Supplemental digital content is available in the text.
Key words/Abbreviations: emotional and behavioral problems, children, British birth cohort, epilepsy/single seizures/febrile seizures, fetal risk indicators, social disadvantage, CI = confidence interval, ESDE = Economic and Social Data Service, EUL = end-user licence, FRI = fetal risk indicators, NCDS = National Child and Development Study, OR = odds ratio
ABSTRACT
Objectives
Emotional/behavioral disorders are often comorbid with childhood epilepsy, but both may be predicted by social disadvantage and fetal risk indicators (FRIs). We used data from a British birth cohort, to assess the association of epilepsy, single unprovoked seizures, and febrile seizures with the later development of emotional/behavioral problems.
Methods
A total of 17,416 children in the 1958 British birth cohort were followed up until age 16 years. Logistic and modified Poisson regression models were used to determine a) the association of social disadvantage at birth and FRI with epilepsy, single unprovoked seizures, and febrile seizures at 7 years, and emotional/behavioral disorders in later childhood, and (ii) the association of childhood seizures by age 7 years with emotional/behavioral disorders in later childhood, after accounting for social disadvantage and FRI.
Results
Higher scores on FRI and social disadvantage were associated with emotional/behavioral problems at 7, 11, and 16 years, but not with seizure disorders at age 7 years. Epilepsy was associated with emotional/behavioral problems at 7 years (odds ratio [OR] = 2.50, 95% confidence interval [CI] = 1.29–4.84), 11 years (OR = 2.00, 95% CI = 1.04–3.81), and 16 years (OR = 5.47, 95% CI = 1.65–18.08), whereas single unprovoked seizures were associated with emotional/behavioral problems at 16 years (OR = 1.44, 95% CI = 1.02–2.01), after adjustment for FRI and social disadvantage. Febrile convulsions were not associated with increased risk for emotional/behavioral problems.
Conclusions
Emotional/behavioral problems in children are related to an earlier diagnosis of epilepsy and single unprovoked seizures after accounting for social disadvantage and FRI, whereas febrile convulsions are not associated with emotional/behavioral problems.
INTRODUCTION
Epilepsy is one of the most common neurologic disorder of childhood (1), known to have significant adverse effects on child development and quality of life (2). A higher prevalence of childhood emotional/behavioral problems, including depression, anxiety, and conduct problems, has been observed in children with epilepsy (3). The higher prevalence of such outcomes may be directly related to the effect of the seizures on the developing brain and shared underlying pathology or represent the consequences of living with a challenging and socially stigmatized condition (2,4). The associations may be bidirectional (5). Also, there may be shared risk factors, for example, genetic susceptibility for both conditions, underlying neurologic impairments, and pregnancy-related factors and social disadvantage. Pregnancy-related factors such as prematurity and low birth weight, preeclampsia, and smoking during pregnancy affect fetal health and development (6). Last, social disadvantage may be associated with the development of both epilepsy and childhood emotional/behavioral problems (7,8). Therefore, research examining the association between childhood seizures and emotional/behavioral problems, adjusting for social disadvantage, is required to clarify the association between the two conditions.
Febrile seizures, a common condition in childhood, are frequently characterized as benign with a relatively favorable prognosis in childhood (9). There are few data on mental health outcomes after febrile seizures (10). Previous studies on the associations between febrile seizures and mental health outcomes in children have been based on clinical samples, which are not representative and may represent children with greater morbidity (10). Single unprovoked seizures seem to have a favorable behavioral or cognitive outcome (11), although there is an increased long-term risk of epilepsy (12).
Previous research in this field has been limited by the use of cross-sectional data (through which the direction of associations cannot be established) (13) and reliance on clinically defined populations (which may be unrepresentative, with more psychiatric comorbidity and poorer prognosis). Given these limitations of previous work, using nationally representative cohort data and structured validated scales to determine childhood psychopathology, we sought to establish the following:
The independent associations of fetal risk indicators and social disadvantage at birth with the development of seizure disorders at 7 years and with childhood emotional/behavioral problems at 7, 11, and 16 years
The association of epilepsy, febrile convulsions, and single unprovoked seizures by age 7 years with the development of childhood emotional/behavioral problems at age 7, 11, and 16 years, after adjusting for fetal risk indicators and social disadvantage at birth
We hypothesized that children born into relative social disadvantage or who had experienced greater fetal risk would be more likely to experience seizure disorders as well as childhood emotional/behavioral problems, at later time points in childhood. Second, we hypothesized that children with a confirmed seizure would be more likely to experience emotional/behavioral problems, and that this elevated emotional/behavioral comorbidity would persist despite adjustment for social disadvantage and fetal risk indicators.
METHODS AND MATERIALS
Study Population
The analysis was based on a cohort of 17,416 babies who participated in the National Child Development Study (NCDS), out of 18,558 eligible children born in England, Scotland, and Wales in the first week of March 1958 (14). We used information collected from parents at birth (baseline survey; 1958) and from parents and teachers at 7 years of age (first sweep; 1965), 11 years (second sweep; 1969), and 16 years (third sweep; 1974).
