Abstract
Aims
This prospective, multicentre study (PRECISION GOLD) evaluated the incidence of asymptomatic cerebral embolism (ACE) after pulmonary vein isolation (PVI) using a new gold multi-electrode radiofrequency (RF) ablation catheter, pulmonary vein ablation catheter (PVAC) GOLD. Also, procedural efficiency of PVAC GOLD was compared with ERACE. The ERACE study demonstrated that a low incidence of ACE can be achieved with a platinum multi-electrode RF catheter (PVAC) combined with procedural manoeuvres to reduce emboli.
Methods and results
A total of 51 patients with paroxysmal atrial fibrillation (AF) (age 57 ± 9 years, CHA2DS2-VASc score 1.4 ± 1.4) underwent AF ablation with PVAC GOLD. Continuous oral anticoagulation using vitamin K antagonists, submerged catheter introduction, and heparinization (ACT ≥ 350 s prior to ablation) were applied. Cerebral magnetic resonance imaging (MRI) scans were performed within 48 h before and 16–72 h post-ablation. Cognitive function assessed by the Mini-Mental State Exam at baseline and 30 days post-ablation. New post-procedural ACE occurred in only 1 of 48 patients (2.1%) and was not detectable on MRI after 30 days. The average number of RF applications per patient to achieve PVI was lower in PRECISION GOLD (20.3 ± 10.0) than in ERACE (28.8 ± 16.1; P = 0.001). Further, PVAC GOLD ablations resulted in significantly fewer low-power (<3 W) ablations (15 vs. 23%, 5 vs. 10% and 2 vs. 7% in 4:1, 2:1, and 1:1 bipolar:unipolar energy modes, respectively). Mini-Mental State Exam was unchanged in all patients.
Conclusion
Atrial fibrillation ablation with PVAC GOLD in combination with established embolic lowering manoeuvres results in a low incidence of ACE. Pulmonary vein ablation catheter GOLD demonstrates improved biophysical efficiency compared with platinum PVAC.
Trial registration
ClinicalTrials.gov NCT01767558.
Keywords: Atrial fibrillation, Ablation, Asymptomatic cerebral embolism, Pulmonary vein isolation, PVAC GOLD
What's new?
This is the first multicentre, prospective clinical study with the new pulmonary vein ablation catheter (PVAC) GOLD ablation catheter.
New PVAC GOLD design avoids key source of micro-emboli (interaction of electrodes 1 and 10).
Study shows a low (2.1%) incidence of ACE with PVAC GOLD catheter, which is among the lowest reported for any ablation technology.
High prevalence of pre-existing cerebral lesions (73%) in patients with paroxysmal AF, which may have an important implication for maintaining sinus rhythm and therapeutic anticoagulation.
Introduction
Pulmonary vein isolation (PVI) is an effective therapy for paroxysmal atrial fibrillation (AF). The pulmonary vein ablation catheter (PVAC) has shown promising clinical results in single-centre studies.1–4 In recent years, several reports have identified the occurrence of new asymptomatic cerebral embolism (ACE) on cerebral magnetic resonance imaging (MRI) following AF ablation with an overall incidence of 1.7–40%, depending on the ablation technology used.5–11 Gaita et al. and Siklódy et al. reported a significantly higher ACE incidence after PVI with the PVAC, when compared with irrigated focal radiofrequency (RF) and cryoballoon ablation.8,9
The prospective, multicentre ERACE study, however, demonstrated a significantly reduced ACE incidence (1.7%) with the implementation of procedural modifications, which included: (1) avoiding proximity of energized electrodes, (2) uninterrupted oral anticoagulation (OAC) and targeted procedural activated clotting time (ACT) ≥350 s, and (3) submerged catheter introduction.6
The next-generation multi-electrode catheter, PVAC GOLD, was designed to mitigate embolus formation and improve the delivery of RF energy compared with the platinum PVAC.12 The purpose of the Phased RF Evaluation of a Cute Pulmonary Vein ISolation In ParOxysmal AF with New GENius UI and PVAC®GOLD (PRECISION GOLD) study was to evaluate the ACE incidence after AF ablation using the new PVAC GOLD. Further, procedural efficiency of PVAC GOLD vs. platinum PVAC was compared based on biophysical data obtained in the ERACE study.
Methods
Patient population
The study population consisted of paroxysmal AF patients enrolled at 10 centres experienced with the PVAC and GENius generator (Phased RF system, Medtronic Ablation Frontiers, Carlsbad, CA, USA). Patients with symptomatic AF between 18 and 70 years of age who had previously failed at least one anti-arrhythmic drug (AAD) were included. Exclusion criteria were prior AF ablation, persistent or permanent AF, invasive cardiac procedure in preceding 90 days, history of stroke or transient ischaemic attack (TIA) within 6 months, contraindication to MRI, contraindication to vitamin K antagonists (VKA), or active thrombus in the left atrium (LA). Patients with heart failure were included based on medical history and were categorized using the New York Heart Association (NYHA) Class I, II, III, or IV. The study was approved by each centre's ethics review board, and all patients provided written informed consent prior to study inclusion. The study was registered on ClinicalTrials.gov NCT01767558.
A total of 56 patients were enrolled, and 51 patients underwent ablation for the primary analysis cohort (Figure 1). Five patients exited prior to ablation due to sub-therapeutic international normalized ratio (INR) (n = 3), or inclusion/exclusion criteria not met (n = 2). Forty eight of the 51 patients were included in the ACE analysis, after refusing post-procedure MRI (n = 2) and undergoing an ablation in the LA with a non-study device (n = 1). See the supplementary material online for subject disposition details.
Figure 1.
