In treating HR+, HER2− metastatic breast cancer, novel agents that enhance endocrine therapy activity but do not worsen quality of life (QoL) are clinically desired. Patient-reported outcomes data from the PALOMA-3 study suggest palbociclib plus fulvestrant allow patients to maintain good QoL in the endocrine resistance setting while experiencing a substantially delayed disease progression.
Keywords: endocrine resistance, palbociclib, patient-reported outcomes, breast cancer, quality of life
Abstract
Background
In the PALOMA-3 study, palbociclib plus fulvestrant demonstrated improved progression-free survival compared with fulvestrant plus placebo in hormone receptor-positive, HER2− endocrine-resistant metastatic breast cancer (MBC). This analysis compared patient-reported outcomes (PROs) between the two treatment groups.
Patients and methods
Patients were randomized 2 : 1 to receive palbociclib 125 mg/day orally for 3 weeks followed by 1 week off (n = 347) plus fulvestrant (500 mg i.m. per standard of care) or placebo plus fulvestrant (n = 174). PROs were assessed on day 1 of cycles 1–4 and of every other subsequent cycle starting with cycle 6 using the EORTC QLQ-C30 and its breast cancer module, QLQ-BR23. High scores (range 0–100) could indicate better functioning/quality of life (QoL) or worse symptom severity. Repeated-measures mixed-effect analyses were carried out to compare on-treatment overall scores and changes from baseline between treatment groups while controlling for baseline. Between-group comparisons of time to deterioration in global QoL and pain were made using an unstratified log-rank test and Cox proportional hazards model.
Results
Questionnaire completion rates were high at baseline and during treatment (from baseline to cycle 14, ≥95.8% in each group completed ≥1 question on the EORTC QLQ-C30). On treatment, estimated overall global QoL scores significantly favored the palbociclib plus fulvestrant group [66.1, 95% confidence interval (CI) 64.5–67.7 versus 63.0, 95% CI 60.6–65.3; P = 0.0313]. Significantly greater improvement from baseline in pain was also observed in this group (−3.3, 95% CI −5.1 to −1.5 versus 2.0, 95% CI −0.6 to 4.6; P = 0.0011). No significant differences were observed for other QLQ-BR23 functioning domains, breast or arm symptoms. Treatment with palbociclib plus fulvestrant significantly delayed deterioration in global QoL (P < 0.025) and pain (P < 0.001) compared with fulvestrant alone.
Conclusion
Palbociclib plus fulvestrant allowed patients to maintain good QoL in the endocrine resistance setting while experiencing substantially delayed disease progression.
Clinical Trial Registration
introduction
Palbociclib (Ibrance®, Pfizer, New York, NY) is an orally bioavailable small-molecule inhibitor of cyclin-dependent kinases (CDKs) with a high level of selectivity for CDK4 and CDK6 versus other CDKs [1]. In preclinical studies, palbociclib is highly active in hormone receptor-positive (HR+) breast cancer cell lines and is synergistic with endocrine therapies [2]. In a randomized phase II study (PALOMA-1) of patients with estrogen receptor-positive (ER+), human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (MBC), palbociclib in combination with letrozole resulted in improved progression-free survival (PFS) that was nearly double that of patients receiving letrozole alone [3]. Because deregulation of CDK4/CDK6 has been implicated in endocrine resistance, palbociclib was investigated in the phase III PALOMA-3 study comparing palbociclib plus fulvestrant with placebo plus fulvestrant in patients with endocrine-resistant, HR+, HER2− advanced breast cancer. The recently reported primary results demonstrated a significant improvement in PFS with palbociclib plus fulvestrant [median 9.2 versus 3.8 months; hazard ratio (HR): 0.42; 95% confidence interval (CI) 0.32–0.56; P < 0.001] [4].
