Abstract
It is now widely recognised that the environment in early life can have important effects on human growth and development, including the “programming” of far reaching effects on the risk of developing common metabolic and other non-communicable diseases in later life. We have shown that greater childhood adiposity is associated with higher maternal adiposity, low maternal vitamin D status, excessive gestational weight gain, and short duration of breastfeeding; maternal dietary patterns in pregnancy and vitamin D status have been linked with childhood bone mineral content and muscle function. Human studies have identified fetal liver blood flow adaptations and epigenetic changes as potential mechanisms that could link maternal influences with offspring body composition. In experimental studies there is now substantial evidence that the environment during early life induces altered phenotypes through epigenetic mechanisms. Epigenetic processes such as DNA methylation, covalent modifications of histones and non-coding RNAs can induce changes in gene expression without a change in DNA base sequence. Such processes are involved in cell differentiation and genomic imprinting, as well as the phenomenon of developmental plasticity in response to environmental influences. Elucidation of such epigenetic processes may enable early intervention strategies to improve early development and growth.
Developmental influences and common non-communicable disorders
Patterns of health, illness and disease are influenced at different stages of the lifecourse by a combination of genetic, epigenetic and environmental factors. Substantial research has demonstrated that during prenatal development, responses to a range of stimuli are likely to ‘programme’ the risk of metabolic and other non-communicable disorders (NCDs), as articulated by the ‘developmental origins’ or ‘DOHaD’ paradigm [1]. Subsequent environmental exposures, including nutritional, social, psychological, physical, lifestyle and occupational factors, during infancy, childhood and adult life can modify or condition this risk of disease.
Research over many years has shown that impaired fetal development, indicated by low birthweight, is associated not only with adverse childhood outcomes, such as stunting and reduced cognitive function, but also with increased morbidity in adult life from type 2 diabetes mellitus, metabolic syndrome, osteoporosis, sarcopenia and coronary heart disease [1]. These findings have been extensively replicated and are known to be independent of adult environmental risk factors for these disorders. It is now known that small body size at birth, in addition to reducing later functional capacity, also conditions later responses to the childhood and adult environment. For example, an increased risk of coronary heart disease, hypertension and type 2 diabetes mellitus is associated with slow growth in utero, coupled with accelerated weight gain during childhood. In contrast, boys and girls who were born short and then gained height poorly during childhood have been found to have an increased risk of hip fracture [2].
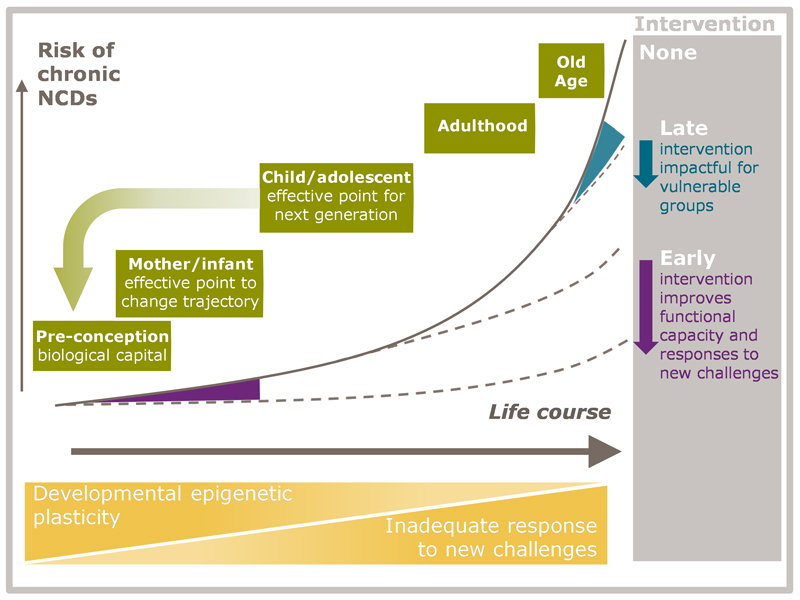
Figure 1 shows the conceptual framework for ongoing research. The risk of NCDs increases across the lifecourse as a result of declining plasticity and accumulative effects of inadequate responses to new challenges (orange triangles). The greatest increase occurs in adult life, but the trajectory is set much earlier, being influenced by factors such as the mother’s diet and body composition before and during pregnancy, and fetal, infant and childhood nutrition. In early life, timely interventions can have a large effect on later disease risk (purple area/arrow), while later intervention can remain impactful for vulnerable groups (blue area/arrow). Intervention in childhood and adolescence increases biological capital, and may have an important impact on the next generation.