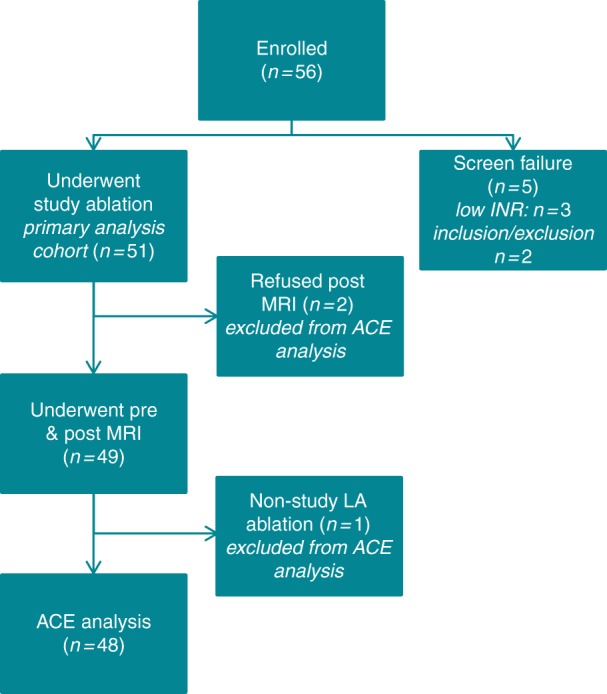
Enrolled patient disposition of primary analysis cohort. Disposition of enrolled patients, including screen failures.
Study design
The study was a prospective, multicentre study to evaluate ACE findings in patients with symptomatic paroxysmal AF undergoing ablation with PVAC GOLD. Patients underwent protocol-specific evaluations at the pre-procedure, discharge, and 1-month visits. Cerebral MRIs were performed at pre- and post-procedure visits (see Cerebral MRI Imaging and Neurological Assessments section). Additionally, cognitive function was assessed by the Mini-Mental State Exam (MMSE) at pre-procedure and 1-month visits. The PRECISION GOLD study was designed similarly to the ERACE study, employing the same embolic lowering procedural manoeuvres.
Peri-procedural anticoagulation
Oral anticoagulation with VKA was initiated at least 3 weeks prior to ablation to achieve an INR of ≥2.0 on the day of ablation and continued for the duration of the study. To rule out LA thrombus, transoesophageal echocardiography (TEE) was performed 48 h before ablation. Heparin was administered prior to and/or immediately after transseptal puncture and sustained throughout the procedure to maintain ACT levels ≥350 s, with monitoring at least every 30 min.
Ablation protocol and procedural techniques
After a single transseptal puncture, a 10Fr or larger sheath was inserted into the LA. Submerged loading of PVAC GOLD into the introducer, as well as continuous flushing of the sheath with heparinized saline, was performed to minimize air ingress. Pulmonary vein ablation catheter GOLD was advanced slowly through the sheath into the LA. Pulmonary veins (PVs) were electrically isolated by targeted ablation of each PV-LA antrum. Pulmonary vein isolation was defined as PV-LA entrance block verified with pacing manoeuvres, as appropriate. Sinus rhythm (SR) was restored by cardioversion at the end of the procedure as needed.
Pulmonary vein ablation catheter technology has been described previously.1,13 Briefly, the GENius generator delivers duty-cycled bipolar and unipolar phased RF energy to all or selected electrode pairs. Radiofrequency is delivered in a temperature-controlled and power-limited fashion (60°C, maximum 10 W) with typical ablation duration of 60 s.
Pulmonary vein ablation catheter GOLD features several modifications to mitigate embolus formation and to improve the efficiency of RF delivery, compared with the predicate platinum electrode PVAC. First, the 10th/proximal electrode was removed to eliminate the potential to produce emboli that can occur when electrodes 1 and 10 are energized in close proximity.14 To maintain the effective arc length, inter-electrode spacing was increased from 3.00 to 3.75 mm. Next, gold electrodes were utilized, due to higher thermal conductivity and potential for reducing coagulum formation and increasing power output vs. platinum electrodes.12,15 Finally, a 20° forward tilt was applied for a more uniform contact across the electrode array (Figure 2).
Figure 2.
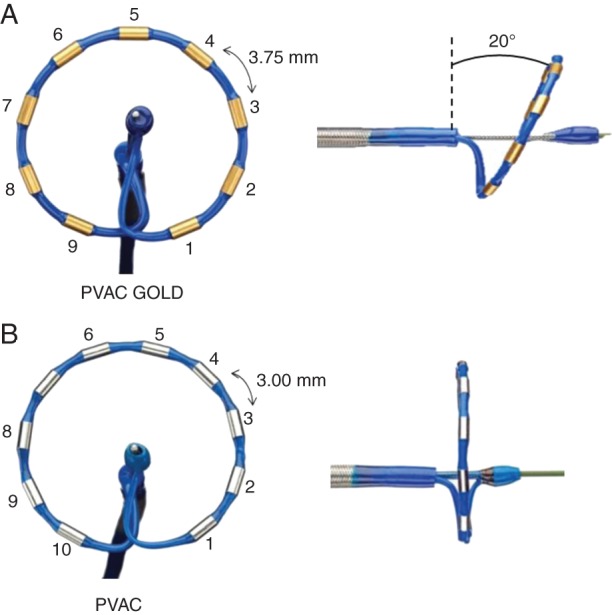
Pulmonary vein ablation catheter GOLD (A) and PVAC (B). Main design changes of PVAC GOLD are 9 (removed electrode 10) gold electrodes, increased inter-electrode spacing to 3.75 mm, and the 20° tilt. See text for further explanation.
Biophysical parameters analysis
The total number of RF applications and the proportion of energy deliveries with low power (<3 W) were calculated. Further, the efficiency of energy transfer was evaluated by measuring effective contact (EC), defined as the number of seconds when an electrode had temperature ≥50°C and power ≥3 W, and effective energy (EE), defined as the total number of Joules delivered for each electrode during EC. The biophysics parameters from the PRECISION GOLD were compared with those collected during the ERACE study.