Determination of Seizure Disorders
A question about a history of seizures was asked at each sweep of the NCDS. A positive response to this screening question at 7 years was used to select case notes from family doctors who attended these children to determine if they had epilepsy or febrile convulsions. Epilepsy was defined as a history of two unprovoked seizures (15). Febrile convulsions were defined as associated with a febrile illness or fever, without evidence of intracranial infection in children 3 months to 5 years (16). Seizures that did not fulfill the criteria for epilepsy were classified as “single nonfebrile seizures.” Definitions for seizures are based on International League Against Epilepsy recommendations (17).
Social Disadvantage
Social disadvantage was measured by four questions regarding parental occupational social class, housing tenure, single-mother family, and financial difficulties. Occupational social class was available at all sweeps, whereas data on financial difficulties and tenure were available for ages 7, 11, and 16 years. Single-mother status was defined as mothers raising children as single parents; this variable was included as a measure for social disadvantage because it may be associated with adverse child health outcomes (18). Occupational social class was classified as a binary variable comprising Social Class IV and Social Class V (manual), versus Social Classes I, II, and III (nonmanual). Housing tenure was categorized into rented/council housing versus owner-occupied homes. Financial difficulties were determined from a question about parental financial ability (“Have you ever experienced financial difficulty?”), with the answer dichotomized into a “Yes” or “No” response. Each social disadvantage indicator was investigated separately, and then a combined variable generated as a summated score of the number of indicators positive in one individual ranging from zero to four. This approach assigned equal weights to each social disadvantage indicator; a similar approach has previously been applied as a measure for cumulative social disadvantage in other work on child health (19). The Φ coefficients between the social disadvantage indicators were significantly positively correlated, supporting our decision to generate aggregated scores.
Fetal Risk Indicators
Pregnancy-related data were derived from family doctor medical notes about the delivery of the child. Prematurity was defined as a delivery before 259 days (20). Low birth weight was defined as less than 2500 grams, regardless of gestation (21). Preeclampsia was assessed as present in mothers who experienced symptoms or signs of high blood pressure, toxemia, or proteinuria, or who had received treatment of eclampsia (22). Mothers were asked if they smoked during pregnancy and responses encoded as “Yes” or “No.” Each fetal risk indicator was investigated separately, and then a combined variable generated as a summated score of the number of positive indicators, ranging from zero to four, this was an ad hoc approach designed to assess the cumulative impact of fetal risk indicators on epilepsy.
Childhood Emotional/Behavioral Problems
Emotional/behavioral problems in childhood are often usefully divided into internalizing disorders (symptoms of depression, anxiety, and social withdrawal) and externalizing problems (disruptive behaviors, including hyperactivity and conduct problems). This conceptual framework was applied in the NCDS, with different age-appropriate tools used to assess emotional/behavioral problems at ages 7 and 11 years (the Bristol Social Adjustment Guide) (23,24), and at 16 years (the Rutter Scale-B) (25). Both assessments were administered to the children's teachers.
The Bristol Social Adjustment Guide assesses 12 syndromes, from which scales for internalizing disorder (unforthcomingness, withdrawal, depression, miscellaneous symptoms for internalizing problems, and nervous symptoms) and externalizing disorders (hostility toward adults, hostility toward children, “writing-off” children and adults, anxiety for acceptance by children and adults, restlessness, and inconsequential behavior) were derived (23).
The teacher version of the Rutter-B scales at age 16 years assessed 18 items. An internalizing problem scale was derived from five items, which were as follows: worries, solitary, miserable, fearful, and fussy (26). An externalizing problem scale was derived by summing reports of restlessness, fidgety, destructiveness, fights, not liked by others, irritable, twitches/mannerisms/tics, sucks thumbs, bites nails, disobedient, poor concentration, lies, and bullies (26). Cases were defined as scores in the top 13th percentile, whereas noncases were those in the lower 87th percentile. This approach has been used by others (27,28), with scores in the top 13% taken to represent children deemed to be most “maladjusted” for either the externalizing or internalizing problem scale (28). Associations were based on 13% of the summed scores for all items because these indicate children with the greatest degree of psychopathology, or caseness for internalizing and externalizing disorders (27,28). The teacher-rated Rutter at age 16 years has been shown to have adequate sensitivity, specificity, and reliability in epidemiologic samples (25). The Bristol Social Adjustment Guide has been shown to closely correlate with the Rutter (28), with good reliability at each of the sweeps it was used (27).
Statistical Analysis
All analyses were performed using STATA, version 11. Pearson χ2 test was used to compare the boys and girls who participated at each stage of the study. Multivariable logistic regression was used to determine the association of social disadvantage at birth and fetal risk indicators with subsequent childhood seizures, specifically epilepsy, febrile convulsions, and single unprovoked seizures mutually adjusting for social disadvantage when fetal risk indicators are the explanatory variables and vice versa. A similar analytical strategy was used to assess the association of social disadvantage indicators and fetal risk indicators with childhood emotional/behavioral problems (internalizing disorders, externalizing disorders, and all emotional/behavioral disorders) at ages 7, 11, and 16 years. Finally, multivariable logistic regression was used to determine the association of seizure disorders by 7 years and childhood emotional/behavioral disorders at 7, 11, and 16 years, after adjusting for social disadvantage at birth, fetal risk indicators, and sex. For continuous variables, tests for linearity and departure from linearity were assessed using likelihood ratio tests. Where there was evidence of a departure from linearity, variables were assessed as categorical exposures, with no assumptions of linearity made.
