render this system susceptible to disturbances. Accordingly, metabolic dysfunction associated with a complete loss of all peroxisomal functions or of individual enzymatic reactions is often linked to perturbation of brain formation, function or maintenance. Thus, pathological aberrations of the nervous system are prominent features in most peroxisomal disorders; the most severe form of PBD has traditionally been designated “cerebro-hepato-renal syndrome” highlighting the apparent brain dysfunction in these patients. The brain pathology in peroxisomal disorders can be grouped into three major classes: i) abnormalities in neuronal migration or differentiation, ii) defects in the formation or maintenance of central white matter, and iii) post-developmental neuronal degeneration [6].
This review summarizes the current knowledge on the contribution of the various peroxisomal pathways to proper brain function with particular consideration of the different cell types of the nervous system.
2. Metabolic functions of peroxisomes
Peroxisomes harbor a variety of enzymes, which either serve to catalyze a single chemical reaction or cooperate with other peroxisomal enzymes in a series of coupled reactions constituting a complete metabolic pathway. A selection of these enzymes, which exert important peroxisomal functions in the context of the brain, is schematically depicted in Fig. 1. For further details on these metabolic pathways, the reader is referred to excellent previous reviews [3] [7].
A prominent example of such a metabolic pathway is the peroxisomal degradation of diverse fatty acids by β-oxidation (Fig. 1, lower part). Here, many different substrates are handled, such as straight-chain saturated VLCFA, unsaturated fatty acids, dicarboxylic acids and a subset of branched-chain fatty acids, but also the side chain of intermediates in bile acid biosynthesis (di- and trihydroxycholestanoic acid; DHCA and THCA) [7]. The β-oxidation cycle is a four-step reaction, executed by three enzymes: an acyl-CoA oxidase (ACOX1 or ACOX2), a bifunctional protein (DBP or LBP) and a thiolase (ACAA1 or SCPx), in which the paralogous/homologous enzymes show different extents of substrate specificity. Each cycle results in a shortening of the acyl-CoA backbone and the release of acetyl-CoA or propionyl-CoA (in case of branched-chain fatty acids). Auxiliary enzymes help to circumvent special properties of unsaturated or branched-chain fatty acids that would be incompatible with continuous β-oxidation. The substrates of β-oxidation are imported into peroxisomes in an activated form, as CoA-ester, via ATP-binding cassette (ABC) transporter proteins (ABCD1, ABCD2 and ABCD3) and the products are further processed either into carnitine esters by carnitine ac(et)yl-transferases (CRAT and CROT) or into free acids by thioesterases (ACOT4 and ACOT8) (Fig. 1, lower part). A subtype of branched-chain acyl-CoA (especially phytanic acid) first has to be oxidatively decarboxylated via the α-oxidation pathway [7]. This process involves hydroxylation of the carbon next to the carboxylate ester (by PHYH) and a subsequent oxidative cleavage to split off the carboxyl group by 2-hydroxyacyl-CoA lyase (2-HACL) releasing an acyl-aldehyde. The subsequent steps involve an oxidation of the aldehyde (fatty aldehyde dehydrogenase; FALDH) and an activation of the generated fatty acid by a still unknown acyl-CoA synthetase.
Furthermore, the early steps of ether phospholipid biosynthesis are exerted by peroxisomal enzymes (Fig. 1, upper part), which reside either inside (DHAPAT, ADHAPS) peroxisomes or at the outer side (FAR1, AADHAPR) [3]. This metabolic pathway consists of a series of reactions; the first, carried out by dihydroxyacetone phosphate acyltransferase (DHAPAT) combines dihydroxyacetone phosphate (DHAP) with a fatty acid, which is then exchanged for an long-chain alcohol by alkyl-DHAP synthase (ADHAPS). This long-chain alcohol is generated from another fatty acid by a fatty acyl-CoA reductase (FAR1) at the outer side of peroxisomes. Finally, the carbonyl group of the original dihydroxyacetone phosphate is reduced by alkyl/acyl-dihydroxyacetone phosphate reductase (AADHAPR) to enable further processing at the endoplasmic reticulum (ER).
Other peroxisomal enzymes can exert their function more independently (Fig. 1, upper part) such as the enzymes of the reactive oxygen species (ROS) detoxification system (peroxiredoxin 1/5, PRDX1/5; superoxide dismutase 1, SOD1; epoxide hydrolase, EPXH2; glutathione-S-transferase kappa 1, GSTK1 and catalase, CAT), which together prevent the accumulation of reactive compounds, as reviewed in [8]. Also several enzymes acting on amino acids and their derivatives (pipecolic acid oxidase, PIPOX; D-aspartate oxidase, DDO; D-amino acid oxidase, DAO; alanine:glyoxylate aminotransferase, AGXT) or other oxidative enzymes like polyamine oxidase (PAO) act in isolation [3]. Furthermore, several proteins with a protease domain have been found in peroxisomes (lon peptidase 2, LONP2; insulin-degrading enzyme, IDE; trypsin domain-containing 1, TYSND1) and some membrane proteins (peroxisomal membrane protein of 22 kDa, PMP22; and peroxisomal membrane protein of 34 kDa, PMP34), which transport a variety of smaller organic compounds such as nicotinamide-adenine-dinucleotides (NAD), CoA, or ATP [9].
The enzymes known to be dysfunctional in patients suffering from inherited peroxisomal disorders are distributed across these pathways (Fig. 1, gray ovals). However, the relative physiological contribution of each enzyme may differ drastically. Consequently, the pathological consequences of their functional loss range from very severe diseases, like D-bifunctional protein (DBP) deficiency (see chapter 5.2.2.), to diseases that affect selective tissues but not the brain, like AGXT deficiency causing primary hyperoxaluria type 1, which involves the kidneys [10].
3. Brain peroxisomes and how they differ from peroxisomes in other tissues
Although peroxisomes are present in all mammalian cell types, except for red blood cells, they contribute to the function of the CNS in specific ways. On the one hand, peroxisomes generate building blocks (intermediates) for the biosynthesis of complex lipids such as ether phospholipids, which are important components of myelin, the membrane processes of oligodendrocytes that ensheath and isolate axons. Moreover, peroxisomes exert the last step in the biosynthesis of the very long-chain polyunsaturated fatty acid docosahexaenoic acid (DHA; C22:6 n-3), which has important roles in the nervous system [11]. This fatty acid is enriched in phospholipids including ether phospholipids and, either directly or after enzymatic conversion to a variety of bioactive derivatives, plays an important role in signaling [12]. On the other hand, peroxisomes degrade toxic compounds that can either interfere with proper brain formation or damage brain structures (e.g., phytanic acid). Furthermore, peroxisomal enzymes degrade D-amino acids such as D-aspartate and D-serine, which modulate synaptic signaling by altering the efficiency of synaptic transmission (Fig. 3A, left panel).
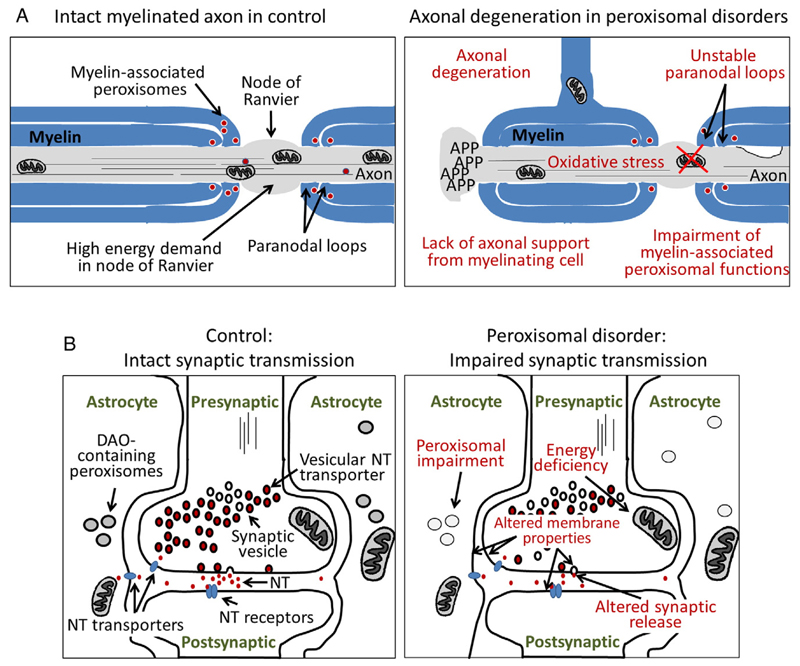
Fig.3.
Schematic representation of abnormalities of myelinated axons and synaptic transmission in peroxisomal deficiencies. (A) The left panel shows a myelinated axon at the level of a node of Ranvier in a healthy control. The myelin sheath of oligodendrocytes (in the CNS) or Schwann cells (in the PNS) surrounds and isolates the axon, except at the node of Ranvier allowing depolarization of the neuronal membrane and propagation of electrical signals. Note that a multitude of ion channels and Na+/K+-ATPases (not indicated) are located at the node of Ranvier and entail a high energy demand. In the right panel, different pathological features are indicated that may contribute to the axonal degeneration frequently observed in peroxisomal disorders, for example, adrenomyeloneuropathy (the late-onset variant of X-ALD). A scenario can be envisaged, where peroxisomal dysfunction and abnormal accumulation of lipid metabolites in myelinating cells lead to unstable paranodal loops and a loss of axonal support resulting in energy deficits and oxidative damage in the axons and progressive axonal degeneration. (B) A normal synapse with the surrounding astrocytes is depicted (left panel), representative for a synapse of any neurotransmitter. D-Amino acid oxidase is indicated for its role in D-serine degradation at e.g. glutamatergic synapses. The right panel shows several possible disturbances of synaptic function (red text) that could lead to altered neurotransmission, as predominantly described in ether lipid deficiency. NT, neurotransmitter; DAO, D-amino acid oxidase
In the brain, peroxisomes appear as electron-dense single membrane-bound organelles that have been detected in all neural cell types, namely in neurons [13], oligodendrocytes [14,15] and astrocytes [13] and microglia and endothelial cells [16]. Brain peroxisomes in general, and neuronal peroxisomes in particular, are smaller than peroxisomes from other tissues and, thus, were termed microperoxisomes [17]. However, for the sake of simplicity, we use the term peroxisomes for all structures within this review. In cultured cells from rat brain, punctate peroxisomal immunoreactivity was found in mixed glial cells and established oligodendrocyte cultures [18], as well as in astrocytes and neurons [19].
The distribution of peroxisomes in the brain has been investigated by different techniques such as cytochemical detection of enzymatic activities restricted to peroxisomes including 3-aminotriazol-sensitive precipitation of diamino-benzidine for catalase, conversion of D-proline for detection of DAO or of D-aspartate for DDO [20]. Moreover, immunohistochemistry, immunofluorescence microscopy and electron microscopy have been used. However, it is important to keep in mind that many studies examined the presence and abundance of a single peroxisomal protein, thus possibly detecting only a subset of peroxisome-positive cells. Therefore, it is necessary to combine the different investigations to obtain an insight into the accurate distribution and abundance of all peroxisomes in the brain. Comparison of DAO and catalase activity revealed that in the locus coeruleus of the rat brain, peroxisomes that stained positive for catalase activity were found in various cell types, whereas DAO activity-positive peroxisomes were restricted to astrocytes [13]. Similar results were obtained in the cerebrum and in the PNS [13]. In the cerebellum, punctate catalase immunoreactivity (characteristic of a peroxisomal localization) was predominantly observed in Bergmann glia (astrocytes), whereas in Purkinje cells, catalase appeared evenly distributed. This finding was recapitulated in explanted cells from the cerebellum, in which catalase appeared cytosolic (not enriched in peroxisomes) in calbindin-positive Purkinje cells but punctate in astrocytes, whereas the peroxisomal membrane protein PEX14 was found punctate in all cell types [21]. Moreover, the abundance of brain peroxisomes differs between brain areas. Although single membrane-bound structures – detectable with different methods to stain peroxisomes – can be found in most regions, some brain areas were reported to contain only modest numbers of peroxisomes [22]. However, peroxisome abundance also changes during development. In the human brain, catalase-positive neurons emerged early in evolutionary old structures such as the basal ganglia, the thalamus and the cerebellum (about 27–28 weeks of gestation), whereas in the frontal cortex, they appeared later (around 35 weeks of gestation) [15]. Similar observations were obtained when investigating the distribution of ACOX1 or thiolase (ACAA1) immunoreactivity [23]. In the deep white matter, catalase-positive glia appeared at 31–32 weeks of gestation, their appearance shifting from the deep to the superficial white matter with increasing age [15]. Interestingly, during rat brain development, peroxisomal activity (as represented by catalase activity) remained constant in the cerebral cortex (a typical gray matter region), whereas in the white matter, the activity changed over time with a clear peak accompanying the phase of myelination (during postnatal days 17–31) [24]. A similar increase in catalase activity was found in extracts from murine cerebellum and brain stem [25], whereas a systematic comparison by western blot analysis and catalase activity measurements found the maximum level two days after birth and at later timepoints, 15 and 49 days postnatally, the levels of peroxisomal enzymes remained comparable [19].
This change in protein abundance is reflected at the mRNA level, where the expression of genes coding for enzymes involved in the same metabolic pathways showed similar temporal profiles. In the murine brain, the mRNA levels of the peroxisomal β-oxidation enzymes (ACOX1, DBP, ACAA1a), the ABC transporters ABCD2 and ABCD3, and the enzymes involved in ether phospholipid biosynthesis increased after birth, reached a maximum during the first weeks and then declined. In contrast, the mRNAs for the enzymes involved in α-oxidation were not detected during the first postnatal weeks; and the ABCD1 mRNA was most highly expressed in the embryonic brain [26–28]. This change in enzyme expression was confirmed in a systematic biochemical investigation of the abundance of peroxisomal enzymes and their activity during mouse brain development. In this study, it was found that peroxisomal activities decreased during postnatal development (P2, P15, P49), irrespective of whether the activity was normalized to the whole brain or to different brain regions (cerebellum, hippocampus, cortex) [19]. This is in agreement with previous findings in rat brain demonstrating that during the first two postnatal weeks, peroxisomes are more abundant than at later time points [22]. However, this general trend contrasts with the reported amount of DAO activity in astrocytes of rat cerebellum, which was only observed in adult rats, whereas no staining was observed in young animals (P3, P13, P16) [13]. This might indicate a more specific contribution of DAO in the adult brain, which could be linked to its function in the modulation of neuronal synaptic transmission (see chapter 6.1.).
In the rat PNS, peroxisomes were described in Schwann cells, which represent the myelinating cells of the PNS, as well as in dorsal root ganglion satellite cells and, less abundantly, in neuronal somata [29]. In neurons of human dorsal root ganglia, peroxisomes were readily detected based on immunohistochemistry for ABCD1 [30]. During early stages of murine peripheral nerve (sciatic nerve) myelination, peroxisomes appear to be diffusely distributed in the myelin sheath of Schwann cells, whereas at later stages, peroxisomes were found to be enriched in the myelin loops of the paranodal region [14]. These axon-glia contact sites flank the nodes of Ranvier, substructures of myelinated neurons, in which highly abundant sodium channels in the axonal membrane enable depolarization and reinitiation of the action potential and thus permit the rapid saltatory propagation of the electrical signal across long distances (Fig. 2A, left panel) [14].
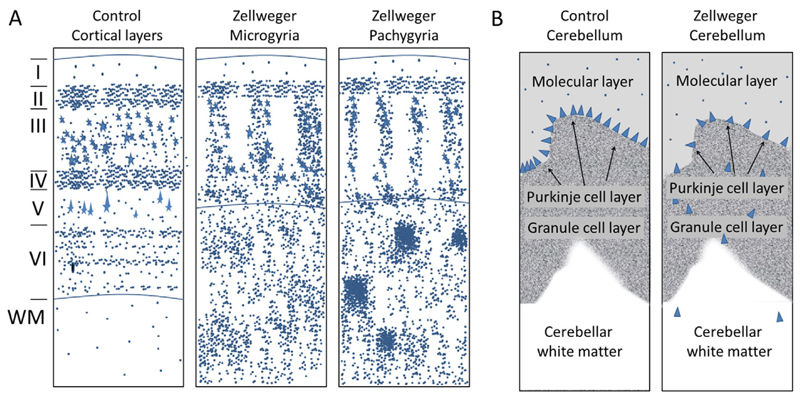
Fig.2.
Schematic representation of neuronal migration defects in peroxisomal biogenesis disorders. (A) In the cerebral cortex (neocortex) of a healthy individual (left panel), the cell bodies of cortical neurons are localized in discrete layers. In comparison, the cortical lamination is severely disturbed and the border to the white matter in microgyric (middle panel) and pachygyric (right panel) brains of cases with Zellweger Syndrome is indicated (horizontal line). Similar abnormalities can also be found in cases of severe D-bifunctional protein deficiency. Roman numerals to the left correspond to normal cortical layers. WM, white matter. (B) In the cerebellum of a healthy individual (left panel), the Purkinje cells (blue triangles) are strictly arranged into a single cell-thick layer at the border of the molecular (outermost) layer and the thick granule cell layer. In Zellweger patients (right panel), many Purkinje cells are mislocalized to the granule cell layer and cerebellar white matter.
4. Peroxisomes, brain and oxidative stress
Oxidative stress is a cellular state characterized by a high level of ROS such as hydrogen peroxide (H2O2) or superoxide anions (O2·–), which are considered to be mediators of the toxic effects associated with oxidative stress. This state often arises as side effect of cellular disturbances and has been amply described in connection with general peroxisomal dysfunction, but also upon specific loss of an individual peroxisomal function [31]. An increase in the concentration of ROS can originate either from overproduction by one or more cellular producers (individual enzymes or whole organelles), a reduction of the detoxifying activity exerted by protective proteins (catalase, glutathione peroxidase, superoxide dismutase, peroxiredoxin) or a shortage of scavenging molecules that normally buffer the emerging ROS molecules (e.g., glutathione, vitamin C and E) [32].
Peroxisomes are known to house a variety of oxidases generating H2O2 and ROS, but they also enclose various ROS-detoxifying enzymes such as catalase, GSTK1, PRDX5 and SOD1 to limit the detrimental effects of local production [8]. However, this detoxification system can be overloaded. Artificial local production of ROS inside peroxisomes can induce apoptosis, which can be rescued by ectopic overexpression of peroxisomal detoxifying enzymes [33]. Exogenously added palmitate can stimulate H2O2 production in peroxisomes of insulin-producing cells [34] and exogenous application of VLCFA to a neuronal cell line induces oxidative stress and mitochondrial damage [35]. Prolonged hyperactivity of peroxisomes has also been linked to the overproduction of H2O2 in the liver of acyl-CoA oxidase-deficient mice [36]. Surprisingly, in patients suffering from acatalasemia, an inherited peroxisomal disorder caused by the loss of functional catalase, no neurological involvement or brain abnormalities have been described, although this enzyme plays such a prominent role in oxygen metabolism [37,38].
Moreover, peroxisomes are involved in the biosynthesis of plasmalogens, which have been suggested as scavenger molecules for H2O2 and ROS [39]. However, this effect is partially disputed, because plasmalogen-deficient mice do not show signs of increased oxidative stress [40]. This issue has been extensively covered in previous reviews [41,42]. Furthermore, the absence of one or more peroxisomal functions can indirectly increase the level of intracellular ROS, because under such conditions, the accumulation of particular compounds such as VLCFAs could be linked to disturbances in mitochondrial integrity, which secondarily increases the production rate of ROS [43,44].
5. Brain dysfunctions in inherited peroxisomal disorders
5.1. Brain pathology under conditions of generalized peroxisome deficiency in man and mice
This section focuses on the brain pathology in disorders of peroxisome biogenesis or assembly, collectively known as peroxisome biogenesis disorders (PBD). In these disorders, peroxisomes are not formed normally, typically leading to deficiency of the entire spectrum of peroxisomal functions. Thus, the observed pathology cannot be attributed to individual peroxisomal metabolic pathways but rather reflects the importance of the entire organelle for brain development and maintenance. In addition, genetically engineered mouse models with tissue- or cell type-specific inactivation of peroxisome biogenesis demonstrate the importance of peroxisomes for the different brain cell types, as well as the significance of peroxisomal functions in peripheral tissues for proper brain development.
5.1.1. Brain pathology in human patients with PBD
The PBD are divided into two types, i) Zellweger spectrum disorders and ii) rhizomelic chondrodysplasia punctata (RCDP) type 1. In our current understanding, the clinical syndromes constituting the Zellweger spectrum (MIM #601539)2 are the Zellweger syndrome, neonatal adrenoleukodystrophy, and infantile Refsum disease, which describe a clinical spectrum of decreasing severity [45]. These were originally described as independent disorders, long before the biochemical and molecular bases of these disorders were understood [45]. In 1992, the first gene defect associated with a PBD was identified [46]. By now, mutations in 13 different peroxin (PEX) genes (PEX1, PEX2, PEX3, PEX5, PEX6, PEX10, PEX11b, PEX12, PEX13, PEX14, PEX16, PEX19, PEX26) have been described in patients of the Zellweger spectrum [45]. Patients with mutations in PEX7 are grouped into PBD because more than one peroxisomal pathway is affected, although the peroxisomal structure remains intact. However, these patients have different clinical symptoms than Zellweger spectrum patients and are clinically not distinguishable from patients suffering from isolated disorders of ether phospholipid synthesis; hence, the associated brain pathology will be discussed in Section 5.5.
The identification of PBD complementation groups and their genetic basis has revealed that there are no clear boundaries between Zellweger syndrome, neonatal adrenoleukodystrophy and infantile Refsum disease, as they can all be caused by mutations in the same gene. However, a genotype-phenotype correlation has been described for PEX gene mutations [45]. The nature and location of the mutations determine whether the mutated peroxin can still contribute to the import machinery and allows residual metabolic functions of the peroxisomes in these patients. In recent years, increasing numbers of patients have been described with a later onset of the disease [47]. In accordance, also the neurological manifestations vary from primarily neurodevelopmental alterations in the most severe phenotypes to mainly degenerative abnormalities in the milder cases [48].
In patients with Zellweger syndrome, the most prominent feature of the brain pathology is a malformation of the cortex, which has been attributed to neuronal migration defects. The abnormalities in the cytoarchitecture of the cerebral cortex are usually bilateral and approximately symmetrical [49,50]. In these patients, often a local thickening of small convolutions (gyri) on the surface of the brain occurs around the central sulcus (centrosylvian pachygyria), causing a reduced depth of the fissions/involutions. Moreover, in these areas, an excess of local convolutions on the surface of the brain is observed (polymicrogyria). The cytoarchitectonic pattern of the cerebral cortex is disturbed in the microgyric and pachygyric areas (Fig. 2A). These abnormalities were characterized in terms of the relative positions of specific neuronal subsets and the patterns of neuronal arrangements into radial groups (Fig. 2A) [50]. In the polymicrogyric cortex, typically a fusion of the molecular layers is associated with a modified distribution of medium to large pyramidal cells originating from the deep cortex. This causes a decrease in the numbers of neurons in the outer layers (layer II and layer III) of the cortex; instead, these neurons are located in the deep cortex and within heterotopias of subcortical white matter (Fig. 2A) [6]. Less severe cerebral migratory abnormalities were reported in neonatal adrenoleukodystrophy [51]. To date, no migration defects have been described in infantile Refsum disease, the least severe form of the Zellweger spectrum. Another striking morphological aberration linked to neuronal migration defects is the heterotopic localization of Purkinje cells in the cerebellar white matter (Fig. 2B) [6,49,50].