Cerebral magnetic resonance imaging and neurological assessments
Cerebral magnetic resonance imagings (MRIs) were performed using a 1.5 T scanner as previously described.5,6 Scans were performed within 48 h pre- and 24 h post-ablation (window 16–72 h) in all patients. In case of a new ACE finding, MRI was repeated after 1 month. The number and size of all ACE findings were recorded. All MRIs were interpreted by a Core Lab, with two independent, blinded, neuro-radiologist reviewers. In cases of disagreement, reviewers reached a consensus prior to final adjudication. The ACE definition used in this trial was identical to that previously described by Verma et al. and Gaita et al.5,6 Briefly, post-procedural ACE findings were defined as any new focal hyperintensities detected on the post-ablation fluid-attenuated inversion recovery (FLAIR) sequences, corresponding to restricted diffusion on the diffusion-weighted (DW) sequences and not ruled out by shine-through artefact on apparent diffusion coefficient (ADC) maps. Pre-ablation abnormalities on cerebral MRI were categorized as an acute pre-existing lesion (PEL) if hyperintensities were detected on the DW sequence, while a chronic PEL if detected only on the FLAIR sequence. The number and size of PELs were recorded. All patients underwent cognitive assessment at the pre-procedure and 1-month visits with the MMSE-2 (PAR, Lutz FL).
Study endpoints
The primary study endpoint was the incidence of new ACE findings on post-procedure cerebral MRI. Secondary endpoints included acute procedural success (APS)—defined as ability to achieve PVI with only PVAC GOLD and restoration of SR with or without cardioversion—as well as all serious adverse events (SAEs) that were related to the procedure and/or ablation system. All SAEs were adjudicated by a Clinical Events Committee. Ancillary objectives included total procedure time, LA dwell time, fluoroscopy time, total energy delivery, number of RF applications per vein, energy modes used, and results of MMSE.
Statistical analysis
Clinically relevant variables are summarized using descriptive statistics, including mean ± standard deviation for continuous variables and percentages and counts for categorical variables. Comparisons were performed using Pearson's χ2 test for categorical variables and Student's t-test for continuous variables. Binomial confidence intervals (95% CI) were calculated using the Clopper–Pearson method. An analysis of covariance model was used to compare changes in MMSE scores from baseline to 1 month, adjusting for baseline score. The relationship between PELs and baseline variables was modelled using ordinal logistic regression. Effective contact of ≥30 s was analysed using logistic regression with generalized estimating equation (GEE) methodology to account for potential correlation between the EC of different electrodes during the same RF ablation. A compound symmetry working correlation was used, and the likelihood of EC was adjusted for the duration each electrode was turned on and energy mode. A similar GEE linear regression was used to analyse EE delivery. A P-value of <0.05 was considered statistically significant. Statistical analyses were conducted with SAS 9.2 (Cary, NC).
Results
Patient characteristics
From December 2013 to July 2014, 51 patients were enrolled and treated in the study with baseline characteristics detailed in Table 1.
Table 1.
Baseline demographics of primary analysis cohort and patients with and without PELs
| Baseline variable | Primary analysis cohort (N = 51) | No PEL (N = 14) | PEL (N = 37) | P-value |
|---|---|---|---|---|
| Age (years) | 57.1 ± 8.8 | 52.4 ± 7.8 | 58.8 ± 8.6 | 0.0171 |
| Gender, male (%) | 40 (78.4%) | 13 (93%) | 27 (73%) | 0.2508 |
| History of AF in years | 3.9 ± 3.7 | 2.6 ± 2.3 | 4.4 ± 4.1 | 0.0517 |
| Number of patients with ≥10 AF episodes in 3 months prior to enrolment (%) | 16 (31.4%) | 8 (57%) | 8 (22%) | 0.0396 |
| Patients with DC cardioversion for AF in past 12 months (%) | 9 (17.6%) | 2 (14%) | 7 (19%) | 1.0000 |
| Patients with hospital admission for AF in past 12 months (%) | 19 (37.3%) | 3 (21%) | 16 (43%) | 0.2023 |
| CHA2DS2-VASc | 1.4 ± 1.4 | 0.5 ± 0.7 | 1.8 ± 1.5 | 0.0001 |
| Hypertension (%) | 16 (31.4%) | 3 (20%) | 13 (35%) | 0.5030 |
| Heart failure (%) | 15 (29.4%) | 2 (14%) | 13 (35%) | 0.1843 |
| Dyslipidemia (%) | 16 (31.4%) | 4 (29%) | 12 (32%) | 1.0000 |
| Diabetes (%) | 1 (2.0%) | 0 (0%) | 1 (3%) | 1.0000 |
| Atrial tachycardia (%) | 2 (3.9%) | 1 (7%) | 1 (3%) | 0.4776 |
| Atrial flutter (%) | 3 (5.9%) | 1 (7%) | 2 (5%) | 1.0000 |
| Coronary artery disease (%) | 5 (9.8%) | 1 (7%) | 4 (11%) | 1.0000 |
Data are presented as mean ± SD or number (%) of patients.
