Abstract
Active maternal smoking has adverse effects on neurobehavioral development of the offspring, with nicotine (Nic) providing much of the underlying causative mechanism. To determine whether the lower exposures caused by second-hand smoke are deleterious, we administered tobacco smoke extract (TSE) to pregnant rats starting preconception and continued through the second postnatal week, corresponding to all 3 trimesters of fetal brain development. Dosing was adjusted to produce maternal plasma Nic concentrations encountered with second-hand smoke, an order of magnitude below those seen in active smokers. We then compared TSE effects to those of an equivalent dose of Nic alone, and to a 10-fold higher Nic dose. Gestational exposure to TSE and Nic significantly disrupted cognitive and behavioral function in behavioral tests given during adolescence and adulthood (postnatal weeks 4–40), producing hyperactivity, working memory deficits, and impairments in emotional processing, even at the low exposure levels corresponding to second-hand smoke. Although TSE effects were highly correlated with those of Nic, the effects of TSE were much larger than could be attributed to just the Nic in the mixture. Indeed, TSE effects more closely resembled those of the 10-fold higher Nic levels, but still exceeded their magnitude. In combination with our earlier findings, this study thus completes the chain of causation to prove that second-hand smoke exposure causes neurodevelopmental deficits, originating in disruption of neurodifferentiation, leading to miswiring of neuronal circuits, and as shown here, culminating in behavioral dysfunction. As low level exposure to Nic alone produced neurobehavioral teratology, ‘harm reduction’ Nic products do not abolish the potential for neurodevelopmental damage.
Keywords: behavioral teratogenesis, nicotine, second-hand smoke, tobacco
Active maternal smoking during pregnancy is a major contributor to perinatal morbidity and mortality, as well as to persistent neurodevelopmental disorders (Gaysina et al., 2013; Pauly and Slotkin, 2008). Nicotine (Nic) contributes in large measure to the adverse behavioral effects, preempting normal developmental signals mediated by acetylcholine acting on nicotinic cholinergic receptors (Pauly and Slotkin, 2008; Slikker et al., 2005; Slotkin, 2008). Much less is known about the effects of second-hand tobacco smoke, in part because of the difficulties involved in documenting the degree and duration of exposure (U.S. Surgeon General, 2006). Prenatal exposure to second-hand smoke is associated with subsequent cognitive impairment (DiFranza et al., 2004; Herrmann et al., 2008; Yolton et al., 2005), with increased externalizing behavior (Liu et al., 2013), with emotional disorders (Bandiera et al., 2011), and with impaired neuromotor development (Evlampidou et al., 2015; Yeramaneni et al., 2015). Nevertheless, a decade ago, the U.S. Surgeon General’s report on second-hand smoke exposure indicated that, ‘The evidence is inadequate to infer the presence or absence of a causal relationship between exposure to secondhand smoke and behavioral problems among children’ (U.S. Surgeon General, 2006).
A major reason for the uncertainty is the dearth of animal studies that could provide mechanistic proof of cause and effect. Most reports have focused on Nic exposures commensurate with active maternal smoking, not the lower levels of second-hand smoke, and there are only a few studies using tobacco smoke as opposed to just Nic. Pre- or perinatal cigarette smoke exposure of rodents or primates in inhalation chambers produces neurodevelopmental defects resembling those of Nic (Amos-Kroohs et al., 2013; Fuller et al., 2012; Golub et al., 2007; Gospe et al., 2009; Slotkin et al., 2006a,b) but these have generally been conducted with simulation of active smoking levels. Additionally, the repetitive, involuntary confinement of animals in a smoke-filled chamber is likely to confound toxicant effects with unavoidable stress. To circumvent these problems, we recently devised a new paradigm for tobacco smoke exposure in developing rats, modeled after the most widely used technique for developmental studies of Nic (Slotkin et al., 2015). In this procedure, tobacco smoke extract (TSE) is prepared under standardized conditions and delivered by osmotic minipumps, implanted prior to mating. TSE is then given throughout gestation and into the first 2 postnatal weeks; because rats are altricial, the postnatal exposure period corresponds to stages of brain development in the late second to third trimester human fetus, so that exposure encompasses brain development corresponding to the entire human fetal period. Notably, TSE lacks some of the volatile components of tobacco smoke, such as carbon monoxide and hydrogen cyanide, and thus the model underestimates the potential for developmental neurotoxicity.
With this technique, we were able to show that TSE exposure mimicking Nic plasma levels commensurate with second-hand smoke exposure, disrupted development of cholinergic, and serotonergic pathways that are essential to cognitive and emotional behavioral function (Slotkin et al., 2015); many of these effects represented a direct impact on neural cell development, since we found similar results with an in vitro model of neurodifferentiation (Slotkin et al., 2014). Equally important, we compared the effects of TSE to equivalent or larger concentrations of Nic alone in these in vitro studies, and found that the adverse effects of TSE exceeded those of Nic, thus indicating additional effects from the thousands of compounds present in tobacco smoke. In this study, we adopted a similar strategy to characterize the neurobehavioral teratogenicity of low-level tobacco smoke exposure. Our findings provide some of the first evidence cementing a cause-and-effect relationship between second-hand smoke exposure and neurobehavioral disorders; further we found distinct roles for other smoke components over and above the effects of Nic.
MATERIALS AND METHODS
Tobacco Smoke Extract
TSE (Arista Laboratories, Richmond, Virginia) was prepared from Kentucky Reference cigarettes (KY3R4F) on a Rotary Smoke Machine under ISO (International Organization for Standardization) smoke conditions. The smoke condensate was collected on 92-mm filter pads, which were then extracted by shaking for 20 min with undiluted dimethyl sulfoxide (DMSO), to obtain a solution of approximately 20 mg of condensate per ml. Condensate aliquots were stored in amber vials at −80 °C until used. Two cigarettes were smoked to produce each ml of extract and the final product contained 0.8 mg/ml Nic (determined by the contract research organization that supplied the TSE, Arista Laboratories, Richmond, Virginia).