In addition to these migration defects, within their first year of life, all patients with Zellweger syndrome display white matter abnormalities in the CNS, which have been observed by histological analyses and brain magnetic resonance tomography (MRT) studies [52–55]. Because myelination is still ongoing during this early period, it cannot be clearly established, whether the lack of peroxisomal functions causes abnormal myelination, early demyelination or both processes simultaneously [56]. Neuropathological examination of brains obtained from three cases of neonatal adrenoleukodystrophy revealed a severe degeneration of the white matter involving both hemispheres of cerebrum and cerebellum, while the axons were preserved [57]. In the cerebellum of one case, overabundance of reactive astrocytes in the white matter was associated with perivascular cuffs of mononuclear cells [57]. Heterotopic Purkinje cells were found to be aggregated in irregular clumps in the subcortical areas of the cerebellar cortex in two of the three cases [57]. In some cases, mild initial symptoms are later followed by severe CNS demyelination and death of the patient [53,55,56]. In the mildest forms of the Zellweger spectrum, patients can survive into adulthood [47,58,59]. In a study of 19 patients (16–35 years old) with such a mild Zellweger spectrum disorder, magnetic resonance imaging (MRI) revealed white matter abnormalities in nine of the patients. These abnormalities were restricted to the cerebellar hilus of the dentate nucleus and/or the peridentate region [47]. During infancy, four of these patients suffered from hypotonia, five from failure to thrive, 12 had a visual handicap due to retinal degeneration and eight presented with hearing impairment. During childhood, all 19 patients had a moderate to severe developmental delay as well as a reduction/loss of visual and hearing abilities and seven did not achieve structured speech. The predominant neurological symptom in the adult patients was a gait disorder, caused by different combinations of cerebellar syndrome, pyramidal tract dysfunction and peripheral neuropathy. Interestingly, at the time of diagnosis, 17 of these patients had a blood metabolite profile typical of a peroxisomal disorder; but at later time points the concentration of many originally accumulating metabolites had declined and, in some patients, even a complete normalization was observed. In particular, the levels of intermediates of bile acid biosynthesis (DHCA, THCA) and of pipecolic acid declined during the observed time period in many patients, whereas VLCFA and plasmalogen levels normalized only in some. This implies that, based on plasma metabolites linked to peroxisomal function, some of these patients would have escaped the diagnosis of a Zellweger spectrum disorder. Other studies reported normal VLCFA levels in plasma of late-onset patients with a PEX2 mutation [59] or normal plasmalogen levels in plasma of patients with PEX16 mutations [58]. Accordingly, for the Zellweger spectrum, separate MIM numbers have been assigned according to the severity of phenotypes (Table 1). These findings indicate that the level of peroxisome-related metabolites in plasma may not necessarily reflect the level of accumulation in tissues. This is of great relevance for the interpretation of alterations of plasmalogen levels in plasma of patients with more common neurological diseases (see Section 6.4.).
5.1.2. Brain pathology in mouse models of the Zellweger spectrum disorders
Currently, several mouse models of the Zellweger spectrum disorders are available, which are represented by mice with targeted deletions in the genes encoding the peroxins PEX2, PEX5 or PEX13 [60–62]. Recently also a knock-in mouse model carrying a missense mutation in the Pex1 gene (Pex1-G844D) was reported, which recapitulates the most frequent mutation in the human Zellweger spectrum disorders with a milder pathology [63]. As Pex7-deficient mice represent a model for RCDP type 1, but not for Zellweger spectrum disorders, we discuss this model in the context of ether phospholipid deficiency (Section 5.5.). Moreover, mouse models with Pex11α [64] and Pex11β [65] deficiencies have been generated.
The phenotype of mice with Pex2, Pex5 and Pex13 deficiency resembles the severe form of human Zellweger syndrome. These mice are born alive, but are growth-retarded and severely hypotonic. Moreover, they do not feed and die within 67#x2013;24 h after birth [60–62]. When the Pex2 mutation was maintained on a mixed genetic background (Swiss Webster × 129Svev), about 25% of the Pex2−/− pups survived for one to two weeks [66]. Furthermore, postnatal survival could be improved by oral bile acid application (9% alive after 30 days) [67]. In all mice with a global peroxisome deficiency (Pex2, Pex5 and Pex13 deficiency), a reduced thickness of the neocortical plate was observed, which reflects abnormal lamination that has been linked to impaired neuronal migration and increased cellular density in the underlying white matter [60–62]. Also cerebellar malformation was explored in all three models of PBD revealing abnormalities in cerebellar foliation. However, because the cerebellum develops largely postnatally in mice, a detailed characterization of cerebellar development was only possible in the longer surviving (Swiss Webster × 129Svev) Pex2−/− mice [68,69]. These studies revealed multiple anomalies affecting the interaction of climbing fibers, granule cells and Purkinje cells and, thus, the cerebellar circuitry. The number of granule cells was reduced due to defects in their migration from the external to the internal granule cell layer and increased apoptotic cell death. The Purkinje cells displayed stunted dendrite trees with abnormal branches and spine morphology. The disturbed dendritic spine compartmentalization reflected a delayed arborization and translocation of the climbing fibers from the inferior olivary nucleus (the major excitatory input to the Purkinje cells from the caudal medulla). In addition, progressive axonal swellings along Purkinje cell axons indicated ongoing dystrophic, neurodegenerative processes.
With regard to the Pex11-related mouse models, it should be noted that in contrast to the other peroxins, the PEX11 family members act as membrane elongation factors during peroxisome proliferation [70]. Whereas PEX11α appears not to be essential for the formation of functional peroxisomes, the absence of PEX11β leads to several pathological features shared by the mouse models of Zellweger syndrome, including neuronal migration defects, enhanced neuronal apoptosis, developmental delay, hypotonia and neonatal lethality [65]. As the import of peroxisomal proteins is not impaired in this mouse model, no accumulation of VLCFA and only a slight decrease in plasmalogen levels were detected in the brain [65]. The mechanism, by which Pex11β deficiency causes Zellweger-like symptoms, in spite of the mild metabolic defects, remains to be resolved.
5.1.3. The importance of peroxisomal Junctions for individual cell types of the brain
The power of mouse genetics provides an opportunity to discriminate the contribution of peroxisomal functions from different cell types to brain development and function. The conditional inactivation of selected genes in specific cell types or tissues has been used to generate mice with a deficiency in all peroxisomal functions restricted to subsets of brain cells. By crossing mice with a “floxed” Pex5 gene (Pex5 flanked by loxP recombination sites), which are susceptible to the removal of the DNA region between the loxP sites by the cyclization recombinase (Cre), and mice expressing Cre in a subset of cells, mouse lines have been generated, in which peroxisomes are selectively absent from different compartments of the CNS according to the specificity of the Cre-driving promoters. When using mice, which express Cre under the nestin promoter (Nestin-Cre driver mice), inactivation of Pex5 occurs in all neural precursor cells at embryonic stages, but not in the microglia lineage. This results in the ablation of peroxisomal functions in the vast majority of neurons, astrocytes and oligodendrocytes of mice already at prenatal stages [71]. These Nestin-Pex5−/− mice appear normal at birth, but develop substantial growth retardation after the first postnatal week. Progressive motor impairments ensue, resulting in lethargy and death before six months of age. In these mice, peroxisome-dependent metabolite levels were deranged (increased VLCFA, decreased plasmalogen levels) in the brain at late embryonic stages, but were normal in the liver. In the developing brain, a defect in neuronal layer formation in the cerebral cortex was observed indicative of neuronal migration defects and, postnatally, delayed cerebellar development including immature foliation and dendritic arborization of Purkinje cells [72]. Marked hypomyelination was detected already during the second to third postnatal week (probably due to insufficient formation of myelin) and was found in all brain regions (later probably also due to demyelination), together with axonal loss, reactive astrocytes as well as activated microglia and macrophages [73]. Also brain-specific (Nestin-Cre-dependent) inactivation of Pex13 in mice resulted in a similar phenotype with impaired cerebellar development, neuronal cell death, astrogliosis and microgliosis as well as signs of mitochondria-mediated oxidative stress [74]. Similarly, selective knockout (KO) of Pex5 in oligodendrocytes by using Cnp-Cre drivers had severe consequences for the adult brain [75]. Interestingly, no developmental defects were observed at birth or after two months although CNPase is expressed in progenitors before myelination as well as in adult oligodendrocytes. However, young adult mice gradually developed impaired motor function and premature death due to axonal degeneration, progressive subcortical demyelination and neuroinflammation, starting at two to six months of age. In contrast, peroxisome ablation in projection neurons of neocortex and hippocampus, obtained with Nex-Cre driver mice [76], or in astrocytes obtained with GFAP-Cre drivers [76], had no obvious deleterious effect on brain development or function. This was surprising, because Pex5 deletion in astrocytes resulted in accumulation of VLCFA as well as reduced plasmalogen levels in the brain. Taken together, these studies indicate that in the murine brain, peroxisomes are most crucial for oligodendrocytes and the myelin compartment.
However, also the selective loss of peroxisomal functions in hepatocytes of the liver, obtained by α-fetoprotein-Cre driver mice [72], results in brain abnormalities including defects in cerebral neuronal migration and cerebellar development (hypotrophy, increased apoptosis, immature foliation, delayed granule cell migration and stunted Purkinje cells). This finding is further corroborated by observations in Pex5-deficient mice (ubiquitous KO), in which liver-specific ectopic expression of Pex5 [77] resulted in partial rescue of the brain defects. These studies indicate a role of brain-extrinsic effects (effects originating from outside the brain) in CNS development in peroxisomal disorders. The mechanisms are not resolved but Faust and collaborators showed that bile acid treatment can partially restore the cerebellar anomalies in Pex2-deficient mice [69]. This treatment partially compensates for the lack of mature C24 bile acids in this mouse model and, thus, restores intestinal absorption of dietary lipids. However, it is unclear, whether the beneficial effect of this treatment on postnatal CNS/cerebellum development is due to an improved metabolic state of the pups because of increased lipid absorption, or prevention of steatorrhoea and cholestasis, or whether the addition of mature bile acids reduces the synthesis of bile acid precursors, which might impair CNS development. However, the absence of developmental problems in the CNS of racemase-deficient mice, in which bile acid precursors accumulate as well [78], renders an exclusive effect of bile acid precursors quite unlikely.
5.2. Brain pathology in peroxisomal β-oxidation disorders in humans and mice
As peroxisomes fulfill a variety of metabolic functions, which are concomitantly ablated upon inactivation of peroxisome biogenesis (in human patients suffering from Zellweger syndrome or in Pex-deficient mice), the attribution of particular aspects of brain pathology cannot be traced back to a single pathway such as β-oxidation. The investigation of single enzyme and transporter deficiencies and mouse models lacking individual peroxisomal enzymes or transporter proteins allows a comparison of the physiological consequences of a selective loss of individual metabolic pathways for brain function. However, even these conditions have limitations, because metabolic pathways such as the peroxisomal β-oxidation handle many different substrates, which renders a direct correlation between the loss of an enzymatic activity and a class of substrates impossible. Peroxisomal β-oxidation can degrade VLCFA, branched-chain fatty acids, bile acid intermediates, long-chain dicarboxylic acids and polyunsaturated fatty acids like tetracosahexaenoic acid (C24:6), which undergoes one cycle of β-oxidation in peroxisomes to produce DHA (C22:6). Moreover, fatty acid-like compounds with signaling activity such as prostaglandins and leukotrienes and some classes of xenobiotics are degraded in peroxisomes [3]. Notably, some activities in the β-oxidation pathway can be executed by more than one isoenzyme (Fig. 1). Human peroxisomes harbor two acyl-CoA oxidases, two bifunctional enzymes and two thiolases, whereas murine peroxisomes are equipped with three acyl-CoA oxidases, two bifunctional enzymes and three thiolases. As most of the human isoenzymes of the peroxisomal β-oxidation have different substrate specificities, some enzyme deficiencies lead to a rather selective accumulation of specific peroxisomal β-oxidation substrates, as will be discussed in the corresponding sections below.
5.2.1. Peroxisomal acyl-CoA oxidase deficiency
Patients with an inactivating mutation in ACOX1 lack peroxisomal acyl-CoA oxidase activity, which is responsible for the degradation of saturated VLCFA, polyunsaturated fatty acids and dicarboxylic acids, but not branched-chain fatty acids or bile acid intermediates (Fig. 1). Still, in many respects, the clinical presentation of acyl-CoA oxidase deficiency (formerly pseudoneonatal adrenoleukodystrophy) resembles Zellweger spectrum disorders, notably neonatal adrenoleukodystrophy [79]. Most patients show neonatal onset of hypotonia, seizures, failure to thrive, hepatomegaly, psychomotor retardation, sensory deafness, absent reflexes, and visual loss with retinopathy and extinguished electroretinograms [80]. Patients may show some early delay in motor development with a typical regression by 273x2013;3 years of age. Brain imaging (MRT and/or CT) revealed cerebral and/or cerebellar white matter abnormalities in all investigated patients in a study involving 12 subjects, of whom three showed neocortical dysplasia [79]. As for the Zellweger spectrum disorders, recently several cases of acyl-CoA oxidase deficiency with less severe clinical phenotypes were reported, which progressively developed neurological symptoms in later childhood [81].
To recapitulate the human disease, a mouse model with generalized Acox1 deficiency was generated [82], in which VLCFA accumulate. In Acox1−/− mice, marked peroxisome proliferation in the liver was observed and accompanied by an increase in H2O2 concentration, leading to the development of hepatic adenomas and carcinomas at 15 months of age [36]. However, no brain pathology has been described for these mice. To date, neither patients with mutations in ACOX2 nor Acox2 or Acox3-deficient mice have been described.
5.2.2. D-Bifunctional protein deficiency
In humans, two peroxisomal bifunctional proteins exist: D-bifunctional protein (DBP; alternatively termed multifunctional protein 2; encoded by HSD17B4) and L-bifunctional protein (LBP; alternatively termed multifunctional protein 1; encoded by EHHADH), both having a catalytic 2-enoyl-CoA hydratase activity and a (3R)-hydroxyacyl-CoA dehydrogenase activity (Fig. 1). All known human patients with bifunctional protein deficiency harbor mutations in the HSDI7B4 gene [83], whereas no patients with a mutation in the EHHADH gene, encoding LBP, have yet been identified. The existence of two enzymatic domains allows the classification of mutations based on the location and the nature of the mutation. Mutations affecting both domains or destabilizing the protein are classified as DBP deficiency type I, those affecting only the hydratase domain as DBP deficiency type II, and those solely affecting the dehydrogenase unit as DBP deficiency type III. However, as both enzymatic steps are essential for peroxisomal β-oxidation, the complete loss of both activities as well as of the individual enzymatic activities causes neurodevelopmental abnormalities and death within the first two years of life [83]. The severe form of DBP deficiency mimics Zellweger syndrome in all aspects including cranio-facial dysmorphism, neuronal migration defects (similar to that depicted in Fig. 2A) and premature death [84]. Also, demyelination of the central white matter is present [83].
Similar to the Zellweger spectrum disorders, recently also patients with unexpected phenotypes of DBP deficiency were identified using next generation sequencing [85]. These patients presented with ovarian dysgenesis, hearing loss, and ataxia comparable to Perrault Syndrome (MIM #233400) demonstrating clinical overlap of DBP deficiency and the genetically heterogeneous Perrault Syndrome [85]. One of the documented patients, who was 27 years old at the last examination [85], had normal levels of VLCFA and phytanic acid [86]. Normal serum VLCFA levels have been reported also in other patients with later clinical onset of DBP deficiency. The correct diagnosis in these cases was initiated by neuroimaging or whole exome sequencing [87,88]. This further demonstrates that peroxisome-related neurological deficits and the level of metabolites linked to peroxisomal functions do not necessarily correlate when measured in the blood of patients.
A mouse model of DBP deficiency (here termed Mfp2 deficiency) has been described, in which VLCFA accumulate specifically in brain, and the degradation of branched-chain fatty acids as well as the maturation of bile acid precursors were disturbed [89]. Mfp2-deficient mice appear quite normal at birth but are severely growth-retarded during the lactation period. Their life span is markedly reduced with a part of the population dying early (at around two weeks of age) [89] while the rest lives for up to six months [90]. Notably, this is much longer than the survival of Pex5-deficient mice (6–24 h) [60]. Moreover, Mfp2-deficient mice do not show signs of neurodevelopmental abnormalities such as migration defects at early time points [90] [91]; but later on, these mice develop cerebellar aberrations and axonal loss, which is reflected by motor impairment and lethargy [92]. Thus, in contrast to the human disorders, where Zellweger syndrome and DBP deficiency are clinically very similar, their respective mouse models are remarkably different. Interestingly, the brain pathology of Mfp2-deficient mice resembles the conditional Nestin-Pex5 mouse model (see 5.1.3), in which functional peroxisomes are absent from all neural cell types of the CNS [71].
5.2.3. 2-Methylacyl-CoA racemase deficiency
The enzyme 2-methylacyl-CoA racemase (AMACR) inverts the steric configuration at the position next to the thioester, resulting in the conversion of (2R)-methyl branched-chain fatty acids into (2S)-methyl branched-chain fatty acids. Only branched-chain acyl-CoAs such as pristanic acid or bile acid intermediates with the 2-methyl branch in the S configuration are substrates for peroxisomal β-oxidation. Accordingly, pristanic acid and the bile acid intermediates DHCA and THCA, but not VLCFA, accumulate in AMACR-deficient patients (Fig. 1) [93]. The phenotype of patients with AMACR deficiency (MIM #614307) often involves adult-onset sensory neuropathy [94] and late-onset cerebellar ataxia [95]. Occasionally, other symptoms and types of pathology have been described such as white matter anomalies [96], relapsing encephalopathy [97] and a more complex adult phenotype including peripheral neuropathy, epilepsy, bilateral thalamic lesions, cataract, pigmentary retinopathy and tremor [98]. Finally, some patients had cholestatic liver disease in the first neonatal weeks [99].
The generation of a racemase-deficient mouse model has been described [78], but so far only the pathological features of peripheral lipid metabolism have been investigated [100]. However, upon phytol supplementation of the diet, the mice developed severe pathology in the brain after 40 days, including demyelination and activation of astroglial cells [101].
5.2.4. SCPx deficiency and gene redundancy of peroxisomal thiolase activity and the consequences for brain function
In man, two enzymes with thiolase activity, acetyl-CoA acyltransferase 1 (ACAA1) and sterol carrier protein X (SCPx), are present in peroxisomes (Fig. 1). However, only for SCPx, the thiolase required for the breakdown of branched-chain fatty acids, a single patient with a deficiency of the enzyme (MIM #613724) has been described so far [102]. Among other clinical features, this adult patient presented with dystonic head tremor and spasmodic torticollis; and cranial MRI showed bilateral hyperintense signals in the thalamus, butterfly-like lesions in the pons and lesions in the occipital region [102].
In the mouse, three enzymes with thiolase activity exist: two closely related proteins (96% amino acid sequence identity) encoded by the differentially regulated genes Acaa1a and Acaa1b, and SCPx. In a classical gene KO model, Acaa1b deficiency showed a very mild phenotype and hardly any accumulation of VLCFA, indicating a compensatory effect from Acaa1a and/or Scpx in mice [103]. In Scpx-deficient mice, methyl-branched-chain fatty acid catabolism is impaired resulting in a mild phenotype under standard conditions [104]. However, high phytol diet treatment led to a much more severe phenotype, in which the mice rapidly lost body weight and acquired an unhealthy appearance and inactivity, reduced muscle tone, ataxia and trembling [104].
5.3. Brain pathology in X-linked adrenoleukodystrophy
Three peroxisomal ATP-binding cassette (ABC) transporters, ABCD1, ABCD2 and ABCD3, mediate the translocation of activated fatty acids and probably other compounds across the peroxisomal membrane, in order to get metabolized within the peroxisomes (Fig. 1). The abundance of these transporter proteins varies between cell types and tissues [28,105–107]. Inherited defects in the ABCD1 (formerly ALD) gene are the genetic basis for X-linked adrenoleukodystrophy (X-ALD; MIM #300100) [108]. X-ALD is the most common peroxisomal disorder with an estimated combined male and female incidence between 1:16,800 [109] and 1:30,000, with similar incidence rates across the world [110]. Human ABCD1 transports CoA-activated saturated straight-chain VLCFA across the peroxisomal membrane for further degradation by the peroxisomal β-oxidation machinery (Fig. 1) [111]. Upon ectopic overexpression in yeast, ABCD1 can mediate the transport of a broader spectrum of substrates [112], and overlapping substrate specificities have been demonstrated for the three peroxisomal ABC transporters [113–115]. Because ABCD2 and ABCD3 as well as the peroxisomal β-oxidation enzymes are intact in X-ALD, only saturated straight-chain VLCFA accumulate, but to a variable extent in different cell types and tissues. This selective substrate transport deficiency as well as the overlapping functions of the peroxisomal ABCD transporters explains why, in contrast to DBP deficiency and acyl-CoA oxidase deficiency, some X-ALD patients can remain pre-symptomatic through more than five decades, even in the complete absence of ABCD1 transporter activity. In addition to the impaired degradation of VLCFA, probably also increased fatty acyl chain elongation of long- to very long-chain acyl-CoA esters contributes to the accumulation of VLCFA (in particular C26:0) in X-ALD. Leading studies of Stephan Kemp's group suggest an important role of the rate-limiting enzyme in this process, elongation of very long-chain fatty acids 1 (ELOVL1), in the homeostasis of VLCFA in X-ALD [116,117]. Among the seven known ELOVL family members, which have different chain length selectivity, ELOVL1 favors saturated and monounsaturated CoA-activated fatty acids with a chain length of 20 to 24 carbons [118,119]. Indeed, upon knockdown of ELOVL1 mRNA in X-ALD fibroblasts, the storage of C26:0 decreased significantly [116].
Although X-ALD does not involve any developmental defect or delay, it is characterized by remarkable clinical heterogeneity. The main phenotypes are adrenomyeloneuropathy (AMN) and cerebral ALD (CALD), the devastating inflammatory and demyelinating form of X-ALD [120]. Both phenotypes can occur within the same kindred [121] and no general genotype–phenotype correlation exists for the severity in X-ALD [122–125]. Adrenal insufficiency represents another major pathological aspect in X-ALD, which often represents the initial symptom and affects 80% of male patients before adulthood but is rare in heterozygous female patients [126]. Virtually all male patients with mutations in the ABCD1 gene eventually develop AMN, a slowly progressive myelopathy with typical onset in the third or fourth decade of life. The earliest symptoms are usually urge incontinence and sensory disturbances in the legs followed by spastic gait. The major neuropathological feature in AMN is a distal dying-back axonopathy, which involves the dorsal columns and corticospinal tracts in the lower thoracic and lumbar regions [127], as well as the more proximal segments of the corticospinal tracts in the internal capsule [128]. The peripheral nerves are also involved, with primary axonal degeneration in most AMN patients [129]. Evidence of myelopathy or peripheral neuropathy was recently observed in more than 80% of women carrying heterozygous ABCD1 mutations and older than 60 years. Thus, female patients develop symptoms similar to those in male AMN patients but at later age [130,131].
