Abstract
Context:
Excessive cardiac long-chain fatty acid (LCFA) metabolism/storage causes cardiomyopathy in animal models of type 2 diabetes. Medium-chain fatty acids (MCFAs) are absorbed and oxidized efficiently. Data in animal models of diabetes suggest MCFAs may benefit the heart.
Objective:
Our objective was to test the effects of an MCFA-rich diet vs an LCFA-rich diet on plasma lipids, cardiac steatosis, and function in patients with type 2 diabetes.
Design:
This was a double-blind, randomized, 2-week matched-feeding study.
Setting:
The study included ambulatory patients in the general community.
Patients:
Sixteen patients, ages 37–65 years, with type 2 diabetes, an ejection fraction greater than 45%, and no other systemic disease were included.
Intervention:
Fourteen days of a diet rich in MCFAs or LCFAs, containing 38% as fat in total, was undertaken.
Main Outcome Measures:
Cardiac steatosis and function were the main outcome measures, with lipidomic changes considered a secondary outcome.
Results:
The relatively load-independent measure of cardiac contractility, S′, improved in the MCFA group (P < .05). Weight-adjusted stroke volume and cardiac output decreased in the LCFA group (both P < .05). The MCFA, but not the LCFA, diet decreased several plasma sphingolipids, ceramide, and acylcarnitines implicated in diabetic cardiomyopathy, and changes in several sphingolipids correlated with improved fasting insulins.
Conclusions:
Although a diet high in MCFAs does not change cardiac steatosis, our findings suggest that the MCFA-rich diet alters the plasma lipidome and may benefit or at least not harm cardiac function and fasting insulin levels in humans with type 2 diabetes. Larger, long-term studies are needed to further evaluate these effects in less-controlled settings.
Type 2 diabetes is a risk factor for the development of left ventricular (LV) dysfunction and failure even in the absence of coronary artery disease (1). This “diabetic cardiomyopathy” is characterized by increased LV mass and dysfunction (1). Metabolic abnormalities likely play a role in its pathogenesis. Increased fat delivery leads to increased myocardial lipid metabolism and accumulation (2). Excess cardiomyocyte lipid causes cell death and cardiac dysfunction in animal models (3–6). In humans, excessive lipid deposition and cardiac dysfunction are also related (7, 8).
Complex lipid species in particular are implicated in this “lipotoxicity.” Sphingolipids are increased in plasma and skeletal muscle in type 2 diabetes (9–11). Sphingomyelins (SMs) and ceramides (Cer) are associated with organ dysfunction in animal models of diabetes (3, 12–14). These lipids can impact organ function through signaling cascades and biophysical effects on cell membranes. Long-chain acylcarnitines are also implicated in diabetes (11, 15).
In contrast to long-chain fatty acids (LCFAs), medium-chain fatty acids (MCFAs, 6–12 carbons long) are readily oxidized, not stored. MCFAs are not implicated in the pathogenesis of steatosis-related diseases. MCFAs improve insulin resistance, reverse steatosis and cardiac dysfunction in animal models (16), and improve glucose disposal in humans (17). Epidemiologic studies suggest diets rich in MCFAs may prevent type 2 diabetes and cardiovascular disease (18–20). Nonetheless, most MCFAs available in foods (eg, coconut oil) are saturated, and saturated fats in general have been linked with increased cardiovascular disease. Whether or not replacement of LCFAs with MCFAs in the diet is detrimental or beneficial for the diabetic human heart is not known.
This study tested the hypothesis that dietary treatment with MCFAs improves relatively load-independent tissue Doppler measures of systolic and diastolic cardiac function as well as cardiac output and stroke volume in humans with type 2 diabetes. To gain insights into possible mechanisms, we also determined if MCFAs have beneficial effects on the plasma lipidome, insulin levels, and tissue fat content. To this end, we performed a randomized, double-blind, controlled feeding study in subjects with diabetes, comparing a diet rich in MCFAs with one high in LCFAs.
Materials and Methods
Study design and subjects
This was a prospective, double-blind, randomized dietary intervention study. The size of this first-in-human pilot study was designed to be similar in size to other animal and human studies of MCFA effects (16, 17). Written informed consent was obtained before participation, which was approved by the Human Research Protection Office at Washington University. Sixteen subjects with type 2 diabetes (37–65 years) completed the study. Subjects underwent a history, physical examination, blood tests, and rest/exercise echocardiography. None took exogenous insulin. Subjects with an ejection fraction lower than 45%, myocardial infarction/ischemia, atrial fibrillation, or who were pregnant, lactating, or smokers were excluded. In addition to study finishers, six dropped out and are not included in the analyses (Supplemental Figure 1).
Experimental procedures and measurements
All subjects underwent body composition measurements before randomization. Other procedures were performed before and within 8 hours after completing the 2-week dietary intervention. All participants reported to the Clinical Research Unit and completed a 24-hour dietary recall and underwent phlebotomy for fasting blood chemistries, glucose, and insulin with each visit. Insulin resistance was calculated as Homeostatic Model Assessment-Insulin Resistance = (fasting insulin × fasting glucose)/22.5. Expert reviewers blinded to diet intervention measured all predetermined study endpoints.
Body composition assessment
Whole-body fat mass and fat-free mass were quantified using a Hologic Discovery (version 12.4) enhanced-array, dual-energy X-ray absorptiometer.
