Abstract
Aims
The low lymphocyte counts and high neutrophil leucocyte fractions have been associated with poor prognosis in chronic heart failure. We hypothesized that the baseline ratio of the neutrophil leucocytes to the lymphocytes (NL ratio) would predict the outcome of chronic heart failure patients undergoing cardiac resynchronization therapy (CRT).
Methods and results
The qualitative blood counts and the serum levels of N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) of 122 chronic heart failure patients and 122 healthy controls were analysed prospectively in this observational study. The 2-year mortality was considered as primary endpoint and the 6-month reverse remodelling (≥15% decrease in the end-systolic volume) as secondary endpoint. Multivariable regression analyses were applied and net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated. The NL ratio was elevated in chronic heart failure patients when compared with the healthy controls [2.93 (2.12–4.05) vs. 2.21 (1.64–2.81), P < 0.0001]. The baseline NL ratio exceeding 2.95 predicted the lack of the 6-month reverse remodelling [n = 63, odds ratio = 0.38 (0.17–0.85), P = 0.01; NRI = 0.49 (0.14–0.83), P = 0.005; IDI = 0.04 (0.00–0.07), P = 0.02] and the 2-year mortality [n = 29, hazard ratio = 2.44 (1.04–5.71), P = 0.03; NRI = 0.63 (0.24–1.01), P = 0.001; IDI = 0.04 (0.00–0.08), P = 0.02] independently of the NT-proBNP levels or other factors.
Conclusion
The NL ratio is elevated in chronic heart failure and predicts outcome after CRT. According to the reclassification analysis, 4% of the patients would have been better categorized in the prediction models by combining the NT-proBNP with the NL ratio. Thus, a single blood count measurement could facilitate the optimal patient selection for the CRT.
Keywords: Chronic heart failure, Resynchronization, Lymphocyte, Neutrophil, Outcome, Reclassification
What's New?
It has been shown previously that the baseline ratio of the neutrophil leucocytes to the lymphocytes (NL ratio) predicts the 6-month reverse remodelling following cardiac resynchronization therapy (CRT).
Our analysis has revealed that the NL ratio is elevated in chronic heart failure and predicts not only the reverse remodelling, but also the 2-year mortality rate of the patients with CRT, independently of the N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels or other factors.
Patients with an NL ratio higher than 2.95 before the CRT implantation experience up to three times higher risk not going under reverse remodelling or to pass away before the 2-year post-implantation period.
The combined use of the NT-proBNP with the NL ratio improves the risk stratification, 4% of the patients would have been better categorized with a simple blood count measurement.
Introduction
The white blood cells (WBCs) or leucocytes are the effectors and coordinators of the chronic cellular inflammatory process in which several WBC subgroups are involved, such as the lymphocytes, monocytes, neutrophils, basophils, and the eosinophils.1 The leucocytosis refers to an increase in the number of the WBC and this phenomenon predicts an increased mortality and hospitalization rate in patients with chronic heart failure.2 The relative decrease of the lymphocytes, termed as lymphocytopenia, has also been associated with disease progression and poor outcome.3 Similarly, the increase in the number of the neutrophils (neutrophilia) predicts death and hospitalization in chronic heart failure.4
The cardiac resynchronization therapy (CRT) is highly effective treatment for the medically refractory chronic heart failure patients with intra- and interventricular conduction delay by biventricular pacing.5,6 Most of the patients respond adequately to the therapy with improvement of the functional status and the reverse remodelling of the failing heart. This latter can be objectively quantified by imaging modalities and based on those several response criteria had been established to assess the success of the therapy.7,8
The N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) is a widely used traditional biomarker in the diagnosis establishment, the treatment guidance and the prognosis prediction in chronic heart failure. The lower levels of the NT-proBNP predict the long-term survival of the patients and also the short-term 6-month response to the CRT.9
It is widely accepted that the chronic heart failure is associated with an ongoing inflammatory response, however, the exact mechanism is not fully described.1 We hypothesized that the ratio of the neutrophil leucocytes to the lymphocytes (NL ratio) is elevated and would predict the 6-month reverse remodelling and the 2-year mortality of the patients independently of the baseline NT-proBNP levels or other factors. We aimed to test the potential clinical utility of our models with reclassification methods.
Methods
Study population and study design
The purpose of this prospective single-centre observational follow-up study was to evaluate the prognostic impact of routine laboratory parameters on the clinical outcome of chronic heart failure patients with CRT. The physicians participating in the study were aware of the blood count of the patients as this is part of the basic laboratory tests before CRT, however, no further calculations were performed (e.g. the NL ratio).