In the human brain, based on immunohistochemical detection, ABCD1 is predominantly expressed in oligodendrocytes, microglia, astrocytes and endothelial cells but not in most neurons, with the exception of a few regions: hypothalamus, basal nucleus of Meynert, periaqueductal gray matter and the locus coeruleus [16,30]. Furthermore, ABCD1 is also highly expressed in dorsal root ganglia, where the neuronal cell bodies of the afferent sensory axons are located, which degenerate in AMN [30]. Thus, pathophysiological involvement is suggested for neurons as well as for oligodendrocytes in the case of axonopathy. By electron microscopy, mitochondrial abnormalities have been observed in neurons of AMN patients [132]. The mitochondrial abnormalities have been confirmed and are believed to be a major pathogenic factor contributing to neurodegeneration in AMN (Fig 3A). Cytosolic deposits of crystalline lamellar lipids were observed in brain macrophages, Schwann cells of peripheral nerves, adrenocortical cells, and Leydig cells of the testes. Cholesterol esters of VLCFA constitute a major component of these crystalline structures. Furthermore, it has been reported that VLCFA can disturb calcium homeostasis and cause mitochondrial dysfunction in neuronal cell cultures as well as toxicity to oligodendrocytes [133].
About 60% of male X-ALD patients develop CALD, the fatal cerebral demyelinating form of the disease. This can occur either in childhood, most commonly between 5 and 10 years of age, before onset of AMN (about 35%) or later in adolescence or adulthood, often on the background of AMN (35%). In children, the first symptoms are emotional lability, hyperactive behavior, school difficulties, impaired auditory discrimination and difficulties in vision [134]. These early clinical symptoms are not specific and often the correct diagnosis of X-ALD is delayed. This phase is followed by a rapidly progressing neurological decline, typically leading to a vegetative state or death within two to five years. For a male patient born with an ABCD1 mutation, it cannot be predicted whether or when the cerebral form will develop. It is currently hypothesized that the cerebral inflammatory phenotype results from a “second hit”, superimposed on the axonal pathology [120]. Based on the lack of a genotype–phenotype correlation in X-ALD, it is likely that a combination of genetic, epigenetic and environmental factors plays an essential role as trigger for the development of CALD. This is also supported by the development of different clinical phenotypes in monozygotic twins [135,136] and the observation that moderate head trauma can initiate cerebral demyelination in AMN patients [137,138]. In magnetic resonance images of the brain of CALD patients, a typical enhancement of the border of the demyelinating lesion is visible after gadolinium administration reflecting an increased permeability of the blood brain barrier due to a marked inflammatory reaction [139]. In this active region, infiltration of macrophages, CD4+ and CD8 + cytotoxic T cells, as well as activated microglia and astrocytes can be observed [140]. This severe neuroinflammation probably causes the loss of oligodendrocytes, which die by cytolysis rather than by apoptosis [140]. Expression of proinflammatory cytokines such as tumor necrosis factor α, interleukin (IL)-1, IL-2, IL-6, IL-12 and interferon-γ and chemokines is increased [141–143]. The importance of microglia in the disease mechanism is supported by the observation of a zone within the perilesional white matter, immediately beyond the actively demyelinating lesion edge, lacking microglia [144]. This might be due to the migration toward the active age of the lesion. In the same study, clusters of activated and apoptotic microglia were detected within the subcortical white matter [144]. Another characteristic of the inflammation in CALD is the resistance to anti-inflammatory therapy. Based on our recent observations, we have suggested that this is due to the intrinsic metabolic defect of macrophages and microglia in X-ALD; these cells cannot degrade VLCFA, which they have taken up by phagocytosing of myelin debris (particular rich in VLCFA in X-ALD), and then fail to support normal immunological brain function [107, 123]. Based on this intrinsic defect, the continuous metabolic stress in the macrophage/microglia populations could also be the reason why only in rare cases a spontaneous arrest of brain inflammation occurs. Allogenic hematopoietic stem cell transplantation [145,146] and autologous stem cell-based gene therapy [147] can arrest the inflammatory demyelinating process with a typical delay of 12–18 months, which has been attributed to the slow replacement of microglia with bone marrow-derived phagocytes [147,148]. It must be noted that, due to the rapid disease progression, hematopoietic stem cell transplantation is only beneficial when performed at an early stage of disease.
Transcriptomic analyses of X-ALD brain tissue have indicated that already in AMN patients, a proinflammatory status prevails [149]. Musolino and coworkers recently demonstrated that inactivation of ABCD1 induces significant alterations in the brain endothelium via c-MYC and may thereby contribute to the increased trafficking of leukocytes across the blood–brain barrier [150]. As the cell-autonomous (intrinsic) metabolic defect in the monocyte–macrophage lineages is also present in AMN patients, together with blood–brain barrier abnormalities, it appears reasonable that this fragile system is predisposed for converting to the inflammatory form of X-ALD, triggered by a broad spectrum of genetic and environmental factors.
In 1997, three independent groups had generated mouse models for X-ALD by targeted inactivation of the Abcd1 gene [151–153]. Although the biochemical phenotype of X-ALD (i.e., accumulation of saturated VLCFA) was well replicated in all three models, Abcd1-deficient mice did not experience brain inflammation and demyelination as seen in humans with CALD. However, after 18 months of age, these mice start to develop a late-onset, mild motor behavior phenotype with resemblance to AMN including sciatic nerve conduction abnormalities and mild signs of axonopathy and myelin instability in the spinal cord [154]. Interestingly, Abcd1 deficiency could further enhance microglia activation and axonal degeneration in mice with mild myelin abnormalities caused by the loss of the myelin-associated glycoprotein [155]. It is intriguing that also Abcd1/Abcd2 double-deficient mice do not develop brain inflammation or demyelination [156], in spite of the finding that Abcd1/Abcd2 double-deficient peritoneal macrophages are metabolically much more severely affected than those from single transporter-deficient mice [157]. Also in these mice, the neuropathology is restricted mainly to axonopathy in the spinal cord and, with the major contribution from Abcd2 deficiency, the dorsal root ganglia resulting in a sensory neuropathy [156]. However, in the double mutant mice these abnormalities develop about six months earlier than upon sole Abcd1 deficiency.
In addition to exploring the effects of therapeutics aimed at normalizing VLCFA levels in vivo, these mouse models have been applied to further characterize the mitochondrial damage noticed in X-ALD. The mitochondrial disturbances are probably not simply secondary effects due to VLCFA accumulation itself [158] but more complex, involving oxidative stress and cell type- and tissue-specific mechanisms that are of particular importance for axonal degeneration in the spinal cord [43,159]. Interestingly, lipoxidative damage was observed early (at three months of age) in the spinal cord of Abcd1-deficient mice, long before the onset of any neuropathological or motoric abnormalities were detected [160].
Evidence for the role of oxidative stress in plasma of X-ALD patients comes from an increased level of thiobarbituric acid reactive species (TBA-RS) reflecting induction of lipid peroxidation, as well as a decrease of plasma total antioxidant reactivity, indicating a deficient capacity to rapidly handle an increase of ROS [161]. Additional evidence comes from the finding of decreased levels of total and reduced glutathione, which were associated with high levels of oxidized glutathione, in lymphocytes of X-ALD (predominantly AMN) patients [162]. Also, decreased plasma thiols and a high level of carbonyls were found, additionally supporting the idea of oxidative stress – at least in blood cells – in X-ALD patients [162]. Encouraging results were obtained from a study applying an antioxidant cocktail consisting of vitamin E, N-acetylcystein and lipoic acid to aging Abcd1-deficient mice; this dietary treatment was sufficient to prevent the onset of locomotor disability and axonal damage [163]. In line with these findings, also oral administration of pioglitazone, an agonist of peroxisome proliferator-activated receptor γ (PPARγ) and inducer of mitochondrial biogenesis and respiration, was able to prevent mitochondrial damage and oxidative stress in Abcd1-deficient mice and could rescue the locomotor disability and axonal damage in the Abcd1/Abcd2 double-deficient mouse model [164].
It has previously been suggested that mitochondrial dysfunction and oxidative stress within the axons are, at least partially, secondary to dysfunctions in the oligodendroglia/myelin compartment resulting in compromised support of axonal integrity [14,165,166]. In this context, it is noteworthy that mice with a sole defect in a myelin protein, such as proteolipid protein or 2′,3′-cyclic nucleotide phosphodiesterase, display axonal dysfunction without demyelination in the spinal cord and brain [167,168]. Most interestingly, mice with oligodendroglia-selective peroxisome deficiency (see also Section 5.1.3.) can also be considered as a phenocopy model for the inflammatory form of X-ALD, recapitulating widespread axonal degeneration, progressive subcortical demyelination and a proinflammatory milieu with B and T cell infiltration of brain lesions [75].
Our current hypothesis envisions the inability to degrade VLCFA combined with the increased elongation of VLCFA, in particular in oligodendrocytes and neurons, as the primary cause of the late-onset, slowly progressing, chronic myeloneuropathy in AMN (Fig. 3A). In heterozygous X-ALD females, a similar disorder develops, but with a later onset and slower progression; most likely random X-inactivation of the intact ABCD1 copy leads to chimerism with a variable extent of ABCD1-deficient cells.
5.4. Brain pathology in α-oxidation deficiency in man and mice
With respect to the peroxisomal fatty acid catabolism, 2-methyl branched-chain fatty acids can directly enter the peroxisomal β-oxidation pathway, whereas 3-methyl branched-chain fatty acids cannot. Instead, 3-methyl branched-chain fatty acids can either be degraded by ω-oxidation (for review see [169]) or by peroxisomal α-oxidation (see Fig. 1). Among the enzymes involved in the α-oxidation pathway, only phytanoyl-CoA hydroxylase (PHYH) has been associated with a human disorder. Mutations in the PHYH gene have been established as the genetic cause for classical Refsum disease (MIM #266500) [170, 171]. The 3-methyl branched-chain fatty acid phytanic acid, solely taken up from dietary sources, accumulates in patients with Refsum disease. Because the disease is caused by the cumulative load of phytanic acid in tissues, the age of onset varies from early childhood to the fourth decade of life [56]. Refsum disease is characterized by progressive retinitis pigmentosa culminating in blindness, peripheral neuropathy and cerebellar ataxia [172]. When phytanic acid levels in the plasma remain low due to dietary restriction or repeated plasmapheresis the progression of the symptoms can be arrested [173,174]. Because phytanoyl-CoA hydroxylase is imported into peroxisomes via its PTS2 motif in a PEX7/PEX5L dependent manner, the α-oxidation pathway is also impaired in RCDP type 1 (PEX7 deficiency, see Section 5.5) and in RCDP type 5 (deficiency in PEX5L).
A mouse model for Refsum disease has been generated by targeted disruption of the Phyh gene [175]. Because standard mouse chow is very low in branched-chain fatty acids, Phyh-deficient mice have an unremarkable phenotype. However, dietary supplementation with 0.25% phytol (the precursor of phytanic acid) for three weeks or 0.1% phytol for six weeks caused ataxia, reflecting Purkinje cell loss and astrogliosis in the cerebellum, and peripheral neuropathy, as revealed by nerve conduction velocity measurements [175].
5.5. Nervous system pathology in ether phospholipid deficiency in man and mice
So far, the biological functions of ether phospholipids (also simply termed ether lipids), especially in the CNS, have not been fully unraveled. However, many clues have been derived from the pathology of ether phospholipid-deficient mice and men. In humans, the lack of these lipids causes the lethal disease RCDP, an autosomal recessively inherited disorder with an estimated incidence of about 1:100,000. On a genetic basis, several different types are distinguished; RCDP type 1 (MIM #215100) is evoked by mutations in the gene encoding PEX7 [176–178], the cytosolic receptor for peroxisomal import of PTS2-containing proteins, whereas RCDP type 2 (MIM #222765) and type 3 (MIM #600121) are caused by mutations in the genes of the first two enzymes for biosynthesis of ether phospholipids, dihydroxyacetone phosphate acyltransferase (DHAPAT, DAPAT; encoded by the GNPAT gene) and alkyl-dihydroxyacetone phosphate synthase (ADHAPS; encoded by the AGPS gene), respectively [179,180]. Due to the fact that not only ether lipid biosynthesis but also peroxisomal α-oxidation is impaired in RCDP type 1, it is classified as a PBD rather than a pure ether lipid biosynthesis defect (see also Section 5.1). However, as the different RCDP types are clinically indistinguishable and the clinical manifestations of Refsum disease (see Section 5.4) are considerably less severe than that of RCDP type 1, we will cover RCDP type 1 in the present section, which focuses exclusively on ether phospholipids. The contribution of α-oxidation deficiency to the clinical phenotype may, however, be more prominent in RCDP type 1 patients with a milder disease course (see below) [181,182]. In addition, it can be speculated that some PEX7 mutations affect proteins with certain PTS2 variants more strongly than others, thereby shifting the impact of the affected pathways on pathology. Recently, two additional subtypes of RCDP were identified based on the strong reduction of plasmalogen levels in the patients and the similarity of their symptoms with “classical” RCDP. First, the disorder of three patients with a deficiency in FAR1, the gene coding for fatty acyl-CoA reductase 1, which generates the fatty alcohols necessary to form the ether bond of the 1-alkyl chain in ether phospholipid biosynthesis [183], was categorized as RCDP type 4 [80]. Second, the disease in patients with mutations specifically affecting the long isoform of PEX5, PEX5L, which is required for efficient transport of cargo-loaded PEX7 to peroxisomes [184], was designated RCDP type 5 [185].
The pathology in all subtypes of RCDP has been more or less exclusively assigned to the lack of plasmalogens, although also other ether phospholipids, like alkylphospholipids (lacking the vinyl ether bond characteristic for plasmalogens) or platelet-activating factor, are depleted in all types of RCDP and, in case of RCDP type 1, elevated plasma levels of phytanic acid have been detected [186]. Irrespective of the affected gene, all RCDP patients share common symptoms. The most typical are the eponymous shortening of the proximal long bones (rhizomelia) and epiphyseal stippling (chondrodysplasia punctata) as well as congenital cataracts, joint contractures and growth and developmental retardation [187]. The severity of the disease varies remarkably, depending strongly on the residual activity of the affected enzyme and, thus, the level of plasmalogens [188–191]. The most severe form of the disease leads to lethality within the first years of life, often due to respiratory failure. In contrast, patients with a less severe disease course present with only some of the characteristic symptoms and can survive into young adulthood [192–194]. In human ether phospholipid deficiency, multiple pathological features affect brain development and function. Mental disability and delayed motor development are hallmarks of RCDP. However, the extent to which the brain is affected varies remarkably between patients and many of the symptoms are restricted to the severer forms of the disease. Frequent delay in brain development is further reflected by the finding of microcephaly in many RCDP cases. Also epileptic seizures are very common but non-specific; seizure type and frequency vary considerably and even affected children with multiple seizure types have been reported [191,192]. The age of onset of seizure activity is reportedly higher in milder cases of RCDP [191].
MRI examination in children with RCDP has revealed varying pathologic features, although some cases, usually with a milder phenotype, with unremarkable MRI results are also found [195]. Consistently, most reports describe enlargement of ventricles and the subarachnoid space, abnormalities in white matter signal intensity and delayed supratentorial white matter myelination with frequent involvement of the parieto-occipital area [195–200]. In line with myelination defects also in the PNS, peripheral neuropathy has been reported in a clinical subset of RCDP patients [201]. The severe form of the disease is usually accompanied by progressive cerebellar atrophy [195], which is caused by a pronounced loss of Purkinje cells and, to a lesser extent, other cell types in the cerebellum [202]. Thus, in addition to peroxisomal β-oxidation, also ether lipid biosynthesis appears to be crucial for cerebellar development and function. Originally, it was assumed that increased levels of phytanic acid contribute to the pathogenesis in the cerebellum [52,202]. However, this hypothesis is strongly weakened by the presence of cerebellar atrophy in a case of RCDP type 3, in which ether lipid deficiency is the only metabolic defect, and, conversely, by the absence of cerebellar atrophy in a case with particularly high phytanic acid levels [191]. Sporadically, also other brain malformations have been observed, like temporal atrophy [197], agenesis of the corpus callosum [203], polymicrogyria [200], and pachygyria [204,205]. Neuronal migration defects never reach the extent of those observed in Zellweger syndrome (see Fig. 2A), but several cases with dysplastic olivary bodies have been reported [202,206]. These findings emphasize the fact that multiple peroxisomal functions are required for proper development of the brain. In addition to the brain pathology, patients suffering from the severe form of RCDP often develop stenosis of the spinal canal (cervical stenosis) [195,207]. From a metabolic point of view, MR spectroscopy of the brain has shown increased levels of myo-inositol, a marker for gliosis, in line with previous reports of gliosis in autopsy cases of RCDP [52,208]. MR spectroscopy has also revealed elevated levels of mobile lipids, most likely caused by accumulation of long-chain acyl-CoAs, as well as a reduction of choline and the presence of acetate [199,209].
More insight into the pathomechanisms of ether lipid deficiency has been gained from the generation of ether lipid-deficient mouse models. Currently, gene KO mouse models exist for RCDP types 1–3 (Pex 7, Gnpat and Agps KO mice, respectively) [210–212], of which the first two models have been extensively characterized. Recently, also a mouse model with inducible inactivation of alkyl/acyl-dihydroxyacetone phosphate reductase (AADHAPR; also named peroxisomal reductase activating PPARγ, PexRAP), the enzyme catalyzing the third step in ether lipid biosynthesis (following the DHAPAT and ADHAPS reactions) and shown to be located at the outer face of the peroxisomal membrane [213], was generated [214]. Furthermore, hypomorphic mouse models with residual transcript levels of the Pex7 [215] or the Agps [216] gene and, consequently, residual levels of ether lipids have been described, which mimic the milder form of the human disease. Many of the phenotypic brain abnormalities described in these ether lipid-deficient mice resemble the observations in humans, with the advantage of animal models being the opportunity to elucidate the underlying molecular processes in more detail. Several studies have reported hypomyelination in different brain areas (neocortex, corpus callosum, cerebellum) of ether lipid-deficient mice [217,218], but no progressive demyelination as seen in several mouse models of Zellweger syndrome [73]. Also myelin of the PNS is affected in ether lipid-deficient mice. Deficiencies in myelination as well as Schwann cell development and differentiation are found [219] resulting in peripheral neuropathy, as judged by reduced motor neuron conduction velocity [220]. Remarkably, hypo- and dysmyelination in the CNS of ether lipid-deficient animals is accompanied by slight loss of axons and mild astrogliosis in some brain areas, whereas microgliosis, which has been reported in several RCDP cases, and the induction of inflammatory cytokines appear to be less pronounced [73]. However, neuroinflammation with marked microglia activation was observed upon combined deficiency of Pex7 and Abcd1 in 11-months-old mice of mixed background (Swiss Webster and C57BL/6J:129S1) [220] suggesting that ether lipid deficiency has little effect on immune cell activation under basal conditions but more drastic consequences in the presence of an additional immunostimulatory factor. This concept is also supported by the finding that plasmalogens suppress microglia activation after systemic injection of lipopolysaccharides (LPS) in mice [221,222]. Furthermore, in murine cell lines, plasmalogens seem to counteract neuronal death elicited by serum starvation [223,224] by utilizing a process, which has been reported to involve protein kinase B (AKT) signaling [224].
Cerebellar pathology is a striking feature in human cases of ether lipid deficiency and has received special attention in the study of the corresponding mouse models. Concordantly, foliation defects with underdevelopment of fissures, particularly affecting foliae VI and VII, have been reported in Gnpat KO mice at different postnatal stages [218,225]. Also, a migration defect of granule cell precursors [218] and increased numbers of apoptotic cells in the external granule layer [225] were found. Furthermore, hypomyelination in cerebellum (and also in cortical areas) of Gnpat KO mice was accompanied by changes in the architecture of the nodes of Ranvier resulting in a delay in the propagation of action potentials [218]. Microscopy studies revealed structural abnormalities in the innervation of Purkinje cells by parallel fibers and climbing fibers as well as axonal swellings with accumulation of smooth ER-like structures in Purkinje cells [218].A detailed review of cerebellar pathology in the context of ether lipid deficiency and other peroxisomal disorders has been published recently [226].
In the first description of the Pex7 KO mouse, neuronal migration defects in the neocortex of mutant embryos were reported; however, these were much less pronounced than the migration deficits in mice completely lacking peroxisomes (e.g. Pex5 KO mice) [210]. Remarkably, although similar techniques were used, no such alterations could be detected in the brains of Gnpat KO mice [225], pointing towards a role of phytanic acid in the development of these migration defects or potential differences in the background strains of the mice used in the different studies. Da Silva and coworkers also speculated that a not yet identified peroxisomal protein harboring a PTS2 (and therefore being dependent on PEX7) may be responsible for these apparently conflicting results [227]. By making use of the progress in the elucidation of PTS2 structure requirements [228], future studies may substantiate this idea.
Involvement of the visual system is another typical feature of RCDP that has been extensively studied in mouse models. Lens anomalies, particularly bilateral cataracts, have been reported in all ether lipid-deficient mouse models, including the hypomorphic mice. Furthermore, hypoplasia of the optic nerve, microphtalmia, a persistent hyaloid artery [211] and abnormal vascularization [229] were detected in Gnpat KO mice. These ocular abnomalities have been covered in detail in previous reviews [217,230].
So far, the molecular bases of mental disability in RCDP and the nervous system pathology upon ether lipid deficiency in mice and men have only been partially unraveled. Recently, a defect in the activation and downstream signaling of AKT was proposed to be responsible for the myelination deficit in the PNS of ether lipid-deficient animals [219]. It remains to be determined, whether a similar disturbance affects the CNS as well. Plasmalogen-deficient myelin might be more prone to oxidative damage [231]; however, this idea is weakened by the observations of reduced rather than elevated levels of malondialdehyde and no substantial change in other oxidative stress markers in the brains of ether lipid-deficient mice [40,73]. Studies in synaptosomes isolated from cortex, mimicking the process of synaptic transmission, have revealed a decrease in calcium-dependent release of the neurotransmitters glutamate and acetylcholine in Gnpat KO mice [40]. This goes along with decreased ATP content and an inability of synaptosomal respiration to adapt to the higher energy requirements of depolarization, thereby offering a potential explanation for the defects in synaptosomal neurotransmitter release [40] (Fig. 3B). Alternatively, changes in membrane properties caused by plasmalogen deficiency might play a role (Fig. 3B). The lack of ethanolamine plasmalogens (by far the most abundant plasmalogen in the brain) is strictly compensated by the structurally similar phospholipid phosphatidylethanolamine [40,232]. However, the characteristic biophysical properties conferred by plasmalogens [233–235] could be essential for neurotransmission, which involves repeated membrane fusion and constriction processes (Fig. 3B). Furthermore, it has been suggested that deficiency in plasmalogens impairs membrane rafts (formerly termed lipid rafts) [211], small membrane domains that organize various cellular processes [236,237] and are enriched in plasmalogens [238]. However, the impact of ether lipid deficiency on neurotransmission in vivo and the underlying molecular mechanism still have to be elucidated in greater detail.
No characterization of the nervous system in hypomorphic mouse models of RCDP has been published so far, which could be due to the milder phenotype of these mice, leaving the nervous system largely unaffected.
6. The contribution of peroxisomes to more common neurological disorders
Besides the fact that inherited peroxisomal disorders have drastic consequences for the nervous system, a dysfunction of peroxisomes or a dysregulation of peroxisomal metabolites has also been described in a variety of other, more common, neurological diseases.