The major clinical challenge of managing HR+, HER2− MBC is that most patients eventually develop resistance to endocrine therapy. Delaying disease progression, even in the absence of an overall survival benefit, may help maintain quality of life (QoL) on treatment at levels that are higher than what would be experienced with disease progression. Yet, addition of novel treatments to existing ones can add toxicities, which could diminish patients' QoL [5, 6]. Therefore, maintaining QoL is particularly relevant in the endocrine resistance setting, for which there are several treatment options including either a combination of endocrine therapy with a targeted agent (e.g. phosphoinositide 3-kinase or mammalian target of rapamycin inhibitors) or a switch to chemotherapy. Previous studies have shown worse QoL with chemotherapy versus endocrine therapy [7]. A novel agent that would enhance endocrine therapy activity and still maintain adequate QoL is clinically desired. Hence, when evaluating new treatments (especially combination regimens), it is important to evaluate the quality of the time gained by delaying disease progression via patient-reported outcomes (PROs) as an integral component of benefit-risk assessments. Previously, we reported top-level results of the impact of palbociclib therapy on global QoL and emotional functioning [4]. Here, we report the detailed PROs results from PALOMA-3.
methods
trial design and participants
PALOMA-3 was an international, multicenter, ongoing double-blind, parallel-group phase III study in which patients were randomized 2 : 1 to receive palbociclib plus fulvestrant or matching placebo plus fulvestrant, respectively. The primary objective was to demonstrate the superiority of palbociclib plus fulvestrant over placebo plus fulvestrant in prolonging investigator-assessed PFS in women with HR+, HER2− MBC whose disease had progressed after prior endocrine therapy.
randomization and study treatments
Randomization was stratified by documented sensitivity to prior hormonal therapy (yes versus no), menopausal status (pre/peri- versus postmenopausal), and presence of visceral metastases (yes versus no). Palbociclib/placebo was orally administered daily on days 1–21, followed by 7 days off-treatment of every 28-day cycle. In cycle 1, fulvestrant was administered intramuscularly on days 1 and 15, every 28 ± 7 days thereafter starting on day 1 of cycle 2. All pre-/perimenopausal women received goserelin for the duration of study treatment. The study was stopped early at the planned interim analyses for PFS. A detailed study design was previously reported [4].
PRO assessment
The PROs were assessed using the European Organisation for Research and Treatment of Cancer Quality-of-Life questionnaire (EORTC QLQ-C30 v3.0) and its breast cancer module (EORTC QLQ-BR23). Patients completed these instruments on day 1 of cycles 1–4, then on day 1 of every other subsequent cycle starting with cycle 6 (e.g. cycles 6, 8, 10, etc.), and at the end-of-treatment visit. Patients were to complete these instruments in the clinic before any tests and/or discussions of their progress with health care personnel at the site. The completed questionnaires were considered source documents and filed accordingly.
The EORTC QLQ-C30 is a 30-item questionnaire composed of a global QoL subscale, 5 multi-item functional subscales (physical, role, emotional, cognitive, and social functioning), 3 multi-item symptom scales (fatigue, nausea/vomiting, and pain), and 5 single-item symptom scales assessing other cancer-related symptoms (dyspnea, sleep disturbance, appetite loss, constipation, and diarrhea). The questionnaire consists of 4-point Likert scale items with responses from ‘not at all’ to ‘very much’ to assess functioning and symptoms and two 7-point Likert scale items for global health and overall QoL [8]. The EORTC QLQ-BR23 is a 23-item breast cancer-specific companion module to the EORTC QLQ-C30 and consists of four functional scales (body image, sexual functioning, sexual enjoyment, future perspective) and four symptom scales (systemic side-effects, breast symptoms, arm symptoms, upset by hair loss). Responses to all items were converted to a 0–100 scale using a standard scoring algorithm [9]. For functional and global QoL scales, higher scores represent a better level of functioning/QoL than lower scores; for symptom-oriented scales, higher scores represent greater symptom severity. For the single items, if two answers were given to a single question, the more severe answer was counted. If ≥50% of the questions were answered for the multi-item scales, the scale score was the mean score. If <50% of the questions in any scale were answered, the score was considered missing.
statistical analysis
All PRO analyses were based on the PRO-evaluable population (i.e. patients in the intent-to-treat population with a baseline and ≥1 postbaseline assessment before the end of study treatment). Completion rates were summarized by cycle. The primary PRO analysis prespecified for comparing the two treatment groups was a longitudinal analysis based on a longitudinal mixed-effect random intercept random slope model. Treatment, time, treatment by time, and baseline were covariates for the model. A restricted maximum likelihood method assuming an unstructured covariance matrix was used. This analysis was carried out based on both the observed values and the changes from baseline for EORTC QLQ-C30 and QLQ-BR23 scales. Analysis of time to deterioration (TTD) in pain scores was prespecified; deterioration was defined as an increase of ≥10 points from baseline.