Figure 1. Conceptual framework illustrating a lifecourse approach to the prevention and treatment of non-communicable diseases.
Early development in relation to childhood adiposity and body composition
Early life may be a critical period when appetite and regulation of energy balance are programmed, with lifelong consequences for the risk of excess adiposity gain. In the UK Southampton Women’s Survey (SWS), we have demonstrated associations of pre-conception, pregnancy and early postnatal factors with childhood body composition, determined using dual x-ray absorptiometry (DXA) at birth and at age 4 and 6-7 years. Using the Institute of Medicine gestational weight gain categorisation [3], excessive gain was associated with greater offspring fat mass from birth to age 6-7 years [4]. Higher maternal adiposity and short duration of breastfeeding were independently associated with greater childhood adiposity, and low maternal vitamin D status in pregnancy with greater postnatal adiposity gain [5]. In relation to other aspects of childhood body composition we have shown that maternal dietary patterns in pregnancy and vitamin D status are linked with childhood bone mineral content and with muscle function [6,7].
Insight into the potential impact of modifying early life risk factors on later adiposity and obesity can be gained by evaluating their combined effects. Examining the three perinatal risk factors for childhood adiposity mentioned above (excess gestational weight gain, low maternal vitamin D status and short duration of breastfeeding), together with smoking during pregnancy and maternal obesity, we found that among SWS children 15% had no early life risk factors, 33% had one, 30% had two, 16% had three and 6% had four or five [8]. At both 4 and 6 years, there were positive graded associations between the number of early life risk factors and adiposity and obesity outcomes. After taking account of confounders, the relative risk of being overweight or obese for children who had 4 or 5 risk factors was 3.99 (95% CI 1.83,8.67) at 4 years and 4.65 (95% CI 2.29,9.43) at 6 years, when compared with children who had none [8]. Other aspects of body composition are also associated with perinatal risk factors; we have identified influences of maternal dietary patterns, fatty acid status, physical activity, smoking and vitamin D status on offspring bone mineral parameters [9].
The long term effects of maternal influences raise the important question of how best to approach modifying such influences. Studies of health behaviours have shown that few women succeeded in complying with nutrition and lifestyle recommendations for planning a pregnancy, and that the diets of infants and toddlers are strongly associated with the mother’s diet before pregnancy [10,11]. Less than 6% of SWS women took the recommended amount of folic acid before pregnancy, but both folate intake and red cell folate increased markedly from before pregnancy to 11 weeks’ gestation [12], suggesting that women take adequate amounts only when they know they are pregnant. Pre-pregnant diet quality, smoking patterns, alcohol consumption and physical activity levels were similar in women who became pregnant within three months of interview to those who did not [10]. In early pregnancy, although smoking, alcohol and caffeine consumption fell, dietary quality (assessed using a ‘prudent diet score’ derived from principal components analysis [10,11]) and consumption of fruit and vegetables hardly changed. Notably, younger and more disadvantaged women were the least likely to modify their behaviours when they became pregnant. These observations are now leading into trials of complex interventions to modify maternal diet and lifestyle.
Postnatal influences on childhood body composition
Few studies have objectively measured physical activity in young children. Using Actiheart devices in collaboration with the MRC Epidemiology Unit, we showed that 4-year-old SWS children who spent more time doing vigorous physical activity had a lower %body fat and fat mass index, but adiposity was not related to sedentary and low-to-moderate–intensity activity [13]. This suggests that activity in young children needs to be vigorous in order to impact on adiposity. Additionally, greater grip strength was found in children who spent fewer hours in sedentary activity each day [14]. These findings have led us to study influences on physical activity, lean mass and muscle (handgrip) strength. We found modest positive associations between maternal vitamin D and n-3 fatty acid status in pregnancy and lean mass in childhood [15,16], but a stronger association between maternal vitamin D status and grip strength at age 4 years, independent of the child’s height and level of physical activity [7]. Our analyses also suggest that the postnatal environment is important. For example, at 4 years of age, we found that lean mass was greater in children whose weaning diets had complied with infant feeding guidance (diet based on fruit, vegetables and home-prepared foods, and longer breastfeeding)[17].
