Abstract
Context:
Roux-en-Y gastric bypass (RYGB) leads to high-turnover bone loss, but little is known about skeletal effects of laparoscopic adjustable gastric banding (LAGB) or mechanisms underlying bone loss after bariatric surgery.
Objective:
To evaluate effects of RYGB and LAGB on fasting and postprandial indices of bone remodeling.
Design and Setting:
Ancillary investigation of a prospective study at 2 academic institutions.
Participants:
Obese adults aged 21–65 years with type 2 diabetes who underwent RYGB (n = 11) or LAGB (n = 8).
Outcomes:
Serum C-terminal telopeptide (CTX), procollagen type 1 N-terminal propeptide (P1NP), and PTH were measured during a mixed meal tolerance test at baseline, 10 days and 1 year after surgery. Changes in 25-hydroxyvitamin D, polypeptide YY (PYY), glucagon-like peptide-1, glucose-dependent insulinotropic peptide, and insulin were also assessed.
Results:
Fasting CTX increased 10 days after RYGB but not LAGB (+69 ± 23% vs +12±12%, P < .001), despite comparable weight loss at that time. By 1 year, fasting CTX and P1NP increased more after RYGB than LAGB (CTX +221 ± 60% vs +15 ± 6%, P<0.001; P1NP +93 ± 25% vs −9 ± 10%, P < .001) and weight loss was greater with RYGB. Changes in CTX were independent of PTH and 25-hydroxyvitamin D but were associated with increases in fasting PYY. Postprandial suppression of CTX was more pronounced after RYGB than LAGB at 10 days and 1 year postoperatively.
Conclusions:
RYGB is accompanied by early increases in fasting indices of bone remodeling, independent of weight loss or changes in PTH or 25-hydroxyvitamin D. LAGB did not affect bone markers. PYY and other enterohormonal signals may play a role in RYGB-specific skeletal changes.
Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric banding (LAGB) are effective bariatric procedures for weight management. RYGB is considered “metabolic” surgery, involving hormonal changes that lead to greater improvements in body weight and obesity comorbidities than LAGB, which is solely a restrictive procedure.
Metabolic bone disease may be an unintended adverse consequence of bariatric procedures. RYGB leads to decline in bone density of about 5%–10% at the hip and 3%–6% at the spine within the first 1–2 years postoperatively (1–9). There are less data concerning bone changes after LAGB, and these have yielded mixed results (9–13). In addition, a large proportion of patients seeking bariatric surgery have type 2 diabetes (T2DM), a condition that is associated with structural bone defects and a 70% increased risk of fracture (14, 15). A recent randomized trial of patients with T2DM documented similar rates of bone loss after RYGB and sleeve gastrectomy as seen in nondiabetic populations (8), whereas another randomized trial of patients with T2DM found numerically more fractures in those who underwent RYGB as compared with those who received intensive medical therapy (16). Therefore, it is important to characterize skeletal consequences of the various bariatric surgery procedures in this population.
The pathophysiology underlying skeletal changes after bariatric surgery is likely multifactorial but remains incompletely understood. In clinical studies to date, the dramatic weight loss observed after bariatric surgery has confounded attempts to differentiate the mechanical unloading mechanisms of bone loss from the weight-independent surgery-specific mechanisms. Although RYGB-induced calcium and vitamin D malabsorption may cause negative skeletal effects, studies suggest that these classical mechanisms may not be the primary determinants of bone loss after bariatric surgery (17).
It is possible that changes in gastrointestinal (GI) hormones resulting from RYGB have direct effects on the skeleton. Specifically, polypeptide YY (PYY), an anorexigenic hormone secreted during feeding that increases after RYGB (18), may have catabolic effects on bone (19–21). Incretins, including glucagon-like peptide-1 (GLP-1), GLP-2, and possibly glucose-dependent insulinotropic peptide (GIP), also increase after RYGB, and may have direct skeletal effects (22–25). Feeding-induced reductions in bone resorption in normal adults (26, 27), and one cross-sectional study showing postprandial alterations in indices of bone remodeling in the setting of RYGB (28) together provide further evidence supporting cross-talk between GI hormones and bone. Alternatively, altered insulin levels after RYGB could contribute to bone loss. Insulin may be an anabolic agent promoting bone formation, and hyperinsulinism is associated with increased bone mass in clinical studies, independent of body mass index (BMI) (29, 30). Therefore, a reduction in basal insulin levels after RYGB and LAGB could be another mechanism contributing to skeletal deterioration, despite higher postprandial insulin peaks.
Our study objectives were to prospectively evaluate the differential effects of RYGB and LAGB on fasting and postprandial indices of bone remodeling in adults with T2DM and to explore potential mechanisms for skeletal changes with feeding and after bariatric surgery. Our primary outcome was postoperative change in serum type 1 cross-linked C-terminal telopeptide (CTX), a product of type 1 collagen degradation and thus a marker of bone resorption, and serum procollagen type 1 N-terminal propeptide (P1NP), which is produced during the formation of the collagen triple helix and is considered a key marker of bone formation. We hypothesized that RYGB is associated with a greater increase in bone resorption than LAGB and that RYGB has weight loss-independent effects on bone, which can be observed even before significant weight loss occurs.
