Abstract
Context:
Insulin resistance impacts virtually all tissues, including pancreatic β cells. Individuals with insulin resistance, but without diabetes, exhibit an increased islet size because of an elevated number of both β and α cells. Neogenesis from duct cells and transdifferentiation of α cells have been postulated to contribute to the β-cell compensatory response to insulin resistance.
Objective:
Our objective was to explore parameters that could potentially predict altered islet morphology.
Methods:
We investigated 16 nondiabetic subjects by a 2-hour hyperglycemic clamp to evaluate β-cell secretory function. We analyzed pancreas samples obtained during pancreatoduodenectomy in the same patients to examine glucagon and insulin double+ cells to assess islet morphology.
Results:
Among all the functional in vivo parameters of insulin secretion that were explored (basal, first phase and total secretion, glucose sensitivity, arginine-stimulated insulin secretion), β-cell glucose sensitivity was unique in exhibiting a significant correlation with both islet size and α-β double+ islet cells.
Conclusions:
Our data suggest that poor β-cell glucose sensitivity is linked to islet transdifferentiation, possibly from α cells to β cells, in an attempt to cope with higher demands for insulin secretion. Understanding the mechanism(s) that underlies the adaptive response of the islet cells to insulin resistance is a potential approach to design tools to enhance functional β-cell mass for diabetes therapy.
Type 2 diabetes (T2D) develops when insulin secretion fails to cope with worsening insulin resistance (1). It has also been shown that β-cell function decline is associated with increasing glucose levels (2), even in patients with normal glucose tolerance, and further worsens with the onset of clinically detectable impaired glucose tolerance and progression to T2D (3). Notably, the absence of overt diabetes in individuals with severe insulin resistance suggests the ability of the islet cells to adapt and secrete insulin to maintain glucose homeostasis. Therefore, to explore whether islet cell plasticity is linked to an organism's ability to compensate for insulin resistance, we have recently examined the mechanisms that maintain glucose homeostasis in response to different metabolic demands. Our findings indicate an increased islet size and an elevated number of both β and α cells (resulting in an altered β-α cell area) as a potential form of compensatory response to insulin resistance that likely delays the onset of overt diabetes (4). In the present study, we built on our previous efforts to examine whether the bihormonal (insulin/glucagon double+) cells observed in human pancreata are associated with changes in β-cell function as examined by a hyperglycemic clamp.
Exploring the relationship between in vivo β-cell function and islet morphology represents a unique opportunity to determine whether β-cell dysfunction directly triggers islet regenerative processes. The aims of the present investigation were to examine β-cell function, modeled from a hyperglycemic clamp, in nondiabetic insulin-resistant patients and to assess the relationship between β-cell function and islet morphology in pancreas sections from surgical (ex vivo) samples.
Research Design and Methods
Subject selection and protocols
For the purpose of this analysis, we included patients from a previous study by our group (4) for whom data from a euglycemic clamp, a hyperglycemic clamp with C-peptide measurements and immunohistochemical analysis of pancreas samples, were already available. Thus, patients scheduled to undergo pylorus-preserving pancreatoduodenectomy were recruited from the Hepato-Biliary Surgery Unit of the Department of Surgery and studied in the Endo-Metabolic Diseases unit (both at the Agostino Gemelli University Hospital, Rome, Italy). The study protocol was approved by the local ethics committee (P/656/CE2010 and 22573/14), and all participants provided written informed consent, which was followed by a comprehensive medical evaluation. Indication for surgery was tumor of the ampulla of Vater. None of the patients enrolled had a family history of diabetes. Patients underwent both a 75-g oral glucose tolerance test and glycated hemoglobin (HbA1c) testing to exclude diabetes, according to the American Diabetes Association criteria (5). Only patients with normal cardiopulmonary and kidney function, as determined by medical history, physical examination, electrocardiography, estimated glomerular filtration rate, and urinalysis were included. Altered serum lipase and amylase levels before surgery, as well as morphologic criteria for pancreatitis, were considered exclusion criteria. Potential patients who had severe obesity (body mass index > 40), uncontrolled hypertension, and/or hypercholesterolemia were excluded. Clinical and metabolic characteristics of patients are shown in Table 1.