Post hoc analyses of TTD in global QoL were carried out using survival analysis methods. Deterioration was defined as a decrease of ≥10 points from baseline, with no subsequent increase above this threshold. The 10-point threshold was chosen based on previously established thresholds for minimal important differences from the perspective of the patient [10]. Patients not meeting deterioration criteria were censored at treatment discontinuation or death (whichever occurred earlier) or time of last available PRO assessment if the patient was alive and continuing treatment. Survival analysis methods included the Kaplan–Meier approach for estimating medians and percentiles, the Brookmeyer and Crawley method for computing 95% CIs, assuming proportional hazards for computing HR, and using the log-rank test (one-sided; α = 0.025) in comparing TTD between the two treatment groups. A repeated-measures model for the subgroup of patients with visceral metastases at baseline was also performed. No adjustments were made for multiple comparisons. All analyses were conducted using Statistical Analysis System software (SAS Institute, Cary, NC) for Windows (Microsoft Corp., Redmond, WA). All P values are two-sided unless stated otherwise.
results
Of 521 patients, 347 were randomized to the palbociclib plus fulvestrant group and 174 to the placebo plus fulvestrant group. Two (0.6% and 1.1%, respectively) patients in each group were randomized but not treated. Baseline characteristics were well balanced across treatment groups (see supplementary Table S1, available at Annals of Oncology online). Median age was 57 years. The majority of patients had visceral metastases (palbociclib plus fulvestrant, 59.4%; placebo plus fulvestrant, 60.3%; see supplementary Table S1, available at Annals of Oncology online). Most patients had ≥2 lines of treatment in the metastatic setting and over one-third had ≥3 disease sites involved. One-third of patients received chemotherapy in the advanced setting (30.8% versus 36.2%).
From baseline to cycle 14, ≥96.9% of patients in the palbociclib plus fulvestrant group and ≥95.8% in the placebo plus fulvestrant group, respectively, completed ≥1 question on the EORTC QLQ-C30 (see supplementary Table S2, available at Annals of Oncology online); ≥93.8% and ≥95.8% completed ≥1 question on the EORTC QLQ-BR23.
global QoL
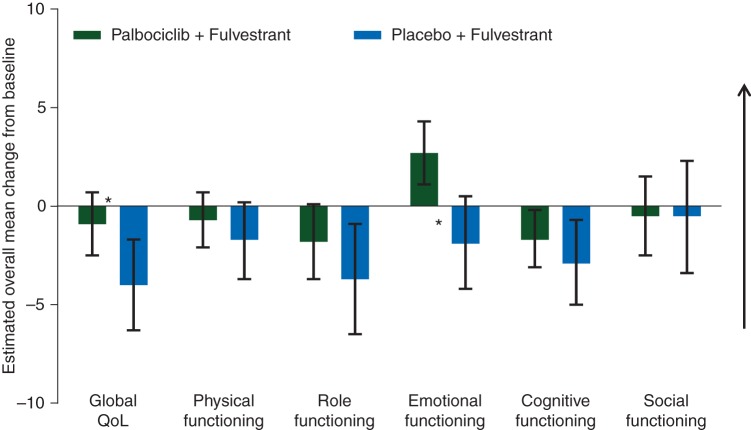
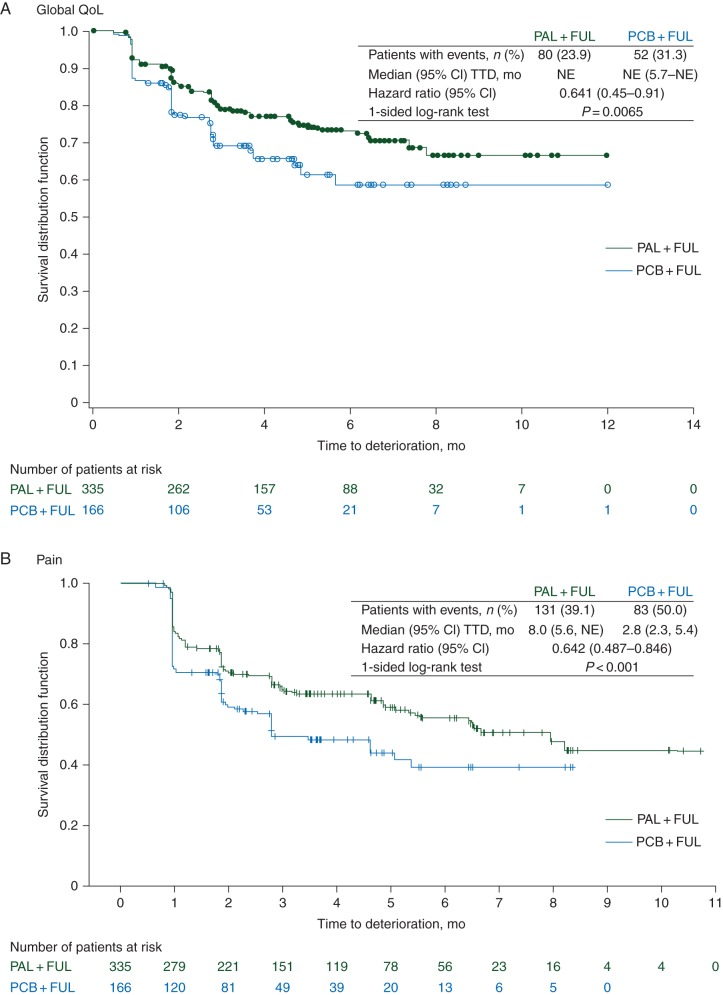
