With 40 years of cancer data from the oldest population-based cancer registry in China, this study evaluates secular time trends in breast cancer incidence and mortality in an urban Chinese population. Our results show a tremendous increase in incidence and a slight increase in mortality with significant age, cohort, and period effects for breast cancer among women in urban Shanghai.
Keywords: breast cancer, incidence, mortality, trends
Abstract
Background
Breast cancer incidence rates are increasing among Asian women, likely due to the changes in risk factors caused by globalization. Trends in breast cancer rates among Chinese women may differ from other Asian regions due to the implementation of a nationwide family planning program and resulting changes in women's reproductive practices. Appraisal of cancer trends can direct cancer control and public health planning, but relevant studies in China are scarce due to a lack of long-term data. We sought to evaluate secular time trends in breast cancer incidence and mortality using 40 years of cancer registry data for women in urban Shanghai.
Materials and methods
Data on invasive breast cancer incidence and mortality were collected by the Shanghai Cancer Registry. Age-standardized rates (ASRs) for incidence and mortality were calculated using the Segi/Doll 1960 world standard population. Age, period, and birth cohort effects were evaluated using age–period–cohort (APC) Poisson regression models. Overall linear trends, interpreted as the estimated annual percentage change (EAPC), were derived from the net drift in age–drift models.
Results
A total of 53 885 breast cancer cases and 17 235 breast cancer-specific deaths were documented among women in urban Shanghai between 1 January 1973 and 31 December 2012. Breast cancer incidence and mortality ASRs increased by 141.2% and 26.6%, respectively. Significant age, cohort, and period effects were identified in both incidence and mortality APC models; cohort effects were pronounced. Overall, a substantial increase in breast cancer incidence (EAPC = 2.96%/year) and a moderate increase in breast cancer mortality (EAPC = 0.87%/year) was observed. A notable downward trend in mortality was identified among younger women born after 1960.
Conclusions
Forty years of cancer registry data document a tremendous increase in incidence and a slight increase in mortality for breast cancer among women in Shanghai. Effective, appropriate, and affordable breast cancer prevention and control strategies are urgently needed in China.
introduction
Breast cancer is the most common cancer and the fifth leading cause of cancer deaths among women worldwide. In 2012, it is estimated that 1.67 million women were diagnosed with breast cancer and 522 000 women died from this disease, corresponding to 25.1% of all cancers, and 14.7% of all cancer deaths in women [1]. Incidence and mortality rates for breast cancer vary considerably across geographic regions [1]. Women in Eastern Asia have a lower breast cancer incidence age-standardized rate (ASR: 27.0/100 000) than women in either Western Europe (91.1/100 000) or Northern America (91.6/100 000) [1]. Breast cancer rates are increasing across almost all regions of the world [2]. This has been attributed to a transition in the risk factors caused by globalization of economies and behaviors [3]. This is particularly apparent among Asian women, as breast cancer incidence has doubled or tripled in Japan, Korea, Hong Kong, and Singapore over the past 40 years [4–6]. Among women in China, changes in reproductive practices, such as lower parity and reduced breastfeeding, may have been influenced not only by globalization, but also by the implementation of a nationwide family planning program in the 1970s. Thus, trends in breast cancer incidence and mortality among Chinese women may differ from women in other developed Asian countries.
Appraisal of cancer trends can direct future cancer control and public health planning. However, relevant studies in China are scarce due to the lack of long-term cancer incidence data. Further, most existing studies on breast cancer trends among Chinese or Asian women have focused on mortality or employed Joinpoint regression [7, 8]. As the oldest population-based cancer registry in China, the Shanghai Cancer Registry (SCR) has accumulated 40 years of data on cancer incidence and mortality. Analyses of such valuable historical data will not only lay the groundwork for future cancer control efforts in Shanghai, but also provide reference for other rapidly developing regions. Therefore, this study was conducted to evaluate secular time trends in breast cancer incidence and mortality, including age, period, and birth cohort effects, among women in urban Shanghai over the last four decades.
