Abstract
Objective:
This clinical practice guideline addresses the diagnosis and treatment of primary adrenal insufficiency.
Participants:
The Task Force included a chair, selected by The Clinical Guidelines Subcommittee of the Endocrine Society, eight additional clinicians experienced with the disease, a methodologist, and a medical writer. The co-sponsoring associations (European Society of Endocrinology and the American Association for Clinical Chemistry) had participating members. The Task Force received no corporate funding or remuneration in connection with this review.
Evidence:
This evidence-based guideline was developed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system to determine the strength of recommendations and the quality of evidence.
Consensus Process:
The evidence used to formulate recommendations was derived from two commissioned systematic reviews as well as other published systematic reviews and studies identified by the Task Force. The guideline was reviewed and approved sequentially by the Endocrine Society's Clinical Guidelines Subcommittee and Clinical Affairs Core Committee, members responding to a web posting, and the Endocrine Society Council. At each stage, the Task Force incorporated changes in response to written comments.
Conclusions:
We recommend diagnostic tests for the exclusion of primary adrenal insufficiency in all patients with indicative clinical symptoms or signs. In particular, we suggest a low diagnostic (and therapeutic) threshold in acutely ill patients, as well as in patients with predisposing factors. This is also recommended for pregnant women with unexplained persistent nausea, fatigue, and hypotension. We recommend a short corticotropin test (250 μg) as the “gold standard” diagnostic tool to establish the diagnosis. If a short corticotropin test is not possible in the first instance, we recommend an initial screening procedure comprising the measurement of morning plasma ACTH and cortisol levels. Diagnosis of the underlying cause should include a validated assay of autoantibodies against 21-hydroxylase. In autoantibody-negative individuals, other causes should be sought. We recommend once-daily fludrocortisone (median, 0.1 mg) and hydrocortisone (15–25 mg/d) or cortisone acetate replacement (20–35 mg/d) applied in two to three daily doses in adults. In children, hydrocortisone (∼8 mg/m2/d) is recommended. Patients should be educated about stress dosing and equipped with a steroid card and glucocorticoid preparation for parenteral emergency administration. Follow-up should aim at monitoring appropriate dosing of corticosteroids and associated autoimmune diseases, particularly autoimmune thyroid disease.
Summary of Recommendations
1.0 Who should be tested and how?
1.1 We recommend diagnostic testing to exclude primary adrenal insufficiency (PAI) in acutely ill patients with otherwise unexplained symptoms or signs suggestive of PAI (volume depletion, hypotension, hyponatremia, hyperkalemia, fever, abdominal pain, hyperpigmentation or, especially in children, hypoglycemia). (1|⊕⊕⊕○)
1.2 We recommend confirmatory testing with the corticotropin stimulation test in patients with clinical symptoms or signs suggesting PAI when the patient's condition and circumstance allow. (1|⊕⊕⊕⊕)
1.3 In patients with severe adrenal insufficiency symptoms or adrenal crisis, we recommend immediate therapy with iv hydrocortisone at an appropriate stress dose prior to the availability of the results of diagnostic tests. (1|⊕⊕⊕○)
2.0 Optimal diagnostic tests
2.1 We suggest the standard dose (250 μg for adults and children ≥2 y of age, 15 μg/kg for infants, and 125 μg for children <2 y of age) iv corticotropin stimulation (30 or 60 min) test over other existing diagnostics tests to establish the diagnosis of adrenal insufficiency. Peak cortisol levels below 500 nmol/L (18 μg/dL) (assay dependent) at 30 or 60 minutes indicate adrenal insufficiency. (2|⊕⊕○○)
2.2 We suggest the low-dose (1 μg) corticotropin test for diagnosis of PAI only when the substance itself is in short supply. (2|⊕⊕○○)
2.3 If a corticotropin stimulation test is not feasible, we suggest using a morning cortisol <140 nmol/L (5 μg/dL) in combination with ACTH as a preliminary test suggestive of adrenal insufficiency (until confirmatory testing with corticotropin stimulation is available). (2|⊕○○○)
2.4 We recommend measurement of plasma ACTH to establish PAI. The sample can be obtained at the same time as the baseline sample in the corticotropin test or paired with the morning cortisol sample. In patients with confirmed cortisol deficiency, a plasma ACTH >2-fold the upper limit of the reference range is consistent with PAI. (1|⊕⊕⊕○)
2.5 We recommend the simultaneous measurement of plasma renin and aldosterone in PAI to determine the presence of mineralocorticoid deficiency. (1|⊕⊕⊕○)
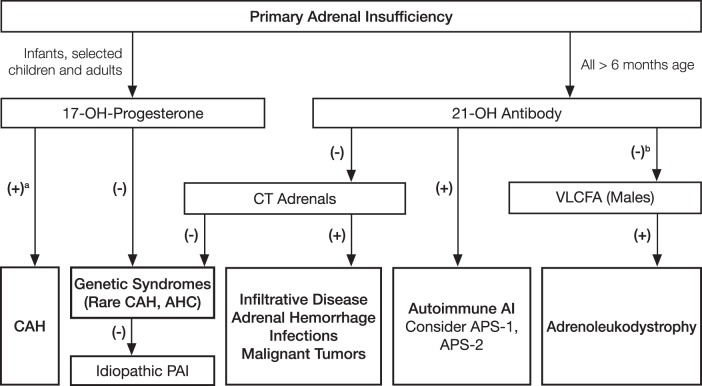
2.6 We suggest that the etiology of PAI should be determined in all patients with confirmed disease. (For diagnostic workup, see Table 2 and Figure 1.) (Ungraded best practice recommendation)
Table 2.
Major Etiologies of PAI and Associated Features
| Etiology | Associated Features |
|---|---|
| Autoimmune | |
| Isolated | Not associated with other autoimmune disorders |
| APS type 1 (APECED) | Chronic cutaneous candidiasis, hypoparathyroidism |
| APS type 2 | Autoimmune thyroid disease, type 1 diabetes |
| Adrenal—infiltration/injury | |
| Adrenal hemorrhage | Associated with sepsis, anticoagulants, anti-cardiolipin/lupus anti-coagulant syndrome |
| Adrenal metastases | Malignancies: lung, breast, colon, melanoma, lymphoma |
| Infections: adrenalitis | Tuberculosis, HIV/AIDS, CMV, candidiasis, histoplasmosis, syphilis, African trypanosomiasis, paracoccidioidomycosis (eg, in South America) |
| Infiltration | Hemochromatosis, primary amyloidosis |
| Bilateral adrenalectomy | Procedure for intractable Cushing's syndrome or bilateral pheochromocytoma |
| CAH: most forms can cause salt loss | Commonest cause of PAI in children (80%); may be diagnosed in older individuals |
| 21-Hydroxylase deficiency | Commonest type of CAH is 21-hydroxylase deficiency, with associated hyperandrogenism |
| 11β-hydroxylase deficiency | Hyperandrogenism, hypertension (in older children and adults) |
| 3β-hydroxysteroid dehydrogenase II deficiency | Ambiguous genitalia in boys, hyperandrogenism in girls |
| P450 side-chain cleavage deficiency (CYP11A1 mutations) | XY sex reversal |
| P450 oxidoreductase deficiency | Skeletal malformations, abnormal genitalia |
| Congenital lipoid adrenal hyperplasia (StAR mutations) | XY sex reversal |
| Adrenal hypoplasia congenita | X-linked NROB1, Xp21 deletion (with Duchenne's muscular deficiency), SF-1 mutations (XY sex reversal), IMAGe syndrome |
| ACTH insensitivity syndromes | Type 1: ACTH receptor, melanocortin 2 receptor gene MC2R |
| Type 2: MRAP | |
| Familial glucocorticoid deficiency (MCM4, NNT, TXNRD2) | |
| TripleA (Allgrove's) syndrome, achalasia, Addison's disease, alacrima, AAAS gene mutation | |
| Drug-induced | Adrenal enzyme inhibitors: mitotane, ketoconazole, metyrapone, etomidate, aminoglutethimide, drugs that may accelerate cortisol metabolism and induce adrenal insufficiency |
| T4 also accelerates cortisol metabolism (at least in part through stimulation of 11β-HSD2) | |
| CTLA-4 inhibitors may enhance autoimmunity and cause PAI | |
| Other metabolic disorders | Mitochondrial disease (rare) |
| Adrenoleukodystrophy in males | |
| Wolman's disease |
Abbreviations: APECED, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy; CMV, cytomegalovirus; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CYP, cytochrome P; HSD, hydroxysteroid dehydrogenase; 11β-HSD2, 11β-hydroxysteroid dehydrogenase type 2; IMAGe, intrauterine growth restriction, metaphyseal dysplasia, adrenal hypoplasia congenital, genital abnormalities; MC2R, melanocortin 2 receptor gene; MCM4, minichromosome maintenance-deficient 4; MRAP, melanocortin receptor accessory protein; NNT, nicotinamide nucleotide transhydrogenase; StAR, steroidogenic acute regulatory protein; TXNRD2, thioredoxin reductase 2. [Derived from E. Charmandari E, et al: Adrenal insufficiency. Lancet. 2014;383:2152–2167 (164), with permission. © Elsevier.]
Figure 1.
Algorithm for the diagnostic approach to the patient with PAI. The most common causes of PAI are autoimmune destruction of the adrenal cortex in adults and CAH in children. These etiologies can be screened for using 21-hydroxylase antibodies and a baseline serum 17-hydroxyprogesterone level. Males with negative 21-hydroxylase antibodies should be tested for adrenoleukodystrophy with plasma VLCFAs. If these diagnoses are excluded, a CT scan of the adrenals may reveal evidence of adrenal infiltrative processes or metastases. The individual's clinical picture and family history may render some steps in the algorithm redundant or suggest specific genetic syndromes. The latter includes subtypes of autoimmune polyglandular syndromes or specific rare genetic disorders where adrenal failure is part of a broader phenotype. A list of differential diagnoses is provided in Table 2. AHC, adrenal hypoplasia congenita; AI, adrenal insufficiency; VLCFA, very long chain fatty acid. a 17-OH-progesterone >1000 ng/dL is diagnostic for 21-OH deficiency (14). b VLCFA should be measured in the initial evaluation of preadolescent boys. [Adapted from E. S. Husebye, et al: Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275:104–115 (181), with permission. © John Wiley & Sons, Inc.]
3.0 Treatment of primary adrenal insufficiency in adults
Glucocorticoid replacement regimen
3.1 We recommend glucocorticoid therapy in all patients with confirmed PAI. (1|⊕⊕⊕⊕)
3.2 We suggest using hydrocortisone (15–25 mg) or cortisone acetate (20–35 mg) in two or three divided oral doses per day; the highest dose should be given in the morning at awakening, the next either in the early afternoon (2 h after lunch; two-dose regimen) or at lunch and afternoon (three-dose regimen). Higher frequency regimens and size-based dosing may be beneficial in individual cases. (2|⊕⊕○○)
3.3 As an alternative to hydrocortisone, we suggest using prednisolone (3–5 mg/d), administered orally once or twice daily, especially in patients with reduced compliance. (2|⊕○○○)
3.4 We suggest against using dexamethasone for the treatment of PAI because of risk of Cushingoid side effects due to difficulties in dose titration. (2|⊕⊕○○)
3.5 We suggest monitoring glucocorticoid replacement using clinical assessment including body weight, postural blood pressure, energy levels, signs of frank glucocorticoid excess. (2|⊕⊕⊕○)
3.6 We suggest against hormonal monitoring of glucocorticoid replacement and to adjust treatment only based on clinical response. (2|⊕⊕⊕○)
Mineralocorticoid replacement in PAI
3.7 We recommend that all patients with confirmed aldosterone deficiency receive mineralocorticoid replacement with fludrocortisone (starting dose, 50–100 μg in adults) and not restrict their salt intake. (1|⊕⊕⊕⊕)
3.8 We recommend monitoring mineralocorticoid replacement primarily based on clinical assessment (salt craving, postural hypotension, or edema), and blood electrolyte measurements. (1|⊕⊕⊕○)
3.9 In patients who develop hypertension while receiving fludrocortisone, we suggest reducing the dose of fludrocortisone. (2|⊕○○○)
3.10 If blood pressure remains uncontrolled, we suggest initiating antihypertensive treatment and continuing fludrocortisone. (2|⊕○○○)
Dehydroepiandrosterone replacement
3.11 We suggest a trial of dehydroepiandrosterone (DHEA) replacement in women with PAI and low libido, depressive symptoms, and/or low energy levels despite otherwise optimized glucocorticoid and mineralocorticoid replacement. (2|⊕⊕○○)
3.12 We suggest an initial period of 6 months of DHEA replacement. If the patient does not report a sustained, beneficial effect of replacement after 6 months, the DHEA should be discontinued. (2|⊕⊕○○)
3.13 We suggest monitoring DHEA replacement by measuring morning serum DHEA sulfate (DHEAS) levels (aiming at the midnormal range) before the intake of the daily DHEA replacement dose. (2|⊕⊕○○)
Treatment during pregnancy
3.14 We suggest that pregnant patients with PAI be monitored for clinical symptoms and signs of glucocorticoid over- and under-replacement (eg, normal weight gain, fatigue, postural hypotension or hypertension, hyperglycemia), with at least one review per trimester. (Ungraded best practice statement)
3.15 We suggest that, based on the individual clinical course, an increase in hydrocortisone dose should be implemented, in particular during the third trimester. (Ungraded best practice statement)
3.16 In pregnant women with PAI, we suggest using hydrocortisone over cortisone acetate, prednisolone, or prednisone (2|⊕⊕○○) and recommend against using dexamethasone because it is not inactivated in the placenta. (1|⊕⊕○○)
3.17 We recommend hydrocortisone stress dosing during the active phase of labor, similar to that used in major surgical stress. (1|⊕⊕○○)
Treatment and monitoring during childhood
3.18 In children with PAI, we suggest treatment with hydrocortisone in three or four divided doses (total starting daily dose of 8 mg/m2 body surface area) over other types of glucocorticoid replacement therapies, with doses adjusted according to individual need. (2|⊕⊕○○)
3.19 In children with PAI, we suggest avoiding synthetic, long-acting glucocorticoids (eg, prednisolone, dexamethasone). (2|⊕⊕○○)
3.20 We suggest monitoring glucocorticoid replacement by clinical assessment, including growth velocity, body weight, blood pressure, and energy levels. (Ungraded best practice statement)
3.21 In children with PAI and confirmed aldosterone deficiency, we recommend treatment with fludrocortisone (starting dosage, 100 μg/d). For infants, we recommend sodium chloride supplements in the newborn period and up to the age of 12 months. (1|⊕⊕○○)
4.0 Management and prevention of adrenal crisis in patients with PAI
4.1 We recommend that patients with suspected adrenal crisis should be treated with an immediate parenteral injection of 100 mg (50 mg/m2 for children) hydrocortisone, followed by appropriate fluid resuscitation and 200 mg (50–100 mg/m2 for children) of hydrocortisone/24 hours (via continuous iv therapy or 6 hourly injection); age- and body surface-appropriate dosing is required in children (see Table 3). (1|⊕⊕⊕○)
Table 3.