A total of 131 consecutive patients with medically refractory heart failure referred to our Heart and Vascular Center between September 2009 and December 2010 for CRT implantation, according to the current guidelines.10 The inclusion criteria included previously diagnosed and medically treated chronic heart failure [New York Heart Association (NYHA) class II–IV] with wide QRS in electrocardiogram (ECG) (>120 ms) and a severely reduced (<35%) left ventricular ejection fraction (LVEF). We considered autoimmune diseases, haematologic diseases, acute or chronic inflammatory diseases, and malignancies as exclusion criteria, and three patients were excluded on this basis. The procedure of the CRT implantation was performed by implantation of a left ventricular lead into the sidebranch of the coronary sinus, a right ventricular lead in a septal position and a right atrial lead where appropriate.
We followed up the patients for 2 years and routine laboratory tests, clinical examinations, ECG, and echocardiographic measurements were carried out. The clinical examinations included heart failure functional assessment with NYHA classification. Echocardiographic measurements were performed using Philips iE33 system to calculate the LVEF with Simpson's biplane method and the left ventricle volumes using Teicholz method.
In the final analysis, 122 chronic heart failure patients were included with complete laboratory and clinical data. The data of 122 age, gender, and BMI matched healthy control subjects were also analysed who participated in the voluntary ‘Budakalász Study’ of our clinic.11 We considered the all-cause 2-year mortality of the chronic heart failure patients as the primary endpoint. The secondary endpoint was the 6-month reverse remodelling [defined as at least 15% absolute decrease in the end-systolic volume (ESV)].7
Prior to the enrolment all patients provided written informed consent. The local Ethics Committee of the Semmelweis University had approved the protocol which was in accordance with the Helsinki Declaration.
Laboratory measurements
We collected serum and ethylenediaminetetraacetic acid plasma samples for biochemical measurements at baseline and 6 months after CRT implantation. The qualitative blood counts were measured on the day of blood sampling as the part of the routine clinical evaluation. Aliquots for the NT-proBNP were processed within 2 h of sampling and were stored frozen at −80°C until the later measurements. Blood cell counting was performed by using the Symex XS-1000i (Kobe, Japan) system with fluorescent flow cytometry technology. The absolute numbers of the WBC subgroups were automatically calculated by the analyser then displayed their relative percentages to the total WBC counts. The levels of the NT-proBNP were measured with electrochemiluminescence technology by Cobas e 411 analyser (Mannheim, Germany) using Roche Elecsys NT-proBNP II kits (Cat. No.: 04842464190, Mannheim, Germany).
Statistical analysis
The parameters reported in this study differed from the normal distributions, thus we used non-parametric tests, and the data are expressed as median values with interquartile ranges (25–75%) and as percentages with event numbers. A two-tailed P-value of <0.05 was considered statistically significant in all cases.
For comparison we applied the Mann–Whitney, the Wilcoxon matched-pairs signed rank and the χ2 tests, as appropriate. The optimum cut-off values were established by using the receiver operating characteristic (ROC) analysis.
The reference models of the reverse remodelling and the survival prediction included the statistically significant variables of the univariate logistic and Cox regression analysis. The NT-proBNP, then the leucocyte parameters were entered into the reference models in a forward stepwise way. The continuous variables were dichotomized based on their best fitting cut-off values.
We aimed to present the exact clinical benefit of the leucocyte parameters in the prediction models, thus we validated and calibrated these models by the Hosmer–Lemeshow test and showed the overall model performance improvement by the changes of the Brier score and the Nagelkerke's R2. Reclassification and discrimination procedures were performed including the c-statistic with DeLong test, the net reclassification improvement (NRI) and the integrated discrimination improvement (IDI) in order to numerically reveal those patients who profit with the new marker.12
The reported statistical analysis was carried out by using IBM SPSS 22 (Apache Software Foundation, USA), Graphpad Prism 6.03 (GraphPad Softwares Inc., USA), PASS 2008 (NCSS, USA), and the open source R software (R version 3.1.2 with PredictABEL and pROC packages).
Results
The baseline characteristics of the study population
The median age of the patients was 67 years, 82% of them were male, 59% had a heart failure of ischaemic origin, 81% had left bundle branch block (LBBB) in the ECG and the median QRS width was 163 ms. The median LVEF was 27% and the functional status showed that 85% of the patients were in NYHA class III or IV (Table 1).
Table 1.