Animal Treatments
All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Duke University Animal Care and Use Committee and in accordance with all federal and state guidelines. Sprague-Dawley rats (Charles River Laboratories, Raleigh, North Carolina) were shipped by climate-controlled truck (transportation time <1 h) and were allowed to acclimate to the housing facility for 2 weeks prior to treatment. Animals were given free access to food and water and were kept on a reverse 12:12-h light/dark schedule. Type 2ML4 Alzet osmotic minipumps (Durect Corp., Cupertino, California) were implanted under anesthesia (60 mg/kg ketamine + 0.15–0.50 mg/kg dexmedetomidine given i.p; followed postimplant by 0.15 mg/kg atipemezole + 5 mg/kg ketoprofen given s.c. and topical bupivicaine) and the animals were allowed to recover for 3 days. Mating was then initiated by including a male rat in the cage for a period of 5 days. Although the pumps are marketed as a 4-week delivery device, it actually takes approximately 39 days for the reservoir to be exhausted completely (information supplied by the manufacturer) and thus the infusions terminated on postnatal day (PN) 12 ± 2 days (the insemination date varied among different mating pairs). In earlier work, we measured plasma Nic levels to confirm the termination of Nic absorption coinciding with the calculated values (Trauth et al., 2000).
There were 4 treatment groups, each comprising 12–14 dams: control (DMSO vehicle; Sigma Chemical Co., St. Louis, Missouri), TSE, and 2 different dose levels of Nic bitartrate (Sigma) dissolved in DMSO, with the Nic groups calibrated to deliver 0.2 or 2 mg/kg/d of Nic free base at the start of the infusion period. Because body weights increased with gestation, the dose rate fell by approximately one-third by the end of gestation and then rose back toward the original values with the postpartum weight loss. Thus, the Nic dose rate for the 2 mg/kg group remained well within the range that produces Nic plasma levels similar to those in moderate smokers, compared with the much lower exposures in the low dose group (Fewell et al., 2001; Trauth et al., 2000). The initial TSE dose rate (based on the Nic concentration in the TSE preparation) corresponded to 0.18 mg/kg/d of Nic, comparable to the levels in the group receiving low dose Nic. Parturition occurred during gestational day 22, which was also taken as PN0, and litters were culled to 8–10 pups to ensure standard nutrition. Because of limitations on the number of animals that could be implanted and mated within our facility, the treatments were delivered, and animals tested, in 7 distinct cohorts, with each treatment group represented within each cohort.
Behavioral Testing
At weaning (PN21), 1 male and 1 female were chosen from each litter in each exposure group to undergo behavioral tests. Males and females were housed in same-sex cages with 3–4 animals in each cage and had free access to food until testing began for the radial-arm maze and the operant visual signal detection task. The rats were also briefly food deprived for 24 h before novelty suppressed feeding tests. Behavioral testing began at PN28 just prior to puberty and continued on a week-to-week basis into full adulthood as follows:
Week 4 (PN28-34) elevated plus maze
Animals were tested on the elevated plus maze (Med Associates, St Albans, Vermont) to assess their anxiety versus risk-taking like behavior. The maze measured 142.2 × 104.1 × 76.2 cm and consisted of 2 arms with enclosed walls and 2 open arms with 2 cm railings. Each rat was assessed on the elevated plus maze for one 5-min session. The time the animal spent in the enclosed arms of the maze was recorded, as well as the number of crossings from the enclosed to open arms. Arm entries were defined as all 4 paws crossing the arm threshold of the maze.
Week 5 (PN35-41) figure-8 maze
Testing for locomotor activity was conducted in an enclosed maze apparatus in the shape of a Figure 8. The maze consisted of a continuous alley that measured 10 × 10 cm, contained within an apparatus measuring 70 × 42 cm. Animals were allowed to freely explore the entire apparatus. Locomotor activity was assessed by the crossing of 8 photobeams located at equal points in the maze alleys. Each locomotor test session lasted 1 h, and photobeam breaks were tallied in 5-min blocks across the entire session.
Week 6 (PN42-48) novelty suppressed feeding
To test for fear responses, animals were tested for suppression of feeding in a novel environment. The rats were food deprived for 24 h prior to novelty suppressed feeding tests. A novel environment was created by placing a plastic rectangular cage (different from the home cage) in the middle of the testing room without a cage top or any bedding in a brightly lit room. Twelve standard rat-chow pellets, each weighed prior to testing, were spread across the floor of the cage in 4 rows of 3 pellets. Each rat was tested for 10 min and the latency to begin eating was recorded. Eating was defined as the act of chewing the food and not merely sniffing, holding, or carrying the food around in the mouth. The food pellets remaining after 10 min were weighed to determine the amount of food eaten during the testing session.
Week 7 (PN49-55) novel object recognition
To test for attention and memory in a low-motivational state recognition of a novel object was assessed. Tests were conducted in opaque plastic enclosures measuring 70 × 41 × 33 cm. Objects consisted of plastic, glass, or ceramic material and were randomized for each animal. Animals were first habituated to the apparatus in 2 consecutive 10 min sessions over the course of 2 days. Testing began on day 3 with a 10 min ‘information’ session in which 2 identical objects (A/A) were placed in the cage for the animal to explore. The A/A session was then followed by a 15 min, 1 h, or 6-h delay period spent in the animal’s home cage. The animal was then placed back in the enclosure with one object from the A/A session and with another, dissimilar, ‘novel’ object (A/B session). The time spent actively exploring each object was recorded. Between sessions, the objects were wiped clean with a solution of 10% acetic acid to avoid odor recognition cues.
Weeks 8–11 (PN56-83) radial-arm maze
To assess spatial learning as well as working and reference spatial memory the rats were tested in the 16-arm radial maze. Tests were conducted on a black, wooden maze consisting of a central platform with 16 arms extending radially from the center. The central platform measured 50 cm in diameter, and each of the 16 arms measured 10 × 60 cm. A food cup was located 2 cm from the distal end of each arm. Visual cues (cardboard shapes) were placed on the walls of the testing room to permit spatial orientation. Each rat was habituated to the maze in two 10-min sessions in which the they were placed on the central platform inside a large, round, opaque cylinder with halves of sugar-coated cereal (Froot Loops; Kellogg’s, Battle Creek, Michigan) available to consume. The food cups of 12 arms of the maze were baited to test working memory performance and the remaining 4 arms were always left unbaited to test reference memory. The baited arms of the maze for each rat remained constant throughout behavioral testing but the choice of arms baited was randomized among all rats. Each trial began by placing the rat on the central platform inside the opaque cylinder for 10 s, after which the cylinder was removed and the rat was allowed to enter any arm. Each testing session lasted 10 min or until the rat had entered all 12-baited arms, whichever came first. Each rat was trained for 18 sessions on the maze and memory errors were assessed. Working memory errors were defined as repeated entries into a baited arm, while reference memory errors were defined as entry into one of the arms that was always unbaited. Latency was calculated as the total session time in seconds divided by the total number of arms entered by the animal. After every session, the maze apparatus was wiped clean with a damp cloth.