Management of PAI in Specific Situations
| Condition | Suggested Action |
|---|---|
| Home management of illness with fever | Hydrocortisone replacement doses doubled (>38°C) or tripled (>39°C) until recovery (usually 2 to 3 d); increased consumption of electrolyte-containing fluids as tolerated |
| Unable to tolerate oral medication due to gastroenteritis or trauma | Adults, im or sc hydrocortisone 100 mg; children, im hydrocortisone 50 mg/m2 or estimate; infants, 25 mg; school-age children, 50 mg; adolescents, 100 mg |
| Minor to moderate surgical stress | Hydrocortisone, 25–75 mg/24 h (usually 1 to 2 d) |
| Children, im hydrocortisone 50 mg/m2 or hydrocortisone replacement doses doubled or tripled | |
| Major surgery with general anesthesia, trauma, delivery, or disease that requires intensive care | Hydrocortisone, 100 mg per iv injection followed by continuous iv infusion of 200 mg hydrocortisone/24h (alternatively 50 mg every 6 h iv or im) |
| Children, hydrocortisone 50 mg/m2 iv followed by hydrocortisone 50–100 mg/m2/d divided q 6 h | |
| Weight-appropriate continuous iv fluids with 5% dextrose and 0.2 or 0.45% NaCl | |
| Rapid tapering and switch to oral regimen depending on clinical state | |
| Acute adrenal crisis | Rapid infusion of 1000 mL isotonic saline within the first hour or 5% glucose in isotonic saline, followed by continuous iv isotonic saline guided by individual patient needs |
| Hydrocortisone 100 mg iv immediately followed by hydrocortisone 200 mg/d as a continuous infusion for 24 h, reduced to hydrocortisone 100 mg/d the following day | |
| Children, rapid bolus of normal saline (0.9%) 20 mL/kg. Can repeat up to a total of 60 mL/kg within 1 h for shock. | |
| Children, hydrocortisone 50–100 mg/m2 bolus followed by hydrocortisone 50–100 mg/m2/d divided q 6 h | |
| For hypoglycemia: dextrose 0.5–1 g/kg of dextrose or 2–4 mL/kg of D25W (maximum single dose 25 g) infused slowly at rate of 2 to 3 mL/min. Alternatively, 5–10 mL/kg of D10W for children <12 y old | |
| Cardiac monitoring: Rapid tapering and switch to oral regimen depending on clinical state |
Abbreviation: D10W, 10% dextrose solution; D25W, 25% dextrose solution. [Adapted from B. Allolio: Extensive expertise in endocrinology: adrenal crisis. Eur J Endocrinol. 2015;172:R115–R124 (126), with permission. © Endocrine Society.]
4.2 If hydrocortisone is unavailable, we suggest prednisolone as an alternative. Dexamethasone is the least-preferred alternative and should only be given if no other glucocorticoid is available. (2|⊕⊕○○)
4.3 For the prevention of adrenal crisis, we suggest adjusting glucocorticoid dose according to severity of illness or magnitude of the stressor. (2|⊕⊕○○)
4.4 We suggest patient education concerning glucocorticoid adjustments in stressful events and adrenal crisis-prevention strategies including parenteral self- or lay-administration of emergency glucocorticoids. (Ungraded best practice statement)
4.5 We recommend that all patients should be equipped with a steroid emergency card and medical alert identification to inform health personnel of the need for increased glucocorticoid doses to avert or treat adrenal crisis and the need of immediate parenteral steroid treatment in the event of an emergency. (Ungraded best practice statement)
4.6 We recommend that every patient should be equipped with a glucocorticoid injection kit for emergency use and be educated on how to use it. (Ungraded best practice statement)
5.0 Additional monitoring requirement
5.1 We suggest that adults and children with PAI be seen by an endocrinologist or a healthcare provider with endocrine expertise at least annually. Infants should be seen at least every 3 to 4 months. (Ungraded best practice statement)
5.2 We suggest that PAI patients be evaluated annually for symptoms and signs of over- and under-replacement. (Ungraded best practice statement)
5.3 We suggest periodic screening for autoimmune diseases known to be more prevalent in PAI patients in whom autoimmune origin of PAI has not been excluded. The optimal frequency of screening is unknown but can be done annually. These conditions include thyroid disease, diabetes mellitus, premature ovarian failure, celiac disease, and autoimmune gastritis with vitamin B12 deficiency. (2|⊕⊕○○)
5.4 We suggest patient education about increasing the dosage of glucocorticoids during intercurrent illness, fever, and stress. This education includes identification of precipitating symptoms and signs and how to act in impending adrenal crisis. (Ungraded best practice statement)
5.5 We suggest genetic counseling for patients with PAI due to monogenic disorders. (Ungraded best practice statement)
General Introduction
PAI is defined by the inability of the adrenal cortex to produce sufficient amounts of glucocorticoids and/or mineralocorticoids. PAI is a severe and potentially life-threatening condition due to the central role of these hormones in energy, salt, and fluid homeostasis. PAI was first described by Thomas Addison (1) and is therefore commonly termed Addison's disease. Cortisol deficiency results in a decrease in feedback to the hypothalamic-pituitary axis and subsequent enhanced stimulation of the adrenal cortex by elevated levels of plasma ACTH. Consequent to disruption of adrenal mineralocorticoid synthesis, renin release by the juxtaglomerular cells of the kidneys increases. This is of clinical, diagnostic, and therapeutic relevance because PAI needs to be distinguished from secondary adrenocortical insufficiency due to insufficient production of ACTH and without impact on the renin-angiotensin-aldosterone system.
The signs of PAI are mainly based on the deficiency of gluco- and mineralocorticoids and the resultant weight loss, orthostatic hypotension due to dehydration, hyponatremia, hyperkalemia, changes in blood count (anemia, eosinophilia, lymphocytosis), and hypoglycemia (summarized in Table 1). Enhanced secretion of ACTH and other pro-opiomelanocortin peptides often leads to the characteristic hyperpigmentation of the skin and mucous membranes. In women, loss of adrenal androgens results in loss of axillary and pubic hair. Except for salt craving, the symptoms of PAI are rather nonspecific and include weakness, fatigue, musculoskeletal pain, weight loss, abdominal pain, depression, and anxiety. As a result, the diagnosis is frequently delayed, resulting in a clinical presentation with an acute life-threatening adrenal crisis (2). Even with treatment, the health-related quality of life (HRQoL) in patients with Addison's disease receiving standard replacement therapy is often reduced (3, 4). Moreover, long-term HRQoL in these patients appears to be inversely related to the delay in establishing the diagnosis after disease onset, emphasizing the importance of recognizing the disease early (5, 6). This is complicated by the fact that PAI is a rare disease with a reported prevalence of about 100 to 140 cases per million and an incidence of 4:1 000 000 per year in Western societies (7–10). Nevertheless, recent health insurance data from Germany report an increasing prevalence, particularly in females (11). The most common cause of PAI is autoimmunity (up to 90% in Western countries), followed by infectious diseases such as tuberculosis, adrenalectomy, neoplasia, and various genetic causes; the last are more likely to be present and diagnosed in children. Moreover, due to the growing number of chronically and severely ill patients requiring chronic intensive care that includes multiple concomitant pharmacological therapies, additional iatrogenic factors (such as adrenal hemorrhage related to anticoagulants, inhibition of cortisol synthesis by aminoglutethimide or etomidate, activation of glucocorticoid metabolism by anticonvulsants like phenytoin or phenobarbital, or antibiotics like rifampicin) increasingly contribute to the ultimate manifestation of PAI (12).
Table 1.
Clinical Features of Adrenal Insufficiency and Adrenal Crisis
| Symptoms | Signs | Routine Laboratory Tests |
|---|---|---|
| Adrenal insufficiency | ||
| Fatigue | Hyperpigmentation (primary only), particularly of sun-exposed areas, skin creases, mucosal membranes, scars, areola of breast | Hyponatremia |
| Weight loss | Low blood pressure with increased postural drop | Hyperkalemia |
| Postural dizziness | Failure to thrive in children | Uncommon: hypoglycemia, hypercalcemia |
| Anorexia, abdominal discomfort | ||
| Adrenal crisis | ||
| Severe weakness | Hyponatremia | |
| Syncope | Hypotension | Hyperkalemia |
| Abdominal pain, nausea, vomiting; may mimic acute abdomen | Abdominal tenderness/guarding | Hypoglycemia |
| Back pain | Reduced consciousness, delirium | Hypercalcemia |
| Confusion |
Most symptoms are nonspecific and present chronically, often leading to delayed diagnosis. Hyponatremia and, later, hyperkalemia are often triggers to diagnosis, requiring biochemical confirmation of adrenal insufficiency. Hyperpigmentation is a specific sign, but it is variably present in individuals and must be compared with the patient's background pigmentation, such as that in siblings. Adrenal crisis is a medical emergency with hypotension, marked acute abdominal symptoms, and marked laboratory abnormalities, requiring immediate treatment. Continuing effort to prevent adrenal crisis is integral to patient management. Additional symptoms and signs may arise from the underlying cause of adrenal insufficiency, eg, associated autoimmune disorders, neurological features of adrenoleukodystrophy, or disorders that may lead to adrenal infiltration.
The diagnosis and management of adrenal insufficiency secondary to hypothalamic-pituitary failure have been recently discussed in an expert review in this journal (13). Also, the management of congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency has been comprehensively discussed, and a current clinical guideline on CAH has been published by the Endocrine Society (14). Therefore, the current guideline focuses on the diagnosis and treatment of PAI alone; issues related to secondary adrenal insufficiency and critical illness-related corticosteroid insufficiency have been excluded. The level of evidence and strength of recommendations published in this guideline were evaluated based on the GRADE system.
Method of Development of Evidence-Based Clinical Practice Guidelines
The Clinical Guidelines Subcommittee (CGS) of the Endocrine Society deemed the management of PAI a priority area in need of practice guidelines and appointed a Task Force to formulate evidence-based recommendations. The Task Force followed the approach recommended by the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) group, an international group with expertise in development of evidence-based guidelines (15). A detailed description of the grading scheme has been published elsewhere (16). The Task Force used the best available research evidence to develop the recommendations. The Task Force also used consistent language and graphical descriptions of both the strength of a recommendation and the quality of evidence. In terms of the strength of the recommendation, strong recommendations use the phrase “we recommend” and the number 1, and weak recommendations use the phrase “we suggest” and the number 2. Cross-filled circles indicate the quality of the evidence, such that ⊕○○○ denotes very low-quality evidence; ⊕⊕○○, low quality; ⊕⊕⊕○, moderate quality; and ⊕⊕⊕⊕, high quality. The Task Force has confidence that persons who receive care according to the strong recommendations will derive, on average, more good than harm. Weak recommendations require more careful consideration of the person's circumstances, values, and preferences to determine the best course of action. Linked to each recommendation is a description of the evidence and the values that panelists considered in making the recommendation; in some instances, there are remarks, a section in which panelists offer technical suggestions for testing conditions, dosing, and monitoring. These technical comments reflect the best available evidence applied to a typical person being treated. Often this evidence comes from the unsystematic observations of the panelists and their values and preferences; therefore, these remarks should be considered suggestions. In this guideline, the Task Force made several statements to emphasize various monitoring and patient education actions needed to prevent the severe morbidity and mortality of adrenal crisis and reduce medication side effects. These were labeled as ungraded best practice statements. Direct evidence for these statements was either unavailable or not systematically appraised and was considered out of the scope of this guideline. The intention of these statements is to draw attention and remind providers of these principles, and these statements should not be considered as graded recommendations (17).
The Endocrine Society maintains a rigorous conflict-of-interest review process for the development of clinical practice guidelines. All Task Force members must declare any potential conflicts of interest, which are reviewed before they are approved to serve on the Task Force and periodically during the development of the guideline. The conflict-of-interest forms are vetted by the CGS before the members are approved by the Society's Council to participate on the guideline Task Force. Participants in the guideline development must include a majority of individuals without conflict of interest in the matter under study. Participants with conflicts of interest may participate in the development of the guideline, but they must have disclosed all conflicts. The CGS and the Task Force have reviewed all disclosures for this guideline and resolved or managed all identified conflicts of interest.
Conflicts of interest are defined by remuneration in any amount from the commercial interest(s) in the form of grants; research support; consulting fees; salary; ownership interest (eg, stocks, stock options, or ownership interest excluding diversified mutual funds); honoraria or other payments for participation in speakers' bureaus, advisory boards, or boards of directors; or other financial benefits. Completed forms are available through the Endocrine Society office.
Funding for this guideline was derived solely from the Endocrine Society, and thus the Task Force received no funding or remuneration from commercial or other entities.
Commissioned systematic reviews
The Task Force commissioned two systematic reviews and developed an a priori protocol that included a specific search strategy, inclusion and exclusion criteria, and methods of evidence synthesis. The two reviews summarized data on patients with both primary and secondary adrenal insufficiency; however, the Task Force used data derived from patients with PAI because this was the target population of this guideline.
The first review compared the diagnostic accuracy of high-dose ACTH vs low-dose ACTH stimulation tests for the initial diagnosis. Only five studies were available to estimate the diagnostic accuracy of the high-dose ACTH stimulation test in PAI, and there were none for the low-dose ACTH stimulation test. The sensitivity of the high-dose ACTH stimulation test for the diagnosis of PAI was 92% (95% confidence interval, 81–97%). Data from 30 studies in patients with secondary adrenal insufficiency showed no statistically significant difference between low-dose and high-dose ACTH stimulation tests. This evidence is indirect for the target population of this guideline, which is PAI patients.
The second review compared various glucocorticoid replacement regimens in patients with adrenal insufficiency and identified 15 relevant observational studies. Data on mortality, bone density, and incidence of adrenal crisis were very sparse or unavailable. In terms of HRQoL, there was no statistically significant difference between regimens using glucocorticoid dosages equal to or higher than 30 mg/d of hydrocortisone vs regimens with dosages <30 mg/d. Very low-quality evidence (comparison across studies, methodological limitations, and substantial heterogeneity) suggested that extended-release and dual-release forms of glucocorticoids may have higher HRQoL scores compared with forms given once, twice, or three times daily. This evidence was considered insufficient for decision-making and required verification in a randomized trial.
Diagnosis of primary adrenal insufficiency
The diagnosis of PAI is traditionally based on low morning cortisol concentrations (measured in serum or plasma) and confirmed by low stimulated cortisol. DHEAS levels (DHEA less so) that are well below the lower limit of normal for age and sex are a useful initial sign of PAI that should not be overlooked, although they cannot be used in isolation to make the diagnosis of PAI because levels may be low in some individuals, especially in older age groups, without PAI. In most cases, the diagnosis is highly likely if the cortisol is <140 nmol/L (5 μg/dL) (18) in combination with an ACTH concentration (measured in plasma) elevated more than 2-fold above the upper limit of the reference interval for the specific assay. An ACTH value >66 pmol/L represents a maximum stimulus for cortisol secretion (19, 20). For confirmation, a corticotropin stimulation test should be performed in most cases unless basal results are absolutely unequivocal.
The corticotropin stimulation test is currently regarded as the diagnostic “gold standard” for the diagnosis of primary (but not secondary) adrenal insufficiency because it has been reasonably well studied and is validated against the insulin tolerance test for diagnostic accuracy (21–25). This test is also known as the cosyntropin test, ACTH test, or short Synacthen test; Synacthen is the trade name of tetracosactide, a synthetic peptide consisting of the first 24 of the 39 amino acids of the endogenous ACTH peptide. However, there is still some ongoing debate over the definition of the cutoff value of cortisol after corticotropin stimulation to exclude PAI (see below). The test is used in clinical practice with different protocols, mainly in the duration of the test procedure, the route of administration (im or iv), and the dose of corticotropin applied (26, 27). Commonly, the standard short corticotropin test is performed measuring cortisol levels before and 30 or 60 minutes after iv (or im) administration of 250 μg corticotropin as bolus injection (28). The standard-dose (250 μg) short corticotropin test is a common diagnostic test in clinical practice with a high degree of validation. Another variation of the cosyntropin test uses a low-dose 1 μg of corticotropin for adrenal stimulation. However, based on the currently available data, the 1-μg test does not provide better diagnostic accuracy than the 250-μg corticotropin test (29). It should be noted that porcine ACTH is used in some countries, although the evidence for its use is much less than with synthetic ACTH analogs such as cosyntropin (30).
The interpretation of the cosyntropin test is based on the peak stimulated serum cortisol concentration. The results of the corticotropin test are not significantly affected by diurnal variations, and the test can therefore be performed without time constraints (31, 32). However, caution is required because adrenocortical function test results may be severely affected by rare conditions such as cortisol-binding globulin (CBG) deficiency, glucocorticoid resistance, and hypersensitivity (33–36).
1.0 Who should be tested and how?
1.1 We recommend diagnostic testing to exclude PAI in acutely ill patients with otherwise unexplained symptoms or signs suggestive of PAI (volume depletion, hypotension, hyponatremia, hyperkalemia, fever, abdominal pain, hyperpigmentation or, especially in children, hypoglycemia). (1|⊕⊕⊕○)
1.2 We recommend confirmatory testing with the corticotropin stimulation test in patients with clinical symptoms or signs suggesting PAI when the patient's condition and circumstance allow. (1|⊕⊕⊕⊕)
1.3 In patients with severe adrenal insufficiency symptoms or adrenal crisis, we recommend immediate therapy with iv hydrocortisone at an appropriate stress dose prior to the availability of the results of diagnostic tests. (1|⊕⊕⊕○)
Evidence
Symptoms of adrenal insufficiency or adrenal crisis are well established by older observational studies and clinical experience and are summarized in Table 1. Delayed treatment of more severe symptoms will increase morbidity and mortality. Treatment should therefore not be delayed by awaiting the results of cosyntropin testing.
