Abstract
Context:
Free T3 (FT3) has been positively associated with body mass index (BMI) in cross-sectional studies in healthy individuals. This is difficult to reconcile with clinical findings in pathological thyroid dysfunction.
Objective:
We aimed to investigate whether childhood adiposity influences FT3 levels.
Design:
Mendelian randomization using genetic variants robustly associated with BMI.
Setting:
Avon Longitudinal Study of Parents and Children, a population-based birth cohort.
Participants:
A total of 3014 children who had thyroid function measured at age 7, who also underwent dual x-ray absorptiometry scans at ages 9.9 and 15.5 years and have genetic data available.
Main Outcome Measures:
FT3.
Results:
Observationally at age 7 years, BMI was positively associated with FT3: β-standardized (β-[std]) = 0.12 (95% confidence interval [CI]: 0.08, 0.16), P = 4.02 × 10−10; whereas FT4 was negatively associated with BMI: β-(std) = −0.08 (95% CI: −0.12, −0.04), P = 3.00 × 10−5. These differences persisted after adjustment for age, sex, and early life environment. Genetic analysis indicated 1 allele change in BMI allelic score was associated with a 0.04 (95% CI: 0.03, 0.04) SD increase in BMI (P = 6.41 × 10−17). At age 7, a genetically determined increase in BMI of 1.89 kg/m2 was associated with a 0.22 pmol/L (95% CI: 0.07, 0.36) increase in FT3 (P = .004) but no substantial change in FT4 0.01 mmol/L, (95% CI: −0.37, 0.40), P = .96.
Conclusion:
Our analysis shows that children with a genetically higher BMI had higher FT3 but not FT4 levels, indicating that higher BMI/fat mass has a causal role in increasing FT3 levels. This may explain the paradoxical associations observed in observational analyses. Given rising childhood obesity levels, this relationship merits closer scrutiny.
The relationship between pathological thyroid dysfunction and body composition is well established with hyperthyroidism associated with weight loss (1) and subsequent hypothyroidism after treatment associated with weight gain (2). There is now increasing awareness that variation in thyroid hormone levels within and just outside the normal population reference range is associated with differences in important health outcomes (3). In particular, cross-sectional studies have shown that variation in thyroid status across the population reference range is associated with substantial differences in body composition (3–6).
However, the nature of the association between nonpathological variation in thyroid status and body composition remains unclear. Longitudinal studies demonstrate that weight gain is accompanied by increased TSH (5) and weight loss is related to decreased TSH and also surprisingly to decreased free T3 (FT3) levels (7). Elevations in TSH and FT3, but not FT4, have also been observed in obese individuals (8, 9). More recently, studies have also shown that in healthy euthyroid adults FT3 is positively associated with body mass index (BMI) and measures of adiposity, whereas FT4 is negatively associated (10–13). This finding is of particular interest, because thyroid hormones, FT3 in particular, increase energy expenditure (14), and hence would be expected to be negatively associated with fat mass. However, because these studies were based on cross-sectional analyses, reverse causation or confounding leading to a spurious association remains a possibility.
Genetic association studies offer an alternative to traditional epidemiological analyses. The random assortment of genes that occurs during gamete formation results in an equal distribution of confounding factors among different genotypes. Furthermore, the direction of causation is from the BMI genetic instruments onto FT3, and cannot be due to reverse causation (a key problem in observational epidemiology) as FT3 levels cannot cause genetic variation. Finally, genetic variants and their effects are subject to relatively little measurement error or bias. A key benefit of using this approach in this analysis is that BMI is associated with a wide range of behavioral, social, and physiological factors that might confound its association with FT3. Our use of genetic variants substantially reduces the risk of this confounding (15). Importantly this approach also enables us to clarify the direction of causation between BMI and FT3 that is to assess whether changes in BMI/fat mass causally affect thyroid status (16) or vice versa. This is information not obtainable using standard epidemiological analysis.
Because alleles are largely passed from parents to offspring independently of the environment, offspring who inherit more alleles associated with BMI are in effect being randomly assigned a higher BMI dosage. Mendelian randomization (MR) can therefore be thought of as analogous to a randomized trial with randomization by genotype taking place at conception. A by-genotype analysis is equivalent to an intention-to-treat analysis in a randomized controlled trial, in which individuals are analyzed according to the group they were randomized into, independent of whether they complied to the treatment regimen or not.
In this study, we undertook MR analyses in a large population-based birth cohort, the Avon Longitudinal Study of Parents and Children (ALSPAC) to examine the nature of the relationship between BMI and FT3. We used 32 independent genetic correlates of BMI, confirmed in a large-scale metaanalysis of genome-wide association studies (17), to assess whether there is a causal pathway between BMI and FT3 levels, which would explain the paradoxical opposing relationship between FT3 and FT4 on BMI in observational studies.
Materials and Methods
Participants
ALSPAC is a prospective birth cohort that enrolled over 13 000 pregnant women in the former County of Avon, United Kingdom, with an expected delivery date between April 1991 and December 1992 (see www.alspac.bris.ac.uk) (18, 19). The study website contains details of all the data that is available through a fully searchable database www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees.
Assessment of height and body composition
Standing height was measured using a wall-mounted Harpenden stadiometer (Holtain Ltd). BMI was calculated as weight (in kilograms) divided by height (in meters) squared. As a measure of adiposity BMI has several limitations (20); in particular, it does not reliably distinguish between fat and lean mass. Therefore total fat mass was also assessed using a Lunar Prodigy narrow fan beam densitometer to perform a whole-body dual x-ray absorptiometry (DXA) scan. Additional details of these measurements, including their reproducibility, are described elsewhere (21). The fat mass index (FMI) was also calculated as total fat mass (in kilograms) divided by height (in meters) squared. Data on BMI were collected at ages 7 and 15 years with anthropometric data from DXA performed at ages 9.9 and 15.5 years.
Laboratory measures
Serum TSH, FT3, and FT4 were measured at age 7 years (median age 89 mo) by chemiluminescent emission using a photomultiplier on Cobas e601 (Roche Diagnostics) in 3014 children who also had genetic data and body composition data available. Repeat thyroid function at age 15 years was performed in 735 of these children (median age 185 mo). Reference ranges for adults are TSH, 0.27–4.2 mU/L; FT3, 3.9–6.7 pmol/L; and FT4, 12–22 pmol/L.