The main analyses are based on cross-sectional assessments of emotional/behavioral disorders at each stage and then for longitudinal incident cases at each stage, whereby earlier cases were removed from the analyses. We reported the effect sizes derived from the logistic regression as odds ratios (ORs) and from the modified Poisson regression models as incident risk ratios (29) and their corresponding 95% confidence intervals (95% CIs). Significance of association was assessed using Wald tests. We did not think it was necessary to perform post hoc correction for multiple comparisons in our study because a) our analyses was based on well-defined hypothesis, b) most multiple tests account for overall inflated errors at the expense of or obscuring well-defined clinical questions of interest, and c) these tests can increase the likelihood of Type II error (30,31).
Ethical Statement
Permission to use the NCDS data for the purposes of secondary analysis was granted according to end-user licence agreement number SN 5565, upon a commitment to adhere to all ethical requirements on data usage, distribution, and safeguarding of participants' confidentiality. A “special licence” for data held under “special conditions” (as specified in section 5 of the Economic and Social Data Service end-user licence) was needed to access additional biomedical data.
RESULTS
Response Rates
Of the original sample of 18,558 children, 83%, 83%, and 79% participated in the first, second, and third surveys, respectively (Fig. 1). Significantly more boys than girls participated in the first survey (48.7% versus 46.4%, p = .018), but not in the second (48.6% versus 46.9%, p = .079) and third (48.5% versus 47.5%, p = .25; Table 1).
FIGURE 1.
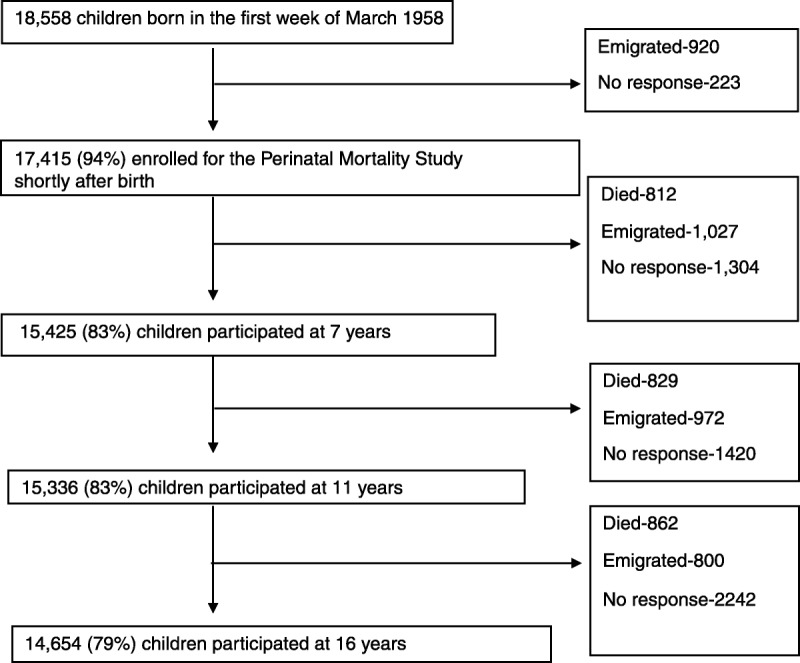
Derivation of study sample. There was relatively minimal attrition across the sweeps and most was due to emigration, nonresponse, and deaths.
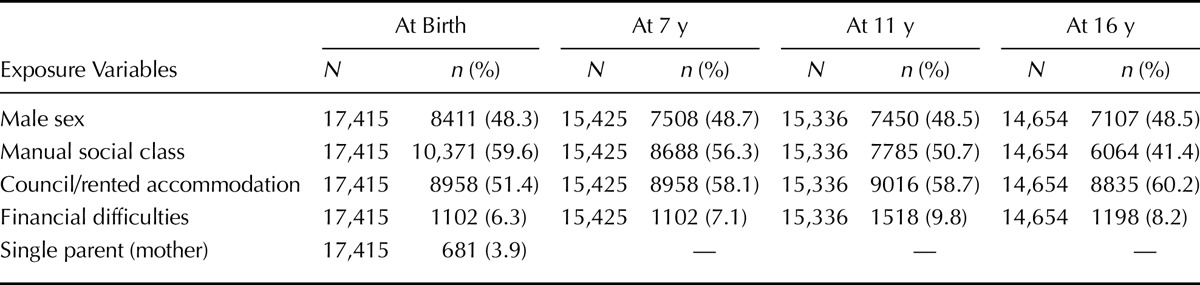
TABLE 1.
