Abstract
Background:
Vitamin D and calcium deficiencies are common worldwide, causing nutritional rickets and osteomalacia, which have a major impact on health, growth, and development of infants, children, and adolescents; the consequences can be lethal or can last into adulthood. The goals of this evidence-based consensus document are to provide health care professionals with guidance for prevention, diagnosis, and management of nutritional rickets and to provide policy makers with a framework to work toward its eradication.
Evidence:
A systematic literature search examining the definition, diagnosis, treatment, and prevention of nutritional rickets in children was conducted. Evidence-based recommendations were developed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system that describe the strength of the recommendation and the quality of supporting evidence.
Process:
Thirty-three nominated experts in pediatric endocrinology, pediatrics, nutrition, epidemiology, public health, and health economics evaluated the evidence on specific questions within five working groups. The consensus group, representing 11 international scientific organizations, participated in a multiday conference in May 2014 to reach a global evidence-based consensus.
Results:
This consensus document defines nutritional rickets and its diagnostic criteria and describes the clinical management of rickets and osteomalacia. Risk factors, particularly in mothers and infants, are ranked, and specific prevention recommendations including food fortification and supplementation are offered for both the clinical and public health contexts.
Conclusion:
Rickets, osteomalacia, and vitamin D and calcium deficiencies are preventable global public health problems in infants, children, and adolescents. Implementation of international rickets prevention programs, including supplementation and food fortification, is urgently required.
Summary of Consensus Recommendations1
Section 1: Defining nutritional rickets and the interplay between vitamin D status and calcium intake
1.1. Definition and diagnosis of nutritional rickets
Nutritional rickets, a disorder of defective chondrocyte differentiation and mineralization of the growth plate and defective osteoid mineralization, is caused by vitamin D deficiency and/or low calcium intake in children. (1⊕⊕⊕)
The diagnosis of nutritional rickets is made on the basis of history, physical examination, and biochemical testing, and is confirmed by radiographs. (1⊕⊕⊕)
1.2. Vitamin D status
- The panel recommends the following classification of vitamin D status, based on serum 25-hydroxyvitamin D (25OHD) levels. (1⊕⊕⊕)
- ■ Sufficiency, >50 nmol/L
- ■ Insufficiency, 30–50 nmol/L
- ■ Deficiency, <30 nmol/L
1.3. Vitamin D toxicity
Toxicity is defined as hypercalcemia and serum 25OHD > 250 nmol/L, with hypercalciuria and suppressed PTH. (1⊕⊕⊕)
1.4. Dietary calcium intake to prevent rickets
For infants 0–6 and 6–12 months of age, the adequate calcium intake is 200 and 260 mg/d, respectively. (1⊕⊕⊕)
For children over 12 months of age, dietary calcium intake of <300 mg/d increases the risk of rickets independent of serum 25OHD levels. (1⊕⊕○)
- For children over 12 months of age, the panel recommends the following classification of dietary calcium intake. (1⊕⊕○)
- ■ Sufficiency, >500 mg/d
- ■ Insufficiency, 300–500 mg/d
- ■ Deficiency, <300 mg/d
1.5. Vitamin D deficiency and fractures
Children with radiographically confirmed rickets have an increased risk of fracture. (1⊕⊕○)
Children with simple vitamin D deficiency are not at increased risk of fracture. (1⊕⊕○)
Section 2: Prevention and treatment of nutritional rickets and osteomalacia
2.1. Vitamin D supplementation for the prevention of rickets and osteomalacia
400 IU/d (10 μg) is adequate to prevent rickets and is recommended for all infants from birth to 12 months of age, independent of their mode of feeding. (1⊕⊕⊕)
Beyond 12 months of age, all children and adults need to meet their nutritional requirement for vitamin D through diet and/or supplementation, which is at least 600 IU/d (15 μg), as recommended by the Institute of Medicine (IOM). (1⊕⊕⊕)
2.2. Target for vitamin D supplementation
In healthy children, routine 25OHD screening is not recommended, and consequently, no specific 25OHD threshold for vitamin D supplementation is targeted in this population. (1⊕⊕⊕)
2.3. Candidates for preventative vitamin D supplementation beyond 12 months of age
In the absence of food fortification, vitamin D supplementation should be given to:
Children with a history of symptomatic vitamin D deficiency requiring treatment. (1⊕⊕⊕)
Children and adults at high risk of vitamin D deficiency, with factors or conditions that reduce synthesis or intake of vitamin D. (1⊕⊕⊕)
Pregnant women (see Section 3.1).
2.4. Dose of vitamin D and calcium for the treatment of nutritional rickets
For treatment of nutritional rickets, the minimal recommended dose of vitamin D is 2000 IU/d (50 μg) for a minimum of 3 months. (1⊕⊕⊕)
Oral calcium, 500 mg/d, either as dietary intake or supplement should be routinely used in conjunction with vitamin D in the treatment regardless of age or weight. (1⊕⊕⊕)
2.5. Appropriate route of administration and duration of therapy
We recommend oral treatment, which more rapidly restores 25OHD levels than IM treatment. (1⊕⊕⊕)
For daily treatment, both D2 and D3 are equally effective. (1⊕⊕⊕)
When single large doses are used, D3 appears to be preferable compared to D2 because the former has a longer half-life. (1⊕⊕⊕)
Vitamin D treatment is recommended for a minimum of 12 weeks, recognizing that some children may require longer treatment duration. (1⊕⊕⊕)
Section 3: Prevention of nutritional rickets/osteomalacia: identification of risk factors
3.1. Dietary practices and nutrient intakes among mothers associated with nutritional rickets in infants
Maternal vitamin D deficiency should be avoided by ensuring that women of childbearing age meet intakes of 600 IU/d recommended by the IOM. (1⊕⊕⊕)
Pregnant women should receive 600 IU/d of vitamin D, preferably as a combined preparation with other recommended micronutrients such as iron and folic acid. (2⊕⊕○)
3.2. Early feeding, supplementation, complementary feeding, and nutrient intake associated with rickets in infants
In addition to an intake of 400 IU/d of vitamin D, complementary foods introduced no later than 26 weeks should include sources rich in calcium. (1⊕⊕⊕)
An intake of at least 500 mg/d of elemental calcium must be ensured during childhood and adolescence. (1⊕⊕⊕)
3.3. Association of sunlight exposure to nutritional rickets
Because UVB rays trigger epidermal synthesis of pre-vitamin D3, restricted exposure to sun increases the risk of vitamin D deficiency and nutritional rickets. (1⊕⊕⊕)
Environmental factors, such as latitude, season, time of day, cloud cover, and pollution affect availability of UVB, whereas personal factors, such as time spent outdoors, skin pigmentation, skin coverage, age, body composition, and genetics affect the dose-response of UVB exposure and circulating 25OHD. (2⊕⊕○)
No safe threshold of UV exposure allows for sufficient vitamin D synthesis across the population without increasing skin cancer risk. (2⊕⊕○)
Section 4: Prevention of osteomalacia during pregnancy and lactation and congenital rickets
4.1. The relationship between vitamin D during pregnancy and infant growth and bone mass
Pregnant women should receive 600 IU/d of supplemental vitamin D. This will ensure adequacy of maternal 25OHD, especially in women at risk of deficiency, to prevent elevated cord blood alkaline phosphatase (ALP), increased fontanelle size, neonatal hypocalcemia and congenital rickets, and to improve dental enamel formation. (2⊕⊕○)
There is little evidence that maternal supplementation with vitamin D will protect or improve birth anthropometry (2⊕○○) and no evidence that supplementation with vitamin D will protect or improve short- or long-term growth or bone mass accretion. (2⊕⊕○)
4.2. The effect of calcium supplementation during pregnancy on infant bone mass
Pregnant women do not need calcium intakes above recommended non-pregnant intakes to improve neonatal bone. (1⊕⊕⊕)
4.3. Influence of calcium or vitamin D supplementation in pregnancy or lactation on breast milk calcium or vitamin D
Lactating women should ensure they meet the dietary recommendations for vitamin D (600 IU/d) for their own needs, but not for the needs of their infant. (1⊕⊕⊕)
Lactating women should not take high amounts of vitamin D as a means of supplementing their infant. (2⊕⊕○)
Pregnant and lactating women should meet the recommended intakes of calcium. Maternal calcium intake during pregnancy or lactation is not associated with breast milk calcium concentrations. (1⊕⊕⊕)
4.4. Causes and therapy of congenital rickets
Supplementing mothers with 600 IU/d of vitamin D and ensuring they receive recommended calcium intakes, or appropriate therapy of maternal conditions predisposing to hypocalcemia or vitamin D deficiency, prevents congenital rickets. (2⊕○○)
Section 5: Assessing the burden of nutritional rickets and public health strategies for prevention
5.1. Assessment of disease burden
The prevalence of rickets should be determined by population-based samples, by case reports from sentinel centers, or by mandatory reporting. (1⊕⊕⊕)
Screening for nutritional rickets should be based on clinical features, followed by radiographic confirmation of suspected cases. (1⊕⊕⊕)
Population-based screening with serum 25OHD, serum ALP, or radiographs is not indicated. (1⊕⊕⊕)
5.2. Public health strategies for rickets prevention
Universally supplement all infants with vitamin D from birth to 12 months of age, independent of their mode of feeding. Beyond 12 months, supplement all groups at risk and pregnant women. Vitamin D supplements should be incorporated into childhood primary health care programs along with other essential micronutrients and immunizations (1⊕⊕⊕), and into antenatal care programs along with other recommended micronutrients. (2⊕⊕○)
Recognize nutritional rickets, osteomalacia, and vitamin D and calcium deficiencies as preventable global public health problems in infants, children, and adolescents. (1⊕⊕⊕)
Implement rickets prevention programs in populations with a high prevalence of vitamin D deficiency and limited vitamin D and/or calcium intakes, and in groups of infants and children at risk of rickets. (1⊕⊕⊕)
Monitor adherence to recommended vitamin D and calcium intakes and implement surveillance for nutritional rickets. (1⊕⊕⊕)
Fortify staple foods with vitamin D and calcium, as appropriate, based on dietary patterns. Food fortification can prevent rickets and improve vitamin D status of infants, children, and adolescents if appropriate foods are used and sufficient fortification is provided, if fortification is supported by relevant legislation, and if the process is adequately monitored. Indigenous food sources of calcium should be promoted or subsidized in children. (1⊕⊕⊕)
Promote addressing the public health impact of vitamin D deficiency as both a clinical and a public health issue. (1⊕⊕⊕)
5.3. Economic cost/benefits of prevention programs
The cost-effectiveness of supplementation and food fortification programs needs further study. (1⊕⊕○)
Nutritional rickets (NR), secondary to vitamin D deficiency and/or dietary calcium deficiency, remains a significant global, public health problem despite the availability of supplementation and numerous published guidelines for its prevention (1–8). This is concerning because NR can have a major impact on the health of infants, children, and adolescents, with ramifications that persist into adulthood. The morbidity and mortality associated with NR can be devastating, with substantial but poorly recognized consequences for society and health economics. Features of NR and osteomalacia include: 1) hypocalcemic seizures and tetanic spasms; 2) life-threatening hypocalcemic cardiomyopathy; 3) bone pain and muscle weakness; 4) limb and pelvic deformities; 5) failure to thrive; 6) developmental delay; and 7) dental anomalies (9, 10). Alarmingly, NR can also lead to death from heart failure caused by hypocalcemic cardiomyopathy, even in developed countries (11). In addition, narrowing of the pelvic outlet after NR in childhood can result in obstructed labor and maternal and fetal death (12).
Despite intense focus around the role of vitamin D status in health and disease, there has been a worldwide failure to implement public health guidance and eradicate the most severe manifestations of vitamin D and calcium deficiency in our most vulnerable population—NR and osteomalacia of childhood. Therefore, the goal of this Consensus Statement is to provide clinicians with clarity and recommendations on the recognition, societal burden, and treatment of NR and osteomalacia, and to enable clinicians and health policy leaders to establish appropriate clinical and public health interventions to prevent this debilitating, costly, and under-recognized global health problem.
Methods
In recognition of the considerable variation in the definition, diagnosis, and management of NR worldwide, the European Society for Pediatric Endocrinology decided to examine current best practice in NR and to formulate evidence-based recommendations. Experts were assembled from the following societies: the Pediatric Endocrine Society (PES), the Asia Pacific Pediatric Endocrine Society (APPES), the Japanese Society for Pediatric Endocrinology (JSPE), the Sociedad Latino-Americana de Endocrinología Pediátrica (SLEP), the Australasian Pediatric Endocrine Group (APEG), the Indian Society for Pediatric and Adolescent Endocrinology (ISPAE), the African Society for Pediatric and Adolescent Endocrinology (ASPAE), the Chinese Society of Pediatric Endocrinology and Metabolism (CSPEM), the British Nutrition Society, and the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN). This consensus paper includes the cumulative evidence up to the end of 2014.
Participants included individuals from Europe, North America (United States and Canada), Latin America, Asia, Africa, and Australia, with a balanced spectrum of professional seniority and expertise. In addition, an expert on the development of evidence-based guidelines served in an advisory capacity. Panel members declared any potential conflict of interest at the initial meeting of the group. Thirty-three participants were assigned to one of five groups to which topics 1–5 were allocated, and a chairperson was designated for each group. Each participant prepared an evidence-based summary of the literature relating to a particular question distributed before the conference (which was held over 3 days in May 2014).
Each group presented the revised summaries for discussion to the full conference. This report is based on the questions addressed. A detailed description of the GRADE classification has been published elsewhere (13). Recommendations were based on published findings and on expert opinion when appropriate.
The best available research evidence was used to develop recommendations. Preference was given to articles written in English, identified by PubMed searches with MeSH terms. For each point, recommendations and evidence are described, with a modification in the grading evidence as follows: 1 = strong recommendation (applies to most patients in most circumstances, benefits clearly outweigh the risk); and 2 = weak recommendation (consensus opinion of working group or should be considered; the best action may depend on circumstances or patient values, benefits, and risks closely balanced or uncertain). Quality of evidence is indicated as follows: ⊕⊕⊕, high quality (prospective cohort studies or randomized controlled trials [RCTs] at low risk of bias); ⊕⊕○, moderate quality (observational studies or trials with methodological flaws, inconsistent or indirect evidence); and ⊕○○, low quality (case series or nonsystematic clinical observations) (13).
The target audience for these guidelines includes general and specialist pediatricians, other professionals providing care for patients with NR, and health policy makers, particularly in countries with developing economies.
1.0. Defining Nutritional Rickets and the Interplay between Vitamin D Status and Calcium Intake
1.1. Definition and diagnosis of nutritional rickets
1.1.1. Recommendations
Nutritional rickets, a disorder of defective chondrocyte differentiation and mineralization of the growth plate and defective osteoid mineralization, is caused by vitamin D deficiency and/or low calcium intake in children. (1⊕⊕⊕)
The diagnosis of nutritional rickets is made on the basis of history, physical examination, and biochemical testing and is confirmed by radiographs. (1⊕⊕⊕)
1.1.2. Evidence
Bone mineralization requires sufficient supply of the essential mineral ions, calcium and phosphate, with vitamin D optimizing their absorption from the gut. With insufficient serum calcium concentration caused by either vitamin D deficiency or inadequate dietary calcium intake, PTH will stimulate osteoclastic bone resorption to release stored bone minerals into the bloodstream and maintain normal serum calcium (14). Bone disease (rickets and osteomalacia) develops once elevated PTH has led to low serum phosphate levels, as a result of impaired renal phosphate conservation (15).