The potential for early life influences to have far reaching effects on adult health are illustrated by ongoing follow up of the Helsinki Birth Cohort Study. A comparison of siblings discordant for duration of breast-feeding has, for example, shown that both short (<2 months) and long (≥8 months) durations were associated with increased BMI and greater % body fat in later life [18] and that higher rates of diabetes were seen in those born before 35 weeks gestation, compared with term births, even adjusting for birthweight [19].
Underlying mechanisms
SWS studies have provided evidence that prenatal developmental adaptations play important roles in the human propensity to deposit fat [20]. Among primates, human neonates have the largest brains but also the highest proportion of body fat. If placental nutrient supply is limited, the fetus faces a dilemma: should resources be allocated to brain growth, or to fat deposition for use as a potential postnatal energy reserve? We hypothesised that resolving this dilemma operates at the level of umbilical blood distribution entering the fetal liver. In uncomplicated third trimester SWS pregnancies we used ultrasound to measure blood flow perfusing the fetal liver, or bypassing it via the ductus venosus to supply the brain and heart [20]. Across the range of fetal size and independent of the mother’s adiposity and parity, greater liver blood flow was associated with greater offspring fat mass measured by DXA, both in the infant at birth and at age 4 years. In contrast, smaller placentas less able to meet fetal demand for essential nutrients were associated with a brain-sparing flow pattern. This led us to propose that humans evolved a developmental strategy to prioritise nutrient allocation for prenatal fat deposition when the supply of conditionally essential nutrients requiring hepatic inter-conversion is limited, switching resource allocation to favour the brain if the supply of essential nutrients is limited. Facilitated placental transfer processes for glucose and other nutrients evolved in environments less affluent than those now prevalent in developed populations, and we proposed that in circumstances of maternal adiposity and nutrient excess these processes now also lead to prenatal fat deposition [20].
Molecular processes
It has been argued that the associations between fetal or infant growth and later adult disease could represent the multiple (pleiotropic) effects of genes transmitted from mother to child. However, the Early Growth Genetics consortium showed only a small genotypic contribution to birthweight [21]. Rather, it appears that maternally mediated environmental modulation of gene expression in offspring and gene-environment interactions may be more important than purely heritable genetic risk. There is also growing evidence that epigenetic mechanisms (DNA methylation, histone modification and non-coding RNAs) are responsible for tissue-specific gene expression during growth and development and that these mechanisms underlie the processes of developmental plasticity. Such ‘tuning’ of phenotype has potential adaptive value and fitness advantage because it adjusts the phenotype to current circumstances and/or matches responses to the predicted later environment [22]. When the phenotype is mismatched to the later environment, e.g. from inaccurate nutritional cues from the mother or placenta, or from rapid environmental change through improved socio-economic conditions, risk of NCDs increases.
Using a transcriptomic approach to examine perinatal influences on gene expression patterns in the umbilical cord, clear differences were found in the transcriptomic pattern of umbilical cords at different gestational ages, even within the normal range [23]. Gestational age dependent expression was enriched for signal transduction pathways (e.g. Hedgehog) and in genes with roles in cytokine signaling and angiogenesis. In contrast, birthweight, even at extremes, was not a major influence on transcriptomic patterns. Transcriptome changes were found to relate to DNA methylation levels, with possible implications for the risk for NCDs later in life [23].