Baseline parameters as predictors of the 6-month reverse remodelling and the 2-year mortality
| Heart failure patients (n = 122) | Six-month reverse remodelling |
Two-year all-cause mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI of OR | χ 2 | P-Value | HR | 95% CI of HR | χ 2 | P-Value | ||
| Clinical variables | |||||||||
| Age (years) | 67 (61–73) | 0.67 | 0.45–0.80 | 3.93 | 0.03 | 0.96 | 0.67–1.37 | 0.03 | 0.84 |
| Male gender | 101 (82) | 0.36 | 0.13–0.99 | 3.77 | 0.04 | 1.35 | 0.47–3.89 | 0.31 | 0.57 |
| BMI (kg/m2) | 27 (25–29) | 1.27 | 0.88–1.83 | 1.63 | 0.20 | 0.87 | 0.60–1.27 | 0.48 | 0.48 |
| Ischaemic | 72 (59) | 0.85 | 0.41–1.75 | 0.18 | 0.66 | 2.04 | 1.04–4.43 | 3.81 | 0.04 |
| LBBB | 100 (81) | 1.69 | 0.66–4.32 | 1.22 | 0.26 | 0.42 | 0.19–0.93 | 4.54 | 0.03 |
| CRT-D | 20 (16) | 2.52 | 0.89–7.08 | 3.09 | 0.07 | 0.53 | 0.16–1.71 | 1.06 | 0.30 |
| QRS (ms) | 163 (139–184) | 1.57 | 1.02–2.48 | 3.83 | 0.04 | 1.03 | 0.72–1.48 | 0.03 | 0.85 |
| LVEF (%) | 27 (23–33) | 0.90 | 0.63–1.30 | 0.27 | 0.60 | 0.93 | 0.64–1.35 | 0.12 | 0.72 |
| ESV (mL) | 210 (147–246) | 1.51 | 1.02–2.24 | 4.33 | 0.03 | 0.99 | 0.68–1.45 | 0.00 | 0.99 |
| EDV (mL) | 303 (242–351) | 1.56 | 1.05–2.31 | 5.01 | 0.02 | 1.02 | 0.71–1.49 | 0.02 | 0.88 |
| NYHA III,IV | 104 (85) | 0.25 | 0.07–0.82 | 5.20 | 0.02 | 2.57 | 0.61–10.81 | 1.65 | 0.19 |
| Hypertension | 65 (53) | 0.92 | 0.45–1.89 | 0.04 | 0.83 | 1.28 | 0.61–2.69 | 0.45 | 0.50 |
| Hyperlipidaemia | 27 (22) | 0.46 | 0.19–1.12 | 2.89 | 0.08 | 0.53 | 0.18–1.53 | 1.35 | 0.24 |
| Diabetes m. | 47 (38) | 0.83 | 0.40–1.74 | 0.22 | 0.63 | 2.82 | 1.33–5.99 | 7.37 | 0.007 |
| ACEi/ARB | 110 (90) | 1.07 | 0.20–5.53 | 0.00 | 0.93 | 0.63 | 0.15–2.69 | 0.37 | 0.54 |
| BB | 116 (95) | 2.31 | 0.65–8.13 | 1.71 | 0.19 | 0.21 | 0.09–0.48 | 13.67 | <0.0001 |
| MRI | 86 (70) | 1.76 | 0.80–3.88 | 2.01 | 0.15 | 0.63 | 0.19–1.34 | 1.41 | 0.23 |
| Laboratory data | |||||||||
| NT-proBNP (pg/mL) | 2626 (1515–5101) | 0.59 | 0.38–0.92 | 5.36 | 0.02 | 1.39 | 1.08–1.80 | 6.56 | 0.01 |
| NL ratio | 2.93 (2.12–4.05) | 0.67 | 0.46–0.99 | 3.88 | 0.04 | 1.48 | 1.13–1.94 | 8.11 | 0.004 |
| Neutrophils (%) | 66.2 (60.0–72.9) | 0.66 | 0.45–0.97 | 4.45 | 0.03 | 1.70 | 1.14–2.53 | 6.80 | 0.009 |
| Lymphocytes (%) | 22.9 (17.6–28.2) | 1.65 | 1.12–2.43 | 6.46 | 0.01 | 0.52 | 0.34–0.79 | 9.54 | 0.002 |
| Monocytes (%) | 6.7 (5.4–8.4) | 0.73 | 0.51–1.07 | 2.49 | 0.11 | 1.15 | 0.81–1.62 | 0.64 | 0.42 |
| Eosinophils (%) | 2.2 (1.4–3.0) | 1.02 | 0.71–1.46 | 0.02 | 0.88 | 1.13 | 0.83–1.55 | 0.64 | 0.42 |
| Basophils (%) | 0.5 (0.3–0.8) | 1.19 | 0.82–1.73 | 0.91 | 0.33 | 0.60 | 0.36–1.00 | 3.75 | 0.05 |
| WBC (×103 µL–1) | 6.9 (5.8–8.1) | 0.85 | 0.59–1.24 | 0.65 | 0.42 | 1.53 | 1.20–1.95 | 9.21 | 0.003 |
Data are expressed as median with interquartile range for continuous variables and as event numbers with percentage for categorical variables. The 6-month reverse remodelling (n = 63, ≥15% decrease in the ESV) was tested by using the univariate logistic regression analysis and the 2-year mortality (n = 29) was assessed by using the univariate Cox regression analysis. The continuous variables were standardized by 1 SD increase. The odds and hazard ratios refer for the presence vs. absence in case of categorical variables and 1 SD increase in case of continuous variables.