Dietary intervention
All subjects completed a 24-hour dietary recall. Subjects were randomized to receive a diet rich in MCFAs or LCFAs. Both contained 46% of calories as carbohydrate, 16% as protein, and 38% as fat, consistent with the macronutrient distribution of a typical Western diet (21). Dieticians used ProNutra software made by Viocare to generate the meal plans for each diet's calorie level. In the MCFA-rich diet, 28% of total calories and 74% of total fat calories were from MCFAs. The diets' duration/composition was based on studies in humans showing that 2 weeks of an MCFA-based diet results in significant changes in cardiac lipid content and energy expenditure (22, 23). MCFAs were provided using the commercially available MCFA triglyceride Delios S (Cognis Corporation) with 33–34% saturated fatty acids, 1–2% monounsaturated fatty acids, and 1–2% polyunsaturated fatty acids. Less than 2% of the fatty acids are C6, approximately 70% are C8, 30% are C10, and less than 1% are C12. In the LCFA diet, LCFAs were provided as a common vegetable oil with roughly 18–21% of the calories from saturated fatty acids, 7–11% from monounsaturated fatty acids, and 3–8% from polyunsaturated fatty acids. All meals were prepared in the metabolic kitchen and provided to subjects every 2–3 days for consumption at home. The diets' energy content was individualized based upon each subject's estimated resting energy expenditure (per the Harris-Benedict equation) and an activity factor. Dieticians monitored dietary compliance and adjusted diets with a goal of weight maintenance, based on subject weights every 3 days. If subjects lost 2 kg or more, unit foods (100 kcal) with the same macronutrient distribution as the overall diet were added. Subjects were instructed to consume all food provided and discouraged from eating other foods. Subjects were encouraged to maintain their level of physical activity and not to change medications during the study.
In the morning of each magnetic resonance spectroscopy (MRS) study, subjects were given a 300-calorie smoothie (containing LCFAs prediet and containing LCFAs or MCFAs at the end of the diet treatment according to their randomization) at 7:00 am and were instructed to finish it by 7:30 am (immediately before their MRS studies) to minimize increases myocardial lipid deposition resulting from prolonged fasting (22, 24).
Indirect calorimetry
Oxygen consumption and carbon dioxide production were measured for 15 minutes by indirect calorimetry (TrueOne 2400, Parvo Medics) in a supine position in the morning after an overnight fast and 30-minute rest period. Resting energy expenditure and carbohydrate and total lipid oxidation rates were determined as previously described (25, 26).
Echocardiography
We quantified LV structure and function using two-dimensional, Doppler, and tissue Doppler imaging (TDI). TDI-derived peak myocardial velocity was obtained by positioning a sample volume on the mitral annulus for measurement of systolic and diastolic myocardial velocities on the lateral and septal mitral valve annulus sites to obtain systolic (S′) and early diastolic myocardial velocity (E′). Three cardiac cycles were obtained at each site and the values averaged; S′ and E′ are reported as the average of the two sites. Stroke volume was determined from the LV outflow tract time-velocity integral × LV outflow tract area. An echocardiography expert (A.W.) blinded to the intervention and pre/post status of the subjects made all of the echocardiographic measurements.
1H-MRS
Myocardial 1H-MRS spectra were acquired at end-systole and end-respiration. A point-resolved spectroscopy sequence was used for region of interest (2.4 ml) localization on the interventricular septum. Data points (n = 512) were acquired with 2-kHz spectral width and 120 signal averages, echo time (TE) = 24 milliseconds and repetition time equal to the length of respiratory cycle. Spectra were analyzed using Advanced Magnetic Resonance fitting algorithm within the jMRUI software package. Hepatic triglyceride (TG) measurements were made using 10 averages for each TE using a 2-second relaxation delay and breath-holds. A 15-mL voxel was placed in the right lobe away from visible vasculature and for subsequent measurements as close to the initial voxel as possible. Data were acquired at TEs of 24, 30, 35, 40, and 50 ms. Spectra were analyzed using the Advanced Magnetic Resonance fitting algorithm and jMRUI. TG contents are reported as the ratio of TG to water extrapolated to TE = 0.
Lipidomic analysis
Lipids in plasma samples were analyzed by liquid chromatography-tandem mass spectroscopy as previously described (27). The analytes measured included the following lipid classes: acylcarnitines, SMs, and Cer. Heat maps for representation of the lipidomic data were generated as described (27). An expert in liquid chromatography-tandem mass spectroscopy made all of the measurements blinded to subject treatment.
Statistical analyses
Subject characteristics at baseline are presented as mean ± SE and compared by Student's t test or χ2 tests as appropriate. Within group changes (ie, delta score) and between group differences in the heart function and lipidomic measures were analyzed by mixed model repeated measures ANOVA that included weight as a time-varying adjustment factor when appropriate. (Weight was not included when evaluating variables normalized to weight). Independent variables included time (pre vs post), group, and the interaction between the two. Model results were used to estimate mean values and change over time for within-group and between-group comparisons. Significance was identified at the P < .05 level. In the lipidomic analyses, a false discovery rate (FDR) of .05 was set and FDR-corrected P values are reported. The linear relationships between the change in heart function (S′) and the change in lipidomic species in the MCFA group were examined by Pearson's correlation coefficient. Analyses were conducted in SAS v9.3 (SAS Institute Inc).
Results
Baseline characteristics.