NR is a disorder of defective growth plate chondrocyte apoptosis and matrix mineralization in children. Osteomalacia is abnormal matrix mineralization in established bone, and although present in children with rickets, it is used to describe bone mineralization defects after completion of growth. Children with an underlying disease such as fat malabsorption, liver disease, renal insufficiency, and illnesses necessitating total parenteral nutrition can also develop NR and are briefly discussed in this review. NR does not include rickets associated with heritable disorders of vitamin D metabolism, including 1-α-hydroxylase deficiency and vitamin D receptor defects, or congenital or acquired hypophosphatemic rickets.
The clinical features and consequences of NR are broad and potentially severe (Table 1) (8). Defective mineralization leads to radiographic growth plate widening as well as metaphyseal cupping and fraying, which confirm the diagnosis of NR.
Table 1.
Clinical Features Associated With Nutritional Rickets
| Osseous signs and symptoms |
| Swelling wrists and ankles |
| Delayed fontanelle closure (normally closed by age 2 y) |
| Delayed tooth eruption (no incisors by age 10 mo, no molars by age 18 mo) |
| Leg deformity (genu varum, genu valgum, windswept deformity) |
| Rachitic rosary (enlarged costochondral joints—felt anteriorly, lateral to the nipple line) |
| Frontal bossing |
| Craniotabes (softening of skull bones, usually evident on palpation of cranial sutures in first 3 mo) |
| Bone pain, restlessness, and irritability |
| Radiographic features |
| Splaying, fraying, cupping, and coarse trabecular pattern of metaphyses |
| Widening of the growth plate |
| Osteopenia |
| Pelvic deformities including outlet narrowing (risk of obstructed labor and death) |
| Long-term deformities in keeping with clinical deformities |
| Minimal trauma fracture |
| Non-osseous features |
| Hypocalcemic seizure and tetany |
| Hypocalcemic dilated cardiomyopathy (heart failure, arrhythmia, cardiac arrest, death) |
| Failure to thrive and poor linear growth |
| Delayed gross motor development with muscle weakness |
| Raised intracranial pressure |
Biochemical testing alone is not sufficient to diagnose NR and may not differentiate whether the primary cause of NR is vitamin D or dietary calcium deficiency because combined deficiencies are common. Typical laboratory findings in NR are decreases in 25OHD, serum phosphorus, serum calcium, and urinary calcium. Conversely, serum PTH, ALP, and urinary phosphorus levels are invariably elevated (10, 16).
Vitamin D status is assessed by measuring blood levels of total 25OHD. Total 25OHD is used, assuming that 25OHD2 and 25OHD3 are of equal biological value (16). There is considerable variability between laboratory methods for measuring serum 25OHD concentrations (17). Immunoassays are popular due to their convenience and high sample throughput; however, cross-reactivity between vitamin D metabolites (25OHD2, 25OHD3, and 24,25(OH)2D) and high levels of estimated bias in several automated assays cast doubt on their reliability, particularly at high and low concentrations of 25OHD (18). Higher-order reference measurement procedures for serum 25OHD have been established by the National Institute of Standards and Technology (NIST) (19) and Ghent University (20) based on isotope dilution liquid chromatography-tandem mass spectrometry. In recent years, the accuracy of 25OHD assays has improved, including through activities of the Vitamin D Standardization Program (21), which aims to standardize the results of different measurement techniques to those of the reference measurement procedures (22). Significant reductions in interlaboratory variation in serum 25OHD are observed using liquid chromatography-tandem mass spectrometry with the application of NIST standard reference materials (23).
Dietary calcium deficiency is diagnosed by obtaining a calcium intake history. Because the sources of calcium will vary by country and region, we recommend that clinicians develop a dietary calcium intake questionnaire specific to their country/region.
1.2. Vitamin D status
1.2.1. Recommendation:
- The panel recommends the following classification of vitamin D status, based on serum 25OHD levels: (1⊕⊕⊕)
- ■ Sufficiency, >50 nmol/L
- ■ Insufficiency, 30–50 nmol/L
- ■ Deficiency, <30 nmol/L
1.2.2. Evidence
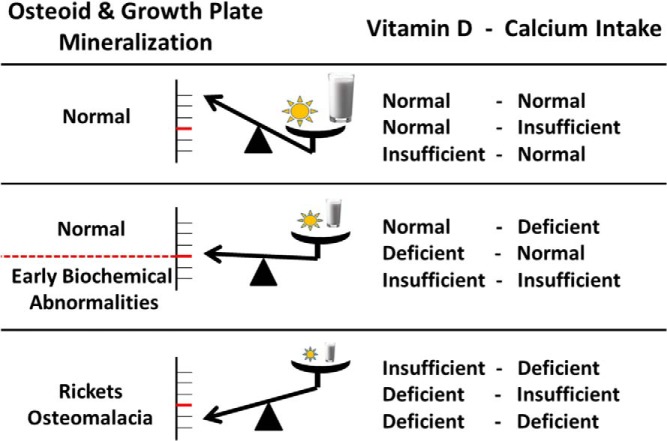
Our definition of vitamin D deficiency in the context of skeletal mineralization and mineral ion metabolism for prevention of NR is based on strong evidence (1, 2, 24–27) supported by the increased incidence of NR with 25OHD concentrations <30 nmol/L (1 ng/mL = 2.5 nmol/L). Our definition is consistent with that of the IOM (28). The potential health impacts of maintaining a serum concentration >50 nmol/L are beyond the scope of this review and are addressed elsewhere (29). It should be noted that NR has been reported in children with 25OHD concentrations >30 nmol/L (2, 5, 6, 30) and that NR may not occur with very low 25OHD concentrations but is more likely to occur with deficiency sustained over time, ie, chronic deficiency. Most children with vitamin D deficiency are asymptomatic (15), highlighting the interplay between serum 25OHD level and dietary calcium intake in maintaining serum calcium concentrations and bone integrity (Figure 1).
Figure 1.
Biochemical disturbances in rickets pathogenesis based on a three-stage classification of vitamin D status (symbolized by the sun) and calcium intake (symbolized by a glass of milk). [Modified from Högler W. Complications of vitamin D deficiency from the foetus to the infant: One cause, one prevention, but who's responsibility? Best Pract Res Clin Endocrinol Metab. 2015;29(3):385–398.(246), with permission. © 2015 Elsevier.]
Although the most significant functional outcome of vitamin D deficiency is the development of osteomalacia and NR, biochemical and bone density associations are also reported. Laboratory observations demonstrate that PTH increases when 25OHD levels drop below 34 nmol/L (7). Moreover, all patients with NR in that study had 25OHD levels < 34 nmol/L, and PTH was elevated in all but one patient. Taken together, the evidence suggests that a 25OHD level at 30–34 nmol/L may be the critical cutoff below which NR is more likely to occur.
Seasonal variations in 25OHD of between 13 and 24 nmol/L (31) emphasize the importance of maintaining 25OHD levels > 50 nmol/L (ie, sufficient), so as to prevent prolonged periods of 25OHD levels < 30 nmol/L, with the risk of developing NR.
1.3. Vitamin D toxicity
1.3.1. Recommendation
Toxicity is defined as hypercalcemia and serum 25OHD > 250 nmol/L with hypercalciuria and suppressed PTH2. (1⊕⊕⊕)
1.3.2. Evidence
Intoxication is predominantly seen in infants and young children after exposure to high doses of vitamin D (240 000 to 4 500 000 IU) (32–37). High 25OHD concentrations can cause hypercalcemia, hypercalciuria, and if prolonged, nephrocalcinosis and renal failure. To allow a large safety margin, the consensus group felt it prudent to use the concentration of 250 nmol/L as the recommended upper limit of serum 25OHD—even if symptomatic toxicity from RCTs has only been reported at levels >500 nmol/L (32). In otherwise healthy infants, hypercalcemia and hypercalciuria in the absence of elevated 25OHD concentrations have been reported and may be related to genetic variation in vitamin D metabolism (38, 39).
1.4. Dietary calcium intake to prevent rickets
1.4.1. Recommendations
For infants 0–6 and 6–12 months of age, the adequate calcium intake is 200 and 260 mg/d, respectively. (1⊕⊕⊕)
For children over 12 months of age, dietary calcium intake of <300 mg/d increases the risk of rickets independent of serum 25OHD levels. (1⊕⊕○)
- For children over 12 months of age, the panel recommends the following classification of dietary calcium intake: (1⊕⊕○)
- ■ Sufficiency, >500 mg/d
- ■ Insufficiency, 300–500 mg/d
- ■ Deficiency, <300 mg/d
1.4.2. Evidence
In developing countries where calcium intake is characteristically very low, with few or no dairy products, dietary calcium deficiency is the main cause of NR among children outside the infant age group.
In 2011, the IOM recommended adequate intakes of calcium for infants, children, and adults (16). The adequate intake for infants was based on breast milk calcium content, which is 200 mg/d and 260 mg/d for babies from 0–6 and 6–12 months of age, respectively. For children 1–18 years of age, the IOM set the daily calcium requirement at 700–1300 mg/d, depending on age (28). There is, however, no true definition of dietary calcium deficiency without a reliable biomarker of calcium intake status, and there are little data to indicate what the lowest calcium intake is that prevents NR. Reports from Nigeria, India, and Bangladesh (6, 30, 40–43) highlighted the role of low dietary calcium intake in the pathogenesis of NR among children. Although some of these children also had suboptimal 25OHD, others had values >50 nmol/L, which points to the interplay between calcium and vitamin D in the pathogenesis of NR (Figure 1). These studies suggest that in children >12 months of age, a dietary calcium intake of <300 mg/d significantly increases the risk of NR independent of serum 25OHD levels, and that at a daily intake of >500 mg, no NR was seen.
1.5. Vitamin D deficiency and fractures
1.5.1. Recommendations
Children with radiographically confirmed rickets have an increased risk of fracture. (1⊕⊕○)
Children with simple vitamin D deficiency are not at increased risk of fracture. (1⊕⊕○)
1.5.2. Evidence
Based on evidence from available observational studies and case reports, children with clinical, biochemical, and radiographic evidence of NR are at increased risk of fracture. A retrospective study found that fractures occurred in seven of 45 (17.5%) infants and toddlers with NR, aged between 2 and 14 months (44). However, fractures only occurred in those who were mobile and had severe radiographic evidence of rickets. Although none of the fractures were considered to be characteristic of nonaccidental injury (child abuse), two infants had lateral or anterior-lateral rib fractures. In a national survey in Canada, 11 of 108 cases of NR (11%) had suffered fractures, although details on the bone sites and numbers of fractures were not provided (1). Fractures also have been reported in cases or case series of NR in toddlers and adolescents (45–50), but details about the number, site, and type of fracture were absent.
It has been suggested that radiographic features of rickets may be mistaken for those characteristic of nonaccidental injury (51, 52), but the necessary biochemical and radiographic data on the cases for validation of the authors' conclusions were absent. In addition, serum 25OHD levels are similar in infants with accidental and nonaccidental injuries (53). Thus, simple vitamin D deficiency, that is, vitamin D deficiency without biochemical or radiological signs of rickets, has not been associated with increased fracture risk in infants and children.
2.0. Prevention and Treatment of Nutritional Rickets and Osteomalacia
2.1. Vitamin D supplementation for the prevention of rickets and osteomalacia
2.1.1. Recommendations
400 IU/d (10 μg) is adequate to prevent rickets and is recommended for all infants from birth to 12 months of age, independent of their mode of feeding. (1⊕⊕⊕)
Beyond 12 months of age, all children and adults need to meet their nutritional requirement for vitamin D through diet and/or supplementation, which is at least 600 IU/d (15 μg), as recommended by the IOM. (1⊕⊕⊕)
2.1.2. Evidence
Few published studies have included the prevention of radiographic or clinical signs of rickets as an outcome. Consequently, we also reviewed studies that assessed the effect of different vitamin D supplementation regimens3 on 25OHD levels and other bone parameters (such as bone mineral density) with the goal of preventing NR by maintaining levels above the rachitic range, ie, >30 nmol/L (54).
In infants and children, 400 IU/d of vitamin D given as a supplement during infancy is sufficient to prevent radiographic signs of rickets in the short term (up to 12 mo) (54). Specifically, an RCT demonstrated that a vitamin D supplement of 400 IU/d was sufficient to prevent radiographic signs of rickets at 6 months of age, even among infants born with vitamin D deficiency (25). Similarly, no cases of radiographically confirmed rickets were seen after administration of 400 IU/d of vitamin D for 12 months, whereas the incidence was 3.8% in Turkish infants and young children who did not receive supplementation (55). In addition, no incident cases of radiographically confirmed rickets were reported in a 2-year surveillance study of Canadian infants who received 400 IU/d of vitamin D (1). Worldwide, there have been no reports of radiographically confirmed rickets in infants or children receiving 400 IU on a regular, daily basis. Furthermore, this dose has been shown in RCTs to achieve 25OHD levels more frequently above the rachitic (severe deficiency) range compared to 100 or 200 IU/d (25).
The prevention of vitamin D deficiency in the absence of NR was also briefly reviewed. In a double-blind RCT of infants without vitamin D deficiency (25OHD > 50 nmol/L), the impact of 400, 800, 1200, and 1600 IU of vitamin D3 per day was assessed (54). Doses of 400 IU/d maintained 25OHD levels > 50 nmol/L in 97% of infants after 12 months; doses of 800 and 1200 IU/d were of no added benefit to bone mineral density parameters, and 1600 IU/d raised concerns about potential toxicity. A study in infants with vitamin D deficiency (25OHD < 25 nmol/L) found that a single dose of 100 000 IU maintained 25OHD levels above 37.5 nmol/L for 3 months without hypercalcemia, whereas higher doses led to unacceptably high 25OHD levels (56).
Among infants and toddlers with 25OHD levels < 50 nmol/L for whom daily vitamin D supplementation may not be ideal, intermittent bolus doses of 50 to 100 000 IU every 3 months hold promise, although a comprehensive understanding of the safety and efficacy of this approach remains to be studied.
2.2. Target for vitamin D supplementation
2.2.1. Recommendation
In healthy children, routine 25OHD screening is not recommended, and consequently, no specific 25OHD threshold for vitamin D supplementation is targeted in this population. (1⊕⊕⊕)
2.2.2. Evidence
No studies have specifically examined the best monitoring approach once supplementation has been given to prevent vitamin D deficiency rickets. 25OHD is a reasonable monitoring parameter to ensure levels > 30–34 nmol/L for prevention of NR. Biochemically, a fall in 25OHD concentration < 34 nmol/L is associated with rising PTH levels, but this intersection point depends on the prevailing calcium intake (7).