Baseline mean (95% CI) scores for global QoL were similar for the palbociclib plus fulvestrant and placebo plus fulvestrant groups [65.9 (63.5–68.2) versus 65.3 (61.9–68.6)]. Baseline scores in the study were within range of reference values published previously [11] in recurrent/MBC patients (Table 1). As shown in Figure 1, the repeated-measures mixed-effect model estimated a significant between-group difference in overall change from baseline score for global QoL, which has been reported previously [4]. The difference between treatment groups in estimated overall global QoL scores was found to be statistically significant favoring palbociclib plus fulvestrant [66.1 (95% CI: 64.5–67.7) versus 63.0 (95% CI: 60.6–65.3); P = 0.0313]. A significantly greater delay in deterioration of QoL was observed in the palbociclib plus fulvestrant versus control (median not reached; HR: 0.641; 95% CI: 0.451–0.910; 1-sided P = 0.0065; Figure 2A).
Table 1.
Baseline scores for EORTC QLQ-C30 and BR-23 scales and reference values (PRO analysis set)
| Domain/scale | Palbociclib + fulvestrant (N = 335) |
Placebo + fulvestrant (N = 166) |
Reference values [11]a | ||
|---|---|---|---|---|---|
| nb | Mean (95% CI) | nb | Mean (95% CI) | Mean (SD) | |
| EORTC QLQ-C30 Global QoL and Functional Scales | |||||
| Global QOL | 334 | 65.9 (63.5–68.2) | 166 | 65.3 (61.9–68.6) | 60.2 (25.5) |
| Physical functioning | 334 | 79.4 (77.3–81.5) | 166 | 78.9 (76.1–81.7) | 81.6 (18.7) |
| Role functioning | 333 | 78.5 (75.7–81.2) | 166 | 77.6 (73.8–81.5) | 67.4 (31.1) |
| Emotional functioning | 334 | 74.6 (72.4–76.8) | 166 | 72.8 (69.7–76.0) | 65.9 (24.6) |
| Cognitive functioning | 334 | 84.8 (82.8–86.8) | 166 | 82.1 (79.2–85.1) | 80.5 (23.2) |
| Social functioning | 334 | 81.3 (78.7–83.9) | 166 | 78.5 (74.7–82.4) | 74.2 (28.4) |
| EORTC QLQ-C30 Symptoms | |||||
| Fatigue | 334 | 32.1 (29.7–34.5) | 166 | 32.2 (28.9–35.5) | 36.3 (27.0) |
| Nausea/vomiting | 335 | 7.4 (5.6–9.1) | 166 | 5.2 (3.4–7.0) | 10.3 (19.7) |
| Pain | 335 | 26.6 (23.9–29.3) | 166 | 27.5 (23.7–31.3) | 30.9 (29.6) |
| Dyspnea | 334 | 15.7 (13.3–18.1) | 166 | 16.5 (13.0–19.9) | 20.4 (28.2) |
| Insomnia | 335 | 26.3 (23.4–29.1) | 166 | 32.9 (28.4–37.5) | 33.1 (32.6) |
| Appetite loss | 335 | 16.8 (14.1–19.5) | 166 | 12.9 (9.4–16.3) | 21.7 (31.0) |
| Constipation | 333 | 13.6 (11.1–16.2) | 166 | 13.7 (10.5–16.8) | 19.2 (28.8) |
| Diarrhea | 332 | 5.4 (3.9–6.9) | 166 | 6.2 (4.1–8.4) | 5.8 (15.2) |
| EORTC QLQ-BR23 Functional | |||||
| Body image | 333 | 74.8 (71.9–77.7) | 164 | 73.8 (69.4–78.3) | 81.9 (22.6) |
| Sexual functioning | 321 | 16.1 (13.7–18.5) | 164 | 13.9 (10.7–17.1) | 19.2 (23.2) |
| Sexual enjoyment | 141 | 44.9 (39.5–50.0) | 64 | 37 (28.4–45.5) | 55.1 (25.6) |
| Future perspective | 333 | 43.1 (39.7–46.6) | 165 | 43.6 (28.9–48.4) | 47.6 (34.1) |
| EORTC QLQ-BR23 Symptoms | |||||
| Systemic therapy side-effects | 335 | 15.7 (14.2–17.1) | 166 | 17.1 (15.1–19.1) | 15.8 (14.3) |
| Breast symptoms | 330 | 10.5 (8.7–12.2) | 166 | 11 (8.5–13.5) | 17.6 (16.7) |
