Abstract
Memory consolidation underpins adaptive behavior and dopaminergic networks may be critical for prolonged, selective information storage. To understand the time course of the dopaminergic contribution to memory consolidation in humans, here we investigate the effect of dopaminergic medication on recall and recognition in the short and longer term in Parkinson disease (PD). Fifteen people with PD were each tested on or off dopaminergic medication during learning/early consolidation (Day 1) and/or late consolidation (Day 2). Fifteen age-matched healthy participants were tested only once. On Day 1 participants learned new information, and early episodic memory was tested after 30 min. Then on Day 2, recall and recognition were retested after a 24-hr delay. Participants on medication on Day 1 recalled less information at 30 min and 24 hr. In contrast, patients on medication on Day 2 (8–24 hr after learning) recalled more information at 24 hr than those off medication. Although recognition sensitivity was unaffected by medication, response bias was dependent on dopaminergic state: Medication during learning induced a more liberal bias 24 hr later, whereas patients off medication during learning were more conservative responders 24 hr later. We use computational modeling to propose possible mechanisms for this change in response bias. In summary, dopaminergic medication in PD patients during learning impairs early consolidation of episodic memory and makes delayed responses more liberal, but enhances late memory consolidation presumably through a dopamine-dependent consolidation pathway that may be active during sleep.
Introduction
Traditionally, memory impairment in neurodegenerative disease has often been considered to be explained by loss of cholinergic neurons (e.g., Bartus, Dean, Beer, & Lippa, 1982). The role of dopamine in memory is more controversial as are the nature and degree of memory impairments in patients with dopaminergic loss such as those with Parkinson disease (PD; Foerde, Braun, & Shohamy, 2013; Foerde & Shohamy, 2011; Shohamy, Myers, Geghman, Sage, & Gluck, 2006; Cools, Barker, Sahakian, & Robbins, 2001). Conventional accounts of memory posit three main stages: encoding (initial learning of the information), consolidation (maintenance of the stored memory over a period of minutes, hours, days, or years), and retrieval (accessing this memory). Dopaminergic effects have been reported across these stages.
Dopamine antagonist infusion during or immediately after encoding worsens delayed recall in animals (Bethus, Tse, & Morris, 2010; O’Carroll, Martin, Sandin, Frenguelli, & Morris, 2006), suggesting a benefit of dopaminergic activity on encoding and perhaps early consolidation. This is also supported by a recent observation that optogenetic stimulation of dopaminergic neurons during learning enhances memory retention (McNamara, Tejero-Cantero, Trouche, Campo-Urriza, & Dupret, 2014). Importantly this recent study found that stimulating the dopaminergic projections from the ventral tegmental area (VTA) to the hippocampal CA1 subfield during learning did not speed up learning but did increase memory at a 1-hr delayed test, as well as increasing the reactivation of memory traces during sleep after learning. The authors suggested that dopaminergic input to the CA1 increases the reactivation of newly formed neuronal assemblies to allow for consolidation of the memories they encode.
Dopamine appears to improve memory consolidation in animals, but only at certain time points after initial learning. A dopamine D1/D5 agonist infused into the CA1 in the hippocampus of rats before inhibitory avoidance learning had no effect when tested 24 hr later, nor when it was infused 9 hr after learning (Bernabeu et al., 1997). However, when it was infused 3 or 6 hr after learning, it improved (increased) the step-down latencies of the rats significantly. A dopamine D1/D5 antagonist had the opposite effect, decreasing the latency only when infused 3 or 6 hr after learning. Adenylyl cyclase activator infusion 3 or 6 hr after learning also increased memory, and a PKA inhibitor decreased it, suggesting that this dopamine-dependent consolidation effect is mediated by the cAMP/PKA pathway in the hippocampus. Further insights into the molecular mechanisms come from Rossato and colleagues, who demonstrated that NMDAR antagonist infusion to the VTA 12 hr after learning impaired memory 14 days later, and this was reversed by dopamine agonist infusion to the CA1 (Rossato, Bevilaqua, Izquierdo, Medina, & Cammarota, 2009). The authors propose that NMDAR activation in the VTA 12 hr after learning excites those cells and causes greater dopaminergic activity projected to the hippocampus, which causes dopamine-dependent consolidation.
Very recent work demonstrated that a D2 inverse agonist (similar to an antagonist, but it decreases the receptor activity below baseline activity) injected postlearning decreased novelty preference 24 hr later (França et al., 2015). Importantly, a putative mechanism for such an effect stems from the findings that the decrease in memory was accompanied by a decrease in CaMKII from 3 to 12 hr after learning, Zif-268 6 hr after learning, and brain-derived neurotrophic factor (BDNF) 12 hr after learning, along with decreased REM sleep. These proteins are all activated at different times after learning, are involved in plasticity and consolidation, and are affected by dopamine just after the time of learning, as well as at their time of activation (Rossato et al., 2009). This suggests that dopamine affects plasticity protein levels, possibly via REM sleep. Perhaps not surprisingly given the known dose dependence of dopamine responses and complexity of dopaminergic networks, somewhat conflicting results have emerged when different experimental paradigms are used (e.g., Furini, Myskiw, Schmidt, Marcondes, & Izquierdo, 2014; Péczely et al., 2014; Rossato et al., 2009, 2013). However, the overall picture is that dopamine-dependent networks have a time-critical role in animal memory consolidation that may to some extent depend on a period of sleep.
One theory of consolidation that incorporates a role of dopamine is the synaptic tagging and capture theory (Clopath, 2012; Redondo & Morris, 2011; Clopath, Ziegler, Vasilaki, Büsing, & Gerstner, 2008; Sajikumar & Frey, 2004; Frey & Morris, 1997, 1998). This theory states that an input to a synapse can cause early synaptic plasticity and can also “tag” the synapse so that when plasticity-related products are synthesized they can be captured by the tagged synapse and used to stabilize the early plasticity changes to allow consolidation to take place. In the computational model of this theory (Clopath et al., 2008), the threshold for production of the plasticity-related products is set by tonic dopamine levels. As this theory states that the tags only last a few hours, which could account for some of the findings mentioned above, it cannot easily account for the consolidation effects of dopamine at later time points found in other studies, without extensions made to the model.
Although these models of memory consolidation have been developed based on animal experimentation, the focus of our work is the contribution of dopamine to human memory. The global application of dopaminergic drugs in humans limits the conclusions that can be drawn and poses a challenge when designing studies in people. However, it is critical to establish how dopamine influences human memory, particularly given that memory loss is such a prominent problem affecting our increasingly elderly population.
Older adults given levodopa, a dopamine precursor, before learning showed no benefits on memory after 2 hr, but did show dose-dependent improvement in scene recognition 6 hr after learning (Chowdhury, Guitart-Masip, Bunzeck, Dolan, & Düzel, 2012). Such an inverted U-shaped correlation between dose and performance perhaps implies that low concentrations were insufficient to have an impact, whereas high concentrations “overdosed” the brain (Cools, 2006; Cools et al., 2001). The delayed beneficial effects of levodopa suggest dopamine might be important for human late memory consolidation, but effects of dopamine over longer timescales in humans have not been investigated.
Genetic studies suggest that increased levels of dopaminergic activity improve memory (Wittmann, Tan, Lisman, Dolan, & Düzel, 2013; De Frias et al., 2004). However, work in humans has not normally fully dissociated encoding, consolidation, and retrieval, and results have conflicted with some studies finding that dopamine replacement medications given before learning improve memory (Chowdhury et al., 2012; Coulthard et al., 2012) whereas another found that they impaired encoding (Macdonald et al., 2013). Thus, it is not clear exactly how dopamine contributes to the earliest stages of memory processing and whether human dopaminergic contributions to memory mirror those observed in animals.
We designed a paradigm to differentiate the effects of dopaminergic activity on encoding, consolidation and retrieval in PD patients. We aimed to see whether exogenous dopamine present during encoding/early consolidation or late consolidation would enhance 24-hr delayed recall and recognition memory in people with PD. Importantly, we were able to distinguish between the stages of memory by probing (i) initial learning, (ii) early consolidation and retrieval (at 30 min), and (iii) late consolidation and retrieval at 24 hr (after overnight consolidation) separately. By withdrawing patients from their dopaminergic medications before learning on Day 1 and/or testing on Day 2, we could see the effects of dopaminergic activity on each component process within the memory system.
Methods
Participants
Fifteen patients with PD and 15 age-matched healthy participants were tested (see Table 1 for details). Patients were identified through movement disorder and general neurology clinics in North Bristol NHS Trust, and all had a clinical diagnosis of PD and no other neurological diagnoses. The PD patients were all on dopaminergic medication (levodopa and/or dopamine agonists) and were not taking cholinesterase inhibitors, monoamine oxidase inhibitors, or anti-psychotic medications or treated with deep brain stimulation or other functional neurosurgery. Aside from Parkinsonian medications, two patients were taking medications for cholesterol (simvastatin), and one of these was also on amlodipine and clopidogrel, which are antihypertensive and antiplatelet medications. One was taking alfuzosin for enlarged prostate, one an SSRI (sertraline) and calcium supplements, one quinine sulfate (for nocturnal leg cramps), and one latanoprast and dorzolamide for glaucoma and salazopyrin for arthritis. Levodopa dose equivalency was calculated to get a measure of total daily dopaminergic medication taken (Tomlinson et al., 2010).