Table 1.
Clinical and Metabolic Characteristics of Studied Patients
| Subject Characteristics | Mean ± sem (n = 16) |
|---|---|
| M/F (n) | 7/9 |
| BMI (kg/m2) | 26.4 ± 1.18 |
| Waist to hip ratio | 0.92 ± 0.05 |
| Glucose uptake during HEC | 4.79 ± 0.33 |
| Triglycerides (mg/dl) | 198 ± 43.6 |
| HDL cholesterol (mg/dl) | 52.5 ± 10.4 |
| LDL cholesterol (mg/dl) | 90.4 ± 14.8 |
| Total cholesterol (mg/dl) | 178 ± 25.7 |
| Fasting glucose (mmol/l) | 5.76 ± 0.33 |
| Fasting insulin (μUI/ml) | 7.83 ± 2.07 |
| HbA1c (mmol/mol) | 37 ± 6.3 |
| HbA1c (%) | 5.57 ± 0.56 |
Data are means ± sem or n (sex distribution).
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; HEC, hyperinsulinemic euglycemic clamp; LDL, low-density lipoprotein.
All subjects underwent a 2-hour euglycemic clamp (insulin infusion rate: 40 mU·minute−1·m−2) and a 2-hour hyperglycemic clamp (with blood glucose acutely raised and maintained at approximately 225 mg/dl) to calculate insulin secretion (from C-peptide deconvolution) as described in the following section.
Hyperglycemic clamp procedure
The plasma glucose was clamped at a stable level of 125 mg/dl above the fasting blood glucose concentration. The hyperglycemic clamp was started with a bolus dose of dextrose 200 mg/ml (150 mg/kg) administered into the antecubital vein. Blood was drawn from a cannulated dorsal hand vein on the opposite arm. Every 5 minutes, venous plasma glucose was analyzed with a glucose analyzer and the infusion of 20% glucose was adjusted to achieve a stable glucose level of 125 mg/dl above the fasting value. Serum samples for insulin and C-peptide were drawn at 0, 2.5, 5, 7.5, 10, 15, 30, 60, 90, 120, 130, 140, and 150 minutes.
Hyperinsulinemic euglycemic clamp procedure
The hyperinsulinemic euglycemic clamp test was performed after a 12-hour overnight fast using insulin 40 mIU/m2·minute of body surface according to DeFronzo and colleagues (6). A primed constant infusion of insulin was administered (Actrapid HM, 40 mIU/m2·minute; Novo Nordisk, Copenhagen, Denmark). The constant rate for the insulin infusion was reached within 10 minutes to achieve steady-state insulin levels; in the meantime, a variable infusion of 20% glucose was started via a separate infusion pump, and the rate was adjusted on the basis of plasma glucose samples drawn every 5 minutes to maintain the plasma glucose concentration at each participant's fasting plasma glucose level. During the last 20 minutes of the clamp procedure, plasma samples from blood drawn at 5- to 10-minute intervals were used to determine glucose and insulin concentrations. Whole-body peripheral glucose utilization was calculated during the last 30-minute period of the steady-state insulin infusion and was measured as the mean glucose infusion rate (as mg·kg−1·minute−1).
Immunohistochemical analysis of surgical pancreas samples
Pancreatic tissue processing and morphometric analysis were performed as described previously (4). The β-, α-, and δ-cell areas were analyzed and expressed as percentage of total pancreas section area. The islet size was calculated as the sum of the individual areas (β-, α-, and δ-cell areas) divided by the number of islets counted in each pancreatic section. The number of insulin and glucagon double+ cells was manually counted in sections costained for insulin or glucagon. A mean (±SE) of 1172 ± 269 endocrine cells per subject was evaluated; resulting data were expressed as percentage of endocrine cells. The double+ cells were confirmed in randomly selected islets by confocal microscopy. We analyzed the distribution of insulin/glucagon double+ cells in islets with less than 50 nuclei (that we considered “small-sized” islets) vs those with more than 50 nuclei, which we considered “big-sized” islets.