The frequent coexistence of dietary calcium deficiency and vitamin D deficiency alters the threshold for rickets development (57). Similarly, monitoring 25OHD concentrations as a public health policy for all individuals is impractical; fortunately, high-risk groups can easily be identified based on clinical profile (Table 2).
Table 2.
Risk Factors for Nutritional Rickets and Osteomalacia and Their Prevention
| Maternal factors |
| Vitamin D deficiency |
| Dark skin pigmentation |
| Full body clothing cover |
| High latitude during winter/spring season |
| Other causes of restricted sun (UVB) exposure, eg, predominant indoor living, disability, pollution, cloud cover |
| Low vitamin D diet |
| Low calcium diet |
| Poverty, malnutrition, special diets |
| Infant/childhood factors |
| Neonatal vitamin D deficiency secondary to maternal deficiency/vitamin D deficiency |
| Lack of infant supplementation with vitamin D |
| Prolonged breast-feeding without appropriate complementary feeding from 6 mo |
| High latitude during winter/spring season |
| Dark skin pigmentation and/or restricted sun (UVB) exposure, eg, predominant indoor living, disability, pollution, cloud cover |
| Low vitamin D diet |
| Low calcium diet |
| Poverty, malnutrition, special diets |
| Risk factors are prevented by: |
| Sun exposure (UVB content of sunlight depends on latitude and season) |
| Vitamin D supplementation |
| Strategic fortification of the habitual food supply |
| Normal calcium intake |
2.3. Candidates for preventative vitamin D supplementation beyond 12 months of age
2.3.1. Recommendations
In the absence of food fortification, vitamin D supplementation should be given to:
Children with a history of symptomatic vitamin D deficiency requiring treatment. (1⊕⊕⊕)
Children and adults at high risk of vitamin D deficiency with factors or conditions that reduce synthesis or intake of vitamin D. (1⊕⊕⊕)
Pregnant women (see Section 3.1).
2.3.2. Evidence
Supplementation is a feasible and acceptable way to ensure adequate vitamin D intake independent of nutrition (58). Consensus guidelines for vitamin D supplementation have been drafted from a variety of pediatric/endocrine groups (16, 59–62). Although there are numerous studies regarding vitamin D deficiency in pediatric populations and primary evaluations of the efficacy of various programs for supplementation of pregnant women, breast-feeding mothers, infants, children, and adolescents, there is more opinion than evidence on many aspects of this topic. However, there is strong, high-quality evidence that vitamin D supplementation should be provided for at-risk groups. All at-risk groups (Table 2) are specifically vulnerable and, in the absence of food fortification, require supplementation.
Vitamin D fortification of infant formula is well established and recommended by all European countries, Australia, New Zealand, and the American Academy of Pediatrics (60). Vitamin D fortification of milk is mandated in Canada, and “enriched milk” is voluntarily fortified in the United States (63).
Children with chronic illnesses and conditions affecting vitamin D synthesis/absorption/metabolism may also benefit from supplementation and may require higher doses (58) but are not in the remit of this consensus on NR.
2.4. Dose of vitamin D and calcium for the treatment of nutritional rickets
2.4.1. Recommendations
For treatment of nutritional rickets, the minimal recommended dose of vitamin D is 2000 IU/d (50 μg) for a minimum of 3 months. (1⊕⊕⊕)
Oral calcium, 500 mg/d, either as dietary intake or supplements, should be routinely used in conjunction with vitamin D in the treatment regardless of age or weight. (1⊕⊕⊕)
2.4.2. Evidence
Most studies claim that the different doses commonly employed to treat vitamin D deficiency are safe, with hypercalcemia and/or hypercalciuria observed as a side effect only in a few individuals and usually seen in the 300 000 to 600 000 IU range (64). In a small study of children with NR (n = 17), doses of 1700 to 4000 IU of vitamin D2 rapidly increased 25OHD concentrations within 1 week and normalized calcium, phosphate, and ALP levels at 10 weeks (65). In another study in children aged 2–36 months with NR (n = 19), 5000 to 10 000 IU of oral vitamin D3 and calcium 0.5 to 1.0 g daily normalized serum PTH, calcium, and phosphate within 3 weeks, although ALP levels remained elevated (66).
Simultaneous administration of calcium with vitamin D appears to be adequate and recommended by several studies (67, 68). In 123 Nigerian children with NR due to calcium deficiency, the combined endpoint of an ALP level < 350 U/L and radiographic evidence of near-complete healing of rickets was seen in a higher percentage of patients who received a combination of calcium and vitamin D (58%) or calcium alone (61%) than in those who received vitamin D alone (19%) (5). Similarly, in 67 Indian children with NR due to combined calcium and vitamin D deficiency, complete healing at 12 weeks was seen in a higher percentage with combined therapy (50%) than with vitamin D (15.7%) or calcium alone (11.7%) (30).
Combined treatment is justified because studies have shown that the diet of children and adolescents with NR is generally low in both vitamin D and calcium (5, 6, 30, 69).
2.5. Appropriate route of administration and duration of therapy
2.5.1. Recommendations
We recommend oral treatment, which more rapidly restores 25OHD levels than IM treatment. (1⊕⊕⊕)
For daily treatment, both D2 and D3 are equally effective. (1⊕⊕⊕)
When single large doses are used, D3 appears to be preferable compared to D2 because the former has a longer half-life. (1⊕⊕⊕)
Vitamin D treatment is recommended for a minimum of 12 weeks, recognizing that some children may require longer treatment duration. (1⊕⊕⊕)
2.5.2. Evidence
Some studies compared IM and oral administration of vitamin D, but most were conducted in adults and, therefore, may not be entirely relevant for children with NR. Oral or IM vitamin D was given to 24 normal volunteers (age, 50–78 y) in a dose of 600 000 IU of D2 or D3 (70). Peak levels of 25OHD were seen at 30 and 120 days in those given oral and IM treatment, respectively. Another study in 92 adults with 25OHD < 75 nmol/L compared 300 000 IU vitamin D3 IM to 50 000 IU D3 orally given on six occasions over 3 months (71). A higher proportion of subjects receiving oral treatment had 25OHD > 75 nmol/L at 3 and 6 months than IM subjects.
One RCT in 61 children with NR compared a single IM dose of 600 000 IU vitamin D3 to a weekly oral dose of 60 000 IU D3 for 10 weeks (72). There were no differences at 1, 4, and 12 weeks between groups in bone profiles, 25OHD concentrations, or side effects. A meta-analysis of studies comparing the administration of vitamin D2 and D3 concluded that, when given as bolus doses, vitamin D3 was more effective at raising 25OHD concentrations, but no significant differences were seen with daily doses (73).
There are no RCTs on the duration of treatment for children with NR, and most of the literature consists of review articles. The review commissioned by the PES recommends that daily oral treatment be given for 8 to 12 weeks (74). Similar durations between 8 and 12 weeks of daily treatment are recommended in reviews from the United Kingdom (75, 76). A duration of 3 months is recommended in a consensus statement from Australia and New Zealand (77). Given the limited evidence, we recommend minimum treatment duration of 12 weeks to achieve a comprehensive healing and normalization of ALP, recognizing that some children may require longer treatment.
Several studies explored the concept of “stoss therapy,” ie, the administration of a large dose given as a single dose or in divided doses over several days. This approach has been advocated for ease of use and compliance with therapy. Three different single oral doses (150 000, 300 000, or 600 000 IU) in 56 Turkish children aged 3–36 months with NR did not affect the rate of improvement of rickets at 30 days (64).
However, eight subjects (two in the 300 000-IU group, six in the 600 000-IU group) developed hypercalcemia. A recent study in India compared single oral doses of 300 000 vs 600 000 IU of vitamin D3 in 76 rachitic children aged 6 months to 5 years (78). At 12 weeks, all children demonstrated radiographic healing with comparable decreases in ALP and PTH. However, hypercalcemia occurred in five children (6.5%)—two receiving 300 000 IU and three receiving 600 000 IU. Several review articles advocate different recommendations with stoss therapy that are not supported by evidence (75–77). The few studies comparing daily treatment to stoss therapy contained groups with different subject characteristics. Although we recommend daily treatment as the first line of management, we recognize that in some situations, stoss therapy may be more practical. Therefore, we provide vitamin D dose recommendations for both treatment options (Table 3). Any treatment needs to be followed by supplementation (see Sections 2.1 and 2.3).
Table 3.
Treatment Doses of Vitamin D for Nutritional Rickets
| Age | Daily Dose for 90 Days, IU | Single Dose, IU | Maintenance Daily Dose, IU |
|---|---|---|---|
| <3 mo | 2000 | N/A | 400 |
| 3–12 mo | 2000 | 50 000 | 400 |
| >12 mo to 12 y | 3000–6000 | 150 000 | 600 |
| >12 y | 6000 | 300 000 | 600 |
Abbreviation: N/A, not available. Reassess response to treatment after 3 months as further treatment may be required. Ensure a daily calcium intake of at least 500 mg. For conversion from IU to μg, divide by 40.
3.0. Prevention of Nutritional Rickets/Osteomalacia: Identification of Risk Factors
3.1. Dietary practices and nutrient intakes among mothers associated with nutritional rickets in infants
3.1.1. Recommendations
Maternal vitamin D deficiency should be avoided by ensuring that women of childbearing age meet the intakes of 600 IU/d recommended by the IOM. (1⊕⊕⊕)
Pregnant women should receive 600 IU/d of vitamin D, preferably as a combined preparation with other recommended micronutrients such as iron and folic acid. (2⊕⊕○)
3.1.2. Evidence
Maternal diet and nutrient intake as a predictor of infantile rickets have not been addressed in the literature as an a priori hypothesis. However, available data have been collected during vitamin D intervention studies, case studies, or case series in women during pregnancy.
Many cases of NR included data on maternal 25OHD and, in some cases, dietary information (79–81). Neonatal vitamin D deficiency is always caused by maternal deficiency and can have life-threatening consequences such as hypocalcemic seizures and dilated cardiomyopathy in unsupplemented infants (11). Hypocalcemia (see Section 4.1.2) or other early biochemical signs of rickets (such as elevated ALP and PTH) are present before radiographic signs of NR occur in unsupplemented neonates and infants (82, 83). A high percentage of mothers of infants with symptomatic vitamin D deficiency are from high-risk groups who are vitamin D deficient and exclusively breast-feeding (11, 24, 83–85).
In a Canadian case series, six First Nation infants presented with hypocalcemic seizures within the first 30 days of life, with suspected or confirmed maternal vitamin D deficiency and a lack of supplementation during pregnancy (86). All were formula fed, which suggests that although their intake of vitamin D would have been sufficient in normal circumstances, in these infants the oral vitamin D supply via formula milk was insufficient to treat their pre-existing severe neonatal deficiency.
Prevention of maternal deficiency is critical, and all mothers should meet their nutritional requirement for vitamin D, which is currently set at 600 IU/d, although this value is not based on direct evidence from RCTs of vitamin D supplementation in pregnant women (28). Potentially, a higher intake of vitamin D may be required to prevent both maternal and neonatal deficiency (87). Prevention of congenital vitamin D deficiency is described in Section 4.4.
3.2. Early feeding, supplementation, complementary feeding, and nutrient intake associated with NR in infants
3.2.1. Recommendations
In addition to an intake of 400 IU/d of vitamin D, complementary foods introduced no later than 26 weeks should include sources rich in calcium. (1⊕⊕⊕)
An intake of at least 500 mg/d of elemental calcium must be ensured during childhood and adolescence. (1⊕⊕⊕)
3.2.2. Evidence
There is abundant, yet low-quality evidence from multiple case reports, case series (11, 48, 49, 88), and observational studies (1, 2, 45, 50, 85, 89–99) that exclusive breast-feeding without vitamin D supplementation is a major risk factor for NR in infants. Furthermore, prolonged breast-feeding with late introduction of complementary feeding is associated with NR in infants not receiving vitamin D supplements (26, 100–105). Abundant observational data (55, 106–111) and one RCT (25) suggest that infants receiving vitamin D supplementation in the first year of life are not at risk of developing NR. Evidence for providing 400 IU/d vitamin D to infants is presented in Section 2.1.
Evidence primarily from developing countries demonstrates that traditional diets low in calcium cause NR (30, 43, 69, 93, 112–114). Therefore, special diets during infancy such as those that avoid milk and dairy products, those using soy or rice milk that are not specifically designed for infants, and/or vegan and macrobiotic diets may predispose infants to NR (115–120). Recommendations on sufficient calcium intake are presented in Section 1.4.
3.3. Association of sunlight exposure to nutritional rickets
3.3.1. Recommendations
Because UVB rays trigger epidermal synthesis of previtamin D3, restricted exposure to sun increases the risk of vitamin D deficiency and nutritional rickets. (1⊕⊕⊕)
Environmental factors such as latitude, season, time of day, cloud cover, and pollution affect availability of UVB, whereas personal factors such as time spent outdoors, skin pigmentation, skin coverage, age, body composition, and genetics affect the dose-response to UVB exposure and circulating 25OHD. (2⊕⊕○)
No safe threshold of UV exposure allows for sufficient vitamin D synthesis across the population without increasing skin cancer risk. (2⊕⊕○)
3.3.2. Evidence
Solar radiation (UVB band of 290 to 315 nm) stimulates synthesis of previtamin D from epidermal 7-dehydrocholesterol, which isomerizes to cholecalciferol and is subsequently metabolized to 25OHD. Sun exposure increases circulating 25OHD (121–123). Assuming UVB availability, an individual's capacity to synthesize vitamin D increases with longer epidermal exposure. However, exposure can be affected by environmental factors such as latitude, altitude, season, time of day, cloud cover, and air quality (123–129) as well as personal factors such as occupation, lifestyle, culture such as clothing, and preference which may modify time spent outdoors and/or the surface area of skin exposed to sunlight (130–133). Finally, the dose-response of circulating 25OHD to cutaneous UVB exposure is dependent on skin pigmentation, age, body composition, genetic factors, and baseline 25OHD levels, among others (121, 131, 134–138).
Abundant global observational data report an association between restricted epidermal exposure and NR as a consequence of vitamin D deficiency (85, 139–143). UV radiation causes skin cancer, and exposure to UV radiation from sunlight and artificial sources early in life elevates the risk of developing skin cancer (144). Without firm evidence to account for variations in age, skin color, latitude, time of day, and time of year, it is currently impractical to provide prescriptive advice on safe solar exposure to the population as a whole. All risk factors are summarized in Table 2.