materials and methods
incidence, mortality, and population data
Data on invasive breast cancer incidence and mortality were available from the SCR, as previously described [9]. Briefly, the SCR was established in 1963, and is the oldest population-based cancer registry in China, and one of the largest cancer registries in the world. Complete incidence and mortality data have been collected since 1973 for urban areas and since 2002 for rural areas. Data for the urban areas, which include 289.4 km2 and an average of 7 million residents, have consistently reached the standards set by the International Agency for Research on Cancer (IARC), and have been published in its quinquennial publications: Cancer Incidence in Five Continents, volumes IV–X. Annual population data were provided by Department of Vital Statistics, Shanghai Municipal Center for Disease Control and Prevention. The study population for the current analysis included an average of 3.1 million female permanent residents in urban Shanghai between 1 January 1973 and 31 December 2012.
statistical analysis
Incidence and mortality ASR were calculated using the Segi/Doll 1960 world standard population [10]. Effects of age (A), calendar period (P), and birth cohort (C, C = P-A) on incidence and mortality were evaluated using age–period–cohort (APC) models [11, 12]. On the basis of Poisson regression, the APC models can be written as log(λ(A·P)) = f(A) + g(P) + h(C), where A, P, and C represent the mean age, period, and birth cohort for the observational units, and λ(A·P) is the incidence or mortality rate at age A in period P for women in birth cohort C. We restricted the APC analyses to women aged 20–84 to avoid statistical instability due to the small numbers of cancer cases among younger women and small populations of older women. Study populations were categorized into thirteen 5-year age groups (20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and 80–84) and eight 5-year calendar period groups (1973–1977, 1978–1982, 1983–1987, 1988–1992, 1993–1997, 1998–2002, 2003–2007, and 2008–2012) according to their age and date of diagnosis, respectively. Twenty 5-year synthetic birth cohort groups were computed by subtracting the midpoint of age groups from the midpoint of period groups. Five submodels were derived in terms of the factors involved in the APC modeling, including age models, age–drift models, age–cohort models, age–period models, and APC models. Model goodness-of-fit was evaluated based on residual deviance statistics. Age, period, and birth cohort effects were derived from pairwise comparisons of the appropriate sub-models. Significance of the pairwise comparisons was examined by comparing the difference in residual deviance using χ2 tests. Overall linear trends, interpreted as estimated annual percentage changes (EAPCs), were derived from the net drift in age–drift models. For visualization of trends, age groupings were also simplified: 20–29, 30–39, 40–49, 50–59, 60–69, and 70–84. All analyses were performed using the APC·fit function in the Epi package [13] in R (version 3.0.1), and used a two-sided probability with a significance level of 0.05.
results
Among women in Shanghai, a total of 53 885 breast cancer cases and 17 235 breast cancer-specific deaths were documented between 1973 and 2012 (Table 1). Overall, the breast cancer incidence ASR increased by 141.2%, with the lowest ASR (17.14/100 000) in the earliest 5-year period, and the highest ASR (41.33/100 000) in the most recent 5-year period. The increase in incidence was observed across the age span, with the greatest increase in age group 40–49 (176.7%). Compared with incidence, the increase in mortality ASR was much smaller (26.6%), with the lowest ASR (7.12/100 000) from 1983 to 1987, and the highest ASR (9.64/100 000) in the most recent 5-year period. The increase in mortality differed by age. The greatest increase was observed in women over 80 (133.3%), followed by moderate increases among women aged 40–79. Notably, mortality decreased among women aged 30–39 across successive periods of time (−18.3%).
Table 1.