| Arm symptoms | 334 | 16 (13.8–18.1) | 166 | 18.1 (14.9–21.3) | 21.0 (21.1) |
| Upset by hair lossc | 104 | 28.5 (22.1–34.9) | 55 | 26.7 (18.5–34.9) | 5.3 (19.3) |
BR23, Breast Cancer Module; C30, core 30 items; CI, confidence interval; EORTC QLQ, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; PRO, patient-reported outcomes; QOL, quality of life; SD, standard deviation.
aReference values for recurrent/metastatic breast cancer patients across all lines of treatment.
bNumber of patients with data available for the corresponding visit.
cOnly patients who experienced hair loss were required to complete this question.
Figure 1.
Overall change from baseline in EORTC QLQ-C30 scores for global QoL and functional scales in the PRO analysis set. Changes from baseline in the patient-reported outcomes analysis population were determined using a repeated-measures mixed-effect model. Arrow denotes direction of improved outcome; changes >0 indicate improvement from baseline. EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 items; PRO, patient-reported outcomes; QoL, quality of life. Asterisks denote that the change from baseline was statistically significantly different between treatment groups.
Figure 2.
Time to deterioration in global QoL (A) and pain (B) in the PRO analysis set. Kaplan–Meier curves of European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 items (EORTC QLQ-C30) scores for the patient-reported outcomes analysis population. CI, confidence interval; NE, not estimable; TTD, time to deterioration; PRO, patient-reported outcomes; QoL, quality of life. Circles and pluses indicate patients censored.
functional scales (QLQ-C30)
Baseline scores for all five QLQ-C30 functional scales were similar between groups with high functioning levels in both. Estimated changes from baseline for the functional scales are presented in Figure 1. Between-group differences in changes from baseline scores were significant only for emotional functioning [2.7 (95% CI 1.1–4.3) versus −1.9 (95% CI −4.2 to 0.5); P = 0.0016) and favored palbociclib plus fulvestrant. The overall change from baseline scores for physical, role, cognitive, and social functioning was not found to be statistically significantly different between the two treatment groups (Figure 1). Within each treatment group, changes from baseline indicated significant improvement (based on interpretation from the 95% CIs) with palbociclib plus fulvestrant in emotional functioning. In contrast, there was significant worsening with placebo plus fulvestrant in role functioning, significant worsening for both treatment groups in cognitive functioning, and no significant change from baseline for either group in the other functional scales.