4.0. Prevention of Osteomalacia During Pregnancy and Lactation and Congenital Rickets
4.1. The relationship between vitamin D during pregnancy and infant growth and bone mass
4.1.1. Recommendations
Pregnant women should receive 600 IU/d of supplemental vitamin D. This will ensure adequacy of maternal 25OHD, especially in women at risk of deficiency, to prevent elevated cord blood ALP, increased fontanelle size, neonatal hypocalcemia and congenital rickets, and to improve dental enamel formation. (2⊕⊕○)
There is little evidence that maternal supplementation with vitamin D will protect or improve birth anthropometry (2⊕○○), and there is no evidence that supplementation with vitamin D will protect or improve short- or long-term growth or bone mass accretion. (2⊕⊕○)
4.1.2. Evidence
There is moderate evidence that low maternal vitamin D status during pregnancy is associated with elevated cord blood ALP and larger fontanelle size at birth (145, 146). Moderate to strong evidence from two RCTs (145, 147), two controlled trials (148, 149), and one observational study (150) indicated that low maternal vitamin D status during pregnancy increases the risk of neonatal hypocalcemia; however, a smaller RCT and a controlled trial did not support these findings (146, 151). A single large controlled trial suggests that vitamin D supplementation during pregnancy improves dental enamel formation of offspring (148).
There are conflicting data about the association between maternal vitamin D status during pregnancy and birth anthropometry. Three RCTs of moderate to high quality using daily dose or single high-dose regimens did not find an association (151–153), whereas two controlled trials of moderate-grade evidence found a positive association (146, 147). Three low- to high-quality RCTs did not find any difference in birth anthropometry in offspring of mothers supplemented with 400, 2000, or 4000 IU/d; none of the studies had a placebo group (87, 154, 155).
There are inconsistent data on the association between maternal serum 25OHD levels and linear growth during the first year of life (152, 157, 162) and insufficient to weak evidence for an association between maternal serum 25OHD levels and bone mass or density at birth (150, 158–161) or in later childhood (162–164).
4.2. The effect of calcium supplementation during pregnancy on infant bone mass
4.2.1. Recommendation
Pregnant women do not need calcium intakes above recommended nonpregnant intakes to improve neonatal bone. (1⊕⊕⊕)
4.2.2. Evidence
Calcium supplementation studies in pregnancy have not had congenital or neonatal rickets as an outcome, but three RCTs of maternal calcium supplementation during pregnancy measured neonatal bone (165–167). These RCTs were conducted in West Africa where typical dietary calcium intakes are 250–300 mg/d (165), in the United States with an average intake of about 2000 mg/d (166), and in a multicenter World Health Organization study in populations with dietary calcium intake of approximately 600 mg/d (Argentina, Peru, India, Egypt, Vietnam, South Africa) (167). Maternal calcium supplementation had no effect on neonatal bone mineral assessed by dual-energy x-ray absorptiometry in the Gambian and US studies, except in the latter study in offspring of women in the lowest quintile of dietary calcium intake (<600 mg/d). There was no effect of maternal calcium supplementation on neonatal or infant anthropometry, a finding consistent with observational studies.
4.3. Influence of calcium or vitamin D supplementation in pregnancy or lactation on breast milk calcium or vitamin D
4.3.1. Recommendations
Lactating women should ensure they meet the dietary recommendations for vitamin D (600 IU/d) for their own needs, but not for the needs of their infant. (1⊕⊕⊕)
Lactating women should not take high amounts of vitamin D as a means of supplementing their infant. (2⊕⊕○)
Pregnant and lactating women should meet the recommended intakes of calcium. Maternal calcium intake during pregnancy or lactation is not associated with breast milk calcium concentrations. (1⊕⊕⊕)
4.3.2. Evidence
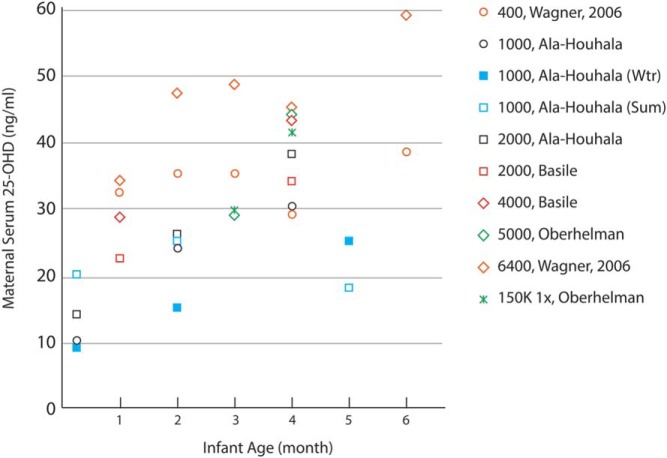
Maternal vitamin D intake during lactation correlates with milk vitamin D activity. Several double-blind RCTs found that high maternal intakes of vitamin D (2000, 4000, and 6400 IU/d) were associated with higher breast milk vitamin D concentration (Figure 2) (168–171).
Figure 2.
Double-blind RCTs have shown that maternal intakes of 1000–6400 IU/d of vitamin D are associated with increased breast milk vitamin D concentrations (156, 168, 169, 171). Lines of similar color represent the same study, and the legend provides the vitamin D supplementation dose (IU/d unless otherwise stated). Oberhelman et al (171) reported milk concentrations of cholecalciferol only.
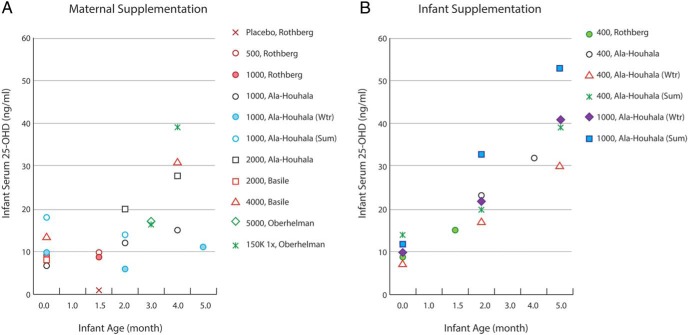
Supplementing mothers with high amounts of vitamin D has been suggested as a means of increasing both maternal (Figure 3) and infant serum 25OHD concentrations (172–175). Maternal vitamin D intakes up to 4000 IU/d are likely safe during pregnancy and lactation (16). However, the finding that infants of mothers supplemented with 2000 IU/d or more have similar serum 25OHD concentrations as infants receiving 400 IU/d (Figure 4), as well as safety concerns, and our own recommendation that all infants receive 400 IU vitamin D per day lead us to advise that mothers should take recommended amounts (600 IU/d) rather than higher doses of vitamin D.
Figure 3.
Double-blind RCTs have shown that maternal serum 25OHD concentrations are increased with vitamin D supplementation (169, 171, 174, 182). Most trials began supplementation shortly after birth. Markers of similar color represent the same study, and the legend provides the vitamin D supplementation dose in IU/d unless otherwise stated. 1 ng/mL ≈ 2.5 nmol/L. Wtr, winter; Sum, summer.
Figure 4.
Infant serum 25OHD concentrations by age in RCTs where either the mother was supplemented with vitamin D (A) or the infant was supplemented (B) (171,173–175–182). Markers of similar color represent the same study, and the legend provides the vitamin D supplementation dose in IU/d unless otherwise stated. 1 ng/mL ≈ 2.5 nmol/L. Wtr, winter; Sum, summer.
Maternal calcium intake during pregnancy or lactation does not influence breast milk calcium concentrations. Only one observational study found a weak association between maternal calcium intakes during pregnancy and breast milk calcium level at day 40 (mature milk) (176). Numerous studies, including two RCTs (177, 178) and two observational studies (179, 180) have not found a relationship between maternal calcium intake and breast milk calcium concentrations.
No studies have investigated the effect of maternal vitamin D intake during pregnancy on either milk calcium or vitamin D concentrations. Two double-blind RCTs found that maternal serum 25OHD concentration or maternal intake of vitamin D (up to 4000 IU/d) during lactation was not associated with milk calcium concentrations (181, 182).
4.4. Causes and therapy of congenital rickets
4.4.1. Recommendation
Supplementing mothers with 600 IU/d of vitamin D and ensuring they receive recommended calcium intakes, or appropriate therapy of maternal conditions predisposing to hypocalcemia or vitamin D deficiency, prevents congenital rickets. (2⊕○○)
4.4.2. Evidence
Approximately 80 cases of congenital rickets, defined as babies presenting within the first 4 weeks of life with biochemical and radiographic signs of rickets, have been described in the medical literature. Typically, mothers of babies with congenital rickets have osteomalacia with severe vitamin D deficiency, low calcium intake, and hypocalcemia at delivery and had not taken vitamin D supplementation during pregnancy (83, 183–197). In rare cases, congenital rickets can occur when mothers have had severe prolonged hypocalcemia not primarily caused by vitamin D deficiency such as poorly treated hypoparathyroidism (198–201), renal failure (202–206), received phosphate-containing enemas (207), or iatrogenic hypermagnesemia (208).
The mechanisms for the development of congenital rickets remain poorly understood, especially how diminished maternal calcium supply as the common primary maternal abnormality in all cases affects fetal mineralization. Clearly, congenital rickets only occurs in extreme metabolic situations. It is fair to state that all reported cases of congenital rickets could have been prevented by vitamin D supplementation, normal calcium intake during pregnancy, and adequate therapy of maternal conditions associated with prolonged hypocalcemia or vitamin D deficiency. Evidence is very limited on the therapy for congenital rickets, but rickets generally is responsive to vitamin D with or without calcium supplementation.
5.0. Assessing the Burden of Nutritional Rickets and Public Health Strategies for Prevention
5.1. Assessment of disease burden
5.1.1. Recommendations
The prevalence of rickets should be determined by population-based samples, by case reports from sentinel centers, or by mandatory reporting. (1⊕⊕⊕)
Screening for nutritional rickets should be based on clinical features, followed by radiographic confirmation of suspected cases. (1⊕⊕⊕)
Population-based screening with serum 25OHD, serum ALP, or radiographs is not indicated. (1⊕⊕⊕)
5.1.2. Evidence
NR has been increasingly reported in high- and low-income countries (55, 209–213). Using different methodology, the incidence of NR has been reported as 2.9, 4.9, 7.5, and 24 per 100 000 in Canada (1), Australia (2), the United Kingdom (3), and the United States (99), respectively. Many studies are hospital based but provide additional insight into the burden of NR. Infants with NR may present with hypocalcemic seizures. The incidence of dilated cardiomyopathy associated with NR and hypocalcemia is unknown, but it is potentially the deadliest and most economically costly complication of NR (11). The methods used for the diagnosis of NR in case reports (102) and small to large case series (104, 214–217) are widely variable and many lack radiographic confirmation (209). Physician-based surveys can estimate the burden of disease, but few have been done (1, 2). Population-based studies provide the most accurate assessment of the disease burden (3, 99, 211, 218–221). Despite differing methodologies, published reports indicate the greatest burden of NR is in Africa, Asia, and the Middle East due to sun avoidance or dietary calcium insufficiency (209, 213).
Even high-income countries have observed a resurgence of NR, mainly among immigrants of African, Asian, or Middle-Eastern origin. This overall increase in the incidence of NR in high-income countries corresponds to an increase in the number of individuals in ethnic minority, immigrant, and refugee groups (1–4, 99, 222). The incidence among established Caucasian populations is stable or decreasing. In regions with a low prevalence of NR, inclusion of NR as a reportable disease is potentially the most cost-effective means of case identification and surveillance (1, 2, 4, 99, 222).
Measurement of serum 25OHD is useful for the diagnosis of vitamin D deficiency in NR, but not for population screening (223, 224). Raised serum ALP has been used as a screening tool for NR (225). However, acute illness, drugs, liver disease, growth spurts, and transient hyperphosphatasemia of infancy and childhood can all elevate ALP values. Because of the invasiveness of venipuncture, high cost, and low positive predictive values, serum ALP and 25OHD cannot be recommended for population screening. Although radiographs of the wrists and knees provide definitive confirmation of active rickets (226), radiation exposure precludes recommending screening radiographs in asymptomatic children.
5.2. Public health strategies for rickets prevention
5.2.1. Recommendation
Universally supplement all infants with vitamin D from birth to 12 months of age, independent of their mode of feeding. Beyond 12 months, supplement all groups at risk and pregnant women. Vitamin D supplements should be incorporated into childhood primary health care programs along with other essential micronutrients and immunizations (1⊕⊕⊕), and into antenatal care programs along with other recommended micronutrients. (2⊕⊕○)
Recognize nutritional rickets, osteomalacia, and vitamin D and calcium deficiencies as preventable global public health problems in infants, children, and adolescents. (1⊕⊕⊕)
Implement rickets prevention programs in populations with a high prevalence of vitamin D deficiency or limited vitamin D and/or calcium intakes and in groups of infants and children at risk of rickets. (1⊕⊕⊕)
Monitor adherence to recommended vitamin D and calcium intakes and implement surveillance for nutritional rickets. (1⊕⊕⊕)
Fortify staple foods with vitamin D and calcium, as appropriate, based on dietary patterns. Food fortification can prevent rickets and improve vitamin D status of infants, children, and adolescents if appropriate foods are used and sufficient fortification is provided, if fortification is supported by relevant legislation, and if the process is adequately monitored. Indigenous food sources of calcium should be promoted or subsidized in children. (1⊕⊕⊕)
Promote addressing the public health impact of vitamin D deficiency as both a clinical and a public health issue. (1⊕⊕⊕)
5.2.2. Evidence
Vitamin D supplementation
Infants aged 0–12 months and adolescents are at increased risk of NR and osteomalacia from vitamin D deficiency due to rapid growth. Vitamin D is found in a limited number of foods, and dietary intakes apart from fortified foods have little impact on overall vitamin D status. Programs that deliver micronutrient supplements provide the fastest improvement in micronutrient status of individuals or targeted populations (227, 228).
Food fortification with vitamin D
Food fortification of commonly consumed staple foods safely provides adequate intake to prevent deficiency at minimal cost. Mandatory fortification of staple foods with vitamin D and calcium ensures nutritional adequacy (229). After vitamin D fortification of milk in North America and of milk, margarine, and cereals in the United Kingdom, the prevalence of NR dramatically declined, so much so that it was considered almost eradicated (16, 63, 228).
Studies in adults and children highlight the need for appropriate foods (230) to be adequately fortified and consumed by the at-risk segments of the population (231) so that vitamin D intakes of most members of a population approach dietary recommendations (232, 233). Because vitamin D fortification of foods rich in calcium is optimal for bone health, dairy products are commonly fortified. In countries where dairy products are not widely consumed, flour, margarine, cooking oil, or soy-based foods can be fortified with vitamin D.
Although several studies have assessed the effectiveness of vitamin D fortification of food to increase 25OHD concentrations in different age groups and communities, relatively few fortification studies have targeted children. A systematic review and meta-analysis concluded from food-based RCTs that vitamin D-fortified foods increase serum 25OHD and reduce the prevalence of deficiency (<30 nmol/L) in adults, provided appropriate vehicles are chosen based on analysis of habitual diet (234). Fortification of chupatty flour (6000 IU/kg) raised 25OHD from approximately 12.5 nmol/L to approximately 48 nmol/L in children over a 6-month period (235). Fortification of fluid milk and margarine was estimated to increase vitamin D intake in 4-year-old children from 176 to 360 IU/d (4.4 to 9 μg/d) and 25OHD concentrations from 55 to 65 nmol/L (236). Milk fortification has also been a successful strategy to improve the vitamin D status of schoolchildren in India (237). Fortification and supplementation requirements may vary with population exposure to sunshine (see Section 3) (238).