Diagnosis of PAI is challenging due to an insidious onset of predominantly nonspecific symptoms over months or years. Therefore, it is essential to keep the threshold for diagnostic evaluation low (2, 37). The exclusion of PAI is mainly based on the determination of cortisol concentrations in the corticotropin stimulation test.
In acutely sick patients with clinical signs and symptoms, treatment should not be delayed awaiting test results. It is important to draw blood for diagnostic purposes before any steroid treatment is given. A single baseline ACTH and cortisol before administration of hydrocortisone is essential for diagnosis, or occasionally even a cosyntropin stimulation test may be performed, but only if safe to do so. Confirmatory cosyntropin testing can be performed after treatment, with temporary cessation of glucocorticoid when the patient's condition is stable (38, 39).
Although it is more elaborate and requires endocrinological expertise, the corticotropin stimulation test is a superior diagnostic test with a higher degree of sensitivity and specificity than determination of morning cortisol and ACTH concentrations and is therefore preferred in all patients considered with the differential diagnosis of PAI (28, 38).
Other relatively frequent conditions predisposing patients to PAI include certain autoimmune disorders (eg, type 1 diabetes mellitus, autoimmune gastritis/pernicious anemia, and vitiligo) as well as infectious diseases (tuberculosis, HIV, cytomegalovirus, candidiasis, histoplasmosis) (Table 2). Adrenal enzyme inhibitors (mitotane, ketoconazole, metyrapone, and etomidate) are examples of agents that may induce adrenal insufficiency. In addition, a number of drugs such as phenytoin and carbamazepine, mitotane, and St John's wort may increase metabolism of cortisol. Therefore, in patients using these agents, the diagnostic threshold should also be kept low (12).
Plasma cortisol is 80% bound to CBG and 10–15% to albumin, so disorders that reduce (inflammation, rare genetic disorders) or increase CBG levels (estrogen, pregnancy, mitotane) need to be considered in interpretation of plasma cortisol levels (36).
The diagnosis of PAI in pregnant women is particularly challenging due to its extreme rarity, overlapping symptoms like nausea and hypotension as well as physiological changes (eg, increased cortisol production during pregnancy; see Section 3.0 and Recommendation 3.14), making the diagnosis difficult. Because untreated PAI in pregnant women is associated with a high mortality, whereas sufficiently treated patients can expect a normal pregnancy course and outcome, early recognition and diagnosis is critical. In addition to a paired sample of cortisol and ACTH, the adrenal reserve is appropriately and safely assessed in pregnancy by corticotropin stimulation, if indicated (40, 41). Interpretation of the diagnostic results requires thorough consideration of pregnancy-associated physiological changes of adrenocortical function (42). A strong recommendation for immediate treatment before the availability of test results (but after the relevant samples have been procured) is driven by placing high value on preventing major harm. A practical diagnostic approach to the patient with PAI is summarized in Figure 1 (14).
Technical remarks on diagnostic recommendations
The range and severity of PAI symptoms can be classified as indicative of adrenal insufficiency or adrenal crisis, as summarized in Table 1.
A heightened level of clinical suspicion of adrenal insufficiency is warranted in patients with compatible symptoms who also have disorders associated with the development of PAI, such as autoimmune disorders or relevant drugs (major etiologies of PAI summarized in Table 2).
There are several situations requiring specific consideration because cortisol levels can be expected to be altered by nonadrenal pathologies. These include critical illness and pregnancy. In addition, reduced CBG levels in illness and elevated levels in pregnancy may alter the interpretation of cortisol levels.
Patients with autoimmune disease and systemic disorders known to affect the adrenal function or who are on drugs (eg, T4) that are known to increase cortisol metabolism are at increased risk of PAI.
2.0 Optimal diagnostic tests
2.1 We suggest the standard dose (250 μg for adults and children ≥2 y of age, 15 μg/kg for infants, and 125 μg for children <2 y of age) iv corticotropin stimulation (30 or 60 min) test over other existing diagnostics tests to establish the diagnosis of adrenal insufficiency. Peak cortisol levels below 500 nmol/L (18 μg/dL) (assay dependent) at 30 or 60 minutes indicate adrenal insufficiency. (2|⊕⊕○○)
Evidence
The exclusion of PAI is mainly based on the determination of cortisol concentrations in the corticotropin stimulation test. There are five primary studies that considered the diagnosis of PAI in their populations and also met the inclusion criteria of the systematic review. Using meta-analysis, the sensitivity of the 250-μg corticotropin stimulation test using a peak response of cortisol (500 nmol/L or 18 μg/dL) was 0.92 (95% confidence interval, 0.81–0.97). The cutoff values for exclusion of adrenal insufficiency may vary according to the assay used. Different immunoassays use different detection antibodies that provide results that may demonstrate significant bias between methods. For example, the low reference limit for cortisol 30 minutes after the corticotropin stimulation ranges from 418 to 574 nmol/L (15.2–20.8 μg/dL) (43, 44). The actual cutpoint used in clinical practice to make the diagnosis should be based on assay-specific normative data. The laboratory providing the result should be able to provide the performance bias of the cortisol method in use. Traditionally, a peak cortisol concentration after acute stimulation with corticotropin exceeding 500 nmol/L (18 μg/dL) is accepted as evidence for sufficient adrenocortical responsiveness (32, 45–47). It is important to consider other factors that will affect interpretation of the result, in particular the factors that alter protein binding of cortisol to CBG (48) and to a lesser extent albumin (49). Importantly, the use of the estrogen-containing oral contraceptives will result in higher CBG with a corresponding rise in cortisol (43, 44). Patients with diseases such as nephrotic syndrome (43) and liver disease (50) as well as those who are in the immediate postoperative period (51) or who require intensive care (52) may have lower CBG and albumin and hence, lower cortisol measurements.
2.2 We suggest the low-dose (1 μg) corticotropin test for diagnosis of PAI only when the substance itself is in short supply. (2|⊕⊕○○)
Evidence
Although the stimulated increase of cortisol after 30 or 60 minutes in healthy individuals is comparable for both low-dose (1 μg) and high-dose (250 μg) corticotropin tests, resulting in a lower cutoff threshold (mean −2SD) of 500 nmol/L (18 μg/dL) (46, 53), the stimulated serum cortisol concentrations differ considerably at 60 minutes between both conditions, with a further rise in the high-dose test and a decrease of cortisol in the low-dose test (54, 55). Cosyntropin-stimulated cortisol levels are slightly higher at 60 vs 30 minutes; however, the 30-minute time point has been validated against the insulin tolerance test (56). Further research is needed to determine whether the traditionally used 60-minute cortisol level is in fact more specific for diagnosis of adrenal insufficiency. Although the low-dose and high-dose corticotropin stimulation tests yield comparable results in the diagnostic workup of PAI and the low-dose test adds no further sensitivity or specificity over the high-dose test (47, 57), evidence has been presented that the low-dose corticotropin stimulation test provides higher sensitivity in the diagnosis of secondary adrenal insufficiency than the high-dose test (18, 29). Although similar arguments for the utility of the low-dose test have been proposed for other special diagnostic challenges such as the detection of adrenal insufficiency in critically ill patients (critical illness-related corticosteroid insufficiency), more evidence is needed to support such a formal recommendation (47, 58, 59).
Thus, for practical reasons, the low-dose test may be applicable to situations when corticotropin is in short supply or for other structural or economic reasons. Although dilution and storage of the lower concentrated solution may be regarded as critical, it is worth noting that diluted tetracosactide (in 0.9% saline at 1 μg/mL) has been reported to be stable at 2–8°C for up to 60 days (60).
Technical remarks
The diagnostic preparation of cosyntropin is supplied in ampules containing 250 μg, and injection of this amount yields a very high stimulus for the adrenal cortex, resulting in reproducible and valid test results. This test is also usually well tolerated by the patient. The low-dose (1 μg) corticotropin test requires dilution of the supplied corticotropin to the required dose, which can introduce dosing errors and sources of contamination into the diagnostic procedure.
2.3 If a corticotropin stimulation test is not feasible, we suggest using a morning cortisol <140 nmol/L (5 μg/dL) in combination with ACTH as a preliminary test suggestive of adrenal insufficiency (until confirmatory testing with corticotropin stimulation is available). (2|⊕○○○)
Evidence
This suggestion is only applicable in acute situations when waiting for the corticotropin stimulation would unnecessarily delay appropriate therapy. It should not be used for community-based screening for PAI. In the absence of exogenous glucocorticoids, a cutoff threshold for basal cortisol concentrations of <140 nmol/L (5 μg/dL) drawn in the morning (6 to 10 am) is suggestive of adrenal insufficiency (18). Unfortunately, most of the primary reports detailing this cutoff value are not based on subjects with PAI (23, 24, 61–64). In addition, the cortisol of 140 nmol/L (5 μg/dL) is typically below or near the lower limit of the range of normal subjects (collected between 6 and 10 am) in reference populations for assays in contemporary clinical laboratories: 113–131 nmol/L (4.1–4.7 μg/dL) (44, 65). As mentioned in Section 2.1, the variation between different cortisol methods will affect the actual cut-point used in clinical practice, and this should be determined in conjunction with the laboratory providing the assay.
It should be noted that the choice of what morning cortisol concentration to utilize to rule out adrenal insufficiency (100% sensitivity) is controversial, with studies arguing levels from >285 nmol/L (10.3 μg/dL) (23) to >480 nmol/L (17 μg/dL) (62). There is no evidence to support the use of random cortisol to rule out adrenal insufficiency.
2.4 We recommend measurement of plasma ACTH to establish PAI. The sample can be obtained at the same time as the baseline sample in the corticotropin test or paired with the morning cortisol sample. In patients with confirmed cortisol deficiency, a plasma ACTH >2-fold the upper limit of the reference range is consistent with PAI. (1|⊕⊕⊕○)
Evidence
A plasma ACTH concentration exceeding 300 ng/L (66 pmol/L) provides maximum stimulation of glucocorticoid synthesis (19), and accordingly, a low cortisol concentration (<140 nmol/L [<5 μg/dL]) found in combination with an elevated concentration of ACTH indicates the inability of the adrenal cortex to respond to ACTH stimulation and is highly predictive for PAI (19, 20, 66, 67). An elevated ACTH concentration in the presence of a cortisol in the normal range can be the first sign of early-stage PAI (68). The challenge with setting a specific cut-point for ACTH levels suggesting PAI is highly influenced by analytical bias demonstrated in ACTH assays (69, 70). Only two studies have reported the ACTH range for PAI at diagnosis with a control reference population, and in these studies the ACTH was typically grossly elevated in PAI. Thus, we have recommended the 2 × the upper limit of the reference interval, but in some cases of PAI it would be only above the quoted reference interval but not >2 × the upper limit of the reference interval (19, 20).
2.5 We recommend the simultaneous measurement of plasma renin and aldosterone in PAI to determine the presence of mineralocorticoid deficiency. (1|⊕⊕⊕○)
Evidence
Determination of renin and aldosterone can be of diagnostic value because in the early phase of evolving PAI, mineralocorticoid deficiency may predominate (71) and may be the only sign (72). Thus, an elevated plasma renin activity or concentration in combination with an (inappropriately) normal or low serum aldosterone concentration is suggestive of PAI (38, 71–74). Both renin and aldosterone measurements have a number of important challenges from the laboratory perspective and should be interpreted based on the reference intervals provided on the report (75). However, in some cases of PAI, eg, in familial glucocorticoid deficiency or patients with milder mutations causing CAH, adrenal mineralocorticoid production may not be compromised.
2.6 We suggest that the etiology of PAI should be determined in all patients with confirmed disease. (For diagnostic workup, see Table 2 and Figure 1) (Ungraded best practice recommendation)
Evidence
When PAI has been confirmed at the hormonal level, it is important for therapeutic reasons to identify the cause of the disease (Figure 1 and Table 2). Autoimmune adrenalitis is the most common cause, accounting for the vast majority of adult cases, and screening for specific autoantibodies against CYP21A2 and for other associated autoimmune diseases is important, keeping in mind that the laboratory tests for the autoantibodies are not standardized and are subject to wide between-method variation (7, 76–79). Finding CYP21A2 autoantibodies, which can be present several years before biochemical and clinical evidence of PAI, strengthens the suspicion that overt PAI is likely to develop. Following CYP21A2 autoantibody-positive individuals revealed that about 30% progressed to overt PAI during a 5-year follow-up (72).
In children with autoimmune PAI, autoimmune polyendocrine syndrome (APS)-1 should be considered with evaluation for hypoparathyroidism and mucocutaneous candidiasis and measurement of antibodies to interferon ω or α that have a high diagnostic sensitivity and specificity (80). Young males and males without autoantibodies should be screened for adrenoleukodystrophy by measuring very-long chain fatty acids (81, 82). Adrenal insufficiency may be the only presenting sign of adrenoleukodystrophy, which most often occurs in boys between 2 and 10 years of age. In CYP21A2 autoantibody-negative individuals with PAI of unknown etiology, we suggest a computer tomography (CT) scan of the adrenals to identify infectious diseases like tuberculosis and tumors. CT scanning for these conditions is generally not specific for the infiltrative disorder, and not all patients with infiltrative adrenal conditions such as tuberculosis causing PAI have enlarged adrenals. Genetic diseases in which ACTH is chronically elevated result in bilateral adrenal enlargement and should be considered in select cases. A variety of rare conditions may require additional diagnostic measures like serological or microbiological testing (12, 83). The nonautoimmune cases of PAI are more frequently seen among children and the elderly. CAH due to 21-hydroxylase deficiency is the most common cause of adrenal insufficiency in infancy (14).
3.0 Treatment of primary adrenal insufficiency in adults
Glucocorticoid replacement regimen
3.1 We recommend glucocorticoid therapy in all patients with confirmed PAI. (1|⊕⊕⊕⊕)
3.2 We suggest using hydrocortisone (15–25 mg) or cortisone acetate (20–35 mg) in two or three divided oral doses per day; the highest dose should be given in the morning at awakening, the next either in the early afternoon (2 h after lunch; two-dose regimen) or at lunch and afternoon (three-dose regimen). Higher frequency regimens and size-based dosing may be beneficial in individual cases. (2|⊕⊕○○)
3.3 As an alternative to hydrocortisone, we suggest using prednisolone (3–5 mg/d), administered orally once or twice daily, especially in patients with reduced compliance. (2|⊕○○○)
3.4 We suggest against using dexamethasone for the treatment of PAI because of risk of Cushingoid side effects due to difficulties in dose titration. (2|⊕⊕○○)
3.5 We suggest monitoring glucocorticoid replacement using clinical assessment including body weight, postural blood pressure, energy levels, signs of frank glucocorticoid excess. (2|⊕⊕⊕○)
3.6 We suggest against hormonal monitoring of glucocorticoid replacement and to adjust treatment only based on clinical response. (2|⊕⊕⊕○)
Evidence
Glucocorticoids are secreted in a pulsatile and circadian rhythm, with the highest peak in the morning, with low levels in the evening, reaching a nadir around midnight (84). Mean cortisol production rates are influenced by age and body composition and have been reported to be about 5–8 mg/m2/d (85–88), which is equivalent to an oral replacement with 15–25 mg/d (with a tendency toward the lower margin to avoid overtreatment) of hydrocortisone or 20–35 mg of cortisone acetate in adults. In most industrialized countries, hydrocortisone is the preferred pharmacological replacement agent, but cortisone acetate is also in widespread use. In a number of countries, only prednisolone is available.
Hydrocortisone and prednisolone are active glucocorticoids, whereas cortisone acetate and prednisone require activation via hepatic 11β-hydroxysteroid dehydrogenase type 1 activity before exerting biological activity. Replacement with inactive precursor glucocorticoids may result in broader interindividual variability of pharmacokinetic parameters (89), but this has not been studied systematically.
Because of the short plasma half-life of hydrocortisone (approximately 90 min) (90), multiple dosing is recommended to mimic physiological conditions. The first and largest dose is suggested to be given upon awakening, the second dose after lunch, and, in case of a three-dose regimen, the last and smallest dose not later than 4–6 hours before bedtime. The rationale for this regimen is to try to mimic the circadian rhythm and also to avoid high doses in the evening, which may compromise sleep and insulin sensitivity (91, 92).