symptom scales (QLQ-C30)
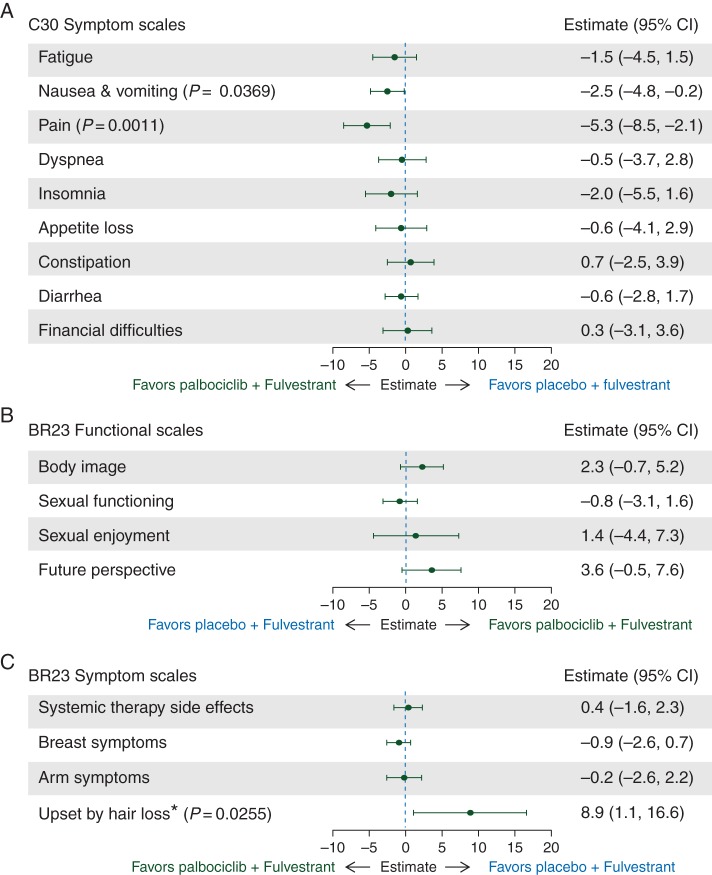
Mean baseline scores for symptoms of the EORTC QLQ-C30 were similar in both treatment groups for all symptoms except insomnia (26.3 versus 32.9 for palbociclib plus fulvestrant versus placebo plus fulvestrant). Baseline symptom scores were on the lower end of the 0–100 score range, indicating low symptom severity in both groups. Differences between treatment comparisons for change from baseline scores for the EORTC QLQ-C30 symptoms are displayed in Figure 3A.
Figure 3.
Between-treatment comparison of changes from baseline in EORTC QLQ-C30 scores for symptom scales (A) and EORTC QLQ-BR23 scores for functional (B) and symptom (C) scales in the PRO analysis set. Changes from baseline in the patient-reported outcomes analysis population were determined using a repeated-measures mixed-effect model. EORTC QLQ-BR23, European Organization for Research and Treatment of Cancer Breast Cancer Module; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 items; PRO, patient-reported outcomes; QoL, quality of life. P values are shown only if significant between-group differences were observed. Asterisk denotes that question was only to be answered by patients who stated they had experienced hair loss, resulting in fewer patients responding to this question compared with other questions.
Significant decrease from baseline in pain was observed with palbociclib plus fulvestrant compared with placebo plus fulvestrant [−3.3 (95% CI −5.1 to −1.5) versus 2.0 (95% CI −0.6 to 4.6); P = 0.0011] and significantly less deterioration from baseline was observed for nausea/vomiting [1.7 (95% CI 0.4–3.0) versus 4.2 (95% CI 2.3–6.1); P = 0.0369]. No significant differences between groups were observed in overall change from baseline scores for any other EORTC QLQ-C30 symptoms (Figure 3A).
The estimated median TTD in pain was 8 months (95% CI 5.6–not estimable) in the palbociclib plus fulvestrant group compared with 2.8 months (95% CI 2.3–5.4) in the placebo plus fulvestrant group. Treatment with palbociclib plus fulvestrant significantly delayed TTD in pain symptoms versus placebo plus fulvestrant [HR, 0.642 (95% CI 0.487–0.846); P < 0.001; Figure 2B].
functional scales (QLQ-BR23)
Mean baseline scores of the functional scales body image, sexual functioning, sexual enjoyment, and future perspective were generally similar in both treatment groups. However, the sample sizes for sexual enjoyment are smaller versus other scales because patients were asked to respond to this question only if they responded that they were sexually active in a previous question. Between-group differences in changes from baseline QLQ-BR23 functional scale scores are displayed in Figure 3B. No significant difference was observed between treatment groups in overall change from baseline scores for any of the breast cancer-specific functional scales. Based on interpretation from the 95% CIs of the overall change from baseline analysis within each treatment group, significant improvement in body image and future perspective was observed in the palbociclib group; significant deterioration in sexual enjoyment was observed in both groups.