Food fortification with calcium
Inadequate dietary calcium intake is a risk factor for NR in children over the age of 12 months with low dairy product intake, a common situation in low-income countries. The IOM recommends a calcium intake of 500 mg/d in children ages 1–3 years when children are at the greatest risk of NR, based on calcium retention in absorption studies (16). In populations with low dairy intake such as in Africa and parts of Asia, indigenous food sources of calcium or fortification of staple foods with calcium can provide adequate calcium intake in children (213, 239). Calcium salts can be used to fortify infant formulas, complementary foods, and staple food in areas where dairy intake is low. Calcium carbonate for food fortification is available at very low cost (227).
Food fortification effectively increased dietary calcium intakes by using calcium-fortified laddoos in the diet of underprivileged Indian toddlers (240) and by calcium fortification of cereal for 7- to 12-year-old children (241). More than 1100 foods are calcium fortified in the United States, yet dairy food makes up more than 65% of adolescents' calcium intake (242). In the United Kingdom, calcium fortification of flour is an important source of calcium intake (16% of total) for young adolescent girls (243). There are limited data from studies on calcium fortification or the acceptability of dietary diversification to include locally available and affordable calcium-rich foods in developing countries. Periodic monitoring for NR is important to determine the effectiveness of fortification and/or supplementation programs in preventing NR.
Health promotion
Education of medical providers and organizations, health insurers, policy makers, governments, public health officials, and the general public is vital to address the public health issue of NR and vitamin D deficiency. They should be provided with guidelines on the importance of adequate vitamin D and calcium intakes in children, adolescents, and pregnant and lactating women (244). National and global public health promotion strategies are essential to raise professional and community awareness, and global action to protect all children from vitamin D and calcium deficiency is imperative.
5.3. Economic cost/benefits of prevention programs
5.3.1. Recommendation
The cost-effectiveness of supplementation and food fortification programs needs further study. (1⊕⊕○)
5.3.2. Evidence
Very weak evidence supports a policy of providing vitamin D supplementation to Asian children in the United Kingdom for the first 2 years of life (245). However, this report had methodological limitations that preclude any conclusions.
Urgent research is required to model the cost-effectiveness of alternative vitamin D supplementation strategies and food fortification programs. Future economic models should include:
Resources associated with different supplementation strategies
Indirect costs of treatment and complications
Resource use of current practice
Effectiveness of different approaches
Expected adherence
Outcomes such as quality of life associated with 25OHD levels, and
Health care costs of disease caused by both skeletal and extraskeletal effects of vitamin D deficiency.
Costs of vitamin D supplementation and/or fortification programs will differ depending on the target 25OHD level and population characteristics. Subgroup analyses targeting high-risk groups, such as people with darkly pigmented skin, limited sun exposure, and low calcium intakes, should be conducted.
Conclusion
Vitamin D deficiency should be considered a major global public health priority. NR can have severe consequences, including death from cardiomyopathy or obstructed labor, myopathy, seizures, pneumonia, lifelong deformity and disability, impaired growth, and pain. NR is the “the tip of the iceberg,” and its resurgence indicates widespread vitamin D deficiency with important public health implications. NR and osteomalacia are fully preventable disorders that are on the rise worldwide and should be regarded as a global epidemic. We advocate for the eradication of NR and osteomalacia through vitamin D supplementation of all infants, pregnant women, and individuals from high-risk groups and the implementation of international food fortification programs to ensure nutritional sufficiency of vitamin D and calcium for the whole population. This consensus document provides policy makers with a reference framework to work toward the global eradication of rickets.
Acknowledgments
Editorial support was provided by Sally Farrand. This guideline was produced by by ESPE, PES, SLEP, ASPAE, ISPAE, CSPEM, JSPE, APEG, APPES, ESPGHAN, and other expert participants. The consensus was financially supported by the above societies and an educational grant (non-participatory) by Danone Nutricia.
Disclosure Summary: M.Z.M. has received honoraria and lecture fees from Nutricia and Alexion. T.D.T. is a consultant for Biomedical Systems. W.H. has received honoraria and lecture fees from Internis and Alexion. No other author has anything to disclose.
Appendix A: Affiliations of Consensus Group Members
Magda Aguiar, Health Economics Unit, University of Birmingham, Birmingham, United Kingdom
Navoda Atapattu, Lady Ridgeway Hospital, Colombo, Sri Lanka
Vijayalakshmi Bhatia, Department of Endocrinology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Uttar Pradesh, India
Christian Braegger, Division of Gastroenterology and Nutrition and Children's Research Center, University Children's Hospital, Zurich, Switzerland
Gary Butler, Department of Paediatric and Adolescent Endocrinology, University College London Hospital, London, United Kingdom
Hamilton Cassinelli, Endocrinology Division, Ricardo Gutierrez Children's Hospital, Buenos Aires, Argentina
Linda A. DiMeglio, Section of Pediatric Endocrinology/Diabetology, Riley Hospital for Children at Indiana University Health, Indianapolis, Indiana, USA
Emma Frew, Health Economics Unit, University of Birmingham, Birmingham, United Kingdom
Junfen Fu, Division of Endocrinology, Children's Hospital of Zhejiang University School of Medicine, Hangzhou, China
Gail Goldberg, Nutrition and Bone Health Research Group, Medical Research Council Human Nutrition Research, Elsie Widdowson Laboratory, Cambridge, United Kingdom
Wolfgang Högler, Department of Endocrinology and Diabetes, Birmingham Children's Hospital; Institute of Metabolism and Systems Research, University of Birmingham, Birmingham, United Kingdom
Elina Hyppönen, School of Population Health and Sansom Research Institute, University of South Australia; South Australian Health and Medical Research Institute, Adelaide, Australia; Population Policy and Practice, University College London Institute of Child Health, London, United Kingdom
Hafsatu Wasagu Idris, Endocrinology and Gastroenterology Unit, Department of Paediatrics, Ahmadu Bello University Teaching Hospital, Zaria, Nigeria
Rajesh Khadgawat, Department of Endocrinology and Metabolism, All India Institute of Medical Sciences, New Delhi, India
Mairead Kiely, Vitamin D Research Group, School of Food and Nutritional Sciences; Irish Centre for Fetal and Neonatal Translational Research [INFANT], University College Cork, Cork, Ireland
Jane Maddock, University College London, Institute of Child Health, London, United Kingdom
Outi Mäkitie, Children's Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
Toshimi Michigami, Department of Bone and Mineral Research, Research Institute, Osaka Medical Center for Maternal and Child Health, Osaka, Japan
M. Zulf Mughal, Department of Paediatric Endocrinology, Royal Manchester Children's Hospital, Manchester, United Kingdom
Craig F. Munns, Department of Endocrinology and Diabetes, The Children's Hospital at Westmead, Sydney, Australia
Abiola Oduwole, College of Medicine University of Lagos, Lagos, Nigeria
Keiichi Ozono, Osaka University Graduate School of Medicine, Department of Pediatrics, Osaka, Japan
John M. Pettifor, Medical Research Council/Wits Developmental Pathways for Health Research Unit, Department of Paediatrics, University of the Witwatersrand, Johannesburg, South Africa
Pawel Pludowski, Department of Biochemistry, Radioimmunology, and Experimental Medicine, The Children's Memorial Health Institute, Warsaw, Poland
Lorna Ramos-Abad, University of the Philippines, College of Medicine, Manila, The Philippines
Lars Sävendahl, Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden
Anju Seth, Division of Pediatric Endocrinology, Lady Hardinge Medical College & Kalawati Saran Children's Hospital, New Delhi, India
Nick Shaw, Department of Endocrinology and Diabetes, Birmingham Children's Hospital, Birmingham, United Kingdom
Bonny L. Specker, Ethel Austin Martin Program, South Dakota State University, Brookings, South Dakota, USA
Tom D. Thacher, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA
Dov Tiosano, Department of Pediatrics, Rambam Medical Center, Haifa, Israel
Ted Tulchinsky, Braun School Public Health, Hebrew University-Hadassah, Jerusalem, Israel; Public Health Reviews, Brussels, Belgium
Leanne Ward, Department of Pediatrics, University of Ottawa and Division of Endocrinology and Metabolism, Children's Hospital of Eastern Ontario, Ottawa, Ontario, Canada
In areas where 25OHD assays are not readily available, suppression of PTH in the presence of hypercalcemia and pharmacological doses of vitamin D may support the diagnosis of vitamin D toxicity. When PTH assay is also unavailable, the possibility of toxicity should be considered in the presence of symptomatic hypercalcemia in association with pharmacological doses of vitamin D.
Note that the term “supplementation” may be interpreted to mean additional vitamin D provided from supplements, general multivitamins, or food fortification. In the context of this article, vitamin D supplementation refers to vitamin D above that which is found in standard dietary sources, with the exception of fortified infant formula.
- ALP
- alkaline phosphatase
- DXA
- dual-energy x-ray absorptiometry
- NR
- nutritional rickets
- 25OHD
- 25-hydroxyvitamin D2 and/or D3 or 25-hydroxycholecalciferol D2 and/or D3
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D2 and/or D3 or 1,25-dihydroxycholecalciferol D2 and/or D3 (calcitriol)
- PTH
- parathyroid hormone
- RCT
- randomized controlled trial
- UVB
- ultraviolet B
- Vitamin D2
- ergocalciferol
- Vitamin D3
- cholecalciferol.
References
- 1. Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D-deficiency rickets among children in Canada. CMAJ. 2007;177(2):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Munns CF, Simm PJ, Rodda CP, et al. Incidence of vitamin D deficiency rickets among Australian children: an Australian Paediatric Surveillance Unit study. Med J Aust. 2012;196(7):466–468. [DOI] [PubMed] [Google Scholar]
- 3. Callaghan AL, Moy RJ, Booth IW, Debelle G, Shaw NJ. Incidence of symptomatic vitamin D deficiency. Arch Dis Child. 2006;91:606–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beck-Nielsen SS, Jensen TK, Gram J, Brixen K, Brock-Jacobsen B. Nutritional rickets in Denmark: a retrospective review of children's medical records from 1985 to 2005. Eur J Pediatr. 2009;168(8):941–949. [DOI] [PubMed] [Google Scholar]
- 5. Thacher TD, Fischer PR, Pettifor JM, et al. A comparison of calcium, vitamin D, or both for nutritional rickets in Nigerian children. N Engl J Med. 1999;341(8):563–568. [DOI] [PubMed] [Google Scholar]
- 6. Balasubramanian K, Rajeswari J, Gulab, et al. Varying role of vitamin D deficiency in the etiology of rickets in young children vs. adolescents in northern India. J Trop Pediatr. 2003;49(4):201–206. [DOI] [PubMed] [Google Scholar]
- 7. Atapattu N, Shaw N, Högler W. Relationship between serum 25-hydroxyvitamin D and parathyroid hormone in the search for a biochemical definition of vitamin D deficiency in children. Pediatr Res. 2013;74(5):552–556. [DOI] [PubMed] [Google Scholar]
- 8. Paxton GA, Teale GR, Nowson CA, et al. Vitamin D and health in pregnancy, infants, children and adolescents in Australia and New Zealand: a position statement. Med J Aust. 2013;198(3):142–143. [DOI] [PubMed] [Google Scholar]
- 9. Pettifor JM. Vitamin D deficiency and nutritional rickets in children. In: Feldman D, Pike JW, Adams J, eds. Vitamin D. 3rd ed London: Elsevier Inc; 2011:1107–1128. [Google Scholar]
- 10. Pettifor JM. Nutritional rickets. In: Glorieux FH, Pettifor JM, Juppner H, eds. Pediatric Bone: Biology and Diseases. 2nd ed Amsterdam, The Netherlands: Elsevier; 2012:625–654. [Google Scholar]
- 11. Maiya S, Sullivan I, Allgrove J, et al. Hypocalcaemia and vitamin D deficiency: an important, but preventable, cause of life-threatening infant heart failure. Heart. 2008;94(5):581–584. [DOI] [PubMed] [Google Scholar]
- 12. Loudon I. Deaths in childbed from the eighteenth century to 1935. Med Hist. 1986;30(1):1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swiglo BA, Murad MH, Schünemann HJ, et al. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. 2008;93(3):666–673. [DOI] [PubMed] [Google Scholar]
- 14. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tiosano D, Hochberg Z. Hypophosphatemia: the common denominator of all rickets. J Bone Miner Metab. 2009;27(4):392–401. [DOI] [PubMed] [Google Scholar]
- 16. Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 17. Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets. 2011;12:19–28. [DOI] [PubMed] [Google Scholar]
- 18. Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem. 2012;58:531–542. [DOI] [PubMed] [Google Scholar]
- 19. Tai SS, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2010;82:1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stepman HC, Vanderroost A, Van Uytfanghe K, Thienpont L. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57:441–448. [DOI] [PubMed] [Google Scholar]
- 21. Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM, Vitamin D Standardization Program (VDSP). Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl. 2012;243:32–40. [DOI] [PubMed] [Google Scholar]
- 22. Carter GD, Phinney KW. Assessing vitamin D status: time for a rethink? Clin Chem. 2014;60(6):809–811. [DOI] [PubMed] [Google Scholar]