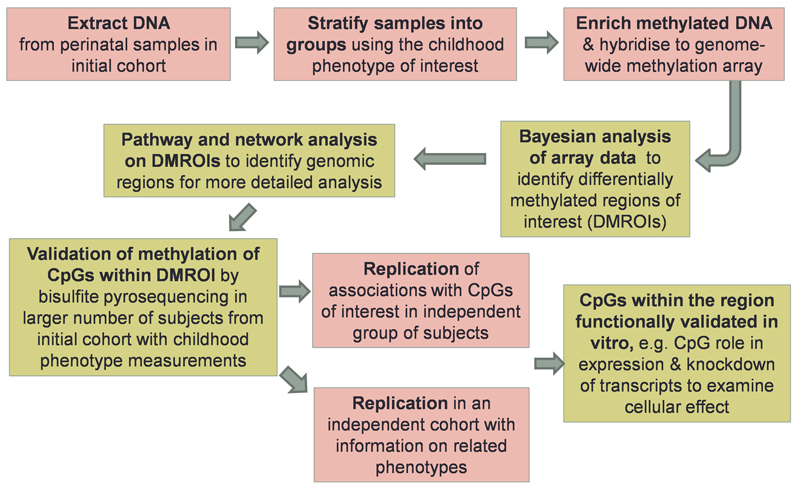
We have shown that epigenetic gene promoter methylation at birth is associated with the child’s later adiposity and measures of bone health [24,25]. In these studies, associations were also observed between levels of RXRA methylation and mothers’ carbohydrate intake [24], supportive of the concept that nutritional conditions in early pregnancy can affect a child’s adiposity in later life. Discovery and validation of perinatal epigenetic biomarkers of metabolic programming is a complex undertaking, in which both replication in independent cohorts and in vitro validation are critical; Figure 2 shows an illustrative work flow for ongoing research that we are undertaking. Alongside systematic genome-wide approaches, insights can also be gained from a candidate approach; recently we have shown that peroxisomal proliferator activated receptor-γ-co-activator-1α promoter methylation in blood at 5–7 years is stable across childhood and associated with adiposity from 9 to 14 years [26].
Figure 2. Illustrative work flow for discovery and validation of perinatal epigenetic biomarkers of metabolic programming.
To examine the relative contributions of genetic and prenatal environmental influences to neonatal methylome variation, samples of umbilical cord DNA from 237 neonates in the Growing Up in Singapore Towards healthy Outcomes cohort study were interrogated on both Illumina Omniexpress+exome genotyping arrays and Illumina Infinium 450K methylation arrays; 958,178 SNPs and 411,107 CpG methylation sites were assayed. Our analysis algorithms identified 1,423 regions for which there was substantial inter-individual variability in DNA methylation, which we termed variably methylated regions (VMRs) [27].
Principal components analysis of the genotypic data resulted in clear separation of Indian neonates from Chinese and Malays on the first principal component, while Chinese and Malay neonates separated on the second principal component; using a similar analysis of the methylome VMR data, the samples did not separate well by ethnicity on the first or second components. The absence of ethnicity driving the methylome in the same manner as observed for the genotype suggests that the genotype plays a subordinate role in specifying methylation levels. We chose nineteen parameters as surrogate measures of the prenatal environment, encompassing the mother’s nutrition, mental health and lifestyle and analysed the data using 39 competing statistical models of genetic polymorphism alone, prenatal environment alone, and genetic differences interacting with the prenatal environment. The results showed that genetic differences alone best explained 25% of the epigenetic variation between neonates, with the remaining 75% best explained by the interaction of genetic differences and the prenatal environment [27]. Focusing on the effects of genotype, the strength of genotype and methylation associations was strongest for SNPs affecting the cytosine-guanosine pairs (CpGs) where DNA methylation typically occurs, intermediate for pairs on the same chromosome (cis) and weakest for pairs on different chromosomes (trans); cis pairs tend towards short distances between the SNP and the CpG, with a mode of 0-10 base pairs, or 50-60 base pairs without the disrupting pairs [28]. The findings have fundamental implications for how epigenetic studies will be conducted in the future and for our understanding of how the mother’s nutrition and lifestyle have long-lasting effects on the health of the offspring.
Conclusion
Non-communicable diseases, including obesity, diabetes, and cardiovascular, chronic lung, mental and neurological disorders, affect all countries, and people of all ages and WHO has identified them as “the world’s biggest killers”. The 2011 High-level Meeting of the United Nations General Assembly on the Prevention and Control of NCDs noted that maternal and child health is inextricably linked with NCDs and their risk factors. It stressed the importance of taking a lifecourse approach to addressing NCDs [28]. Likewise, the UK Department of Health and other agencies now advocate a lifecourse approach to disease prevention from pre-conception through pregnancy, infancy, early years, childhood, adolescence and teenage years, and through to adulthood and preparing for older age. Primary prevention of the non-communicable diseases referred to above requires a deeper understanding of the underlying environmental and societal influences [29] and of the mechanisms underpinning the “memory” of early developmental exposures, alongside determination of whether specific reversal strategies can be achieved. Experimental evidence is accruing that endocrine or nutritional interventions during early postnatal life can reverse epigenetic and phenotypic changes induced, for example, by an unbalanced maternal diet during pregnancy [30]. Elucidation of these epigenetic processes may permit perinatal identification of individuals at risk of later NCD and facilitate a new generation of early intervention strategies to mitigate such risk.