Genotyping
Genotyping in ALSPAC has been previously described (22). Genome wide association study data was generated by Sample Logistics and Genotyping Facilities at the Wellcome Trust Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe. Single nucleotide polymorphisms (SNPs) were removed if the minor allele frequency was less than 1%, the call rate was less than 95% or the P value from an exact test of Hardy-Weinberg equilibrium was less than 5.7 × 10−7. Individual samples were excluded on the basis of incorrect sex assignment, minimal or excessive heterozygosity, high levels of missingness, and cryptic relatedness (16%). Established BMI variants that had not been genotyped directly were imputed with MACH 1.0.16 Markov Chain Haplotyping software (23, 24) using CEPH individuals from HapMap phase 2 (release 22) reference set.
Statistical analysis
Implausible height, weight, BMI, fat mass, lean mass, blood pressure, and thyroid measurement (>4 SD from the mean for the sex and age-specific category) were considered as outliers and recoded to missing. TSH and fat mass were natural log transformed to approximate the normal distribution. Descriptive statistics are presented as backtransformed (geometric) means, SDs, medians, and lower and upper quartiles. All thyroid, body composition, and blood pressure variables were standardized; analyses are therefore presented as per SD.
Analyses were initially performed adjusted for age at measurements and sex (model 1). Where appropriate, 3 further models controlling for key potential confounders were undertaken; model 2 also adjusted for thyroid hormone parameters, model 3 = model 2 adjusted for height, measures of social class and early life environment, including home ownership, maternal age at birth of child, maternal highest educational qualification, maternal smoking in pregnancy, family adversity index, and parents and home score (Supplemental Materials). Height was included in model 3 as although BMI was designed to assess weight independently of height, it remains correlated with height owing to its generalized derivation. Analysis of anthropometric relationships with thyroid hormone status at age 15 also incorporated pubertal status (model 4), which was assessed using a Tanner stage questionnaire (25) at age 13.5 years (pubic hair domain) range from 13.1 to 14.4 years.
Instrumental variable analysis
Speliotes et al previously reported 32 variants to be associated with BMI (Supplemental Table 1) (17). An “allelic score” was then created by summing the dosages for BMI-increasing alleles across all 32 SNPs (26). Here, the dose of the effect allele at each locus was weighted by the effect size of the variant in this independent metaanalysis (17); these doses were then summed to reflect the average number of effect weighted BMI increasing alleles carried by an individual. This combined score was able to explain a greater proportion of variance in BMI than single SNPs (27) and served as an instrumental variable in our MR analysis. This approach has previously been used in ALSPAC to study the relationship between adiposity and physical activity in children (28).
For investigating associations between the allelic score and standardized phenotypes, continuous effects were estimated using linear regression with adjustment for models as above. An additive genetic model was assumed, because there was no evidence for interaction effects among the SNPs combined in the allelic score (17, 29). Although covariates are anticipated to be randomly distributed with respect to genotype (16), we also examined associations between confounders and genotypes to check the core instrumental variable assumption that the genetic instrument (BMI allelic score) is independent of factors that might potentially confound the observational association (30), and allow for comparison with conventional observational epidemiological models.
We then performed a 2-stage least squares regression using the weighted allelic score as an instrument for BMI/adiposity using the “ivreg2” command in Stata. F-statistics from the first-stage regression between genotype and BMI/adiposity were examined to check the assumption that the instrument is sufficiently associated with the exposure to reduce the possibility of weak instrument bias (31). Using the “ivendog' command in Stata the Durbin-Wu-Hausman (DWH) test for endogeneity was performed to compare effect estimates from the second stage of the instrumental variable analysis and estimates from linear regression. Models were repeated as described above.
Sensitivity analyses
To explore the potential distorting effects of pleiotropy in our analysis we repeated our analyses using 2 independent genetic instruments. Pleiotropy occurs when a genetic instrument has an effect on the outcome (FT3) independently of its effect on the exposure (adiposity), which has implications for key assumptions made in MR analyses (32). Similar instrumental variable estimates acquired using 2 independent instruments would provide suggestive evidence against the existence of a pleiotropic effect, as it would be unlikely that both instruments had shared pleiotropy (32). The 2 independent genetic instruments were rs1558902 in FTO (the individual SNP with the largest effect size on BMI identified by Speliotes et al [17]), and a weighted allelic score constructed from the remaining 31 SNPs associated with BMI.
All data analysis was performed using Stata version 12.1 (StataCorp).
Results
Study population and baseline characteristics
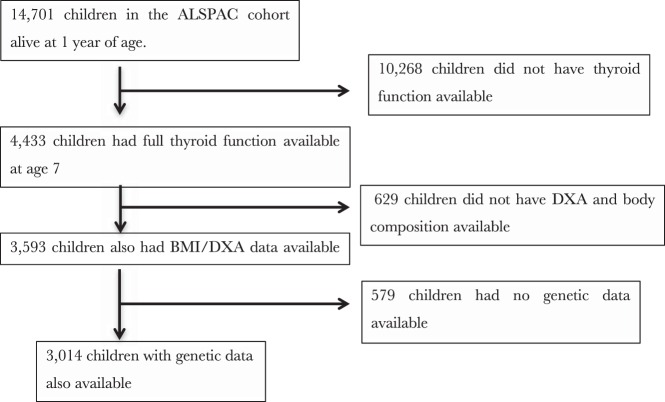
A total of 3014 individuals (1542 males, 1472 females) had relevant thyroid, body composition, and genetic data available (Figure 1). Children in our study dataset were more likely to have lower BMI and fat mass as well as several higher markers of affluence and fewer early life events than the remainder of the ALSPAC cohort (Supplemental Table 2). A total of 473 (16.6%) of children in our study population were obese or overweight at age 7, and 373 were classified as overweight with 100 classified as obese according to cutoffs proposed by the International Obesity Task Force (33).
Figure 1.
Study participants.
Observational analysis of baseline characteristics
There was a good correlation between BMI at age 7 years and FMI at age 9 years (Pearson's correlation coefficient = 0.73). Height and lean mass were both greater in males (126.5 vs 125.7 cm, P < .001) (25.6 vs 23.7 kg, P < .001), respectively; however, BMI and fat mass were greater in females (16.0 vs 16.3 kg/m2; P < .001) (9.46 vs 7.10 kg, P < .001). Baseline characteristics are shown in Table 1.
Table 1.