Quantification of scattered islets and exocrine duct cells positive for insulin
As previously described (7), clusters of less than eight endocrine cells were considered new islets (neogenesis) (7). On sections stained specifically for insulin, clusters with less than eight insulin-positive cells were manually counted and considered as scattered islets, then expressed as the ratio of the number of scattered islets per total pancreas area. Sections costained for pancreatic ductal marker CK19 or insulin were imaged by confocal microscopy and the number of CK19 and insulin-positive cells was manually counted. A mean (±SE) of 1107 ± 475 duct cells were evaluated for each section. The resulting data were expressed as a percentage of duct cells positive for insulin in each pancreas. All data were expressed as mean ±SE for each group.
Calculations
During hyperglycemic clamp, insulin secretion was derived from C-peptide levels by deconvolution (8). The first-phase insulin secretion response was calculated as the mean incremental insulin secretion between 0 and 5 minutes, when the insulin secretion rate had fallen from the initial peak to a nadir in all subjects. Second-phase insulin secretion was calculated as the increment in insulin secretion during the last 20 minutes of the hyperglycemic clamp above basal insulin secretion. An estimate of β-cell glucose sensitivity (βCGS) was obtained as the increment in insulin secretion during the last 20 minutes of the hyperglycemic clamp above basal insulin secretion, divided by the corresponding glucose increment.
To confirm differences in insulin secretion, β-cell function and islet morphology in response to insulin-resistance vs insulin-sensitive states, patients were divided into two groups (insulin resistant and insulin sensitive) according to their insulin sensitivity, as measured with the euglycemic hyperinsulinemic clamp. As previously described (4), the cutoff for insulin sensitivity was the median value of glucose uptake in the overall cohort (4.9 mg·kg−1·minute−1).
Statistics
All data are expressed as mean ±SE, unless otherwise indicated. Since samples were normally distributed, differences in means were tested by two-tailed Student's t test. The relationship between variables was derived with linear regression analysis using SPSS, version 20 (SPSS, Chicago, IL). A P value of less than.05 was considered statistically significant.
Results
Sixteen patients (nine females, seven males; mean age 51 ± 15 years) undergoing pylorus-preserving pancreatoduodenectomy for a tumor of the ampulla of Vater were included in the present analysis. Clinical and metabolic characteristics of study subjects are provided in Table 1.
Islet size and β-cell function
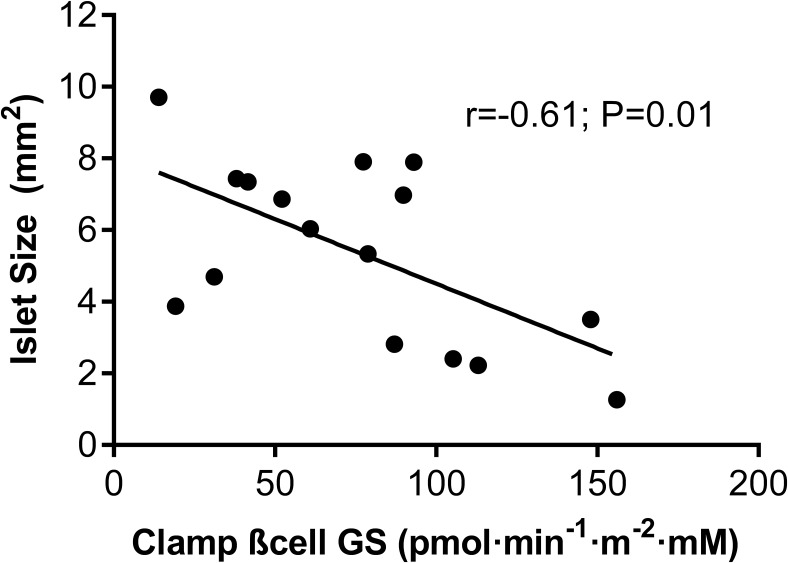
We observed a strong inverse correlation between islet size and βCGS in the entire cohort (r = −0.61; P = .01; Figure 1), which suggests that changes in islet morphology are related to β-cell dysfunction. Further, insulin-resistant individuals displayed a significant reduction in βCGS as compared to insulin-sensitive subjects (51.5 ± 14.3 vs 99.1 ± 18.2 pmol·minute−1 m−2·mM−1, respectively; P < .01). To evaluate whether in vivo functional parameters measured during a hyperglycemic clamp over 2 hours followed by an acute stimulation with L-arginine are directly related to islet morphological changes, we investigated the relationship between insulin secretion pattern and isle size. No relations were observed between islet size and other insulin secretion parameters, ie, basal insulin secretion (r = 0.15; P = .57), incremental first-phase insulin secretion (r = −0.25; P = .28), or total insulin secretion (r = −0.35; P = .18).