OR, odds ratio; HR, hazard ratio; CI, confidence interval; χ2, Chi squared; BMI, body mass index; Ischaemic, ischaemic aetiology of the heart failure; LBBB, left bundle branch block; CRT-D, cardiac resynchronization therapy with implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; ESV, end-systolic volume; EDV, left ventricular end-diastolic volume; NYHA III,IV, New York Heart Association classification III,IV; ACEi/ARB, angiotensin convertase inhibitor/angiotensin receptor blocker; BB, beta blocker; MRI, mineralocorticoid receptor inhibitor; NT-proBNP, N-terminal of the prohormone brain natriuretic peptide.
The NL ratio was elevated in chronic heart failure patients when compared with the healthy controls [2.93 (2.12–4.05) vs. 2.21 (1.64–2.81), P < 0.0001] without significant difference in the total leucocyte counts [6.9 (5.8–8.1) vs. 6.8 (5.8–8.1) × 103 mL–1, P = 0.81, respectively] (Supplementary material online, Table S1).
Changes in echocardiographic and laboratory parameters 6 months after cardiac resynchronization therapy implantation
The CRT has led to a statistically significant increases in the LVEF [27 (23–33) vs. 37 (30–40)%, P < 0.0001] and decreases in the left ventricular end-systolic volume [210 (150–276) vs. 167 (114–238) mL, P < 0.0001] and the left ventricular end-diastolic volume [303 (242–361) vs. 259 (210–326) mL, P < 0.0001] in the cohort.
We have observed statistically significant decreases in the NL ratio [2.93 (2.12–4.05) vs. 2.82 (2.05–3.05), P = 0.04] and the NT-proBNP levels [2626 (1515–5101) vs. 1688 (757–3554) pg/mL, P = 0.001] 6 months after implantation.
The baseline ratio of the neutrophil leucocytes to the lymphocytes in the prediction of the 6-month reverse remodelling
From the aspect of the echocardiographic reverse remodelling following the CRT, 63 patients (52%) were categorized as responders and 59 patients (48%) as non-responders.
Responders at baseline had lower NL ratio [2.62 (1.90–3.71) vs. 3.12 (2.35–4.66), P = 0.01] and lower NT-proBNP levels [2315 (1004–3989) vs. 3305 (1869–6019) pg/mL, P = 0.004, respectively]. Using the ROC analysis, we defined the NL ratio 2.95 [area under the curve (AUC) = 0.63 (0.53–0.73)] and the NT-proBNP 1522 pg/mL [AUC = 0.64 (0.55–0.74)] as optimal cut-off values for the prediction models and their diagnostic accuracy is shown in Table 2.
Table 2.
Diagnostic accuracy of the biomarkers in the prediction models
| Test | Six-month reverse remodelling |
Two-year all-cause mortality |
||
|---|---|---|---|---|
| No | Yes | Yes | No | |
| NL ratio ≥2.95 | 36 | 23 | 21 | 38 |
| NL ratio <2.95 | 23 | 40 | 8 | 55 |
| Sensitivity | 61 (47–73) | 72 (53–87) | ||
| Specificity | 63 (50–75) | 59 (48–69) | ||
| Positive predictive value | 61 (47–73) | 36 (24–49) | ||
| Negative predictive value | 63 (50–75) | 87 (77–94) | ||
| NT-proBNP ≥1522 pg/mL | 51 | 41 | 27 | 65 |
| NT-proBNP <1522 pg/mL | 8 | 22 | 2 | 22 |
| Sensitivity | 86 (75–94) | 93 (77–99) | ||
| Specificity | 35 (23–48) | 30 (21–41) | ||
| Positive predictive value | 55 (45–66) | 29 (20–40) | ||
| Negative predictive value | 73 (54–88) | 93 (78–99) | ||
The values are given as case numbers for the tests. Sensitivity, specificity, positive, and negative predictive values are expressed as percentage with 95% confidence interval.
The univariate logistic regression analysis revealed that the reverse remodelling following the CRT was significantly associated with younger age, female gender, wider QRS, and better NYHA functional status at baseline, thus these variables composed of the later multivariable reference model (Table 1).
We entered the NT-proBNP to the reference model, then the NL ratio in a forward stepwise way and the baseline NL ratio exceeding 2.95 independently predicted the lack of the 6-month reverse remodelling [odds ratio = 0.38 (0.17–0.85), P = 0.01], as shown in Table 3.
Table 3.