Weeks 12–40 (PN84-286) operant visual signal detection task
To assess attentional function the operant visual detection task was used. The task was conducted as described previously in Levin et al. (2014). Briefly, each rat was trained to press 1 of 2 retractable levers inside an operant chamber in response to a visual stimulus in the form of a cue-light that illuminated for a duration of 500 ms. If the cue-light became illuminated (‘signal’ trial), the animal was directed to press 1 of the 2 levers to receive a 20 mg food pellet reward. If the cue-light did not illuminate (‘blank’ trial) the animal was directed to press the opposite lever in the chamber to receive the reward. The choice of ‘signal’ and ‘blank’ levers was randomized among the rats. If the rat made no response within 5 s of insertion of the response levers into the chamber, both levers retracted and a response ‘failure’ was recorded. Each ‘signal’ and ‘blank’ pair was considered 1 test trial, and each test session consisted of 240 trials.
Data Analysis
Data were compiled as means and standard errors (SEs). For each behavioral test, the data were first assessed by global analysis of variance (ANOVA), encompassing all factors and confounds in a single test: the 4 treatment groups, sex, cohort, and depending on the test, time, or test block; the latter factor was considered a repeated measure. Post hoc tests for differences between treated groups and controls, or among the different treatments, were conducted with Fisher’s Least Significant Difference Test. The threshold for assigning significance was P < .05, 2-tailed.
RESULTS
Maternal, Litter and Growth Effects
Animals tested in these studies were littermates of those used in our previous report of TSE and Nic effects on synaptic function (Slotkin et al., 2015). Accordingly, maternal, litter, and growth effects were similar to those published previously and will not be repeated here. Briefly, we did not find any effect on maternal weight gain, litter size, or sex ratio. Earlier, we found a small (4%) but statistically significant reduction in offspring body weights up to PN150, but with inclusion of the later ages in this study, this effect became nonsignificant because of the eventual plateauing of weight (data not shown). Our earlier study also established the bioequivalence of Nic delivered in TSE as compared with Nic alone (Slotkin et al., 2015).
Elevated Plus Maze
Across all groups, ANOVA indicated a main effect of treatment (P < .05), on the percentage of time spent in the open arms (Figure 1A), without any differential effect on males versus females (no treatment × sex interaction). Pairwise comparisons showed that the differences resided solely in the group receiving the lower dose of nicotine, which was statistically distinguishable from the control group and from the group receiving TSE. Likewise, there was a significant main effect of treatment on the number of center crossings (P < .05), and again, these reflected effects of the low dose of Nic, which was distinguishable both from the controls and the TSE group (Figure 1B).
FIG. 1.
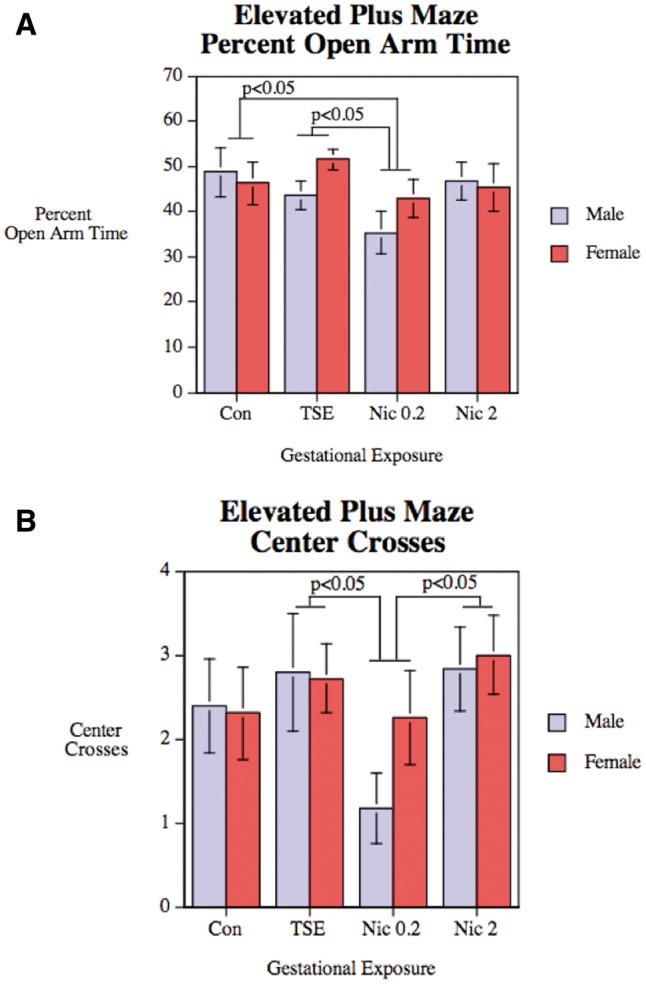
Elevated plus maze performance in control animals (Con), animals exposed to TSE, or animals exposed to Nic at 0.2 or 2 mg/kg/d. Data represent means and SEs from 12 to 14 animals for each sex and treatment. A, Percentage of time spent in the open arms. B, Number of center crossings. ANOVA indicates significant main effects of treatment (P < .05) for both parameters.
Figure-8 Maze
When the animals were tested for locomotor activity during adolescence there was a significant main effect of treatment (P < .03), with TSE causing hyperactivity relative to controls or to animals receiving the lower dose of nicotine (Figure 2A). Animals in the high-dose Nic group showed values that were intermediate between control and TSE, that is, in the same direction (hyperactivity) as TSE, but with a smaller magnitude of effect, and therefore not significant from either the controls or the TSE group. There was also a significant main effect of sex (P < .03), reflecting greater overall activity in females compared with males, but the sex effect was not interactive with treatment. Activity within each of the 5 min blocks is presented in Figure 2B. Although there was a main effect of block (decreasing activity over time, connoting habituation to the apparatus, P < .0005), we did not see a significant treatment × block interaction that would indicate treatment-induced changes in habituation.