Pre-existing cerebral magnetic resonance imaging lesions
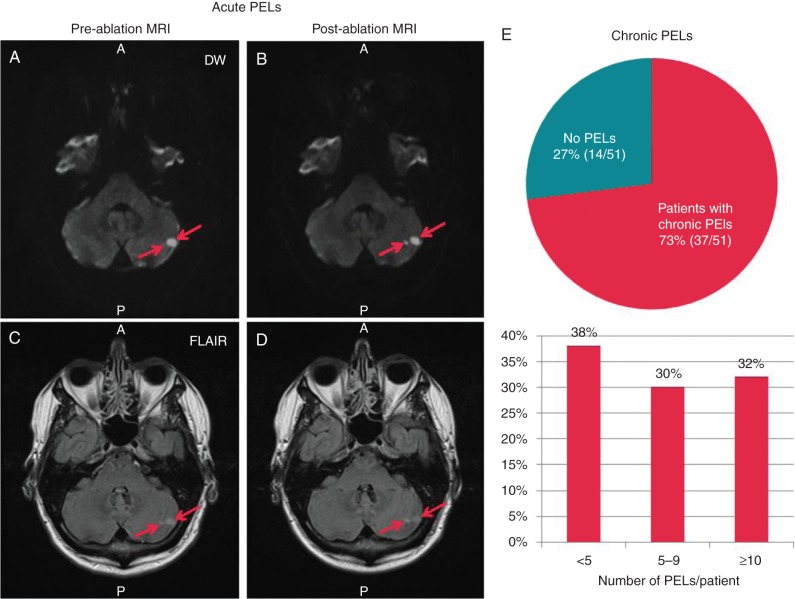
Chronic PELs were identified in 37 of the 51 (73%) patients with 33 (62%) patients having >5 PELs (Figure 3, right panel). These were localized in the frontal (59%), parietal (31%), occipital (6%), and temporal lobes (5%). Patients with chronic MRI findings were older (P = 0.0171), had higher CHA2DS2-VASc (P = 0.0001), but had less episodes of AF (P = 0.0396) in the 3 months prior to ablation (Table 1). Using logistic regression, there was a significant relationship between chronic PELs and CHA2DS2-VASc score, with each point of CHA2DS2-VASc score associated with an odds ratio of 2.00 (95% CI 1.31–3.06, P = 0.001) for a higher level of chronic PELs/patient. In addition to chronic PELs detected on FLAIR, acute PELs were identified on DW for one patient. In this patient, pre-ablation images showed two acute PELs on DW (Figure 3, left cerebellum) as well as >10 chronic PELs on FLAIR. Patients with PELs did not demonstrate cognitive differences with a mean pre-ablation MMSE score of 29.0 ± 1.0 compared with 28.7 ± 1.5 in patients without PELs (P = 0.53).
Figure 3.
Pre-existing lesion burden in study population. One patient had two acute PELs detected (arrows) visible on both pre- and post-ablation DW (A and B) and FLAIR sequences (C and D), as well as >10 chronic PELs (not shown). Most patients (73%) had chronic PELs detected on FLAIR (E). The lower portion of E shows the distribution of PELS/patient.
Procedural parameters
The mean INR was 2.7 ± 0.6 on the day of the procedure, with an average ACT of 462 ± 166 s (Table 2). The majority of patients (78%) were in SR at the start of the procedure. Twenty-nine (57%) patients had rhythm conversions during the course of the ablation (45% spontaneous, 20% electrical, and 14% pharmacological). Pulmonary vein ablation catheter GOLD was able to electrically isolate 97% (197/204) of the targeted PVs, and 94% (48/51) of patients had all accessible PVs isolated, including one patient in which conventional RF was used to complete isolation of one PV and two patients in which one PV was not isolated. In addition, two other patients failed cardioversions despite achieving PVI, resulting in 90% (46/51) APS. The mean total procedure time was 104 ± 31 min with a LA dwell time of 59 ± 18 min and a fluoroscopy time of 16 ± 7 min (Table 2). At the 1-month follow-up visit, 44/51 patients (86%) were in SR.
Table 2.
Procedure and biophysical parameters of primary analysis cohort, in comparison with ERACE
| Procedure parameters | PRECISION GOLD (N = 51) | ERACE (N = 60) | P-value |
|---|---|---|---|
| Mean INR | 2.7 ± 0.6 | 2.4 ± 0.5 | 0.0056 |
| Patients with INR ≥ 2.0 | 94.1% (48) | 88.3% (53) | 0.2888 |
| Mean ACT (s) | 462.3 ± 166.1 | 405 ± 116 | 0.0413 |
| Patients with ≥2 consecutive ACTs <300 | 0.0% (0) | N/A | N/A |
| Patients with ≥2 consecutive ACTs <350 | 9.8% (5) | N/A | N/A |
| ACT—per-patient minimum (s) | 414.5 ± 149.4 | 319 ± 130 | 0.0006 |
| Procedure time (min) | 103.9 ± 31.4 | 100 ± 35 | 0.5375 |
| LA dwell time (min) | 59.5 ± 18.3 | 64 ± 27 | 0.3007 |
| Fluoroscopy time (min) | 15.6 ± 6.9 | N/A | N/A |
| Biophysical parameters | |||
| Total RF applications | 20.3 ± 10.0 | 28.8 ± 16.1 | 0.0010 |
| LSPV RF applications | 6.0 ± 4.5 | 8.2 ± 5.9 | 0.0293 |
| LIPV RF applications | 5.0 ± 3.8 | 6.2 ± 4.1 | 0.1379 |
| RSPV RF applications | 4.8 ± 2.2 | 7.2 ± 4.8 | 0.0006 |
| RIPV RF applications | 4.4 ± 2.9 | 6.2 ± 4.5 | 0.0132 |
| Energy mode 1:1 (per-patient %) | 6.6 ± 18.4 | 6.7 ± 16.3 | 0.9584 |
| Energy mode 2:1 (per-patient %) | 56.6 ± 40.3 | 67.0 ± 38.0 | 0.1707 |
| Energy mode 4:1 (per-patient %) | 36.8 ± 42.1 | 26.5 ± 39.6 | 0.1904 |
Data are presented as mean ± SD or % (n) of patients.
Procedural and biophysical parameters
The PRECISION GOLD procedural and biophysical data were compared with the data from the ERACE study. There were no significant differences in procedural time, LA dwell time, or energy modes between the studies (Table 2). However, mean INR levels before and mean ACT and the average of the minimum ACT/patient during ablation were higher in PRECISION GOLD.
The average number of RF applications per patient in PRECISION GOLD (20.3 ± 10.0) was lower than in ERACE (28.8 ± 16.1; P = 0.001), with significantly less RF applications needed to achieve PVI for the left superior, right superior, and right inferior PVs (Table 2). Pulmonary vein ablation catheter GOLD was also associated with a significant EC increase in 2:1 mode, while all modes showed increased EE and decreased low-power (<3 W) ablations (Figure 4).