The role of the leucocyte parameters in the prediction of the study outcomes
| Six-month reverse remodelling |
P-Value | Two-year all-cause mortality |
P-Value | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI of OR | χ 2 | HR | 95% CI of HR | χ 2 | |||
| NL ratio ≥2.95 | 0.38 | 0.17–0.85 | 5.54 | 0.01 | 2.44 | 1.04–5.71 | 4.24 | 0.03 |
| Neutrophils ≥63.6% | 0.37 | 0.15–0.88 | 5.06 | 0.02 | 2.41 | 1.06–5.46 | 4.49 | 0.03 |
| Lymphocytes ≤22% | 0.41 | 0.18–0.90 | 4.88 | 0.02 | 2.42 | 0.91–6.41 | 3.19 | 0.07 |
The 6-month reverse remodelling (n = 63) was defined as at least 15% decrease in the ESV. The reference multivariable logistic regression analysis included male gender, NYHA class III/IV, age ≥70 years, and QRS ≥160 ms. In a forward stepwise way, we adjusted the NT-proBNP ≥1522 pg/mL to the reference model, then the leucocyte parameters separately. The 2-year mortality (n = 29) was assessed by using multivariable Cox regression. The reference model included ischaemic heart failure aetiology, beta-blocker therapy, LBBB, and diabetes mellitus. We adjusted the NT-proBNP and the leucocyte parameters as described in the logistic regression. The odds ratios (ORs) and the hazard ratios (HRs) refer for the presence or absence of the outcome.
CI, confidence interval; χ2, Wald Chi square; NT-proBNP, N-terminal of the prohormone brain natriuretic peptide; NL ratio, ratio of the neutrophil leucocytes and the lymphocytes.
The baseline ratio of the neutrophil leucocytes to the lymphocytes in the prediction of the 2-year mortality
Up to a median follow-up period of 787 days, 29 (23%) patients died. Those who survived the follow-up period had lower baseline NL ratio [2.70 (2.05–3.68) vs. 3.90 (2.87–5.65), P = 0.001] and lower NT-proBNP levels [2418 (1116–4050) vs. 4959 (2429–6864) pg/mL, P = 0.002]. The ROC analysis showed that the predefined NL ratio of 2.95 [AUC = 0.69 (0.58–0.80)] and the NT-proBNP level of 1522 pg/mL [AUC = 0.70 (0.60–0.80)] were also optimal for the mortality prediction models (Table 2). The Kaplan–Meier survival curves based on the NL ratio are shown in Figure 1.
Figure 1.
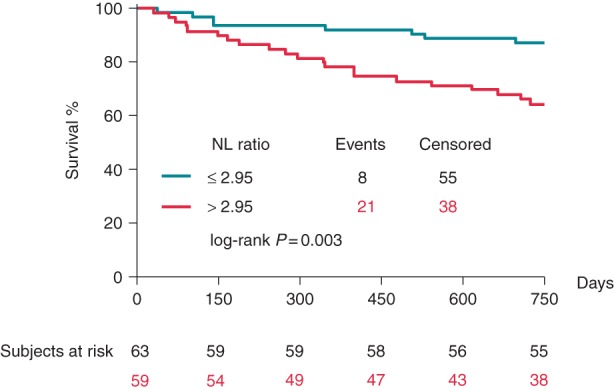
Influence of the baseline NL ratio on the 2-year all-cause mortality of the patients. We compared the Kaplan–Meier survival curves using log-rank test.
The 2-year survival of the patients in the univariate Cox regression analysis was significantly predicted by the LBBB pattern of the ECG, the use of beta-blocker therapy, the absence of diabetes mellitus, and the non-ischaemic aetiology of the heart failure, and consequently these parameters comprising the reference model of the multivariable analysis (Table 1).
Using the multivariable Cox regression analysis, we have adjusted the NT-proBNP to the reference model, then the NL ratio in a forward stepwise way and the NL ratio exceeding 2.95 independently predicted the 2-year mortality of the patients [hazard ratio = 2.44 (1.04–5.71), P = 0.03], as shown in Table 3.
Testing of the prediction models
The regression analyses with a sample size of the 122 patients achieved a power >90% to detect the presented odds and hazard ratios. Building each step of the prediction models achieved statistically significant changes in the Chi scores, thus our models were considered valid (Table 4). The Hosmer–Lemeshow test assesses if the observed event rates match the expected event rates in the prediction steps, and since the test's P values did not differ statistically significant throughout the analysis, the occurred event rates were similar to the expected event rates, in other words the models were calibrated well. The overall performance of the prediction models improved by adding the NL ratio to the reference models because the Nagelkerke's R2 value has increased (which indicates how well the data fit in the model), while the Brier score has decreased (which measures the probability of accidental events in the predictions) in each step, as shown in Table 4.
Table 4.