There were no differences between the groups' baseline diets (Supplemental Table 1). Both consumed a typical “Western” diet (21). Prestudy diets were similar in total % fat to that given in the LCFA and the MCFA diet interventions. There were no significant differences in medications taken by the subjects in the two groups (Supplemental Table 2). No subject changed medication dose or type during the study. There were no significant differences in age, race, weight, waist/hip ratio, glucose control, heart or liver lipid deposition, or lipid or carbohydrate oxidation between the groups (Table 1). All who underwent 1H-MRS had evidence of hepatic steatosis (>5.56%) (24). While total and low-density lipoprotein level were higher in the MCFA group at baseline (P < .04), there were no significant differences in high-density lipoprotein or TG levels.
Table 1.
Body Composition and Metabolic Responses
| LCFA |
P Value LCFA Pre-Post | MCFA |
P Value MCFA Pre-Post | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||
| Age (y) | 52 ± 3 | 48 ± 3 | ||||
| Sex (number M/F) | 3/4 | 1/8 | ||||
| Race (AA, Caucasian) | 4, 3 | 1, 8 | ||||
| Height (cm) | 69 ± 1 | 66 ± 1 | ||||
| Weight (kg) | 101.0 ± 5.9 | 99.8 ± 5.9 | <.01 | 95.6 ± 5.2 | 93.8 ± 5.2 | <.001 |
| Body mass index (kg/m2) | 33.2 ± 1.9 | 32.8 ± 1.9 | <.01 | 34.1 ± 1.7 | 33.5 ± 1.7 | <.0001 |
| Waist/hip | 0.97 ± 0.03 | 1.00 ± 0.03 | NS | 0.96 ± 0.02 | 0.91 ± 0.03 | NS |
| Fat-free mass (kg) | 59.6 ± 3.2 | 52.3 ± 3.1 | ||||
| % Fat | 39.4 ± 2.9 | 44.9 ± 1.7 | ||||
| ALT (units/liter) | 78 ± 18 | 68 ± 18 | NS | 42 ± 15 | 41 ± 16 | NS |
| AST (units/liter) | 48 ± 8 | 40 ± 9 | <.05 | 36 ± 7 | 29 ± 8† | <.05 |
| Glucose (mg/dL) | 169 ± 25 | 146 ± 25 | <.05 | 143 ± 22 | 130 ± 22 | NS |
| Insulin (μU/mL) | 15 ± 6 | 12 ± 6 | NS | 22 ± 5 | 20 ± 5 | NS |
| HbA1c (%) | 7.7 ± 0.7 | 7.9 ± 0.7 | NS | 7.7 ± 0.6 | 7.4 ± 0.6 | NS |
| HbA1c (mmol/mol) | 61 ± 20 | 63 ± 20 | NS | 61 ± 17 | 57 ± 17 | NS |
| TG (mg/dL) | 162 ± 46 | 162 ± 47 | NS | 149 ± 40 | 182 ± 41 | NS |
| Total cholesterol (mg/dL) | 156 ± 10 | 142 ± 10 | NS | 187 ± 9a | 176 ± 9 | <.05 |
| HDL (mg/dL) | 49 ± 6 | 48 ± 6 | NS | 50 ± 5 | 46 ± 5 | NS |
| LDL (mg/dL) | 75 ± 9 | 67 ± 10 | NS | 108 ± 8a | 98 ± 9 | <.05 |
| hsCRP (mg/dL) | 2.7 ± 2.0 | 2.3 ± 2.0 | NS | 7.1 ± 1.8 | 5.7 ± 1.8 | NS |
| Hepatic TG (%) | 19.5 ± 4.5 | 17.6 ± 4.5 | NS | 23.9 ± 3.9 | 23.9 ± 3.9 | NS |
| Cardiac TG (%) | 1.5 ± 0.3 | 1.5 ± 0.4 | NS | 1.8 ± 0.3 | 1.4 ± 0.3 | NS |
| Whole body lipid oxidation (g/kg/day) | 1.5 ± 0.2 | 1.6 ± 0.2 | NS | 1.6 ± 0.2 | 1.4 ± 0.2 | NS |
| Whole body carbohydrate oxidation (g/kg/day) | 1.1 ± 0.4 | 0.7 ± 0.4 | NS | 1.1 ± 0.4 | 1.4 ± 0.4 | NS |
| Resting energy expenditure (MJ/d) | 7.2 ± 0.4 | 6.9 ± 0.4 | NS | 7.7 ± 0.4 | 7.2 ± 0.4 | NS |
Abbreviations: AA, African American; ALT, alanine transaminase; AST, aspartate transaminase; F, female; HDL, high-density lipoprotein; HbA1c, glycated hemoglobin; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; M, male; NS, not significant.
Values are means ± sem adjusted for weight except when weight was the dependent variable. In the LCFA group, one patient postintervention could not be phlebotomized despite multiple attempts. One in each group did not undergo 1H-MRS because of claustrophobia. MCFA preintervention significantly higher than LCFA preintervention:
P < .05.
Baseline cardiac function was similar between the groups. There were no differences in baseline hemodynamics, relative wall thickness, ejection fraction, cardiac output between groups (Figure 1), or the TDI-derived measure of systolic function (mitral annular movement), S′ (Figure 2). TDI-derived diastolic function, as measured by E′, was abnormally low in both groups at baseline but was not different between the groups (Table 2). Although not statistically significant, there were slightly more women in the MCFA group (Table 1). This may have contributed to the observed lower baseline stroke volume (Figure 1) and LV mass (Table 2) in the in the MCFA group (P < .05); LV mass index was not statistically different (P = .08).