FIG. 2.
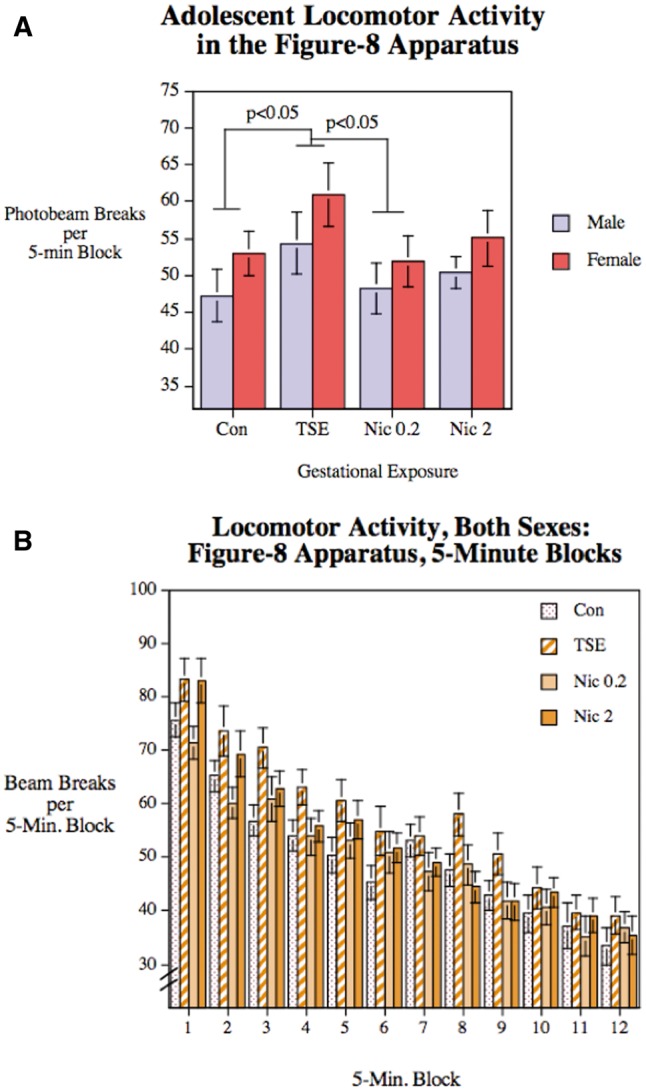
Figure-8 maze performance in Con, animals exposed to TSE, or animals exposed to Nic at 0.2 or 2 mg/kg/d. Data represent means and SEs obtained from 12 to 14 animals for each sex and treatment. A, Average performance over the 60-min test period. B, Performance in each 5-min block. ANOVA indicates significant main effects of treatment (P < .05), sex (P < .05), and block (P < .0005), but no interactions of treatment with the other 2 variables.
Novelty Suppressed Feeding
ANOVA indicated a significant treatment × sex interaction (P < .03) for grams of food eaten. In males, TSE produced a substantial increase in the amount of food consumed during the test, significantly greater than for controls or either of the Nic groups (Figure 3). Again, though, the high dose nicotine group was affected in the same direction as TSE, only to a smaller extent, and therefore was intermediate between control and TSE values. Effects in females were likewise in the same direction (increase for TSE) but of smaller magnitude, and therefore did not achieve statistical significance. There were no significant treatment effects on the latency to begin consuming food (data not shown).
FIG. 3.
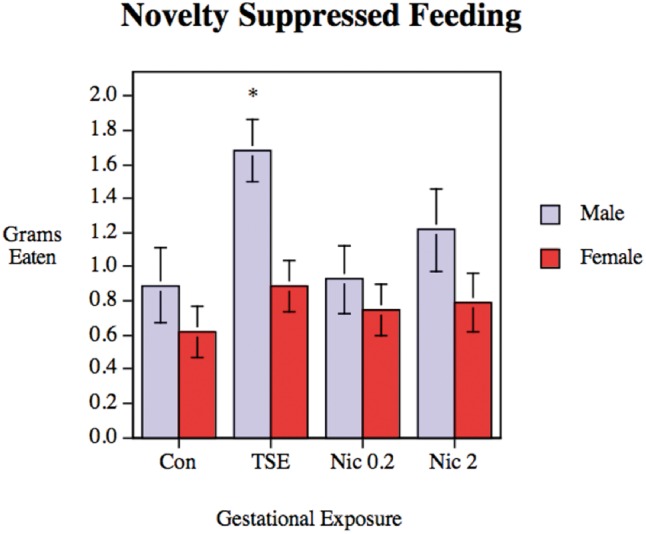
Novelty-suppressed feeding in control animals (Con), animals exposed to TSE, or animals exposed to Nic at 0.2 or 2 mg/kg/d. Data represent means and SEs obtained from 11 to 14 animals for each sex and treatment. ANOVA indicates a significant treatment × sex interaction (P < .05) and the asterisk denotes that, in males, the TSE group is significantly different from all other groups (P < .005 vs. control; P < .005 vs. Nic 0.2; P < .05 vs. Nic 2).
Novel Object Recognition
Preference for the novel object was evaluated as the difference between time spent with the novel versus the familiar object (Figure 4). The treatments reduced the preference for the novel object (main treatment effect, P < .01), with significant effects for the TSE and low-dose Nic groups vs. control. The high-dose nicotine group was just at the margin of significance for the difference from control, and was not distinguishable from the reductions caused by TSE.
FIG. 4.