Testing of the prediction models
| Six-month reverse remodelling |
Two-year all-cause mortality |
|||||
|---|---|---|---|---|---|---|
| Reference model | Reference model + NT-proBNP | Reference model + NT-proBNP + NL ratio | Reference model | Reference model + NT-proBNP | Reference model + NT-proBNP + NL ratio | |
| Validation | ||||||
| Overall χ2 | 12.00 | 16.09 | 21.75 | 25.83 | 29.48 | 36.01 |
| P (overall) | 0.01 | 0.007 | 0.01 | <0.0001 | <0.0001 | <0.0001 |
| Calibration | ||||||
| HL test χ2 | 2.90 | 10.88 | 7.93 | 1.27 | 4.89 | 4.60 |
| P (HL test) | 0.94 | 0.20 | 0.44 | 0.99 | 0.76 | 0.79 |
| Performance | ||||||
| Nagelkerke's R2 | 0.12 | 0.16 | 0.21 | 0.24 | 0.29 | 0.34 |
| Brier score | 0.22 | 0.21 | 0.20 | 0.14 | 0.13 | 0.12 |
| Reclassification | ||||||
| c-Statistic | 0.65 (0.56–0.75) | 0.66 (0.56–0.76) | 0.71 (0.62–0.80) | 0.74 (0.63–0.85) | 0.77 (0.67–0.87) | 0.79 (0.69–0.89) |
| P (c-statistic) | 0.85 | 0.13 | 0.29 | 0.40 | ||
| NRI (95% CI) | 0.42 (0.13–0.72) | 0.49 (0.14–0.83) | 0.46 (0.20–0.72) | 0.63 (0.24–1.01) | ||
| P (NRI) | 0.004 | 0.005 | 0.0005 | 0.001 | ||
| IDI (95% CI) | 0.02 (0.00–0.05) | 0.04 (0.00–0.07) | 0.03 (0.00–0.06) | 0.04 (0.00–0.08) | ||
| P (IDI) | 0.05 | 0.02 | 0.01 | 0.02 | ||
The 6-month reverse remodelling (n = 63) was defined as at least 15% decrease in the ESV. The reference model for the logistic regression analysis included male gender, NYHA class III/IV, age ≥70 years, and QRS ≥160 ms. The reference model for the 2-year mortality (n = 29) using Cox regression analysis included ischaemic heart failure aetiology, beta-blocker therapy, LBBB, and diabetes mellitus. In a forward stepwise way, we adjusted the NT-proBNP ≥1522 pg/mL to the reference models, then the NT ratio ≥2.95.
χ 2, Chi square; HL test, Hosmer–Lemeshow test; 95% CI, 95% confidence interval; NRI, net reclassification improvement; IDI, integrated discrimination improvement; NT-proBNP, N-terminal of the prohormone brain natriuretic peptide; NL ratio, ratio of the neutrophil leucocytes and the lymphocytes.
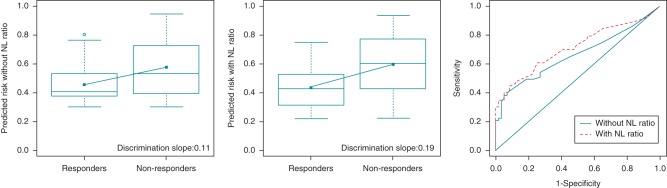
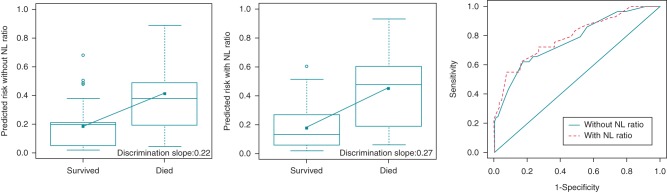
The discriminative power tested by means of the c-statistic demonstrated that the NL ratio has increased the AUC of the prediction (reverse remodelling from 0.66 to 0.71 and mortality from 0.77 to 0.79). We also displayed how the discrimination slope becomes steeper (the reverse remodelling from 0.11 to 0.19 and the mortality from 0.22 to 0.27) by adding the NL ratio to the models (box plots in Figures 2 and 3).
Figure 2.
Improvement in the risk prediction of the 6-month reverse remodelling using the baseline NL ratio. The discrimination slopes are calculated as the difference between the mean predicted probabilities, thus the difference between the slopes is the integrated discrimination index itself. The differences of the receiver operating curves (c-statistic) are demonstrated on the right side.
Figure 3.
Improvement in the risk prediction of the 2-year all-cause mortality using the baseline NL ratio. The discrimination slopes are calculated as the difference between the mean predicted probabilities, thus the difference between the slopes is the integrated discrimination index itself. The differences of the receiver operating curves (c-statistic) are demonstrated on the right side.
We have observed a significant improvement in the NRI [NRI reverse remodelling: 0.49 (0.14–0.83), P = 0.005 and mortality: 0.63 (0.24–1.01), P = 0.001] and also a significant improvement in the IDI [IDI reverse remodelling: 0.04 (0.00–0.07), P = 0.02 and mortality 0.04 (0.00–0.08), P = 0.02] by combining the models with the NL ratio (Table 4).