Breast cancer incidence and mortality age-specific rates and age-standardized rates (1/100 000) among women in urban Shanghai (1973–2012), by period
| Age | Period |
Total | Overall changea (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1973–1977 | 1978–1982 | 1983–1987 | 1988–1992 | 1993–1997 | 1998–2002 | 2003–2007 | 2008–2012 | |||
| Incidence | ||||||||||
| N | 2807 | 3180 | 4160 | 5825 | 6442 | 8590 | 10 765 | 12 116 | 53 885 | – |
| Age-specific rates | ||||||||||
| 0–19 | 0.07 | 0.03 | 0.06 | 0.05 | 0.00 | 0.10 | 0.17 | 0.00 | 0.06 | – |
| 20–29 | 2.01 | 1.75 | 2.38 | 2.94 | 1.48 | 2.41 | 2.95 | 4.02 | 2.49 | 99.7 |
| 30–39 | 14.70 | 17.71 | 17.32 | 22.51 | 26.40 | 23.16 | 19.34 | 24.98 | 21.30 | 69.9 |
| 40–49 | 36.55 | 43.10 | 51.89 | 66.63 | 68.25 | 89.72 | 100.21 | 101.13 | 73.40 | 176.7 |
| 50–59 | 48.78 | 46.41 | 51.44 | 68.83 | 75.96 | 114.48 | 123.68 | 125.77 | 87.13 | 157.8 |
| 60–69 | 57.88 | 54.38 | 61.16 | 67.73 | 79.39 | 99.46 | 125.75 | 138.15 | 86.94 | 138.7 |
| 70–79 | 56.48 | 53.00 | 65.21 | 70.18 | 78.95 | 108.42 | 126.22 | 129.67 | 94.27 | 129.6 |
| ≥80 | 50.68 | 60.10 | 59.78 | 68.85 | 68.80 | 111.83 | 110.13 | 99.73 | 89.23 | 96.8 |
| Age-standardized rate | 17.14 | 17.91 | 20.33 | 25.09 | 27.12 | 35.63 | 39.27 | 41.33 | 29.43 | 141.2 |
| Mortality | ||||||||||
| N | 1210 | 1346 | 1563 | 1969 | 2098 | 2299 | 2810 | 3940 | 17 235 | – |
| Age-specific rates | ||||||||||
| 0–19 | 0.00 | 0.00 | 0.03 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | – |
| 20–29 | 0.27 | 0.28 | 0.33 | 0.56 | 0.18 | 0.34 | 0.14 | 0.28 | 0.31 | 3.5 |
| 30–39 | 3.17 | 3.43 | 3.19 | 3.80 | 4.43 | 2.96 | 2.51 | 2.59 | 3.40 | −18.3 |
| 40–49 | 8.69 | 11.50 | 10.69 | 12.04 | 11.99 | 13.11 | 14.07 | 11.23 | 11.93 | 29.2 |
| 50–59 | 18.67 | 17.04 | 18.13 | 19.81 | 21.95 | 30.29 | 26.68 | 25.87 | 22.63 | 38.5 |
| 60–69 | 33.39 | 26.86 | 25.20 | 26.33 | 29.90 | 28.50 | 29.35 | 33.39 | 28.98 | 0.0 |
| 70–79 | 48.41 | 46.15 | 41.98 | 45.77 | 43.22 | 43.84 | 47.92 | 64.46 | 48.55 | 33.1 |
| ≥80 | 62.56 | 70.61 | 72.92 | 80.00 | 79.43 | 93.60 | 104.46 | 145.95 | 103.57 | 133.3 |
| Age-standardized rate | 7.61 | 7.37 | 7.12 | 7.85 | 8.10 | 9.00 | 8.91 | 9.64 | 8.46 | 26.6 |
aFrom comparing the earliest and latest 5-year periods.
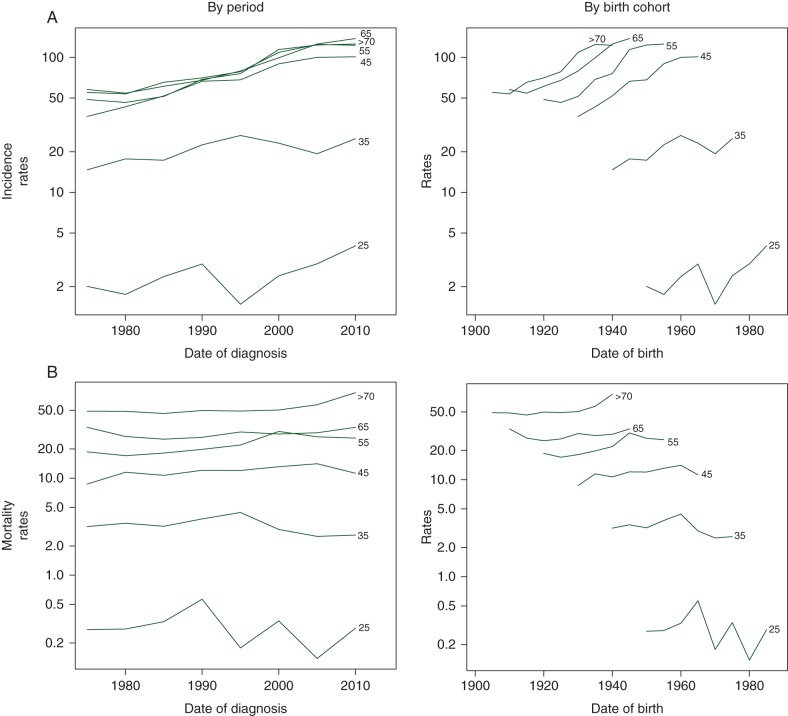
To examine how breast cancer incidence and mortality differed by age, period, and birth cohort, age-specific rates were plotted by date of diagnosis and date of birth (Figure 1). Breast cancer incidence rates increased with advancing age from 20 to 45, and then plateaued after age 45. This pattern was consistent across all periods and birth cohorts. For women in the same age groups, those who were born later or diagnosed later had higher breast cancer incidence rates. On the contrary, breast cancer mortality rates rose with increasing age across the age span. Mortality rates increased slightly with later dates of diagnosis until 1995 or later year of birth until 1960 in all age groups, and then decreased slightly with dates of diagnosis after 1995 or years of birth after 1960 among young women with age <50.