- 23. Yates AM, Bowron A, Calton L, et al. Interlaboratory variation in 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 is significantly improved if common calibration material is used. Clin Chem. 2008;54:2082–2084. [DOI] [PubMed] [Google Scholar]
- 24. Dawodu A, Agarwal M, Sankarankutty M, Hardy D, Kochiyil J, Badrinath P. Higher prevalence of vitamin D deficiency in mothers of rachitic than nonrachitic children. J Pediatr. 2005;147(1):109–111. [DOI] [PubMed] [Google Scholar]
- 25. Specker BL, Ho ML, Oestreich A, et al. Prospective study of vitamin D supplementation and rickets in China. J Pediatr. 1992;120(5):733–739. [DOI] [PubMed] [Google Scholar]
- 26. Majid Molla A, Badawi MH, al-Yaish S, Sharma P, el-Salam RS, Molla AM. Risk factors for nutritional rickets among children in Kuwait. Pediatr Int. 2000;42(3):280–284. [DOI] [PubMed] [Google Scholar]
- 27. Molla AM, Al Badawi M, Hammoud MS, et al. Vitamin D status of mothers and their neonates in Kuwait. Pediatr Int. 2005;47(6):649–652. [DOI] [PubMed] [Google Scholar]
- 28. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aggarwal V, Seth A, Aneja S, et al. Role of calcium deficiency in development of nutritional rickets in Indian children: a case control study. J Clin Endocrinol Metab. 2012;97(10):3461–3466. [DOI] [PubMed] [Google Scholar]
- 31. Benitez-Aguirre PZ, Wood NJ, Biesheuvel C, Moreira C, Munns CF. The natural history of vitamin D deficiency in African refugees living in Sydney. Med J Aust. 2009;190(8):426–428. [DOI] [PubMed] [Google Scholar]
- 32. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99(4):1132–1141. [DOI] [PubMed] [Google Scholar]
- 33. Tau C, Ciriani V, Scaiola E, Acuña M. Twice single doses of 100,000 IU of vitamin D in winter is adequate and safe for prevention of vitamin D deficiency in healthy children from Ushuaia, Tierra Del Fuego, Argentina. J Steroid Biochem Mol Biol. 2007;103:651–654. [DOI] [PubMed] [Google Scholar]
- 34. Joshi R. Hypercalcemia due to hypervitaminosis D: report of seven patients. J Trop Pediatr. 2009;55(6):396–398. [DOI] [PubMed] [Google Scholar]
- 35. Barrueto F, Jr, Wang-Flores HH, Howland MA, Hoffman RS, Nelson L. Acute vitamin D intoxication in a child. Pediatrics. 2005;116(3):e453–e456. [DOI] [PubMed] [Google Scholar]
- 36. Bereket A, Erdogan T. Oral bisphosphonate therapy for vitamin D intoxication of the infant. Pediatrics. 2003;111:899–901. [DOI] [PubMed] [Google Scholar]
- 37. Kara C, Gunindi F, Ustyol A, Aydin M. Vitamin D intoxication due to an erroneously manufactured dietary supplement in seven children. Pediatrics. 2014;133(1):e240–e244. [DOI] [PubMed] [Google Scholar]
- 38. Maalouf J, Nabulsi M, Vieth R, et al. Short- and long-term safety of weekly high-dose vitamin D3 supplementation in school children. J Clin Endocrinol Metab. 2008;93(7):2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vanstone MB, Oberfield SE, Shader L, Ardeshirpour L, Carpenter TO. Hypercalcemia in children receiving pharmacologic doses of vitamin D. Pediatrics. 2012;129(4):e1060–e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pettifor JM, Ross P, Wang J, Moodley G, Couper-Smith J. Rickets in children of rural origin in South Africa: is low dietary calcium a factor? J Pediatr. 1978;92(2):320–324. [DOI] [PubMed] [Google Scholar]
- 41. Legius E, Proesmans W, Eggermont E, Vandamme-Lobaerts R, Bouillon R, Smet M. Rickets due to dietary calcium deficiency. Eur J Pediatr. 1989;148(8):784–785. [DOI] [PubMed] [Google Scholar]
- 42. Eyberg CJ, Pettifor JM, Moodley G. Dietary calcium intake in rural black South African children. The relationship between calcium intake and calcium nutritional status. Hum Nutr Clin Nutr. 1986;40(1):69–74. [PubMed] [Google Scholar]
- 43. Okonofua F, Gill DS, Alabi ZO, Thomas M, Bell JL, Dandona P. Rickets in Nigerian children: a consequence of calcium malnutrition. Metabolism. 1991;40(2):209–213. [DOI] [PubMed] [Google Scholar]
- 44. Chapman T, Sugar N, Done S, Marasigan J, Wambold N, Feldman K. Fractures in infants and toddlers with rickets. Pediatr Radiol. 2010;40(7):1184–1189. [DOI] [PubMed] [Google Scholar]
- 45. Kreiter SR, Schwartz RP, Kirkman HN, Jr, Charlton PA, Calikoglu AS, Davenport ML. Nutritional rickets in African American breast-fed infants. J Pediatr. 2000;137(2):153–157. [DOI] [PubMed] [Google Scholar]
- 46. Hazzazi MA, Alzeer I, Tamimi W, Al Atawi M, Al Alwan I. Clinical presentation and etiology of osteomalacia/rickets in adolescents. Saudi J Kidney Dis Transpl. 2013;24(5):938–941. [DOI] [PubMed] [Google Scholar]
- 47. Agarwal A, Gulati D, Rath S, Walia M. Rickets: a cause of delayed walking in toddlers. Indian J Pediatr. 2009;76(3):269–272. [DOI] [PubMed] [Google Scholar]
- 48. Bloom E, Klein EJ, Shushan D, Feldman KW. Variable presentations of rickets in children in the emergency department. Pediatr Emerg Care. 2004;20(2):126–130. [DOI] [PubMed] [Google Scholar]
- 49. Senniappan S, Elazabi A, Doughty I, Mughal MZ. Case 2: Fractures in under-6-month-old exclusively breast-fed infants born to immigrant parents: nonaccidental injury? (case presentation). Diagnosis: Pathological fractures secondary to vitamin D deficiency rickets in under-6-months-old, exclusively breast-fed infants, born to immigrant parents. Acta Paediatr. 2008;97(7):836–837, 992–993. [DOI] [PubMed] [Google Scholar]
- 50. Mylott BM, Kump T, Bolton ML, Greenbaum LA. Rickets in the dairy state. WMJ. 2004;103(5):84–87. [PubMed] [Google Scholar]
- 51. Paterson CR. Vitamin D deficiency rickets simulating child abuse. J Pediatr Orthop. 1981;1(4):423–425. [DOI] [PubMed] [Google Scholar]
- 52. Paterson CR. Vitamin D deficiency rickets and allegations of non-accidental injury. Acta Paediatr. 2009;98(12):2008–2012. [DOI] [PubMed] [Google Scholar]
- 53. Schilling S, Wood JN, Levine MA, Langdon D, Christian CW. Vitamin D status in abused and nonabused children younger than 2 years old with fractures. Pediatrics. 2011;127(5):835–841. [DOI] [PubMed] [Google Scholar]
- 54. Gallo S, Comeau K, Vanstone C, et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA. 2013;309(17):1785–1792. [DOI] [PubMed] [Google Scholar]
- 55. Beser E, Cakmakci T. Factors affecting the morbidity of vitamin D deficiency rickets and primary prevention. East Afr Med J. 1994;71:(6):358–362. [PubMed] [Google Scholar]
- 56. Zeghoud F, Ben-Mekhbi H, Djeghri N, Garabédian M. Vitamin D prophylaxis during infancy: comparison of the long-term effects of three intermittent doses (15, 5, or 2.5 mg) on 25-hydroxyvitamin D concentrations. Am J Clin Nutr. 1994;60(3):393–396. [DOI] [PubMed] [Google Scholar]
- 57. Pettifor JM. Calcium and vitamin D metabolism in children in developing countries. Ann Nutr Metab. 2014;64(suppl 2):15–22. [DOI] [PubMed] [Google Scholar]
- 58. Holmlund-Suila E, Koskivirta P, Metso T, Andersson S, Mäkitie O, Viljakainen H. Vitamin D deficiency in children with a chronic illness–seasonal and age-related variations in serum 25-hydroxy vitamin D concentrations. PLoS One. 2013;8(4):e60856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. [DOI] [PubMed] [Google Scholar]
- 60. Braegger C, Campoy C, Colomb V, et al. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. 2013;56(6):692–701. [DOI] [PubMed] [Google Scholar]
- 61. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. [DOI] [PubMed] [Google Scholar]
- 62. Vidailhet M, Mallet E, Bocquet A, et al. Vitamin D: still a topical matter in children and adolescents. A position paper by the Committee on Nutrition of the French Society of Paediatrics. Arch Pediatr. 2012;19(3):316–328. [DOI] [PubMed] [Google Scholar]
- 63. Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr. 2004;80:1710S–1716S. [DOI] [PubMed] [Google Scholar]
- 64. Cesur Y, Caksen H, Gündem A, Kirimi E, Odabaş D. Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. J Pediatr Endocrinol Metab. 2003;16(8):1105–1109. [DOI] [PubMed] [Google Scholar]
- 65. Markestad T, Halvorsen S, Halvorsen KS, Aksnes L, Aarskog D. Plasma concentrations of vitamin D metabolites before and during treatment of vitamin D deficiency rickets in children. Acta Paediatr Scand. 1984;73:225–231. [DOI] [PubMed] [Google Scholar]
- 66. Kruse K. Pathophysiology of calcium metabolism in children with vitamin D-deficiency rickets. J Pediatr. 1995;126:736–741. [DOI] [PubMed] [Google Scholar]
- 67. Kutluk G, Cetinkaya F, Başak M. Comparisons of oral calcium, high dose vitamin D and a combination of these in the treatment of nutritional rickets in children. J Trop Pediatr. 2002;48(6):351–353. [DOI] [PubMed] [Google Scholar]
- 68. Oginni LM, Sharp CA, Badru OS, Risteli J, Davie MW, Worsfold M. Radiological and biochemical resolution of nutritional rickets with calcium. Arch Dis Child. 2003;88(9):812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thacher T, Glew RH, Isichei C, et al. Rickets in Nigerian children: response to calcium supplementation. J Trop Pediatr. 1999;45(4):202–207. [DOI] [PubMed] [Google Scholar]
- 70. Cipriani C, Romagnoli E, Pepe J, et al. Long-term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol: implications for treatment and prophylaxis. J Clin Endocrinol Metab. 2013;98(7):2709–2715. [DOI] [PubMed] [Google Scholar]
- 71. Zabihiyeganeh M, Jahed A, Nojomi M. Treatment of hypovitaminosis D with pharmacologic doses of cholecalciferol, oral vs intramuscular; an open labeled RCT. Clin Endocrinol (Oxf). 2013;78(2):210–216. [DOI] [PubMed] [Google Scholar]
- 72. Mondal K, Seth A, Marwaha R, et al. A randomized controlled trial on safety and efficacy of single intramuscular versus staggered oral dose of 600 000 IU Vitamin D in treatment of nutritional rickets. J Trop Pediatr. 2014;60(3):203–210. [DOI] [PubMed] [Google Scholar]
- 73. Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. [DOI] [PubMed] [Google Scholar]
- 75. Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010;340:b5664. [DOI] [PubMed] [Google Scholar]
- 76. Shaw NJ, Mughal MZ. Vitamin D and child health: part 2 (extraskeletal and other aspects). Arch Dis Child. 2013;98(5):368–372. [DOI] [PubMed] [Google Scholar]
- 77. Munns C, Zacharin MR, Rodda CP, et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. Med J Aust. 2006;185(5):268–272. [DOI] [PubMed] [Google Scholar]
- 78. Mittal H, Rai S, Shah D, et al. 300,000 IU or 600,000 IU of oral vitamin D3 for treatment of nutritional rickets: a randomized controlled trial. Indian Pediatr. 2014;51(4):265–272. [DOI] [PubMed] [Google Scholar]
- 79. Elidrissy AT, Sedrani SH, Lawson DE. Vitamin D deficiency in mothers of rachitic infants. Calcif Tissue Int. 1984;36(3):266–268. [DOI] [PubMed] [Google Scholar]
- 80. Bhakhri BK, Debata PK. Nutritional rickets presenting with myelofibrosis. Indian J Pediatr. 2010;77(12):1437–1439. [DOI] [PubMed] [Google Scholar]
- 81. Brinsmead T, Frawley K, Conwell LS. Images in pediatric endocrinology: vitamin D deficiency rickets and other nutritional deficiencies in a 12-month-old infant. J Pediatr Endocrinol Metab. 2011;24:13–14. [DOI] [PubMed] [Google Scholar]
- 82. Sanyal D, Raychaudhuri M. Infants with dilated cardiomyopathy and hypocalcemia. Indian J Endocrinol Metab. 2013;17(suppl 1):S221–S223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Soliman A, Salama H, Alomar S, Shatla E, Ellithy K, Bedair E. Clinical, biochemical, and radiological manifestations of vitamin D deficiency in newborns presented with hypocalcemia. Indian J Endocrinol Metab. 2013;17(4):697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Robinson PD, Högler W, Craig ME, et al. The re-emerging burden of rickets: a decade of experience from Sydney. Arch Dis Child. 2006;91(7):564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Al-Atawi MS, Al-Alwan IA, Al-Mutair AN, Tamim HM, Al-Jurayyan NA. Epidemiology of nutritional rickets in children. Saudi J Kidney Dis Transpl. 2009;20(2):260–265. [PubMed] [Google Scholar]
- 86. Gross ML, Tenenbein M, Sellers EA. Severe vitamin D deficiency in 6 Canadian First Nation formula-fed infants. Int J Circumpolar Health. 2013;72:20244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dawodu A, Saadi HF, Bekdache G, Javed Y, Altaye M, Hollis BW. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J Clin Endocrinol Metab. 2013;98:2337–2346. [DOI] [PubMed] [Google Scholar]
- 88. Brown J, Nunez S, Russell M, Spurney C. Hypocalcemic rickets and dilated cardiomyopathy: case reports and review of literature. Pediatr Cardiol. 2009;30(6):818–823. [DOI] [PubMed] [Google Scholar]
- 89. Ahmed I, Atiq M, Iqbal J, Khurshid M, Whittaker P. Vitamin D deficiency rickets in breast-fed infants presenting with hypocalcaemic seizures. Acta Paediatr. 1995;84(8):941–942. [DOI] [PubMed] [Google Scholar]
- 90. Bachrach S, Fisher J, Parks J. An outbreak of vitamin D deficiency rickets in a susceptible population. Pediatrics. 1979;64(6):871–877. [PubMed] [Google Scholar]
- 91. Binet A, Kooh S. Persistence of vitamin D-deficiency rickets in Toronto in the 1990s. Can J Public Health. 1996;87(4):227–230. [PubMed] [Google Scholar]
- 92. Cesur Y, Yuca SA, Kaya A, Yilmaz C, Bay A. Vitamin D deficiency rickets in infants presenting with hypocalcaemic convulsions. West Indian Med J. 2013;62(3):201–204. [PubMed] [Google Scholar]
- 93. DeLucia MC, Mitnick ME, Carpenter TO. Nutritional rickets with normal circulating 25-hydroxyvitamin D: a call for reexamining the role of dietary calcium intake in North American infants. J Clin Endocrinol Metab. 2003;88(8):3539–3545. [DOI] [PubMed] [Google Scholar]
- 94. Haworth JC, Dilling LA. Vitamin-D-deficient rickets in Manitoba, 1972–84. CMAJ. 1986;134(3):237–241. [PMC free article] [PubMed] [Google Scholar]
- 95. Lazol JP, Cakan N, Kamat D. 10-year case review of nutritional rickets in Children's Hospital of Michigan. Clin Pediatr (Phila). 2008;47(4):379–384. [DOI] [PubMed] [Google Scholar]
- 96. Matsuo K, Mukai T, Suzuki S, Fujieda K. Prevalence and risk factors of vitamin D deficiency rickets in Hokkaido, Japan. Pediatr Int. 2009;51(4):559–562. [DOI] [PubMed] [Google Scholar]
- 97. Peng LF, Serwint JR. A comparison of breastfed children with nutritional rickets who present during and after the first year of life. Clin Pediatr (Phila). 2003;42(8):711–717. [DOI] [PubMed] [Google Scholar]