Materials and Methods
Study subjects
A subset of subjects who were participating in the SLIMM-T2D (Surgery or Lifestyle with Intensive Medical Management of type 2 Diabetes) trials (31, 32) were included in this ancillary investigation. Briefly, adults aged 21–65 years with T2DM and BMI of 30–45 kg/m2 were recruited as part of 2 parallel randomized controlled trials (ClinicalTrials.gov NCT01073020). The first trial randomized participants to RYGB (n = 19) or an intensive multidisciplinary medical diabetes and weight management program (n = 19), Why WAIT (Weight Achievement and Intensive Treatment). A second trial randomized separate participants to LAGB (n = 18) or the Why WAIT (Weight Achievement and Intensive Treatment) program (n = 22). Relevant exclusion criteria included: inflammatory bowel disease, endocrine disorders other than T2DM, malignancy, debilitating medical conditions precluding exercise, lactation, significant weight loss (>3%) within the previous 3 months, history of drug and/or alcohol abuse or smoking within the past 2 months, use of oral steroids, and serum creatinine more than 1.5 mg/dL. For this study, we focused on subjects in the surgical arms of these 2 trials who also participated in a mixed meal tolerance test (MMTT) substudy (RYGB, n = 11; LAGB, n = 8). The SLIMM-T2D (Surgery or Lifestyle with Intensive Medical Management of type 2 Diabetes) trials were approved by the Partners Healthcare Institutional Review Board as well as the Food and Drug Administration. The ancillary metabolic study was approved by both the Partners Healthcare and the Joslin Diabetes Center Institutional Review Boards. All subjects signed informed consent.
Study protocol
Participants were evaluated at baseline (14 d or less before surgery) and at 10 (±2) days and 1 year after surgery. For the MMTT performed at each visit, subjects presented to the clinical research facility in the early morning after 12 hours of fasting. After a baseline blood draw, subjects consumed Ensure (9-g protein, 40-g carbohydrate, 6-g fat) over 5 minutes. Repeat blood draws at 30, 60, and 120 minutes were obtained for biochemical assays. Study visits included measurement of weight (model 0501 electronic scale; ACME) and height using a wall-mounted stadiometer (Holtain), as well as medical history and medication review.
Biochemical assays
Fasting morning serum was collected at each visit and frozen at −80°C for batched analysis. Serum type 1 cross-linked CTX was measured by electrochemiluminescence immunoassay (Roche Diagnostics) with an intra- and interassay coefficient of variation of 2% and 3%. Serum levels of PINP were measured by RIA (Orion Diagnostica), with intra- and interassay coefficients of variation of approximately 4% and 6%. We also assessed serum 25-hydroxyvitamin D via immunochemiluminometric assay (DiaSorin LIAISON), and intact PTH via electrochemiluminescence immunoassay (Roche Diagnostics). Serum calcium, albumin, phosphorus, and hemoglobin A1c (HbA1c) (Quest Diagnostics) were also measured at baseline and 1 year. Corrected calcium was calculated = [0.8 × (4 − albumin (g/dL))] + serum calcium (mg/dL). PYY was assessed by electrochemilumniescence immunoassay (Meso Scale Discovery). The lower limit of detection for PYY is 9.25 pmol/L; intra- and interassay precision for these assays was not provided by the manufacturer. In the RYGB subjects, plasma samples obtained at each time point during the MMTT were additionally assayed for plasma glucose by glucose oxidation, insulin by RIA (Diagnostic Systems Laboratories), total GLP-1 using an antiserum specific for the amidated C terminus, which reacts with intact GLP-1 and the primary (N-terminally truncated) metabolite (33) and is primarily reactive with GLP-1 of intestinal origin, and total GIP using the C-terminally directed antiserum 867, which reacts with intact GIP and the N-terminally truncated metabolite (34). Assay sensitivity for GLP-1 and GIP was below 1 pmol/L, and intraassay coefficient of variation below 6% at 20 pmol/L.
Statistics
Baseline differences were compared using independent t tests or Fisher's exact tests. Longitudinal values are reported as mean ± SEM unless otherwise noted. A longitudinal general linear mixed effects model (SAS PROC MIXED) with a compound symmetry covariance structure was used to compare mean change in outcomes between RYGB and LAGB groups over the 1 year study. The subject-specific intercept was considered a random effect, and time, group, and time by group interaction were considered fixed effects. Associations between percent change in indices of bone remodeling and other outcomes within the gastric bypass group were assessed at day 10 and 1 year; when statistically appropriate, repeated measures analysis was applied to evaluate correlation across the entire study. Postprandial changes in CTX, PYY, GLP-1, and GIP were evaluated using area under the curve (AUC) analyses, calculated according to the trapezoidal rule after subtracting out baseline values. Maximal percent increase or decrease during the MMTT was also calculated. All analyses were performed using SAS 9.2 software (SAS Institute, Inc). P < .05 were considered significant.