Acknowledgements
KMG is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and by the European Union’s Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement n°289346.
Footnotes
Conflict of interest statement
KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone.
References
- 1.Godfrey KM, Inskip HM, Hanson MA. The long term effects of prenatal development on growth and metabolism. Sem Reprod Med. 2011;29:257–265. doi: 10.1055/s-0031-1275518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javaid MK, Eriksson JG, Kajantie E, Forsen T, Osmond C, Barker DJ, Cooper C. Growth in childhood predicts hip fracture risk in later life. Osteoporos Int. 2011;22:69–73. doi: 10.1007/s00198-010-1224-3. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 4.Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, Robinson SM. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women’s Survey. Am J Clin Nutr. 2010;91:1745–1751. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crozier SR, Harvey NC, Inskip HM, Godfrey KM, Cooper C, Robinson SM. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: findings from the Southampton Women’s Survey. Am J Clin Nutr. 2012;96:57–63. doi: 10.3945/ajcn.112.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole Z, Gale C, Javaid MK, Robinson S, Law C, Boucher B, Crozier S, Godfrey K, Dennison E, Cooper C. Maternal dietary patterns during pregnancy and childhood bone mass: a longitudinal study. J Bone Mineral Res. 2009;24:663–668. doi: 10.1359/jbmr.081212. [DOI] [PubMed] [Google Scholar]
- 7.Harvey NC, Moon RJ, Sayer AA, Ntani G, Davies JH, Robinson SM, Godfrey KM, Inskip HM, Cooper C Southampton Women’s Survey Study Group. Maternal antenatal vitamin D status and offspring muscle development: findings from the Southampton Women’s Survey. J Clin Endocrinol Metab. 2014;99:330–7. doi: 10.1210/jc.2013-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson SM, Crozier SR, Harvey NC, Barton BD, Law CM, Godfrey KM, Cooper C, Inskip HM. Modifiable early life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention. Am J Clin Nutr. in press. [DOI] [PMC free article] [PubMed]
- 9.Moon RJ, Harvey NC, Davies JH, Cooper C. Vitamin D and skeletal health in infancy and childhood. Osteoporos Int. 2014;25:2673–84. doi: 10.1007/s00198-014-2783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inskip HM, Crozier SR, Godfrey KM, Borland SE, Cooper C, Robinson SM. Women’s compliance with nutrition and lifestyle recommendations before pregnancy: general population cohort study. BMJ. 2009;338:b481. doi: 10.1136/bmj.b481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisk CM, Crozier SR, Inskip HM, Godfrey KM, Cooper C, Robinson SM Southampton Women’s Survey Study Group. Influences on the quality of young children’s diets: the importance of maternal food choices. Br J Nutr. 2011;105:287–296. doi: 10.1017/S0007114510003302. [DOI] [PubMed] [Google Scholar]
- 12.Blunden CH, Inskip HM, Robinson SM, Cooper C, Godfrey KM, Kendrick TR. Postpartum depressive symptoms: the B-vitamin link. Ment Health Fam Med. 2012;9:5–13. [PMC free article] [PubMed] [Google Scholar]
- 13.Collings PJ, Brage S, Ridgway CL, Harvey NC, Godfrey KM, Inskip HM, Cooper C, Wareham NJ, Ekelund U. Physical activity intensity, sedentary time, and body composition in preschoolers. Am J Clin Nutr. 2013;97:1020–1028. doi: 10.3945/ajcn.112.045088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inskip H, Macdonald-Wallis C, Kapasi T, Robinson S, Godfrey K, Cooper C, Harvey N, Sayer AA. Associations between grip strength of parents and their 4-year-old children: findings from the Southampton Women’s Survey. Paediatr Perinat Epidemiol. 2012;26:27–33. doi: 10.1111/j.1365-3016.2011.01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon RJ, Harvey NC, Robinson SM, Ntani G, Davies JH, Inskip HM, Godfrey KM, Dennison EM, Calder PC, Cooper C. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J Clin Endocrinol Metab. 2013;98:299–307. doi: 10.1210/jc.2012-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey NC, Moon RJ, Sayer AA, Ntani G, Davies JH, Javaid MK, Robinson SM, Godfrey KM, Inskip HM, Cooper C. Maternal antenatal vitamin D status and offspring muscle development: findings from the Southampton Women’s Survey. J Clin Endocrinol Metab. 2014;99:330–337. doi: 10.1210/jc.2013-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson SM, Marriott LD, Crozier SR, Harvey NC, Gale CR, Inskip HM, Baird J, Law CM, Godfrey KM, Cooper C. Variations in infant feeding practice are associated with body composition in childhood: a prospective cohort study. J Clin Endocrinol Metab. 2009;94:2799–2805. doi: 10.1210/jc.2009-0030. [DOI] [PubMed] [Google Scholar]