Baseline Characteristics
| Variable | All (n = 3014) |
Males (n = 1542) |
Females (n = 1472) |
|||
|---|---|---|---|---|---|---|
| Mean or Percentage | SD | Mean or Percentage | SD | Mean or Percentage | SD | |
| BMI age 7 (kg/m2) | 16.1 | 1.89 | 16.0 | 1.76 | 16.3 | 2.09 |
| Height age 7 (cm) | 126.1 | 5.61 | 126.5 | 5.56 | 125.7 | 5.64 |
| Weight age 7 (kg) | 25.8 | 4.34 | 25.7 | 4.16 | 25.9 | 4.51 |
| Fat mass measured by DXA (kg) | 8.26 | 4.77 | 7.10 | 4.53 | 9.46 | 4.71 |
| Body fat percentage (fat mass [kg]/weight [kg]) × 100a | 22.6 | 8.69 | 19.4 | 18.26 | 25.9 | 7.87 |
| Lean mass measured by DXA (kg) | 24.7 | 3.11 | 25.6 | 2.89 | 23.7 | 3.05 |
| Age at DXA assessment (mo) | 90.3 | 3.92 | ||||
| Age 7 FT3 (pmol/L) | 6.28 | 0.62 | 6.22 | 0.62 | 6.34 | 0.62 |
| Age 7 FT4 (pmol/L) | 15.7 | 1.66 | 15.5 | 1.58 | 15.9 | 1.71 |
| Age 7 TSH (mU/L) | 2.28 | 0.91 | 2.36 | 0.90 | 2.20 | 0.90 |
| Age 15 FT3 (pmol/L) | 5.84 | 0.74 | 6.19 | 0.69 | 5.50 | 0.63 |
| Age 15 FT4 (pmol/L) | 15.4 | 1.90 | 15.4 | 1.98 | 15.4 | 1.84 |
| Age 15 TSH (mU/L) | 2.33 | 1.00 | 2.43 | 1.05 | 2.24 | 0.93 |
| Family adversity indexb | 3.71 | 4.45 | 3.73 | 3.87 | 3.69 | 3.80 |
| Home scorec | ||||||
| 0–4 (%) | 3.47 | 2.77 | 4.21 | |||
| 5–8 (%) | 23.9 | 23.2 | 24.6 | |||
| 9–12 (%) | 72.7 | 74.1 | 71.2 | |||
| Housing statusd | ||||||
| Owned/mortgaged (%) | 85.6 | 85.6 | 85.6 | |||
| Privately rented (%) | 11.0 | 11.8 | 10.2 | |||
| Council rented/other (%) | 3.3 | 2.7 | 4.0 | |||
| Maternal age at childbirth (y) | 29.4 | 4.2 | 29.5 | 4.5 | 29.4 | 4.3 |
| Maternal highest educational statuse | ||||||
| Low (%) | 18.4 | 19.3 | 17.4 | |||
| Middle (%) | 34.8 | 35.5 | 34.1 | |||
| High (%) | 46.8 | 45.2 | 48.5 | |||
| Maternal smoking in pregnancyf | ||||||
| None (%) | 84.5 | 84.3 | 84.7 | |||
| Some (%) | 15.5 | 15.7 | 15.2 | |||
| Parityg | ||||||
| 0–1 (%) | 82.1 | 80.9 | 83.4 | |||
| 2–4 (%) | 17.9 | 18.7 | 16.6 | |||
| >5 (%) | 0.2 | 0.28 | 0.07 | |||
% are expressed as column %.
Two children with missing data for weight at age 9.
A total of 323 children with missing data.
A total of 277 children with missing data.
A total of 215 children with missing data.
A total of 224 children with missing data.
A total of 201 children with missing data.
A total of 226 children with missing data.
Relationship between thyroid status and body composition
BMI at age 7 years was positively associated with FT3 at age 7 years β-standardized (β-[std]) = 0.13 (95% confidence interval [CI]: 0.10, 0.16), P = 5.30 × 10−16 and fat mass at age 9 β-(std) = 0.18 (95% CI: 0.15, 0.21), P = 4.32 × 10−25 even after adjustment for confounders (Table 2). FT3 was also positively associated with lean mass β-(std) = 0.10 (95% CI: 0.06, 0.13), P = 6.64 × 10−09. However, adding fat mass to this model attenuated the association (β-[std] = 0.02, 95% CI: −0.02, 0.04; P = .32). In contrast, adjusting for lean mass in the relationship between FT3 and BMI and FT3 and fat mass had no substantial effect on effect estimates β-(std) = 0.07 (95% CI: 0.04, 0.10), P = 7.72 × 10−07 and β-(std) = 0.12 (95% CI: 0.09, 0.16), P = 5.73 × 10−14, respectively.
Table 2.
Associations Between Measures of Adiposity and Thyroid Hormone Parameters at Age 7
| n | TSH (mU/L) |
FT3 (pmol/L) |
FT4 (pmol/L) |
FT3 to FT4 Ratiob |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-(std) | 95% CI | P Valuea | β-(std) | 95% CI | P Valuea | β-(std) | 95% CI | P Valuea | β-(std) | 95% CI | P Valuea | ||
| BMI (age 7) | |||||||||||||
| Model 1 | 3014 | 0.03 | 0.004, 0.06 | .05 | 0.13 | 0.10, 0.16 | 5.30 × 10−16 | −0.05 | −0.08, −0.02 | .001 | 0.15 | 0.12, 0.18 | 1.45 × 10−20 |
| Model 2 | 3014 | 0.02 | −0.01, 0.06 | .13 | 0.16 | 0.13, 0.19 | 6.62 × 10−21 | −0.10 | −0.13, −0.06 | 1.64 × 10−08 | — | — | — |
| Model 3 | 3014 | 0.03 | −0.004, 0.07 | .09 | 0.12 | 0.08, 0.16 | 4.02 × 10−10 | −0.08 | −0.12, −0.04 | .00003 | 0.12 | 0.09, 0.16 | 5.71 × 10−11 |
| Weight (age 7) | |||||||||||||
| Model 1 | 3014 | 0.02 | −0.01, 0.05 | 0.15 | .11 | 0.08, 0.14 | 8.16 × 10−17 | −0.04 | −0.07, 0.02 | .001 | 0.13 | 0.10, 0.15 | 8.46 × 10−22 |
| Model 2 | 3014 | 0.01 | −0.01, 0.04 | 0.35 | .13 | 0.11, 0.16 | 5.20 × 10−22 | −0.08 | −0.11, −0.05 | 6.30 × 10−09 | — | — | — |
| Model 3 | 3014 | 0.02 | −0.001, 0.05 | 0.08 | .09 | 0.06, 0.13 | 5.62 × 10−10 | −0.05 | − 0.08 − 0.03 | .0001 | 0.08 | 0.06, 0.11 | 2.07 × 10−07 |
| Height (age 9) | |||||||||||||
| Model 1 | 3014 | −0.002 | −0.03, 0.03 | 0.92 | .07 | 0.04, 0.10 | 8.07 × 10−06 | −0.03 | −0.06, 0.002 | .03 | 0.09 | 0.06, 0.12 | 2.91 × 10−08 |
| Model 2 | 3014 | −0.01 | −0.04, 0.02 | 0.68 | .09 | 0.05, 0.12 | 1.20 × 10−07 | −0.06 | −0.09, −0.03 | .0003 | — | — | — |