Table 1. Demographics and Questionnaires Scores for Patients and Healthy Participants.
| Group | Number of Missing Patients/HPP | PD Patients | Healthy Participants | One-way ANOVA: Patients vs. Healthy Participants (df, F, p) |
|---|---|---|---|---|
| Number | 15 | 15 | ||
| Age | 71.53 (2.40) | 71.00 (2.63) | (1, 28), 0.076, .785 | |
| Years since diagnosis | 5.20 (1.38) | |||
| Number on levodopa/dopamine agonists/both | 9/1/5 | |||
| Levodopa dose equivalency (mg/day) | 603.00 (71.64) | |||
| MMSE | 27.00 (0.31) | 27.90 (0.34) | (1, 28), 5.000, .033 | |
| UPDRS on meds | 19.90 (3.17) | |||
| UPDRS off meds | 24.90 (3.86) | |||
| DASS | 1/0 | 22.50 (2.57) | 10.30 (3.17) | (1, 27), 10.943, .003 |
| (Depression/ | 6.53 (0.84) | 2.94 (0.82) | (1, 27), 16.079, <.001 | |
| Anxiety/ | 8.18 (1.28) | 3.176 (0.83) | (1, 27), 14.312, .001 | |
| Stress) | 6.41 (0.96) | 5.06 (0.98) | (1, 27), 2.640, .116 | |
| BIS | 1/0 | 63.40 (1.68) | 62.10 (1.37) | (1, 27), 0.736, .398 |
| REI (Rationality/ | 0/4 | 3.33 (0.15) | 3.67 (0.21) | (1, 24), 1.828, .189 |
| Experientiality) | 2.89 (0.12) | 2.91 (0.15) | (1, 24), 0.10, .920 | |
| LARS | −19 (1.56) | −26 (1.32) | (1, 28), 13.094, .001 |
Means with SEM in parentheses unless stated otherwise. MMSE = Mini Mental State Exam; UPDRS = Unified Parkinson Disease Rating Scale; DASS = Depression, Anxiety and Stress Scale; BIS = Barratt Impulsivity Scale; REI = Rational–Experiential Inventory; LARS = Lille Apathy Rating Scale.
Healthy participants were either the spouses of the PD patients who accompanied them to the session or were recruited from the BRACE Centre’s Healthy Volunteer database. Healthy participants taking any dopaminergic or noradrenergic medications were excluded from the study. None had any neurological diagnoses or reported memory problems. Of the healthy participants, one was taking simvastatin and aspirin (cholesterol and antiplatelet medication), one an ACE inhibitor (ramipril), and one fluoxetine (SSRI).
Ethics approval was granted by the North Bristol NHS Trust Research Ethics Committee, and all participants gave written consent in accordance with the Declaration of Helsinki.
Task
The Hopkins Verbal Learning Task-Revised (HVLT-R) is an episodic memory test with immediate and 30-min delayed recall and recognition components (www4.parinc.com/Products/Product.aspx?ProductID=HVLT-R). The experimenter reads aloud 12 words at a rate of one per second, after which the participant recalls as many words as they can in any order. These words are drawn from three semantic categories (four words from each) such as mammals, fuels, and tools. The list is read out twice more in the same order, each time followed by immediate recall to give a total of three immediate recall trials. After a 30-min delay, there is another recall trial, along with a recognition trial where the experimenter reads aloud the 12 target words and 12 new distractor words. Six of these distractors are from the same three semantic categories as the target words (two from each category), and six are not. The words are presented in a randomized order, and no feedback is given. Participants respond “yes” if they think the word was on the learning list and “no” if they think it is a new word. In an addition to the standard HVLT, at 24 hr another recall test was performed along with a further recognition test, which was the same as the 30-min test only with new distractor words. A different version of the task was given in each of the four conditions (Versions 1, 3, 4, 5) in a randomized order between participants. Each word appeared in only one version of the task. As patients completed the HVLT four times, the delayed tests could not be kept a surprise, so all patients were told at the beginning that they would be tested on the list both later on in the current session and in the session the next day.
Procedure
Testing took place over two consecutive days (see Figure 1 for details). On Day 1 participants completed the immediate recall and 30-min delayed recall and recognition tests, along with paper-based questionnaires: Barratt Impulsivity Scale (Patton, Stanford, & Barratt, 1995), Rational–Experiential Inventory (REI; Pacini & Epstein, 1999), Depression Anxiety Stress Scale (DASS; Lovibond & Lovibond, 1995), Mini Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975), and Lille Apathy Rating Scale (LARS; Zahodne et al., 2009; Sockeel et al., 2006). The PD patients also completed a standard motor symptom rating (Unified Parkinson Disease Rating Scale-III; Goetz et al., 2008). These assessments were completed during the 30-min delay.
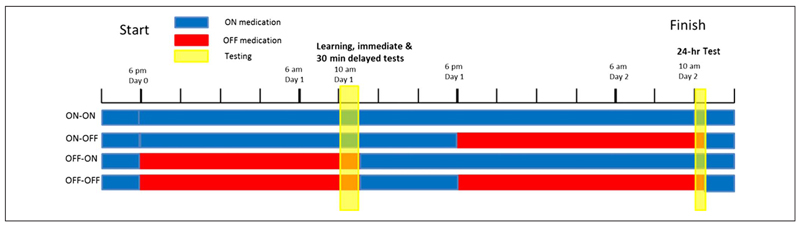
Figure 1.
Timeline of the testing procedure from 6 pm Day 0 to 12 pm Day 2. Blue denotes when patients were on their dopaminergic medication, red when they were withdrawn from it, and yellow the time of testing sessions. To have patients off their medication during the testing at 10 am, patients had to be withdrawn from their dose the night before because of the long washout times of the medications.
On Day 2 participants completed the 24-hr delayed recall and recognition tasks and a sleep questionnaire about their sleep the previous night (St. Mary’s Hospital Sleep Questionnaire; Leigh, Bird, Hindmarch, Constable, & Wright, 1988; Ellis et al., 1981)).
PD patients were on or off their medications on each of the 2 days, giving four conditions for each subject (Day1/Day2 = on/on, on/off, off/on, off/off). When the patients were in the “off” condition, they did not take any dopaminergic medications for a minimum of 15 hr before testing (usually none after 6 pm for testing at 10 am the next day). After the session had finished, they took a dose and then carried on with their usual dose schedule. For example, in the “off–off” condition (bottom bar in Figure 1), patients were on medication until 6 pm Day 0, then off medication until after testing on Day 1 (~11 am), then on medication until 6 pm Day 1, then off medication until after testing on Day 2 (~ 10:30 am), after which they resumed their normal medication schedule. All patients were on their medication for at least a few hours after learning on Day 1 (see Figure 1). This meant that in Day 1 off conditions they took a dose after the session finished and continued their normal dosage until the evening where if they were to be off medication on Day 2 they stopped their doses. This was to minimize discomfort to the patients and to reduce the chance of neuroleptic malignant syndrome, which can occur when dopaminergic medications are stopped (Keyser & Rodnitzky, 1991).
The order of the four conditions was counterbalanced across participants. Healthy participants were tested only once.
Data Analysis
Data were analyzed using within-subject repeated-measures ANOVAs to test the effects of Day 1 and Day 2 medication state on each separate measure in the patient group. Between-subject ANOVAs were used to compare patients and healthy participants. Raw delayed recall scores were measured at 30 min and 24 hr, as well as the percentage retention, which was the delayed recall score divided by the highest of the second and third immediate recall trial scores, multiplied by 100. This was calculated for the 30-min and 24-hr delayed recall scores. The change between the two delayed scores was also expressed as a percentage (24 hr/30 min × 100).
For the recognition tests, the raw measures were number of hits, misses, false alarms, and correct rejections. Signal detection methods (Stanislaw & Todorov, 1999; Macmillan, 1993; Green & Swets, 1966) were used to estimate the d′ measure of sensitivity between the targets and distractors,
| (1) |
where Z is the inverse cumulative density of the standard normal distribution and the hits and false alarms are expressed as probabilities, and response bias (a measure of where the threshold for a “yes” response is set),
| (2) |
Negative response biases indicate that less evidence is needed for a “yes” response, which corresponds to more “yes” responses—a liberal response bias. Positive response biases mean more evidence is needed for a “yes,” which leads to more “no” responses—a conservative response bias. Nonparametric measures of sensitivity and response bias were also calculated (Stanislaw & Todorov, 1999) but are not reported here as they gave the same results as the more common parametric measures.
The response bias score takes into account the proportion of hits and false alarms. We use this measure rather than discussing the raw hits and false alarms scores because there was no clear pattern of results emerging from them.
All SEM bars for figures have been corrected for between-subject variance using the Cousineau–Morey method (O’Brien & Cousineau, 2014; Morey, 2008; Cousineau, 2005), which removes the variance from between-subject differences and only shows the variance due to within-subject differences in a similar manner to the way a repeated-measures ANOVA removes between-subject variance.