Results
Baseline characteristics
At baseline, there were no significant differences between RYGB and LAGB groups in demographics, age, weight, or laboratory measures of diabetes control or bone metabolism (Table 1). None of the participants had a history of bone-modifying disorders. Two subjects in the RYGB group were taking pioglitazone at baseline but both discontinued the medication immediately after surgery; no other known bone-active medications were used by any subjects at any point throughout the study. One subject in the RYGB group withdrew from the study before the 1-year time point.
Table 1.
Baseline Characteristics
| Gastric Bypass, n = 11 | Gastric Banding, n = 8 | P value | |
|---|---|---|---|
| Age (y) | 53 ± 6 | 55 ± 13 | .755 |
| Gender (men/women) | 5 / 6 | 5 / 3 | .650 |
| Ethnicity (Caucasian/African-American/other) | 8/1/2 | 6/2/0 | .864 |
| Weight (kg) | 107 ± 18 | 107 ± 13 | .970 |
| BMI (kg/m2) | 37 ± 4 | 36 ± 3 | .528 |
| HgA1c (%) | 8.0 ± 1.5 | 7.9 ± 1.3 | .746 |
| Fasting glucose (mg/dL) | 139 ± 58 | 155 ± 64 | .568 |
| Creatinine (mg/dL) | 0.77 ± 0.15 | 0.76 ± 0.13 | .860 |
| Corrected calcium (mg/dL)a | 9.0 ± 0.3 | 9.1 ± 0.4 | .648 |
| Phosphorus (mg/dL) | 3.4 ± 0.7 | 3.4 ± 0.5 | .936 |
| 25-hydroxyvitamin D (ng/mL) | 21 ± 11 | 27 ± 15 | .261 |
| PTH (pg/mL) | 43 ± 11 | 40 ± 13 | .576 |
Data are presented as mean ± SD.
Corrected calcium = [0.8 × (4 − albumin (g/dL))] + serum calcium (mg/dL).
Weight loss
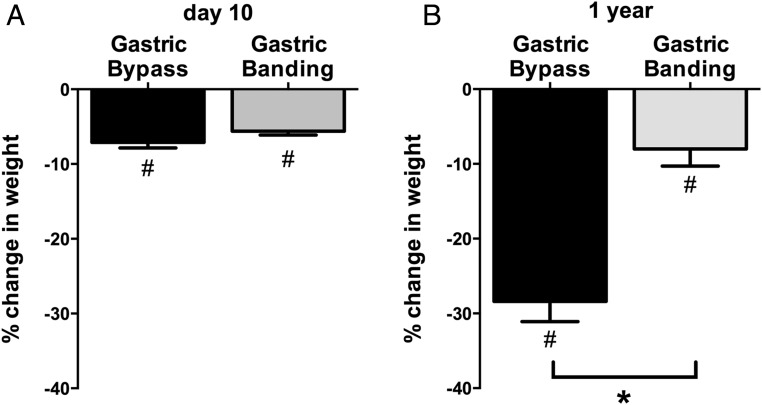
Mean weight loss at 10 days after surgery was similar in RYGB and LAGB groups (−7 ± 1 vs −6 ± 1 kg, P = .672) (Figure 1A) providing an early assessment at comparable weight loss, as intended. By 1 year, the RYGB group lost more weight than those who had undergone LAGB (−27 ± 4 vs −9 ± 2 kg, P < .001) (Figure 1B).
Figure 1.
Mean ± SEM percentage change in weight in gastric bypass (black bar) and gastric banding (gray bar) groups. Percentage weight loss from preoperative baseline is shown at day 10 (A) and 1 year (B) after surgery. *, P < .001 for the comparison of gastric bypass vs gastric banding; #, P < .05 for the within-group comparison against baseline.