- 98. Salama MM, El-Sakka AS. Hypocalcemic seizures in breastfed infants with rickets secondary to severe maternal vitamin D deficiency. Pak J Biol Sci. 2010;13(9):437–442. [DOI] [PubMed] [Google Scholar]
- 99. Thacher TD, Fischer PR, Tebben PJ, et al. Increasing incidence of nutritional rickets: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2013;88(2):176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Akpede GO, Omotara BA, Ambe JP. Rickets and deprivation: a Nigerian study. J R Soc Promot Health. 1999;119(4):216–222. [DOI] [PubMed] [Google Scholar]
- 101. el Hag AI, Karrar ZA. Nutritional vitamin D deficiency rickets in Sudanese children. Ann Trop Paediatr. 1995;15(1):69–76. [DOI] [PubMed] [Google Scholar]
- 102. Kaper BP, Romness MJ, Urbanek PJ. Nutritional rickets: report of four cases diagnosed at orthopaedic evaluation. Am J Orthop. 2000;29:214–218. [PubMed] [Google Scholar]
- 103. Kruger DM, Lyne ED, Kleerekoper M. Vitamin D deficiency rickets. A report on three cases. Clin Orthop Relat Res. 1987;224:277–283. [PubMed] [Google Scholar]
- 104. Mughal MZ, Salama H, Greenaway T, Laing I, Mawer EB. Lesson of the week: florid rickets associated with prolonged breast feeding without vitamin D supplementation. BMJ. 1999;318:39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Siddiqui TS, Rai MI. Presentation and predisposing factors of nutritional rickets in children of Hazara Division. J Ayub Med Coll Abbottabad. 2005;17(3):29–32. [PubMed] [Google Scholar]
- 106. Lerch C, Meissner T. Interventions for the prevention of nutritional rickets in term born children. Cochrane Database Syst Rev. 2007;4:CD006164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ozkan B, Doneray H, Karacan M, et al. Prevalence of vitamin D deficiency rickets in the Eastern part of Turkey. Eur J Pediatr. 2009;168(1):95–100. [DOI] [PubMed] [Google Scholar]
- 108. Pedersen P, Michaelsen KF, Mølgaard C. Children with nutritional rickets referred to hospitals in Copenhagen during a 10-year period. Acta Paediatr. 2003;92(1):87–90. [DOI] [PubMed] [Google Scholar]
- 109. Robertson I. Survey of clinical rickets in the infant population in Cape Town, 1967–1968. S Afr Med J. 1969;43(35):1072–1076. [PubMed] [Google Scholar]
- 110. Strand MA, Peng G, Zhang P, Lee G. Preventing rickets in locally appropriate ways: a case report from north China. Int Q Community Health Educ. 2003;21:297–322. [Google Scholar]
- 111. Tezer H, Siklar Z, Dallar Y, Doğankoç S. Early and severe presentation of vitamin D deficiency and nutritional rickets among hospitalized infants and the effective factors. Turk J Pediatr. 2009;51(2):110–115. [PubMed] [Google Scholar]
- 112. Combs GF, Jr, Hassan N, Dellagana N, et al. Apparent efficacy of food-based calcium supplementation in preventing rickets in Bangladesh. Biol Trace Elem Res. 2008;121(3):193–204. [DOI] [PubMed] [Google Scholar]
- 113. Combs GF, Hassan N. The Chakaria food system study: household-level, case-control study to identify risk factor for rickets in Bangladesh. Eur J Clin Nutr. 2005;59(11):1291–1301. [DOI] [PubMed] [Google Scholar]
- 114. Rudolf M, Arulanantham K, Greenstein R. Unsuspected nutritional rickets. Pediatrics. 1980;66(1):72–76. [PubMed] [Google Scholar]
- 115. Castile RG, Marks LJ, Stickler GB. Vitamin D deficiency rickets. Two cases with faulty infant feeding practices. Am J Dis Child. 1975;129(8):964–966. [PubMed] [Google Scholar]
- 116. Curtis JA, Kooh SW, Fraser D, Greenberg ML. Nutritional rickets in vegetarian children. Can Med Assoc J. 1983;128(2):150–152. [PMC free article] [PubMed] [Google Scholar]
- 117. Fox AT, Du Toit G, Lang A, Lack G. Food allergy as a risk factor for nutritional rickets. Pediatr Allergy Immunol. 2004;15(6):566–569. [DOI] [PubMed] [Google Scholar]
- 118. Imataka G, Mikami T, Yamanouchi H, Kano K, Eguchi M. Vitamin D deficiency rickets due to soybean milk. J Paediatr Child Health. 2004;40(3):154–155. [DOI] [PubMed] [Google Scholar]
- 119. Miyako K, Kinjo S, Kohno H. Vitamin D deficiency rickets caused by improper lifestyle in Japanese children. Pediatr Int. 2005;47(2):142–146. [DOI] [PubMed] [Google Scholar]
- 120. Barreto-Chang OL, Pearson D, Shepard WE, et al. Vitamin D–deficient rickets in a child with cow's milk allergy. Nutr Clin Pract. 2010;25(4):394–398. [DOI] [PubMed] [Google Scholar]
- 121. Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61(3 suppl):638S–645S. [DOI] [PubMed] [Google Scholar]
- 122. Bogh M. Vitamin D production after UVB: aspects of UV-related and personal factors. Scand J Clin Lab Invest Suppl. 2012;243:24–31. [DOI] [PubMed] [Google Scholar]
- 123. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–378. [DOI] [PubMed] [Google Scholar]
- 124. Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, Puliyel JM. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child. 2002;87(2):111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67:1232–1236. [DOI] [PubMed] [Google Scholar]
- 126. Petersen B, Wulf HC, Triguero-Mas M, et al. Sun and ski holidays improve vitamin D status, but are associated with high levels of DNA damage. J Invest Dermatol. 2014;134(11):2806–2813. [DOI] [PubMed] [Google Scholar]
- 127. Richter K, Breitner S, Webb A, et al. Influence of external, intrinsic and individual behaviour variables on serum 25(OH)D in a German survey. J Photochem Photobiol B. 2014;140:120–129. [DOI] [PubMed] [Google Scholar]
- 128. Terushkin V, Bender A, Psaty EL, Engelsen O, Wang SQ, Halpern AC. Estimated equivalency of vitamin D production from natural sun exposure versus oral vitamin D supplementation across seasons at two US latitudes. J Am Acad Dermatol. 2010;62(6):929.e1–e9. [DOI] [PubMed] [Google Scholar]
- 129. Holick MF. McCollum Award Lecture, 1994: vitamin D–new horizons for the 21st century. Am J Clin Nutr. 1994;60(4):619–630. [DOI] [PubMed] [Google Scholar]
- 130. Bodekær M, Petersen B, Thieden E, et al. UVR exposure and vitamin D in a rural population. A study of outdoor working farmers, their spouses and children. Photochem Photobiol Sci. 2014;13(11):1598–1606. [DOI] [PubMed] [Google Scholar]
- 131. Bogh MK, Schmedes AV, Philipsen PA, Thieden E, Wulf HC. Interdependence between body surface area and ultraviolet B dose in vitamin D production: a randomized controlled trial. Br J Dermatol. 2011;164(1):163–169. [DOI] [PubMed] [Google Scholar]
- 132. Kift R, Berry JL, Vail A, Durkin MT, Rhodes LE, Webb AR. Lifestyle factors including less cutaneous sun exposure contribute to starkly lower vitamin D levels in U.K. South Asians compared with the white population. Br J Dermatol. 2013;169(6):1272–1278. [DOI] [PubMed] [Google Scholar]
- 133. Thieden E, Philipsen PA, Heydenreich J, Wulf HC. UV radiation exposure related to age, sex, occupation, and sun behavior based on time-stamped personal dosimeter readings. Arch Derm. 2004;140(2):197–203. [DOI] [PubMed] [Google Scholar]
- 134. Diffey BL. Is casual exposure to summer sunlight effective at maintaining adequate vitamin D status? Photodermatol Photoimmunol Photomed. 2010;26(4):172–176. [DOI] [PubMed] [Google Scholar]
- 135. Farrar MD, Kift R, Felton SJ, et al. Recommended summer sunlight exposure amounts fail to produce sufficient vitamin D status in UK adults of South Asian origin. Am J Clin Nutr. 2011;94(5):1219–1224. [DOI] [PubMed] [Google Scholar]
- 136. Farrar MD, Webb AR, Kift R, et al. Efficacy of a dose range of simulated sunlight exposures in raising vitamin D status in South Asian adults: implications for targeted guidance on sun exposure. Am J Clin Nutr. 2013;97(6):1210–1216. [DOI] [PubMed] [Google Scholar]
- 137. MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Webb AR, Kift R, Durkin MT, et al. The role of sunlight exposure in determining the vitamin D status of the U.K. white adult population. Br J Dermatol. 2010;163(5):1050–1055. [DOI] [PubMed] [Google Scholar]
- 139. Al-Mustafa ZH, Al-Madan M, Al-Majid HJ, Al-Muslem S, Al-Ateeq S, Al-Ali AK. Vitamin D deficiency and rickets in the Eastern Province of Saudi Arabia. Ann Trop Paediatr. 2007;27(1):63–67. [DOI] [PubMed] [Google Scholar]
- 140. Haider N, Nagi AG, Khan KM. Frequency of nutritional rickets in children admitted with severe pneumonia. J Pak Med Assoc. 2010;60(9):729–732. [PubMed] [Google Scholar]
- 141. Ozkan B. Nutritional rickets. J Clin Res Pediatr Endocrinol. 2010;2(4):137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Pettifor JM. Vitamin D &/or calcium deficiency rickets in infants & children: a global perspective. Indian J Med Res. 2008;127(3):245–249. [PubMed] [Google Scholar]
- 143. Teotia SP, Teotia M. Nutritional bone disease in Indian population. Indian J Med Res. 2008;127(3):219–228. [PubMed] [Google Scholar]
- 144. Balk SJ. Ultraviolet radiation: a hazard to children and adolescents. Pediatrics. 2011;127(3):e791–817. [DOI] [PubMed] [Google Scholar]
- 145. Brooke OG, Brown IR, Bone CD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980;280(6216):751–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kalra P, Das V, Agarwal A, Kumar M, Ramesh V, Bhatia E. Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Brit J Nutrit. 2012;108:1052–1058. [DOI] [PubMed] [Google Scholar]
- 147. Marya RK, Rathee S, Dua V, Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian J Med Res. 1988;88:488–492. [PubMed] [Google Scholar]
- 148. Cockburn F, Belton NR, Purvis RJ, et al. Maternal vitamin D intake and mineral metabolism in mothers and their newborn infants. Br Med J. 1980;281(6232):11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Delvin EE, Salle BL, Glorieux FH, Adeleine P, David LS. Vitamin D supplementation during pregnancy: Effect on neonatal calcium homeostasis. J Pediatr. 1986;109:328–334. [DOI] [PubMed] [Google Scholar]
- 150. Congdon P, Horsman A, Kirby PA, Dibble J, Bashir T. Mineral content of the forearms of babies born to Asian and white mothers. Br Med J (Clin Res Ed). 1983;286(6373):1233–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Mallet E, Gügi B, Brunelle P, Hénocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet Gynecol. 1986;68:300–304. [DOI] [PubMed] [Google Scholar]
- 152. Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Br Med J Clin Res Ed. 1981;283(6298):1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol (Oxf). 2009;70:685–690. [DOI] [PubMed] [Google Scholar]
- 154. Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wagner CL, McNeil R, Hamilton SA, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol. 2013;208(2):137.e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Saadi HF, Dawodu A, Afandi BO, Zayed R, Benedict S, Nagelkerke N. Efficacy of daily and monthly high-dose calciferol in vitamin D-deficient nulliparous and lactating women. Am J Clin Nutr. 2007;85:1565–1571. [DOI] [PubMed] [Google Scholar]
- 157. Leffelaar ER, Vrijkotte TG, van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. Brit J Nutrit. 2010;104:108–117. [DOI] [PubMed] [Google Scholar]
- 158. Weiler H, Fitzpatrick-Wong S, Veitch R, Kovacs H, Schellenberg J, McCloy U. Vitamin D deficiency and whole-body and femur bone mass relative to weight in healthy newborns. CMAJ. 2005;172:757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Viljakainen HT, Saarnio E, Hytinantti T, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95(4):1749–1757. [DOI] [PubMed] [Google Scholar]
- 160. Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N. Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res. 2010;25:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ioannou C, Javaid MK, Mahon P, et al. The effect of maternal vitamin D concentration on fetal bone. J Clin Endocrinol Metab. 2012;97:E2070–E2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. [DOI] [PubMed] [Google Scholar]
- 163. Lawlor DA, Wills AK, Fraser A, Sayers A, Fraser WD, Tobias JH. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: a prospective cohort study. Lancet. 2013;381:2176–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Sayers A, Tobias J. Estimated maternal ultraviolet B exposure levels in pregnancy influence skeletal development of the child. J Clin Endocrinol Metab. 2009;94:765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jarjou LM, Prentice A, Sawo Y, et al. Randomized, placebo-controlled, calcium supplementation study in pregnant Gambian women: effects on breast-milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. Am J Clin Nutr. 2006;83(3):657–666. [DOI] [PubMed] [Google Scholar]
- 166. Koo WW, Walters JC, Esterlitz J, Levine RJ, Bush AJ, Sibai B. Maternal calcium supplementation and fetal bone mineralization. Obstet Gynecol. 1999;94:577–582. [DOI] [PubMed] [Google Scholar]
- 167. Villar J, Abdel-Aleem H, Merialdi M, et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. Am J Obstet Gynecol. 2006;194:639–649. [DOI] [PubMed] [Google Scholar]
- 168. Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80(6 suppl):1752S–1758S. [DOI] [PubMed] [Google Scholar]
- 169. Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med. 2006;1(2):59–70. [DOI] [PubMed] [Google Scholar]
- 170. Saadi HF, Dawodu A, Afandi B, et al. Effect of combined maternal and infant vitamin D supplementation on vitamin D status of exclusively breastfed infants. Matern Child Nutr. 2009;5:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Oberhelman SS, Meekins ME, Fischer PR, et al. Maternal vitamin D supplementation to improve the vitamin D status of breast-fed infants: a randomized controlled trial. Mayo Clin Proc. 2013;88(12):1378–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Basile LA, Taylor SN, Wagner CL, Horst RL, Hollis BW. The effect of high-dose vitamin D supplementation on serum vitamin D levels and milk calcium concentration in lactating women and their infants. Breastfeed Med. 2006;1:27–35. [DOI] [PubMed] [Google Scholar]
- 173. Rothberg AD, Pettifor JM, Cohen DF, Sonnendecker EW, Ross FP. Maternal-infant vitamin D relationships during breast-feeding. J Pediatr. 1982;101:500–503. [DOI] [PubMed] [Google Scholar]
- 174. Ala-Houhala M. 25-Hydroxyvitamin D levels during breast-feeding with or without maternal or infantile supplementation of vitamin D. J Pediatr Gastroenterol Nutr. 1985;4:220–226. [DOI] [PubMed] [Google Scholar]
