Abstract
Scarlet fever notifications surged across the United Kingdom in spring 2014. Molecular epidemiologic investigation of Streptococcus pyogenes infections in North-West London highlighted increased emm4 and emm3 infections coincident with the upsurge. Unlike outbreaks in other countries, antimicrobial resistance was uncommon, highlighting an urgent need to better understand the drivers of scarlet fever activity.
Keywords: scarlet fever, Streptococcus pyogenes, bacteria, streptococci, antimicrobial resistance, superantigens, genotype, respiratory infections, notifications, London, England
An unprecedented rise in scarlet fever occurred in England in spring 2014, with >13,000 notifications, for an overall population rate of 24.5/100,000 persons (1,2). We analyzed clinical notification data for North-West London (population ≈1,900,400) during 2009–2014 and determined emm genotypes of Streptococcus pyogenes causing upper respiratory tract (URT) infections during 2009–2014. We focused on peak periods of scarlet fever notification.
The Study
During weeks 10–20 (March–May) 2014, scarlet fever notifications in North-West London increased 3–8-fold compared with the same period in previous years (Figure, panel A). Although Health Protection regulations in England require clinicians to report suspected cases of scarlet fever, molecular surveillance of noninvasive S. pyogenes is not feasible because testing for S. pyogenes is not routinely advised for patients with a sore throat in the United Kingdom (3). Nonetheless, a limited number of URT swab specimens are submitted by clinicians for culture. Since 2009, we have stored all S. pyogenes URT isolates identified in our West London diagnostic laboratory, which serves a population of ≈2 million, overlapping with the North-West London region.
Figure.
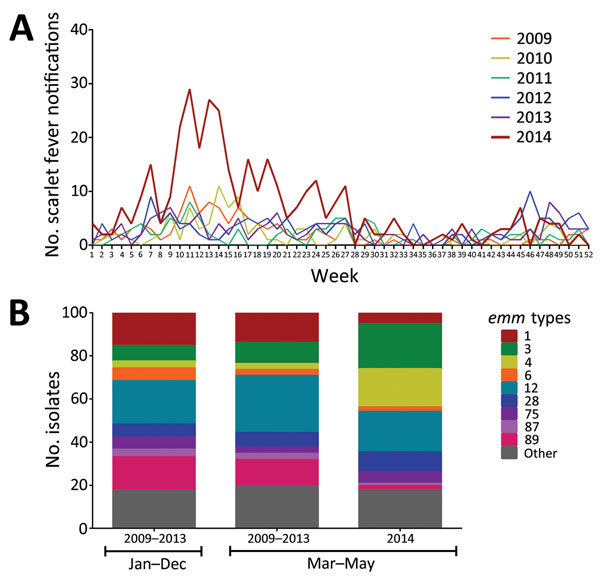
Increase in North-West London scarlet fever notifications and association with emm4 and emm3, 2014. A) Weekly scarlet fever notifications in North-West London during 2009–2014. During weeks 10–20 (March–May) 2014, the number of notifications substantially increased. B) emm genotyping of 404 upper respiratory tract Streptococcus pyogenes isolates. Isolates were available from March 2009 through May 2014, inclusive. A total of 308 isolates were from 2009–2013; however, of these, 134 were from 2009, and 174 were from 2010–2013, reflecting a fall in submission rates and affecting the availability of strains for study. Thus, isolates from 2009–2013 were considered a single group (January–December) and the following emm-types were identified: emm12 20% (62/308), emm89 16% (48/308), emm1 15% (47/308), emm3 7% (22/308), emm28 6% (19/308), emm4 3% (10/308). A similar pattern was observed during March–May in 2009–2013 (n = 72). A total 96 isolates were available from 2014, all of which were from March–May, submitted following alerts to clinicians regarding scarlet fever activity. During March–May 2014, isolates typed as emm4 increased significantly, from 3% (2/72) to 17% (17/96), (χ2(1df) = 9.478. p = 0.0021). A borderline significant increase occurred in emm3, from 10% (7/72) in 2009–2013 to 20% (20/96) in 2014 (χ2(1df) = 3.766, p = 0.0523). This constituted a 3-fold increase in emm3 and emm4 combined, from 13% in 2009–2013 to 37% in 2014 (March–May).