Changes in fasting indices of bone metabolism
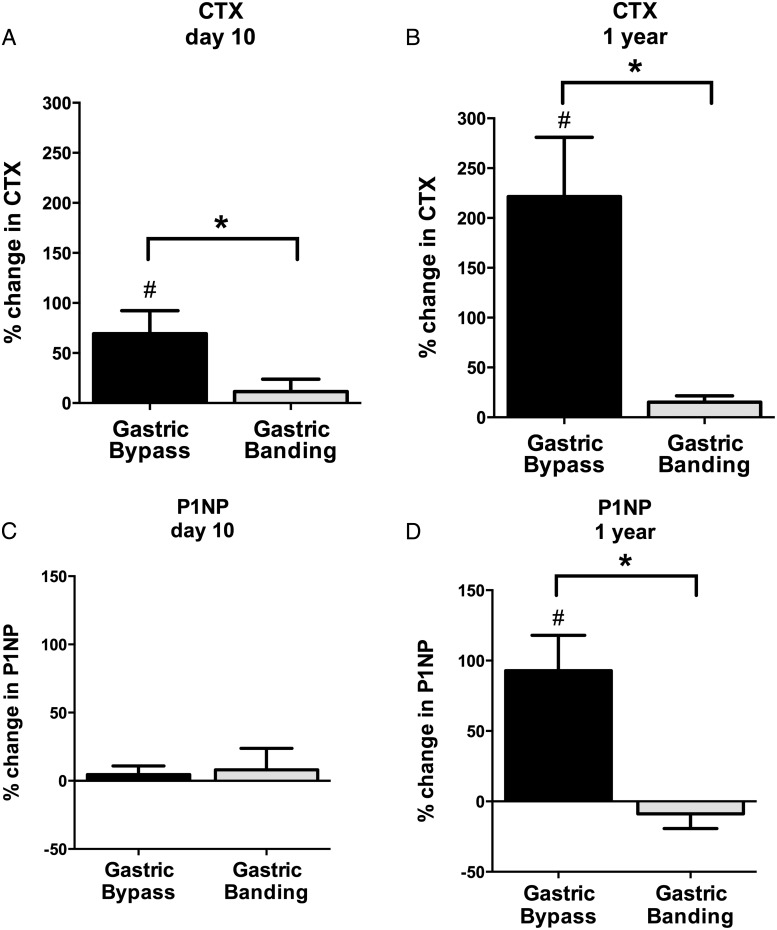
Despite equivalent weight loss at day 10, mean increase in fasting CTX was significantly higher after gastric bypass than gastric banding (+69 ± 23% vs +12 ± 12%, P < .001) (Figure 2A). By 1 year, fasting CTX had increased further after RYGB, as compared with no change after LAGB (+221 ± 60% vs +15 ± 6%, P < .001) (Figure 2B). Within-group analyses confirmed a linear increase in CTX above baseline in the RYGB group (P ≤ .001), as compared with no detectable change in the LAGB group (P = not significant [NS]). P1NP was essentially unchanged at day 10 after both surgeries (P = NS, Figure 2C) but significantly higher after RYGB than LAGB at 1 year (+93 ± 25% vs −9 ± 10%, P < .001) (Figure 2D). Within-group analyses again confirm an increase in P1NP above baseline only in the RYGB group at 1 year (P < .001) and no change in P1NP in the LAGB group throughout the study (P = NS).
Figure 2.
Mean ± SEM percentage change in CTX and P1NP in gastric bypass (black bar) and gastric banding (gray bar) groups. Percentage change from preoperative baseline is shown for CTX and P1NP at day 10 (A and C, respectively) and 1 year (B and D, respectively) after surgery. *, P < .001 for the comparison of gastric bypass vs gastric banding; #, P < .05 for the within-group comparison against baseline.
Fasting serum levels of corrected calcium and phosphorus were unchanged and similar in the 2 surgical groups over 1 year (Table 2). 25-hydroxyvitamin D levels were unchanged after LAGB and early after RYGB, but increased +151 ± 83% at 1 year after RYGB (P < .001). Of note, 78% of subjects in the RYGB group reported an increase in vitamin D supplements during the year after surgery, as compared with 38% of subjects in the gastric banding group. Although mean PTH levels did not change after RYGB over the 1-year period, mean PTH levels were higher in the RYGB group than the LAGB group at day 10 due to a transient PTH decline in the LAGB group at this early time point (Table 2). By 1 year, PTH levels were similar in both groups.
Table 2.
Calciotropic Hormones
| Baseline | 10 Days | 1 Year | ||
|---|---|---|---|---|
| Corrected calcium (mg/dL)a | Gastric Bypass: | 8.9 ± 0.3 | N/A | 8.9 ± 0.3 |
| Gastric Banding: | 9.0 ± 0.4 | N/A | 9.1 ± 0.3 | |
| Phosphorus | Gastric Bypass: | 3.4 ± 0.7 | N/A | 3.5 ± 0.4 |
| Gastric Banding: | 3.4 ± 0.5 | N/A | 3.6 ± 0.4 | |
| 25-hydroxyvitamin D (ng/mL) | Gastric Bypass: | 22 ± 10 | 25 ± 8 | 36 ± 8b |
| Gastric Banding: | 29 ± 15 | 32 ± 12 | 19 ± 8 | |
| PTH (pg/mL) | Gastric Bypass: | 43 ± 11 | 47 ± 17b | 45 ± 4 |
| Gastric Banding: | 40 ± 13 | 30 ± 10 | 39 ± 7 |
Data are presented as mean ± SD.
Corrected calcium = [0.8 × (4 − albumin (g/dL))] + serum calcium (mg/dL).
P < .05 for comparison of gastric bypass vs gastric banding groups.