Figure 1.
In vivo β cell function impacts islet morphology. Correlation between β-cell function index β cell glucose sensitivity (βCGS) and islet size is shown in all subjects. r = −0.61, P = .01.
Islet double+ cells and β-cell function
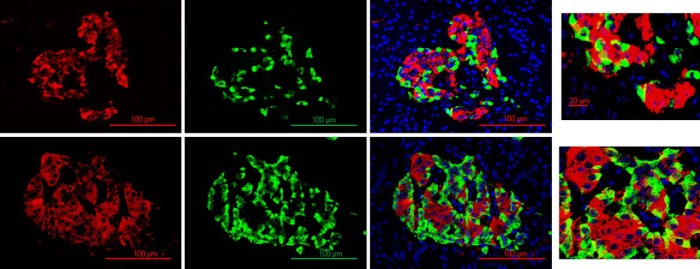
As previously observed, the percentage of insulin/glucagon double+ cells is statistically different between insulin-sensitive and insulin-resistant groups (insulin sensitive 9.11 ± 1.90 vs insulin resistant 16.18 ± 2.3%, P = .03; Figure 3). Interestingly, we observed a significant increase in the percentage of double+ cells in islets with more than 50 nuclei compared to islets with less than 50 nuclei (% double+ cells insulin sensitive: 5.23 ± 0.98 vs insulin resistant 14.9 ± 1.12%, P < .001).
Figure 3.
Confocal microscopy analysis of insulin (red) and glucagon (green) immunostaining. 25× objective, scale bar 100 μm (1, 2, 3, 4, 5, 6); 3× amplification, scale 20 μm (7, 8). Insulin sensitive (upper panel) and insulin resistant (lower panel).
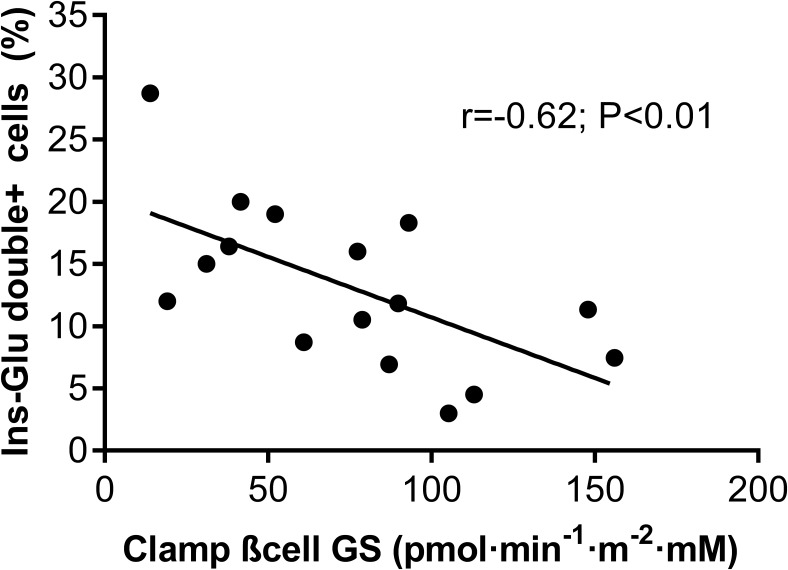
We also analyzed the relationship between β-cell function parameters and neogenesis and transdifferentiation markers. βCGS strongly correlated with transdifferentiation expressed as percent of insulin-glucagon double+ cells (r = −0.62; P < .01; Figure 2), but not with the number of scattered islets (r = 0.20, P = .60) or with the percentage of CK19/insulin double+ cells (r = −0.13, P = .51). Furthermore, we explored whether other in vivo insulin secretion parameters were related to transdifferentiation. Transdifferentiation, expressed as % of insulin-glucagon double+ cells, did not correlate with basal insulin secretion (r = 0.01; P = .84), incremental first phase insulin secretion (r = −0.40; P = .09) or total insulin secretion (r = −0.21; P = .07). Finally, we noted that arginine-stimulated insulin secretion (used as a functional surrogate of β-cell mass) was not correlated with islet size (r = −0.16; P = .53) or transdifferentiation (as % insulin-glucagon double+ cells, r = 0.22; P = .40).