Methods of Simulation
To illustrate what changes in synaptic connections could underlie the observed effects of dopaminergic medication on recognition memory (i.e., higher tendency to classify both previously seen and unseen stimuli as familiar when on medication during encoding; see Figure 4B), we used a simple anti-Hebbian model of familiarity discrimination (Bogacz & Brown, 2003a). The intuitive description of model is provided in the Results section, whereas here we describe details of the model (it is recommended to read the Results section before these details).
Figure 4.
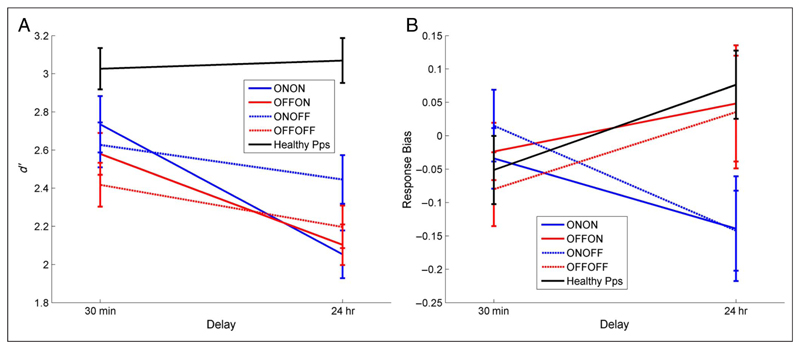
Recognition memory. (A) d′ measure of sensitivity and (B) response bias for 30-min and 24-hr delayed tests (SEM bars). Healthy participants show no decrease in d′ across the delay whereas patients do (F(1, 14) = 14.089, p = .002). Response bias has a significant interaction of Day 1 medication state and delay (F(1, 14) = 15.083, p = .002), with patients on dopamine on Day 1 (blue lines) showing a decrease in response bias over 24 hr (more “yes” responses) and patients off dopamine during learning (red lines) and healthy participants showing an increase in response bias (more “no” responses) irrespective of Day 2 medication (solid/dashed lines).
The model is a simple layer network with N input neurons and novelty neurons in separate layers. For the version we used, we simplified the model to only have one novelty neuron and N = 100 input neurons. The model assumes the novelty neuron receives connections from all input neurons. The weights of the connections from input neuron j to the novelty neuron are denoted as wj. The activity of each input neuron is denoted by xj, and it is 1 if the neuron is active and 0 otherwise. The activity of the novelty neuron (h) is thus calculated,
| (3) |
The change in weights after presentation of a novel stimulus is calculated as
| (4) |
where η is the learning rate and a is the sparseness parameter controlling the fraction of input neurons active in the patterns. In particular for each input pattern (x), the number of active neurons is determined by the sparseness parameter a such that
| (5) |
In all our simulations, parameters were fixed at a = 0.2 and η = 0.2. After learning a series of randomly generated patterns, we present the now-familiar patterns and some randomly generated novel patterns. For each presented pattern, the neuron’s activity is compared against a threshold T: if h < T it is classified as familiar, and if h > T as novel. We further denote the thresholds on 2 days of testing by T1 and T2, respectively. In all our simulations, T1 = 1.
To simulate the effects of high and low dopaminergic state, we proposed two versions of the model. In the first version (the decay model), dopaminergic medications would affect the decay of the weights. Dopamine has been implicated in consolidation in animals (Bethus et al., 2010; Rossato et al., 2009; Bernabeu et al., 1997), and models have been proposed in which dopamine sets the threshold for consolidation of weight changes at synapses (Clopath et al., 2008; Frey & Morris, 1997). We model this with two coefficients that control how much of the weight change is expressed:
| (6) |
There is a separate coefficient for the decreases in weights caused by coactivation of the input and novelty neurons and the increase in weights caused by the activation of the input neurons alone. This change was only applied after the 30-min test as consolidation takes time to have an effect. This model has four parameters that have been optimized: α+ and α− for PD patients on and off medication on Day 1, respectively (α+,On, α−,Off, α+,Off, α−,Off). In this model, the thresholds are assumed to be the same on both days T2 = T1 = 1.
In the second version of the model (the threshold model), the dopamine state during learning does not affect the decay of the weight changes but instead the threshold T2 for the 24-hr test. We assumed that T2 depends upon Day 1 dopamine state; thus, the model has four free parameters, two of which correspond to the value of T2 when the dopamine was on and off during Day 1 (T2,On, T2,Off). This is akin to a consolidation or decay of the threshold inputs dependent on dopamine 30–120 min after learning. Two other free parameters correspond to α+ and α−, but these were the same for Day 1 on and off conditions.
We simulated the models and then compared the recognition accuracy d′ and response bias measures from the model with the behavioral data (Figure 4) using root mean square deviation (RMSD),
| (7) |
where yi is the behavioral data, di is the simulated data for measure i, and n is the number of measures (n = 8; 30 min and 24 hr d′ and response bias on and off medications on Day 1).
We used 12 familiar patterns, each presented three times in the same order (as in the HVLT-R), and for the recognition tests all 12 familiar patterns along with 12 novel patterns were presented (different novel patterns were used for each test). We repeated this 1000 times with newly generated patterns and random starting weights each time.
We used Matlab’s fminsearch function to find free parameters of each model that minimize this RMSD. The initial values of the free parameters (i.e., the starting point for the search) were randomly generated from uniform distributions between 0 and 2. For each model, the whole optimization was repeated 300 times with different randomly generated initial sets of parameters to avoid local minima.
Results
No Effects of Dopaminergic Medication on Immediate Recall
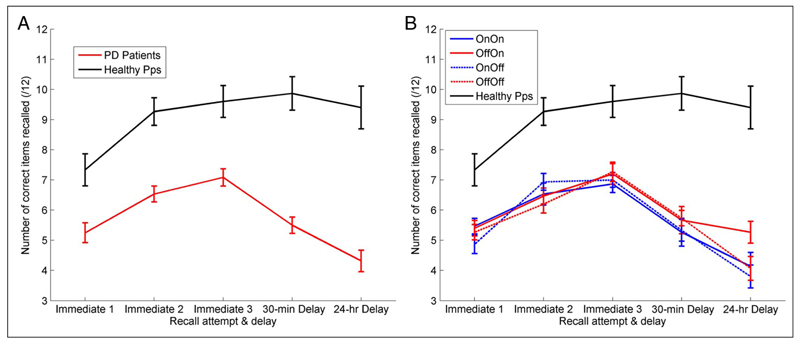
Patients and healthy participants show an increase in the number of words recalled over the three immediate recall trials (Figure 2A), although healthy participants recall significantly more words at each trial than patients (oneway multivariate ANOVAs, p < .001). Healthy participants only show a change in number of correctly recalled words between the first and second recalls (one-way repeated-measures ANOVA with Bonferroni-corrected pairwise comparisons, p = .005), with no further increases or decreases (p = 1). A two-way repeated-measures ANOVA (Day 1 medication state × Time) on the PD patients’ immediate recall trials revealed a significant effect of Time (F(2, 28) = 24.870, p < .001) but no significant effect of Day 1 medication state (F(1, 14) = .008, p = .931; see Figure 2B). PD patients take longer to learn information and retain less information than healthy participants, with their final immediate recall score about the same as the healthy participants’ first, and show no dopamine medication effects.
Figure 2.
The mean number of correct words recalled at each recall test. (A) The red line is the patients’ raw recall scores averaged across all conditions (SEM bars). Healthy participants showed significantly higher accuracy on all recall trials (p < .001 for all) and no decrease over the delays (F(1, 14) = 2.507, p = .136). PD patients learn more slowly and forget more over 30-min and 24 hr-delays. (B) Each PD condition separately, there were no significant differences for the immediate recall trials.
Medication Impairs Early Consolidation but Improves Late Consolidation
Healthy participants retain information across both 30-min and 24-hr delays (Figure 2A). Patients, however, show a decrease across both delays: 30-min delayed recall is significantly lower than the third immediate recall (one-way repeated-measures ANOVA with Bonferroni-corrected pairwise comparisons; p = .001), and 24-hr recall score is significantly lower than both the third immediate recall score and the 30-min delayed recall score (p < .001 for both). On average, 1–1.5 words are lost across both 30-min and 24-hr delays in patients.