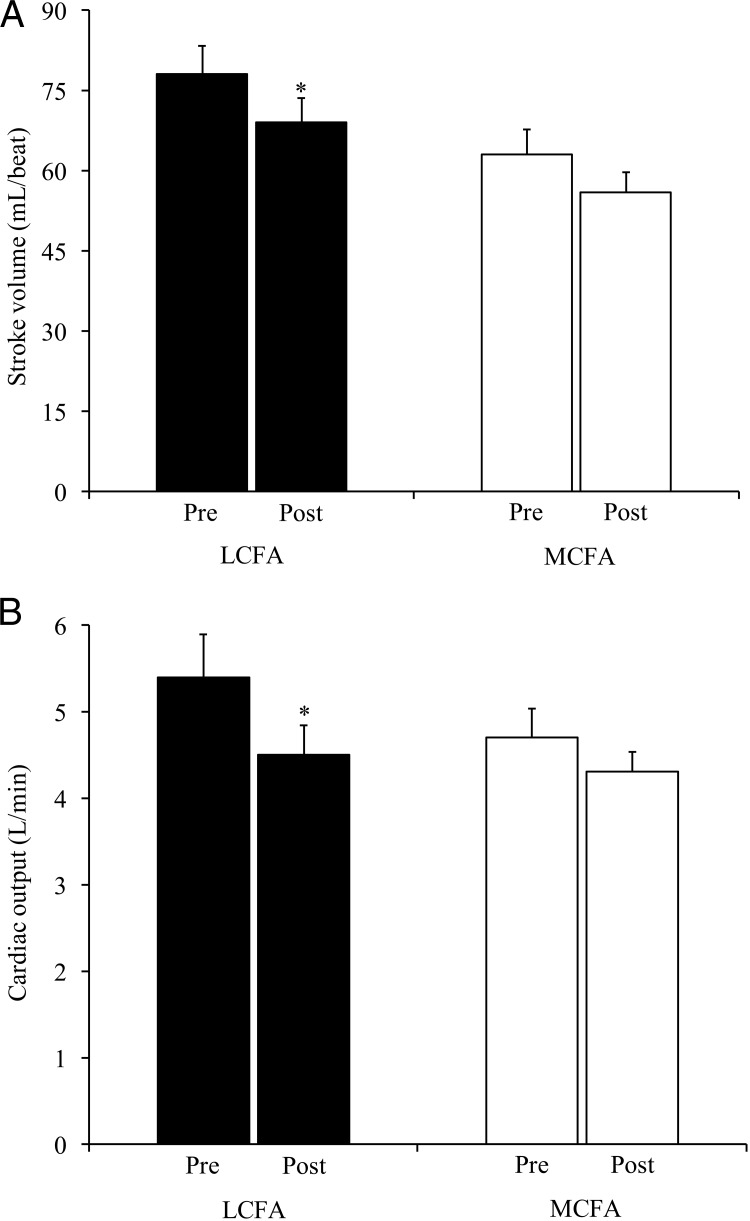
Figure 1.
Stroke volume and cardiac output are decreased following an LCFA-enriched diet. Stroke volume (A) and cardiac output (B) are shown for subjects assigned to the LCFA- (black bars) or MCFA-enriched (open bars) diets. Values are weight-adjusted and reported are means ± SEM. *P < .05 for pre vs postdiet values. The interactions between time and group were not significant.
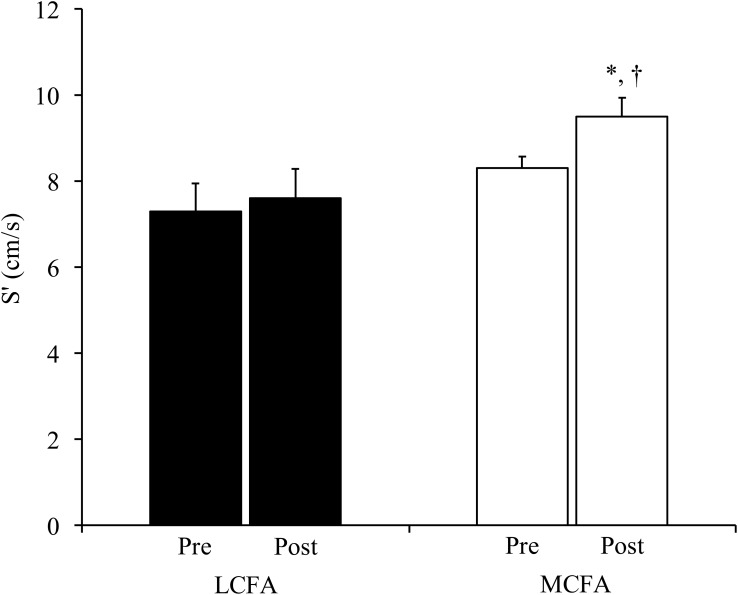
Figure 2.
Systolic function improves following MCFA-enriched diet. Tissue Doppler-derived movement of the mitral valve annulus toward the transducer in systole (S′) was quantified during transthoracic echocardiography in subjects assigned to LCFA (black bars) or MCFA (open bars) dietary intervention. Values are weight-adjusted and reported are means ± SEM. *P = .01 for pre vs postintervention measure in the MCFA treatment group; †P < .05 for the difference between the post-MCFA S′ and the post-LCFA S′. (As a point of reference, a nonweight-adjusted S′ value in a healthy 50-year-old man is approximately 9 cm/s; range, 7–12 cm/s) (39). The interactions between time and group were not significant (P = .13).