Figure 2.
In vivo β-cell function is linked to transdifferentiation. Correlation between beta cell function index β-cell glucose sensitivity (βCGS) and percentage of double+ (Ins-Glu) cells is shown in all subjects. r = −0.62, P < .01.
Discussion
We previously observed that islet morphology is largely remodeled in nondiabetic, insulin-resistant subjects, and suggested that neogenesis and transdifferentiation could be potential underlying mechanisms. Our previous study, however, did not include an accurate measure of β-cell function. We therefore undertook the present analysis to specifically focus on investigating the relationship between β-cell function parameters and morphologic features of islets obtained ex vivo from nondiabetic subjects.
The key finding of our study is that βCGS was strongly and inversely correlated with both islet size (r = −0.61; P = .01) and insulin-glucagon double+ cells (r = −0.62; P < .01). This suggests that the increase in islet mass and altered morphology induced by insulin resistance are accompanied by some impairment of β-cell function, even in nondiabetic individuals
Along with increased mean islet size and increased number of “big-sized” islets in insulin resistant patients, we also observed a higher percentage of double+ cells inside larger islets, suggesting that transdifferentiation participates in the islet adaptation response to insulin resistance.
In this small cross-sectional study, the interplay between insulin sensitivity, β-cell function, and islet morphology cannot be fully elucidated. In our previous work (4), increased islet size and insulin-glucagon double+ cells were inversely associated with insulin sensitivity; in this study, we show a similar association with β-cell glucose sensitivity, and the causal mechanistic link requires further investigation. However, we hypothesize that insulin resistance, together with a relative loss of βCGS, together activate islet plasticity (including α to β-cell transdifferentiation) to eventually lead to an increase in islet size and compensate for the higher insulin demands.
Indeed, we observed a decrease in βCGS in insulin-resistant subjects, confirming the previous in vivo finding that hypersecretion of insulin resulting from insulin resistance may coexist with decreased βCGS (8, 9) in the progression toward glucose intolerance. In agreement with previous reports on both humans and transgenic models of insulin resistance (reviewed by Goldfine and Kulkarni (10)), our observations on β-cell function could be explained through an altered insulin action at the β-cell level, which determines both altered islet morphology and relative loss of βCGS.
Although we do not exclude that other mechanisms may have directly contributed to our findings, such as β-cell dedifferentiation and transdifferentiation of β to α cells, the current study, along with our previous report, supports the hypothesis that nondiabetic subjects compensate for insulin resistance by increasing the number of insulin-producing cells. Although additional studies using isolated cell samples will be necessary to examine how exactly the double+ islet cells contribute to insulin secretory capacity, we speculate that an increasing number of these double+ cells in insulin-resistant subjects represents one possible approach to increase insulin secretion to compensate for the decreasing glucose sensitivity.
It is worth noting that the relationship between islet morphology and β-cell function was specific for βCGS and did not apply to other insulin secretion parameters obtained from the hyperglycemic clamp and arginine stimulation, suggesting that a specific functional impairment accompanies, and eventually drives the islet morphological changes. Furthermore, because βCGS has been shown to be a key regulator of glucose homeostasis (9), alterations in islet morphology may be a crucial factor in glucose intolerance.
Although previous studies have reported morphological analyses of human pancreata on autopsied samples, none has systematically coupled the data with accurate metabolic and hormonal profiling in the same patients (11, 12). Thus, despite the limitations, to our knowledge our study is the first to suggest that islet morphology is related to β-cell dysfunction.