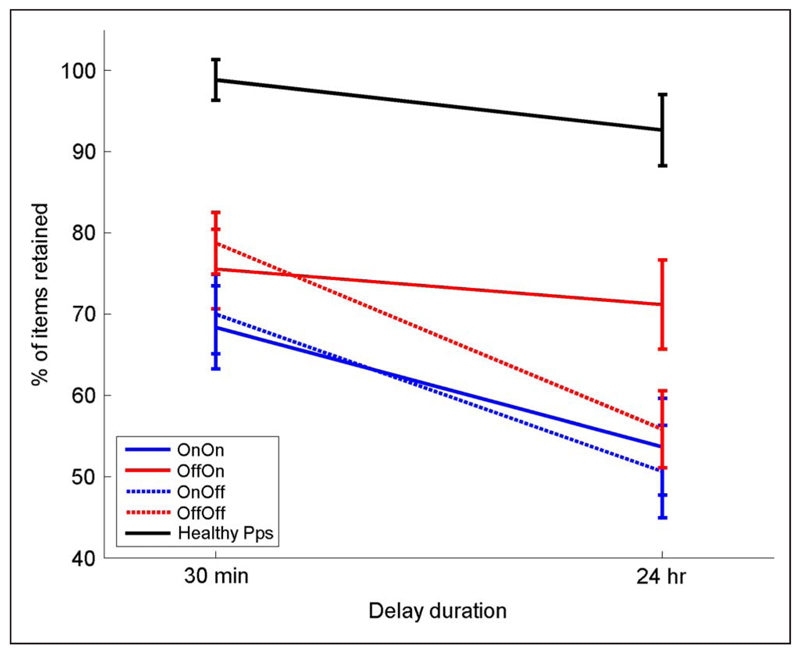
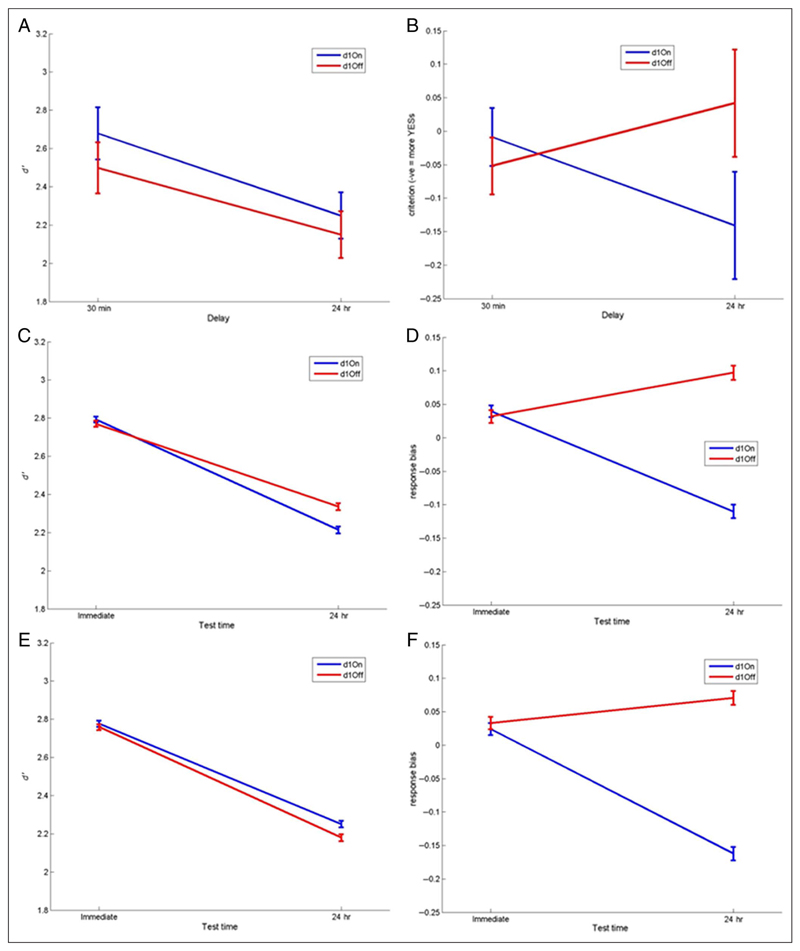
The recall retention scores in different medication conditions are shown in Figure 3, and the patients were statistically analyzed with a three-way repeated-measures ANOVA (Delay × Day 1 medication state × Day 2 medication state). The recall scores after 24 hr were significantly lower than scores after 30 min (effect of delay: F(1, 14) = 49.855, p < .0001). Patients who were on dopaminergic medications on Day 1 recalled less at both 30-min and 24-hr recall, which can be seen in Figure 3 where both blue lines are below the red lines (effect of Day 1 medication state: F(1, 14) = 7.320, p = .017). In stark contrast, patients on dopaminergic medication on Day 2 retained significantly more words between 30 min and 24 hr, which can be seen in Figure 3 where both solid lines are flatter than the dashed lines (interaction of delay and Day 2 medication: F(1, 14) = 11.4, p = .005; note that Day 2 medication effect could only be an interaction, as Day 2 medication could not possibly affect the 30-min recall score on Day 1). Thus, dopaminergic medication impairs delayed memory when present during learning (without affecting immediate recall) but improves it when present overnight after learning and during testing the next day.
Figure 3.
The mean percentage retention over 30-min and 24-hr delays. Healthy participants (black line) do not show a significant decrease over 24 hr (F(1, 14) = 2.507, p = .136). For PD patients (red and blue lines), there is a significant effect of delay (F(1, 14) = 49.885, p < .0001) and a clear effect of Day 1 medication state (F(1, 14) = 7.329, p = .017), with patients off medication on Day 1 during learning (red lines) having higher retention of words at 30 min and 24 hr than patients on medication on Day 1 (blue lines). It also shows a significant interaction of Delay and Day 2 medication state (F(1, 14) = 11.4, p = .005), with patients on medication on Day 2 (solid lines) having higher retention on Day 2 than patients off dopamine on Day 2 (dashed lines). All bars show SEM.
Looking at Figure 3B, it seems that the Delay × Day 2 medication state effects may be driven mostly by the off–on condition and that this may be significantly different to the other three conditions by itself. However, the three-way interaction from the ANVOA (Delay × Day 1 medication state × Day 2 medication state) did not return a significant result (F(1, 14) = 1.274, p = .278), which means that the significant differences are not due just to the off–on condition. There is only a significant effect when delay and Day 2 medication state are factors, meaning that whereas the off–on condition may contribute majorly to the effect, the on–on condition does also.
Response Bias, but Not Recognition Sensitivity, Is Affected by Dopamine
As shown in Figure 4A, the patients had significantly lower sensitivity (d′) on the 24-hr recognition task than on the 30-min test (effect of delay: F(1, 14) = 14.089, p = .002), but there was no significant effect of medication state. Healthy participants have the same d′ scores at both time delays (paired t test: T = 0.363, p = .722), which are much higher than the patients’ scores in both cases.
Figure 4B shows that when patients were on medication on Day 1 they had a more liberal (negative) response bias after 24 hr, but when patients were off medication on Day 1 they had a more conservative (positive) bias 24 hr later, regardless of Day 2 medication (interaction of time and Day 1 medication: F(1, 14) = 15.083, p = .002). In other words, dopaminergic medication during learning and early consolidation led to more “yes” responses 24 hr later, whereas a lack of dopaminergic medication during learning led to more “no” responses 24 hr later. In contrast, response bias of healthy participants increased over 24 hr; they were more conservative at the 24-hr recognition task, responding “no” more often and decreasing the number of hits and false alarms. This is the same pattern as PD patients off medication on Day 1.
Modeling the Response Bias Effects
The response bias effects were the most unexpected results we found, and we were unable to come up with a simple explanation, so we turned to computational models of familiarity discrimination to see if an interaction of delay and Day 1 medication state could be found in the models.
We have used an abstract recognition model to attempt to replicate our recognition memory results. The anti-Hebbian model (Bogacz & Brown, 2002, 2003b; Kohonen, Oja, & Rouhonen, 1974) was originally developed to capture the finding that perirhinal cortex neurons that discriminate on the basis of familiarity show high firing activity for novel, unfamiliar stimuli, but low firing activity for familiar stimuli (Brown & Aggleton, 2001). For example, when presenting the same stimulus twice, in the first time the neurons will fire wildly, and in the second time the neurons will fire at a lower rate. This differential activity allows discrimination between novel and familiar stimuli.
In the model, when a novel stimulus is presented as a pattern of activity to the novelty neuron, it elicits an output activity (Figure 5A). Consequently, the synaptic weights from the activated inputs are decreased, which results in lower output activity for the same stimulus presented again (Figure 5B; see Banks, Bashir, & Brown, 2012, for review of experimental evidence, suggesting that such anti-Hebbian synaptic plasticity in the perirhinal cortex underlies familiarity discrimination). Additionally, during learning the weights from the inactivate inputs are increased to balance the overall excitability for the neuron. When a new novel pattern is presented, it is likely to return a similar level of output activity as the original novel pattern (here shown by the output number 6; Figure 5C), which would not be the case without this increase. The new novel pattern has some overlapping input activity because of the finite number of inputs. The output activity of the novelty neuron in the model is a simple number, as this is a phenomenological model, but could correspond to either the number of spikes fired, the level of depolarization, or the probability of firing (if the model were to include more than one novelty neuron).
Figure 5.