6.1. Involvement of D-amino acid oxidase in amyotrophic lateral sclerosis and schizophrenia
Across evolution, D-amino acid oxidase (D-AAO, DAO) serves as a tool to access the nutritional supply; however, in the brain, this enzyme and its more specific counterpart D-aspartate oxidase (DDO) exert a regulatory function in modulating the amount of the neuroactive D-amino acids D-serine and D-aspartate, respectively [239]. DAO is peroxisomal [13,240], contains a functional PTS1 motif [241] and interacts with PEX5 [242] and also human DDO was shown to be located in peroxisomes [243,244]. These enzymes are flavin adenine dinucleotide (FAD)-containing flavoenzymes that oxidize certain amino acids, thereby generating H2O2 as side product. DAO activity is abundant in various human brain areas, but in the murine brain, some of the corresponding regions contain markedly lower activity [245].
D-Serine binds to a specific extracellular site on the N-methyl-D-aspartate (NMDA) receptor, which responds to glutamate as neurotransmitter, and further increases the signaling strength [246]. In the brain, D-serine is generated in astrocytes and released into the synaptic cleft [247], but also taken up from the synaptic cleft and degraded in astrocytic peroxisomes (see Fig. 3B).
Genetic linkage between the DAO locus and schizophrenia was first described by Chumakov and collaborators [248] and has since been observed by several groups. In a later study, also elevated activity of DAO was linked to schizophrenia [249], corresponding to reduced D-serine levels and NMDA receptor hypoactivity. An increased activity of DAO has been found in the cortex of patients [250] and the protein level was increased in cerebellum and cortex [251], while reduced levels of D-serine were observed in the cerebrospinal fluid of patients [252]. Thus, inhibitors of DAO have been suggested as a therapeutic option for schizophrenia [253]. However, it is unclear, whether the relevant DAO activity is entirely peroxisomal in the astrocytes of patients.
Furthermore, a mutation in DAO was recently linked to a familiar form of amyotrophic lateral sclerosis (ALS) [254], which is a fatal human disease with neurodegenerative aspects affecting predominantly motor neurons. The link to D-amino acid metabolism was supported by the finding that in a mouse model for the familial form of ALS (SOD1G93A), DAO activity in the spinal cord was reduced and, consequently, D-serine levels were increased [255].
6.2. The link between ether lipid biosynthesis and autism
In 2013, a study applying whole exome sequencing identified a link between mutations in PEX7 (RCDP type 1) and autism spectrum disorder, a range of neurodevelopmental conditions characterized by deficits in communication and social interaction and repetitive behavior, in a family with three affected children [256]. This observation supports previous work showing an association between single nucleotide polymorphisms in the PEX7 gene and autism [257]. Prompted by their findings, the authors reviewed previously reported RCDP cases for potential signs of autism and found two further patients, which had later been diagnosed with neurodevelopmental conditions (one with autism, the other with attention deficit hyperactivity disorder) [256]. Additional consolidation of the proposed link between plasmalogen deficiency and autism comes from the finding of reduced levels of plasmalogens in plasma and red blood cells of autistic patients [258,259]. The mechanism, by which these two phenomena are connected, is still fully unexplored. Autistic features have, so far, not been regarded as a typical symptom of RCDP (or other peroxisomal disorders), although one similar case was already mentioned in 1999 [260]. The reasons for this might be that many children affected by the disease do not reach a stage, in which symptoms of autism manifest, and that treatment of patients with RCDP focuses on other, more vital aspects. However, in the future, clinicians may pay more attention to signs of autism in RCDP patients, especially those suffering from the milder form of the disease, which should help to substantiate a relationship between deficiency in plasmalogens and autism.
6.3. Peroxisomes and Alzheimer’s disease
Several links have been found between peroxisomes and Alzheimer’s disease (AD), the most common form of dementia affecting several millions of people worldwide. Most prominently, a role of plasmalogen deficiency in the etiology of AD is considered. Many studies have confirmed a severe depletion of ethanolamine plasmalogens (ethanolamine is by far the most abundant plasmalogen head group in the CNS), in post mortem brain tissue of AD patients [261–266]; but also contradictory results exist [267]. The decrease in brain plasmalogens emerges early in the disease course [262,268] and, at least in gray matter, corresponds well with the deterioration of cognitive function [262]. Although, to a lesser extent, also associated with normal aging [269], depletion of brain plasmalogens appears to be rather specific for AD and not a general feature of neurodegeneration. Similar abnormalities could not be found in several other neurodegenerative diseases like Parkinson’s disease or Huntington’s disease [270]. Remarkably, deficiency of ethanolamine plasmalogens was also repeatedly detected in peripheral blood of AD patients [271] rendering these lipids potential as biomarkers for early and easy detection of the disease [272].
The origin of plasmalogen deficiency in AD is currently still unknown. Grimm and coworkers suggested that a dysregulation of AGPS expression by the amyloid precursor protein intracellular domain and oxidative damage by amyloid-beta (Aβ) peptides lead to instability and loss of activity at the protein (ADHAPS) level causing decreased plasmalogen biosynthesis in AD [266]. Others speculate that an Aβ-mediated increase in the activity of plasmalogen-selective phospholipase A2 (PLA2), as detected in certain brain regions of AD patients [273], depletes plasmalogens, thereby leading to excessive vesicular fusion and, finally, synaptic failure [274]. Other alternative explanations include increased oxidation of plasmalogens, in line with their proposed role as radical scavengers or excessive membrane degradation. However, also impaired generalized function of peroxisomes could contribute to the disturbance of plasmalogen homeostasis. This hypothesis is supported by findings of altered levels also of other peroxisomal metabolites in the context of AD. For example, increased VLCFA levels were detected in cortical tissues [263,264] and in peripheral blood [275] of AD patients (although the latter results still require confirmation). Also, a decrease in DHA, whose endogenous de novo production requires peroxisomes, in brain and liver [276] and a regional decrease in catalase activity in the temporal lobe [277] were reported. Furthermore, our group has previously identified an accumulation of peroxisomes in the somata of neurons in the gyrus frontalis of AD patients accompanied by a lack of peroxisomes in dendrites with abnormally phosphorylated Tau protein, which might prevent the transport of peroxisomes into these processes [263]. These results are complemented by studies in mouse models of AD, which imply that the number and protein content of peroxisomes are strongly modulated by the disease course [278,279], and that these alterations are possibly triggered by excessive oxidative stress and/or mitochondrial dysfunction.
As the etiology of AD is still unresolved, also the role of peroxisomal dysfunction in the disease process is debated. It might be speculated, though, that a decrease in peroxisomal activity, even if not the primary cause of the observed pathology, aggravates oxidative stress and neurodegeneration in the AD brain. This fits the observation that treatment with a PPARα agonist, presumably by increasing the number of peroxisomes, protects cultured rat hippocampal neurons from Aβ-mediated cell death [280]. In line with this, based on the changes in expression of peroxisomal enzymes in rat cortical neuron cultures upon different treatment regimens with Aβ, it has also been hypothesized that peroxisomes represent an important defense mechanism against oxidative stress triggered by Aβ [281]. In addition, elevated levels of VLCFA and reduced levels of plasmalogens, both markers of peroxisomal dysfunction, have been suggested to stimulate the production of Aβ peptides [282,283]. Detailed reviews covering the potential roles of plasmalogen depletion [284] or peroxisomal impairment [285] in the context of AD have been published in recent years.
An independent connection between peroxisomes and AD is provided by insulin-degrading enzyme (IDE; see Fig. 1), a Zn2+-dependent endopeptidase, whose name-giving enzymatic activity was described already in 1949 [286]. This peptidase can degrade a variety of physiologically relevant peptides reaching from glucagon via Aβ to insulin-like growth factors (for review see [287]). IDE has been found in peroxisomes of cultured cells upon overexpression; and in rat liver, a fraction of the protein was localized to peroxisomes [288–291]. Moreover, this peptidase can degrade N-terminal peptides derived from PTS2-carrying pre-proteins upon processing inside peroxisomes [288]. However, IDE was also described at many other subcellular locations such as mitochondria [292], the nucleus [293], the plasma membrane [294] or the extracellular space [295].
Because IDE can also degrade Aβ [295], this protease has been suggested as a candidate for a modulatory function in the pathophysiology of AD [296]. In line with an important contribution of Aβ degradation by IDE to the pathology of AD, an early genetic investigation described a genetic link between late-onset AD and a region of chromosome 10 that includes the IDE locus [297]. However, some subsequent studies using various single nucleotide polymorphisms found evidence for a linkage while others did not. Moreover, a recent meta-analysis could not confirm indications for a contribution of individual single nucleotide polymorphisms [298]; and in genome-wide association studies, the locus has not been identified [299]. In the mouse, the deletion of Ide (by gene KO) was accompanied by reduced degradation of insulin and higher levels of Aβ in the brain [300]. Moreover, the neuron-specific ectopic expression of IDE in a mouse model for AD (APPSwe/Ind) [301] was sufficient to relieve the burden of amyloid plaque-related pathology [302]. Consequently, the upregulation of IDE activity has been amply suggested for therapeutic purposes. However, it has to be stressed that the occurrence of IDE in various subcellular compartments renders the attribution of specific contributions of IDE functions to one location like peroxisomes rather difficult.
6.4. Peroxisomes and other neurological diseases
In recent years, with increasing progress in lipidomic techniques, changes in the amounts of plasmalogens either in the brain or in the periphery have been described in a variety of neurological diseases. These include Parkinson’s disease [303], schizophrenia [304,305], Down syndrome [306,307], Pelizaeus–Merzbacher disease [308], and lysosomal storage disorders like Gaucher’s disease [309] or neuronal ceroid lipofuscinosis [310]. However, in many cases the detected changes might be too small to be physiologically relevant and are likely to be counteracted by compensatory lipid changes [232]. Additionally, changes of plasmalogen levels may be secondary to the plethora of degenerative and pathologic processes in these diseases. Special caution is warranted for the interpretation of altered levels of plasmalogens in peripheral blood, as even in the milder forms of peroxisomal disorders some cases are known with normal plasmalogen levels in plasma (see Section 5.1.1.). Therefore, only limited conclusions about pathology can be drawn from these values; this can also be deduced from the observation that lipid levels in blood and brain in disease often correlate poorly [311].
It should be noted in the context of peroxisomes and neurodegenerative disorders that in all three Zellweger mouse models (Pex2, Pex5 and Pex13 deficiency [60–62]), increased α-synuclein oligomerization was observed in brain tissues [312]. This is of particular interest, as in Parkinson’s disease and related synucleinopathies, the normally presynaptic protein α-synuclein aggregates intraneuronally to form Lewy bodies, the neuropathological hallmark of these diseases. When α-synuclein was overexpressed in murine fibroblasts, the oligomerization and phosphorylation of α-synuclein was markedly higher in Pex5-deficient cells than in control fibroblasts [312]. In this study, it was suggested that α-synuclein oligomerization and aggregation correlate with lipid alterations rather than with mitochondrial dysfunction or oxidative stress.
Based on the observations that in murine experimental autoimmune encephalomyelitis (EAE) several peroxisomal functions appear to be impaired [313] and that peroxisome deficiency goes along with severe neuroinflammation, a contribution of peroxisomal dysfunction to the pathology in multiple sclerosis, a chronic inflammatory demyelinating disease of the CNS, was postulated [314]. Gray and coworkers further supported their hypothesis by showing decreased expression of ABCD3 mRNA together with a reduction of ABCD3/PMP70 immunoreactivity in gray matter within and outside of lesions, as well as a slight elevation of VLCFA levels in post mortem cortical gray matter from MS patients [314]. Contrary to the idea of a general impairment of peroxisomal functions, the results of a recent report indicate a tendency towards increased plasmalogen levels in the serum of multiple sclerosis patients [315]. However, as discussed above, peripheral lipid levels may be of limited relevance for the interpretation of pathological processes in the brain.
7. Concluding remarks
The functionality of the nervous system critically depends on the ability of peroxisomes to provide biosynthetic intermediates and to degrade undesired compounds that interfere with brain formation, brain function or brain preservation. Moreover, peroxisomes participate in the maintenance of metabolites in the appropriate concentration ranges (e.g., D-amino acid or ROS levels). A lack of each of these functions causes structural abnormalities of the brain, in particular in the cortex or the cerebellum, defects in the intercellular communication of neurons affecting electrical propagation rates along the axon and synaptic transmission efficiency or inappropriate onset of aging and inflammatory processes.
The spectrum of neurological symptoms observed in patients suffering from PBD or single enzyme and transporter deficiencies is surprisingly broad. This variability can be traced back to: (i) residual activity of the affected protein causing milder pathologies (PBD), (ii) the genetic background of patients rendering them more susceptible or resilient, or (iii) environmental factors ranging from disease-inducing occurrences to protection by nutrition deprived of detrimental compounds (Refsum disease). This implies that patients with mild variants of PBD might even escape correct diagnosis. Moreover, the observations that peroxisomes tightly interact with other organelles and that peroxisomal dysfunction secondarily affects their functionality suggest that patients suffering from inherited diseases originating from peroxisomes may benefit from therapeutic approaches targeting such secondary sites.
Finally, increasing evidence indicates that peroxisomes also exert a modulatory role in more common neurodegenerative disease such as AD, autism, ALS or schizophrenia. This is partially supported by linkage analyses, but also by comparative measurements of enzymatic activities, expression levels of mRNAs or proteins or changes in metabolite concentrations linked to peroxisomal functions between cohorts of patients and healthy controls. Future work applying steadily improving bioanalytical tools will help to decipher the relationship between the accumulation or deprivation of biomolecules linked to peroxisomes (e.g., DHA, plasmalogens or D-amino acids) and certain physiological or pathophysiological conditions.
Supplementary Material
The Transparency document associated with this article can be found, in online version,
Acknowledgments
This work was funded by the Austrian Science Fund (FWF): P26112-B19, P24843-B24 and I2738-B26. The authors thank Christoph Wiesinger for his contribution to Fig. 1.
Footnotes
Abbreviations: Aβ, amyloid-β; ABC, ATP-binding cassette; ACAA, acetyl-CoA acyltransferase; ACOX, acyl-CoA oxidase; AD, Alzheimer’s disease; ADHAPS, alkyl-dihydroxyacetone phosphate synthase; ALS, amyotrophic lateral sclerosis; AMACR, 2-methylacyl-CoA racemase; AMN, adrenomyeloneuropathy; CALD, cerebral X-ALD; CNS, central nervous system; CT, computed tomography; DAO, D-amino acid oxidase; DBP, D-bifunctional protein; DDO, D-aspartate oxidase; DHA, docosahexaenoic acid; DHAPAT, dihydroxyacetone phosphate acyltransferase; DHCA/THCA, di-/trihydroxycholestanoic acid; ER, endoplasmic reticulum; FAR, fatty acyl-CoA reductase; IDE, insulin-degrading enzyme; KO, knockout; MRI, magnetic resonance imaging; PBD, peroxisome biogenesis disorders; PEX, peroxin; PHYH, phytanoyl-CoA hydroxylase; PMP, peroxisomal membrane protein; PNS, peripheral nervous system; PTS, peroxisomal targeting signal, RCDP, rhizomelic chondrodysplasia punctata; ROS, reactive oxygen species; SCPx, sterol carrier protein X; VLCFA, very long-chain fatty acids; X-ALD, X-linked adrenoleukodystrophy.
When MIM numbers are indicated within this manuscript, we always refer to phenotype MIM numbers (online source: www.omim.org) characterizing the respective disorder.
References
- 1.Hruban Z, Rechcigl M., Jr Microbodies and related particles. Morphology, biochemistry, and physiology. Int Rev Cytol. 1969;(Suppl. 1):1–296. [PubMed] [Google Scholar]
- 2.Wiese S, Gronemeyer T, Ofman R, Kunze M, Grou CP, Almeida JA, Eisenacher M, Stephan C, Hayen H, Schollenberger L, Korosec T, et al. Proteomics characterization of mouse kidney peroxisomes by tandem mass spectrometry and protein correlation profiling. Mol Cell Proteomics. 2007;6:2045–2057. doi: 10.1074/mcp.M700169-MCP200. [DOI] [PubMed] [Google Scholar]
- 3.Wanders RJ, Waterham HR. Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem. 2006;75:295–332. doi: 10.1146/annurev.biochem.74.082803.133329. [DOI] [PubMed] [Google Scholar]
- 4.Waterham HR, Ebberink MS. Genetics and molecular basis of human peroxisome biogenesis disorders. Biochim Biophys Acta. 2012;1822:1430–1441. doi: 10.1016/j.bbadis.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Mi J, Kirchner E, Cristobal S. Quantitative proteomic comparison of mouse peroxisomes from liver and kidney. Proteomics. 2007;7:1916–1928. doi: 10.1002/pmic.200600638. [DOI] [PubMed] [Google Scholar]
- 6.Powers JM, Moser HW. Peroxisomal disorders: genotype, phenotype, major neuropathologic lesions, and pathogenesis. Brain Pathol. 1998;8:101–120. doi: 10.1111/j.1750-3639.1998.tb00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Veldhoven PP. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J Lipid Res. 2010;51:2863–2895. doi: 10.1194/jlr.R005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fransen M, Nordgren M, Wang B, Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim Biophys Acta. 2012;1822:1363–1373. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Antonenkov VD, Hiltunen JK. Transfer of metabolites across the peroxisomal membrane. Biochim Biophys Acta. 2012;1822:1374–1386. doi: 10.1016/j.bbadis.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Salido E, Pey AL, Rodriguez R, Lorenzo V. Primary hyperoxalurias: disorders of glyoxylate detoxification. Biochim Biophys Acta. 2012;1822:1453–1464. doi: 10.1016/j.bbadis.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 13.Arnold G, Liscum L, Holtzman E. Ultrastructural localization of D-amino acid oxidase in microperoxisomes of the rat nervous system. J Histochem Cytochem. 1979;27:735–745. doi: 10.1177/27.3.39097. [DOI] [PubMed] [Google Scholar]
- 14.Kassmann CM, Quintes S, Rietdorf J, Mobius W, Sereda MW, Nientiedt T, Saher G, Baes M, Nave KA. A role for myelin-associated peroxisomes in maintaining paranodal loops and axonal integrity. FEBS Lett. 2011;585:2205–2211. doi: 10.1016/j.febslet.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 15.Houdou S, Kuruta H, Hasegawa M, Konomi H, Takashima S, Suzuki Y, Hashimoto T. Developmental immunohistochemistry of catalase in the human brain. Brain Res. 1991;556:267–270. doi: 10.1016/0006-8993(91)90314-l. [DOI] [PubMed] [Google Scholar]
- 16.Fouquet F, Zhou JM, Ralston E, Murray K, Troalen F, Magal E, Robain O, Dubois-Dalcq M, Aubourg P. Expression of the adrenoleukodystrophy protein in the human and mouse central nervous system. Neurobiol Dis. 1997;3:271–285. doi: 10.1006/nbdi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 17.Holtzman E, Teichberg S, Abrahams SJ, Citkowitz E, Crain SM, Kawai N, Peterson ER. Notes on synaptic vesicles and related structures, endoplasmic reticulum, lysosomes and peroxisomes in nervous tissue and the adrenal medulla. J Histochem Cytochem. 1973;21:349–385. doi: 10.1177/21.4.349. [DOI] [PubMed] [Google Scholar]