Figure 4.
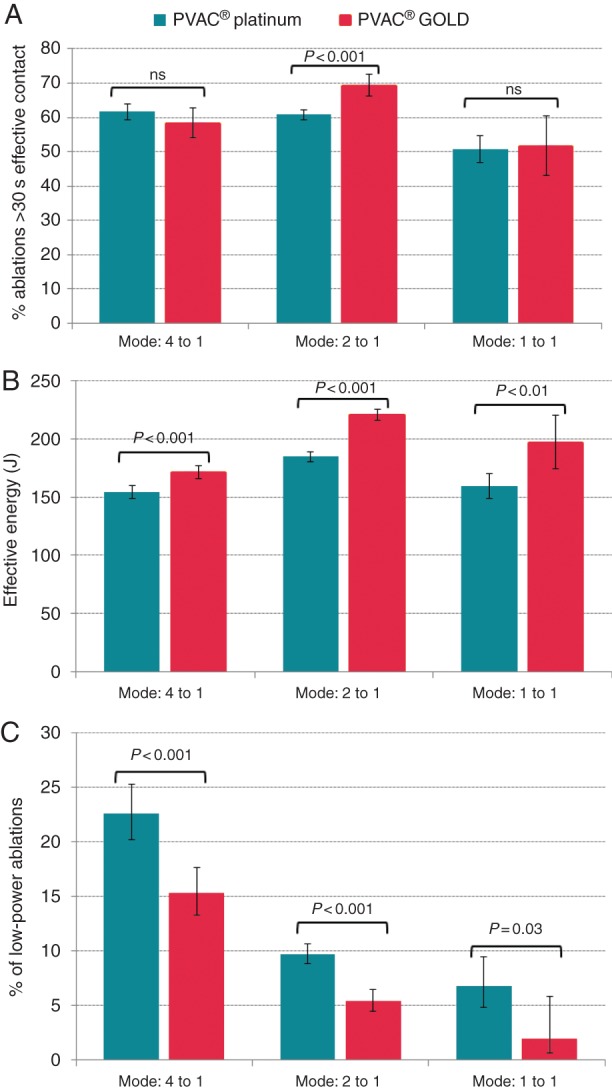
Comparison of EC, EE, and proportion of low-power ablations between PVAC and PVAC GOLD. The percentage of ablations with >30 s of EC time (for the 2:1 mode) and EE (for all modes) was higher during PVAC GOLD ablations (A and B). Also, the number of low-power ablations was reduced (C).
Incidence of ACE and neurological findings post-ablation
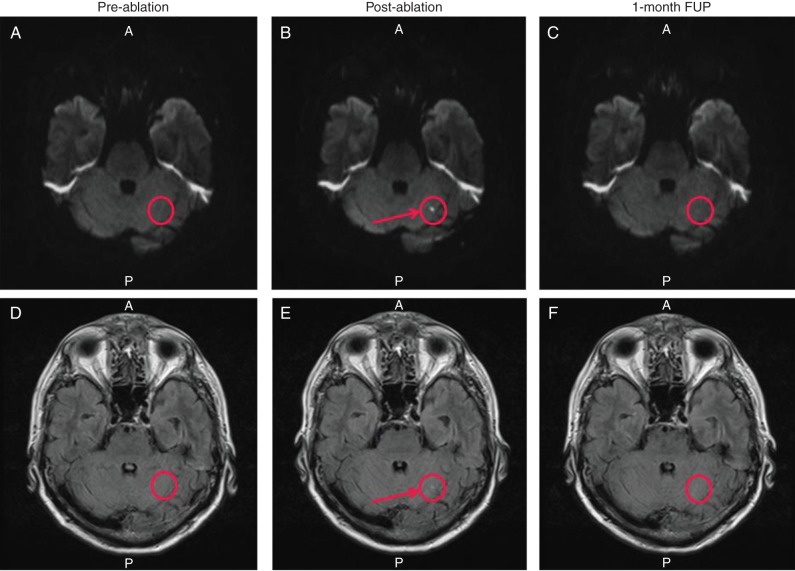
Three ablated patients from the primary analysis cohort were excluded from ACE analysis for refusing post-ablation MRI (n = 2) or undergoing treatment with a non-study device (n = 1). Evaluation of post-ablation MRIs demonstrated one patient with one new ACE finding (2.1%, 1/48, 95% CI = 0.1–11.1%), located in the left cerebellum (4 × 5 mm). This ACE finding was no longer detectable on the 1-month MRI (Figure 5). For all patients, there was no significant change in MMSE from baseline to the 1-month visit with the mean difference of 0.31 (95% CI −0.02 to 0.63; P = 0.06).
Figure 5.
Pre-, post-ablation, and 1-month follow-up cerebral MRIs from single patient with ACE (S-I view). The top panels (A–C) are DW images, and the bottom panels (D–F) are FLAIR images. The right column (A and D) shows pre-ablation DW and FLAIR MRI sequences showing no PEL in the region of the ACE finding (circled). The ACE finding (circle) is identified on the immediate post-ablation MRI as a restricted diffusion hyperintensity on both DW and FLAIR MRI sequences denoted by an arrow (B and E). Panels C and F show that at 1 month, the ACE finding is no longer detected on DW or FLAIR MRI sequences (circle).
Serious adverse events
One patient developed pericarditis, and one patient had a groin pseudo-aneurysm. Both events resolved without medical intervention. No other SAEs were noted.
Discussion
Main findings
Main observations of the study are (1) ACE incidence after PVI using the PVAC GOLD in combination with embolic lowering procedural manoeuvres is 2.1%; (2) paroxysmal AF patients exhibit a high burden of cerebral PELs, despite a low estimated stroke risk; and (3) PVAC GOLD demonstrates improved biophysical efficiency compared with platinum PVAC, with a low complication rate.