Only small studies have compared dosing regimens. Using the fluctuating normal range of cortisol throughout the 24-hour day as a goal, Peacey et al (93) and Howlett (94) independently recommended 10 mg hydrocortisone on awakening, 5 mg at lunchtime, and 5 mg in the early evening. Some patients appear to require higher glucocorticoid doses, but these must be used with caution. In single-dose morning studies of adrenal insufficiency patients, hydrocortisone adjusted by body surface area (5.5 mg/m2) or by weight (0.12 mg/kg) produced integrated cortisol levels over 6 hours reliably within the healthy control 95% confidence intervals, whereas fixed dosing at 10 mg hydrocortisone did not (95). Hence, dose adjustment by weight or body surface area may produce more physiological cortisol levels in PAI patients than fixed dose regimens. Laureti et al (96) and Barbetta et al (97) found that thrice-daily cortisone acetate lowered ACTH levels and gave 24-hour cortisol curves more similar to the endogenous cortisol rhythm compared with a two-dose regimen. One double-blind, randomized, crossover study evaluating two-dose vs four-dose hydrocortisone treatments (98) concluded that cortisol pharmacokinetics were more physiological on the four-dose regimen; surprisingly, participating patients preferred this regimen. Conversely, Alonso et al (99) found that HRQoL scores were similar and on some parameters worse on thrice-daily compared with twice-daily hydrocortisone. Taken together, because few and mostly underpowered short-term studies have been performed, it is difficult to make strong global recommendations on these different regimens. Based on prevailing evidence and clinical experience, three or four daily doses give more physiological cortisol profiles that may be beneficial to counter long-term complications of glucocorticoid replacement. Weight-adjusted dosing will increase the chance of obtaining a cortisol value within the reference range. However, outcome studies confirming this hypothesis are lacking, and less-frequent dosing may be associated with better compliance (100).
Rigorous pharmacokinetic studies have only compared hydrocortisone vs cortisone acetate in adrenal insufficiency. The absorption curve of cortisone acetate is less steep and is delayed compared with that of hydrocortisone (89, 101), which might be favorable considering the short half-life of hydrocortisone. Although clinical experience supports the recommendation of both drugs, individual patients may prefer one over the other.
When PAI patients fail to recover in terms of HRQoL and working capacity or have difficulty adhering to a multiple-dose regimen, prednisolone, 3–5 mg/d administered in one or two doses, can be prescribed. Retrospective studies of patients taking higher doses of glucocorticoids in some cases, including prednisolone or dexamethasone, appear to show a tendency to adverse metabolic consequences including weight gain, dyslipidemia, and diabetes mellitus (102). However, prospective studies comparing the safety and efficacy of prednisolone and hydrocortisone over time are not available. It is the experience of some physicians that higher doses of prednisolone achieve good results is some patients. Dexamethasone should be avoided because Cushingoid side effects frequently appear (103). Alternatively, a newly marketed dual-release hydrocortisone preparation can be administered once daily (104). However, whereas this dual-release hydrocortisone preparation was shown to slightly reduce blood pressure and HbA1c (104), such results may not always be desirable in patients with PAI. Moreover, such variability can reflect differences in glucocorticoid bioavailability between the dual-release formulation and the hydrocortisone control dosing regimen. Other slow-release preparations of hydrocortisone are in clinical development (see Perspectives and Demand for Future Research below). Additional, preferably double-blind, studies are needed to fully evaluate possible advantages of these preparations over current hydrocortisone and cortisone acetate regimens.
Monitoring glucocorticoid replacement relies primarily on clinical assessment. Symptoms and signs of over-replacement are weight gain, insomnia, and peripheral edema. Insufficient dosing is characterized by nausea, poor appetite, weight loss, lethargy, and hyperpigmentation. Detailed questioning about the patient's daily habits, working patterns (eg, shift work), general feelings of energy, mental concentration, daytime somnolence, and dips in energy can help fine-tune when tablets should be taken, how often, and at what dose. Compliance and use of extra doses should be mapped. In cases when malabsorption is suspected, serum or salivary cortisol day curve monitoring may be useful to guide dosing.
Measurement of plasma ACTH to guide glucocorticoid replacement doses is not recommended because patients who receive appropriate replacement often have elevated ACTH levels, due to disturbance of the normal close relationship between ACTH and cortisol secretion and negative feedback. Use of ACTH levels to adjust glucocorticoid replacement is clinically known to lead to over-replacement.
Mineralocorticoid replacement in PAI
3.7 We recommend that all patients with confirmed aldosterone deficiency receive mineralocorticoid replacement with fludrocortisone (starting dose, 50–100 μg in adults) and not restrict their salt intake. (1|⊕⊕⊕⊕)
3.8 We recommend monitoring mineralocorticoid replacement primarily based on clinical assessment (salt craving, postural hypotension, or edema), and blood electrolyte measurements. (1|⊕⊕⊕○)
3.9 In patients who develop hypertension while receiving fludrocortisone, we suggest reducing the dose of fludrocortisone. (2|⊕○○○)
3.10 If blood pressure remains uncontrolled, we suggest initiating antihypertensive treatment and continuing fludrocortisone. (2|⊕○○○)
Evidence
Mineralocorticoids are vital for maintaining water and electrolyte homeostasis, and thereby blood pressure. The synthetic mineralocorticoid 9α-fludrocortisone is used as replacement therapy, but its use in PAI has not been studied systematically. Fludrocortisone is routinely taken once daily in the morning, the rationale being that aldosterone level is highest at this time because it follows a circadian rhythm similar to cortisol (105). The fludrocortisone dose is related to individual fluid and electrolyte intake and losses. A daily dose of 0.05–0.2 mg is usually sufficient in adults and adolescents with PAI. In newborns and children, mineralocorticoid sensitivity is lower, thereby usually requiring higher fludrocortisone doses compared to adults (106). Temporary dose increments of 50–100% or increased salt intake can be recommended in a hot climate and conditions that promote excessive sweating. In addition, the patients should be advised not to restrict their salt intake. Patients on prednisolone may require more fludrocortisone than those on hydrocortisone because prednisolone has less mineralocorticoid activity. Dexamethasone does not exert any mineralocorticoid activity.
Mineralocorticoid replacement is assessed clinically by inquiring about salt craving or light-headedness, measuring blood pressure in the sitting and standing position, and identifying the presence of peripheral edema, although the latter is of low sensitivity. General well-being, electrolytes within the normal range, and normal blood pressure without evidence of postural hypotension indicate adequate mineralocorticoid replacement. Furthermore, plasma renin activity in the upper reference range has been found to be a useful marker for a correct mineralocorticoid dose (38, 107, 108). Licorice and grapefruit juice potentiate the mineralocorticoid effect of hydrocortisone and should be avoided (109). Phenytoin has been reported to increase fludrocortisone metabolism, leading to a need for higher replacement (110).
Primary hypertension may also be present in PAI (111). Assessment of the hypertensive PAI patient should involve an evaluation of not only the fludrocortisone dose but also the glucocorticoid dose because overtreatment with either preparation can lead to hypertension. If hypertension prevails after adjustment and the patient is euvolemic, angiotensin II receptor blockers or angiotensin-converting enzyme blockers may be used to counter the vasoconstrictive effects of elevated angiotensin II (112). Second-line treatment can include a dihydropyridine calcium blocker. Diuretics should be avoided. Aldosterone receptor blockers such as spironolactone and eplerenone are contraindicated.
Dehydroepiandrosterone replacement
3.11 We suggest a trial of DHEA replacement in women with PAI and low libido, depressive symptoms, and/or low energy levels despite otherwise optimized glucocorticoid and mineralocorticoid replacement. (2|⊕⊕○○)
3.12 We suggest an initial period of 6 months of DHEA replacement. If the patient does not report a sustained, beneficial effect of replacement after 6 months, the DHEA should be discontinued. (2|⊕⊕○○)
3.13 We suggest monitoring DHEA replacement by measuring morning serum DHEAS levels (aiming at the midnormal range) before the intake of the daily DHEA replacement dose. (2|⊕⊕○○)
Evidence
In women, adrenal production of the androgen precursors DHEA and androstenedione is a major source of androgen production. Consequently, adrenal insufficiency is frequently associated with androgen deficiency in female patients. Serum DHEAS concentrations physiologically peak between ages 20 and 30 years, followed by a gradual decline that is independent of menopause (113). The adrenal androgen precursor DHEA is activated to sex steroids in a wide variety of peripheral tissues and in the gonads, but it has also been shown to have neurosteroidal properties with potential antidepressive action in the brain. DHEA replacement in PAI with a single oral dose has been shown to restore circulating levels of androgen precursors and androgens back to the normal range (3, 114). In addition, some (but not all) studies have shown that DHEA replacement in adrenal insufficiency may improve HRQoL and mood, with reduced depression and anxiety scores (114–116). A systematic review and meta-analysis of randomized placebo controls of DHEA treatment have not shown any substantial clinical benefit, suggesting that the current evidence is insufficient to support routine use of DHEA in women with adrenal insufficiency (117).
A number of studies have documented positive effects on libido, and DHEA replacement restores pubarche in adolescent adrenal insufficiency patients (118). Studies on long-term outcomes of chronic DHEA supplementation in women with PAI are lacking and are unlikely to be available soon due to a lack of commercial interest and the ready availability of DHEA over the counter or through internet orders.
DHEA replacement (25–50 mg as a single oral dose in the morning) may be considered in premenopausal women with PAI and in the presence of reduced or absent libido, depression, anxiety, and reduced energy levels despite otherwise optimized glucocorticoid and mineralocorticoid replacement. In addition to looking at clinical efficacy and potential side effects, the appropriateness of the DHEA dose during treatment should be monitored by a morning blood sample for serum DHEAS, before taking the next dose of DHEA, aiming at the midrange of a premenopausal female reference cohort.
Treatment during pregnancy
3.14 We suggest that pregnant patients with PAI be monitored for clinical symptoms and signs of glucocorticoid over- and under-replacement (eg, normal weight gain, fatigue, postural hypotension or hypertension, hyperglycemia), with at least one review per trimester. (Ungraded best practice statement)
3.15 We suggest that, based on the individual clinical course, an increase in hydrocortisone dose should be implemented, in particular during the third trimester. (Ungraded best practice statement)
3.16 In pregnant women with PAI, we suggest using hydrocortisone over cortisone acetate, prednisolone, or prednisone (2|⊕⊕○○) and recommend against using dexamethasone because it is not inactivated in the placenta. (1|⊕⊕○○)
3.17 We recommend hydrocortisone stress dosing during the active phase of labor, similar to that used in major surgical stress. (1|⊕⊕○○)
Evidence
During normal pregnancy, circulating cortisol concentrations are increased 2- to 3-fold, with a continuous increase from the first trimester onward due to increases in CBG levels (40, 42). From week 22 of gestation onward, free cortisol levels also increase significantly, with a further rise immediately preterm due to a fall in CBG (42, 119); cortisol levels return to normal after delivery (40). Adrenal crisis due to insufficient glucocorticoid dose adjustment during pregnancy has been reported (120). Although little evidence exists on the exact regimen of optimized glucocorticoid replacement in pregnancy, one common approach is to increase hydrocortisone dose by 20–40% from the 24th week onward to reflect the physiological increase in free cortisol. Glucocorticoid preparations that can be used in pregnancy are hydrocortisone, cortisone acetate, prednisolone, and prednisone; dexamethasone is usually contraindicated because it is not inactivated by placental 11β-hydroxysteroid dehydrogenase type 2 and thus crosses the placenta to the fetus.
The diagnosis of new-onset adrenal insufficiency in pregnancy is challenging because symptoms are nonspecific and often are not different from those commonly present in pregnancy itself, such as fatigue, nausea, and vomiting. The cosyntropin stimulation test is the test of choice in pregnant women if adrenal insufficiency is suspected. In a small cohort of healthy pregnant women, the peak total cortisol response after ACTH injection was significantly higher in comparison to the nonpregnant state (median, 1000 nmol/L [37 μg/dL]) in the second and third trimesters, whereas their responses returned to prepregnancy levels during the postpartum period (median, 700 nmol/L [26 μg/dL]) (41). Thus, it has been suggested to use higher diagnostic cortisol cutoffs of 700 nmol/L (25 μg/dL), 800 nmol/L (29 μg/dL), and 900 nmol/L (32 μg/dL) for the first, second, and third trimesters, respectively (40).
Mineralocorticoid requirements during pregnancy are more difficult to assess, again due to nonspecific symptoms overlapping with those observed in physiological pregnancy, such as edema or postural hypotension. Sodium and potassium can be monitored in blood and urine, whereas plasma renin physiologically increases during pregnancy and therefore cannot be used for monitoring purposes. There is some evidence that aldosterone increases during normal pregnancy (41) and serum progesterone steadily increases throughout pregnancy, exerting some anti-mineralocorticoid effect; hence, fludrocortisone dose adjustments are sometimes required (121). However, in most cases this will be covered by the increase in glucocorticoid replacement dose in the later stages of pregnancy.
A hydrocortisone dose equivalent to that used for major surgical stress should be initiated at the onset of active labor (cervix dilation > 4 cm and/or contractions every 5 min for the last hour) with a bolus injection of 100 mg hydrocortisone iv followed by continuous infusion of 200 mg hydrocortisone/24 hours (40, 122). After delivery, hydrocortisone can be quickly tapered back to prepregnancy doses.
Treatment and monitoring during childhood
3.18 In children with PAI, we suggest treatment with hydrocortisone in three or four divided doses (total starting daily dose of 8 mg/m2 body surface area) over other types of glucocorticoid replacement therapies, with doses adjusted according to individual need. (2|⊕⊕○○)
3.19 In children with PAI, we suggest avoiding synthetic, long-acting glucocorticoids (eg, prednisolone, dexamethasone). (2|⊕⊕○○)
3.20 We suggest monitoring glucocorticoid replacement by clinical assessment, including growth velocity, body weight, blood pressure, and energy levels. (Ungraded best practice statement)
3.21 In children with PAI and confirmed aldosterone deficiency, we recommend treatment with fludrocortisone (starting dosage, 100 μg/d). For infants, we recommend sodium chloride supplements in the newborn period and up to the age of 12 months. (1|⊕⊕○○)
Evidence
There are no published randomized, controlled trials of various treatment regimens for PAI in children. Treatment in children is aimed at controlling the symptoms of adrenal insufficiency with a dose that allows adequate growth and pubertal development. No data are available to compare the long-term effects of various formulations of glucocorticoid. However, hydrocortisone has a short half-life and is easier to titrate in children. This minimizes the adverse side effects compared with the more potent longer-acting glucocorticoids. Most data available on the treatment of children with PAI are in children with adrenal insufficiency due to CAH. However, patients with adrenal insufficiency due to CAH have the additional risk of hyperandrogenism with undertreatment resulting in the need for higher glucocorticoid therapy; this risk is not present in PAI due to other causes. Estimates of normal cortisol secretion rate can be used to determine an initial hydrocortisone dose (85). The daily dose is usually divided into two or preferably three doses, with a typical daily dose of about 8 mg/m2. Cortisone acetate may be used instead of hydrocortisone; however, caution should be used because 11β-hydroxysteroid dehydrogenase type 1 activity is variable in childhood (123), and it is uncertain whether the hydrocortisone dose equivalency used in adults applies in children.
Overtreatment must be avoided, and daily dose should be adjusted depending on clinical status and growth. The glucocorticoid dose will need to be increased to account for a child's increasing body surface area. Excessive weight gain with decreased height velocity or other symptoms or signs of Cushing syndrome indicate excessive glucocorticoid replacement. In patients with CAH, glucocorticoid dosages exceeding 20 mg/m2/d in infants and 15 to 17 mg/m2/d in adolescents have been shown to result in loss of height and shorter adult stature (124, 125). Growth suppression at lower dosages is also possible because dosages above 8 mg/m2/d may exceed the physiological range; thus, close follow-up of growth and weight velocities and general clinical well-being are most important for dose adjustments. Measurement of plasma ACTH is typically above the normal range and is not useful for routine monitoring. Inadequate weight gain, fatigue, anorexia, and hyperpigmentation suggest the need for increased medication dose.