Distribution of Sex and Social Disadvantage Indicators Across the Sweeps
Social Disadvantage Indicators and Perinatal Complications
Across the four sweeps, the percentage of parents who reported manual occupational social class ranged from 41.4% to 59.6% (Table 1), rented accommodation from 51.4% to 60.2%, and financial difficulty from 7.1% to 9.8% (Table 1). Six hundred eighty-one mothers (3.9%) delivered when they were single. During pregnancy, 5783 (33.2%) of 17,415 mothers smoked and 5268 (30.3%) of 17,415 had symptoms compatible with either preeclampsia and/or eclampsia. There were 2773 (15.9%) of 17,415 premature deliveries and 1909 (11.0%) of 17,415 babies with low birth weight.
There were no data on house tenure and financial difficulty in Sweep 1, which was assumed to have been similar with that collected at Sweep 2. The proportion without rented accommodation had privately owned accommodation.
Seizure Disorders
Nine hundred eighty-three (2.2%) of 15,425 children were reported as having had seizures or convulsions at the first sweep at 7 years. Of these 983 with seizures, 65 (6.6%) were classified as having epilepsy, whereas 244 (24.8%) had a history suggestive of febrile convulsions. The remainder had single unprovoked seizures not classified as either epilepsy or febrile seizures. Based on the 15,425 children in the first sweep, the overall prevalence of epilepsy, febrile convulsions, and single unprovoked seizures was 0.4% (95% CI = 0.3%–0.5%), 1.6% (95% CI = 1.4%–1.8%), and 4.4% (95% CI = 4.3%–4.5%), respectively.
Emotional/Behavioral Disorders
Overall, emotional/behavioral disorders were present in 4121 (26.7%) of 15,425 at 7 years, 3688 (24.1%) of 15,336 at 11 years, and 3105 (26.8%) of 11,572 at 16 years.
Internalizing disorders were present in 2676 (17.3%) of 15,425 at 7 years, 2354 (15.3%) of 15,336 at 11 years, and 1666 (14.3%) of 11,572 at 16 years. Externalizing disorders in the clinical range were present in 2190 (14.2%) of 15,425 at 7 years, 2072 (13.5%) of 15,336 at 11 years, and 2010 (17.4%) of 11,572 at 16 years.
Association of Social Disadvantage and Perinatal Complications With Seizures and Emotional/Behavioral Disorders
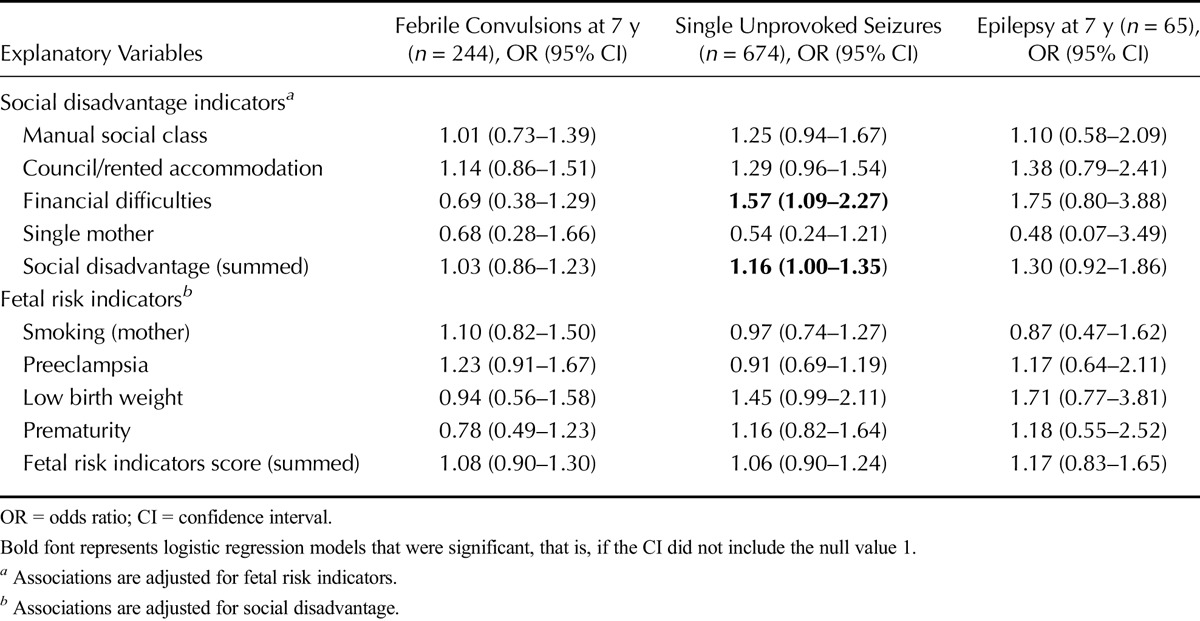
Summed scores of social disadvantage or fetal risk indicators were associated neither with epilepsy nor febrile convulsions (Table 2). However, there was some evidence to support an association between mothers reporting financial difficulties and single unprovoked seizures at age 7 years, with a similar but nonsignificant trend for epilepsy at age 7 years (Table 2). Similarly, there was a sizeable but nonsignificant trend indicative of children with low birth weight being more likely to experience single unprovoked seizures or epilepsy by the age of 7 years (Table 2).