The IDI = 0.04 value for the reverse remodelling and also for the mortality demonstrates that 4% of the patients were better categorized in these prediction models by adding the NL ratio to the NT-proBNP adjusted reference models. Thus, 5 patients of the 122 cases had lower baseline NT-proBNP levels than 1522 pg/mL and still did not experience reverse remodelling, or died 2 years later. On the other hand, all of these patients had an elevated NL ratio.
Discussion
Synopsis of key findings
We have found that the baseline NL ratio is elevated in chronic heart failure and the NL ratio predicts the 6-month reverse remodelling and the 2-year mortality of the patients undergoing CRT independently of the NT-proBNP levels or other factors.
Patients with an NL ratio higher than 2.95 before the CRT implantation experience up to three times higher risk not going under reverse remodelling or to pass away in the post-implantation period. According to the reclassification analysis, the combined use of the NT-proBNP with the NL ratio improves the risk stratification: 5 patients of the 122 cases were better categorized with a simple blood count measurement.
Possible mechanisms and explanations
The lymphocytes (T cells, B cells, and NK cells) are the effectors of the adaptive and the innate immune system.1 The lymphocytopenia is common finding among chronic heart failure patients associated with adverse outcome.3 The neurohormonal activation, oxidative stress, and the chronic inflammation in heart failure increase the catecholamine release13 and the plasma cortisol levels14 leading to bone marrow suppression and down-regulation of the lymphocyte proliferation and differentiation14 with aggravated lymphocyte apoptosis.15
The natriuretic peptides such as the NT-proBNP are up-regulated in heart failure in order to compensate the disease progression by natriuresis, vasodilation, and the suppression of the sympathetic nervous activation.16 Experimental data suggest that the natriuretic peptides exert a significant impact on the WBCs: the atrial natriuretic peptide inhibits the expression of adhesion molecules and reduce the production of various lymphocyte subgroups,17 while the BNP overexpression after acute myocardial infarction facilitates the neutrophil infiltration in rat model.18
The neutrophils release reactive oxygen species, activate phagocytosis, and pro-inflammatory agents such as the C-reactive protein, tumour necrosis factor-alpha, interleukin-6, and trigger myocardial injury.1 On the other hand, the interleukin-6 directly promotes the neutrophil release to the circulation from the bone marrow.19 These inflammatory mediators are commonly elevated in chronic heart failure patients which could lead to the neutrophilia.20
The role of the NL ratio in the CRT has been recently investigated by Agacdiken et al.,21 who included 70 patients in their study and demonstrated that the echocardiographic response to the CRT was associated with lower baseline NL ratio. Our results confirm and expand those preliminary findings, as we were the first to reveal the value of the NL ratio in the mortality prediction as well.
The CRT is one of the most important advances in the therapy of the severe chronic heart failure in the past decade. However, even with our increasing knowledge on the therapy, the rate of the non-responders is still high. Thus, the need for the appropriate patient selection in the CRT is outmost important. With analysing the landmark trials several predictors had been identified, such as age, gender, heart failure aetiology, LBBB or the QRS width, and implemented in the guidelines.10 Besides their success, the need for identifying different predictors that characterize different aspects of the heart failure, for instance the inflammatory markers is still there.
On the other hand, the prediction studies commonly leave out the comparison of the new marker to standard risk markers and the discrimination analysis itself, which could actually prove that the patients do really have higher risk with the new marker than without the new marker.22 The gold standard for the discrimination processes used to be the c-statistic, but novel studies showed its lack in sensitivity,23 thus new methods have been developed, such as the NRI and IDI.12 Their positive value shows an improvement in the discriminations quality. In our analysis, both NRI and IDI were positive, so the combined use of the NL ratio with the traditional heart failure biomarker, the NT-proBNP improved the risk prediction.
Strengths and limitations
Modern, automated qualitative blood count systems can easily determine the fractions of the lymphocytes and the neutrophils with great precision and most importantly with low cost. The main strength of our study is that all patients have blood count data in the clinical practice, thus with a little effort the NL ratio could be calculated (or at least estimated) and an elevated NL ratio should draw attention to a possible adverse outcome.
Since the reference models included the most important prognostic factors (age, gender, LBBB, aetiology, and QRS width) and were adjusted to the traditional predictive marker, the NT-proBNP, the results are therefore independent of their influence.
Although we numerically determined the advantage of the NL ratio with appropriate power, clinical application could be recommended only after establishment its usefulness in larger population of heart failure patients with CRT. The low patient and event numbers in this study resulted in low sensitivity and specificity values for the NL ratio, but an acceptable negative predictive value was achieved, indicating its possible role in identifying low-risk patients. In this line, our results should be considered only as preliminary and hypothesis-generating. Further prospective trials are clearly needed to test this promising new biomarker.
Conclusions
The NL ratio was found to predict the 6-month reverse remodelling and the 2-year mortality in the CRT of chronic heart failure. A single blood count measurement could facilitate the optimal patient selection for the CRT independently of traditional biomarkers or other factors.