Children with PAI require mineralocorticoid replacement with fludrocortisone. A typical daily dose is 100 μg. The mineralocorticoid dose does not require adjustment by body surface area and often remains the same throughout life. However, infants, especially in the first 6 months of life, require sodium chloride supplementation of 1 to 2 g/d (17 to 34 mmol/d), divided in several feedings due to mineralocorticoid resistance in the immature infant kidney and the relatively low sodium content of formula and breast milk (14).
Signs and symptoms of inadequate mineralocorticoid replacement include poor weight gain, salt craving, dehydration, hyponatremia with hyperkalemia, and elevated plasma renin activity or concentration. Excessive mineralocorticoid replacement results in hypertension and suppressed plasma renin. Monitoring of blood pressure should be performed routinely. Assessment of plasma renin should be done periodically in response to changes in clinical status or if compliance is in question.
Infants require frequent assessment and should be evaluated at a minimum every 3 to 4 months to assess growth, blood pressure, and general well-being. Over the first months of life, sensitivity to mineralocorticoid increases; thus, it is especially important to monitor blood pressure during the first year of life.
4.0 Management and prevention of adrenal crisis in patients with PAI
4.1 We recommend that patients with suspected adrenal crisis should be treated with an immediate parenteral injection of 100 mg (50 mg/m2 for children) hydrocortisone, followed by appropriate fluid resuscitation and 200 mg (50–100 mg/m2 for children) of hydrocortisone/24 hours (via continuous iv therapy or 6 hourly injection); age- and body surface-appropriate dosing is required in children (Table 3). (1|⊕⊕⊕○)
4.2 If hydrocortisone is unavailable, we suggest prednisolone as an alternative. Dexamethasone is the least-preferred alternative and should only be given if no other glucocorticoid is available. (2|⊕⊕○○)
Evidence
Patients with PAI are at risk of life-threatening adrenal crises. Adrenal crisis occurs when the adrenal glands cannot produce sufficient cortisol in response to an increased need. The major clinical features of adrenal crisis are hypotension and volume depletion. Combined glucocorticoid and mineralocorticoid deficiency results in urinary sodium loss, hyponatremia, hyperkalemia, increased serum urea, and hypoglycemia; the latter is most relevant in children, but rarely occurs in adults.
Under-dosing of glucocorticoids in an adrenal crisis is potentially hazardous. However, there are no systematic dose-response-studies, and therefore the glucocorticoid doses recommended for the treatment of adrenal crisis are largely on an empiric basis.
Adrenal crisis in patients with known PAI is best prevented by patient education and increasing the glucocorticoid dosage in situations of stressors known to increase cortisol requirements (126).
In a retrospective analysis of 444 patients with adrenal insufficiency, the frequency of adrenal crises in adult patients with PAI was 6.6 adrenal crises/100 patient years (120). The main precipitating factors were gastrointestinal diseases (32.6%) and other infectious disease (24.3%). Similarly, a postal survey of over 1000 patients with PAI from four countries (United Kingdom, Canada, Australia, New Zealand) found 8% of patients experienced an adrenal crisis annually, and gastrointestinal infection and flu-like illnesses were the two most common triggers (127). In a first prospective study comprising 768 patient years, 8.3 crises/100 patient years were reported (128). Based on these data, about one in 12 patients will experience a life-threatening crisis in the coming year. Furthermore, in this first prospective study, a mortality rate from crisis of 0.5/100 patient years was reported. Adrenal crisis is also a common occurrence in patients with CAH, although detailed data are only available from pediatric patients. In a cross-sectional questionnaire-based study of 122 patients with PAI due to classic CAH, the reported frequency of adrenal crises was 5.8 adrenal crises/100 patient years (4.9 adrenal crises/100 patient years after correction for a neonatal salt-wasting crisis) (129). An age-related pattern emerged; respiratory infections were the main trigger in early childhood, whereas gastrointestinal infections were the main cause at older ages. The median time from recognition of the first symptoms to overt adrenal crisis was 1 day.
The addition of co-medication that alters cortisol clearance could also trigger an adrenal crisis, and consideration of a glucocorticoid dose increase and re-evaluation should occur. Initiation of T4 replacement may induce adrenal crisis due to increased cortisol metabolism (130). Medications that induce the drug-metabolizing enzyme CYP3A4 (eg, carbamazepine, mitotane, St John's wort) also increase cortisol clearance, necessitating a higher replacement dose (131). Similarly, glucocorticoid replacement with dexamethasone without concurrent fludrocortisone can trigger an adrenal crisis because dexamethasone has no mineralocorticoid activity. Based on traditional studies, 40 mg hydrocortisone are regarded as equivalent to 100 μg fludrocortisone (132–134), suggesting that higher hydrocortisone doses are likely to provide replacement levels of mineralocorticoid activity.
High-intensity exercise for 20 minutes did not require additional hydrocortisone in CAH patients (135). Additional hydrocortisone (5 to 10 mg) has been discussed for prolonged intensive fitness training in patients with PAI (136). However, there is no evidence for a general recommendation.
Values and preferences
The proposed glucocorticoid regimen in the management of adrenal crisis places a higher value on the prevention of underdosage than on reducing potential negative effects of short-term overdosage.
4.3 For the prevention of adrenal crisis, we suggest adjusting glucocorticoid dose according to severity of illness or magnitude of the stressor. (2|⊕⊕○○)
Evidence
Studies provide evidence for an endogenous increase in cortisol during anesthesia, surgery, trauma, and critical illness, with great interindividual variation (137–141). Enhanced glucocorticoid secretion in critical illness is considered necessary to prevent defense mechanisms (eg, cytokine release) from overshooting with concomitant detrimental toxicity (142, 143). There are no randomized controlled studies evaluating glucocorticoid dose requirements in patients with PAI during times of increased cortisol need. Glucocorticoid dose is typically based on the severity and duration of the stressor. Traditionally, it is estimated that adults secrete 75–100 mg of cortisol/d in response to major surgery and 50 mg/d in response to minor surgery (144). The concept for giving these high doses is not to mimic median values of normal subjects during surgery, but also to cover unexpected additional needs if complications occur. In addition, harm from these doses has not been shown, and direct studies indicating that lower doses are safe do not exist. In a review of perioperative adrenal insufficiency in glucocorticoid-treated patients, cortisol secretion in the first 24 hours after surgery rarely exceeded 200 mg, and the secretion rate correlated with the duration and extent of surgery (144). Lower doses of hydrocortisone (25–75 mg/24 h) for surgical stress have been advocated in patients with secondary adrenal insufficiency (144, 145). This has not been studied in patients with PAI.
Reduced cortisol metabolism has been shown during critical illness (146). In a study of 158 intensive care unit patients and 64 matched controls, total and free circulating cortisol levels were higher in the patients than in controls due to a reduction in cortisol clearance of more than 50% and reduced inactivation of cortisol to cortisone. The implications of this reduced cortisol metabolism on glucocorticoid dosing during critical illness in patients with PAI has not been studied.
During febrile illness, the glucocorticoid dose is typically taken orally at double or triple the usual daily dose, until recovery, usually of 2- to 3-day duration (Table 3). If the patient is unable to tolerate oral medication due to vomiting or trauma, early parenteral (iv, im, or sc) injection of 100 mg hydrocortisone is indicated. Mineralocorticoid replacement is not required if the hydrocortisone dose exceeds 50 mg/24 hours.
In a single-center, open-label, randomized, crossover study of 12 patients with PAI, sc and im injection of hydrocortisone had similar pharmacokinetics; however, sc injection required two single injections (vs one im injection) and had slower time to reach a cortisol level >1000 nmol/L (>36 μg/dL) (22 vs 11 min) (147). Subcutaneous self-injection was the route of administration preferred by the patients, and the time delay in reaching peak cortisol concentrations compares favorably with the time from contacting emergency help until eventual glucocorticoid administration by health professionals (148). Rectal suppositories (prednisolone 100 mg suppository) or enemas (prednisolone 20 mg/100 mL or hydrocortisone acetate enema 10%) have been successfully used but should not be used with diarrhea; they have not been extensively studied and are not widely available (eg, not available in the United States).
Assumed values and preferences
The proposed recommendation places a higher value on prevention of adrenal crisis than on reducing the potential negative effect of short-term overtreatment.
Technical remarks
See Table 3 for details. Relevant conditions include surgery, trauma, critical illness, and delivery.
4.4 We suggest patient education concerning glucocorticoid adjustments in stressful events and adrenal crisis-prevention strategies including parenteral self- or lay-administration of emergency glucocorticoids. (Ungraded best practice statement)
4.5 We recommend that all patients should be equipped with a steroid emergency card and medical alert identification to inform health personnel of the need for increased glucocorticoid doses to avert or treat adrenal crisis and the need of immediate parenteral steroid treatment in the event of an emergency. (Ungraded best practice statement)
4.6 We recommend that every patient should be equipped with a glucocorticoid injection kit for emergency use and be educated on how to use it. (Ungraded best practice statement)
Evidence
Mortality of patients with PAI was increased in some (149, 150), but not all studies (7). However, in all these reports, adrenal crisis was a significant cause of death. Thus, patient education for prevention of adrenal crisis and the use of emergency glucocorticoids is greatly needed (151). However, it has been shown that a high percentage of patients (46%) were not sufficiently skilled in steroid management with physical stress (152). Thus, repeated education efforts should be part of outpatient visits. A large international survey found that approximately one-third of all medical emergencies in patients with PAI occurred outside the home. Only 12% of patients with an adrenal crisis in the previous year gave themselves an injection; over two-thirds relied on medical personnel for their first line of treatment (127). In a survey study of 26 patients with Addison's disease in the United Kingdom (153), only two patients could self-administer parenteral hydrocortisone, and 10 responders claimed never to have received injection teaching, although instruction was provided as unit policy. In a survey of 60 caregivers of children with CAH in the United States (154), 70% had received written guidelines regarding stress dosing, and half received injection teaching. In a survey of 254 patients with PAI in Germany, only 63% felt well informed about stress dosing (120).
Still, many patients meet physicians who are unaware of the need for immediate treatment of an adrenal crisis. A steroid card can help overcome this hurdle. Recently, a new Swedish steroid card was introduced, with simple instructions in Swedish and English, and several European countries are now introducing the same card with the national language on one side and English on the other (155). A number of different steroid cards are in circulation (for examples, see http://www.ese-hormones.org/professional/docs/ExistingEmergencyCards.pdf), issued by departments of endocrinology, patient organizations, and the pharmaceutical industry. Such cards should be issued to all patients so as to inform health care providers about the diagnosis of PAI and how to provide treatment for an adrenal crisis; a medical alert bracelet or necklace is also useful. All patients should be provided with injectable hydrocortisone for emergency use. Training in self-injection is likely to lower the threshold for parenteral hydrocortisone use. Rectal suppositories (prednisolone 100 mg) are an alternative for crisis prevention in the absence of diarrhea. Strong recommendations for patient education and emergency care were made based on placing a high value on reducing the morbidity and mortality of adrenal crisis and the knowledge of the existing low awareness levels of patients and health professionals. Measures to prevent an adrenal crisis are summarized in Table 4 (122).
Table 4.
Measures for Prevention of Adrenal Crisis
| Action Point | Intervention |
|---|---|
| Identify and define the problem | Steroid emergency card (check that card is available and up to date) |
| Medical alert bracelet or necklace: “Adrenal insufficiency – needs steroids!” | |
| Educate patient (and partner/parents) | Sick day rule 1: need to double the routine oral glucocorticoid dose when the patient experiences fever or illness requiring bed rest; when requiring antibiotics for an infection; or before a small outpatient procedure (eg, dental work) |
| Sick day rule 2: need to inject a glucocorticoid preparation im or iv in case of severe illness, trauma, persistent vomiting, when fasting for a procedure (colonoscopy!), or during surgical intervention. | |
| 100 mg hydrocortisone iv, im, or sc followed by 200 mg hydrocortisone per continuous iv infusion, alternatively repeated bolus doses (iv or im) every 6 h | |
| Give special attention to: | Explaining the rationale for dose adjustment in stress/sickness |
| Discussing the situations requiring dose adjustment | |
| Discussing symptoms and signs of emergent adrenal crisis | |
| Teaching parenteral self-administration of glucocorticoid preparation | |
| Enforcing the need to go to hospital after emergency injection | |
| Provide patient with: | Sufficient supply of hydrocortisone and fludrocortisone (accounting for possible sick days) |
| Hydrocortisone emergency injection kit prescription (vials of 100 mg hydrocortisone sodium, syringes, needles; alternatively, also hydrocortisone or prednisolone suppositories) | |
| Leaflet with information on adrenal crisis and hospitalization to be shown to health care staff; clearly advise regarding the need to inject 100 mg hydrocortisone immediately iv or im, followed by continuous infusion of 200 mg/24 h | |
| Emergency phone number of endocrine specialist team | |
| Follow-up | Reinforce education and confirm understanding during each follow-up visit (at least annually in a patient without specific problems or recent crises; otherwise, more frequently) |
Adapted from I. Bancos, et al: Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3:216–226 (122), with permission. © Elsevier Limited.
5.0 Additional monitoring requirement
5.1 We suggest that adults and children with PAI be seen by an endocrinologist or a healthcare provider with endocrine expertise at least annually. Infants should be seen at least every 3 to 4 months. (Ungraded best practice statement)
5.2 We suggest that PAI patients be evaluated annually for symptoms and signs of over- and under-replacement. (Ungraded best practice statement)
5.3 We suggest periodic screening for autoimmune diseases known to be more prevalent in PAI patients in whom autoimmune origin of PAI has not been excluded. The optimal frequency of screening is unknown but can be done annually. These conditions include thyroid disease, diabetes mellitus, premature ovarian failure, celiac disease, and autoimmune gastritis with vitamin B12 deficiency. (2|⊕⊕○○)
5.4 We suggest patient education about increasing the dosage of glucocorticoids during intercurrent illness, fever, and stress. This education includes identification of precipitating symptoms and signs and how to act in impending adrenal crisis. (Ungraded best practice statement)
5.5 We suggest genetic counseling for patients with PAI due to monogenic disorders. (Ungraded best practice statement)
Evidence
The goal of treatment and follow-up of patients with PAI is to restore normal well-being, stable weight, normal sexual function, and full professional activity. Regular medical examinations allow evaluation of the physical condition of patients with regard to over- or under-replacement, the correct dosage of the replacement therapy, and HRQoL. Questions on family relations, professional duties, and self-esteem are relevant. Any adrenal crisis should be addressed.
The monitoring of replacement therapy is mainly clinical. Most patients will attain a normal pigmentation on sufficient replacement therapy. Arterial blood pressure should be normal and the weight stable. Orthostatic hypotension indicates insufficient mineralocorticoid therapy or low salt intake.
Routine laboratory analyses should include serum sodium and potassium determinations. Although not recommended for routine monitoring of glucocorticoid replacement, measurement of cortisol can be useful to obtain evidence of adequate cortisol uptake. Drugs that increase the metabolism of cortisol may influence half-life. Plasma renin activity or renin substrate is useful to monitor fludrocortisone replacement, which should aim at the upper normal reference range or a slightly elevated value.
Increased prevalence of other autoimmune disorders may provide a rationale for surveillance, especially autoimmune thyroid disease, which is seen in half of females and 25% of males with PAI (7). Type 1 diabetes is present in 10–15% of patients in Scandinavia (7), but is less frequent in other populations (156–158). Therefore, annual assay of TSH, free T4, and HbA1c can help in the identification and treatment of these conditions. Females should be informed of the risk of premature ovarian insufficiency, which was seen in 8% of females in a national Norwegian survey (7). In an Italian cohort of 258 women with PAI, 20.2% had premature ovarian insufficiency; premature ovarian insufficiency was diagnosed in 20 of 49 (40.8%) with APS-1, six of 18 (33.3%) with APS-4, 26 of 163 (16%) with APS-2, but none of 28 with isolated PAI (159). Additional testing can include measurement of CYP11A1 autoantibodies (160), the presence of which is correlated to premature ovarian insufficiency, although the protein is expressed in all steroidogenic tissues. However, the predictive value of CYP11A1 autoantibodies has not been assessed. Using the less sensitive immunofluorescence assay of steroid-cell autoantibodies, Reato et al (159) found that three of 13 seropositive patients developed premature ovarian insufficiency during a mean observation period of 8 years, indicating that the predictive value is not that high. Unlike premature ovarian insufficiency caused by nonautoimmune conditions, follicular function seems to be retained for up to several years after diagnosis in PAI patients (161). This creates a window of opportunity to sample ovarian tissue for cryopreservation for further in vitro maturation and subsequent in vitro fertilization. Although this approach is currently experimental, further refinement and improved performance of these technologies can be expected for the near to midterm future.