Figure 1.
Age-specific breast cancer (A) incidence and (B) mortality rates (1/100 000) among women in urban Shanghai (1973–2012), by period and birth cohort.
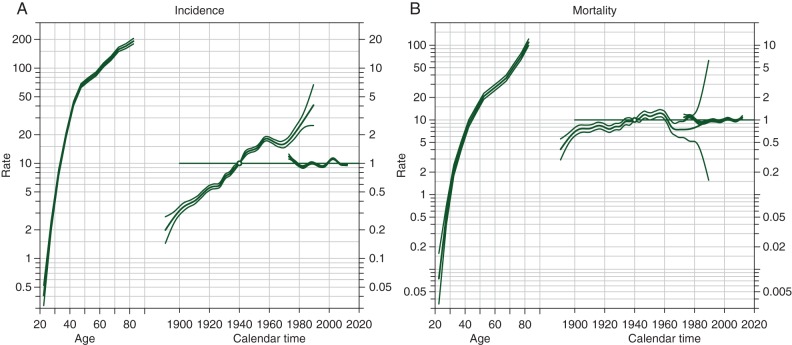
Effects of age, period, and birth cohort on breast cancer incidence and mortality were further evaluated using APC Poisson regression models (Table 2). The EAPC in incidence was considerable (2.96%/year), whereas mortality rose less sharply (0.87%/year). Comparisons of APC submodels yielded significant age, cohort, and period effects for both incidence and mortality (P < 0.001); cohort effects were notably larger than period effects. By examining residual deviance and change in deviance in APC models, cohort effects seemed to explain the majority of the changes in breast cancer incidence and mortality. Estimated age, period, and birth cohort effects are plotted in Figure 2. While incidence showed a striking increasing trend, an increase in mortality was less apparent. Further, a downward trend in mortality was observed among women in younger generations that were born after 1960.
Table 2.
APC models for breast cancer incidence and mortality among women in urban Shanghai (1973–2012)
| Submodel | Goodness of fit |
Model comparison |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Residual df | Residual deviance | P | Comparison | Interpretation | Change in df | Change in deviance | P | EAPC (95% CI) | |
| Incidence | |||||||||
| 1. Age | 507 | 6753.10 | <0.001 | ||||||
| 2. Age–drift | 506 | 1422.80 | <0.001 | 2 versus 1 | Trend (drift) | 1 | 5330.30 | <0.001 | 2.96 (2.88, 3.05) |
| 3. Age–cohort | 487 | 954.30 | <0.001 | 3 versus 2 | Nonlinear cohort effect | 19 | 468.50 | <0.001 | |
| 4. Age–period | 499 | 1213.10 | <0.001 | 4 versus 2 | Nonlinear period effect | 7 | 209.70 | <0.001 | |
| 5. Age–period–cohort | 480 | 762.00 | <0.001 | 5 versus 3 | Period effect adjusted for cohort | 7 | 192.30 | <0.001 | |
| 5 versus 4 | Cohort effect adjusted for period | 19 | 451.10 | <0.001 | |||||
| Mortality | |||||||||
| 1. Age | 507 | 989.76 | <0.001 | ||||||
| 2. Age–drift | 506 | 844.61 | <0.001 | 2 versus 1 | Trend (drift) | 1 | 145.15 | <0.001 | 0.87 (0.73, 1.01) |
| 3. Age–cohort | 487 | 713.27 | <0.001 | 3 versus 2 | Nonlinear cohort effect | 19 | 131.34 | <0.001 | |
| 4. Age–period | 499 | 813.57 | <0.001 | 4 versus 2 | Nonlinear period effect | 7 | 31.03 | <0.001 | |
| 5. Age–period–cohort | 480 | 674.43 | <0.001 | 5 versus 3 | Period effect adjusted for cohort | 7 | 38.84 | <0.001 | |
| 5 versus 4 | Cohort effect adjusted for period | 19 | 139.15 | <0.001 | |||||
df, degrees of freedom; EAPC, estimated annual percentage change (%/year); CI, confidence interval.