- 18.Singh I, Carillo O, Namboodiri A. Isolation and biochemical characterization of peroxisomes from cultured rat glial cells. Neurochem Res. 2000;25:197–203. doi: 10.1023/a:1007563201595. [DOI] [PubMed] [Google Scholar]
- 19.Ahlemeyer B, Neubert I, Kovacs WJ, Baumgart-Vogt E. Differential expression of peroxisomal matrix and membrane proteins during postnatal development of mouse brain. J Comp Neurol. 2007;505:1–17. doi: 10.1002/cne.21448. [DOI] [PubMed] [Google Scholar]
- 20.Fahimi HD, Baumgart E. Current cytochemical techniques for the investigation of peroxisomes. A review. J Histochem Cytochem. 1999;47:1219–1232. doi: 10.1177/002215549904701001. [DOI] [PubMed] [Google Scholar]
- 21.Nagase T, Shimozawa N, Takemoto Y, Suzuki Y, Komori M, Kondo N. Peroxisomal localization in the developing mouse cerebellum: implications for neuronal abnormalities related to deficiencies in peroxisomes. Biochim Biophys Acta. 2004;1671:26–33. doi: 10.1016/j.bbagen.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Arnold G, Holtzman E. Microperoxisomes in the central nervous system of the postnatal rat. Brain Res. 1978;155:1–17. doi: 10.1016/0006-8993(78)90300-1. [DOI] [PubMed] [Google Scholar]
- 23.Houdou S, Takashima S, Suzuki Y. Immunohistochemical expression of peroxisomal enzymes in developing human brain. Mol Chem Neuropathol. 1993;19:235–248. doi: 10.1007/BF03160002. [DOI] [PubMed] [Google Scholar]
- 24.Adamo AM, Aloise PA, Pasquini JM. A possible relationship between concentration of microperoxisomes and myelination. Int J Dev Neurosci. 1986;4:513–517. doi: 10.1016/0736-5748(86)90003-1. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs WJ, Faust PL, Keller GA, Krisans SK. Purification of brain peroxisomes and localization of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Eur J Biochem. 2001;268:4850–4859. doi: 10.1046/j.0014-2956.2001.02409.x. [DOI] [PubMed] [Google Scholar]
- 26.Knoll A, Sargueil F, Salles J, Cassagne C, Garbay B. Gene expression of peroxisomal beta-oxidation enzymes in rat brain. Brain Res Mol Brain Res. 1999;74:217–220. doi: 10.1016/s0169-328x(99)00252-1. [DOI] [PubMed] [Google Scholar]
- 27.Huyghe S, Casteels M, Janssen A, Meulders L, Mannaerts GP, Declercq PE, Van Veldhoven PP, Baes M. Prenatal and postnatal development of peroxisomal lipid-metabolizing pathways in the mouse. Biochem J. 2001;353:673–680. doi: 10.1042/0264-6021:3530673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger J, Albet S, Bentejac M, Netik A, Holzinger A, Roscher AA, Bugaut M, Forss-Petter S. The four murine peroxisomal ABC-transporter genes differ in constitutive, inducible and developmental expression. Eur J Biochem. 1999;265:719–727. doi: 10.1046/j.1432-1327.1999.00772.x. [DOI] [PubMed] [Google Scholar]
- 29.Citkowitz E, Holtzman E. Peroxisomes in dorsal root ganglia. J Histochem Cytochem. 1973;21:34–41. doi: 10.1177/21.1.34. [DOI] [PubMed] [Google Scholar]
- 30.Hoftberger R, Kunze M, Weinhofer I, Aboul-Enein F, Voigtlander T, Oezen I, Amann G, Bernheimer H, Budka H, Berger J. Distribution and cellular localization of adrenoleukodystrophy protein in human tissues: implications for X-linked adrenoleukodystrophy. Neurobiol Dis. 2007;28:165–174. doi: 10.1016/j.nbd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Trompier D, Vejux A, Zarrouk A, Gondcaille C, Geillon F, Nury T, Savary S, Lizard G. Brain peroxisomes. Biochimie. 2014;98:102–110. doi: 10.1016/j.biochi.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Lismont C, Nordgren M, Van Veldhoven PP, Fransen M. Redox interplay between mitochondria and peroxisomes. Front Cell Dev Biol. 2015;3:35. doi: 10.3389/fcell.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B, Van Veldhoven PP, Brees C, Rubio N, Nordgren M, Apanasets O, Kunze M, Baes M, Agostinis P, Fransen M. Mitochondria are targets for peroxisome-derived oxidative stress in cultured mammalian cells. Free Radic Biol Med. 2013;65:882–894. doi: 10.1016/j.freeradbiomed.2013.08.173. [DOI] [PubMed] [Google Scholar]
- 34.Elsner M, Gehrmann W, Lenzen S. Peroxisome-generated hydrogen peroxide as important mediator of lipotoxicity in insulin-producing cells. Diabetes. 2011;60:200–208. doi: 10.2337/db09-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarrouk A, Vejux A, Nury T, El Hajj HI, Haddad M, Cherkaoui-Malki M, Riedinger JM, Hammami M, Lizard G. Induction of mitochondrial changes associated with oxidative stress on very long chain fatty acids (C22:0, C24:0, or C26:0)-treated human neuronal cells (SK-NB-E) Oxidative Med Cell Longev. 2012;2012:623257. doi: 10.1155/2012/623257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan CY, Pan J, Usuda N, Yeldandi AV, Rao MS, Reddy JK. Steatohepatitis, spontaneous peroxisome proliferation and liver tumors in mice lacking peroxisomal fatty acyl-CoA oxidase. Implications for peroxisome proliferator-activated receptor alpha natural ligand metabolism. J Biol Chem. 1998;273:15639–15645. doi: 10.1074/jbc.273.25.15639. [DOI] [PubMed] [Google Scholar]
- 37.van der Knaap MS, Valk J. The MR spectrum of peroxisomal disorders. Neuroradiology. 1991;33:30–37. doi: 10.1007/BF00593330. [DOI] [PubMed] [Google Scholar]
- 38.Eaton JW, Ma M. Acatalasemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 1995. pp. 2371–2383. [Google Scholar]
- 39.Brosche T, Platt D. The biological significance of plasmalogens in defense against oxidative damage. Exp Gerontol. 1998;33:363–369. doi: 10.1016/s0531-5565(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 40.Brodde A, Teigler A, Brugger B, Lehmann WD, Wieland F, Berger J, Just WW. Impaired neurotransmission in ether lipid-deficient nerve terminals. Hum Mol Genet. 2012;21:2713–2724. doi: 10.1093/hmg/dds097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lessig J, Fuchs B. Plasmalogens in biological systems: their role in oxidative processes in biological membranes, their contribution to pathological processes and aging and plasmalogen analysis. Curr Med Chem. 2009;16:2021–2041. doi: 10.2174/092986709788682164. [DOI] [PubMed] [Google Scholar]
- 42.Wallner S, Schmitz G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem Phys Lipids. 2011;164:573–589. doi: 10.1016/j.chemphyslip.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Fourcade S, Lopez-Erauskin J, Ruiz M, Ferrer I, Pujol A. Mitochondrial dysfunction and oxidative damage cooperatively fuel axonal degeneration in X-linked adrenoleukodystrophy. Biochimie. 2014;98:143–149. doi: 10.1016/j.biochi.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinberg SJ, Dodt G, Raymond GV, Braverman NE, Moser AB, Moser HW. Peroxisome biogenesis disorders. Biochim Biophys Acta. 2006;1763:1733–1748. doi: 10.1016/j.bbamcr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Shimozawa N, Tsukamoto T, Suzuki Y, Orii T, Shirayoshi Y, Mori T, Fujiki Y. A human gene responsible for Zellweger syndrome that affects peroxisome assembly. Science. 1992;255:1132–1134. doi: 10.1126/science.1546315. [DOI] [PubMed] [Google Scholar]
- 47.Berendse K, Engelen M, Ferdinandusse S, Majoie CB, Waterham HR, Vaz FM, Koelman JH, Barth PG, Wanders RJ, Poll-The BT. Zellweger spectrum disorders: clinical manifestations in patients surviving into adulthood. J Inherit Metab Dis. 2015 doi: 10.1007/s10545-015-9880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barth PG, Majoie CB, Gootjes J, Wanders RJ, Waterham HR, van der Knaap MS, de Klerk JB, Smeitink J, Poll-The BT. Neuroimaging of peroxisome biogenesis disorders (Zellweger spectrum) with prolonged survival. Neurology. 2004;62:439–444. doi: 10.1212/01.wnl.0000106943.40848.03. [DOI] [PubMed] [Google Scholar]
- 49.Volpe JJ, Adams RD. Cerebro-hepato-renal syndrome of Zellweger: an inherited disorder of neuronal migration. Acta Neuropathol. 1972;20:175–198. doi: 10.1007/BF00686900. [DOI] [PubMed] [Google Scholar]
- 50.Evrard P, Caviness VS, Jr, Prats-Vinas J, Lyon G. The mechanism of arrest of neuronal migration in the Zellweger malformation: an hypothesis bases upon cytoarchitectonic analysis. Acta Neuropathol. 1978;41:109–117. doi: 10.1007/BF00689761. [DOI] [PubMed] [Google Scholar]
- 51.Kelley RI, Datta NS, Dobyns WB, Hajra AK, Moser AB, Noetzel MJ, Zackai EH, Moser HW. Neonatal adrenoleukodystrophy: new cases, biochemical studies, and differentiation from Zellweger and related peroxisomal polydystrophy syndromes. Am J Med Genet. 1986;23:869–901. doi: 10.1002/ajmg.1320230404. [DOI] [PubMed] [Google Scholar]
- 52.Powers JM. The pathology of peroxisomal disorders with pathogenetic considerations. J Neuropathol Exp Neurol. 1995;54:710–719. [PubMed] [Google Scholar]
- 53.Weller S, Rosewich H, Gartner J. Cerebral MRI as a valuable diagnostic tool in Zellweger spectrum patients. J Inherit Metab Dis. 2008;31:270–280. doi: 10.1007/s10545-008-0856-3. [DOI] [PubMed] [Google Scholar]
- 54.Poll-The BT, Gartner J. Clinical diagnosis, biochemical findings and MRI spectrum of peroxisomal disorders. Biochim Biophys Acta. 2012;1822:1421–1429. doi: 10.1016/j.bbadis.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Barkovich AJ, Peck WW. MR of Zellweger syndrome. AJNR Am J Neuroradiol. 1997;18:1163–1170. [PMC free article] [PubMed] [Google Scholar]
- 56.Baes M, Aubourg P. Peroxisomes, myelination, and axonal integrity in the CNS. Neuroscientist. 2009;15:367–379. doi: 10.1177/1073858409336297. [DOI] [PubMed] [Google Scholar]
- 57.Aubourg P, Scotto J, Rocchiccioli F, Feldmann-Pautrat D, Robain O. Neonatal adrenoleukodystrophy. J Neurol Neurosurg Psychiatry. 1986;49:77–86. doi: 10.1136/jnnp.49.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ebberink MS, Csanyi B, Chong WK, Denis S, Sharp P, Mooijer PA, Dekker CJ, Spooner C, Ngu LH, De Sousa C, Wanders RJ, et al. Identification of an unusual variant peroxisome biogenesis disorder caused by mutations in the PEX16 gene. J Med Genet. 2010;47:608–615. doi: 10.1136/jmg.2009.074302. [DOI] [PubMed] [Google Scholar]
- 59.Sevin C, Ferdinandusse S, Waterham HR, Wanders RJ, Aubourg P. Autosomal recessive cerebellar ataxia caused by mutations in the PEX2 gene, Orphanet. J Rare Dis. 2011;6:8. doi: 10.1186/1750-1172-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baes M, Gressens P, Baumgart E, Carmeliet P, Casteels M, Fransen M, Evrard P, Fahimi D, Declercq PE, Collen D, van Veldhoven PP, et al. A mouse model for Zellweger syndrome. Nat Genet. 1997;17:49–57. doi: 10.1038/ng0997-49. [DOI] [PubMed] [Google Scholar]
- 61.Faust PL, Hatten ME. Targeted deletion of the PEX2 peroxisome assembly gene in mice provides a model for Zellweger syndrome, a human neuronal migration disorder. J Cell Biol. 1997;139:1293–1305. doi: 10.1083/jcb.139.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maxwell M, Bjorkman J, Nguyen T, Sharp P, Finnie J, Paterson C, Tonks I, Paton BC, Kay GF, Crane DI. Pex13 inactivation in the mouse disrupts peroxisome biogenesis and leads to a Zellweger syndrome phenotype. Mol Cell Biol. 2003;23:5947–5957. doi: 10.1128/MCB.23.16.5947-5957.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hiebler S, Masuda T, Hacia JG, Moser AB, Faust PL, Liu A, Chowdhury N, Huang N, Lauer A, Bennett J, Watkins PA, et al. The Pex1-G844D mouse: a model for mild human Zellweger spectrum disorder. Mol Genet Metab. 2014;111:522–532. doi: 10.1016/j.ymgme.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Baumgart E, Dong GX, Morrell JC, Jimenez-Sanchez G, Valle D, Smith KD, Gould SJ. PEX11alpha is required for peroxisome proliferation in response to 4-phenylbutyrate but is dispensable for peroxisome proliferator-activated receptor alpha-mediated peroxisome proliferation. Mol Cell Biol. 2002;22:8226–8240. doi: 10.1128/MCB.22.23.8226-8240.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Baumgart E, Morrell JC, Jimenez-Sanchez G, Valle D, Gould SJ. PEX11 beta deficiency is lethal and impairs neuronal migration but does not abrogate peroxisome function. Mol Cell Biol. 2002;22:4358–4365. doi: 10.1128/MCB.22.12.4358-4365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faust PL, Su HM, Moser A, Moser HW. The peroxisome deficient PEX2 Zellweger mouse: pathologic and biochemical correlates of lipid dysfunction. J Mol Neurosci. 2001;16:289–297. doi: 10.1385/JMN:16:2-3:289. discussion 317-221. [DOI] [PubMed] [Google Scholar]
- 67.Keane MH, Overmars H, Wikander TM, Ferdinandusse S, Duran M, Wanders RJ, Faust PL. Bile acid treatment alters hepatic disease and bile acid transport in peroxisome-deficient PEX2 Zellweger mice. Hepatology. 2007;45:982–997. doi: 10.1002/hep.21532. [DOI] [PubMed] [Google Scholar]
- 68.Faust PL. Abnormal cerebellar histogenesis in PEX2 Zellweger mice reflects multiple neuronal defects induced by peroxisome deficiency. J Comp Neurol. 2003;461:394–413. doi: 10.1002/cne.10699. [DOI] [PubMed] [Google Scholar]
- 69.Faust PL, Banka D, Siriratsivawong R, Ng VG, Wikander TM. Peroxisome biogenesis disorders: the role of peroxisomes and metabolic dysfunction in developing brain. J Inherit Metab Dis. 2005;28:369–383. doi: 10.1007/s10545-005-7059-y. [DOI] [PubMed] [Google Scholar]
- 70.Koch J, Pranjic K, Huber A, Ellinger A, Hartig A, Kragler F, Brocard C. PEX11 family members are membrane elongation factors that coordinate peroxisome proliferation and maintenance. J Cell Sci. 2010;123:3389–3400. doi: 10.1242/jcs.064907. [DOI] [PubMed] [Google Scholar]
- 71.Hulshagen L, Krysko O, Bottelbergs A, Huyghe S, Klein R, Van Veldhoven PP, De Deyn PP, D’Hooge R, Hartmann D, Baes M. Absence of functional peroxisomes from mouse CNS causes dysmyelination and axon degeneration. J Neurosci. 2008;28:4015–4027. doi: 10.1523/JNEUROSCI.4968-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krysko O, Hulshagen L, Janssen A, Schutz G, Klein R, De Bruycker M, Espeel M, Gressens P, Baes M. Neocortical and cerebellar developmental abnormalities in conditions of selective elimination of peroxisomes from brain or from liver. J Neurosci Res. 2007;85:58–72. doi: 10.1002/jnr.21097. [DOI] [PubMed] [Google Scholar]
- 73.Bottelbergs A, Verheijden S, Van Veldhoven PP, Just W, Devos R, Baes M. Peroxisome deficiency but not the defect in ether lipid synthesis causes activation of the innate immune system and axonal loss in the central nervous system. J Neuroinflammation. 2012;9:61. doi: 10.1186/1742-2094-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muller CC, Nguyen TH, Ahlemeyer B, Meshram M, Santrampurwala N, Cao S, Sharp P, Fietz PB, Baumgart-Vogt E, Crane DI. PEX13 deficiency in mouse brain as a model of Zellweger syndrome: abnormal cerebellum formation, reactive gliosis and oxidative stress. Dis Model Mech. 2011;4:104–119. doi: 10.1242/dmm.004622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kassmann CM, Lappe-Siefke C, Baes M, Brugger B, Mildner A, Werner HB, Natt O, Michaelis T, Prinz M, Frahm J, Nave KA. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat Genet. 2007;39:969–976. doi: 10.1038/ng2070. [DOI] [PubMed] [Google Scholar]
- 76.Bottelbergs A, Verheijden S, Hulshagen L, Gutmann DH, Goebbels S, Nave KA, Kassmann C, Baes M. Axonal integrity in the absence of functional peroxisomes from projection neurons and astrocytes. Glia. 2010;58:1532–1543. doi: 10.1002/glia.21027. [DOI] [PubMed] [Google Scholar]
- 77.Janssen A, Gressens P, Grabenbauer M, Baumgart E, Schad A, Vanhorebeek I, Brouwers A, Declercq PE, Fahimi D, Evrard P, Schoonjans L, et al. Neuronal migration depends on intact peroxisomal function in brain and in extraneuronal tissues. J Neurosci. 2003;23:9732–9741. doi: 10.1523/JNEUROSCI.23-30-09732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Savolainen K, Kotti TJ, Schmitz W, Savolainen TI, Sormunen RT, Ilves M, Vainio SJ, Conzelmann E, Hiltunen JK. A mouse model for alpha-methylacyl-CoA racemase deficiency: adjustment of bile acid synthesis and intolerance to dietary methyl-branched lipids. Hum Mol Genet. 2004;13:955–965. doi: 10.1093/hmg/ddh107. [DOI] [PubMed] [Google Scholar]
- 79.Ferdinandusse S, Denis S, Hogenhout EM, Koster J, van Roermund CW, Moser , Wanders RJ, Waterham HR. Clinical, biochemical, and mutational spectrum of peroxisomal acyl-coenzyme A oxidase deficiency. Hum Mutat. 2007;28:904–912. doi: 10.1002/humu.20535. [DOI] [PubMed] [Google Scholar]
- 80.Wanders RJ, Poll-The BT. Role of peroxisomes in human lipid metabolism and its importance for neurological development. Neurosci Lett. 2015 doi: 10.1016/j.neulet.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 81.Ferdinandusse S, Barker S, Lachlan K, Duran M, Waterham HR, Wanders RJ, Hammans S. Adult peroxisomal acyl-coenzyme A oxidase deficiency with cerebellar and brainstem atrophy. J Neurol Neurosurg Psychiatry. 2010;81:310–312. doi: 10.1136/jnnp.2009.176255. [DOI] [PubMed] [Google Scholar]
- 82.Fan CY, Pan J, Chu R, Lee D, Kluckman KD, Usuda N, Singh I, Yeldandi AV, Rao MS, Maeda N, Reddy JK. Targeted disruption of the peroxisomal fatty acyl-CoA oxidase gene: generation of a mouse model of pseudoneonatal adrenoleukodystrophy. Ann N Y Acad Sci. 1996;804:530–541. doi: 10.1111/j.1749-6632.1996.tb18643.x. [DOI] [PubMed] [Google Scholar]
- 83.Ferdinandusse S, Denis S, Mooyer PA, Dekker C, Duran M, Soorani-Lunsing RJ, Boltshauser E, Macaya A, Gartner J, Majoie CB, Barth PG, et al. Clinical and biochemical spectrum of D-bifunctional protein deficiency. Ann Neurol. 2006;59:92–104. doi: 10.1002/ana.20702. [DOI] [PubMed] [Google Scholar]
- 84.Ferdinandusse S, Ylianttila MS, Gloerich J, Koski MK, Oostheim W, Waterham HR, Hiltunen JK, Wanders RJ, Glumoff T. Mutational spectrum of D-bifunctional protein deficiency and structure-based genotype–phenotype analysis. Am J Hum Genet. 2006;78:112–124. doi: 10.1086/498880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pierce SB, Walsh T, Chisholm KM, Lee MK, Thornton AM, Fiumara A, Opitz JM, Levy-Lahad E, Klevit RE, King MC. Mutations in the DBP-deficiency protein HSD17B4 cause ovarian dysgenesis, hearing loss, and ataxia of Perrault Syndrome. Am J Hum Genet. 2010;87:282–288. doi: 10.1016/j.ajhg.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fiumara A, Sorge G, Toscano A, Parano E, Pavone L, Opitz JM. Perrault syndrome: evidence for progressive nervous system involvement. Am J Med Genet A. 2004;128A:246–249. doi: 10.1002/ajmg.a.20616. [DOI] [PubMed] [Google Scholar]
- 87.Khan A, Wei XC, Snyder FF, Mah JK, Waterham H, Wanders RJ. Neurodegeneration in D-bifunctional protein deficiency: diagnostic clues and natural history using serial magnetic resonance imaging. Neuroradiology. 2010;52:1163–1166. doi: 10.1007/s00234-010-0768-4. [DOI] [PubMed] [Google Scholar]
- 88.McMillan HJ, Worthylake T, Schwartzentruber J, Gottlieb CC, Lawrence SE, Mackenzie A, Beaulieu CL, Mooyer PA, Consortium FC, Wanders RJ, Majewski J, et al. Specific combination of compound heterozygous mutations in 17beta-hydroxysteroid dehydrogenase type 4 (HSD17B4) defines a new subtype of D-bifunctional protein deficiency. Orphanet J Rare Dis. 2012;7:90. doi: 10.1186/1750-1172-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baes M, Huyghe S, Carmeliet P, Declercq PE, Collen D, Mannaerts GP, Van Veldhoven PP. Inactivation of the peroxisomal multifunctional protein-2 in mice impedes the degradation of not only 2-methyl-branched fatty acids and bile acid intermediates but also of very long chain fatty acids. J Biol Chem. 2000;275:16329–16336. doi: 10.1074/jbc.M001994200. [DOI] [PubMed] [Google Scholar]
- 90.Huyghe S, Schmalbruch H, De Gendt K, Verhoeven G, Guillou F, Van Veldhoven PP, Baes M. Peroxisomal multifunctional protein 2 is essential for lipid homeostasis in Sertoli cells and male fertility in mice. Endocrinology. 2006;147:2228–2236. doi: 10.1210/en.2005-1571. [DOI] [PubMed] [Google Scholar]
- 91.Baes M, Dewerchin M, Janssen A, Collen D, Carmeliet P. Generation of Pex5-loxP mice allowing the conditional elimination of peroxisomes. Genesis. 2002;32:177–178. doi: 10.1002/gene.10047. [DOI] [PubMed] [Google Scholar]
- 92.Huyghe S, Schmalbruch H, Hulshagen L, Veldhoven PV, Baes M, Hartmann D. Peroxisomal multifunctional protein-2 deficiency causes motor deficits and glial lesions in the adult central nervous system. Am J Pathol. 2006;168:1321–1334. doi: 10.2353/ajpath.2006.041220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferdinandusse S, Denis S, Clayton PT, Graham A, Rees JE, Allen JT, McLean BN, Brown AY, Vreken P, Waterham HR, Wanders RJ. Mutations in the gene encoding peroxisomal alpha-methylacyl-CoA racemase cause adult-onset sensory motor neuropathy. Nat Genet. 2000;24:188–191. doi: 10.1038/72861. [DOI] [PubMed] [Google Scholar]
- 94.Smith EH, Gavrilov DK, Oglesbee D, Freeman WD, Vavra MW, Matern D, Tortorelli S. An adult onset case of alpha-methyl-acyl-CoA racemase deficiency. J Inherit Metab Dis. 2010;33(Suppl. 3):S349–S353. doi: 10.1007/s10545-010-9183-6. [DOI] [PubMed] [Google Scholar]
- 95.Dick D, Horvath R, Chinnery PF. AMACR mutations cause late-onset autosomal recessive cerebellar ataxia. Neurology. 2011;76:1768–1770. doi: 10.1212/WNL.0b013e31821a4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clarke CE, Alger S, Preece MA, Burdon MA, Chavda S, Denis S, Ferdinandusse S, Wanders RJ. Tremor and deep white matter changes in alpha-methylacyl-CoA racemase deficiency. Neurology. 2004;63:188–189. doi: 10.1212/01.wnl.0000132841.81250.b7. [DOI] [PubMed] [Google Scholar]