Anti-Hebbian model with one novelty neuron. Numbers to the left show the activity of inputs (for simplicity just four are shown), the numbers over the four connections indicate their synaptic weights, and the number on the right is the level of neuron’s activity. (A) A novel input pattern is presented to the neuron, and it outputs an activity of 6. Anti-Hebbian learning takes place decreasing the weights of the active connections and increasing the inactive weights to balance overall excitation leading to the weights shown in B. (B) The same pattern (now familiar) is presented again, this time eliciting a lower output activity. (C) Presenting a new novel pattern returns the same output as the original novel pattern because of the increased weights of the inactive connections balancing the excitation. (D) Simulated PD patients on dopamine during learning show decays in the weight increase for the inactive connections, but the activated connections remain decreased so the output for the familiar pattern remains the same. (E) Presentation of the novel pattern from C would now elicit a lowered output, meaning it is more likely to be accepted as familiar. (F) Simulated PD patients off their medication during learning show decays in both increased and decreased weights, leading to higher activity for the familiar stimuli and (G) regular activity for novel patterns, corresponding to an increased response bias.
We considered two ways of modeling the effect of medication to which we refer as the decay and threshold models. The decay model assumes that PD patients on medication on Day 1 would show a decay in the increased weights (Figure 5D), which does not affect the output for familiar stimuli but decreases it for novel stimuli (Figure 5E). This would lead to more false alarms and a lower response bias. PD patients off medication on Day 1 were expected to have a decay in all weight changes (Figure 5F), which heightens the activity from familiar stimuli and overall keeps it the same for novel patterns (Figure 5G), meaning more rejections of familiar stimuli (i.e., a more conservative, positive response bias).
The threshold model assumes that the dopaminergic medications on Day 1 affect the threshold for the novelty decision on Day 2. In the threshold model, there is still a decay of the weight changes (Equation 6), but this is the same for the two simulated medication conditions. The threshold can be thought of as either the spiking threshold for the novelty neuron or for a “decision neuron” that receives input from the novelty neuron. Either way, dopamine-dependent consolidation mechanisms could affect the threshold.
We simulated each model with a variety of randomly generated parameters and minimized the RMSD between the simulated data and the PD patients’ behavioral data. The best fitting parameters (with the lowest RMSD) for each model are shown along with the simulated d′ data in Figure 6. We compare the two models directly using the RMSD as both models have the same number of parameters, meaning that measures like the Akaike Information Criterion are not needed.
Figure 6.
The behavioral and simulated d′ and response bias results for the patients and decay and threshold models. The results of the model fittings; (A) Behavioral d′ and (B) response bias data for PD patients split by Day 1 medications state (Day 1 on vs. Day 1 off). (C) The simulated d′ for the best fitting decay model (blue lines are simulated PD Day 1 on medication patients, and red lines are Day 1 off medication). (D) The simulated response biases for the best fitting decay model (RMSD = 0.0778), showing the same pattern as the behavioral data. The best fitting parameters were as follows: Day 1 on: α+ = .1515, α− = 0.8787; Day 1 off: α+ = 0.4079, α− = 0.8702. (E) The simulated d′ for the best fitting threshold model (RMSD = 0.0620) and (D) the simulated response bias results. The best fitting parameters were as follows: α+ = 0.3685, α− = 0.7562, Day 1 on: T2 = 1.1594; Day 1 off: T2 = 1.1108. The threshold model has a better fit to the data, based on the lower RMSD, which may be due to Day 1 on d′ being slightly higher than Day 1 off, as in the behavioral data.
Both models can provide good fits to the patient data (Figure 6A and B), reproducing the similar decrease in d′ in both conditions (Figure 6C and E), but with Day 1 on condition leading to a lower, more liberal response bias and Day 1 off medication leading to a higher, more conservative response bias (Figure 6D and F). Although the RMSD for the threshold model is slightly lower than for the decay model, the behavior of participants does not differ from the predictions of either model; thus, given the uncertainty in exact values of experimental data, we feel that it is not justified to categorically say that one of the models fit better.
The best fitting parameters for the decay model were α+,On = 0.1515, α−,On = 0.8787, α+,Off = 0.4079 and α−,Off = 0.8702. This represents greater decay of positive weight changes and slightly lesser decay of negative weight changes for PD on medication on Day 1 when compared to PD Day 1 off. This means that PD patients on dopamine during learning may be better preserving the weight decreases associated with the active inputs from the patterns, but at the cost of poorer consolidation of the weight increases for the inactive inputs.
The best fitting parameters for the threshold model were T2,On = 1.1594, T2,Off = 1.1108, α+ = 0.3685, α− = 0.7562. This means that, even though this model is nominally the threshold model, there were only very slight differences between the two conditions’ thresholds. The slightly higher threshold when on medication on Day 1 means that 24 hr later patterns with higher (more novel) activities are accepted as familiar, leading to a more liberal response bias. Both conditions shared the same decay parameters (α+ and α−), but these two were not the same, meaning that both on and off conditions had a large decrease in inactive connection weight increases and a smaller decrease in active connection weight decreases, which would decrease the novelty neuron’s activity and then the slight differences in thresholds would be enough to separate the two response biases.
In summary, both models can account for the current data, and we mention in the Discussion additional experiments that could distinguish between the models.
Questionnaires
A one-way ANOVA was run on the questionnaire data (PD vs. healthy participants), which revealed that PD patients scored significantly lower on the MMSE (see Table 1 for p values) and higher on the DASS and LARS than healthy participants. The DASS breaks down into subscores for depression, anxiety, and stress, and there were significant differences only for the depression and anxiety subscores, not for stress.
The groups did not differ significantly on Rational–Experiential Inventory, Barratt Impulsivity Scale, or age. This means that the PD patients were more apathetic, depressed, and anxious and had poorer memory as compared to the healthy participants, which may have biased the between-group comparisons, but the critical results from this study are within subjects.
From the St. Mary’s Hospital Sleep Questionnaire administered on every Day 2 session, we had self-reported measures of sleep latency, sleep quality, number of hours of sleep during the night and day, and the total number of hours. We compared each of these measures with a two-way repeated-measures ANOVA (Day 1 medication state × Day 2 medication state). There were no significant effects of Day 1 or Day 2 medication states on any of the sleep measures.
Repetition Effects
As the patients completed the HVLT four times, albeit with different versions of the HVLT each time, it was possible that the practice improved their recall and recognition scores so that they performed better during their fourth condition than their first. We ran repeated-measures ANOVAs on the 30-min and 24-hr delayed recall and recognition tests as well as the learning score (third immediate recall trial – first) to test for this. The ANOVAs revealed that there were no significant effects of order on 30-min recognition (F(3, 42) = 0.035, p = .991), 24-hr recognition (F(3, 42) = 2.671, p = .06), 30-min recall (F(3, 42) = 0.887, p = .456), 24-hr recall (F(3, 42) = 2.516, p = .071), or learning score (F(3, 42) = 1.444, p = .897). The two 24-hr scores show p values approaching significance, but this is not due to an increase in accuracy with repetition but instead a “U”-shaped curve with the final value similar to the first.
Discussion
We investigated the effect of dopaminergic medication on memory consolidation in PD patients tested on and off dopaminergic medications during learning/early consolidation and late consolidation/recall. Compared to age-matched healthy participants, PD patients retain less information over 30 min and 24 hr than healthy elderly participants. Remarkably, for free recall, dopamine during learning impaired recall at 30 min and 24 hr (but not immediate memory), whereas dopamine between 8 and 24 hr after learning (including a period of sleep) enhanced recall at 24 hr. Thus, we have demonstrated a benefit of dopaminergic medication on long-term memory storage over 24 hr in PD patients. Although dopaminergic medication state had no overall effect on recognition sensitivity, dopamine during learning led to a more liberal response bias 24 hr after learning regardless of dopamine state during recognition.
Dopaminergic Medication during Learning Impairs Early Consolidation of Memory
PD patients on medication during learning retained less after 30-min and 24-hr delays, suggesting that dopaminergic medication is interfering with early consolidation of information within 30 min of encoding and that this deleterious effect persists despite subsequent changes to dopamine levels. The effects of dopaminergic medication are unlikely to reflect a role in encoding as there were no effects of dopamine state on immediate memory, which would be expected if encoding were compromised by dopaminergic drugs. Also, we used the retention scores for the ANOVAs on the delayed memory tests, which normalize the number of words recalled by the maximum number recalled in the immediate recall trials. This should have removed any effects of worse encoding of words. The results are not consistent with a Day 1 effect of dopaminergic medication purely on retrieval processes; such an effect would be similar across immediate recall and 30 min and would not affect performance after a 24-hr delay. Thus, the critical time point at which dopaminergic activity appears to impair both 30-min and 24-hr recall is early consolidation (within 30 min of encoding). Note that this memory impairment on dopamine medication is seen despite improvement of motoric symptoms (see Unified Parkinson’s Disease Rating Scale in Table 1) and so does not represent a general deleterious effect of dopamine medication on the patients’ overall function, rather dopaminergic medication appears to specifically interfere with early consolidation of new information.