TABLE 2.
The Association of Social Disadvantage at Birth and Fetal Risk Indicators With Febrile Convulsions and Seizures at 7 Years
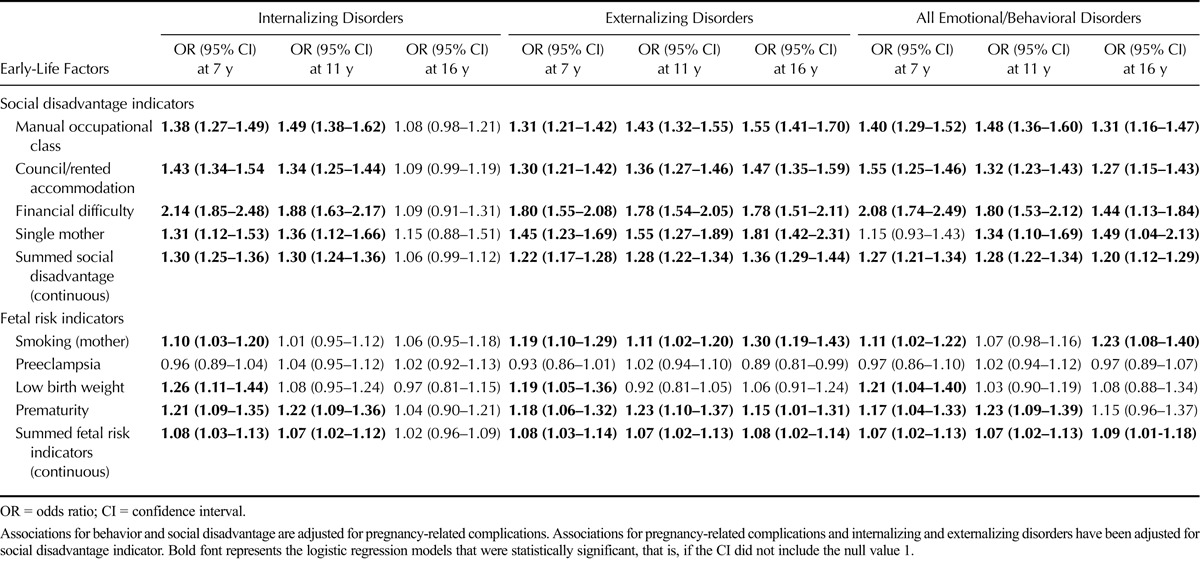
Each additional 1-unit increase in the summed score for social disadvantage was associated with emotional/behavioral disorders at age 7 years (OR = 1.27, 95% CI = 1.21–1.34), 11 years (OR = 1.28, 95% CI = 1.22–1.34), and 16 years (OR = 1.20, 95% CI = 1.12–1.29, all p < .001[trend]), after adjusting for fetal risk indicators (Table 3). Other associations of social disadvantage with behavioral disorders are displayed in Table 3. For each additional fetal risk indicator, ORs for the association with emotional/behavioral disorders were also elevated (at age 7 years: OR = 1.09, 95% CI = 1.04–1.14, p = .009 [trend]; at age 11 years: OR = 1.11, 95% CI = 1.05–1.16, p = .010 [trend]; and at age 16 years: OR = 1.07, 95% CI = 1.01–1.13, p = .027 [trend]), after controlling for social disadvantage. Other specific pregnancy-related associations with emotional/behavioral disorders are outlined in Table 3.
TABLE 3.