Other testing to be considered during the annual evaluation of patients with PAI is a complete blood count. Vitamin B12 deficiency due to autoimmune gastritis is common (7, 156), and vitamin B12 levels can also be monitored annually. If vitamin B12 deficiency is suspected, further diagnostic workup is warranted (eg, determination of holotranscobalamin, homocysteine, methylmalonic acid, and/or autoantibodies against parietal cells and intrinsic factor). Because the prevalence of celiac disease in PAI is about 5% (156, 162, 163), screening for tissue transglutaminase 2 autoantibodies and total IgA can be done occasionally, even when abdominal symptoms are absent. Vitiligo and alopecia areata are frequent signs and are considered as markers of autoimmunity.
Other less frequent autoimmune disorders have to be taken into account in some patients with suggestive clinical features. Several forms of PAI are familial. Ten percent of Norwegian PAI patients have a relative with autoimmune PAI. PAI in the context of adrenoleukodystrophy, congenital hypogonadotropic hypogonadism, and congenital adrenal hypoplasia follow X-linked inheritance, whereas CAH and APS-1 are autosomal recessive (164).
Perspectives and Demand for Future Research
Diagnostic procedures and treatment strategies for PAI are still far from being optimal. There are a number of unresolved questions regarding the validity of adrenal function tests in PAI. In particular, confounding factors relate to defining adrenal dysfunction during critical illness and monitoring adrenal steroids in relation to underlying diseases and concurrent medications. Interpreting abnormal stress responses in a range of clinical situations will remain challenging until appropriate research can identify factors that are more diagnostic than cortisol. Two current developments may be of benefit. First, salivary cortisol has been investigated in a number of settings and is showing some potential as a biomarker. There are some limitations from the collection and analysis perspective, but these can be dealt with (165). Second, the introduction of more specific diagnostic testing involving liquid chromatography/tandem mass spectrometry (LC-MS/MS) should provide better standardization in the measurement of cortisol. Such methods are relatively free from analytical interferences associated with medications and dietary constituents and are not hampered by the cross-reactivity issues associated with immunoassays. Although isobaric interferences can occur with LC-MS/MS, particularly for some steroids with identical elemental formulae, such sources of interference can be avoided by appropriate chromatographic separation and use of multiple mass transitions for identification. Another major advantage of LC-MS/MS over immunoassays is its ability to quantify in a single analysis multiple steroids, in some methods up to 15 or more, thereby substantially broadening identification of different forms and underlying causes of adrenal insufficiency (166).
Regarding treatment of PAI, there are even more unresolved issues. Despite optimal steroid replacement including glucocorticoids, mineralocorticoids, and androgens as depicted above, a significant number of patients continue to have objective and subjective complaints. This includes symptoms of over- or under-substitution with glucocorticoids, reduced vitality and perception of general health, and reduced physical function, especially in women (3), which translates into reduced working capacity. There are also a number metabolic or cardiovascular complications including hypertension (167).
Current replacement strategies do not restore the physiological feedback regulation of an intact hypothalamic-pituitary-adrenal axis. Circadian and pulsatile hormone secretion is not normalized. There is an impairment of adrenomedullary function including the regulation of catecholamines and neuropeptides (168). This has been shown to correlate with cardiovascular instability, hypoglycemia, and physical activity in patients with PAI due to CAH (169).
Other modified and delayed-release formulations of hydrocortisone in clinical development aim to mimic the cortisol circadian rhythm (170, 171). Dual and slow-release formulations of cortisol may better mimic circadian hormone release (104) but do not mimic the physiological pulsatile release of cortisol. Cortisol replacement by means of continuous sc infusion using insulin pumps provides a similar circadian rhythm, also mimicking the early morning increase in cortisol (172, 173). Subcutaneous infusion utilizing an insulin pump can reconstitute the circadian variation and was associated with improvement of HRQoL in an open crossover study (173). In a smaller double-blind study, no impact on HRQoL was seen (174). Pump treatment is more cumbersome than conventional treatment and should be reserved for patients who encounter major difficulties with conventional treatment. Because the late night nadir and the early morning rise in cortisol is attained, ACTH levels can be used as a biomarker to assess correct dosing (173). Recently, the feasibility of pulsatile sc cortisol replacement was studied in healthy dexamethasone-suppressed individuals (175). There is a great need for large, randomized clinical trials to increase the evidence base on what is the best glucocorticoid replacement therapy.
At diagnosis, most PAI patients retain a certain cortisol production (176). Treatment of newly diagnosed autoimmune PAI patients with rituximab (176) and established PAI patients with depot tetracosactide (177) has demonstrated that regeneration of cortisol production is possible. Further exploration of the regenerative potential of adrenocortical stem cells combined with immunomodulatory treatment to stop the autoimmune destruction could potentially provide the ultimate treatment option in autoimmune PAI.
Gene therapy may potentially restore the defect in certain forms of monogenic PAI (178), and there is one report in the literature on successful adrenal transplantation (179). A great potential may involve novel cell replacement and encapsulation technologies (180). This may potentially allow a restoration of hypothalamic-pituitary-adrenal axis function and a possible cure for PAI. However, to be successful with such an endeavor, a major interdisciplinary effort of physicians, cell biologists, immunologists, and material scientists will be required.
Financial Disclosures of the Task Force*
Stefan R. Bornstein, MD, PhD, (chair)—Financial or Business/Organizational Interests: Novo Nordisk, Boehringer Ingelheim; Significant Financial Interest or Leadership Position: none declared. Bruno Allolio, MD—Financial or Business/Organizational Interests: European Society of Endocrinology; Significant Financial Interest or Leadership Position: Boehringer Ingelheim (Consultant). Wiebke Arlt, MD, DSc, FRCP, FMedSci—Financial or Business/Organizational Interests: European Society of Endocrinology, Society for Endocrinology, Diurnal Limited, Janssen Pharmaceutical, Atterocor; Significant Financial Interest or Leadership Position: none declared. Andreas Barthel, MD, MSc—Financial or Business/Organizational Interests: DGE (German Endocrine Society); Significant Financial Interest or Leadership Position: none declared. Andrew Don-Wauchope, MB, BCh, MD, FRCP Edin, FCPath, FRCPath, FRCPC—Financial or Business/Organizational Interests: Institute for Quality Management in Healthcare, Canadian Society for Clinical Chemistry, American Association of Clinical Chemistry; Significant Financial Interest or Leadership Position: Canadian Association of Medical Biochemists (Past President). Gary D. Hammer, MD, PhD—Financial or Business/Organizational Interests: ISIS Pharmaceutical, Orphagen, HRA Pharma, Embara; Significant Financial Interest or Leadership Position: Atterocor (Founder, Owner, Consultant). Eystein Husebye, MD, PhD—Financial or Business/Organizational Interests: none declared; Significant Financial Interest or Leadership Position: ViroPharma/Shire (Lecturer). Deborah P. Merke, MS, MD—Financial or Business/Organizational Interests: Diurnal Limited; Significant Financial Interest or Leadership Position: none declared. M. Hassan Murad, MD, MPH**—Financial or Business/Organizational Interests: Mayo Clinic, Division of Preventive Medicine; Significant Financial Interest or Leadership Position: none declared. Constantine A. Stratakis, MD, DSc—Financial or Business/Organizational Interests: none declared; Significant Financial Interest or Leadership Position: none declared. David J. Torpy, MBBS, PhD, FRACP—Financial or Business/Organizational Interests: Novartis; Significant Financial Interest or Leadership Position: None declared.
* Financial, business, and organizational disclosures of the Task Force cover the year prior to publication. Disclosures prior to this time period are archived.
Acknowledgments
The authors of the Diagnosis and Treatment of Primary Adrenal Insufficiency Clinical Practice Guideline dedicate the guideline to Professor Dr. Bruno Allolio, who suddenly died on August 16, 2015. Bruno was a highly respected endocrinologist and brilliant expert in the adrenal field. Improving the care of patients diagnosed with adrenal insufficiency was a major driver of his clinical activities. We will miss him very much and will keep him in our memories as an outstanding colleague and friend.
Cosponsoring Associations: European Society of Endocrinology and the American Association for Clinical Chemistry.
** Evidence-based reviews for this guideline were prepared under contract with the Endocrine Society.
Footnotes
- APS
- autoimmune polyendocrine syndrome
- CAH
- congenital adrenal hyperplasia
- CBG
- cortisol-binding globulin
- CT
- computer tomography
- DHEA
- dehydroepiandrosterone
- DHEAS
- DHEA sulfate
- HRQoL
- health-related quality of life
- LC-MS/MS
- liquid chromatography/tandem mass spectrometry
- PAI
- primary adrenal insufficiency.
References
- 1. Addison T. On the Constitutional and Local Effects of Disease of the Supra-renal Capsules. London, UK: Samuel Highley; 1855. [Google Scholar]
- 2. Bleicken B, Hahner S, Ventz M, Quinkler M. Delayed diagnosis of adrenal insufficiency is common: a cross-sectional study in 216 patients. Am J Med Sci. 2010;339:525–531. [DOI] [PubMed] [Google Scholar]
- 3. Løvås K, Loge JH, Husebye ES. Subjective health status in Norwegian patients with Addison's disease. Clin Endocrinol (Oxf). 2002;56:581–588. [DOI] [PubMed] [Google Scholar]
- 4. Hahner S, Allolio B. Therapeutic management of adrenal insufficiency. Best Pract Res Clin Endocrinol Metab. 2009;23:167–179. [DOI] [PubMed] [Google Scholar]
- 5. Reisch N, Arlt W. Fine tuning for quality of life: 21st century approach to treatment of Addison's disease. Endocrinol Metab Clin North Am. 2009;38:407–418, ix–x. [DOI] [PubMed] [Google Scholar]
- 6. Meyer G, Hackemann A, Penna-Martinez M, Badenhoop K. What affects the quality of life in autoimmune Addison's disease? Horm Metab Res. 2013;45:92–95. [DOI] [PubMed] [Google Scholar]
- 7. Erichsen MM, Løvås K, Fougner KJ, et al. Normal overall mortality rate in Addison's disease, but young patients are at risk of premature death. Eur J Endocrinol. 2009;160:233–237. [DOI] [PubMed] [Google Scholar]
- 8. Wallace I, Cunningham S, Lindsay J. The diagnosis and investigation of adrenal insufficiency in adults. Ann Clin Biochem. 2009;46:351–367. [DOI] [PubMed] [Google Scholar]
- 9. Chakera AJ, Vaidya B. Addison disease in adults: diagnosis and management. Am J Med. 2010;123:409–413. [DOI] [PubMed] [Google Scholar]
- 10. Laureti S, Vecchi L, Santeusanio F, Falorni A. Is the prevalence of Addison's disease underestimated? J Clin Endocrinol Metab. 1999;84:1762. [DOI] [PubMed] [Google Scholar]
- 11. Meyer G, Neumann K, Badenhoop K, Linder R. Increasing prevalence of Addison's disease in German females: health insurance data 2008–2012. Eur J Endocrinol. 2014;170:367–373. [DOI] [PubMed] [Google Scholar]
- 12. Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009;360:2328–2339. [DOI] [PubMed] [Google Scholar]
- 13. Grossman AB. Clinical review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010;95:4855–4863. [DOI] [PubMed] [Google Scholar]
- 14. Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swiglo BA, Murad MH, Schünemann HJ, et al. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. 2008;93:666–673. [DOI] [PubMed] [Google Scholar]
- 17. Guyatt GH, Schünemann HJ, Djulbegovic B, Akl EA. Guideline panels should not GRADE good practice statements. J Clin Epidemiol. 2015;68:597–600. [DOI] [PubMed] [Google Scholar]
- 18. Kazlauskaite R, Evans AT, Villabona CV, et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008;93:4245–4253. [DOI] [PubMed] [Google Scholar]
- 19. Oelkers W, Boelke T, Bähr V. Dose-response relationships between plasma adrenocorticotropin (ACTH), cortisol, aldosterone, and 18-hydroxycorticosterone after injection of ACTH-(1–39) or human corticotropin-releasing hormone in man. J Clin Endocrinol Metab. 1988;66:181–186. [DOI] [PubMed] [Google Scholar]
- 20. Lee MK, Vasikaran S, Doery JC, Wijeratne N, Prentice D. Cortisol: ACTH ratio to test for primary hypoadrenalism: a pilot study. Postgrad Med J. 2013;89:617–620. [DOI] [PubMed] [Google Scholar]
- 21. Wood JB, Frankland AW, James VH, Landon J. A rapid test of adrenocortical function. Lancet. 1965;1:243–245. [DOI] [PubMed] [Google Scholar]
- 22. Abdu TA, Elhadd TA, Neary R, Clayton RN. Comparison of the low dose short synacthen test (1 microg), the conventional dose short synacthen test (250 microg), and the insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab. 1999;84:838–843. [DOI] [PubMed] [Google Scholar]
- 23. Schmidt IL, Lahner H, Mann K, Petersenn S. Diagnosis of adrenal insufficiency: evaluation of the corticotropin-releasing hormone test and basal serum cortisol in comparison to the insulin tolerance test in patients with hypothalamic-pituitary-adrenal disease. J Clin Endocrinol Metab. 2003;88:4193–4198. [DOI] [PubMed] [Google Scholar]
- 24. Deutschbein T, Unger N, Mann K, Petersenn S. Diagnosis of secondary adrenal insufficiency in patients with hypothalamic-pituitary disease: comparison between serum and salivary cortisol during the high-dose short synacthen test. Eur J Endocrinol. 2009;160:9–16. [DOI] [PubMed] [Google Scholar]
- 25. Cho HY, Kim JH, Kim SW, et al. Different cut-off values of the insulin tolerance test, the high-dose short Synacthen test (250 μg) and the low-dose short Synacthen test (1 μg) in assessing central adrenal insufficiency. Clin Endocrinol (Oxf). 2014;81:77–84. [DOI] [PubMed] [Google Scholar]
- 26. Chatha KK, Middle JG, Kilpatrick ES. National UK audit of the short synacthen test. Ann Clin Biochem. 2010;47:158–164. [DOI] [PubMed] [Google Scholar]
- 27. Reynolds RM, Stewart PM, Seckl JR, Padfield PL. Assessing the HPA axis in patients with pituitary disease: a UK survey. Clin Endocrinol (Oxf). 2006;64:82–85. [DOI] [PubMed] [Google Scholar]
- 28. Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79:923–931. [DOI] [PubMed] [Google Scholar]
- 29. Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139:194–204. [DOI] [PubMed] [Google Scholar]
- 30. Gundgurthi A, Garg MK, Dutta MK, Pakhetra R. Intramuscular ACTH stimulation test for assessment of adrenal function. J Assoc Physicians India. 2013;61:320–324. [PubMed] [Google Scholar]