The only theory that predicts poorer performance when dopamine is restored in PD is the dopamine overdose hypothesis (Cools et al., 2001). This posits that the brain regions that are relatively spared from the dopaminergic cell loss in PD are flooded with dopamine from the medication, and this impairs normal function. Thus, a possible explanation is that encoding/early consolidation mechanisms in brain regions spared the dopaminergic depletion in PD and are therefore harmed by excess dopamine replacement therapy. Although the VTA is usually implicated in situations such as these because of its relatively preserved dopaminergic projections in PD (when compared to the substantia nigra; Wittmann et al., 2013; Cools, 2006; Gasbarri, Sulli, & Packard, 1997; Agid et al., 1989), in this case the VTA is thought to underpin improved memory performance when on medication overnight (see below). Although the VTA can underlie both encoding/early consolidation and sleep consolidation mechanisms, we would expect that dopamine would exert either positive or negative effects on both, not the different directions of effects seen here. Therefore, although the dopamine overdose hypothesis may fit with the finding of impaired learning when on dopaminergic medication, the exact underlying neural substrates necessary for this effect are unknown. Neuroimaging experiments offer a way to investigate this by examining blood flow changes in brain regions during learning when on and off dopaminergic medication.
Dopaminergic Activity Improves Late Consolidation of Memory
We found that the number of items retained between 30 min and 24 hr decreased in the patients. However, patients who were on medication between 8 and 24 hr after learning showed a smaller decrease, irrespective of Day 1 medication state. Thus, dopaminergic medication during late consolidation and retrieval enhances memory in PD patients. It appears more likely that dopamine is exerting effects during consolidation rather than retrieval, as retrieval was also required on Day 1 tests, when dopaminergic medication did not enhance performance. Although this is the first demonstration in humans of an effect of dopamine on memory consolidation over 24 hr, the finding is consistent with animal work (Bethus et al., 2010; O’Carroll et al., 2006).
When coming off medication, patients spend a minimum of 15 hr without dopaminergic medications before testing, meaning they are off medication overnight during sleep. In addition, on Day 1 all patients are on their medications from 11 am until 6 pm regardless of Day 1 or two condition (see Figure 1), meaning that the only time Day 2 off patients differed from Day 2 on patients was from 6 pm on Day 1 until testing on Day 2 (around 10 am). This suggests that any dopaminergic medication effects on 24-hr recall are occurring within this window (8–24 hr after learning), which coincides with a period of sleep.
It has been proposed that sleep plays a role in the consolidation of memories, and therefore, a lack of dopamine during sleep could impair this process. PD patients often complain of sleep disturbances (Dhawan, Healy, Pal, & Chaudhuri, 2006), and dopamine has been suggested to play a role in the sleep–wake cycle (Rye, 2004) possibly because of the increased phasic firing of VTA neurons during REM sleep (Lima, 2013; Dahan et al., 2007). Indeed, D2 antagonist infusion has been found to decrease REM sleep as well as the levels of plasticity related proteins and memory performance (França et al., 2015), and the VTA has been shown to activate the dopaminergic projections to the hippocampus and increase BDNF levels there (Rossato et al., 2009). McNamara et al. (2014) also found dopaminergic effects on sleep, but during SWS. They found that optogenetic stimulation of VTA neurons or their axons onto hippocampal CA1 cells increased memory performance on a spatial learning task. This coincided with SWS reactivation of the firing patterns present during awake exploration. This effect was removed when a D1/D5 antagonist was infused before the exploration, suggesting that dopaminergic activity during learning can affect later SWS consolidation via VTA–hippocampal connections. It may be that a similar process occurs when dopamine is present during sleep itself. So dopaminergic consolidation effects have been found in both REM and SWS; an interesting focus for future work would be unpicking the relative contribution of these two sleep stages to memory consolidation.
If dopaminergic activity is restored during postlearning sleep, this would allow the VTA firing to have its full effect and, because of the VTA-hippocampal projections, affect consolidation via increased dopamine release onto the hippocampus. Our self-reported sleep questionnaires did not yield any significant effects of Day 2 medication state on sleep (the night when consolidation takes place after learning); thus, it seems that, although dopamine may effect overnight consolidation, it does this without affecting overall self-reported sleep measures such as duration and quality.
An alternative although not mutually exclusive mechanism of overnight dopamine could involve selection of memories to be consolidated. Evidence for this comes from a placebo-controlled dopamine agonist study in humans, which found that a dopamine D2 receptor agonist given overnight after learning increased memory for pictures associated with low value rewards to the level of accuracy seen for pictures associated with high value rewards (Feld, Besedovsky, Kaida, Münte, & Born, 2014). This suggests that the greater dopaminergic activity mimicked the dopamine-dependent consolidation usually only seen with items associated with rewards (Wittmann et al., 2005). In other words, the greater dopaminergic activity selected the low-reward stimuli to be consolidated in the same way that the high-reward stimuli were selected by the dopamine bursts associated with the rewards. Although our words were not associated with rewards, it may be that the increased dopaminergic activity overnight is still selecting these words as though they had been reward related and thus consolidating them.
One possible molecular mechanism for the overnight consolidation effect of dopamine is suggested by the synaptic-tagging and capture model (Clopath, 2012; Clopath et al., 2008; Frey & Morris, 1997). In this model, synaptic activity induces potentiation or depression of the synapse, and in order for this to persist, the number of “tagged” synapses must exceed the threshold that is set by dopamine levels. This triggers protein synthesis and causes consolidation of the synaptic weight change. In this TagTriC model, dopamine lowers the threshold for the synthesis of a consolidation protein. In the model, there is only a fairly short time window for this protein to be synthesized—before the number of tagged synapses has decayed to below the dopamine threshold (<4 hr). However, dopamine consolidation over these longer timescales could operate similarly to that proposed by the TagTriC model, perhaps through a protein with a much slower decay rate, slower genetic modifications to synapses, or downstream proteins that do not depend on the number of tagged synapses (e.g., BDNF), perhaps depending instead on the previous protein levels or the change in weight which has already occurred. Extending the current models to cover the effects seen at longer timescales could help to explain our findings.
One interesting thing is to see which PD medication condition is most similar to healthy participants’ behavior. We might expect that the on–on condition would have the best overall performance as this condition has the most dopamine over the 2 days and should be most similar to the conditions in healthy participants. However, Figure 3 shows that the off–on condition (solid red line) is closest to the healthy participants. This condition shows the smallest decrease between the 30-min and 24-hr delayed tests, a comparable gradient to healthy participants. This fits with the significant effects we found; that no medication on Day 1 and medication on Day 2 both improve recall. The fact that this condition was more similar to the behavior in healthy participants suggests that the picture is not just as simple as “more dopamine is better,” but rather that timings of dopamine are critical. This potentially has important implications for the timing of dopamine replacement therapies in PD.
Dopamine Does Not Affect Recognition Accuracy but Response Bias
Dopamine did not affect overall recognition sensitivity. Interestingly though, there was an effect of Day 1 medication on Day 2 response bias score. Patients off medication on Day 1 had more conservative bias scores 24 hr later, which corresponds to more “no” responses, more rejections. When patients were on medication on Day 1 they had more liberal bias scores on Day 2 recognition test, meaning more “yes” responses. This suggests that dopamine may set the threshold for a word to be perceived as familiar and that this happens soon after learning.
One would perhaps expect no effect of dopamine on recognition as recognition memory is primarily based in the perirhinal cortex (Brown & Aggleton, 2001), which does not receive much dopaminergic input from the substantia nigra (Edelstyn, Mayes, Condon, Tunnicliffe, & Ellis, 2007). So, increasing the dopaminergic activity of those cells would have little effect on recognition. Recall, however, is more hippocampus based, an area with more dopamine receptors (Meador-Woodruff et al., 1994) receiving projections from the VTA (Lisman & Grace, 2005), which therefore would function better when supplied with dopamine. This makes our finding of dopamine during learning leading to a more liberal response bias 24 hr later even more intriguing; we have demonstrated that dopaminergic activity affects some aspect of recognition after all. Although there were no effects of dopamine on the response bias after 30 min, Day 1 dopamine did influence the response bias on Day 2. As Day 1 off patients go back on their medication after Day 1 session (and Day 1 on are already on their medications), both groups will be in a high dopaminergic state around 1 hr after the end of the session (the medications take around 1 hr to reach maximum concentrations). This means that any Day 1 effects are likely happening before this time, so within 120 min of learning. Thus, we suggest that the impact of dopamine on response bias is between 30 and 120 min after learning.
The results of the computational modeling provide possible mechanisms for this effect. One is that dopamine affects the decay of the weight changes associated with active and inactive inputs during learning. The best fitting parameters suggested that PD patients on medication during learning had greater consolidation of weight changes associated with learning from the active inputs of the “targets,” but at the cost of poorer consolidation of the inactive inputs that were upregulated to maintain overall excitability. The model simulations showed that this is able to capture the interaction between dopamine state during learning and delay seen behaviorally in PD patients, suggesting it is a plausible mechanism. This could be tested via electrophysiological recordings from the perirhinal cortex to see the effects of dopamine (or dopamine agonists) on late LTP/LTD.
A threshold model was also fit to the data and provided a slightly better fit. In this model, Day 1 dopamine state sets the threshold for Day 2. Simulated Day 1 on patients had a higher threshold 24 hr later than Day 2 off patients, which corresponded with a more liberal response bias (here lower activity means a familiar stimulus so higher thresholds are more likely to class stimuli as familiar). The threshold model predicts a change in excitability of either the novelty neuron or a neuron that receives inputs from the novelty neuron, which could be tested in in vitro experiments involving current injection into the neurons. Either way, dopaminergic consolidation 30–120 min after learning could affect this threshold by affecting the expression of receptors in the synapses of either neuron.