Association Between Social Disadvantage at Birth and Fetal Risk Indicators With Later Childhood Emotional/Behavioral Disorders (at Ages 7, 11, and 16 Years)
Association of Seizure Disorders With Emotional/Behavioral Disorders
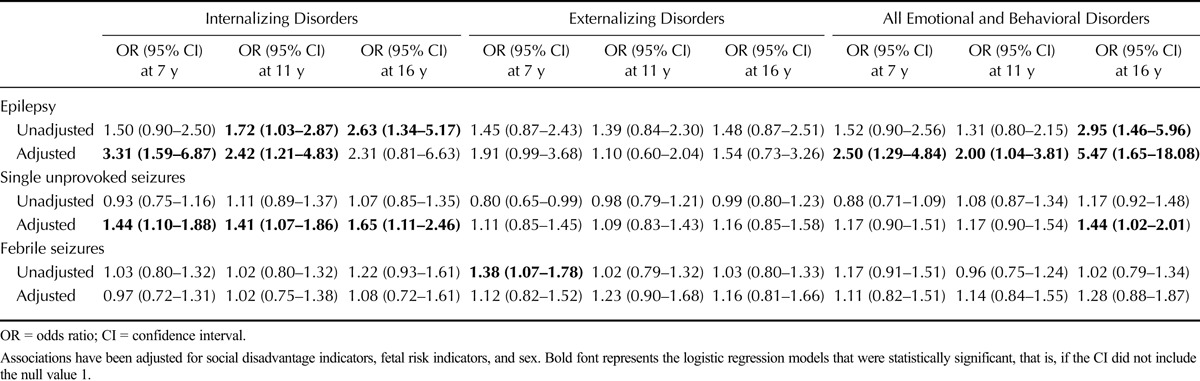
Table 4 displays the relative odds of emotional/behavioral disorders at ages 7, 11, and 16 years for children classified as having epilepsy, single unprovoked seizures, and febrile convulsions by the age of 7 years compared with those without any of these conditions, after adjusting for social disadvantage, fetal risk indicators, and sex. The most striking mental health associations were with epilepsy, with the association with all emotional/behavioral problems being significant at 16 years (OR = 5.47, 95% CI = 1.68–18.08). Most of the excess risk of emotional/behavioral disorders associated with epilepsy was accounted for by internalizing disorders. Similar association between mental health problems and epilepsy has been reported in a previous study (32). Single unprovoked seizures were also significantly associated with internalizing disorders at all ages. Otherwise, no substantial or statistically significant associations were observed between febrile convulsions and any emotional/behavioral disorder outcomes (for details, see Table 4). Based on incident cases of emotional/behavioral problems at each stage, none of the seizure disorders significantly increased the risk for emotional/behavioral problems (see Table S1, Supplemental Digital Content 1, http://links.lww.com/PSYMED/A268, which summarizes associations of seizure disorders with incident cases of emotional/behavioral problems).
TABLE 4.
The Association of Epilepsy, Febrile Convulsions, and Single Unprovoked Seizures at Age 7 Years With Childhood Emotional/Behavioral Disorders at Ages 7, 11, and 16 Years
DISCUSSION
This study examined the risk for emotional/behavioral problems in children after a history of epilepsy, febrile convulsions, or single unprovoked seizures, adjusting for social disadvantage and indicators of fetal risk. Social disadvantage and fetal risk indicators consistently predicted the development of emotional/behavioral disorders across all ages studied, but not seizures in children. Epilepsy was associated with emotional/behavioral problems across all ages studied, even after accounting for disadvantaged social circumstances and fetal risk indicators.
Seizures and Emotional/Behavioral Disorders
Our findings support the hypothesis that epilepsy is a risk factor for the development of emotional/behavioral problems in childhood, consistent with previous studies (33). The increased prevalence of psychopathology may be related to the epilepsy or may be a consequence of psychosocial disadvantage associated with having to live with epilepsy (34). The risk of emotional/behavioral problems after epilepsy was greater at 16 years than at 7 years, suggesting an increasing risk over time possibly related to accumulating brain damage, or the cumulative effects of living with the illness. Hormonal changes and maturation during adolescence of brain regions and networks involved in the neuropathology underlying epilepsy and in psychopathology might also play a role in these findings. At the ages of 11 and 16 years (1969 and 1974), a fifth of the children with epilepsy in this cohort were in special schools (15), a marker both of the cognitive impairment that may have shared risk factors with epilepsy and the historical failure to integrate such children within the mainstream. Most of the childhood psychological ill health associated with epilepsy was accounted for by an increased risk for internalizing disorders, which is consistence with findings in the literature (2).
Like previous studies (35,36), febrile convulsions in the first 5 years of life were not associated with emotional or behavioral problems. However, single unprovoked seizures were associated with internalizing disorders at all ages and emotional/behavioral disorders at 16 years. More research is needed into underlying etiological mechanisms; it is possible that neurologic pathology or genetic susceptibility may have predisposed children to seizures and emotional problems (11), or, alternatively, single seizures may have been associated with greater family disruption and child distress leading to emotional problems.
Social Disadvantage and Fetal Risk Indicators as Risk Factors for Seizures and Emotional/Behavioral Disorders
A summed score of the social disadvantage variable was not associated with an increased risk for seizures disorders at 7 years, supporting an Icelandic case-control study (37), but contrasting a prospective study in England (7). We did find an association between financial difficulties and single unprovoked seizures, and a strong but nonsignificant trend toward such an association with epilepsy. Similarly, low birth weight seemed to be associated with single unprovoked seizures and possibly epilepsy; it is possible that the study was not sufficiently powered to detect differences for these analyses. A lack of association between fetal risk indicators and seizures contrasts some earlier studies (38), but these studies also did not account for social disadvantage. We examined epilepsy at 7 years; however, social disadvantage and fetal risk indicators may still be associated with the onset of epilepsy in subsequent years.
Both social disadvantage and fetal risk indicators independently increased the risk for emotional/behavioral problems, suggesting, at least to some extent, separate mechanisms. Prematurity and low birth weight are associated with neuropathologies (38), whereas smoking may impair fetal blood supply resulting in neurodevelopment disorders (39).