Table 2.
Hemodynamics, Cardiac Structure, and Function: Response to Dietary Intervention
| LCFA |
P Value LCFA Pre-Post | MCFA |
P Value MCFA Pre-Post | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||
| Hemodynamics | ||||||
| Heart rate (bpm) | 69 ± 3 | 65 ± 3 | NS | 75 ± 2a | 79 ± 2 | NS |
| Systolic blood pressure (mmHg) | 138 ± 7 | 137 ± 7 | NS | 128 ± 6 | 127 ± 6 | NS |
| Diastolic blood pressure (mmHg) | 80 ± 4 | 79 ± 4 | NS | 75 ± 4 | 75 ± 4 | NS |
| LV structure | ||||||
| Mass (g) | 221 ± 12 | 216 ± 12 | NS | 190 ± 11a | 179 ± 11 | NS |
| Mass (g/BSA) | 50.1 ± 3.8 | 49.3 ± 3.8 | NS | 46.8 ± 3.3 | 43.9 ± 3.4 | NS |
| Relative wall thickness | 0.6 ± 0.1 | 0.6 ± 0.1 | NS | 0.5 ± 0.1 | 0.5 ± 0.1 | NS |
| LV diastolic function | ||||||
| E′ average (cm/s) | 8.4 ± 0.7 | 8.5 ± 0.8 | NS | 8.4 ± 0.7 | 8.4 ± 0.7 | NS |
| E/e′ (septal) | 11.6 ± 1.6 | 12.0 ± 1.6 | NS | 9.9 ± 1.4 | 9.1 ± 1.4 | NS |
| Mitral valve deceleration time (ms) | 238 ± 15 | 225 ± 15 | NS | 204 ± 13 | 214 ± 13 | NS |
| LV systolic function | ||||||
| Ejection fraction (%) | 66 ± 2 | 64 ± 2 | NS | 65 ± 2 | 65 ± 2 | NS |
Abbreviation: NS, not significant.
Values are means ± SE. MCFA pre significantly different from LCFA pre:
P < .05. Measures of systolic function other than ejection fraction are shown as figures.
Effects of dietary interventions on whole-body metabolic measures (Table 1).
In general, subjects tolerated the diets well. One subject in the MCFA group had transient gastrointestinal upset with one of the foods, which subsided after food substitution. Study dieticians weighed all returned uneaten food. Subjects in the MCFA group ate 96.5%; the LCFA group ate 95.8% of provided food, indicating no difference in overall excellent dietary compliance. There were no reported changes in physical activity.
Despite estimating daily calories for weight maintenance, close caloric monitoring, and preplanned addition/subtraction of 100 cal “unit foods” to maintain weight, both groups lost weight. Accordingly, we controlled for weight change in our analyses. Aspartate transferase decreased in both groups. Fasting plasma glucose decreased in response to the LCFA diet, but was not significantly lower in the MCFA group. In contrast, fasting insulin tended to decrease (P < .06) in response to the MCFA diet, but not to the LCFA diet. All other measures of glucose and lipid homeostasis and metabolism did not change in response to either diet.
Effects of dietary interventions on cardiac function.
There were no significant changes in hemodynamics or cardiac chamber size in response to either diet (Table 2). Stroke volume and cardiac output, overall measures of cardiac performance, decreased in response to LCFA; however, these parameters did not significantly change in response to MCFA (Figure 1, A and B). LV ejection fraction also did not significantly change in either group. The more load-independent myocardial wall velocity during systole (S′) increased significantly (P = .02) in response to MCFA and was significantly greater (P = .04) postintervention compared to the postintervention S′ in the LCFA group (Figures 2 and 3). There were no changes in diastolic function measures with either diet (Table 2).
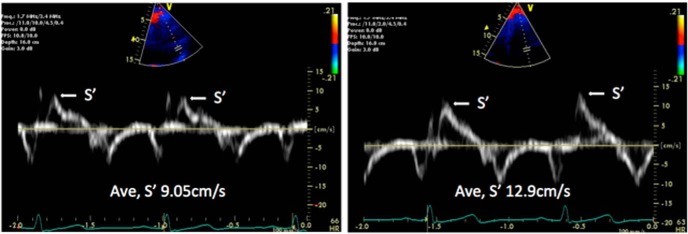
Figure 3.
An example of tissue Doppler-derived systolic function (S′) pre- and post-MCFA diet intervention in one subject.
Effects of dietary interventions on cardiac and hepatic lipid content.
Despite excellent compliance with the diets, modest weight loss in both groups, and improvement in cardiac function in the MCFA group, neither cardiac nor hepatic lipid content changed in either diet group (Table 1).
Effects of dietary interventions on the plasma lipidome.