| Model3c | 3014 | 0.002 | −0.03, 0.03 | 0.99 | .08 | 0.05, 0.12 | 1.29 × 10−06 | −0.06 | −0.10, −0.02 | .001 | 0.09 | 0.06, 0.12 | 1.47 × 10−07 |
| Fat mass (age 9) | |||||||||||||
| Model 1 | 3014 | 0.01 | −0.02, 0.04 | 0.74 | .18 | 0.15, 0.21 | 4.32 × 10−25 | −0.04 | −0.08, −0.01 | .02 | 0.18 | 0.15, 0.21 | 1.86 × 10−25 |
| Model 2 | 3014 | −0.003 | −0.03, 0.03 | 0.87 | .21 | 0.17, 0.24 | 1.17 × 10−30 | −0.10 | −0.13, −0.06 | 2.67 × 10−08 | — | — | — |
| Model 3d | 2538 | −0.008 | −0.04, 0.03 | 0.64 | .12 | 0.09, 0.16 | 4.02 × 10−12 | −0.07 | −0.10, −0.03 | .002 | 0.11 | 0.08, 0.14 | 8.06 × 10−11 |
| FMI (age 9) | |||||||||||||
| Model 1 | 3014 | 0.01 | −0.03, 0.04 | 0.75 | .17 | 0.13, 0.20 | 1.56 × 10−22 | −0.04 | −0.07, 0.002 | .04 | 0.17 | 0.14, 0.20 | 1.54 × 10−22 |
| Model 2 | 3014 | −0.003 | −0.04, 0.03 | 0.84 | .19 | 0.16, 0.23 | 2.16 × 10−27 | −0.10 | −0.13, −0.06 | 2.42 × 10−07 | — | — | — |
| Model 3d | 2538 | −0.01 | −0.04, 0.02 | 0.65 | .13 | 0.09, 017 | 3.99 × 10−12 | −0.07 | −0.11, −0.03 | .002 | 0.12 | 0.08, 0.15 | 7.98 × 10−11 |
| Lean mass (age 9) | |||||||||||||
| Model 1 | 3014 | 0.01 | −0.02, 0.06 | 0.18 | .10 | 0.06, 0.13 | 6.64 × 10−09 | −0.05 | −0.09, −0.02 | .002 | 0.12 | 0.09, 0.16 | 1.73 × 10−13 |
| Model 2 | 3014 | 0.02 | −0.02, 0.05 | 0.35 | .12 | 0.09, 0.15 | 3.46 × 10−12 | −0.09 | −0.12, −0.05 | 8.91 × 10−07 | — | — | — |
| Model 3d | 2538 | 0.02 | −0.01, 0.04 | 0.11 | .01 | −0.02, 0.03 | .56 | −0.03 | −0.05, −0.01 | .01 | 0.02 | −0.0001, 0.04 | .05 |
Model 1 adjusted for age and sex model 2 adjusted for model 1 and other thyroid hormone parameters. Model 3 adjusted for model 2 and height, markers of social class, and early life environment (home ownership, maternal age at birth of child, maternal highest educational qualification, maternal smoking in pregnancy, family adversity index, and parents and home score). P, strength of evidence against the null hypothesis of no association.
Calculated using the Wald test.
Not adjusted for other thyroid hormone parameters.
Not adjusted for height.
A total of 476 individuals with missing data.
FT4 was negatively associated with fat mass β-(std) = −0.04 (95% CI: −0.08, −0.01), P = .02 and lean mass β-(std) = −0.05 (95% CI: −0.09, −0.02), P = .002 as well as height and weight (Table 2). No clear associations between TSH and body composition were observed even after adjusting for confounders and other thyroid hormone parameters.
Similar cross-sectional associations were also observed with thyroid hormone parameters at age 15 years and BMI at age 15 years for FT3 and FT3 to FT4 ratio; however, effect estimates were weaker (Supplemental Table 3). Much weaker or no associations were observed with other components of body composition. Analysis revealed that for every 0.20-kg/m2 increase in FMI between ages 9 and 15 years, there was a 0.10 pmol/L increase in FT3 (P = .005).
Genotypic associations
The allelic score for BMI was normally distributed with a mean of 29.1 (SD 3.87 range, 15.1–45.0) (Supplemental Figure 1) and explained 2.29% of the variance in standardized BMI and 2.93% of the variance in standardized FMI. A per allele change in allelic score was associated with a 0.04 (95% CI: 0.03, 0.04) SD increase in BMI (P = 6.41 × 10−17) at age 7 years, a 0.04 SD increase (95% CI: 0.04, 0.05) in FMI (P = 4.87 × 10−21) at age 9 years, and a 0.05 SD increase (95% CI: 0.04, 0.06) in BMI at age 15 years (P = 1.16 × 10−16). In contrast to measures of adiposity, confounding factors were not associated with the allelic score in this cohort aside from a weak association for maternal smoking in pregnancy, likely representing a type-1 error (Supplemental Table 4).
Mendelian randomization
Genetic analysis demonstrated that a genetically higher BMI of 1 SD (1.89 kg/m2) at age 7 years was associated with a 0.22 pmol/L (95% CI: 0.07, 0.36) increase in FT3 (P = .004) (Table 3). We did not observe strong evidence of a departure of instrumental-variable-derived estimates from observational results, as demonstrated by DWH tests (P = .08), indicating similarity between observational and MR estimates in the effect of BMI on FT3. Point estimates for standardized effect sizes from the instrumental variable analysis were greater (0.30 vs 0.13) than those derived from basic observational analyses. Similar results were observed when FMI at age 9 years was instrumented (Table 3) and when assessing FT3 and adiposity data for individuals at age 15 years (Supplemental Tables 5 and 6) with no evidence of difference in the regression estimates at ages 7 and 15 years (P = .88).
Table 3.