symptom scales (QLQ-BR23)
Because the question on hair loss was addressed only to patients who experienced hair loss, sample sizes for the symptom scale ‘upset by hair loss’ are much lower than those for other scales. Hair loss was reported in 14.8% versus 5.8% of patients per Common Terminology Criteria for Adverse Events version 4.0. Nearly all events were grade 1 (hair thinning); 1.2% of patients receiving palbociclib plus fulvestrant and no patients receiving placebo plus fulvestrant reported grade 2 (complete hair loss). The between-treatment comparison in overall change from baseline QLQ-BR23 symptom scale scores are displayed in Figure 3C. Significantly greater deterioration from baseline was observed with palbociclib for upset by hair loss [2.9 (95% CI −1.7 to 7.4) versus −6.0 (95% CI −12.3 to 0.3); P = 0.0255]. No significant between-treatment difference was observed in any of the other breast cancer-specific symptoms. The overall changes within each group, based on interpretation from the 95% CIs of the change from baseline analysis (not adjusted for multiple comparisons), indicate a significant improvement in breast symptoms in the palbociclib group, a significant improvement in arm symptoms in both groups, and a significant worsening of systemic therapy side-effects in both groups.
The subgroup analyses results for patients with visceral metastases at baseline (palbociclib plus fulvestrant, n = 199; placebo plus fulvestrant, n = 101) were consistent with the primary analyses in the overall population and showed a significant difference between the treatment groups favoring palbociclib plus fulvestrant in global QoL, emotional functioning, nausea/vomiting, and pain. In addition, a significant difference between treatment groups was observed for role functioning favoring palbociclib plus fulvestrant.
discussion
Given that treatment for MBC is palliative and not curative in nature, when introducing new therapies with increased efficacy it is critical to demonstrate that patient QoL is not compromised and there is no significant deterioration in functioning or symptoms compared with the current standard of care. The recently published European Society for Medical Oncology Magnitude of Clinical Benefit Scale guidance emphasized the importance of a holistic assessment of the value of medicine that includes PROs in addition to efficacy and safety. Presented herein is detailed information about PROs with palbociclib that, together with the efficacy data, could assist with this assessment and argue for high scores for the palbociclib combination on this scale. We show that the favorable efficacy achieved by addition of palbociclib to fulvestrant is accompanied by a delay in deterioration of QoL and pain symptoms. Patients maintained good QoL when an active treatment was added to a well-tolerated endocrine agent, whereas their QoL deteriorated on the endocrine agent alone. No significant difference was observed between the two treatment groups in deterioration of systemic side-effects, breast symptoms, and arm symptoms.
Based on TTD in global QoL, addition of palbociclib to endocrine therapy resulted in a 36% reduction in risk of QoL deterioration. Delayed deterioration in global QoL and pain symptoms with palbociclib corresponds to the delay in disease progression that was previously reported [4]. As disease progression may negatively impact patient QoL, delaying progression could delay QoL deterioration barring any significant detrimental effect due to treatment-related toxicity. Our results further support the positive risk-benefit profile of palbociclib in combination with fulvestrant and show that addition of palbociclib does not impose toxicities that interfere with patient QoL. Pain has been shown to have a significant negative impact on QoL in advanced/MBC patients [12]. Consequently, reducing or delaying pain symptoms is likely to have a positive impact on overall patient functioning and QoL.
We analyzed the impact of palbociclib therapy on parameters considered important by the patients themselves, including emotional functioning and pain. Emotional functioning is reportedly affected by aspects of uncertainty associated with survival in MBC patients and depression and anxiety have been reported in MBC patients [13]. Delaying progression in a clinically meaningful way could potentially decrease the uncertainty associated with prognosis and survival and have a positive impact on emotional functioning. However, the differences in myelosuppression rates between the treatment arms could have influenced the patient scoring and may limit the interpretation of emotional functional scores.
In a recent cross-sectional study by Hollen et al. [14], breast cancer patients rated maintaining QoL, independence, performing normal activities, and controlling pain as more important than control of breast cancer symptoms. Among the symptom scales examined, significantly higher scores in upset by hair loss were observed among patients reporting hair loss while receiving palbociclib. This is consistent with the correspondingly higher rates of alopecia reported in this group (14.8%) versus the placebo plus fulvestrant group (5.8%).