There were no differences between the two groups in preintervention plasma levels of any acylcarnitine or ceramide species, although the MCFA group had higher levels of several SMs in pretreatment (Supplemental Table 3). All of the lipid species in Figure 4 and Supplemental Table 3 significantly decreased after the MCFA diet even after applying a FDR correction and including body weight as a time-varying adjustment factor. In contrast, no lipid species was different after the LCFA diet. There were no interactions between treatment group assignment and time (pre vs post) in the prediction of any lipid species change. Moreover, the decrease in several SM species correlated well with decreased fasting plasma insulin level: SM, 12:0 (r = 0.60, P = .03); SM, 14:0 (r = 0.63, P < .05), 15:0 (r = 0.62, P = .02), 16:0 (r = 0.61, P < .05), 20:0 (r = 0.73, P < .005, Supplemental Figure 2), 21:0 (r = 0.70, P < .01), and 23:1 (r = 0.66, P < .01) were all related to decreased insulin. Homeostatic Model Assessment-Insulin Resistance decreases related significantly to decreases in these same SMs and to a decrease in SM 14:1, 16:1, 21:2, 18:0, 20:1; 21:1, 22:1, 23:0, and 24:0 (all P < .05 or less). Although the MCFA diet was rich in C8 and C10, there were no significant differences in the AC8:0 and AC10:0 species in the plasma. There were no correlations between the changes in lipid species of interest (Figure 4, Supplemental Table 3) and changes in stroke volume or S′.
Figure 4.
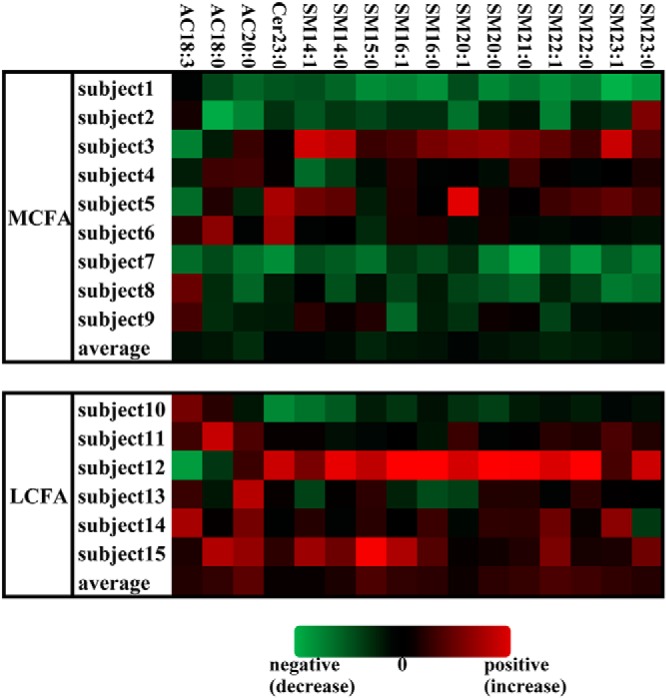
Lipidomic changes with dietary intervention. This “heat map” depicts the changes in acylcarnitine (AC), Cer, and SM species that were statistically significantly different from pre- to postintervention in the MCFA diet intervention group (as a whole) after FDR correction (*P < .05, †P < .01, ‡P < .001, §P < .005). For preparation of heat maps, we calculated the % change for a given analyte for each subject: [(post values − pre values)/pre values] × 100%. For each lipid species, we calculated the mean and SD of the % change for all MCFA and LCFA subjects. To generate the Z-score transformations, [(%change-mean % change)/SD] was calculated for each analyte for each subject. Data are shown for each subject in the MCFA and LCFA intervention group and the average value for each group, with green and red shades representing decreases or increases, respectively, for each lipid. Among the LCFA diet group, there were no significant changes in these or other lipid species.
Discussion
In this double-blind, randomized study, we found marked changes in the plasma lipidome and improvements in the relatively load-independent measure of cardiac contractility, TDI-derived S′, in patients with type 2 diabetes who consumed an MCFA-enriched diet compared to those who consumed a standard Western diet high in LCFAs. This improvement was independent of changes in hemodynamics, cardiac structure, or spectroscopy-detectable myocardial lipid deposition. Cardiac output and stroke volume, more load-dependent measures of function, worsened after a diet high in LCFAs but were unchanged on the MCFA-enriched diet. Even though the MCFA-enriched diet was higher in saturated fats than the LCFA-enriched diet, there were no significant changes in total cholesterol, high- or low-density lipoproteins, or TGs following either diet. The plasma lipidome, however, demonstrated marked changes after the MCFA diet intervention. Specifically, several sphingolipids, acylcarnitines, and a ceramide—all species implicated in the development of cardiomyopathy—decreased after a diet high in MCFAs. There were no changes in the plasma lipidome in patients on the high LCFA diet, likely reflecting the similarity of fat composition between the LCFA-enriched diet and the subjects' prestudy diet.
The major finding of this study was that cardiac S′ improved in patients with diabetes treated with a diet rich in MCFAs after only 14 days. S′ is a sensitive marker of cardiac function, with a higher S′ velocity indicating increased contractility (28). The more load-dependent indices, stroke volume and cardiac output, were not different after the MCFA diet, but decreased after the LCFA. Our study's small sample size may have contributed to the MCFA group's lack of a difference in these measures. It is possible that the changes in load-dependent measures are due to changes in preload because afterload, as estimated by the blood pressure and wall thickness, was unchanged. Subjects in both groups lost weight, which is often accompanied by decreased plasma volume. The sum of our data suggests a salutary effect of MCFAs on function and a neutral to possibly negative effect of LCFAs. We did not observe a change in diastolic function after the MCFA diet, and it may be that a larger sample size is needed. Our findings extend those of studies in rodent models of lipotoxic cardiomyopathy (3, 4, 16). In one model with cardiac-restricted overexpression of peroxisome proliferator-alpha, lipid deposition in the myocardium accompanied cardiac dysfunction—especially following consumption of a diet high in LCFAs. In these same peroxisome proliferator-α–overexpressing mice, a diet rich in MCFAs rescued cardiac dysfunction and steatosis (16).