Figure 2.
Age, period, and cohort effects for breast cancer (A) incidence and (B) mortality among women in urban Shanghai (1973–2012).
discussion
Using IARC quality cancer registry data, we found a substantial increase in breast cancer incidence among permanent female residents in urban Shanghai over the last four decades. The ASR reached 44.0/100 000 in 2012, which is nearly double the rate reported for all of China (22.1/100 000) [1]. On the basis of APC modeling, the increase in incidence was driven by age and cohort effects, such that older women and those born in more recent generations have higher risks of developing breast cancer. These effects are likely attributable to lifestyle and energy-related changes that have occurred, including dietary changes, reduced physically activity, and an increased prevalence of obesity [4]. Results from the China Health and Nutrition Survey show increased energy intake and a transition toward high-fat and energy-dense diets among Chinese adults over the past two decades [14]. Simultaneously, the age-adjusted prevalence of obesity increased from 4.6% in 1991 to 11.0% in 2011 among women in China [15]. Changes in menstrual and reproductive factors are also likely to contribute to the increase in breast cancer incidence identified [16]. Compared with older generations, younger women generally have earlier ages at menarche, later ages at menopause, delayed childbearing, lower parity, and reduced breastfeeding practices [4]. These changes are particularly relevant in China, where the Chinese Family Planning Program was implemented in the late 1970s. The total fertility rate, which measures the average number of children born per woman, has decreased from 6.1 to 1.5 over the past 50 years [17]. According to a recent large-scale birth cohort study that compared Chinese women born in the 1930s and 1970s, age at menarche decreased by 1.8 years (16.1 versus 14.3), age at first birth increased by 6 years (urban: 19.0 versus 25.9; rural: 18.3 versus 23.8), duration of breastfeeding declined by 4–5 months (urban: 16 versus 11; rural: 18 versus 14), and age at menopause increased by 1.4 years (47.9 versus 49.3) [18]. As generations who were affected by the family planning program have not reached the peak age of incidence (45) by the end of our study period (2012), the effect of the family planning program and resulting transitions in breast cancer risk factors may not be fully reflected in changes in breast cancer incidence at the present time, and a greater increase in breast cancer incidence is expected in the future. Therefore, breast cancer prevention and control strategies are urgently needed for women in China.
While incidence markedly increased, breast cancer mortality rose only slightly among women in urban Shanghai, with significant cohort and age effects. With an ASR of 9.4/100 000 in 2012, breast cancer mortality in Shanghai was higher than all of China (5.4/100 000) [1]. Although overall mortality increased, we did find evidence of a downward trend among recent generations of women. Women that were born after 1960 or that were <50 years old after 1995 had lower breast cancer mortality than those born before 1960 or were aged 50 and above before 1995. In Western countries, breast cancer mortality has been declining since the 1980s, likely due to cancer screening and improved treatments over time [19]. In Asia, declining breast cancer mortality trends have been observed in Hong Kong and Singapore [7]. However, these declines occurred before local screening programs were initiated [7]. The slight decline in breast cancer mortality among young women found in this study is also ∼10 years before breast cancer screening programs were initiated in Shanghai, and is most likely attributable to the increased use of diagnostic imaging and improved biopsy methods [20]. Data on stage of disease at diagnosis have been collected by the SCR since 2002. Among breast cancers diagnosed in urban Shanghai during this time, the ASR of early (stage I) breast cancer increased 59.0%, while that of more advanced (stage II and higher) breast cancer remained relatively stable (−2.4%), and breast cancer with unknown stage decreased 17.8% (supplementary Table S1, available at Annals of Oncology online). These changes in stage of disease at presentation are supportive of improved diagnostic approaches. On the other hand, adjuvant therapy has also significantly improved the prognosis of breast cancer patients in the last few decades [21]. Taken together, the decline in breast cancer mortality observed in Asian regions is most likely attributable to improved cancer diagnosis and prognosis, rather than screening. Notably, in other developed Asian regions, including Taiwan, Japan, and Korea, where organized mammography screening programs were initiated in the late 1990s and early 2000s, breast cancer mortality rates have continued to rise [7]. Given that screening benefits take at least 10 years to be reflected in reduced mortality rates [22], it will be important to continue to evaluate breast cancer mortality trends in Asian regions in the future.