- 97.Thompson SA, Calvin J, Hogg S, Ferdinandusse S, Wanders RJ, Barker RA. Relapsing encephalopathy in a patient with alpha-methylacyl-CoA racemase deficiency. J Neurol Neurosurg Psychiatry. 2008;79:448–450. doi: 10.1136/jnnp.2007.129478. [DOI] [PubMed] [Google Scholar]
- 98.Haugarvoll K, Johansson S, Tzoulis C, Haukanes BI, Bredrup C, Neckelmann G, Boman H, Knappskog PM, Bindoff LA. MRI characterisation of adult onset alpha-methylacyl-coA racemase deficiency diagnosed by exome sequencing. Orphanet J Rare Dis. 2013;8:1. doi: 10.1186/1750-1172-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Setchell KD, Heubi JE, Bove KE, O’Connell NC, Brewsaugh T, Steinberg SJ, Moser A, Squires RH., Jr Liver disease caused by failure to racemize trihydroxycholestanoic acid: gene mutation and effect of bile acid therapy. Gastroenterology. 2003;124:217–232. doi: 10.1053/gast.2003.50017. [DOI] [PubMed] [Google Scholar]
- 100.Selkala EM, Kuusisto SM, Salonurmi T, Savolainen MJ, Jauhiainen M, Pirila PL, Kvist AP, Conzelmann E, Schmitz W, Alexson SE, Kotti TJ, et al. Metabolic adaptation allows Amacr-deficient mice to remain symptom-free despite low levels of mature bile acids. Biochim Biophys Acta. 2013;1831:1335–1343. doi: 10.1016/j.bbalip.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Selkala EM, Nair RR, Schmitz W, Kvist AP, Baes M, Hiltunen JK, Autio KJ. Phytol is lethal for Amacr-deficient mice. Biochim Biophys Acta. 2015;1851:1394–1405. doi: 10.1016/j.bbalip.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 102.Ferdinandusse S, Kostopoulos P, Denis S, Rusch H, Overmars H, Dillmann U, Reith W, Haas D, Wanders RJ, Duran M, Marziniak M. Mutations in the gene encoding peroxisomal sterol carrier protein X (SCPx) cause leukencephalopathy with dystonia and motor neuropathy. Am J Hum Genet. 2006;78:1046–1052. doi: 10.1086/503921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chevillard G, Clemencet MC, Latruffe N, Nicolas-Frances V. Targeted disruption of the peroxisomal thiolase B gene in mouse: a new model to study disorders related to peroxisomal lipid metabolism. Biochimie. 2004;86:849–856. doi: 10.1016/j.biochi.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 104.Seedorf U, Raabe M, Ellinghaus P, Kannenberg F, Fobker M, Engel T, Denis S, Wouters F, Wirtz KW, Wanders RJ, Maeda N, et al. Defective peroxisomal catabolism of branched fatty acyl coenzyme A in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes Dev. 1998;12:1189–1201. doi: 10.1101/gad.12.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Troffer-Charlier N, Doerflinger N, Metzger E, Fouquet F, Mandel JL, Aubourg P. Mirror expression of adrenoleukodystrophy and adrenoleukodystrophy related genes in mouse tissues and human cell lines. Eur J Cell Biol. 1998;75:254–264. doi: 10.1016/S0171-9335(98)80121-0. [DOI] [PubMed] [Google Scholar]
- 106.Langmann T, Mauerer R, Zahn A, Moehle C, Probst M, Stremmel W, Schmitz G. Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clin Chem. 2003;49:230–238. doi: 10.1373/49.2.230. [DOI] [PubMed] [Google Scholar]
- 107.Weber FD, Wiesinger C, Forss-Petter S, Regelsberger G, Einwich A, Weber WH, Kohler W, Stockinger H, Berger J. X-linked adrenoleukodystrophy: very long-chain fatty acid metabolism is severely impaired in monocytes but not in lymphocytes. Hum Mol Genet. 2014;23:2542–2550. doi: 10.1093/hmg/ddt645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser H, Poustka AM, Mandel JL, Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- 109.Bezman L, Moser AB, Raymond GV, Rinaldo P, Watkins PA, Smith KD, Kass NE, Moser HW. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol. 2001;49:512–517. [PubMed] [Google Scholar]
- 110.Wiesinger C, Eichler FS, Berger J. The genetic landscape of X-linked adrenoleukodystrophy: inheritance, mutations, modifier genes, and diagnosis. Appl Clin Genet. 2015;8:109–121. doi: 10.2147/TACG.S49590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wiesinger C, Kunze M, Regelsberger G, Forss-Petter S, Berger J. Impaired very long-chain acyl-CoA beta-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dysfunction. J Biol Chem. 2013;288:19269–19279. doi: 10.1074/jbc.M112.445445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van Roermund CW, Visser WF, Ijlst L, van Cruchten A, Boek M, Kulik W, Waterham HR, Wanders RJ. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008;22:4201–4208. doi: 10.1096/fj.08-110866. [DOI] [PubMed] [Google Scholar]
- 113.van Roermund CW, Ijlst L, Wagemans T, Wanders RJ, Waterham HR. A role for the human peroxisomal half-transporter ABCD3 in the oxidation of dicarboxylic acids. Biochim Biophys Acta. 2014;1841:563–568. doi: 10.1016/j.bbalip.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 114.Kemp S, Wei HM, Lu JF, Braiterman LT, McGuinness MC, Moser AB, Watkins PA, Smith KD. Gene redundancy and pharmacological gene therapy: implications for X-linked adrenoleukodystrophy. Nat Med. 1998;4:1261–1268. doi: 10.1038/3242. [DOI] [PubMed] [Google Scholar]
- 115.Netik A, Forss-Petter S, Holzinger A, Molzer B, Unterrainer G, Berger J. Adrenoleukodystrophy-related protein can compensate functionally for adrenoleukodystrophy protein deficiency (X-ALD): implications for therapy. Hum Mol Genet. 1999;8:907–913. doi: 10.1093/hmg/8.5.907. [DOI] [PubMed] [Google Scholar]
- 116.Ofman R, Dijkstra IM, van Roermund CW, Burger N, Turkenburg M, van Cruchten A, van Engen CE, Wanders RJ, Kemp S. The role of ELOVL1 in very long-chain fatty acid homeostasis and X-linked adrenoleukodystrophy. EMBO Mol Med. 2010;2:90–97. doi: 10.1002/emmm.201000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schackmann MJ, Ofman R, Dijkstra IM, Wanders RJ, Kemp S. Enzymatic characterization of ELOVL1, a key enzyme in very long-chain fatty acid synthesis. Biochim Biophys Acta. 2015;1851:231–237. doi: 10.1016/j.bbalip.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 118.Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: Their regulation and roles in metabolism. Prog Lipid Res. 2006;45:237–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 119.Ohno Y, Suto S, Yamanaka M, Mizutani Y, Mitsutake S, Igarashi Y, Sassa T, Kihara A. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci U S A. 2010;107:18439–18444. doi: 10.1073/pnas.1005572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kemp S, Berger J, Aubourg P. X-linked adrenoleukodystrophy: Clinical, metabolic, genetic and pathophysiological aspects. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbadis.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 121.Berger J, Molzer B, Fae I, Bernheimer H. X-linked adrenoleukodystrophy (ALD): a novel mutation of the ALD gene in 6 members of a family presenting with 5 different phenotypes. Biochem Biophys Res Commun. 1994;205:1638–1643. doi: 10.1006/bbrc.1994.2855. [DOI] [PubMed] [Google Scholar]
- 122.Berger J, Gartner J. X-linked adrenoleukodystrophy: clinical, biochemical and pathogenetic aspects. Biochim Biophys Acta. 2006;1763:1721–1732. doi: 10.1016/j.bbamcr.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 123.Berger J, Forss-Petter S, Eichler FS. Pathophysiology of X-linked adrenoleukodystrophy. Biochimie. 2014;98:135–142. doi: 10.1016/j.biochi.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moser HW, Kemp S, Smith KD. Mutational analysis and the pathogenesis of variant X-linked adrenoleukodystrophy phenotypes. Arch Neurol. 1999;56:273–275. doi: 10.1001/archneur.56.3.273. [DOI] [PubMed] [Google Scholar]
- 125.Kemp S, Pujol A, Waterham HR, van Geel BM, Boehm CD, Raymond GV, Cutting GR, Wanders RJ, Moser HW. ABCD1 mutations and the X-linked adrenoleukodystrophy mutation database: role in diagnosis and clinical correlations. Hum Mutat. 2001;18:499–515. doi: 10.1002/humu.1227. [DOI] [PubMed] [Google Scholar]
- 126.Dubey P, Raymond GV, Moser AB, Kharkar S, Bezman L, Moser HW. Adrenal insufficiency in asymptomatic adrenoleukodystrophy patients identified by very long-chain fatty acid screening. J Pediatr. 2005;146:528–532. doi: 10.1016/j.jpeds.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 127.Powers JM, DeCiero DP, Cox C, Richfield EK, Ito M, Moser AB, Moser HW. The dorsal root ganglia in adrenomyeloneuropathy: neuronal atrophy and abnormal mitochondria. J Neuropathol Exp Neurol. 2001;60:493–501. doi: 10.1093/jnen/60.5.493. [DOI] [PubMed] [Google Scholar]
- 128.Dubey P, Fatemi A, Barker PB, Degaonkar M, Troeger M, Zackowski K, Bastian A, Smith SA, Pomper MG, Moser HW, Raymond GV. Spectroscopic evidence of cerebral axonopathy in patients with “pure” adrenomyeloneuropathy. Neurology. 2005;64:304–310. doi: 10.1212/01.WNL.0000149514.13580.84. [DOI] [PubMed] [Google Scholar]
- 129.van Geel BM, Koelman JH, Barth PG, Ongerboer de Visser BW. Peripheral nerve abnormalities in adrenomyeloneuropathy: a clinical and electrodiagnostic study. Neurology. 1996;46:112–118. doi: 10.1212/wnl.46.1.112. [DOI] [PubMed] [Google Scholar]
- 130.Engelen M, Barbier M, Dijkstra IM, Schur R, de Bie RM, Verhamme C, Dijkgraaf MG, Aubourg PA, Wanders RJ, van Geel BM, de Visser M, et al. X-linked adrenoleukodystrophy in women: a cross-sectional cohort study. Brain. 2014;137:693–706. doi: 10.1093/brain/awt361. [DOI] [PubMed] [Google Scholar]
- 131.Horn MA, Retterstol L, Abdelnoor M, Skjeldal OH, Tallaksen CM. Age-dependent penetrance among females with X-linked adrenoleukodystrophy. Brain. 2015;138:e325. doi: 10.1093/brain/awu232. [DOI] [PubMed] [Google Scholar]
- 132.Powers JM. Adreno-leukodystrophy: a personal historical note. Acta Neuropathol. 2005;109:124–127. doi: 10.1007/s00401-004-0961-9. [DOI] [PubMed] [Google Scholar]
- 133.Hein S, Schonfeld P, Kahlert S, Reiser G. Toxic effects of X-linked adrenoleukodystrophy-associated, very long chain fatty acids on glial cells and neurons from rat hippocampus in culture. Hum Mol Genet. 2008;17:1750–1761. doi: 10.1093/hmg/ddn066. [DOI] [PubMed] [Google Scholar]
- 134.Engelen M, Kemp S, de Visser M, van Geel BM, Wanders RJ, Aubourg P, Poll-The BT. X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis. 2012;7:51. doi: 10.1186/1750-1172-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Korenke GC, Fuchs S, Krasemann E, Doerr HG, Wilichowski E, Hunneman DH, Hanefeld F. Cerebral adrenoleukodystrophy (ALD) in only one of monozygotic twins with an identical ALD genotype. Ann Neurol. 1996;40:254–257. doi: 10.1002/ana.410400221. [DOI] [PubMed] [Google Scholar]
- 136.Di Rocco M, Doria-Lamba L, Caruso U. Monozygotic twins with X-linked adrenoleukodystrophy and different phenotypes. Ann Neurol. 2001;50:424. doi: 10.1002/ana.1220. [DOI] [PubMed] [Google Scholar]
- 137.Berger J, Pujol A, Aubourg P, Forss-Petter S. Current and future pharmacological treatment strategies in X-linked adrenoleukodystrophy. Brain Pathol. 2010;20:845–856. doi: 10.1111/j.1750-3639.2010.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raymond GV, Seidman R, Monteith TS, Kolodny E, Sathe S, Mahmood A, Powers JM. Head trauma can initiate the onset of adreno-leukodystrophy. J Neurol Sci. 2010;290:70–74. doi: 10.1016/j.jns.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 139.Miller WP, Mantovani LF, Muzic J, Rykken JB, Gawande RS, Lund TC, Shanley RM, Raymond GV, Orchard PJ, Nascene DR. Intensity of MRI gadolinium enhancement in cerebral adrenoleukodystrophy: a biomarker for inflammation and predictor of outcome following transplantation in higher risk patients. AJNR Am J Neuroradiol. 2015 doi: 10.3174/ajnr.A4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ito M, Blumberg BM, Mock DJ, Goodman AD, Moser AB, Moser HW, Smith KD, Powers JM. Potential environmental and host participants in the early white matter lesion of adreno-leukodystrophy: morphologic evidence for CD8 cytotoxic T cells, cytolysis of oligodendrocytes, and CD1-mediated lipid antigen presentation. J Neuropathol Exp Neurol. 2001;60:1004–1019. doi: 10.1093/jnen/60.10.1004. [DOI] [PubMed] [Google Scholar]
- 141.Paintlia AS, Gilg AG, Khan M, Singh AK, Barbosa E, Singh I. Correlation of very long chain fatty acid accumulation and inflammatory disease progression in childhood X-ALD: implications for potential therapies. Neurobiol Dis. 2003;14:425–439. doi: 10.1016/j.nbd.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 142.Powers JM, Pei Z, Heinzer AK, Deering R, Moser AB, Moser HW, Watkins PA, Smith KD. Adreno-leukodystrophy: oxidative stress of mice and men. J Neuropathol Exp Neurol. 2005;64:1067–1079. doi: 10.1097/01.jnen.0000190064.28559.a4. [DOI] [PubMed] [Google Scholar]
- 143.Powers JM, Liu Y, Moser AB, Moser HW. The inflammatory myelinopathy of adreno-leukodystrophy: cells, effector molecules, and pathogenetic implications. J Neuropathol Exp Neurol. 1992;51:630–643. doi: 10.1097/00005072-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 144.Eichler FS, Ren JQ, Cossoy M, Rietsch AM, Nagpal S, Moser AB, Frosch MP, Ransohoff RM. Is microglial apoptosis an early pathogenic change in cerebral X-linked adrenoleukodystrophy? Ann Neurol. 2008;63:729–742. doi: 10.1002/ana.21391. [DOI] [PubMed] [Google Scholar]
- 145.Peters C, Charnas LR, Tan Y, Ziegler RS, Shapiro EG, DeFor T, Grewal SS, Orchard PJ, Abel SL, Goldman AI, Ramsay NK, et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104:881–888. doi: 10.1182/blood-2003-10-3402. [DOI] [PubMed] [Google Scholar]
- 146.Aubourg P, Blanche S, Jambaque I, Rocchiccioli F, Kalifa G, Naud-Saudreau C, Rolland MO, Debre M, Chaussain JL, Griscelli C, et al. Reversal of early neurologic and neuroradiologic manifestations of X-linked adrenoleukodystrophy by bone marrow transplantation. New Engl J Med. 1990;322:1860–1866. doi: 10.1056/NEJM199006283222607. [DOI] [PubMed] [Google Scholar]
- 147.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 148.Cartier N, Aubourg P. Hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy in X-linked adrenoleukodystrophy. Brain Pathol. 2010;20:857–862. doi: 10.1111/j.1750-3639.2010.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schluter A, Espinosa L, Fourcade S, Galino J, Lopez E, Ilieva E, Morato L, Asheuer M, Cook T, McLaren A, Reid J, et al. Functional genomic analysis unravels a metabolic-inflammatory interplay in adrenoleukodystrophy. Hum Mol Genet. 2012;21:1062–1077. doi: 10.1093/hmg/ddr536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Musolino PL, Gong Y, Snyder JM, Jimenez S, Lok J, Lo EH, Moser AB, Grabowski EF, Frosch MP, Eichler FS. Brain endothelial dysfunction in cerebral adrenoleukodystrophy. Brain. 2015 doi: 10.1093/brain/awv250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Forss-Petter S, Werner H, Berger J, Lassmann H, Molzer B, Schwab MH, Bernheimer H, Zimmermann F, Nave KA. Targeted inactivation of the X-linked adrenoleukodystrophy gene in mice. J Neurosci Res. 1997;50:829–843. doi: 10.1002/(SICI)1097-4547(19971201)50:5<829::AID-JNR19>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 152.Lu JF, Lawler AM, Watkins PA, Powers JM, Moser AB, Moser HW, Smith KD. A mouse model for X-linked adrenoleukodystrophy. Proc Natl Acad Sci U S A. 1997;94:9366–9371. doi: 10.1073/pnas.94.17.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kobayashi T, Shinnoh N, Kondo A, Yamada T. Adrenoleukodystrophy protein-deficient mice represent abnormality of very long chain fatty acid metabolism. Biochem Biophys Res Commun. 1997;232:631–636. doi: 10.1006/bbrc.1997.6340. [DOI] [PubMed] [Google Scholar]
- 154.Pujol A, Hindelang C, Callizot N, Bartsch U, Schachner M, Mandel JL. Late onset neurological phenotype of the X-ALD gene inactivation in mice: a mouse model for adrenomyeloneuropathy. Hum Mol Genet. 2002;11:499–505. doi: 10.1093/hmg/11.5.499. [DOI] [PubMed] [Google Scholar]
- 155.Dumser M, Bauer J, Lassmann H, Berger J, Forss-Petter S. Lack of adrenoleukodystrophy protein enhances oligodendrocyte disturbance and microglia activation in mice with combined Abcd1/Mag deficiency. Acta Neuropathol. 2007;114:573–586. doi: 10.1007/s00401-007-0288-4. [DOI] [PubMed] [Google Scholar]
- 156.Pujol A, Ferrer I, Camps C, Metzger E, Hindelang C, Callizot N, Ruiz M, Pampols T, Giros M, Mandel JL. Functional overlap between ABCD1 (ALD) and ABCD2 (ALDR) transporters: a therapeutic target for X-adrenoleukodystrophy. Hum Mol Genet. 2004;13:2997–3006. doi: 10.1093/hmg/ddh323. [DOI] [PubMed] [Google Scholar]
- 157.Muneer Z, Wiesinger C, Voigtlander T, Werner HB, Berger J, Forss-Petter S. Abcd2 Is a strong modifier of the metabolic impairments in peritoneal macrophages of Abcd1-deficient mice. PLoS One. 2014;9:e108655. doi: 10.1371/journal.pone.0108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Oezen I, Rossmanith W, Forss-Petter S, Kemp S, Voigtlander T, Moser-Thier K, Wanders RJ, Bittner RE, Berger J. Accumulation of very long-chain fatty acids does not affect mitochondrial function in adrenoleukodystrophy protein deficiency. Hum Mol Genet. 2005;14:1127–1137. doi: 10.1093/hmg/ddi125. [DOI] [PubMed] [Google Scholar]
- 159.Galea E, Launay N, Portero-Otin M, Ruiz M, Pamplona R, Aubourg P, Ferrer I, Pujol A. Oxidative stress underlying axonal degeneration in adrenoleukodystrophy: a paradigm for multifactorial neurodegenerative diseases? Biochim Biophys Acta. 2012;1822:1475–1488. doi: 10.1016/j.bbadis.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 160.Fourcade S, Lopez-Erauskin J, Galino J, Duval C, Naudi A, Jove M, Kemp S, Villarroya F, Ferrer I, Pamplona R, Portero-Otin M, et al. Early oxidative damage underlying neurodegeneration in X-adrenoleukodystrophy. Hum Mol Genet. 2008;17:1762–1773. doi: 10.1093/hmg/ddn085. [DOI] [PubMed] [Google Scholar]
- 161.Vargas CR, Wajner M, Sirtori LR, Goulart L, Chiochetta M, Coelho D, Latini A, Llesuy S, Bello-Klein A, Giugliani R, Deon M, et al. Evidence that oxidative stress is increased in patients with X-linked adrenoleukodystrophy. Biochim Biophys Acta. 2004;1688:26–32. doi: 10.1016/j.bbadis.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 162.Petrillo S, Piemonte F, Pastore A, Tozzi G, Aiello C, Pujol A, Cappa M, Bertini E. Glutathione imbalance in patients with X-linked adrenoleukodystrophy. Mol Genet Metab. 2013;109:366–370. doi: 10.1016/j.ymgme.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lopez-Erauskin J, Fourcade S, Galino J, Ruiz M, Schluter A, Naudi A, Jove M, Portero-Otin M, Pamplona R, Ferrer I, Pujol A. Antioxidants halt axonal degeneration in a mouse model of X-adrenoleukodystrophy. Ann Neurol. 2011;70:84–92. doi: 10.1002/ana.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Morato L, Galino J, Ruiz M, Calingasan NY, Starkov AA, Dumont M, Naudi A, Martinez JJ, Aubourg P, Portero-Otin M, Pamplona R, et al. Pioglitazone halts axonal degeneration in a mouse model of X-linked adrenoleukodystrophy. Brain. 2013;136:2432–2443. doi: 10.1093/brain/awt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nave KA, Edgar J, Griffiths IR, Jansen K, Kassmann CM, Klugmann M, Lappe-Siefke C, Meyer-zu-Horste G, Mobius W, Sereda MH, Werner H. Oligodendrocytes and Schwann cells support axon function and survival independent of their role in myelination: implications for multiple sclerosis. J Neuroimmunol. 2006;178:36–36. [Google Scholar]
- 166.Kassmann CM, Nave KA. Oligodendroglial impact on axonal function and survival—a hypothesis. Curr Opin Neurol. 2008;21:235–241. doi: 10.1097/WCO.0b013e328300c71f. [DOI] [PubMed] [Google Scholar]
- 167.Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 168.Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, Schneider A, Zimmermann F, McCulloch M, Nadon N, Nave KA. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- 169.Wanders RJ, Komen J, Ferdinandusse S. Phytanic acid metabolism in health and disease. Biochim Biophys Acta. 2011;1811:498–507. doi: 10.1016/j.bbalip.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 170.Jansen GA, Ofman R, Ferdinandusse S, Ijlst L, Muijsers AO, Skjeldal OH, Stokke O, Jakobs C, Besley GT, Wraith JE, Wanders RJ. Refsum disease is caused by mutations in the phytanoyl-CoA hydroxylase gene. Nat Genet. 1997;17:190–193. doi: 10.1038/ng1097-190. [DOI] [PubMed] [Google Scholar]
- 171.Jansen GA, Hogenhout EM, Ferdinandusse S, Waterham HR, Ofman R, Jakobs C, Skjeldal OH, Wanders RJ. Human phytanoyl-CoA hydroxylase: resolution of the gene structure and the molecular basis of Refsum’s disease. Hum Mol Genet. 2000;9:1195–1200. doi: 10.1093/hmg/9.8.1195. [DOI] [PubMed] [Google Scholar]
- 172.Wanders RJA, Jakobs C, Skjeldal OH. Refsum disease. In: Scriver CR, Beaudet AL, Valle D, Sly WS, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 3303–3321. [Google Scholar]
- 173.Baldwin EJ, Gibberd FB, Harley C, Sidey MC, Feher MD, Wierzbicki AS. The effectiveness of long-term dietary therapy in the treatment of adult Refsum disease. J Neurol Neurosurg Psychiatry. 2010;81:954–957. doi: 10.1136/jnnp.2008.161059. [DOI] [PubMed] [Google Scholar]
- 174.Ruether K, Baldwin E, Casteels M, Feher MD, Horn M, Kuranoff S, Leroy BP, Wanders RJ, Wierzbicki AS. Adult Refsum disease: a form of tapetoretinal dystrophy accessible to therapy. Surv Ophthalmol. 2010;55:531–538. doi: 10.1016/j.survophthal.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 175.Ferdinandusse S, Zomer AW, Komen JC, van den Brink CE, Thanos M, Hamers FP, Wanders RJ, van der Saag PT, Poll-The BT, Brites P. Ataxia with loss of Purkinje cells in a mouse model for Refsum disease. Proc Natl Acad Sci U S A. 2008;105:17712–17717. doi: 10.1073/pnas.0806066105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Braverman N, Steel G, Obie C, Moser A, Moser H, Gould SJ, Valle D. Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat Genet. 1997;15:369–376. doi: 10.1038/ng0497-369. [DOI] [PubMed] [Google Scholar]