Associations Between BMI/FMI and FT3 and FT4 Levels at Age 7 as Tested Both by Conventional Epidemiological Approaches and Through the Application of Instrumental Variable Analysis Using a 32-SNP Weighted Allelic BMI Score as an Instrument
| Adiposity | Model | n | Observational Linear Regression |
Instrumental Variable Regression (Weighted Allelic Score With 32 SNPs) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | β-(std) (95% CI) | P Value | F-Statistic | Partial R2 | B (95% CI) | β-(std) (95% CI) | P Value | P Value (DWH) | |||
| FT3 age 7 | |||||||||||
| BMI (kg/m2) | Model 1 | 3014 | 0.05 (0.04, 0.06) | 0.13 (0.10, 0.16) | 5.30 × 10−16 | 72.4 | 0.009 | 0.22 (0.07, 0.36) | 0.35 (0.11, 0.58) | .004 | .08 |
| Age 7 | Model 2 | 3014 | 0.05 (0.04, 0.06) | 0.16 (0.13, 0.19) | 6.61 × 10−21 | 73.7 | 0.009 | 0.22 (0.08, 0.36) | 0.35 (0.143 0.57) | .002 | .09 |
| Model 3a | 2538 | 0.04 (0.03, 0.05) | 0.12 (0.08, 0.16) | 4.02 × 10−13 | 56.6 | 0.08 | 0.24 (0.06, 0.42) | 0.38 (0.09, 0.68) | .01 | .06 | |
| FMI (kg/m2) | Model 1 | 3014 | 0.22 (0.17, 0.26) | 0.17 (0.13 0.20) | 1.56 × 10−22 | 100.4 | 0.03 | 0.19 (0.07, 0.31) | 0.30 (0.10, 0.50) | .002 | .21 |
| Age 9 | Model 2 | 3014 | 0.23 (0.19, 0.27) | 0.19 (0.16, 0.23) | 2.16 × 10−27 | 100.7 | 0.11 | 0.19 (0.08, 0.31) | 0.31 (0.12, 0.50) | .001 | .22 |
| Model 3a | 2538 | 0.17 (0.12, 0.22) | 0.13 (0.09, 0.17) | 3.99 × 10−12 | 79.5 | 0.09 | 0.20 (0.05, 0.35) | 0.32 (0.08, 0.56) | .008 | .14 | |
| FT4 age 7 | |||||||||||
| BMI (kg/m2) | Model 1 | 3014 | −0.07 (−0.10, −0.03) | −0.05 (−0.08, −0.02) | .001 | 72.4 | 0.01 | 0.01 (−0.37, 0.40) | 0.01 (−0.23, 0.24) | .96 | .58 |
| Age 7 | Model 2 | 3014 | −0.12 (−0.16, −0.08) | −0.10 (−0.13, −0.06) | 1.64 × 10−08 | 67.6 | 0.11 | −0.18 (−0.56, 0.20) | −0.11 (−0.34, 0.12) | .35 | .92 |
| Model 3a | 2538 | −0.09 (−0.14, −0.05) | −0.08 (−0.12, −0.04) | .00003 | 46.8 | 0.10 | −0.11 (−0.58, 0.38) | −0.06 (−0.36, 0.23) | .68 | .92 | |
| FMI (kg/m2) | Model 1 | 3014 | −0.01 (−0.02, −0.001) | −0.04 (−0.07, −0.002) | .04 | 100 | 0.02 | −0.01 (−0.33, 0.32) | −0.004 (−0.21, 0.20) | .97 | .81 |
| Age 9 | Model 2 | 3014 | −0.03 (−0.04, −0.02) | −0.09 (−0.13, −0.06) | 2.42 × 10−07 | 90.3 | 0.11 | −0.18 (−0.51, 0.14) | −0.11 (−0.32, 0.09) | .28 | .76 |
| Model 3a | 2538 | −0.02 (−0.03, −0.01) | −0.07 (−0.11, −0.03) | .002 | 74.1 | 0.11 | −0.07 (−0.47, 0.34) | −0.04 (−0.28, 0.21) | .75 | .76 | |
Model 1 adjusted for age and sex model 2 adjusted for model 1 and other thyroid hormone parameters. Model 3 adjusted for model 2 and height, markers of social class, and early life environment (home ownership, maternal age at birth of child, maternal highest educational qualification, maternal smoking in pregnancy, family adversity index, and parents and home score). B, β-coefficient; P, strength of evidence against the null hypothesis of no association; P (DWH), P value of the Durbin form of the DWH test, which examines the difference between the estimates from linear regression and instrumental variable analysis.
A total of 476 individuals with missing data.
Genetic analysis demonstrated that a genetically higher BMI of 1 SD did not reveal any substantial evidence of an association with FT4 β-(std) = 0.01 (95% CI: −0.23, 0.24), P = .96 (Table 3).
MR analysis using multiple independent instruments
Results of genetic analysis using rs1558902 in FTO (the individual SNP with the largest effect size on BMI) were compared with those of a weighted allelic score consisting of the remaining 31 genetic variants (Table 4) and both revealed a positive association with individuals with a genetically higher BMI having higher FT3 levels making pleiotropy unlikely. Again no evidence of association was observed with FT4 levels (Table 4).
Table 4.