- 175. Ala-Houhala M, Koskinen T, Terho A, Koivula T, Visakorpi J. Maternal compared with infant vitamin D supplementation. Arch Dis Child. 1986;61:1159–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ortega RM, Martínez RM, Quintas ME, López-Sobaler AM, Andrés P. Calcium levels in maternal milk: relationships with calcium intake during the third trimester of pregnancy. Br J Nutr. 1998;79:501–507. [DOI] [PubMed] [Google Scholar]
- 177. Prentice A, Jarjou LM, Cole TJ, Stirling DM, Dibba B, Fairweather-Tait S. Calcium requirements of lactating Gambian mothers: effects of a calcium supplement on breast-milk calcium concentration, maternal bone mineral content, and urinary calcium excretion. Am J Clin Nutr. 1995;62:58–67. [DOI] [PubMed] [Google Scholar]
- 178. Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The effect of calcium supplementation on bone density during lactation and after weaning. N Engl J Med. 1997;337:523–528. [DOI] [PubMed] [Google Scholar]
- 179. Vaughan LA, Weber CW, Kemberling SR. Longitudinal changes in the mineral content of human milk. Am J Clin Nutr. 1979;32:2301–2306. [DOI] [PubMed] [Google Scholar]
- 180. Kirksey A, Ernst JA, Roepke JL, Tsai TL. Influence of mineral intake and use of oral contraceptives before pregnancy on the mineral content of human colostrum and of more mature milk. Am J Clin Nutr. 1979;32:30–39. [DOI] [PubMed] [Google Scholar]
- 181. Prentice A, Yan L, Jarjou LM, et al. Vitamin D status does not influence the breast-milk calcium concentration of lactating mothers accustomed to a low calcium intake. Acta Paediatr. 1997;86:1006–1008. [DOI] [PubMed] [Google Scholar]
- 182. Basile LA, Taylor SN, Wagner CL, Horst RL, Hollis BW. The effect of high-dose vitamin D supplementation on serum vitamin D levels and milk calcium concentration in lactating women and their infants. Breastfeed Med. 2006;1(1):27–35. [DOI] [PubMed] [Google Scholar]
- 183. Begum R, Coutinho ML, Dormandy TL, Yudkin S. Maternal malabsorption presenting as congenital rickets. Lancet. 1968;1(7551):1048–1052. [DOI] [PubMed] [Google Scholar]
- 184. Ford JA. Proceedings: aetiology of Asian rickets and osteomalacia in the United Kingdom. Arch Dis Child. 1973;48(10):827–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Moncrieff M, Fadahunsi TO. Congenital rickets due to maternal vitamin D deficiency. Arch Dis Child. 1974;49:810–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Russell JG, Hill LF. True fetal rickets. Br J Radiol. 1974;47:732–734. [DOI] [PubMed] [Google Scholar]
- 187. Zeidan S, Bamford M. Congenital rickets with maternal pre-eclampsia. J R Soc Med. 1984;77(5):426–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Park W, Paust H, Kaufmann HJ, Offermann G. Osteomalacia of the mother–rickets of the newborn. Eur J Pediatr. 1987;146(3):292–293. [DOI] [PubMed] [Google Scholar]
- 189. Mittal M, Kumar A, Ramji S, Narula S, Thirupuram S. Congenital rickets. Indian Pediatr. 1990;27(8):857–859. [PubMed] [Google Scholar]
- 190. Teotia M, Teotia SP, Nath M. Metabolic studies in congenital vitamin D deficiency rickets. Indian J Pediatr. 1995;62(1):55–61. [DOI] [PubMed] [Google Scholar]
- 191. Sann L, David L, Frederich A. Congenital rickets. Study of the evolution of secondary hyperparathyroidism. Acta Paediatr Scand. 1977;66(3):323–327. [DOI] [PubMed] [Google Scholar]
- 192. Ramavat L. Vitamin D deficiency rickets at birth in Kuwait. Indian J Pediatr. 1999;66(1):37–43. [DOI] [PubMed] [Google Scholar]
- 193. Innes AM, Seshia MM, Prasad C, et al. Congenital rickets caused by maternal vitamin D deficiency. Paediatr Child Health. 2002;7(7):455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Maiyegun SO, Malek AH, Devarajan LV, Dahniya MH. Severe congenital rickets secondary to maternal hypovitaminosis D: a case report. Ann Trop Paediatr. 2002;22(2):191–195. [DOI] [PubMed] [Google Scholar]
- 195. Mohapatra A, Sankaranarayanan K, Kadam SS, Binoy S, Kanbur WA, Mondkar JA. Congenital rickets. J Trop Pediatr. 2003;49(2):126–127. [DOI] [PubMed] [Google Scholar]
- 196. Erdeve O, Atasay B, Arsan S, Siklar Z, Ocal G, Berberoğlu M. Hypocalcemic seizure due to congenital rickets in the first day of life. Turk J Pediatr. 2007;49(3):301–303. [PubMed] [Google Scholar]
- 197. Tiwari S, Kumar R, Singla S, Dudeja A, Nangia S, Saili A. Congenital rickets presenting as refractory respiratory distress at birth. Indian J Pediatr. 2014;81(8):800–802. [DOI] [PubMed] [Google Scholar]
- 198. Gradus D, Le Roith D, Karplus M, Zmora E, Grief M, Bar-Ziv J. Congenital hyperparathyroidism and rickets: secondary to maternal hypoparathyroidism and vitamin D deficiency. Isr J Med Sci. 1981;17(8):705–708. [PubMed] [Google Scholar]
- 199. Glass EJ, Barr DG. Transient neonatal hyperparathyroidism secondary to maternal pseudohypoparathyroidism. Arch Dis Child. 1981;56(7):565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Loughead JL, Mughal Z, Mimouni F, Tsang RC, Oestreich AE. Spectrum and natural history of congenital hyperparathyroidism secondary to maternal hypocalcemia. Am J Perinatol. 1990;7(4):350–355. [DOI] [PubMed] [Google Scholar]
- 201. Demirel N, Aydin M, Zenciroglu A, et al. Hyperparathyroidism secondary to maternal hypoparathyroidism and vitamin D deficiency: an uncommon cause of neonatal respiratory distress. Ann Trop Paediatr. 2009;29(2):149–154. [DOI] [PubMed] [Google Scholar]
- 202. Kirk J. Congenital rickets – a case report. Aust Paediatr J. 1982;18(4):291–293. [PubMed] [Google Scholar]
- 203. Levin TL, States L, Greig A, Goldman HS. Maternal renal insufficiency: a cause of congenital rickets and secondary hyperparathyroidism. Pediatr Radiol. 1992;22(4):315–316. [DOI] [PubMed] [Google Scholar]
- 204. Wang LY, Hung HY, Hsu CH, Shih SL, Lee YJ. Congenital rickets–a patient report. J Pediatr Endocrinol Metab. 1997;10(4):437–441. [DOI] [PubMed] [Google Scholar]
- 205. al-Senan K, al-Alaiyan S, al-Abbad A, LeQuesne G. Congenital rickets secondary to untreated maternal renal failure. J Perinatol. 2001;21(7):473–475. [DOI] [PubMed] [Google Scholar]
- 206. Samson GR. Skeletal dysplasias with osteopenia in the newborn: the value of alkaline phosphatase. J Matern Fetal Neonatal Med. 2005;17(3):229–231. [DOI] [PubMed] [Google Scholar]
- 207. Rimensberger P, Schubiger G, Willi U. Connatal rickets following repeated administration of phosphate enemas in pregnancy: a case report. Eur J Pediatr. 1992;151(1):54–56. [DOI] [PubMed] [Google Scholar]
- 208. Lamm CI, Norton KI, Murphy RJ, Wilkins IA, Rabinowitz JG. Congenital rickets associated with magnesium sulfate infusion for tocolysis. J Pediatr. 1988;113(6):1078–1082. [DOI] [PubMed] [Google Scholar]
- 209. Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: causes and future directions. Ann Trop Paediatr. 2006;26:1–16. [DOI] [PubMed] [Google Scholar]
- 210. Bener A, Al-Ai M, Hoffmann GF. Vitamin D deficiency in healthy children in a sunny country: associated factors. Int J Food Sci Nutr. 2009;60(suppl 5):60–70. [DOI] [PubMed] [Google Scholar]
- 211. Harris NS, Crawford PB, Yangzom Y, Pinzo L, Gyaltsen P, Hudes M. Nutritional and health status of Tibetan children living at high altitudes. N Engl J Med. 2001;344:341–347. [DOI] [PubMed] [Google Scholar]
- 212. Muhe L, Lulseged S, Mason KE, Simoes EA. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349:1801–1804. [DOI] [PubMed] [Google Scholar]
- 213. Thacher TD, Fischer PR, Isichei CO, Zoakah AI, Pettifor JM. Prevention of nutritional rickets in Nigerian children with dietary calcium supplementation. Bone. 2012;50(5):1074–1080. [DOI] [PubMed] [Google Scholar]
- 214. Fischer PR, Rahman A, Cimma JP, et al. Nutritional rickets without vitamin D deficiency in Bangladesh. J Trop Pediatr. 1999;45:291–293. [DOI] [PubMed] [Google Scholar]
- 215. Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Manaster BJ, Reading JC. Radiographic scoring method for the assessment of the severity of nutritional rickets. J Trop Pediatr. 2000;46:132–139. [DOI] [PubMed] [Google Scholar]
- 216. Cesur Y, Doğan M, Ariyuca S, et al. Evaluation of children with nutritional rickets. J Pediatr Endocrinol Metab. 2011;24:35–43. [DOI] [PubMed] [Google Scholar]
- 217. Weisberg P, Scanlon KS, Li R, Cogswell ME. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. Am J Clin Nutr. 2004;80(6 suppl):1697S–1705S. [DOI] [PubMed] [Google Scholar]
- 218. Uush T. Prevalence of classic signs and symptoms of rickets and vitamin D deficiency in Mongolian children and women. J Steroid Biochem Mol Biol. 2013;136:207–210. [DOI] [PubMed] [Google Scholar]
- 219. Bener A, Hoffmann G. Nutritional rickets among children in a sun rich country. Int J Pediatr Endocrinol. 2010;2010:410502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Tserendolgor U, Mawson JT, Macdonald AC, Oyunbileg M. Prevalence of rickets in Mongolia. Asia Pac J Clin Nutr. 1998;7:325–328. [PubMed] [Google Scholar]
- 221. Underwood P, Margetts B. High levels of childhood rickets in rural North Yemen. Soc Sci Med. 1987;24:37–41. [DOI] [PubMed] [Google Scholar]
- 222. Goldacre M, Hall N, Yeates DG. Hospitalisation for children with rickets in England: a historical perspective. Lancet. 2014;383(9917):597–598. [DOI] [PubMed] [Google Scholar]
- 223. Gordon CM, Williams AL, Feldman HA, et al. Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrinol Metab. 2008;93(7):2716–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Strand MA, Perry J, Jin M, et al. Diagnosis of rickets and reassessment of prevalence among rural children in northern China. Pediatr Int. 2007;49:202–209. [DOI] [PubMed] [Google Scholar]
- 225. Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin D among infants breastfed for prolonged periods. Pediatrics. 2010;125:105–111. [DOI] [PubMed] [Google Scholar]
- 226. Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Isichei CO, Chan GM. Case-control study of factors associated with nutritional rickets in Nigerian children. J Pediatr. 2000;137:367–373. [DOI] [PubMed] [Google Scholar]
- 227. World Health Organization. Guidelines on food fortification with micronutrients. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 228. Allen L. New approaches for designing and evaluating food fortification programs. J Nutr. 2006;136:1055–1058. [DOI] [PubMed] [Google Scholar]
- 229. Flynn M. Recommendations for a national policy on vitamin D supplementation for infants in Ireland. Dublin, Ireland: Food Safety Authority of Ireland; 2007. [Google Scholar]
- 230. Engle-Stone R, Ndjebayi AO, Nankap M, Brown K. Consumption of potentially fortifiable foods by women and young children varies by ecological zone and socio-economic status in Cameroon. J Nutr. 2012;142(3):555–565. [DOI] [PubMed] [Google Scholar]
- 231. Babu US, Calvo MS. Modern India and the vitamin D dilemma: evidence for the need of a national food fortification program. Mol Nutri Food Res. 2010;54(8):1134–1147. [DOI] [PubMed] [Google Scholar]
- 232. Kiely M, Black L. Dietary strategies to maintain adequacy of circulating 25-hydroxyvitamin D concentrations. Scand J Clin Lab Invest Suppl. 2012;243:14–23. [DOI] [PubMed] [Google Scholar]
- 233. Lamberg-Allardt C, Brustad M, Meyer H, Steingrimsdottir L. Vitamin D - a systematic literature review for the 5th edition of the Nordic Nutrition Recommendations. Food Nutr Res. 2013;3:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Black LJ, Seamans KM, Cashman KD, Kiely M. An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J Nutr. 2012;142(6):1102–1108. [DOI] [PubMed] [Google Scholar]
- 235. Pietrek J, Preece MA, Windo J, et al. Prevention of vitamin-D deficiency in Asians. Lancet. 1976;1(7970):1145–1148. [DOI] [PubMed] [Google Scholar]
- 236. Piirainen T, Laitinen K, Isolauri E. Impact of national fortification of fluid milks and margarines with vitamin D on dietary intake and serum 25-hydroxyvitamin D concentration in 4-year-old children. Eur J Clin Nutr. 2007;61(1):123–128. [DOI] [PubMed] [Google Scholar]
- 237. Khadgawat R, Marwaha RK, Garg MK, et al. Impact of vitamin D fortified milk supplementation on vitamin D status of healthy school children aged 10–14 years. Osteoporos Int. 2013;24(8):2335–2343. [DOI] [PubMed] [Google Scholar]
- 238. Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics. 2009;124(3):e362–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Thacher TD, Fischer PR, Pettifor JM. Vitamin D treatment in calcium-deficiency rickets: a randomised controlled trial. Arch Dis Child. 2014;99(9):807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Ekbote VH, Khadilkar AV, Chiplonkar SA, Hanumante NM, Khadilkar VV, Mughal MZ. A pilot randomized controlled trial of oral calcium and vitamin D supplementation using fortified laddoos in underprivileged Indian toddlers. Eur J Clin Nutr. 2011;65(4):440–446. [DOI] [PubMed] [Google Scholar]
- 241. Nieman DC, Henson DA, Sha W. Ingestion of micronutrient fortified breakfast cereal has no influence on immune function in healthy children: a randomized controlled trial. Nutr J. 2011; 10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Rafferty K, Watson P, Lappe J. The selection and prevalence of natural and fortified calcium food sources in the diets of adolescent girls. J Nutr Educ Behav. 2011;43(2):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Hackett AF, Rugg-Gunn AJ, Allinson M, Robinson CJ, Appleton DR, Eastoe JE. The importance of fortification of flour with calcium and the sources of Ca in the diet of 375 English adolescents. Br J Nutr. 1984;51(2):193–197. [DOI] [PubMed] [Google Scholar]
- 244. Greer F. Defining vitamin D deficiency in children: beyond 25-OH vitamin D serum concentrations. Pediatrics. 2009;124:1471–1473. [DOI] [PubMed] [Google Scholar]
- 245. Zipitis CS, Markides GA, Swann IL. Vitamin D deficiency: prevention or treatment? Arch Dis Child. 2006;91:1011–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Högler W. Complications of vitamin D deficiency from the foetus to the infant: One cause, one prevention, but who's responsibility? Best Pract Res Clin Endocrinol Metab. 2015;29(3):385–398. [DOI] [PubMed] [Google Scholar]