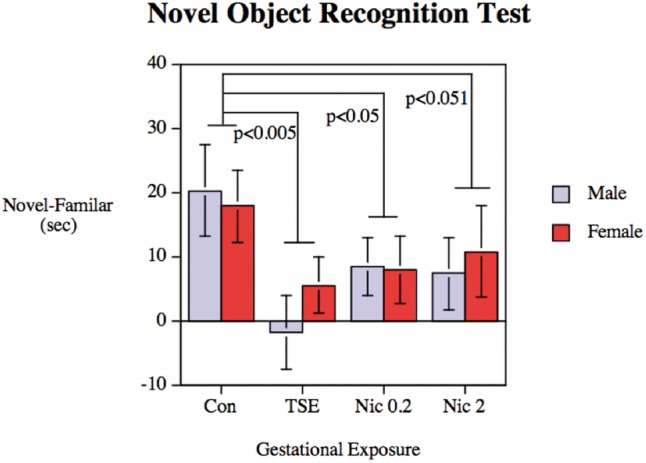
Preference for the novel object in the Novel Object Recognition Test, assessed in Con, animals exposed to TSE, or animals exposed to Nic at 0.2 or 2 mg/kg/d. Data represent means and SEs obtained from 11 to 14 animals for each sex and treatment. ANOVA indicates a main treatment effect (P < .01), with the TSE and Nic 0.2 groups differing significantly from control, and the Nic 2 group at the margin of significance; the reductions caused by TSE, Nic 0.2, and Nic 2 were not distinguishable from each other.
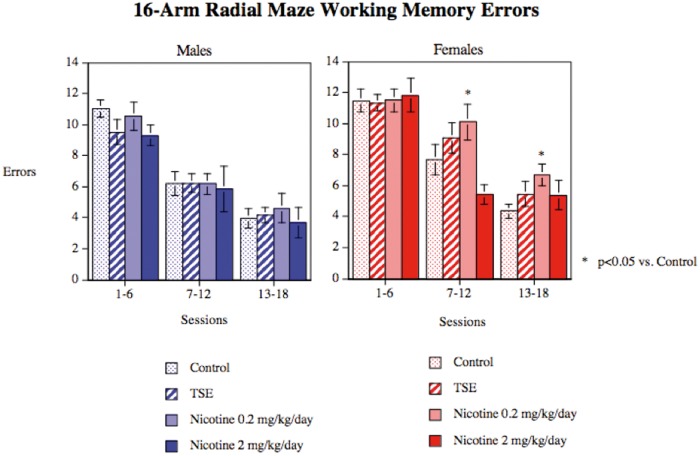
Radial-Arm Maze
During the 18 sessions of acquisition training in the 16-arm radial maze there was a significant main effect of treatment on working memory errors (P < .03) but the treatment effects interacted with both sex and test session (treatment × sex × session, P < .05). For males, we did not find any statistically significant treatment effects on performance, whereas females did show significant changes that emerged over the course of training (Figure 5). In females, all groups performed equivalently in the initial testing blocks. As training proceeded and error rates declined, the low dose Nic group showed slower learning, with significantly increased working memory errors. By the end of testing, the TSE and high-dose nicotine groups showed an intermediate increase in errors, so that the values were significant neither from controls nor from the low dose Nic group. We did not find significant treatment differences in reference memory performance or in response latency (data not shown).
FIG. 5.
Working memory errors in the radial arm maze, assessed in Con, animals exposed to TSE, or animals exposed to Nic at 0.2 or 2 mg/kg/d. Data represent means and SEs obtained from 12 to 14 animals for each sex and treatment. ANOVA indicates a main treatment effect (P < .03) and an interaction of treatment × sex × session (P < .05). Males did not show significant treatment effects, whereas females did; asterisks denote groups that differ from the corresponding control.
Visual Signal Detection Task
There was no significant effect of any of the treatments on choice accuracy in the visual signal detect task (Figure 6); males performed better than females overall (main effect of sex, P < .05). We also did not find any interaction of treatment with session block in this task (data not shown).
FIG. 6.
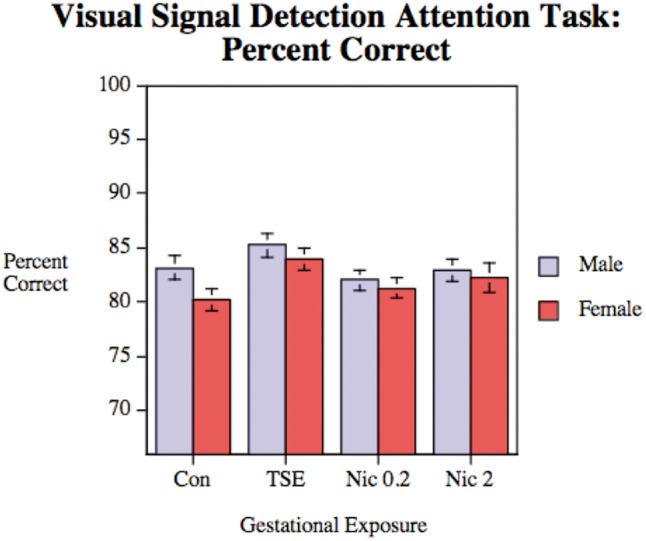
Visual attention performance, assessed in Con, animals exposed to TSE, or animals exposed to Nic at 0.2 or 2 mg/kg/d. Data represent means and SEs obtained from 12 to 14 animals for each sex and treatment. There were no significant treatment effects but there was a main effect of sex (P < .05), reflecting better overall performance in males.
DISCUSSION
There are 2 main findings in this study. First, TSE exposure comparable to second-hand smoke produces neurobehavioral impairments. Second, the effects are consonant with those elicited by nicotine alone, but the magnitude exceeds that of Nic, thus showing that the other components of tobacco smoke contribute to the adverse outcomes. We reached the same 2 basic conclusions in our earlier study delineating the impact of TSE on neurochemical parameters and synaptic function (Slotkin et al., 2015). This study thus completes the chain of causation to prove that second-hand smoke exposure causes neurodevelopmental deficits, originating in disruption of neurodifferentiation (Slotkin et al., 2014), leading to miswiring of neuronal circuits (Slotkin et al., 2015), and as shown here, culminating in behavioral dysfunction.