Strengths and Limitations of the Study
The strengths of this study are in the use of a large cohort with minimal attrition in childhood surveys and the use of structured and validated tools to assess psychological morbidity. An additional advantage was in the use of medical records to confirm the diagnosis of seizure disorders. The associations discussed are based on cross-sectional measures in each stage. The lower estimate of epilepsy is probably because only one screening question for seizures was used in the second survey of 1965. The lack of longitudinal data for seizures may have affected findings of some associations; for example, some cases of epilepsy may have resolved at later time points or single unprovoked seizures progressed into epilepsy. It cannot be known if the children who did not participate in the study were possible cases of either emotional/behavioral disorders or seizures. Despite a large cohort of more than 17,000 children, some of the analyses may have been underpowered. In addition, our measures for social disadvantage were relatively coarse and may not have adequately captured the relevant aspects of poverty-induced disadvantage. Like any observational study, our analysis may have suffered from residual confounding by genetic and nongenetic influences not investigated in this study (36). Our comparison or reference group comprised those without seizures. The high prevalence of emotional/behavioral problems may be a reflection of the tools used, which led to a dimensional count of symptoms as opposed to categorical diagnoses. Use of different scales for specific age-groups may lead to differences in prevalence of mental health problems at these ages. Because scales used to assess emotional/behavioral problems in this study are no longer in use in clinical practice, future studies could attempt to replicate these findings using scales in current practice.
CONCLUSIONS
Development of emotional/behavioral disorders in children is related to an earlier diagnosis of seizures after accounting for social disadvantage and fetal risk indicators. Social disadvantage and fetal risk indicators were independently associated with the later development of emotional/behavioral problems, and possible associations with seizure onset in subsequent years cannot be excluded. The results suggest that the association of epilepsy with emotional/behavioral problems remains significant even after accounting for indicators of social disadvantage and fetal life. These findings suggest that social disadvantage and a history of fetal vulnerability should not be a barrier to accessing care in children with mental health problems.
Acknowledgments
J.D.M. is a Health Foundation/Academy of Medical Sciences Clinician Scientist Fellow. The analyses in this work are based wholly on analysis of data from the National Child and Development Study. The data were deposited at the UK Data Archive by the Centre for Longitudinal Studies at the Institute of Education, University of London.
Source of Funding and Conflicts of Interest: The National Child Development Study, the source of this analysis data set, is funded by the Economic and Social Research Council. The British Commonwealth Scholarship Commission and Wellcome Trust supported the master of science studies for S.M.K. at King's College London between 2010 and 2011 when this analysis was conceived and performed. J.D. is funded by the Health Foundation, working with the Academy of Medical Sciences. The authors declare no conflicts of interests.
Footnotes
Supplemental Content
REFERENCES
- 1.Camfield CS, Camfield PR, Gordon K, Wirrell E, Dooley JM. Incidence of epilepsy in childhood and adolescence: a population-based study in Nova Scotia from 1977 to 1985. Epilepsia 1996;37:19–23. [DOI] [PubMed] [Google Scholar]
- 2.Rodenburg R, Stams GJ, Meijer AM, Aldenkamp AP, Deković M. Psychopathology in children with epilepsy: a meta-analysis. J Pediatr Psychol 2005;30:453–68. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger AB, Weisbrot DM, Nolan EE, Gadow KD, Vitale SA, Andriola MR, Lenn NJ, Novak GP, Hermann BP. Symptoms of depression and anxiety in pediatric epilepsy patients. Epilepsia 1998;39:595–9. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal N, Govender S. Epilepsy and neuropsychiatric comorbidities. Adv Psychiatr Treat 2011;17:44–53. [Google Scholar]
- 5.Adelow C, Andersson T, Ahlbom A, Tomson T. Hospitalization for psychiatric disorders before and after onset of unprovoked seizures/epilepsy. Neurology 2012;78:396–401. [DOI] [PubMed] [Google Scholar]
- 6.Whitehead E, Dodds L, Joseph KS, Gordon KE, Wood E, Allen AC, Camfield P, Dooley JM. Relation of pregnancy and neonatal factors to subsequent development of childhood epilepsy: a population-based cohort study. Pediatrics 2006;117:1298–306. [DOI] [PubMed] [Google Scholar]
- 7.Heaney DC, MacDonald BK, Everitt A, Stevenson S, Leonardi GS, Wilkinson P, Sander JW. Socioeconomic variation in incidence of epilepsy: prospective community based study in south east England. BMJ 2002;325:1013–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalff AC, Kroes M, Vles JS, Hendriksen JG, Feron FJ, Steyaert J, van Zeben TM, Jolles J, van Os J. Neighbourhood level and individual level SES effects on child problem behaviour: a multilevel analysis. J Epidemiol Community Health 2001;55:246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verity CM, Greenwood R, Golding J. Long-term intellectual and behavioral outcomes of children with febrile convulsions. N Engl J Med 1998;338:1723–8. [DOI] [PubMed] [Google Scholar]