However, we did not find that a MCFA-rich diet improved cardiac function by decreasing myocardial steatosis. Although our subjects did have evidence of myocardial steatosis (normal values are approximately 0.46 ± 0.3%), myocardial fat content even in patients with diabetes is relatively low (eg, compared to the liver). This may have contributed to our inability to detect a difference after a major diet change (and some weight loss). However, we also did not find a significant change in the very high hepatic TG levels (approximately 20%) with the MCFA-rich diet. In a study of marked diet-induced weight loss in human subjects with type 2 diabetes, improvement in diastolic function and cardiac steatosis were observed but were not correlated (29). Some studies in cultured cells and transgenic mouse models of metabolic cardiomyopathy have instead suggested that, although TG accumulation in lipid droplets in nonadipose tissues is the hallmark of overnutrition, the TG itself may not be detrimental (30). Our findings are consistent with the notion that fat storage in heart may be a marker, rather than a direct cause, of lipotoxicity.
Plasma lipids that were decreased in the MCFA-rich diet, including ceramides, SMs, and acylcarnitines, are species that have been implicated previously in lipotoxicity and the development of diabetic cardiomyopathy. Cardiac ceramides are elevated in models of diabetic cardiomyopathy and inhibition of de novo ceramide synthesis ameliorates cardiac function (3, 31). Ceramides can contribute to cell dysfunction through promotion of apoptosis, altered cellular signaling, inflammation, and oxidative stress, all of which could negatively affect organ function (3, 32–35). SMs, which can be hydrolyzed to produce ceramides, were significantly decreased in the plasma after the MCFA diet intervention but not after the LCFA intervention. The significance of higher baseline values for some SM in the MCFA group at baseline is unclear. Although subjects were randomized and generally well-matched across groups, differences in sex distribution may have contributed to the differences in baseline SMs. Acylcarnitines have also been implicated in lipotoxicity and are thought to be markers of incomplete mitochondrial oxidation (11). Although we observed decreases in each of these lipid species following the MCFA-enriched diet, our study was not designed to determine whether these changes are related to decreased production or increased catabolism/elimination. Our study was also not designed to correlate these lipidomic changes in the plasma with those in cardiac tissue.
Although the MCFA diet had a higher percentage of saturated fatty acids, it did not result in a significantly worse cholesterol profile. This is consistent with epidemiologic studies of Tokelau islanders, who consume a diet extremely high in saturated MCFAs (40–50% of calories are from saturated fats) but have cholesterol panels similar to New Zealanders who consume far less saturated fat (36). In our study, the MCFA diet group's fasting insulin levels trended lower after the intervention, a finding reminiscent of a 5-day MCFA diet intervention study that increased insulin sensitivity in patients with type 2 diabetes (17). The decreases we observed in several SM species correlated with the improvement in insulin levels, suggesting that SMs may contribute to the pathogenesis of and may have potential to serve as biomarkers in diabetes.
The current pilot study has limitations; it is relatively small, which may have contributed to our not finding a difference in some variables, such as cardiac lipid deposition. Future larger studies are needed to confirm the effects of MCFA vs LCFA diets on cardiac function. Larger, longer-term studies would also be helpful to definitively rule out an effect of MCFA diet on cardiac lipid deposits, the cholesterol profile, and diastolic function. In addition, although the dietary intervention was as long or longer than many studies of MCFA effects in humans (23, 37, 38), whether our findings extend to even longer dietary interventions with MCFAs requires future study. Our study was also not designed to evaluate the effects of sex hormones on the effects of the diet and/or lipidome.
Our study suggests that that not all saturated fatty acids are equally detrimental to the heart. In this tightly controlled dietary study in subjects with type 2 diabetes, we demonstrated that MCFAs have possible cardiac benefits relative to LCFAs despite the former having a higher saturated fat content. Neither diet was associated with a change in cardiac steatosis. However, this does not rule out a “lipotoxic” effect of LCFAs that is independent of cardiac steatosis. Finally, plasma lipids previously implicated in lipotoxicity were lowered to a greater extent in MCFA-treated subjects. More studies are needed to determine if plasma SM, ceramide, and acylcarnitine species will have utility as biomarkers to assess lipotoxic pathways, diabetes treatments, and/or to identify individuals at risk for diabetic cardiomyopathy.
Acknowledgments
The authors thank Ava Ysaguirre for her assistance in manuscript preparation and Dr. Julio Pérez and Joshua Leibowitz for assistance with the study. The Human Imaging and the Cardiovascular Imaging Research Cores were instrumental in completing this study.
This work was funded by grants from the Diabetic Cardiovascular Disease Center (St. Louis, Missouri) and the National Institutes of Health (P20 HL113444, P30 DK 020579, P30 DK 056341, UL1 TR000448). Cognis contributed some of the medium chain triglycerides for the study. Cognis did not have access to our data or the manuscript before publication. Cognis has since been acquired by BASF.
Trial registration: ClinicalTrials.gov: NCT01373814.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Cer
- ceramide
- E′
- early diastolic myocardial velocity
- FDR
- false discovery rate
- LCFA
- long-chain fatty acid
- LV
- left ventricular
- MCFA
- medium-chain fatty acid
- MRS
- magnetic resonance spectroscopy
- S′
- early systolic myocardial velocity
- SM
- sphingomyelin
- TDI
- tissue Doppler imaging
- TE
- echo time
- TG
- triglyceride.