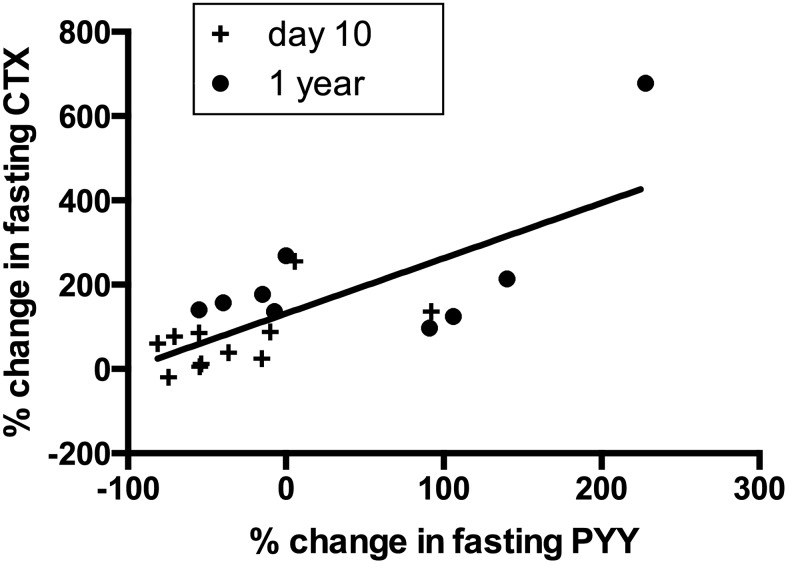
Fasting PYY was unchanged in the 2 surgical groups at 10 days, but increased more at 1 year after RYGB in contrast to LAGB (+50 ± 32% vs −15 ± 16%, P = .030). Within the RYGB group, the change in fasting PYY was significantly correlated with increases in fasting CTX (r = 0.70, P < .001) (Figure 3) throughout the study. A similar correlation was observed between fasting PYY and fasting P1NP at 1 year (r = 0.77, P = .014). In contrast, there was no change in fasting GIP and GLP1 after RYGB, and no subsequent correlations with change in CTX or P1NP.
Figure 3.
Scatterplot of percentage change in fasting CTX and PYY within the gastric bypass group at day 10 (+) and 1 year (●). Increases in CTX were directly correlated with change in fasting PYY (r = 0.70, P < .001).
In the RYGB group, improvements in HbA1c (−17 ± 6%, P = .010) and fasting insulin (−58 ± 12%, P < .001) were noted at 1 year as compared with baseline. Changes in HbA1c were inversely correlated with changes in fasting CTX (r = −0.74, P = .023). There were no significant associations between change in fasting insulin and CTX. In the LAGB group, the mean HbA1c decreased from 7.9 ± 1.3% to 7.1 ± 1.1%, but this did not reach statistical significance (P = .168). Finally, there were no significant associations between change in weight and changes in either CTX or P1NP in the RYGB or LAGB group at either time point.
Postprandial changes in indices of bone metabolism
At presurgical baseline, there was a similar and mild postprandial reduction in serum CTX in both the RYGB and LAGB groups. After RYGB, CTX declined more dramatically in response to a mixed meal than after LAGB (P < .001 for both) (Figure 4). For example, by 1 year, maximal postprandial CTX suppression was −35 ± 4% after RYGB, as compared with −21 ± 7% after LAGB (P < .001). This more aggressive postprandial suppression of CTX after RYGB occurred despite overall higher absolute levels of CTX at day 10 and 1 year. There were no significant differences between surgical groups in postprandial PTH or calcium at any time point.
Figure 4.
Mean ± SEM percentage change in postprandial CTX in gastric bypass (solid line) and gastric banding (dotted line) groups. Postprandial changes in CTX were assessed during a 2-hour MMTT at baseline (A), day 10 (B), and 1 year (C) after surgery. Although fasting CTX increased after gastric bypass, postprandial declines in CTX were more pronounced after gastric bypass than gastric banding at day 10 and 1 year (P < .001 for both).
Postprandial PYY increased more after RYGB than after LAGB at both day 10 and at 1 year (P < .001) (Supplemental Figure 1). In addition, postprandial increases in GLP1 were greater after RYGB as compared with presurgical baseline (P < .001), whereas postprandial responses to GIP did not change. In the RYGB group, we observed no significant change in the total glucose AUC during the MMTT at 10 days or 1 year postoperatively compared with the baseline. Kinetic patterns of postprandial insulin secretion differed after RYGB, but insulin AUC did not differ from baseline at 10 days or 1 year. Change in postprandial CTX was not associated with postprandial change in PYY, GLP1, GIP, or insulin at either time point (all P > .05). Similarly, postprandial change in CTX was not significantly associated with weight loss.
Discussion
In this prospective study, we demonstrate differential changes in fasting and postprandial indices of bone remodeling after RYGB and LAGB in patients with obesity and T2DM, including at an early postoperative time point when weight loss was comparable in the 2 groups. RYGB was accompanied by large increases in fasting markers of bone resorption (CTX) as well as bone formation (P1NP) over 1 year, whereas LAGB did not lead to significant changes in either bone marker. A rise in fasting CTX after RYGB was detectable as early as 10 days after surgery, and appears to be independent of either weight loss or changes in PTH and 25-hydroxyvitamin D. In addition, we show that reductions in postprandial CTX are exaggerated after RYGB.