- 18.O'Tierney PF, Barker DJ, Osmond C, Kajantie E, Eriksson JG. Duration of breast-feeding and adiposity in adult life. J Nutr. 2009;139:422S–425S. doi: 10.3945/jn.108.097089. [DOI] [PubMed] [Google Scholar]
- 19.Kajantie E, Osmond C, Barker DJ, Eriksson JG. Preterm birth--a risk factor for type 2 diabetes? The Helsinki birth cohort study. Diabetes Care. 2010;33:2623–2625. doi: 10.2337/dc10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfrey KM, Haugen G, Kiserud T, Inskip HM, Cooper C, Harvey NC, Crozier SR, Robinson SM, Davies L, Hanson MA. Fetal liver blood flow distribution: role in human developmental strategy to prioritize fat deposition versus brain development. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041759. e41759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horikoshi M, Yaghootkar H, Mook-Kanamori DO, Sovio U, Taal HR, et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat Genet. 2013;45:76–82. doi: 10.1038/ng.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Non-imprinted epigenetics in fetal and postnatal development and growth. Nestle Nutr Inst Workshop Ser. 2013;71:57–63. doi: 10.1159/000342552. [DOI] [PubMed] [Google Scholar]
- 23.Stünkel W, Pan H, Chew SB, Tng E, Tan JH, Chen L, Joseph R, Cheong CY, Ong ML, Lee YS, Chong YS, et al. Transcriptome changes affecting Hedgehog and cytokine signalling in the umbilical cord: implications for disease risk. PLoS One. 2012;7:e39744. doi: 10.1371/journal.pone.0039744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Burdge GC, McLean C, Rodford J, Slater-Jefferies JL, Garratt E, Crozier SR, Emerald BS, et al. Epigenetic gene promoter methylation at birth is associated with child's later adiposity. Diabetes. 2011;60:1528–34. doi: 10.2337/db10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey NC, Sheppard A, Godfrey KM, McLean C, Garratt E, Ntani G, Davies L, Murray R, Inskip HM, Gluckman PD, Hanson MA, et al. Childhood bone mineral content is associated with methylation status of the RXRA promoter at birth. J Bone Miner Res. 2014;29:600–7. doi: 10.1002/jbmr.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke-Harris R, Wilkin TJ, Hosking J, Pinkney J, Jeffery AN, Metcalf BS, Godfrey KM, Voss LD, Lillycrop KA, Burdge GC. PGC1α promoter methylation in blood at 5-7 years predicts adiposity from 9 to 14 years (EarlyBird 50) Diabetes. 2014;63:2528–37. doi: 10.2337/db13-0671. [DOI] [PubMed] [Google Scholar]
- 27.Teh AL, Pan H, Chen L, Ong ML, Dogra S, Wong J, MacIsaac JL, Mah SM, McEwen LM, Saw SM, Godfrey KM, et al. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Res. 2014;24:1064–74. doi: 10.1101/gr.171439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.United Nations. Political declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases 2011. United Nations; 2011. [Google Scholar]
- 29.Inskip H, Baird J, Barker M, Briley A, Chrousos GP, d’Angelo S, Grote V, Lawrence W, Manios Y, Moschonis G, Poston L, et al. Influences on adherence to diet and physical activity recommendations in women and children; insights from six European studies. Ann Nutr Metab. 2014;64:332–9. doi: 10.1159/000365042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanson MA, Godfrey KM, Lillycrop KA, Burdge GC, Gluckman P. Developmental plasticity and developmental origins of non-communicable disease: theoretical considerations and epigenetic mechanisms. Prog Biophys Mol Biol. 2011;106:272–280. doi: 10.1016/j.pbiomolbio.2010.12.008. [DOI] [PubMed] [Google Scholar]