Several national breast cancer screening programs have been initiated by the Chinese government since the late 2000s, such as the ‘Chinese One Million Women Breast Cancer Screening’, the ‘Breast Cancer Screening Program’, and the ‘Countryside Women Breast and Cervical Cancer Screening Program’ [23, 24]. The Shanghai government has also been providing free biennial screening to retired and low-income women, and requiring employers to provide screening coverage for female workers since 2005. While these large-scale efforts are promising, the effectiveness of screening modalities employed in these screening programs is largely limited by budget constraints. Currently, the budget for breast and cervical cancer screening provided by the government ranges from 35 to 100 Chinese Yuan per woman, which is far from sufficient for covering mammography or ultrasonography. Due to a low cost, clinical breast examination (CBE) is the primary breast cancer screening modality currently used in China [25]. However, the efficacy of CBE as a screening modality is not known; its performance and effectiveness has only been indirectly determined from studies in conjunction with mammography [26]. Further, the coverage of these screening programs for the general population is limited. According to a report in 2010, the coverage of breast cancer screening was only 27.4% in Shanghai and 21.7% for all of China [27]. Given the low population coverage and utilization of CBE as the primary screening modality, results of this study reflect trends in breast cancer incidence and mortality that occurred largely in the absence of screening programs.
Strengths of this study include the large population size of Shanghai (3.1 million) and a long time span (40 years). The use of APC modeling allowed us to examine age, period, and birth cohort effects. However, as calendar year minus age equals birth year (C = P-A), it is impossible to truly disentangle their effects [28]. For example, the cohort effect in breast cancer mortality seen as a downtrend among women born after 1960, would best be described as an age-specific period effect resulting in a decline in mortality among young women (age <50) after 1995. Additional strengths of this study include evaluating both incidence and mortality data from the SCR, which has been shown to have high validity and good coverage of the population of Shanghai [29]. Thus, our results provide the most accurate estimates available for secular trends in breast cancer incidence and mortality among women in urban Shanghai. However, as an ecologic study, our analysis was unable to quantify the relative contributions of different factors that may have resulted in changes in breast cancer incidence or mortality over time.
In summary, this analysis documented a tremendous increase in breast cancer incidence and a modest increase in breast cancer mortality in an urban Chinese population over 40 years. Our results attest to an urgent need for breast cancer prevention and control strategies in China. Identifying and employing effective, appropriate, and affordable approaches for breast cancer screening in China is a critical cancer control priority.
funding
This work was supported by the Vanderbilt-Shanghai Chronic Disease Research Training Program grant from the Fogarty International Center (D43 TW008313 to ZH); the Shanghai Municipal Commission of Health and Family Planning [15GWZK0801 (PI: Fan Wu) to ZH]; the Shanghai Public Health Talented Professional Overseas Training Program to YZ; and the Building Interdisciplinary Research Careers in Women's Health (BIRCWH) K12 Scholars Program [NIH/NICHD 5 K12 HD 043483-05 (PI: Katherine Hartmann) to ABF].
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank registrars from the Shanghai Cancer Registry and staff members of the research teams for their contributions and support for the study.