Molecular testing, with standard DNA extraction, emm typing, and superantigen typing methods (4), was performed on all 404 viable S. pyogenes URT isolates identified. Of all isolates obtained from March through May 2009–2013 (n = 72), predominant emm genotypes were emm12 (26%), emm89 (13%), and emm1 (14%) (Figure, panel B). These proportions were almost identical to proportions for all 308 isolates obtained throughout 2009–2013 (Figure, panel B). In contrast, during March–May 2014 (n = 96), dominating emm types changed, with a borderline significant increase in emm3 (from 10% in March–May 2009–2013 to 20% in 2014; χ2(1df) = 3.766, p = 0.0523), and a significant >5-fold increase in emm4 (from 3% to 17%; χ2(1df) = 9.478, p = 0.0021). Among 96 URT samples submitted in March–May 2014, a total of 42 were from children ages ≤5 years. Emm4 was significantly associated with age ≤5 years (χ2(1df) = 6.046, p = 0.0139), and the rise was largely attributable to disease in this age group (12/17 emm4 isolates).
Isolates from March–May 2014 were categorized by at least one of the following clinical features (provided by submitting physician): 1) tonsillitis, pharyngitis, or sore throat and no mention of scarlet fever (n = 44); 2) any mention of scarlet fever, regardless of other information (n = 16); 3) any other illness (n = 6); and 4) no details provided (n = 30). The 16 scarlet fever–associated isolates were limited to patients ages 1.25–11 years, a significant proportion of whom were ≤5 years (12/16; χ2(1df) = 7.619, p = 0.0058); 7/16 were emm3 and 3/16 were emm4. The remainder were emm12 (3), emm28 (2), and emm87 (1). On the basis of these limited data, emm3 was significantly associated with scarlet fever in 2014 (χ2(1df) = 5.964, p = 0.0146).
Clinical data were not collected in earlier years routinely, although in 2009 a total of 3/3 isolates from scarlet fever case-patients were emm3. Scarlet fever–associated emm4 strains from 2014 (n = 3) carried superantigens speC, ssa, and smeZ; the same superantigen profile was found in emm4 strains from patients for whom scarlet fever was not mentioned (n = 14). All 7 scarlet fever–associated emm3 strains carried speA, ssa, speG, and a known mutation in smeZ.
Antimicrobial drug resistance was identified in 10/96 URT isolates from 2014; however, none of these isolates were associated with scarlet fever and none were emm4. Erythromycin resistance was found in 2/20 non–scarlet fever emm3 isolates, in combination with clindamycin resistance in 1 isolate.
Conclusions
An increase in emm3 and emm4 S. pyogenes URT isolates was detected in North-West London, during the period in 2014 when scarlet fever notifications peaked. The increase in emm4 infections was also found predominantly in 4- to 5-year-old children, the group we and others found to be most at risk for scarlet fever (1). The percentage of children 4 years old in North-West London (an urban population) is similar to the national average of 1.3%; therefore, our findings are probably relevant to the rest of the United Kingdom.
Emm4 isolates accounted for only 3/16 cases in which scarlet fever was mentioned, although, because of the study’s retrospective nature and paucity of clinical data supplied, we cannot dismiss the possibility that other emm4 isolates were also associated with scarlet fever. On the basis of the limited analysis of isolates from infections in which scarlet fever was mentioned, we found an association between scarlet fever and S. pyogenes emm3 strains.
The results of our historical comparison must be interpreted with caution; obtaining swab samples from patients with URT infections in England is not routine. Thus, the 2009–2013 samples may reflect persistent infections, in contrast to 2014 samples, when clinicians were encouraged to submit swab specimens for scarlet fever case-patients. Furthermore, the number of strains available for emm typing was limited. Nonetheless, this was the only collection of strains available to us that permitted historical comparison.