These results extend our current understanding of the differential effects of bariatric surgical procedures on the skeleton. In contrast to neutral effects of LAGB on bone, RYGB increases bone resorption markers soon after surgery, and in a manner that persists over at least 1 year. The delayed increase in bone formation markers after RYGB could potentially result from the physiologic coupling of osteoclasts and osteoblasts in the setting of a longstanding high bone turnover state. In contrast, the lack of change in biochemical parameters of bone metabolism after LAGB is perhaps reassuring in terms of the impact of this procedure on bone health. Our findings are similar to another study that did not find any change in bone turnover markers after LAGB over a 2-year period (9), however, contrast with 2 other reports of increases in bone resorption after LAGB (10, 11). It is possible the differences in our findings may be due to varying amounts of weight loss; we documented an average 8% weight loss 1 year after LAGB, whereas in other studies weight loss was more than 15%. Note, however, that we did not find an association between weight loss and changes in indices of bone remodeling in either group. Furthermore, effects of LAGB on bone density remain unclear, with studies reporting conflicting data on bone mineral density (9, 11–13). As for fracture implications, 1 cohort of bariatric surgery patients (60% LAGB, 29% RYGB) found no short-term increase in fracture risk (35), whereas a long-term study of RYGB patients documented a 3- to 5-fold increased risk of spine and hip fractures (36). Larger studies are required to determine whether LAGB has a truly neutral effect on the skeleton, and to verify whether RYGB increases fracture risk.
Our study also raises intriguing questions about mechanisms underlying skeletal change after RYGB. Previous studies also document increases in bone turnover markers after RYGB (2–4, 9, 37), but this is the first study to examine early postsurgical changes, and to have a comparator group with similar magnitudes of weight loss. We demonstrate increases in CTX as early as 10 days after RYGB but not LAGB, even though weight loss was comparable in the 2 groups at this time point. The differential CTX response this early after RYGB suggests the early increases in bone resorption after RYGB are not due to mechanical unloading from weight loss. Furthermore, weight loss was not associated with changes in bone turnover markers at any point during the study. Although mechanical unloading is likely to play a role in the long-term bone loss observed after RYGB, these data suggest additional contributions of other mechanisms, and are consistent with animal studies that have shown weight loss-independent mechanisms of bone loss after RYGB (38, 39).
We also observed increased bone resorption after RYGB despite unchanged PTH and calcium levels, and with an increase in serum 25-hydroxyvitamin D, which was likely due to increased supplement use. Although RYGB-induced calcium and vitamin D malabsorption have been documented (40, 41), appropriate postoperative supplementation can prevent secondary hyperparathyroidism in many cases. The elevation in bone resorption markers despite unchanged PTH levels in this and other studies (6–10) suggests that calcium and vitamin D deficiencies are not the primary drivers of changes in bone turnover after RYGB.
An alternative hypothesis is that RYGB induces changes in the GI tract that have a direct influence on the skeleton. Along these lines, we found significant associations between changes in fasting bone markers and PYY, an anorexigenic hormone that is secreted from intestinal L cells. Although association does not prove causality, preclinical and clinical studies suggest that PYY stimulates osteoclastic bone resorption, has a negative effect on osteoblastic activity, and is inversely correlated with bone mass (19, 20). These catabolic effects appear to be mediated by the Y1 receptor, which is present on osteoblasts (42, 43). GLP-1 and GIP may also have direct skeletal effects (22–24), but we did not find any associations between changes in these GI hormones and bone marker outcomes. We did find, however, that postprandial changes in CTX were exaggerated after RYGB, which is consistent with findings from a cross-sectional study that compared RYGB recipients with nonoperated weight-matched controls (28). Although we cannot rule out that postprandial changes are due to transient increases in the clearance of bone markers, the acute skeletal response to feeding seems to suggest that GI signals have a direct impact on bone through mechanisms that have not yet been elucidated. Thus the changing enterohormonal profile after RYGB may modulate postprandial effects on bone turnover markers. Nevertheless, despite the exaggerated postprandial suppression of CTX after RYGB, levels of this bone resorption marker remain elevated at all time points, consistent with an overall negative bone balance after RYGB.
We also found that increases in CTX were significantly associated with improvements in HbA1c. Hyperglycemia is thought to have negative effects on bone, perhaps through direct effects on bone cells and/or skeletal accumulation of advanced glycosylation end-products (15). Therefore, improvements in hyperglycemia are unlikely to be causally related to increases in bone resorption after RYGB. Instead, this association may be confounded by improvements in other metabolic factors that may have effects on bone. Although we hypothesized that reductions in hyperinsulinism after RYGB might lead to increased bone resorption, we did not find a significant association between changes in insulin and bone turnover markers.