Associations Between BMI/FMI and FT3 and FT4 Levels at Age 7 as Tested Both by Conventional Epidemiological Approaches and Through the Application of Instrumental Variable Analyses
| Adiposity | Model | n | Linear Regression |
Instrumental Variable Regression (Weighted Allelic Score With 31 SNPs) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | β-(std) (95% CI) | P Value | F-Statistic | Partial R2 | B (95% CI) | β-(std) (95% CI)a | P Value | P Value (DWH) | |||
| 31 SNPs (all SNPs excluding FTO) | |||||||||||
| FT3 age 7 | |||||||||||
| BMI (kg/m2) | Model 1 | 3014 | 0.05 (0.04, 0.06) | 0.13 (0.10, 0.16) | 5.30 × 10−16 | 73.6 | 0.008 | 0.19 (0.04, 0.33) | 0.30 (0.07, 0.53) | .01 | .17 |
| Age 7 | Model 2 | 3014 | 0.05 (0.04, 0.06) | 0.16 (0.13, 0.19) | 6.61 × 10−21 | 75.1 | 0.10 | 0.19 (0.05, 0.32) | 0.30 (0.08, 0.52) | .007 | .21 |
| Model 3a | 2538 | 0.04 (0.03, 0.05) | 0.12 (0.08, 0.16) | 3.08 × 10−13 | 57.9 | 0.07 | 0.20 (0.03, 0.38) | 0.33 (0.04, 0.61) | .02 | .14 | |
| FMI (kg/m2)a | Model 1 | 3014 | 0.22 (0.17, 0.27) | 0.17 (0.13, 0.20) | 1.56 × 10−22 | 98.1 | 0.04 | 0.16 (0.04, 0.29) | 0.26 (0.06, 0.46) | .01 | .45 |
| Age 9 | Model 2 | 3014 | 0.23 (0.19, 0.27) | 0.19 (0.16, 0.23) | 2.16 × 10−18 | 98.6 | 0.12 | 0.17 (0.05, 0.28) | 0.27 (0.08, 0.46) | .06 | .43 |
| Model 3a | 2538 | 0.17 (0.12, 0.22) | 0.13 (0.09, 0.17) | 3.59 × 10−18 | 77.6 | 0.10 | 0.17 (0.03, 0.32) | 0.28 (0.04, 0.51) | .02 | .26 | |
| FT4 age 7 | |||||||||||
| BMI (kg/m2) | Model 1 | 3014 | −0.07 (−0.10, −0.03) | −0.05 (−0.08, −0.02) | .001 | 71.9 | 0.01 | 0.03 (−0.35, 0.41) | 0.02 (−0.21, 0.25) | .88 | .51 |
| Age 7 | Model 2 | 3014 | −0.12 (−0.16, −0.08) | −0.10 (−0.13, −0.06) | 1.64 × 10−08 | 69.9 | 0.11 | −0.14 (−0.51, 0.23) | −0.09 (−0.31, 0.14) | .46 | .90 |
| Model 3a | 2538 | −0.09 (−0.14, −0.05) | −0.08 (−0.12, −0.04) | .00003 | 48.7 | 0.10 | −0.06 (−0.54 0.41) | −0.04 (−0.33, 0.25) | .79 | .79 | |
| FMI (kg/m2)a | Model 1 | 3014 | −0.01 (−0.02, −0.001) | −0.04 (−0.07, −0.002) | .04 | 105 | 0.01 | −0.003 (−0.33, 0.32) | −0.01 (−0.20, 0.20) | .99 | .70 |
| Age 9 | Model 2 | 3014 | −0.03 (−0.04, −0.02) | −0.09 (−0.13, −0.06) | 2.42 × 10−07 | 103 | 0.11 | −0.16 (−0.49, 0.17) | −0.10 (−0.29, 0.10) | .35 | .99 |
| Model 3a | 2538 | −0.02 (−0.03, −0.01) | −0.07 (−0.11, −0.03) | .002 | 74.2 | 0.10 | −0.05 (−0.45, 0.35) | −0.03 (−0.27, 0.21) | .80 | .70 | |
| FTO SNP alone | |||||||||||
| FT3 age 7 | |||||||||||
| BMI (kg/m2) | Model 1 | 3014 | 0.05 (0.04, 0.06) | 0.13 (0.10, 0.16) | 5.30 × 10−16 | 10.6 | 0.64 | 0.61 (0.12, 1.09) | 0.97 (0.20, 1.75) | .01 | .006 |
| Age 7 | Model 2 | 3014 | 0.05 (0.04, 0.06) | 0.16 (0.13, 0.19) | 6.61 × 10−21 | 11.8 | 0.61 | 0.63 (0.15, 1.11) | 1.02 (0.25, 21.80) | .01 | .003 |
| Model 3a | 2538 | 0.04 (0.03, 0.05) | 0.12 (0.08, 0.16) | 3.08 × 10−13 | 9.52 | 0.67 | 0.72 (0.02, 1.43) | 1.17 (0.03, 2.30) | .04 | .007 | |
| FMI (kg/m2) | Model 1 | 3014 | 0.22 (0.17, 0.27) | 0.17 (0.13, 0.20) | 1.56 × 10−22 | 9.87 | 0.31 | 0.48 (0.14, 0.82) | 0.78 (0.23, 1.32) | .006 | .01 |
| Age 9 | Model 2 | 3014 | 0.23 (0.19, 0.27) | 0.19 (0.16, 0.23) | 2.16 × 10−78 | 15.9 | 0.26 | 0.50 (0.17, 0.84) | 0.82 (0.28, 1.36) | .003 | .008 |
| Model 3a | 2538 | 0.17 (0.12, 0.22) | 0.13 (0.09, 0.17) | 3.59 × 10−18 | 14.6 | 0.24 | 0.52 (0.10, 0.94) | 0.84 (0.16, 1.52) | .008 | .02 | |
| FT4 age 7 | |||||||||||
| BMI (kg/m2) | Model 1 | 3014 | −0.07 (−0.10, −0.03) | −0.05 (−0.08, −0.02) | .001 | 10.6 | 0.01 | −0.21 (−1.20, 0.79) | −0.13 (−0.73, 0.47) | .68 | .82 |
| Age 7 | Model 2 | 3014 | −0.12 (−0.16, −0.08) | −0.10 (−0.13, −0.06) | 1.64 × 10−08 | 7.95 | 0.02 | −0.77 (−1.96, 0.41) | −0.47 (−1.18, 0.25) | .20 | .28 |
| Model 3a | 2538 | −0.09 (−0.14, −0.05) | −0.08 (−0.12, −0.04) | .00003 | 5.27 | 0.01 | −0.68 (−2.16, 0.83) | −0.41 (−1.33, 0.50) | .37 | .45 | |
| FMI (kg/m2) | Model 1 | 3014 | −0.01 (−0.02, −0.001) | −0.04 (−0.07, −0.002) | .04 | 18.1 | 0.01 | −0.20 (−1.00, 0.60) | −0.12 (−0.61, 0.31 | .63 | .74 |
| Age 9 | Model 2 | 3014 | −0.03 (−0.04, −0.02) | −0.09 (−0.13, −0.06) | 2.42 × 10−07 | 14.2 | 0.03 | −0.65 (−1.56, 0.26) | −0.39 (−0.94, 0.16) | .16 | .27 |
| Model 3a | 2538 | −0.02 (−0.03, −0.01) | −0.07 (−0.11, −0.03) | .002 | 11.2 | 0.07 | −0.50 (−1.55, 0.56) | −0.30 (−0.93, 0.35) | .36 | .49 | |
P (DWH) is the P value of the Durbin form of the DWH test, which examines the difference between the estimates from linear regression and instrumental variable analysis. Model 1 adjusted for age and sex; model 2 adjusted for model 1 and other thyroid hormone parameters; model 3 adjusted for model 2 and height markers of social class and early life environment (home ownership, maternal age at birth of child, maternal highest educational qualification, maternal smoking in pregnancy, family adversity index, and parents and home score). B, β-coefficient; P, strength of evidence against the null hypothesis of no association.