ACE incidence and PVAC ablation
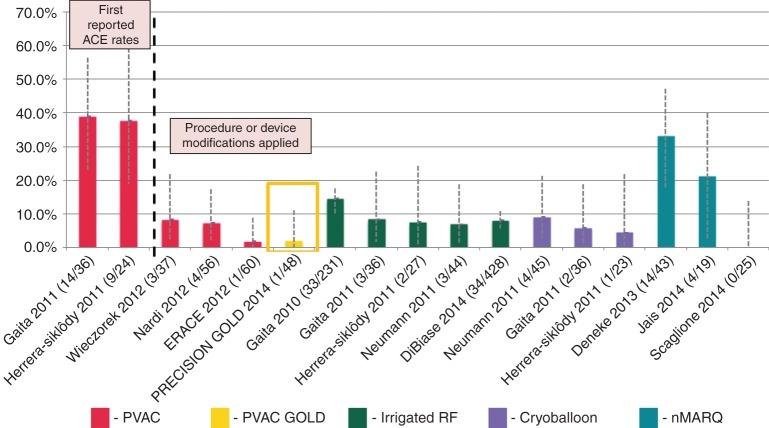
As illustrated in Figure 6, ACE detected by DW MRI following AF ablation has been reported at widely varying rates between 1.7 and almost 40%. Initial studies by Gaita et al. and Siklódy et al. raised a safety concern for PVAC with a significantly higher rate of ACE findings after PVAC ablation (38.9 and 37.5%, respectively) vs. irrigated RF ablation (8.3 and 7.4%, respectively) and cryoballoon (5.6 and 4.3%, respectively) (Figure 6).8,9
Figure 6.
Summary of ACE studies using various technologies. The 2.1% ACE rate in the current study is one of the lowest reported for any technology. Error bars represent the 95% CI of the point estimates for each study. The two PVAC studies left of the dotted line represent the first reported ACE studies that did not utilize embolic lowering manoeuvres, which were implemented in the phased RF studies right of the dotted line.
Preclinical research to identify potential causes of ACE has demonstrated that the most significant source of micro-emboli (gas or particle) for PVAC occurs during an electrode-1-and-10 interaction (E1–E10), which can occur when electrodes are energized in close proximity. This creates a bipolar short circuit, resulting in excessive tissue and blood heating. Furthermore, air ingress during introduction into the transseptal sheath was a source of gaseous emboli.14 Wieczorek et al. showed a significantly lower ACE incidence in patients without vs. with an E1–E10 interaction (11 vs. 44%).7 Subsequently, the multicentre ERACE study showed that submerged capture to avoid air entrapment, plus de-activation of E1 or E10, could reduce ACE incidence to 1.7%, which is one of the lowest rates ever published.6
Importantly, uninterrupted OAC with warfarin coupled with an elevated procedural heparinization (target ACT >350 s) likely contributed to the marked ACE reduction in this study. Recent evidence has shown the importance of maintaining therapeutic peri-procedural OAC to mitigate thromboemboli.16–18 Di Biase et al. demonstrated in the randomized COMPARE study that OAC maintenance vs. bridging with low-molecular-weight heparin can significantly reduce peri-procedural cerebral events, 0.25 vs. 5%, respectively.16 In a similar study using irrigated tip catheters, the ACE incidence was 2.1% for patients maintaining OAC vs. 14.2% for those who received heparin bridging.17 Additionally, maintaining high ACT levels during ablation may further lower embolic risk.19 Scaglione et al. found that higher mean intraprocedural ACT values are protective, with no ACE occurring when mean ACT value was >320 s.19
The primary change to PVAC GOLD for mitigating emboli formation is the removal of the 10th/proximal electrode, thereby completely eliminating the potential for E1–10 interaction. Combined with the same embolic lowering manoeuvres as in ERACE, the current study shows a similarly low ACE rate of 2.1%.
Pre-existing lesions: sources and impact
Several studies have shown a high prevalence of chronic PELs in AF patients prior to ablation, ranging from 12.3 to 92%.20,21 In the current study, a high chronic PEL prevalence (73%) and burden (≥5 PEL in 62% of patients) was observed, despite a low thromboembolism risk (CHA2DS2-VASc score 1.4 ± 1.4). This finding is also consistent with the ERACE study, which found PELs in 60% of a similar patient cohort.
In the present study, one patient had two acute PELs in the left cerebellum, 5 × 5 and 9 × 6 mm. For studies with silent embolism endpoints, this finding illustrates the importance of pre-ablation imaging, as its absence would have doubled the observed post-ablation ACE incidence in the present study. As 1-month MRI was not performed for this patient, it is not known whether these acute PELs would have disappeared similarly to the majority of post-ablation ACE findings ≤10 mm in size which are not visible during follow-up imaging.7,21 The longer-term course of spontaneous acute PELs vs. chronic PELs, and their clinical significance, requires further longer-term evaluation.
Although the aetiology of chronic PELs is currently unknown, several factors may contribute including a history of AF, diabetes, sub-therapeutic anticoagulation, hypertension, and microvascular disease. A recent meta-analysis demonstrated that AF is associated with a great that two-fold increase in the odds for PEL.22 Of note, in the present study, older age and a higher CHA2DS2-VASc score were the main predictors of PELs and not the number of AF episodes in the last 3 months before ablation. Though not seen in this study, other reports have found a correlation between the prevalence of PELs and increased risk of stroke, dementia, and/or cognitive decline.23
Post-ablation ACE findings differ from chronic PELs in their transient nature with a low rate of chronic persistence (>30 days).6,21 In the current study, the only new ACE finding detected post-ablation was no longer detectable after 1 month. This transient nature suggests that gaseous or sub-mm particle emboli may be the primary contributors to post-ablation ACE causing a temporary interruption of blood flow, but not resulting in a permanent detectable scar. The potential impact of transient ACE on cerebral function might therefore be less significant, when compared with permanent chronic PELs. As such, performing ablations earlier in the course of AF may confer clinical benefits through a decrease in AF burden, stroke risk, PEL incidence, and cognitive decline. Larger clinical studies with longer-term follow-up are needed to confirm this hypothesis.