This study is limited by the small size of the cohorts. Despite this, we had sufficient power to detect significant increases in indices of bone remodeling after RYGB. Another limitation was that weight loss in the LAGB group was less than seen in some studies, which could explain a lack of skeletal change at 1 year. We do not have information about physical activity levels in the acute postoperative period after RYGB and LAGB, but at our institution, patients are typically ambulatory within 24 hours of both surgeries and discharged home within 2–3 days. Furthermore, the acute increases in serum CTX we observed after RYGB are double that seen in complete bedrest studies (44), and therefore we do not believe that minor differences in activity level can explain the large increases we observed in CTX after RYGB but not LAGB. We also lacked direct measurement of bone density, and therefore cannot determine whether the observed changes in bone markers resulted in loss of bone mass. Lastly, our study population included only adults with T2DM, and therefore the results may not be generalizable to a nondiabetic population. Although both RYGB and LAGB can lead to diabetes remission, it is not clear whether and when there is resolution of the skeletal fragility that accompanies longstanding diabetes. Thus, it is possible that the response to bone remodeling differs in the presence of diabetes, which itself is a disorder associated with osteoblastic dysfunction. Major strengths of this research include the prospective design and the comparison of 2 common bariatric surgery procedures within a well-matched population. Importantly, our early postsurgical time point (d 10) was chosen to have equivalent weight loss between RYGB and LAGB groups, and to allow us to examine weight loss-independent effects of the 2 surgeries. Finally, the inclusion of the MMTT allowed us to examine the effects of RYGB on postprandial changes in bone remodeling, and the differential responses in postprandial bone turnover markers and GI hormones after the 2 bariatric procedures.
In summary, RYGB leads to an early and weight loss-independent increase in a marker of bone resorption, whereas LAGB did not affect indices of bone remodeling over a 1-year period among adults with T2DM. The elevation in CTX after RYGB is independent of weight loss or changes in PTH, and may be associated with changes in PYY. Future studies should further investigate connections between GI hormones and bone as a potential mechanism of skeletal changes after RYGB surgery. Finally, it will be important to conduct large prospective studies to examine differential changes in bone density after bariatric surgery. These studies will provide clinically important information for physicians and patients who are weighing potential risks of these highly effective weight loss surgeries.
Acknowledgments
We thank the Clinical Laboratory Research Core at the Massachusetts General Hospital for batch laboratory testing.
This work was supported by National Institutes of Health Grants K23-DK093713, RC1-DK086918, R56-DK095451, and P30-DK036836; Harvard Catalyst and The Harvard Clinical and Translational Science Center (National Center for Research Resources and National Center for Advancing Translational Sciences, Award 8UL1TR000170–05; KL2/MeRIT Program Award UL1 RR 025758; and financial contributions from Harvard University and its affiliated academic healthcare centers); the Joslin Clinical Research Center and its philanthropic donors; the Marietta Blau Grant ICM-2010–02797 from the Österreichischer Austausdienst; the Herbert Graetz Fund; Covidien; Lifescan, a division of Johnson and Johnson; Nestle; and NovoNordisk.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CTX
- C-terminal telopeptide
- GI
- gastrointestinal
- GIP
- glucose-dependent insulinotropic peptide
- GLP-1
- glucagon-like peptide-1
- HbA1c
- hemoglobin A1c
- LAGB
- laparoscopic adjustable gastric banding
- MMTT
- mixed meal tolerance test
- NS
- not significant
- P1NP
- procollagen type 1 N-terminal propeptide
- PYY
- polypeptide YY
- RYGB
- Roux-en-Y gastric bypass
- T2DM
- type 2 diabetes.
References
- 1. Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19:41–46. [DOI] [PubMed] [Google Scholar]
- 2. Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93:3735–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu EW, Bouxsein ML, Putman MS, et al. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2015;100:1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stein EM, Carrelli A, Young P, et al. Bariatric surgery results in cortical bone loss. J Clin Endocrinol Metab. 2013;98:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carlin AM, Rao DS, Yager KM, Parikh NJ, Kapke A. Treatment of vitamin D depletion after Roux-en-Y gastric bypass: a randomized prospective clinical trial. Surg Obes Relat Dis. 2009;5:444–449. [DOI] [PubMed] [Google Scholar]
- 6. Vilarrasa N, San José P, García I, et al. Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3-year follow-up. Obes Surg. 2011;21:465–472. [DOI] [PubMed] [Google Scholar]
- 7. Muschitz C, Kocijan R, Marterer C, et al. Sclerostin levels and changes in bone metabolism after bariatric surgery. J Clin Endocrinol Metab. 2015;100:891–901. [DOI] [PubMed] [Google Scholar]
- 8. Maghrabi AH, Wolski K, Abood B, et al. Two-year outcomes on bone density and fracture incidence in patients with T2DM randomized to bariatric surgery versus intensive medical therapy. Obesity (Silver Spring). Published online ahead of print July 20, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53:918–921. [DOI] [PubMed] [Google Scholar]
- 10. Riedl M, Vila G, Maier C, et al. Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. J Clin Endocrinol Metab. 2008;93:2307–2312. [DOI] [PubMed] [Google Scholar]