- 31. Bhansali A, Subrahmanyam KA, Talwar V, Dash RJ. Plasma cortisol response to 1 microgram adrenocorticotropin at 0800 h & 1600 h in healthy subjects. Indian J Med Res. 2001;114:173–176. [PubMed] [Google Scholar]
- 32. Dickstein G, Shechner C, Nicholson WE, et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab. 1991;72:773–778. [DOI] [PubMed] [Google Scholar]
- 33. Charmandari E, Kino T. Chrousos syndrome: a seminal report, a phylogenetic enigma and the clinical implications of glucocorticoid signalling changes. Eur J Clin Invest. 2010;40:932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Charmandari E, Kino T, Chrousos GP. Primary generalized familial and sporadic glucocorticoid resistance (Chrousos syndrome) and hypersensitivity. Endocr Dev. 2013;24:67–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Torpy DJ, Bachmann AW, Grice JE, et al. Familial corticosteroid-binding globulin deficiency due to a novel null mutation: association with fatigue and relative hypotension. J Clin Endocrinol Metab. 2001;86:3692–3700. [DOI] [PubMed] [Google Scholar]
- 36. Gagliardi L, Ho JT, Torpy DJ. Corticosteroid-binding globulin: the clinical significance of altered levels and heritable mutations. Mol Cell Endocrinol. 2010;316:24–34. [DOI] [PubMed] [Google Scholar]
- 37. Jones DA, Miras A, Tringham JR. Addison's disease: a diagnostic challenge. Br J Hosp Med (Lond). 2008;69:M192–M195. [DOI] [PubMed] [Google Scholar]
- 38. Oelkers W, Diederich S, Bähr V. Diagnosis and therapy surveillance in Addison's disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab. 1992;75:259–264. [DOI] [PubMed] [Google Scholar]
- 39. Oelkers W. Adrenal insufficiency. N Engl J Med. 1996;335:1206–1212. [DOI] [PubMed] [Google Scholar]
- 40. Lebbe M, Arlt W. What is the best diagnostic and therapeutic management strategy for an Addison patient during pregnancy? Clin Endocrinol (Oxf). 2013;78:497–502. [DOI] [PubMed] [Google Scholar]
- 41. Suri D, Moran J, Hibbard JU, Kasza K, Weiss RE. Assessment of adrenal reserve in pregnancy: defining the normal response to the adrenocorticotropin stimulation test. J Clin Endocrinol Metab. 2006;91:3866–3872. [DOI] [PubMed] [Google Scholar]
- 42. Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96:1533–1540. [DOI] [PubMed] [Google Scholar]
- 43. Klose M, Lange M, Rasmussen AK, et al. Factors influencing the adrenocorticotropin test: role of contemporary cortisol assays, body composition, and oral contraceptive agents. J Clin Endocrinol Metab. 2007;92:1326–1333. [DOI] [PubMed] [Google Scholar]
- 44. El-Farhan N, Pickett A, Ducroq D, et al. Method-specific serum cortisol responses to the adrenocorticotrophin test: comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin Endocrinol (Oxf). 2013;78:673–680. [DOI] [PubMed] [Google Scholar]
- 45. May ME, Carey RM. Rapid adrenocorticotropic hormone test in practice. Retrospective review. Am J Med. 1985;79:679–684. [DOI] [PubMed] [Google Scholar]
- 46. Oelkers W. The role of high- and low-dose corticotropin tests in the diagnosis of secondary adrenal insufficiency. Eur J Endocrinol. 1998;139:567–570. [DOI] [PubMed] [Google Scholar]
- 47. Magnotti M, Shimshi M. Diagnosing adrenal insufficiency: which test is best–the 1-microg or the 250-microg cosyntropin stimulation test? Endocr Pract. 2008;14:233–238. [DOI] [PubMed] [Google Scholar]
- 48. Dhillo WS, Kong WM, Le Roux CW, et al. Cortisol-binding globulin is important in the interpretation of dynamic tests of the hypothalamic–pituitary–adrenal axis. Eur J Endocrinol. 2002;146:231–235. [DOI] [PubMed] [Google Scholar]
- 49. Mueller UW, Potter J. Binding of cortisol to human albumin and serum: the effect of protein concentration. Biochem Pharmacol. 1981;30:727–733. [DOI] [PubMed] [Google Scholar]
- 50. Vincent RP, Etogo-Asse FE, Dew T, Bernal W, Alaghband-Zadeh J, le Roux CW. Serum total cortisol and free cortisol index give different information regarding the hypothalamus-pituitary-adrenal axis reserve in patients with liver impairment. Ann Clin Biochem. 2009;46:505–507. [DOI] [PubMed] [Google Scholar]
- 51. le Roux CW, Chapman GA, Kong WM, Dhillo WS, Jones J, Alaghband-Zadeh J. Free cortisol index is better than serum total cortisol in determining hypothalamic-pituitary-adrenal status in patients undergoing surgery. J Clin Endocrinol Metab. 2003;88:2045–2048. [DOI] [PubMed] [Google Scholar]
- 52. Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629–1638. [DOI] [PubMed] [Google Scholar]
- 53. Pura M, Kreze A, Jr, Kentos P, Vanuga P. The low-dose (1 microg) cosyntropin test (LDT) for primary adrenocortical insufficiency: defining the normal cortisol response and report on first patients with Addison disease confirmed with LDT. Exp Clin Endocrinol Diabetes. 2010;118:151–157. [DOI] [PubMed] [Google Scholar]
- 54. Mayenknecht J, Diederich S, Bähr V, et al. Comparison of low and high dose corticotropin stimulation tests in patients with pituitary disease. J Clin Endocrinol Metab. 1998;83:1558–1562. [DOI] [PubMed] [Google Scholar]
- 55. Chitale A, Musonda P, McGregor AM, Dhatariya KK. Determining the utility of the 60 min cortisol measurement in the short synacthen test. Clin Endocrinol (Oxf). 2013;79:14–19. [DOI] [PubMed] [Google Scholar]
- 56. Lindholm J, Kehlet H. Re-evaluation of the clinical value of the 30 min ACTH test in assessing the hypothalamic-pituitary-adrenocortical function. Clin Endocrinol (Oxf). 1987;26:53–59. [DOI] [PubMed] [Google Scholar]
- 57. Dekkers OM, Timmermans JM, Smit JW, Romijn JA, Pereira AM. Comparison of the cortisol responses to testing with two doses of ACTH in patients with suspected adrenal insufficiency. Eur J Endocrinol. 2011;164:83–87. [DOI] [PubMed] [Google Scholar]
- 58. Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348:727–734. [DOI] [PubMed] [Google Scholar]
- 59. Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–1949. [DOI] [PubMed] [Google Scholar]
- 60. Anantharaman R, Menezes G, Yusuf R, Ganapathi B, Ayyar SV, Srinivasan R. The 1 μg cosyntropin test in normal individuals: a reappraisal. Indian J Endocrinol Metab. 2013;17:693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Watts NB, Tindall GT. Rapid assessment of corticotropin reserve after pituitary surgery. JAMA. 1988;259:708–711. [PubMed] [Google Scholar]
- 62. Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83:2350–2354. [DOI] [PubMed] [Google Scholar]
- 63. Jones SL, Trainer PJ, Perry L, Wass JA, Bessser GM, Grossman A. An audit of the insulin tolerance test in adult subjects in an acute investigation unit over one year. Clin Endocrinol (Oxf). 1994;41:123–128. [DOI] [PubMed] [Google Scholar]
- 64. Le Roux CW, Meeran K, Alaghband-Zadeh J. Is a 0900-h serum cortisol useful prior to a short Synacthen test in outpatient assessment? Ann Clin Biochem. 2002;39:148–150. [DOI] [PubMed] [Google Scholar]
- 65. Endert E, Ouwehand A, Fliers E, Prummel MF, Wiersinga WM. Establishment of reference values for endocrine tests. Part IV: Adrenal insufficiency. Neth J Med. 2005;63:435–443. [PubMed] [Google Scholar]
- 66. Jenkins D, Forsham PH, Laidlaw JC, Reddy WJ, Thorn GW. Use of ACTH in the diagnosis of adrenal cortical insufficiency. Am J Med. 1955;18:3–14. [DOI] [PubMed] [Google Scholar]
- 67. Hägg E, Asplund K, Lithner F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol (Oxf). 1987;26:221–226. [DOI] [PubMed] [Google Scholar]
- 68. Baker PR, Baschal EE, Fain PR, et al. Haplotype analysis discriminates genetic risk for DR3-associated endocrine autoimmunity and helps define extreme risk for Addison's disease. J Clin Endocrinol Metab. 2010;95:E263–E270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Van Rijn JL, Van Landeghem BA, Haima P, Goldschmidt HM. Evaluation of ACTH immunoradiometric assays. Clin Biochem. 1996;29:93–95. [DOI] [PubMed] [Google Scholar]
- 70. Pecori Giraldi F, Saccani A, Cavagnini F. Assessment of ACTH assay variability: a multicenter study. Eur J Endocrinol. 2011;164:505–512. [DOI] [PubMed] [Google Scholar]
- 71. Saenger P, Levine LS, Irvine WJ, et al. Progressive adrenal failure in polyglandular autoimmune disease. J Clin Endocrinol Metab. 1982;54:863–867. [DOI] [PubMed] [Google Scholar]
- 72. Coco G, Dal Pra C, Presotto F, et al. Estimated risk for developing autoimmune Addison's disease in patients with adrenal cortex autoantibodies. J Clin Endocrinol Metab. 2006;91:1637–1645. [DOI] [PubMed] [Google Scholar]
- 73. De Bellis A, Bizzarro A, Rossi R, et al. Remission of subclinical adrenocortical failure in subjects with adrenal autoantibodies. J Clin Endocrinol Metab. 1993;76:1002–1007. [DOI] [PubMed] [Google Scholar]
- 74. Betterle C, Scalici C, Presotto F, et al. The natural history of adrenal function in autoimmune patients with adrenal autoantibodies. J Endocrinol. 1988;117:467–475. [DOI] [PubMed] [Google Scholar]
- 75. Rehan M, Raizman JE, Cavalier E, Don-Wauchope AC, Holmes DT. Laboratory challenges in primary aldosteronism screening and diagnosis. Clin Biochem. 2015;48:377–387. [DOI] [PubMed] [Google Scholar]
- 76. Conrad K, Roggenbuck D, Reinhold D, Sack U. Autoantibody diagnostics in clinical practice. Autoimmun Rev. 2012;11:207–211. [DOI] [PubMed] [Google Scholar]
- 77. Winqvist O, Karlsson FA, Kämpe O. 21-Hydroxylase, a major autoantigen in idiopathic Addison's disease. Lancet. 1992;339:1559–1562. [DOI] [PubMed] [Google Scholar]
- 78. Laureti S, De Bellis A, Muccitelli VI, et al. Levels of adrenocortical autoantibodies correlate with the degree of adrenal dysfunction in subjects with preclinical Addison's disease. J Clin Endocrinol Metab. 1998;83:3507–3511. [DOI] [PubMed] [Google Scholar]
- 79. Laureti S, Aubourg P, Calcinaro F, et al. Etiological diagnosis of primary adrenal insufficiency using an original flowchart of immune and biochemical markers. J Clin Endocrinol Metab. 1998;83:3163–3168. [DOI] [PubMed] [Google Scholar]
- 80. Meager A, Visvalingam K, Peterson P, et al. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3:e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Laureti S, Casucci G, Santeusanio F, Angeletti G, Aubourg P, Brunetti P. X-linked adrenoleukodystrophy is a frequent cause of idiopathic Addison's disease in young adult male patients. J Clin Endocrinol Metab. 1996;81:470–474. [DOI] [PubMed] [Google Scholar]
- 82. Horn MA, Erichsen MM, Wolff AS, et al. Screening for X-linked adrenoleukodystrophy among adult men with Addison's disease. Clin Endocrinol (Oxf). 2013;79:316–320. [DOI] [PubMed] [Google Scholar]
- 83. Vita JA, Silverberg SJ, Goland RS, Austin JH, Knowlton AI. Clinical clues to the cause of Addison's disease. Am J Med. 1985;78:461–466. [DOI] [PubMed] [Google Scholar]
- 84. Knutsson U, Dahlgren J, Marcus C, et al. Circadian cortisol rhythms in healthy boys and girls: relationship with age, growth, body composition, and pubertal development. J Clin Endocrinol Metab. 1997;82:536–540. [DOI] [PubMed] [Google Scholar]
- 85. Linder BL, Esteban NV, Yergey AL, Winterer JC, Loriaux DL, Cassorla F. Cortisol production rate in childhood and adolescence. J Pediatr. 1990;117:892–896. [DOI] [PubMed] [Google Scholar]
- 86. Esteban NV, Loughlin T, Yergey AL, et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab. 1991;72:39–45. [DOI] [PubMed] [Google Scholar]
- 87. Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, Rogol AD. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab. 1993;76:1505–1510. [DOI] [PubMed] [Google Scholar]
- 88. Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab. 2004;89:281–287. [DOI] [PubMed] [Google Scholar]
- 89. Allolio B, Winkelmann W, Fricke U, Heesen D, Kaulen D. Cortisol plasma concentration in patients with primary adrenal cortex insufficiency during substitution therapy with cortisone acetate [in German]. Verh Dtsch Ges Inn Med. 1978;84:1456–1458. [PubMed] [Google Scholar]
- 90. Czock D, Keller F, Rasche FM, Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44:61–98. [DOI] [PubMed] [Google Scholar]
- 91. Plat L, Leproult R, L'Hermite-Baleriaux M, et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84:3082–3092. [DOI] [PubMed] [Google Scholar]
- 92. Simon N, Castinetti F, Ouliac F, Lesavre N, Brue T, Oliver C. Pharmacokinetic evidence for suboptimal treatment of adrenal insufficiency with currently available hydrocortisone tablets. Clin Pharmacokinet. 2010;49:455–463. [DOI] [PubMed] [Google Scholar]
- 93. Peacey SR, Guo CY, Robinson AM, et al. Glucocorticoid replacement therapy: are patients over treated and does it matter? Clin Endocrinol (Oxf). 1997;46:255–261. [DOI] [PubMed] [Google Scholar]
- 94. Howlett TA. An assessment of optimal hydrocortisone replacement therapy. Clin Endocrinol (Oxf). 1997;46:263–268. [DOI] [PubMed] [Google Scholar]
- 95. Mah PM, Jenkins RC, Rostami-Hodjegan A, et al. Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin Endocrinol (Oxf). 2004;61:367–375. [DOI] [PubMed] [Google Scholar]
- 96. Laureti S, Falorni A, Santeusanio F. Improvement of treatment of primary adrenal insufficiency by administration of cortisone acetate in three daily doses. J Endocrinol Invest. 2003;26:1071–1075. [DOI] [PubMed] [Google Scholar]
- 97. Barbetta L, Dall'Asta C, Re T, Libè R, Costa E, Ambrosi B. Comparison of different regimens of glucocorticoid replacement therapy in patients with hypoadrenalism. J Endocrinol Invest. 2005;28:632–637. [DOI] [PubMed] [Google Scholar]
- 98. Ekman B, Bachrach-Lindström M, Lindström T, Wahlberg J, Blomgren J, Arnqvist HJ. A randomized, double-blind, crossover study comparing two- and four-dose hydrocortisone regimen with regard to quality of life, cortisol and ACTH profiles in patients with primary adrenal insufficiency. Clin Endocrinol (Oxf). 2012;77:18–25. [DOI] [PubMed] [Google Scholar]
- 99. Alonso N, Granada ML, Lucas A, et al. Evaluation of two replacement regimens in primary adrenal insufficiency patients. Effect on clinical symptoms, health-related quality of life and biochemical parameters. J Endocrinol Invest. 2004;27:449–454. [DOI] [PubMed] [Google Scholar]
- 100. Forss M, Batcheller G, Skrtic S, Johannsson G. Current practice of glucocorticoid replacement therapy and patient-perceived health outcomes in adrenal insufficiency - a worldwide patient survey. BMC Endocr Disord. 2012;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fariss BL, Hane S, Shinsako J, Forsham PH. Comparison of absorption of cortisone acetate and hydrocortisone hemisuccinate. J Clin Endocrinol Metab. 1978;47:1137–1140. [DOI] [PubMed] [Google Scholar]
- 102. Filipsson H, Monson JP, Koltowska-Häggström M, Mattsson A, Johannsson G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab. 2006;91:3954–3961. [DOI] [PubMed] [Google Scholar]
- 103. Fadeev VV, Gitel EP, Mel'nichenko GA. The diurnal rhythm of adrenocorticotropic hormone secretion in the assessment of the adequacy of replacement therapy in primary chronic adrenal failure. Neurosci Behav Physiol. 2001;31:237–242. [DOI] [PubMed] [Google Scholar]
- 104. Johannsson G, Nilsson AG, Bergthorsdottir R, et al. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dual-release formulation. J Clin Endocrinol Metab. 2012;97:473–481. [DOI] [PubMed] [Google Scholar]