references
- 1.Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Report 2014. Lyon: International Agency for Research on Cancer, 2014. [Google Scholar]
- 3.Vineis P, Wild CP. Global cancer patterns: causes and prevention. Lancet 2014; 383(9916):549–557. [DOI] [PubMed] [Google Scholar]
- 4.Porter P. ‘Westernizing’ women's risks? Breast cancer in lower-income countries. N Engl J Med 2008; 358(3):213–216. [DOI] [PubMed] [Google Scholar]
- 5.Wong IOL, Schooling CM, Cowling BJ, Leung GM. Breast cancer incidence and mortality in a transitioning Chinese population: current and future trends. Br J Cancer 2015; 112(1):167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virani S, Sriplung H, Rozek LS, Meza R. Escalating burden of breast cancer in southern Thailand: analysis of 1990–2010 incidence and prediction of future trends. Cancer Epidemiol 2014; 38(3):235–243. [DOI] [PubMed] [Google Scholar]
- 7.Shin H-R, Boniol M, Joubert C et al. Secular trends in breast cancer mortality in five East Asian populations: Hong Kong, Japan, Korea, Singapore and Taiwan. Cancer Sci 2010; 101(5):1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi X-J, Au WW, Wu K-S et al. Mortality characteristics and prediction of female breast cancer in China from 1991 to 2011. Asian Pac J Cancer Prev 2014; 15(6):2785–2791. [DOI] [PubMed] [Google Scholar]
- 9.Jin F, Devesa SS, Chow WH et al. Cancer incidence trends in urban shanghai, 1972–1994: an update. Int J Cancer 1999; 83(4):435–440. [DOI] [PubMed] [Google Scholar]
- 10.Doll R, Cook P. Summarizing indices for comparison of cancer incidence data. Int J Cancer 1967; 2(3):269–279. [DOI] [PubMed] [Google Scholar]
- 11.Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: age-period and age-cohort models. Stat Med 1987; 6(4): 449–467. [DOI] [PubMed] [Google Scholar]
- 12.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: age-period-cohort models. Stat Med 1987; 6(4): 469–481. [DOI] [PubMed] [Google Scholar]
- 13.Carstensen B, Plummer M, Hills M, Laara E. Epi: A Package for Statistical Analysis in Epidemiology. R package version 2.0. http://CRAN.R-project.org/package=Epi. [Google Scholar]
- 14.Pan K, Smith LP, Batis C et al. Increased energy intake and a shift towards high-fat, non-staple high-carbohydrate foods amongst Chinas older adults, 1991–2009. J Aging Res Clin Pract 2014; 3(2):107–115. [PMC free article] [PubMed] [Google Scholar]
- 15.Mi Y-J, Zhang B, Wang H-J et al. Prevalence and secular trends in obesity among Chinese adults, 1991–2011. Am J Prev Med 2015; 49(5): 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi S, Sugiura H, Ando Y et al. Reproductive history and breast cancer risk. Breast Cancer Tokyo Jpn 2012; 19(4):302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Population Prospects, the 2010 Revision. New York: United Nations, Department of Economic and Social Affairs, Population Division, 2011. [Google Scholar]
- 18.Lewington S, Li L, Murugasen S et al. Temporal trends of main reproductive characteristics in ten urban and rural regions of China: the China Kadoorie Biobank study of 300 000 women. Int J Epidemiol 2014; 43(4):1252–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermon C, Beral V. Breast cancer mortality rates are levelling off or beginning to decline in many western countries: analysis of time trends, age-cohort and age-period models of breast cancer mortality in 20 countries. Br J Cancer 1996; 73(7): 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B-L, Sivasubramaniam PG, Zhang Q et al. Trends in radical surgical treatment methods for breast malignancies in China: a multicenter 10-year retrospective study. Oncologist 2015; 20(9):1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Q, Luo T, He P et al. Trends and present treatment patterns of early breast cancer in Southwest China. Pathol Oncol Res 2015; 21(2):367–378. [DOI] [PubMed] [Google Scholar]
- 22.Jatoi I, Miller AB. Why is breast-cancer mortality declining? Lancet Oncol 2003; 4(4):251–254. [DOI] [PubMed] [Google Scholar]
- 23.The Breast Cancer Screening Project was launched in Tianjin on March 11, 2008. http://www.caca.org.cn/system/2009/01/23/010020029.shtml (1 September 2015, date last accessed).
- 24.The plight of China's breast cancer screening program. [http://www.chinatoday.com.cn/ctenglish/se/txt/2009-03/30/content_188455.htm] (1 September 2015, date last accessed).
- 25.Zheng Y. Approaches to Breast Cancer Early Detection and Screening in China, Chicago: The Breast Health Global Initiative, 2010. [Google Scholar]
- 26.Sankaranarayanan R, Ramadas K, Thara S et al. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst 2011; 103(19):1476–1480. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, He M, Wang L et al. Breast cancer screening among adult women in China, 2010. Prev Chronic Dis 2013; 10:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien RM. The age–period–cohort conundrum as two fundamental problems. Qual Quant 2010; 45(6):1429–1444. [Google Scholar]
- 29.Forman D, Bray F, Brewster DH et al. Cancer Incidence in Five Continents. Vol. X, Lyon, France: WHO Press, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.