References
- 1. Peterson LR, McKenzie CR, Schaffer JE. Diabetic cardiovascular disease: getting to the heart of the matter. J Cardiovasc Transl Res. 2012;5:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peterson LR, Saeed IM, McGill JB, et al. Sex and type 2 diabetes: obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity (Silver Spring). 2012;20:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chiu HC, Kovacs A, Ford DA, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finck BN, Lehman JJ, Leone TC, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiu HC, Kovacs A, Blanton RM, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–233. [DOI] [PubMed] [Google Scholar]
- 7. Szczepaniak LS, Dobbins RL, Metzger GJ, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–423. [DOI] [PubMed] [Google Scholar]
- 8. Hammer S, van der Meer RW, Lamb HJ, et al. Short-term flexibility of myocardial triglycerides and diastolic function in patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2008;295:E714–E718. [DOI] [PubMed] [Google Scholar]
- 9. Straczkowski M, Kowalska I, Baranowski M, et al. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366–2373. [DOI] [PubMed] [Google Scholar]
- 10. Amati F, Dube JJ, Alvarez-Carnero E, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60:2588–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mihalik SJ, Goodpaster BH, Kelley DE, et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring). 2010;18:1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitsutake S, Zama K, Yokota H, et al. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J Biol Chem. 2011;286:28544–28555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park TS, Hu Y, Noh HL, et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russo SB, Baicu CF, Van Laer A, et al. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest. 2012;122:3919–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adams SH, Hoppel CL, Lok KH, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finck BN, Han X, Courtois M, et al. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eckel RH, Hanson AS, Chen AY, Berman JN, Yost TJ, Brass EP. Dietary substitution of medium-chain triglycerides improves insulin-mediated glucose metabolism in NIDDM subjects. Diabetes. 1992;41:641–647. [PubMed] [Google Scholar]
- 18. Prior IA, Davidson F, Salmond CE, Czochanska Z. Cholesterol, coconuts, and diet on Polynesian atolls: a natural experiment: the Pukapuka and Tokelau island studies. Am J Clin Nutr. 1981;34:1552–1561. [DOI] [PubMed] [Google Scholar]
- 19. Stanhope JM, Sampson VM, Prior IA. The Tokelau Island Migrant Study: serum lipid concentration in two environments. J Chronic Dis. 1981;34:45. [DOI] [PubMed] [Google Scholar]
- 20. Dayrit CS. Coconut oil: atherogenic or not? Phillipp J Cardiol. 2003;31:97. [Google Scholar]
- 21. Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J. 2007;406:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reingold JS, McGavock JM, Kaka S, Tillery T, Victor RG, Szczepaniak LS. Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: reproducibility and sensitivity of the method. Am J Physiol Endocrinol Metab. 2005;289:E935–E939. [DOI] [PubMed] [Google Scholar]
- 23. St-Onge MP, Jones PJ. Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. J Nutr. 2002;132:329–332. [DOI] [PubMed] [Google Scholar]
- 24. Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. [DOI] [PubMed] [Google Scholar]
- 25. Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- 26. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:628–634. [DOI] [PubMed] [Google Scholar]
- 27. Fan M, Sidhu R, Fujiwara H, et al. Identification of Niemann-Pick C1 disease biomarkers through sphingolipid profiling. J Lipid Res. 2013;54:2800–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waggoner AD, Bierig SM. Tissue Doppler imaging: a useful echocardiographic method for the cardiac sonographer to assess systolic and diastolic ventricular function. J Am Soc Echocardiogr. 2001;14:1143–1152. [DOI] [PubMed] [Google Scholar]
- 29. Hammer S, Snel M, Lamb HJ, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol. 2008;52:1006–1012. [DOI] [PubMed] [Google Scholar]
- 30. Listenberger LL, Han X, Lewis SE, Cases S, Farese RVJ, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–14895. [DOI] [PubMed] [Google Scholar]
- 32. Bieberich E. Ceramide signaling in cancer and stem cells. Future Lipidol. 2008;3:273–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teichgraber V, Ulrich M, Endlich N, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14:382–391. [DOI] [PubMed] [Google Scholar]
- 34. Carracedo A, Geelen MJ, Diez M, Hanada K, Guzman M, Velasco G. Ceramide sensitizes astrocytes to oxidative stress: protective role of cannabinoids. Biochem J. 2004;380:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin CH, Kurup S, Herrero P, et al. Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity (Silver Spring). 2011;19:1804–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wessen J. Migration and Health in a Small Society: The Case of Tokelau (Research Monographs in Human Population Biology). New York, NY: Oxford University Press; 1992. [Google Scholar]
- 37. White MD, Papamandjaris AA, Jones PJ. Enhanced postprandial energy expenditure with medium-chain fatty acid feeding is attenuated after 14 d in premenopausal women. Am J Clin Nutr. 1999;69:883–889. [DOI] [PubMed] [Google Scholar]
- 38. Scalfi L, Coltorti A, Contaldo F. Postprandial thermogenesis in lean and obese subjects after meals supplemented with medium-chain and long-chain triglycerides. Am J Clin Nutr. 1991;53:1130–1133. [DOI] [PubMed] [Google Scholar]
- 39. Kasner M, Westermann D, Steendijk P, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. [DOI] [PubMed] [Google Scholar]