- 10.Baram TZ, Shinnar S. Febrile Seizures. San Diego: Academic Press; 2002. [Google Scholar]
- 11.Sogawa Y, Masur D, O'Dell C, Moshe SL, Shinnar S. Cognitive outcomes in children who present with a first unprovoked seizure. Epilepsia 2010;51:2432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira C, Resende C, Fineza I, Robalo C. A 15-year follow-up of first unprovoked seizures: a prospective study of 200 children. Epileptic Disord 2014;16:50–5. [DOI] [PubMed] [Google Scholar]
- 13.Berg AT, Caplan R, Hesdorffer DC. Psychiatric and neurodevelopmental disorders in childhood-onset epilepsy. Epilepsy Behav 2011;20:550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Power C, Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study). Int J Epidemiol 2006;35:34–41. [DOI] [PubMed] [Google Scholar]
- 15.Ross EM, Peckham CS, West PB, Butler NR. Epilepsy in childhood: findings from the National Child Development Study. Br Med J 1980;280:207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verity CM, Butler NR, Golding J. Febrile convulsions in a national cohort followed up from birth. II—medical history and intellectual ability at 5 years of age. Br Med J (Clin Res Ed) 1985;290:1311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ILAE. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1989;30:389–99. [DOI] [PubMed] [Google Scholar]
- 18.Brown SL. Marriage and child well-being: research and policy perspectives. J Marriage Fam 2010;72:1059–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauman LJ, Silver EJ, Stein RE. Cumulative social disadvantage and child health. Pediatrics 2006;117:1321–8. [DOI] [PubMed] [Google Scholar]
- 20.Henderson JL. The definition of prematurity. BJOG 1945;52:29–35. [Google Scholar]
- 21.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts JM, Redman CWG. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet 1993;341:1447–51. [DOI] [PubMed] [Google Scholar]
- 23.Scott D. The Social Adjustment of Children. 3rd ed London, England: University of London Press; 1969. [Google Scholar]
- 24.McKenzie DJ, Wilson PE. A pilot study of the bristol social adjustment guides used with pre-school children. Aust Psychol 1969;3:177–80. [Google Scholar]
- 25.Elander J, Rutter M. Use and development of the Rutter parents' and teachers' scales. Int J Methods Psychiatr Res 1996;6:63–78. [Google Scholar]
- 26.Rutter M, Graham P, Yule W. A Neuropsychiatric Study in Childhood. London: Heinemann Medical; 1970. [Google Scholar]
- 27.Clark C, Rodgers B, Caldwell T, Power C, Stansfeld S. Childhood and adulthood psychological ill health as predictors of midlife affective and anxiety disorders: the 1958 British birth cohort. Arch Gen Psychiatry 2007;64:668–78. [DOI] [PubMed] [Google Scholar]
- 28.Ghodsian M, Fogelman K, Lambert L, Tibbenham A. Changes in behaviour ratings of a national sample of children. Br J Soc Clin Psychol 1980;19:247–56. [DOI] [PubMed] [Google Scholar]
- 29.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ 1995;310:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook RJ, Farewell VT. Multiplicity considerations in the design and analysis of clinical trials. J R Stat Soc Ser A 1996;159:93–110. [Google Scholar]
- 32.Tegethoff M, Belardi A, Stalujanis E, Meinlschmidt G. Association between mental disorders and physical diseases in adolescents from a nationally representative cohort. Psychosom Med 2015;77:319–32. [DOI] [PubMed] [Google Scholar]
- 33.Davies S, Heyman I, Goodman R. A population survey of mental health problems in children with epilepsy. Dev Med Child Neurol 2003;45:292–5. [DOI] [PubMed] [Google Scholar]
- 34.Caplan R. Psychopathology and epilepsy: a two-way relationship. Epilepsy Curr 2012;12:201–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang Y-C, Guo N-W, Huang C-C, Wang S-T, Tsai J-J. Neurocognitive attention and behavior outcome of school-age children with a history of febrile convulsions: a population study. Epilepsia 2000;41:412–20. [DOI] [PubMed] [Google Scholar]
- 36.Visser AM, Jaddoe VW, Ghassabian A, Schenk JJ, Verhulst FC, Hofman A, Tiemeier H, Moll HA, Arts WF. Febrile seizures and behavioural and cognitive outcomes in preschool children: the Generation R study. Dev Med Child Neurol 2012;54:1006–11. [DOI] [PubMed] [Google Scholar]
- 37.Hesdorffer DC, Tian H, Anand K, Hauser WA, Ludvigsson P, Olafsson E, Kjartansson O. Socioeconomic status is a risk factor for epilepsy in Icelandic adults but not in children. Epilepsia 2005;46:1297–303. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y, Vestergaard M, Pedersen CB, Christensen J, Basso O, Olsen J. Gestational age, birth weight, intrauterine growth, and the risk of epilepsy. Am J Epidemiol 2008;167:262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stene-Larsen K, Borge AI, Vollrath ME. Maternal smoking in pregnancy and externalizing behavior in 18-month-old children: results from a population-based prospective study. J Am Acad Child Adolesc Psychiatry 2009;48:283–9. [DOI] [PubMed] [Google Scholar]