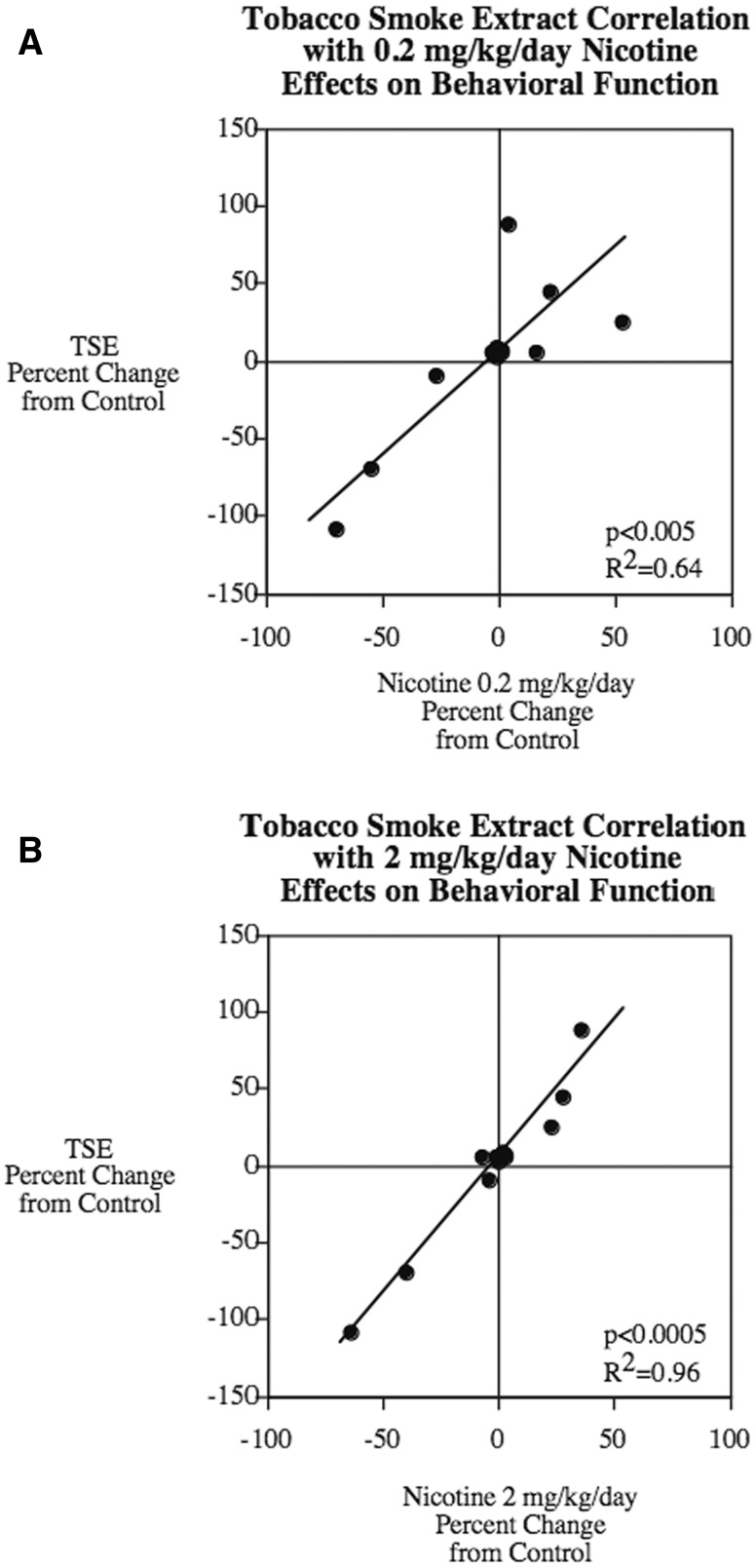
In general, the impact of TSE on behavioral performance mimicked that of nicotine. However, if Nic alone accounts for the effect of TSE, then it would be expected that the TSE group would match the group receiving the comparable nicotine dose, 0.2 mg/kg/d. Instead, though, the effects were more consonant with those of a 10-fold higher dose of Nic, and actually exceeded those effects. To quantitate these relationships, we performed regression analysis across all the behavioral tests, evaluating the degree to which Nic can account for the overall effects of TSE, and found significant correlation of TSE with either dose of nicotine (Figure 7). Whereas the lower dose of Nic accounted for the majority of the TSE effect (R2 = 0.64), the higher dose actually encompassed virtually all of the effect (R2 = 0.96). Nevertheless, it would be incorrect to conclude that TSE simply behaves like a higher dose of Nic, since if that were the case, the slope of the regression line would equal 1. In fact, the slope significantly exceeded that value (1.6 ± 0.1, P < .001). This indicates that the thousands of compounds in tobacco smoke contribute to the adverse neurodevelopmental effects both by augmenting the effect of Nic, and by converging on the same endpoints as nicotine, so as to produce a similar pattern with a much larger magnitude of effect. Again, these conclusions recapitulate those from our earlier neurochemical studies of TSE both in vivo (Slotkin et al., 2015) and in vitro (Slotkin et al., 2014). Indeed, we have already shown that at least one component of tobacco smoke, benzo[a]pyrene, synergizes the effect of Nic on neurodifferentiation (Slotkin et al., 2013).
FIG. 7.
Concordance plots demonstrating the dependence of TSE behavioral effects on Nic. A, 0.2 mg/kg/d. B, 2 mg/kg/d. Data were taken from Figures 1–6 and converted to percent change from control to enable all tests to contribute equally. For the elevated plus maze, we used only the data in Figure 1A so as not to inflate the contribution of this test. Likewise, for working memory, we used the data from only one set of blocks (the last set, reflecting persistent effects on working memory). Each test contributed 2 points to the regression plot, one for males and one for females.
Although the overall conclusions of the present behavioral study and the earlier neurochemical study (Slotkin et al., 2015) are consonant, there are 2 notable differences in the dependence of TSE effects on nicotine. First, the behavioral correlations are better for the behavioral tasks than they were for the synaptic biomarkers. Second, the neurochemistry showed much greater dependence of TSE effects on Nic for males than for females, whereas here, we were unable to show a difference in regression relationships between the 2 sexes; ie, although there were sex differences in individual behavioral tests, the differences for TSE were paralleled by differences for nicotine, so that the correlation between the 2 treatments remained the same for both sexes. Indeed, both males (P < .0005, R2 = 0.98 and females (P < .0005, R2 = 0.97) showed very tight correlations between gestational TSE and Nic (2 mg/kg/d) effects on behavioral function in the offspring. However, it would be surprising if there were complete quantitative agreement for sex differences between the neurochemical and behavioral studies.