Another way of looking at the data is that it is the dopaminergic state at time of retesting on Day 1 (the 30-min recognition test), and not learning, that is important. Dopamine receptors have been found to be involved in the destabilization and reconsolidation of object recognition memory during reactivation of the memory in rats (Rossato et al., 2015; Maroun & Akirav, 2009). It is possible that a high dopamine state during the recognition test (despite no feedback and the distractors not being repeated in the next day’s test) affects the reconsolidation of the activated memory and improves the stability of it, leading to a stronger memory, and also stronger activation of random connections associated with the distractors. At the 24-hr test, this corresponds to more hits and false alarms. If dopamine state is low during the first test, the reconsolidation is impaired and the memory traces for targets is weakened, along with those connections activated by the distractors, which leads to more misses and correct rejections (i.e., more conservative response bias).
It should also be mentioned that the PD patients off their medication on Day 1 showed the same pattern here as the healthy participants; a more conservative response bias 24 hr later. This finding was unexpected as we would expect the dopaminergic medication to revert the patients back to healthy behavior, but the opposite is true. This may be another example of dopaminergic medications impairing specific memory phenomena despite improving others, similar to the dissociation between learning from positive and negative feedback shown in reward learning (Frank, Seeberger, & O’reilly, 2004). Again this could tie in with the dopamine overdose hypothesis and may suggest that whatever mechanism is mediating this dopaminergic consolidation effect on response bias is in a relatively intact part of the PD brain, although the neuroanatomical substrate for this is not currently clear.
The similarity between healthy participants and PD patients off their medication raises an important point about the samples used in this study. Although the healthy participants were age-matched with the PD patients, they showed some differences on the questionnaires used, with higher memory performance on the MMSE, lower depression and anxiety on the DASS, and lower apathy ratings on the LARS. These may have contributed to the differences seen between the two populations. Although PD patients had higher apathy scores than healthy participants, both averages were under the cutoff for classification as apathetic (Zahodne et al., 2009; Sockeel et al., 2006). The PD patients were not diagnosed with anxiety disorders or major depressive disorder, so these differences were subclinical and were unsurprising given the higher incidence of depression in PD populations, but an effect on behavioral results is not possible to exclude (Veiga et al., 2009; Reijnders, Ehrt, Weber, Aarsland, & Leentjens, 2008).
We note that care should be taken when comparing PD patients and healthy participants as PD patients have large dopaminergic loss, which is partially remediated with their medications (Shulman, De Jager, & Feany, 2011; Agid et al., 1989), but dopaminergic activity on medication is unlikely to be a perfect restoration of normal function. As absolute dopamine levels are hard to measure in humans, it is not known whether dopamine replacement therapy restores dopamine concentrations to healthy levels or even higher. The levodopa dose PD patients are given is determined by the corresponding amelioration of motor symptoms, which should correlate with dopamine loss, but is not an exact match because of confounds such as non-dopaminergic cell death and compensatory mechanisms. Furthermore, even healthy elderly people show a decrease in dopamine levels with age (Frank & Kong, 2008; Schott et al., 2007). Although this is not thought to be clinically relevant or routinely treated, the effect of such age-related dopamine loss on cognitive findings in experimental paradigms is uncertain. In short, caution should thus be taken when extrapolating these findings about the effects of dopaminergic medication in PD to the effects of dopamine in the general population. However, dopaminergic cell loss in PD and treatment with medications are widely used as human models of dopaminergic depletion and restoration respectively (e.g., Foerde et al., 2013; Shiner et al., 2012; Smittenaar et al., 2012; Moustafa, Sherman, & Frank, 2008; Cools, Lewis, Clark, Barker, & Robbins, 2007; Frank, Samanta, Moustafa, & Sherman, 2007; Cools, 2006; Frank et al., 2004). When combined with evidence from other research modalities, such patient work significantly enhances our understanding of dopaminergic contribution to cognition.
Conclusion
In conclusion, PD patients are impaired at delayed recall, and dopamine during learning further impairs performance. Dopaminergic medications during learning also lead to a more liberal response bias 24 hr later without affecting recognition accuracy. Dopaminergic medications 8–24 hr after learning improve recall memory in PD, but not recognition. These findings suggest a distinct dopamine-dependent pathway for information consolidation that is active between 8 and 24 hr after information is encoded, coinciding with a period of sleep. In addition to enhancing our understanding of the role of dopamine in memory consolidation, this work has clinical implications for the use and timing of dopaminergic therapies in neurodegenerative and psychiatric disorders.
Acknowledgments
This work was supported by the Wellcome Trust [SJ1102], and BRACE Charity. We would also like to thank the two reviewers of this article for their helpful comments on earlier drafts of this manuscript.
Footnotes
Reprint requests should be sent to John Grogan, ReMemBr Group, Bristol BRAIN Centre, Elgar House, Southmead Hospital, Southmead Road, Bristol, BS10 5NB, UK, or via e-mail: John. Grogan@bristol.ac.uk.
References
- Agid Y, Cervera P, Hirsch E, Javoy-agid F, Lehency S, Raisman R, et al. Biochemistry of Parkinson’s disease 28 years later: A critical review. Movement Disorders. 1989;4(Suppl 1):S126, S144. doi: 10.1002/mds.870040514. [DOI] [PubMed] [Google Scholar]
- Banks PJ, Bashir ZI, Brown MW. Recognition memory and synaptic plasticity in the perirhinal and prefrontal cortices. Hippocampus. 2012;22:2012–2031. doi: 10.1002/hipo.22067. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–417. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proceedings of the National Academy of Sciences, USA. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethus I, Tse D, Morris RGM. Dopamine and memory: Modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. Journal of Neuroscience. 2010;30:1610–1618. doi: 10.1523/JNEUROSCI.2721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogacz R, Brown MW. The restricted influence of sparseness of coding on the capacity of familiarity discrimination networks. Network. 2002;13:457–485. [PubMed] [Google Scholar]
- Bogacz R, Brown MW. An anti-Hebbian model of familiarity discrimination in the perirhinal cortex. Neurocomputing. 2003a;52–54:1–6. [Google Scholar]
- Bogacz R, Brown MW. Comparison of computational models of familiarity discrimination in the perirhinal cortex. Hippocampus. 2003b;13:494–524. doi: 10.1002/hipo.10093. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Guitart-Masip M, Bunzeck N, Dolan RJ, Düzel E. Dopamine modulates episodic memory persistence in old age. Journal of Neuroscience. 2012;32:14193–14204. doi: 10.1523/JNEUROSCI.1278-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopath C. Synaptic consolidation: An approach to long-term learning. Cognitive Neurodynamics. 2012;6:251–257. doi: 10.1007/s11571-011-9177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopath C, Ziegler L, Vasilaki E, Büsing L, Gerstner W. Tag-trigger-consolidation: A model of early and late long-term-potentiation and depression. PLoS Computational Biology. 2008;4:e1000248. doi: 10.1371/journal.pcbi.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neuroscience and Biobehavioral Reviews. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cerebral Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Lewis SJG, Clark L, Barker RA, Robbins TW. L-DOPA disrupts activity in the nucleus accumbens during reversal learning in Parkinson’s disease. Neuropsychopharmacology. 2007;32:180–189. doi: 10.1038/sj.npp.1301153. [DOI] [PubMed] [Google Scholar]
- Coulthard EJ, Bogacz R, Javed S, Mooney LK, Murphy G, Keeley S, et al. Distinct roles of dopamine and subthalamic nucleus in learning and probabilistic decision making. Brain. 2012;135:3721–3734. doi: 10.1093/brain/aws273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutorials in Quantitative Methods for Psychology. 2005;1:42–45. [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- De Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson L-G. COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behavior Genetics. 2004;34:533–539. doi: 10.1023/B:BEGE.0000038491.06972.8c. [DOI] [PubMed] [Google Scholar]
- Dhawan V, Healy DG, Pal S, Chaudhuri KR. Sleep-related problems of Parkinson’s disease. Age and Ageing. 2006;35:220–228. doi: 10.1093/ageing/afj087. [DOI] [PubMed] [Google Scholar]
- Edelstyn NMJ, Mayes AR, Condon L, Tunnicliffe M, Ellis SJ. Recognition, recollection, familiarity and executive function in medicated patients with moderate Parkinson’s disease. Journal of Neuropsychology. 2007;1:131–147. doi: 10.1348/174866407x182565. [DOI] [PubMed] [Google Scholar]
- Ellis BW, Johns MW, Lancaster R, Raptopoulos P, Angelopoulos N, Priest RG. The St. Mary’s Hospital Sleep Questionnaire: A study of reliability. Sleep. 1981;4:93–97. doi: 10.1093/sleep/4.1.93. [DOI] [PubMed] [Google Scholar]