- 11. Giusti V, Gasteyger C, Suter M, Heraief E, Gaillard RC, Burckhardt P. Gastric banding induces negative bone remodelling in the absence of secondary hyperparathyroidism: potential role of serum C telopeptides for follow-up. Int J Obes Relat Metab Disord. 2005;29:1429–1435. [DOI] [PubMed] [Google Scholar]
- 12. Dixon JB, Strauss BJG, Laurie C, O'Brien PE. Changes in body composition with weight loss: obese subjects randomized to surgical and medical programs. Obesity (Silver Spring). 2007;15:1187–1198. [DOI] [PubMed] [Google Scholar]
- 13. Nadler EP, Reddy S, Isenalumhe A, et al. Laparoscopic adjustable gastric banding for morbidly obese adolescents affects android fat loss, resolution of comorbidities, and improved metabolic status. J Am Coll Surg. 2009;209:638–644. [DOI] [PubMed] [Google Scholar]
- 14. Yu EW, Putman MS, Derrico N, Abrishamanian-Garcia G, Finkelstein JS, Bouxsein ML. Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos Int. 2015;26:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. J Bone Miner Res. 2012;27:2231–2237. [DOI] [PubMed] [Google Scholar]
- 16. Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomised, controlled trial. Lancet Diabetes Endocrinol. 2015;3:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu EW. Bone metabolism after bariatric surgery. J Bone Miner Res. 2014;29:1507–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–407. [DOI] [PubMed] [Google Scholar]
- 19. Wong IPL, Driessler F, Khor EC, et al. Peptide YY regulates bone remodeling in mice: a link between gut and skeletal biology. PLoS One. 2012;7:e40038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Utz AL, Lawson EA, Misra M, et al. Peptide YY (PYY) levels and bone mineral density (BMD) in women with anorexia nervosa. Bone. 2008;43:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027–1033. [DOI] [PubMed] [Google Scholar]
- 22. Xie D, Zhong Q, Ding KH, et al. Glucose-dependent insulinotropic peptide-overexpressing transgenic mice have increased bone mass. Bone. 2007;40:1352–1360. [DOI] [PubMed] [Google Scholar]
- 23. Yamada C, Yamada Y, Tsukiyama K, et al. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008;149:574–579. [DOI] [PubMed] [Google Scholar]
- 24. Henriksen DB, Alexandersen P, Hartmann B, et al. Four-month treatment with GLP-2 significantly increases hip BMD: a randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD. Bone. 2009;45:833–842. [DOI] [PubMed] [Google Scholar]
- 25. Nissen A, Christensen M, Knop FK, Vilsbøll T, Holst JJ, Hartmann B. Glucose-dependent insulinotropic polypeptide inhibits bone resorption in humans. J Clin Endocrinol Metab. 2014;99:E2325–E2329. [DOI] [PubMed] [Google Scholar]
- 26. Bjarnason NH, Henriksen EE, Alexandersen P, Christgau S, Henriksen DB, Christiansen C. Mechanism of circadian variation in bone resorption. Bone. 2002;30:307–313. [DOI] [PubMed] [Google Scholar]
- 27. Qvist P, Christgau S, Pedersen BJ, Schlemmer A, Christiansen C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone. 2002;31:57–61. [DOI] [PubMed] [Google Scholar]
- 28. Valderas JP, Padilla O, Solari S, Escalona M, González G. Feeding and bone turnover in gastric bypass. J Clin Endocrinol Metab. 2014;99:491–497. [DOI] [PubMed] [Google Scholar]
- 29. Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haffner SM, Bauer RL. The association of obesity and glucose and insulin concentrations with bone density in premenopausal and postmenopausal women. Metabolism. 1993;42:735–738. [DOI] [PubMed] [Google Scholar]
- 31. Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149:716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ding SA, Simonson DC, Wewalka M, et al. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab. 2015;100:2546–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–539. [DOI] [PubMed] [Google Scholar]
- 34. Lindgren O, Carr RD, Deacon CF, et al. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J Clin Endocrinol Metab. 2011;96:2519–2524. [DOI] [PubMed] [Google Scholar]
- 35. Lalmohamed A, de Vries F, Bazelier MT, et al. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ. 2012;345:e5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporos Int. 2014;25:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Casagrande DS, Repetto G, Mottin CC, et al. Changes in bone mineral density in women following 1-year gastric bypass surgery. Obes Surg. 2012;22:1287–1292. [DOI] [PubMed] [Google Scholar]
- 38. Abegg K, Gehring N, Wagner CA, et al. Roux-en-Y gastric bypass surgery reduces bone mineral density and induces metabolic acidosis in rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R999–R1009. [DOI] [PubMed] [Google Scholar]
- 39. Stemmer K, Bielohuby M, Grayson BE, et al. Roux-en-Y gastric bypass surgery but not vertical sleeve gastrectomy decreases bone mass in male rats. Endocrinology. 2013;154:2015–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity. 2006;14:1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schafer AL, Weaver CM, Black DM, et al. Intestinal calcium absorption decreases dramatically after gastric bypass surgery despite optimization of vitamin D status. J Bone Miner Res. 2015;30:1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee NJ, Nguyen AD, Enriquez RF, et al. Osteoblast specific Y1 receptor deletion enhances bone mass. Bone. 2011;48:461–467. [DOI] [PubMed] [Google Scholar]
- 43. Wong IP, Driessler F, Khor EC, et al. Peptide YY regulates bone remodeling in mice: a link between gut and skeletal biology. PLoS One. 2012;7:e40038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Belavy DL, Baecker N, Armbrecht G, et al. Serum sclerostin and DKK1 in relation to exercise against bone loss in experimental bed rest. J Bone Miner Metab. Published online ahead of print June 9, 2015. [DOI] [PubMed] [Google Scholar]