- 177.Motley AM, Hettema EH, Hogenhout EM, Brites P, ten Asbroek AL, Wijburg FA, Baas F, Heijmans HS, Tabak HF, Wanders RJ, Distel B. Rhizomelic chondrodysplasia punctata is a peroxisomal protein targeting disease caused by a non-functional PTS2 receptor. Nat Genet. 1997;15:377–380. doi: 10.1038/ng0497-377. [DOI] [PubMed] [Google Scholar]
- 178.Purdue PE, Zhang JW, Skoneczny M, Lazarow PB. Rhizomelic chondrodysplasia punctata is caused by deficiency of human PEX7, a homologue of the yeast PTS2 receptor. Nat Genet. 1997;15:381–384. doi: 10.1038/ng0497-381. [DOI] [PubMed] [Google Scholar]
- 179.Wanders RJ, Schumacher H, Heikoop J, Schutgens RB, Tager JM. Human dihydroxyacetonephosphate acyltransferase deficiency: a new peroxisomal disorder. J Inherit Metab Dis. 1992;15:389–391. doi: 10.1007/BF02435984. [DOI] [PubMed] [Google Scholar]
- 180.Wanders RJ, Dekker C, Hovarth VA, Schutgens RB, Tager JM, Van Laer P, Lecoutere D. Human alkyldihydroxyacetonephosphate synthase deficiency: a new peroxisomal disorder. J Inherit Metab Dis. 1994;17:315–318. doi: 10.1007/BF00711817. [DOI] [PubMed] [Google Scholar]
- 181.Horn MA, van den Brink DM, Wanders RJ, Duran M, Poll-The BT, Tallaksen CM, Stokke OH, Moser H, Skjeldal OH. Phenotype of adult Refsum disease due to a defect in peroxin 7. Neurology. 2007;68:698–700. doi: 10.1212/01.wnl.0000255960.01644.39. [DOI] [PubMed] [Google Scholar]
- 182.Nanetti L, Pensato V, Leoni V, Rizzetto M, Caccia C, Taroni F, Mariotti C, Gellera C. PEX7 mutations cause congenital cataract retinopathy and late-onset ataxia and cognitive impairment: report of two siblings and review of the literature. J Clin Neurol. 2015;11:197–199. doi: 10.3988/jcn.2015.11.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Buchert R, Tawamie H, Smith C, Uebe S, Innes AM, Al Hallak B, Ekici AB, Sticht H, Schwarze B, Lamont RE, Parboosingh JS, et al. A peroxisomal disorder of severe intellectual disability, epilepsy, and cataracts due to fatty acyl-CoA reductase 1 deficiency. Am J Hum Genet. 2014;95:602–610. doi: 10.1016/j.ajhg.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kunze M, Malkani N, Maurer-Stroh S, Wiesinger C, Schmid JA, Berger J. Mechanistic insights into PTS2-mediated peroxisomal protein import: the co-receptor PEX5L drastically increases the interaction strength between the cargo protein and the receptor PEX7. J Biol Chem. 2015;290:4928–4940. doi: 10.1074/jbc.M114.601575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Baroy T, Koster J, Stromme P, Ebberink MS, Misceo D, Ferdinandusse S, Holmgren A, Hughes T, Merckoll E, Westvik J, Woldseth B, et al. A novel type of rhizomelic chondrodysplasia punctata, RCDP5, is caused by loss of the PEX5 long isoform. Hum Mol Genet. 2015;24:5845–5854. doi: 10.1093/hmg/ddv305. [DOI] [PubMed] [Google Scholar]
- 186.Hoefler G, Hoefler S, Watkins PA, Chen WW, Moser A, Baldwin V, McGillivary B, Charrow J, Friedman JM, Rutledge L, et al. Biochemical abnormalities in rhizomelic chondrodysplasia punctata. J Pediatr. 1988;112:726–733. doi: 10.1016/s0022-3476(88)80689-9. [DOI] [PubMed] [Google Scholar]
- 187.Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 188.Noguchi M, Honsho M, Abe Y, Toyama R, Niwa H, Sato Y, Ghaedi K, Rahmanifar A, Shafeghati Y, Fujiki Y. Mild reduction of plasmalogens causes rhizomelic chondrodysplasia punctata: functional characterization of a novel mutation. J Hum Genet. 2014;59:387–392. doi: 10.1038/jhg.2014.39. [DOI] [PubMed] [Google Scholar]
- 189.Nuoffer JM, Pfammatter JP, Spahr A, Toplak H, Wanders RJ, Schutgens RB, Wiesmann UN. Chondrodysplasia punctata with a mild clinical course. J Inherit Metab Dis. 1994;17:60–66. doi: 10.1007/BF00735395. [DOI] [PubMed] [Google Scholar]
- 190.Braverman N, Chen L, Lin P, Obie C, Steel G, Douglas P, Chakraborty PK, Clarke JT, Boneh A, Moser A, Moser H, et al. Mutation analysis of PEX7 in 60 probands with rhizomelic chondrodysplasia punctata and functional correlations of genotype with phenotype. Hum Mutat. 2002;20:284–297. doi: 10.1002/humu.10124. [DOI] [PubMed] [Google Scholar]
- 191.Bams-Mengerink AM, Koelman JH, Waterham H, Barth PG, Poll-The BT. The neurology of rhizomelic chondrodysplasia punctata. Orphanet J Rare Dis. 2013;8:174. doi: 10.1186/1750-1172-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.White AL, Modaff P, Holland-Morris F, Pauli RM. Natural history of rhizomelic chondrodysplasia punctata. Am J Med Genet A. 2003;118A:332–342. doi: 10.1002/ajmg.a.20009. [DOI] [PubMed] [Google Scholar]
- 193.Motley AM, Brites P, Gerez L, Hogenhout E, Haasjes J, Benne R, Tabak HF, Wanders RJ, Waterham HR. Mutational spectrum in the PEX7 gene and functional analysis of mutant alleles in 78 patients with rhizomelic chondrodysplasia punctata type 1. Am J Hum Genet. 2002;70:612–624. doi: 10.1086/338998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Poll-The BT, Maroteaux P, Narcy C, Quetin P, Guesnu M, Wanders RJ, Schutgens RB, Saudubray JM. A new type of chondrodysplasia punctata associated with peroxisomal dysfunction. J Inherit Metab Dis. 1991;14:361–363. doi: 10.1007/BF01811703. [DOI] [PubMed] [Google Scholar]
- 195.Bams-Mengerink AM, Majoie CB, Duran M, Wanders RJ, Van Hove J, Scheurer CD, Barth PG, Poll-The BT. MRI of the brain and cervical spinal cord in rhizomelic chondrodysplasia punctata. Neurology. 2006;66:798–803. doi: 10.1212/01.wnl.0000205594.34647.d0. discussion 789. [DOI] [PubMed] [Google Scholar]
- 196.Sztriha L, Al-Gazali LI, Wanders RJ, Ofman R, Nork M, Lestringant GG. Abnormal myelin formation in rhizomelic chondrodysplasia punctata type 2 (DHAPAT-deficiency) Dev Med Child Neurol. 2000;42:492–495. doi: 10.1017/s0012162200000918. [DOI] [PubMed] [Google Scholar]
- 197.Karabayir N, Keskindemirci G, Adal E, Korkmaz O. A case of rhizomelic chondrodysplasia punctata in newborn. Case Rep Med. 2014;2014:879679. doi: 10.1155/2014/879679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sastrowijoto SH, Vandenberghe K, Moerman P, Lauweryns JM, Fryns JP. Prenatal ultrasound diagnosis of rhizomelic chondrodysplasia punctata in a primigravida. Prenat Diagn. 1994;14:770–776. doi: 10.1002/pd.1970140821. [DOI] [PubMed] [Google Scholar]
- 199.Viola A, Confort-Gouny S, Ranjeva JP, Chabrol B, Raybaud C, Vintila F, Cozzone PJ. MR imaging and MR spectroscopy in rhizomelic chondrodysplasia punctata. AJNR Am J Neuroradiol. 2002;23:480–483. [PMC free article] [PubMed] [Google Scholar]
- 200.Goh S. Neuroimaging features in a neonate with rhizomelic chondrodysplasia punctata. Pediatr. Neurol. 2007;37:382–384. doi: 10.1016/j.pediatrneurol.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 201.van den Brink DM, Brites P, Haasjes J, Wierzbicki AS, Mitchell J, Lambert-Hamill M, de Belleroche J, Jansen GA, Waterham HR, Wanders RJ. Identification of PEX7 as the second gene involved in Refsum disease. Am J Hum Genet. 2003;72:471–477. doi: 10.1086/346093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Powers JM, Kenjarski TP, Moser AB, Moser HW. Cerebellar atrophy in chronic rhizomelic chondrodysplasia punctata: a potential role for phytanic acid and calcium in the death of its Purkinje cells. Acta Neuropathol. 1999;98:129–134. doi: 10.1007/s004010051060. [DOI] [PubMed] [Google Scholar]
- 203.Itzkovitz B, Jiralerspong S, Nimmo G, Loscalzo M, Horovitz DD, Snowden A, Moser A, Steinberg S, Braverman N. Functional characterization of novel mutations in GNPAT and AGPS, causing rhizomelic chondrodysplasia punctata (RCDP) types 2 and 3. Hum Mutat. 2012;33:189–197. doi: 10.1002/humu.21623. [DOI] [PubMed] [Google Scholar]
- 204.Spranger JW, Opitz JM, Bidder U. Heterogeneity of Chondrodysplasia punctata. Humangenetik. 1971;11:190–212. doi: 10.1007/BF00274739. [DOI] [PubMed] [Google Scholar]
- 205.Yakovac WC. Calcareous chondropathies in the newborn infant. AMA Arch Pathol. 1954;57:62–79. [PubMed] [Google Scholar]
- 206.Poulos A, Sheffield L, Sharp P, Sherwood G, Johnson D, Beckman K, Fellenberg AJ, Wraith JE, Chow CW, Usher S, et al. Rhizomelic chondrodysplasia punctata: clinical, pathologic, and biochemical findings in two patients. J Pediatr. 1988;113:685–690. doi: 10.1016/s0022-3476(88)80378-0. [DOI] [PubMed] [Google Scholar]
- 207.Khanna AJ, Braverman NE, Valle D, Sponseller PD. Cervical stenosis secondary to rhizomelic chondrodysplasia punctata. Am J Med Genet. 2001;99:63–66. doi: 10.1002/1096-8628(20010215)99:1<63::aid-ajmg1117>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 208.Agamanolis DP, Novak RW. Rhizomelic chondrodysplasia punctata: report of a case with review of the literature and correlation with other peroxisomal disorders. Pediatr Pathol Lab Med. 1995;15:503–513. doi: 10.3109/15513819509026986. [DOI] [PubMed] [Google Scholar]
- 209.Alkan A, Kutlu R, Yakinci C, Sigirci A, Aslan M, Sarac K. Delayed myelination in a rhizomelic chondrodysplasia punctata case: MR spectroscopy findings. Magn Reson Imaging. 2003;21:77–80. doi: 10.1016/s0730-725x(02)00625-2. [DOI] [PubMed] [Google Scholar]
- 210.Brites P, Motley AM, Gressens P, Mooyer PA, Ploegaert I, Everts V, Evrard P, Carmeliet P, Dewerchin M, Schoonjans L, Duran M, et al. Impaired neuronal migration and endochondral ossification in Pex7 knockout mice: a model for rhizomelic chondrodysplasia punctata. Hum Mol Genet. 2003;12:2255–2267. doi: 10.1093/hmg/ddg236. [DOI] [PubMed] [Google Scholar]
- 211.Rodemer C, Thai TP, Brugger B, Kaercher T, Werner H, Nave KA, Wieland F, Gorgas K, Just WW. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum Mol Genet. 2003;12:1881–1895. doi: 10.1093/hmg/ddg191. [DOI] [PubMed] [Google Scholar]
- 212.Liegel RP, Ronchetti A, Sidjanin DJ. Alkylglycerone phosphate synthase (AGPS) deficient mice: models for rhizomelic chondrodysplasia punctate type 3 (RCDP3) malformation syndrome. Mol Genet Metab Rep. 2014;1:299–311. doi: 10.1016/j.ymgmr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lodhi IJ, Yin L, Jensen-Urstad AP, Funai K, Coleman T, Baird JH, El Ramahi MK, Razani B, Song H, Fu-Hsu F, Turk J, et al. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell Metab. 2012;16:189–201. doi: 10.1016/j.cmet.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lodhi IJ, Wei X, Yin L, Feng C, Adak S, Abou-Ezzi G, Hsu F, Link DC, Semenkovich CF. Peroxisomal lipid synthesis regulates inflammation by sustaining neutrophil membrane phospholipid composition and viability. Cell Metab. 2015;21:51–64. doi: 10.1016/j.cmet.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Braverman N, Zhang R, Chen L, Nimmo G, Scheper S, Tran T, Chaudhury R, Moser A, Steinberg S. A Pex7 hypomorphic mouse model for plasmalogen deficiency affecting the lens and skeleton. Mol Genet Metab. 2010;99:408–416. doi: 10.1016/j.ymgme.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liegel R, Chang B, Dubielzig R, Sidjanin DJ. Blind sterile 2 (bs2), a hypomorphic mutation in Agps, results in cataracts and male sterility in mice. Mol Genet Metab. 2011;103:51–59. doi: 10.1016/j.ymgme.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gorgas K, Teigler A, Komljenovic D, Just WW. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim Biophys Acta. 2006;1763:1511–1526. doi: 10.1016/j.bbamcr.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 218.Teigler A, Komljenovic D, Draguhn A, Gorgas K, Just WW. Defects in myelination, paranode organization and Purkinje cell innervation in the ether lipid-deficient mouse cerebellum. Hum Mol Genet. 2009;18:1897–1908. doi: 10.1093/hmg/ddp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.da Silva TF, Eira J, Lopes AT, Malheiro AR, Sousa V, Luoma A, Avila RL, Wanders RJ, Just WW, Kirschner DA, Sousa MM, et al. Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J Clin Invest. 2014;124:2560–2570. doi: 10.1172/JCI72063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Brites P, Mooyer PA, El Mrabet L, Waterham HR, Wanders RJ. Plasmalogens participate in very-long-chain fatty acid-induced pathology. Brain. 2009;132:482–492. doi: 10.1093/brain/awn295. [DOI] [PubMed] [Google Scholar]
- 221.Ifuku M, Katafuchi T, Mawatari S, Noda M, Miake K, Sugiyama M, Fujino T. Anti-inflammatory/anti-amyloidogenic effects of plasmalogens in lipopolysaccharide-induced neuroinflammation in adult mice. J Neuroinflammation. 2012;9:197. doi: 10.1186/1742-2094-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Katafuchi T, Ifuku M, Mawatari S, Noda M, Miake K, Sugiyama M, Fujino T. Effects of plasmalogens on systemic lipopolysaccharide-induced glial activation and beta-amyloid accumulation in adult mice. Ann N Y Acad Sci. 2012;1262:85–92. doi: 10.1111/j.1749-6632.2012.06641.x. [DOI] [PubMed] [Google Scholar]
- 223.Yamashita S, Kanno S, Nakagawa K, Kinoshita M, Miyazawa T. Extrinsic plasmalogens suppress neuronal apoptosis in mouse neuroblastoma Neuro-2A cells: importance of plasmalogen molecular species. RSC Adv. 2015;5:61012–61020. [Google Scholar]
- 224.Hossain MS, Ifuku M, Take S, Kawamura J, Miake K, Katafuchi T. Plasmalogens rescue neuronal cell death through an activation of AKT and ERK survival signaling. PLoS One. 2013;8:e83508. doi: 10.1371/journal.pone.0083508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Krysko O, Bottelbergs A, Van Veldhoven P, Baes M. Combined deficiency of peroxisomal beta-oxidation and ether lipid synthesis in mice causes only minor cortical neuronal migration defects but severe hypotonia. Mol Genet Metab. 2010;100:71–76. doi: 10.1016/j.ymgme.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 226.De Munter S, Verheijden S, Regal L, Baes M. Peroxisomal Disorders: A Review on Cerebellar Pathologies. Brain Pathol. 2015;25:663–678. doi: 10.1111/bpa.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.da Silva TF, Sousa VF, Malheiro AR, Brites P. The importance of etherphospholipids: a view from the perspective of mouse models. Biochim Biophys Acta. 2012;1822:1501–1508. doi: 10.1016/j.bbadis.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 228.Kunze M, Neuberger G, Maurer-Stroh S, Ma J, Eck T, Braverman N, Schmid JA, Eisenhaber F, Berger J. Structural requirements for interaction of peroxisomal targeting signal 2 and its receptor PEX7. J Biol Chem. 2011;286:45048–45062. doi: 10.1074/jbc.M111.301853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Saab S, Buteau B, Leclere L, Bron AM, Creuzot-Garcher CP, Bretillon L, Acar N. Involvement of plasmalogens in post-natal retinal vascular development. PLoS One. 2014;9:e101076. doi: 10.1371/journal.pone.0101076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Saab S, Mazzocco J, Creuzot-Garcher CP, Bron AM, Bretillon L, Acar N. Plasmalogens in the retina: from occurrence in retinal cell membranes to potential involvement in pathophysiology of retinal diseases. Biochimie. 2014;107(Pt A):58–65. doi: 10.1016/j.biochi.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 231.Luoma AM, Kuo F, Cakici O, Crowther MN, Denninger AR, Avila RL, Brites P, Kirschner DA. Plasmalogen phospholipids protect internodal myelin from oxidative damage. Free Radic Biol Med. 2015;84:296–310. doi: 10.1016/j.freeradbiomed.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 232.Dorninger F, Brodde A, Braverman NE, Moser AB, Just WW, Forss-Petter S, Brugger B, Berger J. Homeostasis of phospholipids—the level of phosphatidylethanolamine tightly adapts to changes in ethanolamine plasmalogens. Biochim Biophys Acta. 2015;1851:117–128. doi: 10.1016/j.bbalip.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Glaser PE, Gross RW. Plasmenylethanolamine facilitates rapid membrane fusion: a stopped-flow kinetic investigation correlating the propensity of a major plasma membrane constituent to adopt an HII phase with its ability to promote membrane fusion. Biochemistry. 1994;33:5805–5812. doi: 10.1021/bi00185a019. [DOI] [PubMed] [Google Scholar]
- 234.Thai TP, Rodemer C, Jauch A, Hunziker A, Moser A, Gorgas K, Just WW. Impaired membrane traffic in defective ether lipid biosynthesis. Hum Mol Genet. 2001;10:127–136. doi: 10.1093/hmg/10.2.127. [DOI] [PubMed] [Google Scholar]
- 235.Hermetter A, Rainer B, Ivessa E, Kalb E, Loidl J, Roscher A, Paltauf F. Influence of plasmalogen deficiency on membrane fluidity of human skin fibroblasts: a fluorescence anisotropy study. Biochim Biophys Acta. 1989;978:151–157. doi: 10.1016/0005-2736(89)90510-5. [DOI] [PubMed] [Google Scholar]
- 236.Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 237.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 238.Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 239.Pollegioni L, Piubelli L, Sacchi S, Pilone MS, Molla G. Physiological functions of D-amino acid oxidases: from yeast to humans. Cell Mol Life Sci. 2007;64:1373–1394. doi: 10.1007/s00018-007-6558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Usuda N, Yokota S, Hashimoto T, Nagata T. Immunocytochemical localization of D-amino acid oxidase in the central clear matrix of rat kidney peroxisomes. J Histochem Cytochem. 1986;34:1709–1718. doi: 10.1177/34.12.2878022. [DOI] [PubMed] [Google Scholar]
- 241.Sacchi S, Caldinelli L, Cappelletti P, Pollegioni L, Molla G. Structure-function relationships in human D-amino acid oxidase. Amino Acids. 2012;43:1833–1850. doi: 10.1007/s00726-012-1345-4. [DOI] [PubMed] [Google Scholar]
- 242.Ghosh D, Berg JM. A proteome-wide perspective on peroxisome targeting signal 1(PTS1)-Pex5p affinities. J Am Chem Soc. 2010;132:3973–3979. doi: 10.1021/ja9109049. [DOI] [PubMed] [Google Scholar]
- 243.Van Veldhoven PP, Brees C, Mannaerts GP. D-aspartate oxidase, a peroxisomal enzyme in liver of rat and man. Biochim Biophys Acta. 1991;1073:203–208. doi: 10.1016/0304-4165(91)90203-s. [DOI] [PubMed] [Google Scholar]
- 244.Zaar K, Kost HP, Schad A, Volkl A, Baumgart E, Fahimi HD. Cellular and subcellular distribution of D-aspartate oxidase in human and rat brain. J Comp Neurol. 2002;450:272–282. doi: 10.1002/cne.10320. [DOI] [PubMed] [Google Scholar]
- 245.Sasabe J, Suzuki M, Imanishi N, Aiso S. Activity of D-amino acid oxidase is widespread in the human central nervous system. Front Synaptic Neurosci. 2014;6:14. doi: 10.3389/fnsyn.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Oliet SH, Mothet JP. Regulation of N-methyl-D-aspartate receptors by astrocytic D-serine. Neuroscience. 2009;158:275–283. doi: 10.1016/j.neuroscience.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 247.Oliet SH, Mothet JP. Molecular determinants of D-serine-mediated gliotransmission: from release to function. Glia. 2006;54:726–737. doi: 10.1002/glia.20356. [DOI] [PubMed] [Google Scholar]
- 248.Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci U S A. 2002;99:13675–13680. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Verrall L, Burnet PW, Betts JF, Harrison PJ. The neurobiology of D-amino acid oxidase and its involvement in schizophrenia. Mol Psychiatry. 2010;15:122–137. doi: 10.1038/mp.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R. Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr Res. 2008;101:76–83. doi: 10.1016/j.schres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 251.Verrall L, Walker M, Rawlings N, Benzel I, Kew JN, Harrison PJ, Burnet PW. D-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. Eur J Neurosci. 2007;26:1657–1669. doi: 10.1111/j.1460-9568.2007.05769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H, Agam G. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr Res. 2007;90:41–51. doi: 10.1016/j.schres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 253.Sacchi S, Rosini E, Pollegioni L, Molla G. D-amino acid oxidase inhibitors as a novel class of drugs for schizophrenia therapy. Curr Pharm Des. 2013;19:2499–2511. doi: 10.2174/1381612811319140002. [DOI] [PubMed] [Google Scholar]
- 254.Mitchell J, Paul P, Chen HJ, Morris A, Payling M, Falchi M, Habgood J, Panoutsou S, Winkler S, Tisato V, Hajitou A, et al. Familial amyotrophic lateral sclerosis is associated with a mutation in D-amino acid oxidase. Proc Natl Acad Sci U S A. 2010;107:7556–7561. doi: 10.1073/pnas.0914128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sasabe J, Miyoshi Y, Suzuki M, Mita M, Konno R, Matsuoka M, Hamase K, Aiso S. D-amino acid oxidase controls motoneuron degeneration through D-serine. Proc Natl Acad Sci U S A. 2012;109:627–632. doi: 10.1073/pnas.1114639109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yu TW, Chahrour MH, Coulter ME, Jiralerspong S, Okamura-Ikeda K, Ataman B, Schmitz-Abe K, Harmin DA, Adli M, Malik AN, D'Gama AM, et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013;77:259–273. doi: 10.1016/j.neuron.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ro M, Park J, Nam M, Bang HJ, Yang J, Choi KS, Kim SK, Chung JH, Kwack K. Association between peroxisomal biogenesis factor 7 and autism spectrum disorders in a Korean population. J Child Neurol. 2012;27:1270–1275. doi: 10.1177/0883073811435507. [DOI] [PubMed] [Google Scholar]
- 258.Wiest MM, German JB, Harvey DJ, Watkins SM, Hertz-Picciotto I. Plasma fatty acid profiles in autism: a case–control study. Prostaglandins Leukot Essent Fat Acids. 2009;80:221–227. doi: 10.1016/j.plefa.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 259.Bell JG, MacKinlay EE, Dick JR, MacDonald DJ, Boyle RM, Glen AC. Essential fatty acids and phospholipase A2 in autistic spectrum disorders. Prostaglandins Leukot Essent Fat Acids. 2004;71:201–204. doi: 10.1016/j.plefa.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 260.Moser HW. Genotype-phenotype correlations in disorders ofperoxisome biogenesis. Mol Genet Metab. 1999;68:316–327. doi: 10.1006/mgme.1999.2926. [DOI] [PubMed] [Google Scholar]