Comparison of procedural efficiency and safety between PVAC GOLD and PVAC
Compared with PVAC in the ERACE study, no differences were observed in procedure time, LA dwell times, or mix of energy modes. Importantly, PVAC GOLD was associated with a similarly low rate of complications and comparable rate of PVI (94 vs. 100% in ERACE). In contrast, less RF applications per patient and per PV (except for the left inferior PV) were needed to achieve isolation in PRECISION GOLD. Contributing factors likely include the increased heat transfer of gold electrode material, as reflected by a significant increase in EC and EE and a 50% reduction in low-power ablations, and the 20° forward tilt, which provides more uniform contact with the PV antrum. Overall, this may reflect improved lesion formation with PVAC GOLD as Haines et al. recently demonstrated that gold electrodes allow more efficient cooling and deeper lesions with phased RF ablations than platinum.12 De Greef et al. demonstrated that increased rates of low-power ablations (<3 W) with PVAC were associated with acute PV reconnection and clinical AF recurrence.24 In a temperature-controlled RF ablation, low-power ablations can be caused by inadequate circulatory cooling at the electrode–tissue interface, limiting the lesion depth. Kardos et al. showed that cavotricuspid isthmus ablation was faster and required fewer RF applications using 8 mm gold vs. platinum electrodes.25 Whether better RF biophysics results in improved clinical outcome with PVAC GOLD remains to be proved.
Study limitations
The present study includes a moderate number of patients without a control group. The observed ACE incidence in this study cannot be extrapolated to different peri-procedural anticoagulation strategies or newer agents, as well as, persistent or longstanding persistent AF patients. There are limitations to comparing the procedural and biophysical data from the PRECISION GOLD and ERACE studies, including the impact of different investigators and different software versions for the GENius generator. Lastly, subtle changes in cognitive function may not be detected by the MMSE.
Conclusions
The PRECISION GOLD study builds upon the preclinical work by Haines et al., and the ERACE study providing evidence that AF ablations performed with the new-generation PVAC GOLD result in some of the lowest reported ACE rates (2.1%) for any technology. This study shows that despite a low thromboembolic risk score and inclusion of only paroxysmal AF patients, 73% of patients had pre-existing cerebral lesions. Finally, the current study demonstrates that the new PVAC GOLD with associated design changes demonstrate improved biophysics with similar acute safety, efficacy, and incidence of ACE as the predicate PVAC.
Funding
The PRECISION GOLD trial was sponsored by Medtronic, Inc. Funding to pay the Open Access publication charges for this article was provided by Medtronic, Inc.
Supplementary material
Supplementary Material
Acknowledgements
The PRECISION GOLD study was sponsored by Medtronic.
Conflict of interest: L.D. is a consultant for and receives funding for research from Medtronic. L.B. is a consultant for Medtronic and a prior stockholder of Ablation Frontiers. The Cardiology Department has received grant support for research from Medtronic and Ablation Frontiers, Inc. S.M. is a consultant to Medtronic and Boston Scientific and received honoraria from St Jude and Biosense Webster. M.W. is a consultant to and received speakers honoraria from Medtronic. S.S. received research support from Ablation Frontiers and Medtronic. N.D. is a consultant for Medtronic. M.H. is a stockholder of Cardio Insight and received grants from Biosense Webster, Medtronic, and St Jude Medical. J.C.G. is a consultant for St Jude Medical, Biosense Webster, Boston Scientific, Medtronic, AstraZeneca, and Pfizer and has received speaker fees from St Jude Medical, Boston Scientific, Medtronic, Biotronik, AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Novartis, Meda, Pfizer, and Sanofi Aventis. Z.C. is a consultant for and has received speaker fees from Medtronic, Biotronik, Boehringer Ingelheim, and Bayer. He has also received research support from Medtronic.
References
- 1. Boersma LV, Wijffels MC, Oral H, Wever EF, Morady F. Pulmonary vein isolation by duty-cycled bipolar and unipolar radiofrequency energy with a multielectrode ablation catheter. Heart Rhythm 2008;5:1635–42. [DOI] [PubMed] [Google Scholar]
- 2. De Greef Y, Buysschaert I, Schwagten B, Stockman D, Tavernier R, Duytschaever M. Duty-cycled multi-electrode radiofrequency vs. conventional irrigated point-by-point radiofrequency ablation for recurrent atrial fibrillation: comparative 3-year data Europace 2014;16:820–5. [DOI] [PubMed] [Google Scholar]
- 3. McCready J, Chow AW, Lowe MD, Segal OR, Ahsan S, de Bono J et al. Safety and efficacy of multipolar pulmonary vein ablation catheter vs. irrigated radiofrequency ablation for paroxysmal atrial fibrillation: a randomized multicentre trial. Europace 2014;16:1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wasmer K, Foraita P, Leitz P, Güner F, Pott C, Lange PS et al. Safety profile of multielectrode-phased radiofrequency pulmonary vein ablation catheter and irrigated radiofrequency catheter. Europace 2016;18:78–84. [DOI] [PubMed] [Google Scholar]
- 5. Gaita F, Caponi D, Pianelli M, Scaglione M, Toso E, Cesarani F et al. Radiofrequency catheter ablation of AF: A cause of silent thromboembolism? Circulation 2010;122:1667–73. [DOI] [PubMed] [Google Scholar]