- 105. Williams GH, Cain JP, Dluhy RG, Underwood RH. Studies of the control of plasma aldosterone concentration in normal man. I. Response to posture, acute and chronic volume depletion, and sodium loading. J Clin Invest. 1972;51:1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Oelkers W, L'Age M. Control of mineralocorticoid substitution in Addison's disease by plasma renin measurement. Klin Wochenschr. 1976;54:607–612. [DOI] [PubMed] [Google Scholar]
- 108. Flad TM, Conway JD, Cunningham SK, McKenna TJ. The role of plasma renin activity in evaluating the adequacy of mineralocorticoid replacement in primary adrenal insufficiency. Clin Endocrinol (Oxf). 1996;45:529–534. [DOI] [PubMed] [Google Scholar]
- 109. Methlie P, Husebye EE, Hustad S, Lien EA, Løvås K. Grapefruit juice and licorice increase cortisol availability in patients with Addison's disease. Eur J Endocrinol. 2011;165:761–769. [DOI] [PubMed] [Google Scholar]
- 110. Keilholz U, Guthrie GP., Jr Adverse effect of phenytoin on mineralocorticoid replacement with fludrocortisone in adrenal insufficiency. Am J Med Sci. 1986;291:280–283. [DOI] [PubMed] [Google Scholar]
- 111. Ross IL, Bergthorsdottir R, Levitt N, et al. Cardiovascular risk factors in patients with Addison's disease: a comparative study of South African and Swedish patients. PLoS One. 2014;9:e90768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Inder WJ, Meyer C, Hunt PJ. Management of hypertension and heart failure in patients with Addison's disease. Clin Endocrinol (Oxf). 2015;82:789–792. [DOI] [PubMed] [Google Scholar]
- 113. Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. [DOI] [PubMed] [Google Scholar]
- 114. Arlt W, Callies F, van Vlijmen JC, et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999;341:1013–1020. [DOI] [PubMed] [Google Scholar]
- 115. Hunt PJ, Gurnell EM, Huppert FA, et al. Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison's disease in a randomized, double blind trial. J Clin Endocrinol Metab. 2000;85:4650–4656. [DOI] [PubMed] [Google Scholar]
- 116. Christiansen JJ, Bruun JM, Christiansen JS, Jørgensen JO, Gravholt CH. Long-term DHEA substitution in female adrenocortical failure, body composition, muscle function, and bone metabolism: a randomized trial. Eur J Endocrinol. 2011;165:293–300. [DOI] [PubMed] [Google Scholar]
- 117. Alkatib AA, Cosma M, Elamin MB, et al. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. J Clin Endocrinol Metab. 2009;94:3676–3681. [DOI] [PubMed] [Google Scholar]
- 118. Binder G, Weber S, Ehrismann M, et al. Effects of dehydroepiandrosterone therapy on pubic hair growth and psychological well-being in adolescent girls and young women with central adrenal insufficiency: a double-blind, randomized, placebo-controlled phase III trial. J Clin Endocrinol Metab. 2009;94:1182–1190. [DOI] [PubMed] [Google Scholar]
- 119. Allolio B, Hoffmann J, Linton EA, Winkelmann W, Kusche M, Schulte HM. Diurnal salivary cortisol patterns during pregnancy and after delivery: relationship to plasma corticotrophin-releasing-hormone. Clin Endocrinol (Oxf). 1990;33:279–289. [DOI] [PubMed] [Google Scholar]
- 120. Hahner S, Loeffler M, Bleicken B, et al. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol. 2010;162:597–602. [DOI] [PubMed] [Google Scholar]
- 121. Zelissen PM, Croughs RJ, van Rijk PP, Raymakers JA. Effect of glucocorticoid replacement therapy on bone mineral density in patients with Addison disease. Ann Intern Med. 1994;120:207–210. [DOI] [PubMed] [Google Scholar]
- 122. Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3:216–226. [DOI] [PubMed] [Google Scholar]
- 123. Wiegand S, Richardt A, Remer T, et al. Reduced 11β-hydroxysteroid dehydrogenase type 1 activity in obese boys. Eur J Endocrinol. 2007;157:319–324. [DOI] [PubMed] [Google Scholar]
- 124. Bonfig W, Pozza SB, Schmidt H, Pagel P, Knorr D, Schwarz HP. Hydrocortisone dosing during puberty in patients with classical congenital adrenal hyperplasia: an evidence-based recommendation. J Clin Endocrinol Metab. 2009;94:3882–3888. [DOI] [PubMed] [Google Scholar]
- 125. Grigorescu-Sido A, Bettendorf M, Schulze E, Duncea I, Heinrich U. Growth analysis in patients with 21-hydroxylase deficiency influence of glucocorticoid dosage, age at diagnosis, phenotype and genotype on growth and height outcome. Horm Res. 2003;60:84–90. [DOI] [PubMed] [Google Scholar]
- 126. Allolio B. Extensive expertise in endocrinology: adrenal crisis. Eur J Endocrinol. 2015;172:R115–R124. [DOI] [PubMed] [Google Scholar]
- 127. White K, Arlt W. Adrenal crisis in treated Addison's disease: a predictable but under-managed event. Eur J Endocrinol. 2010;162:115–120. [DOI] [PubMed] [Google Scholar]
- 128. Hahner S, Spinnler C, Fassnacht M, et al. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab. 2015;100:407–416. [DOI] [PubMed] [Google Scholar]
- 129. Reisch N, Willige M, Kohn D, et al. Frequency and causes of adrenal crises over lifetime in patients with 21-hydroxylase deficiency. Eur J Endocrinol. 2012;167:35–42. [DOI] [PubMed] [Google Scholar]
- 130. Fonseca V, Brown R, Hochhauser D, Ginsburg J, Havard CW. Acute adrenal crisis precipitated by thyroxine. Br Med J (Clin Res Ed). 1986;292:1185–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chortis V, Taylor AE, Schneider P, et al. Mitotane therapy in adrenocortical cancer induces CYP3A4 and inhibits 5α-reductase, explaining the need for personalized glucocorticoid and androgen replacement. J Clin Endocrinol Metab. 2013;98:161–171. [DOI] [PubMed] [Google Scholar]
- 132. Goldfien A, Laidlaw JC, Haydar NA, Renold AE, Thorn GW. Fluorohydrocortisone and chlorohydrocortisone, highly potent derivatives of compound F. N Engl J Med. 1955;252:415–421. [DOI] [PubMed] [Google Scholar]
- 133. Renold AE, Haydar NA, Reddy WJ, Goldfien A, St Marc JR, Laidlaw JC. Biological effects of fluorinated derivatives of hydrocortisone and progesterone in man. Ann NY Acad Sci. 1955;61:582–590. [DOI] [PubMed] [Google Scholar]
- 134. Thorn GW, Renold AE, Morse WI, Goldfien A, Reddy WJ. Highly potent adrenal cortical steroids: structure and biological activity. Ann Intern Med. 1955;43:979–1000. [DOI] [PubMed] [Google Scholar]
- 135. Weise M, Drinkard B, Mehlinger SL, et al. Stress dose of hydrocortisone is not beneficial in patients with classic congenital adrenal hyperplasia undergoing short-term, high-intensity exercise. J Clin Endocrinol Metab. 2004;89:3679–3684. [DOI] [PubMed] [Google Scholar]
- 136. Quinkler M, Hahner S. What is the best long-term management strategy for patients with primary adrenal insufficiency? Clin Endocrinol (Oxf). 2012;76:21–25. [DOI] [PubMed] [Google Scholar]
- 137. Chernow B, Alexander HR, Smallridge RC, et al. Hormonal responses to graded surgical stress. Arch Intern Med. 1987;147:1273–1278. [PubMed] [Google Scholar]
- 138. Udelsman R, Norton JA, Jelenich SE, et al. Responses of the hypothalamic-pituitary-adrenal and renin-angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J Clin Endocrinol Metab. 1987;64:986–994. [DOI] [PubMed] [Google Scholar]
- 139. Rains PC, Rampersad N, De Lima J, et al. Cortisol response to general anaesthesia for medical imaging in children. Clin Endocrinol (Oxf). 2009;71:834–839. [DOI] [PubMed] [Google Scholar]
- 140. Hsu AA, von Elten K, Chan D, et al. Characterization of the cortisol stress response to sedation and anesthesia in children. J Clin Endocrinol Metab. 2012;97:E1830–E1835. [DOI] [PubMed] [Google Scholar]
- 141. Taylor LK, Auchus RJ, Baskin LS, Miller WL. Cortisol response to operative stress with anesthesia in healthy children. J Clin Endocrinol Metab. 2013;98:3687–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. [DOI] [PubMed] [Google Scholar]
- 143. Koniaris LG, Wand G, Wright TM. TNF mediates a murine model of Addison's crisis. Shock. 2001;15:29–34. [DOI] [PubMed] [Google Scholar]
- 144. Salem M, Tainsh RE, Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219:416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Glowniak JV, Loriaux DL. A double-blind study of perioperative steroid requirements in secondary adrenal insufficiency. Surgery. 1997;121:123–129. [DOI] [PubMed] [Google Scholar]
- 146. Boonen E, Vervenne H, Meersseman P, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013;368:1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hahner S, Burger-Stritt S, Allolio B. Subcutaneous hydrocortisone administration for emergency use in adrenal insufficiency. Eur J Endocrinol. 2013;169:147–154. [DOI] [PubMed] [Google Scholar]
- 148. Hahner S, Hemmelmann N, Quinkler M, Beuschlein F, Spinnler C, Allolio B. Timelines in the management of adrenal crisis - targets, limits and reality. Clin Endocrinol (Oxf). 2015;82:497–502. [DOI] [PubMed] [Google Scholar]
- 149. Bergthorsdottir R, Leonsson-Zachrisson M, Odén A, Johannsson G. Premature mortality in patients with Addison's disease: a population-based study. J Clin Endocrinol Metab. 2006;91:4849–4853. [DOI] [PubMed] [Google Scholar]
- 150. Bensing S, Brandt L, Tabaroj F, et al. Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin Endocrinol (Oxf). 2008;69:697–704. [DOI] [PubMed] [Google Scholar]
- 151. Repping-Wuts HJ, Stikkelbroeck NM, Noordzij A, Kerstens M, Hermus AR. A glucocorticoid education group meeting: an effective strategy for improving self-management to prevent adrenal crisis. Eur J Endocrinol. 2013;169:17–22. [DOI] [PubMed] [Google Scholar]
- 152. Harsch IA, Schuller A, Hahn EG, Hensen J. Cortisone replacement therapy in endocrine disorders - quality of self-care. J Eval Clin Pract. 2010;16:492–498. [DOI] [PubMed] [Google Scholar]
- 153. Braatvedt GD, Newrick PG, Corrall RJ. Patients' self administration of hydrocortisone. BMJ. 1990;301:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Fleming LK, Rapp CG, Sloane R. Caregiver knowledge and self-confidence of stress dosing of hydrocortisone in children with congenital adrenal hyperplasia. J Pediatr Nurs. 2011;26:e55–e60. [DOI] [PubMed] [Google Scholar]
- 155. Quinkler M, Dahlqvist P, Husebye ES, Kämpe O. A European Emergency Card for adrenal insufficiency can save lives. Eur J Intern Med. 2015;26:75–76. [DOI] [PubMed] [Google Scholar]
- 156. Fichna M, Fichna P, Gryczyńska M, et al. Screening for associated autoimmune disorders in Polish patients with Addison's disease. Endocrine. 2010;37:349–360. [DOI] [PubMed] [Google Scholar]
- 157. Zelissen PM, Bast EJ, Croughs RJ. Associated autoimmunity in Addison's disease. J Autoimmun. 1995;8:121–130. [DOI] [PubMed] [Google Scholar]
- 158. Betterle C, Scarpa R, Garelli S, et al. Addison's disease: a survey on 633 patients in Padova. Eur J Endocrinol. 2013;169:773–784. [DOI] [PubMed] [Google Scholar]
- 159. Reato G, Morlin L, Chen S, et al. Premature ovarian failure in patients with autoimmune Addison's disease: clinical, genetic, and immunological evaluation. J Clin Endocrinol Metab. 2011;96:E1255–E1261. [DOI] [PubMed] [Google Scholar]
- 160. Winqvist O, Gustafsson J, Rorsman F, Karlsson FA, Kämpe O. Two different cytochrome P450 enzymes are the adrenal antigens in autoimmune polyendocrine syndrome type I and Addison's disease. J Clin Invest. 1993;92:2377–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Falorni A, Brozzetti A, Aglietti MC, et al. Progressive decline of residual follicle pool after clinical diagnosis of autoimmune ovarian insufficiency. Clin Endocrinol (Oxf). 2012;77:453–458. [DOI] [PubMed] [Google Scholar]
- 162. Myhre AG, Aarsetøy H, Undlien DE, Hovdenak N, Aksnes L, Husebye ES. High frequency of coeliac disease among patients with autoimmune adrenocortical failure. Scand J Gastroenterol. 2003;38:511–515. [DOI] [PubMed] [Google Scholar]
- 163. Betterle C, Lazzarotto F, Spadaccino AC, et al. Celiac disease in North Italian patients with autoimmune Addison's disease. Eur J Endocrinol. 2006;154:275–279. [DOI] [PubMed] [Google Scholar]
- 164. Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383:2152–2167. [DOI] [PubMed] [Google Scholar]
- 165. Raff H. Utility of salivary cortisol measurements in Cushing's syndrome and adrenal insufficiency. J Clin Endocrinol Metab. 2009;94:3647–3655. [DOI] [PubMed] [Google Scholar]
- 166. Keevil BG. Novel liquid chromatography tandem mass spectrometry (LC-MS/MS) methods for measuring steroids. Best Pract Res Clin Endocrinol Metab. 2013;27:663–674. [DOI] [PubMed] [Google Scholar]
- 167. Giordano R, Marzotti S, Balbo M, et al. Metabolic and cardiovascular profile in patients with Addison's disease under conventional glucocorticoid replacement. J Endocrinol Invest. 2009;32:917–923. [DOI] [PubMed] [Google Scholar]
- 168. Bornstein SR, Breidert M, Ehrhart-Bornstein M, Kloos B, Scherbaum WA. Plasma catecholamines in patients with Addison's disease. Clin Endocrinol (Oxf). 1995;42:215–218. [DOI] [PubMed] [Google Scholar]
- 169. Merke DP, Chrousos GP, Eisenhofer G, et al. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med. 2000;343:1362–1368. [DOI] [PubMed] [Google Scholar]
- 170. Newell-Price J, Whiteman M, Rostami-Hodjegan A, et al. Modified-release hydrocortisone for circadian therapy: a proof-of-principle study in dexamethasone-suppressed normal volunteers. Clin Endocrinol (Oxf). 2008;68:130–135. [DOI] [PubMed] [Google Scholar]
- 171. Mallappa A, Sinaii N, Kumar P, et al. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100:1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Løvås K, Husebye ES. Continuous subcutaneous hydrocortisone infusion in Addison's disease. Eur J Endocrinol. 2007;157:109–112. [DOI] [PubMed] [Google Scholar]
- 173. Oksnes M, Björnsdottir S, Isaksson M, et al. Continuous subcutaneous hydrocortisone infusion versus oral hydrocortisone replacement for treatment of Addison's disease: a randomized clinical trial. J Clin Endocrinol Metab. 2014;99:1665–1674. [DOI] [PubMed] [Google Scholar]
- 174. Gagliardi L, Nenke MA, Thynne TR, et al. Continuous subcutaneous hydrocortisone infusion therapy in Addison's disease: a randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2014;99:4149–4157. [DOI] [PubMed] [Google Scholar]
- 175. Russell GM, Durant C, Ataya A, et al. Subcutaneous pulsatile glucocorticoid replacement therapy. Clin Endocrinol (Oxf). 2014;81:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Pearce SH, Mitchell AL, Bennett S, et al. Adrenal steroidogenesis after B lymphocyte depletion therapy in new-onset Addison's disease. J Clin Endocrinol Metab. 2012;97:E1927–E1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Gan EH, MacArthur K, Mitchell AL, et al. Residual adrenal function in autoimmune Addison's disease: improvement after tetracosactide (ACTH1–24) treatment. J Clin Endocrinol Metab. 2014;99:111–118. [DOI] [PubMed] [Google Scholar]
- 178. Tajima T, Okada T, Ma XM, Ramsey W, Bornstein S, Aguilera G. Restoration of adrenal steroidogenesis by adenovirus-mediated transfer of human cytochrome P450 21-hydroxylase into the adrenal gland of 21-hydroxylase-deficient mice. Gene Ther. 1999;6:1898–1903. [DOI] [PubMed] [Google Scholar]
- 179. Grodstein E, Hardy MA, Goldstein MJ. A case of human intramuscular adrenal gland transplantation as a cure for chronic adrenal insufficiency. Am J Transplant. 2010;10:431–433. [DOI] [PubMed] [Google Scholar]
- 180. Ludwig B, Reichel A, Steffen A, et al. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci USA. 2013;110:19054–19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275:104–115. [DOI] [PubMed] [Google Scholar]