A total of 476 individuals with missing data.
The instrumented effect for FTO on FT3 showed some difference to observational estimates, especially for both BMI and FMI, where the instrumental variable analysis produced larger effect estimates than the observational analysis (DWH P ≤ .01). There was also some evidence that the instrumented effects of BMI on FT3 were higher for FTO than the other 31 SNPs. An additional analysis identified that independent pairs of variants from the 32 SNPs have instrumental variable effects that are normally distributed (Supplemental Figure 2). However, pairs of variants which included FTO are at the upper end of this distribution, indicating that although variation in FTO produces a substantially higher impact on FT3 than the average instrumental variable effect it is not an outlier.
Discussion
Our observational analyses clearly show that FT3 and FT4 have opposing strong correlations with body composition in childhood; with FT3 being positively associated and FT4 negatively associated with fat mass and BMI. We therefore utilized a MR approach to investigate whether BMI (and FMI) have a causal role in increasing FT3 levels in children. Analysis using our genetic instrument revealed that individuals with a genetically higher BMI/fat mass had higher FT3 levels in keeping with observational estimates. In contrast there was no evidence of association between individuals with a genetically higher BMI/fat mass and FT4 levels. Interestingly the observational positive association between BMI and FT3 appears to be substantially weaker at age 15; however, instrumental variable analysis effect estimates were more comparable. Furthermore, the effect also still appears to be present in adults as previous studies also identified a positive association between FT3 and BMI in healthy euthyroid men aged between 25–45 years (10, 13). Therefore repeating this MR analysis in adults would be particularly informative as would further studies comparing the biology of fat in children and adults in this regard.
Taken together, our data suggests that higher levels of BMI and adiposity cause an increase in serum FT3 levels but do not appear to influence FT4 levels. This would explain the paradoxical opposing relationship of FT3 (positive) and FT4 (negative) with BMI and fat mass in observational studies. It is also notable that our overall genetic effect estimates of fat mass on FT3 were substantially higher than the observational analysis. This may indicate that the higher FT3 generated from higher fat mass has a negative effect on fat mass, but this is a much weaker overall effect than the positive effect of fat mass on FT3. This is in keeping with a recent study, which identified obese children have higher FT3 levels (34).
Although our genetic analyses indicate the nature of the relationship between fat mass and FT3 the mechanism of action for increased fat mass increasing FT3 remains unclear. A simplistic assumption could be that increased fat mass results in increased generation of FT3 from FT4 and this increased FT3 production in fat leads to increased FT3 in serum. However, this would require increased Deiodinase 2 conversion of FT4 to FT3 in fat and this enzyme is expressed in brown but is not substantially expressed in white adipose tissue (35). Alternatively, increased fat mass may result in other alterations in the hypothalamic-pituitary-thyroid axis as longitudinal analysis in ALSPAC has indicated that FT3 is less influenced by TSH levels than FT4 and has greater intraindividual variation (our unpublished data). Alternatively, observed changes in FT3 may relate in part to excess carbohydrate in the diet of obese individuals (36). In addition our analysis showed children have higher FT3 levels than adults with almost 25% of children having a FT3 above the adult reference range. This implies that other factors influence FT3 levels in young children although fat mass may still have a substantial role. Further insight may be available from whole genome sequence analysis of body mass and thyroid function; however, they are unlikely to substantially increase the variance explained at present (37).
Although the MR method is more resistant to reverse causation and confounding than traditional observational epidemiological studies, there are limitations to this approach. These include population stratification, pleiotropy (genes influencing multiple phenotypes), canalization (the ability of a population to produce the same phenotype regardless of variability of its environment or genotype), inadequate power and linkage disequilibrium (16, 38). Polygenic score analyses for TSH and FT4 have not revealed a common genetic determination with metabolic and anthropometric measures (37); although FT3 has not been studied, it is unlikely that it shares a common genetic architecture (pleiotropy) to fat mass. Our use of 32 independent alleles in determining the gene score, substantially reduced the risk of shared pleiotropy and linkage-disequilibrium-induced confounding (32). Furthermore, our use of 2 separate genetic instruments substantially reduced the risk of pleiotropy. Another limitation was that we did not have data on thyroid function, body composition or genetic architecture in a substantial number of children in the ALSPAC cohort. However, this would only lead to bias if the causal effects of higher fat mass increasing FT3 levels are different in the children not studied in this study. Although we cannot fully exclude this there was no substantial difference in our models after adjustment for relevant socioeconomic confounders. Furthermore, positive associations between adiposity and FT3 were also observed when using different instrument combinations, suggesting that there is not a systematic and biasing effect of pleiotropy in this case. With regard to measurement of free thyroid hormones, biases have been reported (39), whereas measured levels of free thyroid hormones in our study may not be entirely independent from thyroid-binding globulin levels, the striking difference observed in the associations between BMI allelic score and FT3 and FT4 makes a substantial impact from thyroid-binding globulin in our genetic analysis unlikely. Previous analyses have also identified the association between thyroid hormone parameters and body composition was largely independent of thyroid-binding globulin (11). Potentially, FT3 levels may be reduced in children with recent illness before blood sampling, but this would have likely biased our genetic associations to the null.
As well as providing insight into the regulation of FT3 in children, our findings are potentially clinically relevant. Childhood obesity is common and rising (40), and increased FT3 levels arising from increasing fat mass may have long-term consequences, particularly at the population level as even modest variation in thyroid status within the population reference range has adverse phenotypic effects (3).
In conclusion, our analysis has indicated that BMI and adiposity causally increase FT3 levels in children. More research is required to identify the causal mechanisms for this and the consequences of childhood obesity on this relationship.
Acknowledgments
We thank all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
This work was supported by The United Kingdom Medical Research Council and the Wellcome Trust Grant 102215/2/13/2 the University of Bristol (core support for ALSPAC); thyroid function was supported by the Above and Beyond Charitable Trust, the British Thyroid Foundation, and the BUPA Research Foundation. P.N.T. is funded through the Welsh Clinical Academic Trainee scheme.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALSPAC
- Avon Longitudinal Study of Parents and Children
- BMI
- body mass index
- DWH
- Durbin-Wu-Hausman
- DXA
- dual x-ray absorptiometry
- FMI
- fat mass index
- FT3
- free T3
- MR
- Mendelian randomization
- SNP
- single nucleotide polymorphism
- β-(std)
- β-standardized.