The observed PROs support the concept that the greater efficacy and favorable safety profile of palbociclib plus fulvestrant translates to relatively better QoL compared with placebo plus fulvestrant. This is demonstrated by a significant delay in deterioration of global QoL and pain symptoms, a significantly greater improvement from baseline in emotional functioning and pain, and no significant increase in systemic therapy side-effects. When palbociclib was added to an already well-tolerated endocrine agent (fulvestrant), the combination allowed patients to maintain good QoL in the endocrine resistance setting while experiencing substantially delayed disease progression.
funding
This study was sponsored by Pfizer Inc. No grant number is applicable.
disclosure
NH received honoraria from Amgen, Celgene, Genomic Health, NanoString Technologies, Novartis, Pfizer, and Roche; has served as a consultant/advisor to AstraZeneca, Celgene, Novartis, Roche/Genentech, Pfizer, and Sandoz; and her institution has received research funding from Boehringer Ingelheim, Novartis, Pfizer, and Roche/Genentech. NCT received advisory board honoraria and research funding from Pfizer. MC received honoraria from Nanostring and Agendia, and has served as a consultant/advisor to NewMonics, Dompé, and Cynvenio. JR has served as a consultant/advisor to Nippon Kayaku. FA received honoraria from Novartis and AstraZeneca and has received research funding from and served as a consultant/advisor to Novartis, AstraZeneca, and Pfizer. SLoi received research funding from Pfizer. SV received honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, and Roche/Genentech and has served as a consultant/advisor to Amgen, Astra Zeneca, Eisai, Lilly, Novartis, Pfizer, and Roche/Genentech. HI received honoraria from AstraZeneca, Eisai, Daiichi-Sankyo, Chugai, and Pfizer. SL's institution has received honoraria and research funding from Pfizer. SI, HB, KPT, and CHB are employees of and own stock in Pfizer.
Supplementary Material
acknowledgements
We thank all of the patients, investigators, nurses, and site staff who participated in the PALOMA-3 study. We also thank Ke Zhang, from Pfizer Clinical Statistics, for input and review of the data analyses. Editorial assistance was provided by Johna Van Stelten of Complete Healthcare Communications, LLC, and was funded by Pfizer Inc.
references
- 1.Toogood PL, Harvey PJ, Repine JT et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem 2005; 48: 2388–2406. [DOI] [PubMed] [Google Scholar]
- 2.Finn RS, Dering J, Conklin D et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009; 11: R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finn RS, Crown JP, Lang I et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16: 25–35. [DOI] [PubMed] [Google Scholar]
- 4.Turner NC, Ro J, Andre F et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 2015; 373: 209–219. [DOI] [PubMed] [Google Scholar]
- 5.Butters DJ, Ghersi D, Wilcken N. Addition of drug/s to a chemotherapy regimen for metastatic breast cancer. Cochrane Database Syst Rev 2010; CD003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miles D, von Minckwitz G, Seidman AD. Combination versus sequential single-agent therapy in metastatic breast cancer. Oncologist 2002; 7(Suppl 6): 13–19. [PubMed] [Google Scholar]
- 7.Gupta S, Zhang J, Jerusalem G. The association of chemotherapy versus hormonal therapy and health outcomes among patients with hormone receptor-positive, HER2-negative metastatic breast cancer: experience from the patient perspective. Expert Rev Pharmacoecon Outcomes Res 2014; 14: 929–940. [DOI] [PubMed] [Google Scholar]
- 8.Aaronson NK, Ahmedzai S, Bergman B et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]
- 9.Fayers PM, Aaronson NK, Bjordal K et al. EORTC QLQ-C30 Scoring Manual, 3rd edition. Brussels: European Organisation for Research and Treatment of Cancer 2001. [Google Scholar]
- 10.Osoba D, Rodrigues G, Myles J et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998; 16: 139–144. [DOI] [PubMed] [Google Scholar]
- 11.Scott NW, Fayers PM, Aaronson NK et al. EORTC QLQ-C30 Reference Values. Brussels: European Organisation for Research and Treatment of Cancer; 2008. [Google Scholar]
- 12.Cleeland CS, Mayer M, Dreyer NA et al. Impact of symptom burden on work-related abilities in patients with locally recurrent or metastatic breast cancer: results from a substudy of the VIRGO observational cohort study. Breast 2014; 23: 763–769. [DOI] [PubMed] [Google Scholar]
- 13.Warren M. Uncertainty, lack of control and emotional functioning in women with metastatic breast cancer: a review and secondary analysis of the literature using the critical appraisal technique. Eur J Cancer Care (Engl) 2010; 19: 564–574. [DOI] [PubMed] [Google Scholar]
- 14.Hollen PJ, Msaouel P, Gralla RJ. Determining issues of importance for the evaluation of quality of life and patient-reported outcomes in breast cancer: results of a survey of 1072 patients. Breast Cancer Res Treat 2015; 151: 679–686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.