- 6. Verma A, Debruyne P, Nardi S, Deneke T, DeGreef Y, Spitzer S et al. ERACE Investigators. Evaluation and reduction of asymptomatic cerebral embolism in ablation of AF, but high prevalence of chronic silent infarction: Results of the ERACE trial. Circ Arrhythm Electrophysiol 2013;6:835–42. [DOI] [PubMed] [Google Scholar]
- 7. Wieczorek M, Lukat M, Hoeltgen R, Condie C, Hilje T, Missler U et al. Investigation into causes of abnormal cerebral MRI findings following PVAC duty-cycled, phased RF ablation of AF. J Cardiovasc Electrophysiol 2013;24:121–8. [DOI] [PubMed] [Google Scholar]
- 8. Gaita F, Leclerq JF, Schumacher B, Scaglione M, Toso E, Halimi F et al. Incidence of silent cerebral thrombomembolic lesions after AF ablation may change according to technology used. J Cardiovasc Electrophysiol 2011;22:961–8. [DOI] [PubMed] [Google Scholar]
- 9. Herrera Siklódy C, Deneke T, Hocini M, Lehrmann H, Shin DI, Miyazaki S et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation: comparison of different AF ablation technologies in a multicenter study. J Am Coll Cardiol 2011;58:681–8. [DOI] [PubMed] [Google Scholar]
- 10. Martinek M, Sigmund E, Lemes C, Derndorfer M, Aichinger J, Winter S et al. Asymptomatic cerebral lesions during pulmonary vein isolation under uninterrupted oral anticoagulation. Europace 2013;15:325–31. [DOI] [PubMed] [Google Scholar]
- 11. Neumann T, Kuniss M, Conradi G, Janin S, Berkowitsch A, Wojcik M et al. MEDAFI-Trial (Micro-embolization during ablation of atrial fibrillation): comparison of pulmonary vein isolation using cryoballoon technique vs. radiofrequency energy. Europace 2011;13:37–44. [DOI] [PubMed] [Google Scholar]
- 12. Haines DE, Strunk AR, Novichenok A, Kirchhof N, Stewart MT. The biophysics of passive convective cooling during catheter ablation with gold versus platinum electrodes and multielectrode phased radiofrequency energy delivery. J Cardiovasc Electrophysiol 2015;26:1257–61. [DOI] [PubMed] [Google Scholar]
- 13. Scharf C, Boersma L, Davies W, Kanagaratnam P, Peters NS, Paul V et al. Ablation of persistent AF using multielectrode catheters and duty-cycled radiofrequency energy. J Am Coll Cardiol 2009;54:1450–6. [DOI] [PubMed] [Google Scholar]
- 14. Haines DE, Stewart MT, Dahlberg S, Barka ND, Condie C, Fiedler GR et al. Microembolism and catheter ablation I: A comparison of irrigated radiofrequency and multielectrode phased radiofrequency catheter ablation of pulmonary vein ostia. Circ Arrhythm Electrophysiol 2013;6:16–22. [DOI] [PubMed] [Google Scholar]
- 15. Lewalter T, Weiss C, Spencker S, Jung W, Haverkamp W, Willems S et al. AURUM 8 Study Investigators. Gold vs. Platinum-iridium tip catheters for cavotricuspid isthmus ablation: the AURUM 8 study. Europace 2011;13:102–8. [DOI] [PubMed] [Google Scholar]
- 16. Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of AF with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in AF (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation 2014;129:2638–44. [DOI] [PubMed] [Google Scholar]
- 17. Di Biase L, Gaita F, Toso E, Santangeli P, Mohanty P, Rutledge N et al. Does periprocedural anticoagulation management of AF affect the prevalence of silent thromboembolic lesion detected by diffusion cerebral magnetic resonance imaging in patients undergoing radiofrequency AF ablation with open irrigated catheters? Results from a prospective multicenter study. Heart Rhythm 2014;11:791–8. [DOI] [PubMed] [Google Scholar]
- 18. Sticherling C, Marin F, Birnie D, Boriani G, Calkins H, Dan GA et al. Antithrombotic management in patients undergoing electrophysiological procedures: a European Heart Rhythm Association (EHRA) position document endorsed by the ESC Working Group Thrombosis, Heart Rhythm Society (HRS), and Asia Pacific Heart Rhythm Society (APHRS). Europace 2015;17:1197–214. [DOI] [PubMed] [Google Scholar]
- 19. Scaglione M, Blandino A, Raimondo C, Caponi D, Di Donna P, Toso E et al. Impact of ablation catheter irrigation design on silent cerebral embolism after radiofrequency catheter ablation of AF: results from a pilot study. J Cardiovasc Electrophysiol 2012;23:801–5. [DOI] [PubMed] [Google Scholar]
- 20. Gaita F, Corsinovi L, Anselmino M, Raimondo C, Pianelli M, Toso E et al. Prevalence of silent cerebral ischemia in paroxysmal and persistent AF and correlation with cognitive function. J Am Coll Cardiol 2013;62:1990–7. [DOI] [PubMed] [Google Scholar]
- 21. Deneke T, Shin DI, Balta O, Bünz K, Fassbender F, Mügge A et al. Postablation asymptomatic cerebral lesions: Long-term follow-up using magnetic resonance imaging. Heart Rhythm 2011;8:1705–11. [DOI] [PubMed] [Google Scholar]
- 22. Kalantarian S, Ay H, Gollub RL, Lee H, Retzepi K, Mansour M et al. Association between atrial fibrillation and silent cerebral infarctions: a systematic review and meta-analysis. Ann Intern Med 2014;161:650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vermeer SE, Prins ND, Den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–22. [DOI] [PubMed] [Google Scholar]
- 24. De Greef Y, Tavernier R, Schwagten B, De Keulenaer G, Stockman D, Duytschaever M. Impact of radiofrequency characteristics on acute pulmonary vein reconnection and clinical outcome after PVAC ablation. J Cardiovasc Electrophysiol 2013;24:290–6. [DOI] [PubMed] [Google Scholar]
- 25. Kardos A, Foldesi C, Mihalcz A, Szili-Torok T. Cavotricuspid isthmus ablation with large-tip gold alloy versus platinum-iridium-tip electrode catheters. Pacing Clin Electrophysiol 2009;32(Suppl. 1):S138–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.