Both emm3 and emm4 S. pyogenes strains have been associated with scarlet fever (5). In the Far East, emm1 and emm4 isolates were the leading causes of scarlet fever in the late 1990s (6), although more recently, antimicrobial drug–resistant emm12 S. pyogenes has dominated in this region (7–9). We found that the proportion of emm12 isolates fell during the scarlet fever surge and found no antimicrobial drug resistance among emm3 or emm4 isolates associated with scarlet fever.
Emm4 isolates are associated with pharyngitis in children (10,11); these isolates are entirely acapsular, a phenotype linked to enhanced adhesion to surfaces (12). Whether this characteristic can increase persistence and transmission is unknown. Surges in scarlet fever are believed to require a population susceptible to pharyngeal infection with specific strain types and specific superantigens. Both emm3 and emm4 strains in our study possessed 2 prophage-associated superantigens, either SPEA and SSA, or SPEC and SSA. Although these toxin genes were found in emm3 and emm4 strains not associated with scarlet fever, the probability of triggering scarlet fever may be enhanced through production of 2 such superantigens. An association between these superantigens and scarlet fever has been reported (13).
Periodic increases in scarlet fever are well recognized, although the magnitude of the upsurge in the United Kingdom was unexpected. Consultation rates for sore throat diminished in the 1990s (14), and the 2008 UK national guidelines advise against diagnostic testing and recommend a policy of nonprescribing or delayed prescribing for sore throat when the Centor score is <3 (3). These recommendations contrast with those of North America and of some European countries (15). Whether exceeding a threshold level of community S. pyogenes transmission is required for such a marked upsurge is unclear; increased scarlet fever activity was not reported elsewhere in Europe, to our knowledge. Apart from natural fluctuations in population immunity, emergence of hypertransmissible lineages, acquisition of novel phage-encoded toxins, or antimicrobial drug resistance may contribute to scarlet fever surges (6,7). Notably, isolates we found associated with scarlet fever were not resistant to common antimicrobial agents.
As part of the national response, clinicians were advised to treat scarlet fever to minimize complications and reduce transmission. Whether use of more refined molecular diagnostics could assist future community prevention and management of S. pyogenes infection will require careful evaluation. Increased scarlet fever activity has continued in England in 2015 and 2016, underscoring the need for ongoing surveillance and further investigation.
Acknowledgments
We are grateful to Hugo Donaldson and staff of the local diagnostic laboratory of Imperial College Healthcare National Health Service Trust, who performed identification and susceptibility testing, and to the clinical staff, who reported cases of scarlet fever.
This project was funded by the National Institute for Health Research Biomedical Research Centre awarded to Imperial College Healthcare NHS Trust and the UK Clinical Research Collaboration (UKCRC, Centre for Infection Prevention and Management).
Biography
Dr. Turner is a Junior Research Fellow in the Department of Medicine, Imperial College London. Her primary research interests are streptococcal pathogenesis and bacterial population genomics.
Footnotes
Suggested citation for this article: Turner CE, Pyzio M, Song B, Lamagni T, Meltzer M, Chow JY, et al. Scarlet fever upsurge in England—molecular-genetic analysis in North-West London, 2014. Emerg Infect Dis. 2016 Jun [date cited]. http://dx.doi.org/10.3201/eid2206.151726
Current affiliation: Royal Sussex County Hospital, Brighton, UK.