For the neurochemistry, we evaluated only 2 neurotransmitter systems, acetylcholine and serotonin, which, although undoubtedly contributing to the behavioral performance measures, are not the sole determinants of those behaviors. Although developmental TSE and Nic exposures may cause equal damage to these circuits in males and females, the 2 sexes differ substantially in their subsequent adaptation to the adverse effects, with females generally restoring function better than males (Jacobsen et al., 2007; Slotkin et al., 2007a,b). Future work should address the mechanisms by which males and females differ in their adaptive responses to TSE-induced injury.
Superimposed on these overall conclusions, there were effects on several of the behavioral tests that provide important cues as to specific neurobehavioral targets of TSE and nicotine. Exposure to TSE during development produced hyperactivity in the Figure-8 maze during adolescence when compared with exposure to vehicle or Nic alone. No significant difference in locomotor activity was observed as a result of exposure to either the low or high dose of Nic alone compared with vehicle exposure. There are numerous previous studies examining the effect of prenatal Nic on locomotor activity in rodent models, often with variable and conflicting results (Huang et al., 2007; LeSage et al., 2006; Levin et al., 1993, 1996; Romero and Chen, 2004; Schneider et al., 2011; Vaglenova et al., 2004). The findings of this study suggest that other components contained in the TSE are acting in concert with Nic to produce a hyperactive behavioral phenotype in adolescence.
Animals exposed to TSE exhibited significant impairments in working memory in the novel object recognition task. Nic alone also caused a significant reduction in novel object recognition. In contrast to the substantial impairment caused by TSE in this task, no TSE-induced impairments were seen in the radial-arm maze and visual signal detection tasks. One critical difference between these tasks is that of appetitive motivation. The novel object recognition test is a simple test of novelty exploration with no explicit reward or punishment. In contrast, the radial-arm maze and the visual signal detection task both used appetitive reinforcement while the rats were on a restricted feeding schedule. There are other differences between these tasks, but it is interesting that a low-motivation task like novel object recognition, which includes aspects of both attention and memory, was sensitive to the persisting TSE impairment, while the higher motivation memory and attention tasks did not show a TSE-induced impairment. Nonetheless, it does appear from our study and others that developmental second-hand exposure appears to affect specific domains of cognitive function, rather than a more generalized impairment.
With some behavioral tests significant effects were seen with the lower 0.2 mg/kg/d dose but not for the higher 2 mg/kg/d nicotine dose. In some cases, this was merely an incidence of the higher dose just missing statistical significance such as with the novel object recognition test. In other tests the enhanced effect of the lower Nic dose does seem to reflect real differences in biological effect. This may be due to the multiple effects of nicotine stimulating nicotinic receptors at lower doses and having more long-lasting desensitizing effects at higher doses, possibly attenuating its impact.
In conclusion, this findings show that TSE is a neurobehavioral teratogen even at exposures extending down to levels commensurate with second-hand smoke, and further, that the effects exceed those that can be attributed to just the Nic present in the mixture. Indeed, TSE effects resembled more closely those of a 10-fold higher nicotine levels, and in fact, exceeded their magnitude. Thus, the effects of TSE appear to be a combination of an intensified response to Nic, as well as contributions from the thousands of other chemicals present in tobacco smoke. In combination with our earlier work demonstrating a direct impact of TSE on neurodifferentiation (Slotkin et al., 2014), leading to miswiring of brain circuitry (Slotkin et al., 2015), our findings provide a complete chain of causation from initial exposure to adverse functional outcomes. The fact that TSE has much greater effects than comparable exposure to nicotine indicate that substitution of Nic for tobacco is likely to provide harm reduction in terms of neurodevelopmental outcomes. Nevertheless, we did find significant adverse effects of low doses of Nic alone, indicating that ‘harm reduction’ is not the same thing as ‘harm elimination’. Thus, our results reinforce the need to avoid second-hand smoke exposure in pregnancy, while at the same time they also highlight the potential adverse effects of electronic Nic delivery devices and shisha, which likewise expose users and bystanders to significant amounts of Nic (Ballbe et al., 2014).
ACKNOWLEDGMENTS
T.A.S. has received consultant income in the past 3 years from the following firms: Acorda Therapeutics (Ardsley New York), Carter Law (Peoria Illinois), Pardieck Law (Seymour, Indiana), Tummel & Casso (Edinburg, Texas), Taylor, Reams, Tilson & Harrison (Morristown, New Jersey) and Chaperone Therapeutics (Research Triangle Park, North Carolina).
FUNDING
This work was supported by the National Institutes of Health (ES022831) and by the U.S. Environmental Protection Agency (83543701). EPA support does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. National Institute of Environmental Health Sciences (‘ES022831’) Environmental Protection Agency, (‘83543701’).