- 261.Guan ZZ, Wang YA, Cairns NJ, Lantos PL, Dallner G, Sindelar PJ. Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:740–747. doi: 10.1097/00005072-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 262.Han X, Holtzman DM, McKeel DW., Jr Plasmalogen deficiency in early Alzheimer's disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem. 2001;77:1168–1180. doi: 10.1046/j.1471-4159.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 263.Kou J, Kovacs GG, Hoftberger R, Kulik W, Brodde A, Forss-Petter S, Honigschnabl S, Gleiss A, Brugger B, Wanders R, Just W, et al. Peroxisomal alterations in Alzheimer's disease. Acta Neuropathol. 2011;122:271–283. doi: 10.1007/s00401-011-0836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wood PL, Barnette BL, Kaye JA, Quinn JF, Woltjer RL. Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer's disease subjects. Acta Neuropsychiatr. 2015;27:270–278. doi: 10.1017/neu.2015.18. [DOI] [PubMed] [Google Scholar]
- 265.Igarashi M, Ma K, Gao F, Kim HW, Rapoport SI, Rao JS. Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer's disease prefrontal cortex. J Alzheimers Dis. 2011;24:507–517. doi: 10.3233/JAD-2011-101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Grimm MO, Kuchenbecker J, Rothhaar TL, Grosgen S, Hundsdorfer B, Burg VK, Friess P, Muller U, Grimm HS, Riemenschneider M, Hartmann T. Plasmalogen synthesis is regulated via alkyl-dihydroxyacetonephosphate-synthase by amyloid precursor protein processing and is affected in Alzheimer's disease. J Neurochem. 2011;116:916–925. doi: 10.1111/j.1471-4159.2010.07070.x. [DOI] [PubMed] [Google Scholar]
- 267.Pettegrew JW, Panchalingam K, Hamilton RL, McClure RJ. Brain membrane phospholipid alterations in Alzheimer's disease. Neurochem Res. 2001;26:771–782. doi: 10.1023/a:1011603916962. [DOI] [PubMed] [Google Scholar]
- 268.Han X. Lipid alterations in the earliest clinically recognizable stage of Alzheimer's disease: implication of the role of lipids in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:65–77. doi: 10.2174/1567205052772786. [DOI] [PubMed] [Google Scholar]
- 269.Rouser G, Yamamoto A. Curvilinear regression course of human brain lipid composition changes with age. Lipids. 1968;3:284–287. doi: 10.1007/BF02531202. [DOI] [PubMed] [Google Scholar]
- 270.Ginsberg L, Rafique S, Xuereb JH, Rapoport SI, Gershfeld NL. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer's disease brain. Brain Res. 1995;698:223–226. doi: 10.1016/0006-8993(95)00931-f. [DOI] [PubMed] [Google Scholar]
- 271.Goodenowe DB, Cook LL, Liu J, Lu Y, Jayasinghe DA, Ahiahonu PW, Heath D, Yamazaki Y, Flax J, Krenitsky KF, Sparks DL, et al. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer’s disease and dementia. J Lipid Res. 2007;48:2485–2498. doi: 10.1194/jlr.P700023-JLR200. [DOI] [PubMed] [Google Scholar]
- 272.Wood PL, Mankidy R, Ritchie S, Heath D, Wood JA, Flax J, Goodenowe DB. Circulating plasmalogen levels and Alzheimer Disease Assessment Scale-Cognitive scores in Alzheimer patients. J Psychiatry Neurosci. 2010;35:59–62. doi: 10.1503/jpn.090059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Farooqui AA. Studies on plasmalogen-selective phospholipase A2 in brain. Mol Neurobiol. 2010;41:267–273. doi: 10.1007/s12035-009-8091-y. [DOI] [PubMed] [Google Scholar]
- 274.Bennett SA, Valenzuela N, Xu H, Franko B, Fai S, Figeys D. Using neurolipidomics to identify phospholipid mediators of synaptic (dys)function in Alzheimer’s Disease. Front Physiol. 2013;4:168. doi: 10.3389/fphys.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zarrouk A, Riedinger JM, Ahmed SH, Hammami S, Chaabane W, Debbabi M, Ben Ammou S, Rouaud O, Frih M, Lizard G, Hammami M. Fatty acid profiles in demented patients: identification of hexacosanoic acid (c26:0) as a blood lipid biomarker of dementia. J Alzheimers Dis. 2015;44:1349–1359. doi: 10.3233/JAD-142046. [DOI] [PubMed] [Google Scholar]
- 276.Astarita G, Jung KM, Berchtold NC, Nguyen VQ, Gillen DL, Head E, Cotman CW, Piomelli D. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer’s disease. PLoS One. 2010;5:e12538. doi: 10.1371/journal.pone.0012538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Marcus DL, Thomas C, Rodriguez C, Simberkoff K, Tsai JS, Strafaci JA, Freedman ML. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp Neurol. 1998;150:40–44. doi: 10.1006/exnr.1997.6750. [DOI] [PubMed] [Google Scholar]
- 278.Cimini A, Moreno S, D’Amelio M, Cristiano L, D’Angelo B, Falone S, Benedetti E, Carrara P, Fanelli F, Cecconi F, Amicarelli F, et al. Early biochemical and morphological modifications in the brain of a transgenic mouse model of Alzheimer’s disease: a role for peroxisomes. J Alzheimers Dis. 2009;18:935–952. doi: 10.3233/JAD-2009-1199. [DOI] [PubMed] [Google Scholar]
- 279.Fanelli F, Sepe S, M DA, Bernardi C, Cristiano L, Cimini A, Cecconi F, Ceru MP, Moreno S. Age-dependent roles of peroxisomes in the hippocampus of a transgenic mouse model of Alzheimer’s disease. Mol Neurodegener. 2013;8:8. doi: 10.1186/1750-1326-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Santos MJ, Quintanilla RA, Toro AS, Grandy R, Dinamarca MC, Godoy JA, Inestrosa NC. Peroxisomal proliferation protects from beta-amyloid neurodegeneration. J Biol Chem. 2005;280:41057–41068. doi: 10.1074/jbc.M505160200. [DOI] [PubMed] [Google Scholar]
- 281.Cimini A, Benedetti E, D’Angelo B, Cristiano L, Falone S, Di Loreto S, Amicarelli F, Ceru MP. Neuronal response of peroxisomal and peroxisome-related proteins to chronic and acute Abeta injury. Curr Alzheimer Res. 2009;6:238–251. doi: 10.2174/156720509788486518. [DOI] [PubMed] [Google Scholar]
- 282.Shi R, Zhang Y, Shi Y, Shi S, Jiang L. Inhibition of peroxisomal beta-oxidation by thioridazine increases the amount of VLCFAs and Abeta generation in the rat brain. Neurosci Lett. 2012;528:6–10. doi: 10.1016/j.neulet.2012.08.086. [DOI] [PubMed] [Google Scholar]
- 283.Rothhaar TL, Grosgen S, Haupenthal VJ, Burg VK, Hundsdorfer B, Mett J, Riemenschneider M, Grimm HS, Hartmann T, Grimm MO. Plasmalogens inhibit APP processing by directly affecting gamma-secretase activity in Alzheimer’s disease. ScientificWorldJournal. 2012;2012:141240. doi: 10.1100/2012/141240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Han X. Multi-dimensional mass spectrometry-based shotgun lipidomics and the altered lipids at the mild cognitive impairment stage of Alzheimer’s disease. Biochim Biophys Acta. 2010;1801:774–783. doi: 10.1016/j.bbalip.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lizard G, Rouaud O, Demarquoy J, Cherkaoui-Malki M, Iuliano L. Potential roles of peroxisomes in Alzheimer’s disease and in dementia of the Alzheimer’s type. J Alzheimers Dis. 2012;29:241–254. doi: 10.3233/JAD-2011-111163. [DOI] [PubMed] [Google Scholar]
- 286.Mirsky IA, Broh-Kahn RH. The inactivation of insulin by tissue extracts; the distribution and properties of insulin inactivating extracts. Arch Biochem Biophys. 1949;20:1–9. [PubMed] [Google Scholar]
- 287.Fernandez-Gamba A, Leal MC, Morelli L, Castano EM. Insulin-degrading enzyme: structure–function relationship and its possible roles in health and disease. Curr Pharm Des. 2009;15:3644–3655. doi: 10.2174/138161209789271799. [DOI] [PubMed] [Google Scholar]
- 288.Authier F, Bergeron JJ, Ou WJ, Rachubinski RA, Posner BI, Walton PA. Degradation of the cleaved leader peptide of thiolase by a peroxisomal proteinase. Proc Natl Acad Sci U S A. 1995;92:3859–3863. doi: 10.1073/pnas.92.9.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chesneau V, Perlman RK, Li W, Keller GA, Rosner MR. Insulin-degrading enzyme does not require peroxisomal localization for insulin degradation. Endocrinology. 1997;138:3444–3451. doi: 10.1210/endo.138.8.5344. [DOI] [PubMed] [Google Scholar]
- 290.Morita M, Kurochkin IV, Motojima K, Goto S, Takano T, Okamura S, Sato R, Yokota S, Imanaka T. Insulin-degrading enzyme exists inside of rat liver peroxisomes and degrades oxidized proteins. Cell Struct Funct. 2000;25:309–315. doi: 10.1247/csf.25.309. [DOI] [PubMed] [Google Scholar]
- 291.Kuo WL, Gehm BD, Rosner MR, Li W, Keller G. Inducible expression and cellular localization of insulin-degrading enzyme in a stably transfected cell line. J Biol Chem. 1994;269:22599–22606. [PubMed] [Google Scholar]
- 292.Leissring MA, Farris W, Wu X, Christodoulou DC, Haigis MC, Guarente L, Selkoe DJ. Alternative translation initiation generates a novel isoform of insulindegrading enzyme targeted to mitochondria. Biochem J. 2004;383:439–446. doi: 10.1042/BJ20041081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Udrisar DP, Wanderley MI, Porto RC, Cardoso CL, Barbosa MC, Camberos MC, Cresto JC. Androgen- and estrogen-dependent regulation of insulin-degrading enzyme in subcellular fractions of rat prostate and uterus. Exp Biol Med (Maywood) 2005;230:479–486. doi: 10.1177/153537020523000706. [DOI] [PubMed] [Google Scholar]
- 294.Bulloj A, Leal MC, Surace EI, Zhang X, Xu H, Ledesma MD, Castano EM, Morelli L. Detergent resistant membrane-associated IDE in brain tissue and cultured cells: relevance to Abeta and insulin degradation. Mol Neurodegener. 2008;3:22. doi: 10.1186/1750-1326-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V, Rosner MR, Selkoe DJ. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J Neurosci. 2000;20:1657–1665. doi: 10.1523/JNEUROSCI.20-05-01657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Malito E, Hulse RE, Tang WJ. Amyloid beta-degrading cryptidases: insulin degrading enzyme, presequence peptidase, and neprilysin. Cell Mol Life Sci. 2008;65:2574–2585. doi: 10.1007/s00018-008-8112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Bertram L, Blacker D, Mullin K, Keeney D, Jones J, Basu S, Yhu S, McInnis MG, Go RC, Vekrellis K, Selkoe DJ, et al. Evidence for genetic linkage of Alzheimer’s disease to chromosome 10q. Science. 2000;290:2302–2303. doi: 10.1126/science.290.5500.2302. [DOI] [PubMed] [Google Scholar]
- 298.Zhang Y, Wang B, Wan H, Zhou Q, Li T. Meta-analysis of the insulin degrading enzyme polymorphisms and susceptibility to Alzheimer’s disease. Neurosci Lett. 2013;541:132–137. doi: 10.1016/j.neulet.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 299.Chouraki V, Seshadri S. Genetics of Alzheimer’s disease. Adv Genet. 2014;87:245–294. doi: 10.1016/B978-0-12-800149-3.00005-6. [DOI] [PubMed] [Google Scholar]
- 300.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- 303.Dragonas C, Bertsch T, Sieber CC, Brosche T. Plasmalogens as a marker of elevated systemic oxidative stress in Parkinson’s disease. Clin Chem Lab Med. 2009;47:894–897. doi: 10.1515/CCLM.2009.205. [DOI] [PubMed] [Google Scholar]
- 304.Kaddurah-Daouk R, McEvoy J, Baillie R, Zhu H, J KY, Nimgaonkar VL, Buckley PF, Keshavan MS, Georgiades A, Nasrallah HA. Impaired plasmalogens in patients with schizophrenia. Psychiatry Res. 2012;198:347–352. doi: 10.1016/j.psychres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 305.Wood PL, Unfried G, Whitehead W, Phillipps A, Wood JA. Dysfunctional plasmalogen dynamics in the plasma and platelets of patients with schizophrenia. Schizophr Res. 2015;161:506–510. doi: 10.1016/j.schres.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 306.Murphy EJ, Schapiro MB, Rapoport SI, Shetty HU. Phospholipid composition and levels are altered in Down syndrome brain. Brain Res. 2000;867:9–18. doi: 10.1016/s0006-8993(00)02205-8. [DOI] [PubMed] [Google Scholar]
- 307.Bueno AA, Brand A, Neville MM, Lehane C, Brierley N, Crawford MA. Erythrocyte phospholipid molecular species and fatty acids of Down syndrome children compared with non-affected siblings. Br J Nutr. 2014;113:72–81. doi: 10.1017/S0007114514003298. [DOI] [PubMed] [Google Scholar]
- 308.Wood PL, Smith T, Pelzer L, Goodenowe DB. Targeted metabolomic analyses of cellular models of Pelizaeus–Merzbacher disease reveal plasmalogen and myoinositol solute carrier dysfunction. Lipids Health Dis. 2011;10:102. doi: 10.1186/1476-511X-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Moraitou M, Dimitriou E, Dekker N, Monopolis I, Aerts J, Michelakakis H. Gaucher disease: plasmalogen levels in relation to primary lipid abnormalities and oxidative stress. Blood Cells Mol Dis. 2014;53:30–33. doi: 10.1016/j.bcmd.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 310.Kohlschutter A, Schade B, Blomer B, Hubner C. Low erythrocyte plasmalogen and plasma docosahexaenoic acid (DHA) in juvenile neuronal ceroid-lipofuscinosis (JNCL) J Inherit Metab Dis. 1993;16:299–304. doi: 10.1007/BF00710270. [DOI] [PubMed] [Google Scholar]
- 311.Cunnane SC, Schneider JA, Tangney C, Tremblay-Mercier J, Fortier M, Bennett DA, Morris MC. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2012;29:691–697. doi: 10.3233/JAD-2012-110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Yakunin E, Moser A, Loeb V, Saada A, Faust P, Crane DI, Baes M, Sharon R. alpha-Synuclein abnormalities in mouse models of peroxisome biogenesis disorders. J Neurosci Res. 2010;88:866–876. doi: 10.1002/jnr.22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Singh I, Paintlia AS, Khan M, Stanislaus R, Paintlia MK, Haq E, Singh AK, Contreras MA. Impaired peroxisomal function in the central nervous system with inflammatory disease of experimental autoimmune encephalomyelitis animals and protection by lovastatin treatment. Brain Res. 2004;1022:1–11. doi: 10.1016/j.brainres.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 314.Gray E, Rice C, Hares K, Redondo J, Kemp K, Williams M, Brown A, Scolding N, Wilkins A. Reductions in neuronal peroxisomes in multiple sclerosis grey matter. Mult Scler. 2014;20:651–659. doi: 10.1177/1352458513505691. [DOI] [PubMed] [Google Scholar]
- 315.Senanayake VK, Jin W, Mochizuki A, Chitou B, Goodenowe DB. Metabolic dysfunctions in multiple sclerosis: implications as to causation, early detection, and treatment, a case control study. BMC Neurol. 2015;15:154. doi: 10.1186/s12883-015-0411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Reuber BE, Germain-Lee E, Collins CS, Morrell JC, Ameritunga R, Moser HW, Valle D, Gould SJ. Mutations in PEX1 are the most common cause of peroxisome biogenesis disorders. Nat Genet. 1997;17:445–448. doi: 10.1038/ng1297-445. [DOI] [PubMed] [Google Scholar]
- 317.Shimozawa N, Imamura A, Zhang Z, Suzuki Y, Orii T, Tsukamoto T, Osumi T, Fujiki Y, Wanders RJ, Besley G, Kondo N. Defective PEX gene products correlate with the protein import, biochemical abnormalities, and phenotypic heterogeneity in peroxisome biogenesis disorders. J Med Genet. 1999;36:779–781. doi: 10.1136/jmg.36.10.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Muntau AC, Mayerhofer PU, Paton BC, Kammerer S, Roscher AA. Defective peroxisome membrane synthesis due to mutations in human PEX3 causes Zellweger syndrome, complementation group G. Am J Hum Genet. 2000;67:967–975. doi: 10.1086/303071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Dodt G, Braverman N, Wong C, Moser A, Moser HW, Watkins P, Valle D, Gould SJ. Mutations in the PTS1 receptor gene, PXR1, define complementation group 2 of the peroxisome biogenesis disorders. Nat Genet. 1995;9:115–125. doi: 10.1038/ng0295-115. [DOI] [PubMed] [Google Scholar]
- 320.Yahraus T, Braverman N, Dodt G, Kalish JE, Morrell JC, Moser HW, Valle D, Gould SJ. The peroxisome biogenesis disorder group 4 gene, PXAAA1, encodes a cytoplasmic ATPase required for stability of the PTS1 receptor. EMBO J. 1996;15:2914–2923. [PMC free article] [PubMed] [Google Scholar]
- 321.Matsumoto N, Tamura S, Moser A, Moser HW, Braverman N, Suzuki Y, Shimozawa N, Kondo N, Fujiki Y. The peroxin Pex6p gene is impaired in peroxisomal biogenesis disorders of complementation group 6. J Hum Genet. 2001;46:273–277. doi: 10.1007/s100380170078. [DOI] [PubMed] [Google Scholar]
- 322.Warren DS, Morrell JC, Moser HW, Valle D, Gould SJ. Identification of PEX10, the gene defective in complementation group 7 of the peroxisome-biogenesis disorders. Am J Hum Genet. 1998;63:347–359. doi: 10.1086/301963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Chang CC, Lee WH, Moser H, Valle D, Gould SJ. Isolation of the human PEX12 gene, mutated in group 3 of the peroxisome biogenesis disorders. Nat Genet. 1997;15:385–388. doi: 10.1038/ng0497-385. [DOI] [PubMed] [Google Scholar]
- 324.Gootjes J, Skovby F, Christensen E, Wanders RJ, Ferdinandusse S. Reinvestigation of trihydroxycholestanoic acidemia reveals a peroxisome biogenesis disorder. Neurology. 2004;62:2077–2081. doi: 10.1212/01.wnl.0000127576.26352.d1. [DOI] [PubMed] [Google Scholar]
- 325.Shimozawa N, Suzuki Y, Zhang Z, Imamura A, Toyama R, Mukai S, Fujiki Y, Tsukamoto T, Osumi T, Orii T, Wanders RJ, et al. Nonsense and temperature-sensitive mutations in PEX13 are the cause of complementation group H of peroxisome biogenesis disorders. Hum Mol Genet. 1999;8:1077–1083. doi: 10.1093/hmg/8.6.1077. [DOI] [PubMed] [Google Scholar]
- 326.Liu Y, Bjorkman J, Urquhart A, Wanders RJ, Crane DI, Gould SJ. PEX13 is mutated in complementation group 13 of the peroxisome-biogenesis disorders. Am J Hum Genet. 1999;65:621–634. doi: 10.1086/302534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Shimozawa N, Tsukamoto T, Nagase T, Takemoto Y, Koyama N, Suzuki Y, Komori M, Osumi T, Jeannette G, Wanders RJ, Kondo N. Identification of a new complementation group of the peroxisome biogenesis disorders and PEX14 as the mutated gene. Hum Mutat. 2004;23:552–558. doi: 10.1002/humu.20032. [DOI] [PubMed] [Google Scholar]
- 328.Honsho M, Tamura S, Shimozawa N, Suzuki Y, Kondo N, Fujiki Y. Mutation in PEX16 is causal in the peroxisome-deficient Zellweger syndrome of complementation group D. Am J Hum Genet. 1998;63:1622–1630. doi: 10.1086/302161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Matsuzono Y, Kinoshita N, Tamura S, Shimozawa N, Hamasaki M, Ghaedi K, Wanders RJ, Suzuki Y, Kondo N, Fujiki Y. Human PEX19: cDNA cloning by functional complementation, mutation analysis in a patient with Zellweger syndrome, and potential role in peroxisomal membrane assembly. Proc Natl Acad Sci U S A. 1999;96:2116–2121. doi: 10.1073/pnas.96.5.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Matsumoto N, Tamura S, Furuki S, Miyata N, Moser A, Shimozawa N, Moser HW, Suzuki Y, Kondo N, Fujiki Y. Mutations in novel peroxin gene PEX26 that cause peroxisome-biogenesis disorders of complementation group 8 provide a genotype-phenotype correlation. Am J Hum Genet. 2003;73:233–246. doi: 10.1086/377004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Ebberink MS, Koster J, Visser G, Spronsen F, Stolte-Dijkstra I, Smit GP, Fock JM, Kemp S, Wanders RJ, Waterham HR. A novel defect of peroxisome division due to a homozygous non-sense mutation in the PEX11beta gene. J Med Genet. 2012;49:307–313. doi: 10.1136/jmedgenet-2012-100778. [DOI] [PubMed] [Google Scholar]
- 332.Thoms S, Gartner J. First PEX11beta patient extends spectrum of peroxisomal biogenesis disorder phenotypes. J Med Genet. 2012;49:314–316. doi: 10.1136/jmedgenet-2012-100899. [DOI] [PubMed] [Google Scholar]
- 333.Poll-The BT, Roels F, Ogier H, Scotto J, Vamecq J, Schutgens RB, Wanders RJ, van Roermund CW, van Wijland MJ, Schram AW, et al. A new peroxisomal disorder with enlarged peroxisomes and a specific deficiency of acyl-CoA oxidase (pseudo-neonatal adrenoleukodystrophy) Am J Hum Genet. 1988;42:422–434. [PMC free article] [PubMed] [Google Scholar]
- 334.Watkins PA, Chen WW, Harris CJ, Hoefler G, Hoefler S, Blake DC, Jr, Balfe A, Kelley RI, Moser AB, Beard ME, et al. Peroxisomal bifunctional enzyme deficiency. J Clin Invest. 1989;83:771–777. doi: 10.1172/JCI113956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ferdinandusse S, Jimenez-Sanchez G, Koster J, Denis S, Van Roermund CW, Silva-Zolezzi I, Moser AB, Visser WF, Gulluoglu M, Durmaz O, Demirkol M, et al. A novel bile acid biosynthesis defect due to a deficiency of peroxisomal ABCD3. Hum Mol Genet. 2015;24:361–370. doi: 10.1093/hmg/ddu448. [DOI] [PubMed] [Google Scholar]
- 336.Mihalik SJ, Morrell JC, Kim D, Sacksteder KA, Watkins PA, Gould SJ. Identification of PAHX, a Refsum disease gene. Nat Genet. 1997;17:185–189. doi: 10.1038/ng1097-185. [DOI] [PubMed] [Google Scholar]
- 337.Carlton VE, Harris BZ, Puffenberger EG, Batta AK, Knisely AS, Robinson DL, Strauss KA, Shneider BL, Lim WA, Salen G, Morton DH, et al. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genet. 2003;34:91–96. doi: 10.1038/ng1147. [DOI] [PubMed] [Google Scholar]
- 338.Danpure CJ, Jennings PR. Peroxisomal alanine:glyoxylate aminotransferase deficiency in primary hyperoxaluria type I. FEBS Lett. 1986;201:20–24. doi: 10.1016/0014-5793(86)80563-4. [DOI] [PubMed] [Google Scholar]
- 339.Ogata M. Acatalasemia. Hum Genet. 1991;86:331–340. doi: 10.1007/BF00201829. [DOI] [PubMed] [Google Scholar]
- 340.De Laurenzi V, Rogers GR, Hamrock DJ, Marekov LN, Steinert PM, Compton JG, Markova N, Rizzo WB. Sjogren–Larsson syndrome is caused by mutations in the fatty aldehyde dehydrogenase gene. Nat Genet. 1996;12:52–57. doi: 10.1038/ng0196-52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.