References
- 1.Guy R, Williams C, Irvine N, Reynolds A, Coelho J, Saliba V, et al. Increase in scarlet fever notifications in the United Kingdom, 2013/2014. Euro Surveill. 2014;19:20749. 10.2807/1560-7917.ES2014.19.12.20749 [DOI] [PubMed] [Google Scholar]
- 2.Public Health England. Group A streptococcal infections: seasonal activity, 2014/15. Health Protection Report. 2014;8 [cited 2015 Sep 28]. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/377520/hpr4414_SF.pdf
- 3.National Institute for Health and Care Excellence. Respiratory tract infections: antibiotic prescribing. Prescribing antibiotics for self-limiting respiratory tract infections in adults and children in primary care (NICE guideline), July 2008. [cited 2015 Sep 28]. https://www.nice.org.uk/guidance/CG69/chapter/1-Guidance [PubMed]
- 4.Turner CE, Dryden M, Holden MT, Davies FJ, Lawrenson RA, Farzaneh L, et al. Molecular analysis of an outbreak of lethal postpartum sepsis caused by Streptococcus pyogenes. J Clin Microbiol. 2013;51:2089–95. 10.1128/JCM.00679-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perks EM, Mayon-White RT. The incidence of scarlet fever. J Hyg Camb. 1983;91:203–9. 10.1017/S0022172400060204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan JJ, Liu CC, Ko WC, Hsu SY, Wu HM, Lin YS, et al. Molecular analysis of group A streptococcal isolates associated with scarlet fever in southern Taiwan between 1993 and 2002. J Clin Microbiol. 2003;41:4858–61. 10.1128/JCM.41.10.4858-4861.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tse H, Bao JY, Davies MR, Maamary P, Tsoi HW, Tong AH, et al. Molecular characterization of the 2011 Hong Kong scarlet fever outbreak. J Infect Dis. 2012;206:341–51. 10.1093/infdis/jis362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiou CS, Wang YW, Chen PL, Wang WL, Wu PF, Wei HL. Association of the shuffling of Streptococcus pyogenes clones and the fluctuation of scarlet fever cases between 2000 and 2006 in central Taiwan. BMC Microbiol. 2009;9:115. 10.1186/1471-2180-9-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luk EYY, Lo JYC, Li AZL, Lau MCK, Cheung TKM, Wong AYM, et al. Scarlet fever epidemic, Hong Kong, 2011. Emerg Infect Dis. 2012;18:1658–61. 10.3201/eid1810.111900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaggi P, Tanz RR, Beall B, Shulman ST. Age influences the emm type distribution of pediatric group A streptococcal pharyngeal isolates. Pediatr Infect Dis J. 2005;24:1089–92. 10.1097/01.inf.0000190023.89759.96 [DOI] [PubMed] [Google Scholar]
- 11.Shulman ST, Tanz RR, Dale JB, Beall B, Kabat W, Kabat K, et al. Seven-year surveillance of North American pediatric group A streptococcal pharyngitis isolates. Clin Infect Dis. 2009;49:78–84. 10.1086/599344 [DOI] [PubMed] [Google Scholar]
- 12.Turner CE, Abbott J, Lamagni T, Holden MT, David S, Jones MD, et al. Emergence of a new highly successful acapsular group A Streptococcus clade of genotype emm89 in the United Kingdom. MBio. 2015;6:e00622. 10.1128/mBio.00622-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva-Costa C, Carrico JA, Ramirez M, Melo-Cristino J. Scarlet fever is caused by a limited number of Streptococcus pyogenes lineages and is associated with the exotoxin genes ssa, speA and speC. Pediatr Infect Dis J. 2014;33:306–1. 10.1097/INF.0000000000000088 [DOI] [PubMed] [Google Scholar]
- 14.Ashworth M, Latinovic R, Charlton J, Cox K, Rowlands G, Gulliford M. Why has antibiotic prescribing for respiratory illness declined in primary care? A longitudinal study using the General Practice Research Database. J Public Health (Oxf). 2004;26:268–74. 10.1093/pubmed/fdh160 [DOI] [PubMed] [Google Scholar]
- 15.Chiappini E, Regoli M, Bonsignori F, Sollai S, Parretti A, Galli L, et al. Analysis of different recommendations from international guidelines for the management of acute pharyngitis in adults and children. Clin Ther. 2011;33:48–58. 10.1016/j.clinthera.2011.02.001 [DOI] [PubMed] [Google Scholar]