In conclusion, we posit that transdifferentiation represents a key process in islet plasticity. Given that our data demonstrate that islet plasticity is related to βCGS, further studies aimed at examining signaling pathways in human islet cells, using phospho-proteomics and RNA-sequencing approaches, have the potential to identify potential regulators linking βCGS to the transdifferentiation processes. Investigating the modifications that occur in β-cell dysfunction as a consequence of ambient insulin resistance provides an excellent opportunity to identify possible targets to prevent and possibly slow the progression of glucometabolic derangements by promoting plasticity of islet cells favoring an increase in functional β cells to enhance effective compensation.
Acknowledgments
We thank J. Hu, PhD (Joslin Diabetes Center) for advice and assistance with histological examination of the samples, Francesca Cinti for the many hours of thoughtful discussion, and Serena Rotunno for assistance with editing.
This study was supported by grants from the Università Cattolica del Sacro Cuore (Fondi AteneoLinea D.3.2); the Italian Ministry of Education, University and Research (PRIN 2010JS3PMZ 011); and by an European Foundation for the Study of Diabetes award supported by AstraZeneca (to A.G.). T.M., G.P.S., and C.M.A. are the recipients of a fellowship prize from Diabete Ricerca. Some of the reagents used in the study were supported by National Institutes of Health Grants RO1DK67536 and NIH R01DK103215 (to R.N.K.) and Grant P30 DK36386 (Joslin Diabetes Research Center).
Author Contributions: T.M., A.G., and R.N.K. are guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of data analyses. T.M. generated the data and wrote the manuscript. R.N.K., A.M., and A.G. reviewed/edited the manuscript. G.P.S., C.C., and A.P. contributed to discussion and reviewed/edited the manuscript. C.M.A.C., S.M., and V.A.S. researched data and A.M. generated the data. R.N.K supervised the work at the Joslin Diabetes Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- βCGS
- β-cell glucose sensitivity
- HbA1c
- glycated hemoglobin
- T2D
- type 2 diabetes.
References
- 1. Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378(9786):169–181. [DOI] [PubMed] [Google Scholar]
- 2. Giaccari A, Sorice G, Muscogiuri G. Glucose toxicity: the leading actor in the pathogenesis and clinical history of type 2 diabetes - mechanisms and potentials for treatment. Nutr Metab Cardiovasc Dis. 2009;19(5):365–377. [DOI] [PubMed] [Google Scholar]
- 3. Meier JJ, Bonadonna RC. Role of reduced β-cell mass versus impaired β-cell function in the pathogenesis of type 2 diabetes. Diabetes Care. 2013;36(Suppl 2):S113–S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mezza T, Muscogiuri G, Sorice GP, et al. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes. 2014;63(3):994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2015;38(Suppl):S8–S16. [DOI] [PubMed] [Google Scholar]
- 6. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 7. Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both b-cell replication and neogenesis, resulting in increased b-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48(12):2270–2276. [DOI] [PubMed] [Google Scholar]
- 8. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41(3):368–377. [DOI] [PubMed] [Google Scholar]
- 9. Mari A, Tura A, Natali A, et al. RISC Investigators. Impaired beta cell glucose sensitivity rather than inadequate compensation for insulin resistance is the dominant defect in glucose intolerance. Diabetologia. 2010;53(4):749–756. [DOI] [PubMed] [Google Scholar]
- 10. Goldfine AB, Kulkarni RN. Modulation of β-cell function: a translational journey from the bench to the bedside. Diabetes Obes Metab. 2012;14(Suppl 3):152–160. [DOI] [PubMed] [Google Scholar]
- 11. Cnop M, Vidal J, Hull RL, et al. Progressive loss of beta-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care. 2007;30:677–682. [DOI] [PubMed] [Google Scholar]
- 12. Ehrmann DA, Breda E, Cavaghan MK, et al. Insulin secretory responses to rising and falling glucose concentrations are delayed in subjects with impaired glucose tolerance. Diabetologia. 2002;45:509–517. [DOI] [PubMed] [Google Scholar]