Supplementary material
Funding
This work was supported by the National Development Agency of Hungary [‘Semmelweis Egyetem Híd Projekt’ (TÁMOP-4.2.2-08/1/KMR-2008-0004), ‘Semmelweis Egyetem Magiszter Program’ (TÁMOP-4.2.2./B10/1.-210-0013)], the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (to G.S. and L.G.), and the Hungarian Scientific Research Fund (OTKA K 105555). Funding to pay the Open Access publication charges for this article was provided by Arrhythmia Foundation.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Epelman S, Mann DL. Communication in the heart: the role of the innate immune system in coordinating cellular responses to ischemic injury. J Cardiovasc Transl Res 2012;5:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cooper HA, Exner DV, Waclawiw MA, Domanski MJ. White blood cell count and mortality in patients with ischemic and nonischemic left ventricular systolic dysfunction (an analysis of the Studies Of Left Ventricular Dysfunction [SOLVD]). Am J Cardiol 1999;84:252–7. [DOI] [PubMed] [Google Scholar]
- 3. Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation 1998;97:19–22. [DOI] [PubMed] [Google Scholar]
- 4. Arruda-Olson AM, Reeder GS, Bell MR, Weston SA, Roger VL. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcomes 2009;2:656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daubert JC, Saxon L, Adamson PB, Auricchio A, Berger RD, Beshai JF et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace 2012;14:1236–86. [DOI] [PubMed] [Google Scholar]
- 6. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013;15:1070–118. [DOI] [PubMed] [Google Scholar]
- 7. Sebag FA, Martins RP, Defaye P, Hidden-Lucet F, Mabo P, Daubert JC et al. Reverse electrical remodeling by cardiac resynchronization therapy: prevalence and clinical impact. J Cardiovasc Electrophysiol 2012;23:1219–27. [DOI] [PubMed] [Google Scholar]
- 8. Lellouche N, De Diego C, Boyle NG, Wiener I, Akopyan G, Child JS et al. Relationship between mechanical and electrical remodelling in patients with cardiac resynchronization implanted defibrillators. Europace 2011;13:1180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fruhwald FM, Fahrleitner-Pammer A, Berger R, Leyva F, Freemantle N, Erdmann E et al. Early and sustained effects of cardiac resynchronization therapy on N-terminal pro-B-type natriuretic peptide in patients with moderate to severe heart failure and cardiac dyssynchrony. Eur Heart J 2007;28:1592–7. [DOI] [PubMed] [Google Scholar]
- 10. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2006;8:746–837. [DOI] [PubMed] [Google Scholar]
- 11. Bagyura Z, Kiss L, Edes E, Lux A, Polgar L, Soos P et al. Cardiovascular screening programme in the Central Hungarian region. The Budakalasz Study. Orv Hetil 2014;155:1344–52. [DOI] [PubMed] [Google Scholar]
- 12. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation 1990;82:1730–6. [DOI] [PubMed] [Google Scholar]
- 14. Thomson SP, McMahon LJ, Nugent CA. Endogenous cortisol: a regulator of the number of lymphocytes in peripheral blood. Clin Immunol Immunopathol 1980;17:506–14. [DOI] [PubMed] [Google Scholar]
- 15. Mooren FC, Bloming D, Lechtermann A, Lerch MM, Volker K. Lymphocyte apoptosis after exhaustive and moderate exercise. J Appl Physiol (1985) 2002;93:147–53. [DOI] [PubMed] [Google Scholar]
- 16. Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res 2006;69:318–28. [DOI] [PubMed] [Google Scholar]
- 17. De Vito P. Atrial natriuretic peptide: an old hormone or a new cytokine? Peptides 2014;58c:108–16. [DOI] [PubMed] [Google Scholar]
- 18. Kawakami R, Saito Y, Kishimoto I, Harada M, Kuwahara K, Takahashi N et al. Overexpression of brain natriuretic peptide facilitates neutrophil infiltration and cardiac matrix metalloproteinase-9 expression after acute myocardial infarction. Circulation 2004;110:3306–12. [DOI] [PubMed] [Google Scholar]
- 19. Suwa T, Hogg JC, English D, Van Eeden SF. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol 2000;279:H2954–60. [DOI] [PubMed] [Google Scholar]
- 20. Tracchi I, Ghigliotti G, Mura M, Garibaldi S, Spallarossa P, Barisione C et al. Increased neutrophil lifespan in patients with congestive heart failure. Eur J Heart Fail 2009;11:378–85. [DOI] [PubMed] [Google Scholar]
- 21. Agacdiken A, Celikyurt U, Sahin T, Karauzum K, Vural A, Ural D. Neutrophil-to-lymphocyte ratio predicts response to cardiac resynchronization therapy. Med Sci Monit 2013;19:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 2009;119:2408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol 2004;159:882–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.