References
- 1. Hoogwerf BJ, Nuttall FQ. Long-term weight regulation in treated hyperthyroid and hypothyroid subjects. Am J Med. 1984;76:963–970. [DOI] [PubMed] [Google Scholar]
- 2. Dale J, Daykin J, Holder R, Sheppard MC, Franklyn JA. Weight gain following treatment of hyperthyroidism. Clin Endocrinol (Oxf). 2001;55:233–239. [DOI] [PubMed] [Google Scholar]
- 3. Taylor PN, Razvi S, Pearce SH, Dayan CM. Clinical review: a review of the clinical consequences of variation in thyroid function within the reference range. J Clin Endocrinol Metab. 2013;98:3562–3571. [DOI] [PubMed] [Google Scholar]
- 4. Asvold BO, Bjøro T, Vatten LJ. Association of serum TSH with high body mass differs between smokers and never-smokers. J Clin Endocrinol Metab. 2009;94:5023–5027. [DOI] [PubMed] [Google Scholar]
- 5. Fox CS, Pencina MJ, D'Agostino RB, et al. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med. 2008;168:587–592. [DOI] [PubMed] [Google Scholar]
- 6. Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–4024. [DOI] [PubMed] [Google Scholar]
- 7. Wolters B, Lass N, Reinehr T. TSH and free triiodothyronine concentrations are associated with weight loss in a lifestyle intervention and weight regain afterwards in obese children. Eur J Endocrinol. 2013;168:323–329. [DOI] [PubMed] [Google Scholar]
- 8. De Pergola G, Ciampolillo A, Paolotti S, Trerotoli P, Giorgino R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clin Endocrinol (Oxf). 2007;67:265–269. [DOI] [PubMed] [Google Scholar]
- 9. Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316:165–171. [DOI] [PubMed] [Google Scholar]
- 10. Roef GL, Rietzschel ER, Van Daele CM, et al. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid. 2014;24:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roef G, Lapauw B, Goemaere S, et al. Thyroid hormone status within the physiological range affects bone mass and density in healthy men at the age of peak bone mass. Eur J Endocrinol. 2011;164:1027–1034. [DOI] [PubMed] [Google Scholar]
- 12. Alevizaki M, Saltiki K, Voidonikola P, Mantzou E, Papamichael C, Stamatelopoulos K. Free thyroxine is an independent predictor of subcutaneous fat in euthyroid individuals. Eur J Endocrinol. 2009;161:459–465. [DOI] [PubMed] [Google Scholar]
- 13. Roef G, Lapauw B, Goemaere S, Zmierczak HG, Toye K, Kaufman JM, Taes Y. Body composition and metabolic parameters are associated with variation in thyroid hormone levels among euthyroid young men. Eur J Endocrinol. 2012;167:719–726. [DOI] [PubMed] [Google Scholar]
- 14. Silvestri E, Schiavo L, Lombardi A, Goglia F. Thyroid hormones as molecular determinants of thermogenesis. Acta Physiol Scand. 2005;184:265–283. [DOI] [PubMed] [Google Scholar]
- 15. Smith GD, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med. 2007;4:e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
- 17. Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyd A, Golding J, Macleod J, et al. Cohort profile: the ‘children of the 90s’–the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fraser A, Macdonald-Wallis C, Tilling K, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–147. [DOI] [PubMed] [Google Scholar]
- 21. Tobias JH, Steer CD, Emmett PM, Tonkin RJ, Cooper C, Ness AR. Bone mass in childhood is related to maternal diet in pregnancy. Osteopor Int. 2005;16:1731–1741. [DOI] [PubMed] [Google Scholar]
- 22. Paternoster L, Zhurov AI, Toma AM, et al. Genome-wide association study of three-dimensional facial morphology identifies a variant in PAX3 associated with nasion position. Am J Hum Genet. 2012;90:478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Willer C, Sanna S, Abecasis G. Genotype imputation. Annu Rev Genomics Hum Genet. 2009;10:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sayers A, Tobias JH. Fat mass exerts a greater effect on cortical bone mass in girls than boys. J Clin Endocrinol Metab. 2010;95:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janssens AC, Aulchenko YS, Elefante S, Borsboom GJ, Steyerberg EW, van Duijn CM. Predictive testing for complex diseases using multiple genes: fact or fiction? Genet Med. 2006;8:395–400. [DOI] [PubMed] [Google Scholar]
- 27. Palmer TM, Lawlor DA, Harbord RM, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21:223–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richmond RC, Davey Smith G, Ness AR, den Hoed M, McMahon G, Timpson NJ. Assessing causality in the association between child adiposity and physical activity levels: a Mendelian randomization analysis. PLoS Med. 2014;11:e1001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wei WH, Hemani G, Gyenesei A, et al. Genome-wide analysis of epistasis in body mass index using multiple human populations. Eur J Hum Genet. 2012;20:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–1163. [DOI] [PubMed] [Google Scholar]
- 31. Staiger DO, Stock JH. Instrumental Variables Regression With Weak Instruments. Cambridge, MA: National Bureau of Economic Research; 1994. [Google Scholar]
- 32. Davey Smith G. Use of genetic markers and gene-diet interactions for interrogating population-level causal influences of diet on health. Genes Nutr. 2011;6:27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karavani G, Strich D, Edri S, Gillis D. Increases in thyrotropin within the near-normal range are associated with increased triiodothyronine but not increased thyroxine in the pediatric age group. J Clin Endocrinol Metab. 2014;99:E1471–E1475. [DOI] [PubMed] [Google Scholar]
- 35. Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116:2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Danforth E, Jr, Horton ES, O'Connell M, et al. Dietary-induced alterations in thyroid hormone metabolism during overnutrition. J Clin Invest. 1979;64:1336–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taylor PN, Porcu E, Chew S, et al. Whole-genome sequence-based analysis of thyroid function. Nat Commun. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33:30–42. [DOI] [PubMed] [Google Scholar]
- 39. Yue B, Rockwood AL, Sandrock T, La'ulu SL, Kushnir MM, Meikle AW. Free thyroid hormones in serum by direct equilibrium dialysis and online solid-phase extraction–liquid chromatography/tandem mass spectrometry. Clin Chem. 2008;54:642–651. [DOI] [PubMed] [Google Scholar]
- 40. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]