- Feld GB, Besedovsky L, Kaida K, Münte TF, Born J. Dopamine D2-like receptor activation wipes out preferential consolidation of high over low reward memories during human sleep. Journal of Cognitive Neuroscience. 2014;26:2310–2320. doi: 10.1162/jocn_a_00629. [DOI] [PubMed] [Google Scholar]
- Foerde K, Braun EK, Shohamy D. A trade-off between feedback-based learning and episodic memory for feedback events: Evidence from Parkinson’s disease. Neuro-Degenerative Diseases. 2013;11:93–101. doi: 10.1159/000342000. [DOI] [PubMed] [Google Scholar]
- Foerde K, Shohamy D. The role of the basal ganglia in learning and memory: Insight from Parkinson’s disease. Neurobiology of Learning and Memory. 2011;96:624–636. doi: 10.1016/j.nlm.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical state method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- França ASC, Muratori L, Nascimento G, Winne J, Pereira CM, Jeronimo SMB, et al. D2 dopamine receptor regulation of learning, sleep and plasticity. European Neuropsychopharmacology. 2015;25:493–504. doi: 10.1016/j.euroneuro.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Kong L. Learning to avoid in older age. Psychology and Aging. 2008;23:392–398. doi: 10.1037/0882-7974.23.2.392. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: Impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’reilly RC. By carrot or by stick: Cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging: Implications for late maintenance of hippocampal long-term potentiation. Trends in Neurosciences. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RGM. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Furini CRG, Myskiw JC, Schmidt BE, Marcondes LA, Izquierdo I. D1 and D5 dopamine receptors participate on the consolidation of two different memories. Behavioural Brain Research. 2014;271:212–217. doi: 10.1016/j.bbr.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1997;21:1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Movement Disorders. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Keyser DL, Rodnitzky RL. Neuroleptic malignant syndrome in Parkinson’s disease after withdrawal or alteration of dopaminergic therapy. Archives of Internal Medicine. 1991;151:794–796. [PubMed] [Google Scholar]
- Kohonen T, Oja E, Rouhonen M. Adaptation of a linear system to a finite set of patterns occurring in an arbitrarily varying order. Acta Polytechnica Scandinavica: Electrical Engineering. 1974;25 [Google Scholar]
- Leigh TJ, Bird HA, Hindmarch I, Constable PD, Wright V. Factor analysis of the St. Mary’s Hospital Sleep Questionnaire. Sleep. 1988;11:448–453. doi: 10.1093/sleep/11.5.448. [DOI] [PubMed] [Google Scholar]
- Lima MMS. Sleep disturbances in Parkinson’s disease: The contribution of dopamine in REM sleep regulation. Sleep Medicine Reviews. 2013;17:367–375. doi: 10.1016/j.smrv.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2nd ed. Sydney: Psychology Foundation; 1995. [Google Scholar]
- Macdonald AA, Seergobin KN, Owen AM, Tamjeedi R, Monchi O, Ganjavi H, et al. Differential effects of Parkinson’s disease and dopamine replacement on memory encoding and retrieval. PloS One. 2013;8:e74044. doi: 10.1371/journal.pone.0074044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA. Signal detection theory as data analysis method and psychological decision model. In: Keren G, Lewis C, editors. A handbook for data analysis in the behavioural sciences: Methodological issues. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1993. pp. 21–57. [Google Scholar]
- Maroun M, Akirav I. Differential involvement of dopamine D1 receptor and MEK signaling pathway in the ventromedial prefrontal cortex in consolidation and reconsolidation of recognition memory. Learning & Memory. 2009;16:243–247. doi: 10.1101/lm.1245009. [DOI] [PubMed] [Google Scholar]
- McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nature Neuroscience. 2014;17:1658–1660. doi: 10.1038/nn.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Grandy DK, Van Tol HH, Damask SP, Little KY, Civelli O, et al. Dopamine receptor gene expression in the human medial temporal lobe. Neuropsychopharmacology. 1994;10:239–248. doi: 10.1038/npp.1994.27. [DOI] [PubMed] [Google Scholar]
- Morey RD. Confidence intervals from normalized data: A correction to Cousineau (2005) Tutorials in Quantitative Methods for Psychology. 2008;4:61–64. [Google Scholar]
- Moustafa AA, Sherman SJ, Frank MJ. A dopaminergic basis for working memory, learning and attentional shifting in parkinsonism. Neuropsychologia. 2008;46:3144–3156. doi: 10.1016/j.neuropsychologia.2008.07.011. [DOI] [PubMed] [Google Scholar]
- O’Brien F, Cousineau D. Representing error bars in within-subject designs in typical software packages. The Quantitative Methods for Psychology. 2014;10:56–67. [Google Scholar]
- O’Carroll CM, Martin SJ, Sandin J, Frenguelli B, Morris RGM. Dopaminergic modulation of the persistence of one-trial hippocampus-dependent memory. Learning & Memory. 2006;13:760–769. doi: 10.1101/lm.321006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini R, Epstein S. The relation of rational and experiential information processing styles to personality, basic beliefs, and the ratio-bias phenomenon. Journal of Personality and Social Psychology. 1999;76:972–987. doi: 10.1037//0022-3514.76.6.972. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Péczely L, Ollmann T, László K, Kovács A, Gálosi R, Szabó A, et al. Effects of ventral pallidal D1 dopamine receptor activation on memory consolidation in morris water maze test. Behavioural Brain Research. 2014;274:211–218. doi: 10.1016/j.bbr.2014.07.031. [DOI] [PubMed] [Google Scholar]
- Redondo RL, Morris RGM. Making memories last: The synaptic tagging and capture hypothesis. Nature Reviews Neuroscience. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- Reijnders JSAM, Ehrt U, Weber WEJ, Aarsland D, Leentjens AFG. A systematic review of prevalence studies of depression in Parkinson’s disease. Movement Disorders. 2008;23:183–189. doi: 10.1002/mds.21803. quiz 313. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LRM, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325:1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Köhler CA, Radiske A, Lima RH, Bevilaqua LRM, Cammarota M. State-dependent effect of dopamine D1/D5 receptors inactivation on memory destabilization and reconsolidation. Behavioural Brain Research. 2015;285:194–199. doi: 10.1016/j.bbr.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Radiske A, Kohler CA, Gonzalez C, Bevilaqua LR, Medina JH, et al. Consolidation of object recognition memory requires simultaneous activation of dopamine D1/D5 receptors in the amygdala and medial prefrontal cortex but not in the hippocampus. Neurobiology of Learning and Memory. 2013;106:66–70. doi: 10.1016/j.nlm.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Rye DB. The two faces of Eve: Dopamine’s modulation of wakefulness and sleep. Neurology. 2004;63(Suppl 3):S2–S7. doi: 10.1212/wnl.63.8_suppl_3.s2. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiology of Learning and Memory. 2004;82:12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Schott BH, Niehaus L, Wittmann BC, Schütze H, Seidenbecher CI, Heinze H-J, et al. Ageing and early-stage Parkinson’s disease affect separable neural mechanisms of mesolimbic reward processing. Brain. 2007;130:2412–2424. doi: 10.1093/brain/awm147. [DOI] [PubMed] [Google Scholar]
- Shiner T, Seymour B, Wunderlich K, Hill C, Bhatia KP, Dayan P, et al. Dopamine and performance in a reinforcement learning task: Evidence from Parkinson’s disease. Brain. 2012;135:1871–1883. doi: 10.1093/brain/aws083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Geghman KD, Sage J, Gluck MA. L-dopa impairs learning, but spares generalization, in Parkinson’s disease. Neuropsychologia. 2006;44:774–784. doi: 10.1016/j.neuropsychologia.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman JM, De Jager PL, Feany MB. Parkinson’s disease: Genetics and pathogenesis. The Annual Review of Pathology: Mechanisms of Disease. 2011;6:193–224. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- Smittenaar P, Chase HW, Aarts E, Nusselein B, Bloem BR, Cools R. Decomposing effects of dopaminergic medication in Parkinson’s disease on probabilistic action selection—Learning or performance? European Journal of Neuroscience. 2012;35:1144–1151. doi: 10.1111/j.1460-9568.2012.08043.x. [DOI] [PubMed] [Google Scholar]
- Sockeel P, Dujardin K, Devos D, Denève C, Destée A, Defebvre L. The Lille Apathy Rating Scale (LARS), a new instrument for detecting and quantifying apathy: Validation in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77:579–584. doi: 10.1136/jnnp.2005.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Movement Disorders. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Veiga BA, Borges V, Silva SM, Goulart FDO, Cendoroglo MS, Ferraz HB. Depression in Parkinson’s disease: Clinical-epidemiological correlates and comparison with a controlled group of non-parkinsonian geriatric patients. Revista Brasileira de Psiquiatria. 2009;31:39–42. doi: 10.1590/s1516-44462009000100010. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze H-J, Düzel E. Reward-related fMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Tan GC, Lisman JE, Dolan RJ, Düzel E. DAT genotype modulates striatal processing and long-term memory for items associated with reward and punishment. Neuropsychologia. 2013;51:2184–2193. doi: 10.1016/j.neuropsychologia.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Young S, Kirsch-darrow L, Nisenzon A, Fernandez HH, Okun MS, et al. Examination of the Lille Apathy Rating Scale in Parkinson disease. Movement Disorders. 2009;24:677–683. doi: 10.1002/mds.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]