REFERENCES
- Amos-Kroohs R. M., Williams M. T., Braun A. A., Graham D. L., Webb C. L., Birtles T. S., Greene R. M., Vorhees C. V., Pisano M. M. (2013). Neurobehavioral phenotype of C57BL/6J mice prenatally and neonatally exposed to cigarette smoke. Neurotoxicol. Teratol. 35, 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballbe M., Martinez-Sanchez J. M., Sureda X., Fu M., Perez-Ortuno R., Pascual J. A., Salto E., Fernandez E. (2014). Cigarettes vs. e-cigarettes: passive exposure at home measured by means of airborne marker and biomarkers. Environ. Res. 135, 76–80. [DOI] [PubMed] [Google Scholar]
- Bandiera F. C., Richardson A. K., Lee D. J., He J. P., Merikangas K. R. (2011). Secondhand smoke exposure and mental health among children and adolescents. Arch. Pediatr. Adolesc. Med. 165, 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza J. R., Aligne C. A., Weitzman M. (2004). Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics 113, 1007–1015. [PubMed] [Google Scholar]
- Evlampidou I., Bagkeris M., Vardavas C., Koutra K., Patelarou E., Koutis A., Chatzi L., Kogevinas M. (2015). Prenatal second-hand smoke exposure measured with urine cotinine may reduce gross motor development at 18 months of age. J. Pediatr. 167, 246–252. [DOI] [PubMed] [Google Scholar]
- Fewell J. E., Smith F. G., Ng V. K. Y. (2001). Threshold levels of maternal nicotine impairing protective responses of newborn rats to intermittent hypoxia. J. Appl. Physiol. 90, 1968–1976. [DOI] [PubMed] [Google Scholar]
- Fuller B. F., Cortes D. F., Landis M. K., Yohannes H., Griffin H. E., Stafflinger J. E., Bowers M. S., Lewis M. H., Fox M. A., Ottens A. K. (2012). Exposure of rats to environmental tobacco smoke during cerebellar development alters behavior and perturbs mitochondrial energetics. Environ. Health Perspect. 120, 1684–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaysina D., Fergusson D. M., Leve L. D., Horwood J., Reiss D., Shaw D. S., Elam K. K., Natsuaki M. N., Neiderhiser J. M., Harold G. T. (2013). Maternal smoking during pregnancy and offspring conduct problems: Evidence from three independent genetically-sensitive research designs. JAMA Psychiat. 70, 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub M. S., Slotkin T. A., Pinkerton K. E. (2007). Visual recognition memory and auditory brainstem response in infant rhesus monkeys exposed perinatally to environmental tobacco smoke. Brain Res. 1151, 102–106. [DOI] [PubMed] [Google Scholar]
- Gospe S. M., Jr, Joyce J. A., Siebert J. R., Jack R. M., Pinkerton K. E. (2009). Exposure to environmental tobacco smoke during pregnancy in rats yields less effect on indices of brain cell number and size than does postnatal exposure. Reprod. Toxicol. 27, 22–27. [DOI] [PubMed] [Google Scholar]
- Herrmann M., King K., Weitzman M. (2008). Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr. Opin. Pediatr. 20, 184–190. [DOI] [PubMed] [Google Scholar]
- Huang L. P. Z., Liu X. H., Griffith W. H., Winzer-Serhan U. H. (2007). Chronic neonatal nicotine increases anxiety but does not impair cognition in adult rats. Behav. Neurosci. 121, 1342–1352. [DOI] [PubMed] [Google Scholar]
- Jacobsen L. K., Slotkin T. A., Mencl W. E., Frost S. J., Pugh K. R. (2007). Gender specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology 32, 2453–2464. [DOI] [PubMed] [Google Scholar]
- LeSage M. G., Gustaf E., Dufek M. B., Pentel P. R. (2006). Effects of maternal intravenous nicotine administration on locomotor behavior in pre-weanling rats. Pharmacol. Biochem. Behav. 85, 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. D., Briggs S. J., Christopher N. C., Rose J. E. (1993). Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol. Teratol. 15, 251–260. [DOI] [PubMed] [Google Scholar]
- Levin E. D., Hao I., Burke D. A., Cauley M, Hall B. J., Rezvani A. H. (2014). Effects of tobacco smoke constituents, anabasine and anatabine, on memory and attention in female rats. J. Psychopharmacol. 28, 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. D., Wilkerson A., Jones J. P., Christopher N. C., Briggs S. J. (1996). Prenatal nicotine effects on memory in rats: Pharmacological and behavioral challenges. Dev. Brain Res. 97, 207–215. [DOI] [PubMed] [Google Scholar]
- Liu J., Leung P. W. L., McCauley L., Ai Y., Pinto-Martin J. (2013). Mother’s environmental tobacco smoke exposure during pregnancy and externalizing behavior problems in children. Neurotoxicology 34, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly J. R., Slotkin T. A. (2008). Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Pædiatr. 97, 1331–1337. [DOI] [PubMed] [Google Scholar]
- Romero R. D., Chen W. J. A. (2004). Gender-related response in open-field activity following developmental nicotine exposure in rats. Pharmacol. Biochem. Behav. 78, 675–681. [DOI] [PubMed] [Google Scholar]
- Schneider T., Ilott N., Brolese G., Bizarro L., Asherson P. J., Stolerman I. P. (2011). Prenatal exposure to nicotine impairs performance of the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology 36, 1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W., Xu Z. A., Levin E. D., Slotkin T. A. (2005). Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction—developmental neurotoxicity of nicotine. Crit. Rev. Toxicol. 35, 703–711. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A. (2008). If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol. Teratol. 30, 1–19. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Card J., Seidler F. J. (2013). Adverse benzo[a]pyrene effects on neurodifferentiation are altered by other neurotoxicant coexposures: Interactions with dexamethasone, chlorpyrifos, or nicotine in PC12 cells. Environ. Health Perspect. 121, 825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Card J., Stadler A., Levin E. D., Seidler F. J. (2014). Effects of tobacco smoke on PC12 cell neurodifferentiation are distinct from those of nicotine or benzo[a]pyrene. Neurotoxicol. Teratol. 43, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., MacKillop E. A., Rudder C. L., Ryde I. T., Tate C. A., Seidler F. J. (2007a). Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at six months of age. Neuropsychopharmacology 32, 1082–1097. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Pinkerton K. E., Seidler F. J. (2006a). Perinatal environmental tobacco smoke exposure in Rhesus monkeys: critical periods and regional selectivity for effects on brain cell development and lipid peroxidation. Environ. Health Perspect. 114, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Pinkerton K. E., Tate C. A., Seidler F. J. (2006b). Alterations of serotonin synaptic proteins in brain regions of neonatal Rhesus monkeys exposed to perinatal environmental tobacco smoke. Brain Res. 1111, 30–35. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Ryde I. T., Seidler F. J. (2007b). Separate or sequential exposure to nicotine prenatally and in adulthood: persistent effects on acetylcholine systems in rat brain regions. Brain Res. Bull. 74, 91–103. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Skavicus S., Card J., Stadler A., Levin E. D., Seidler F. J. (2015). Developmental neurotoxicity of tobacco smoke directed toward cholinergic and serotonergic systems: more than just nicotine. Toxicol. Sci. 147, 178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth J. A., Seidler F. J., Slotkin T. A. (2000). An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 867, 29–39. [DOI] [PubMed] [Google Scholar]
- U.S. Surgeon General. (2006). The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. U.S. Department of Health and Human Services, Rockville. [Google Scholar]
- Vaglenova J., Birru S., Pandiella N. M., Breese C. R. (2004). An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav. Brain Res. 150, 159–170. [DOI] [PubMed] [Google Scholar]
- Yeramaneni S., Dietrich K. N., Yolton K., Parsons P., Aldous K. M., Haynes E. N. (2015). Secondhand tobacco smoke exposure and neuromotor function in rural children. J. Pediatr. 167, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton K., Dietrich K., Auinger P., Lanphear B. P., Hornung R. (2005). Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ. Health Perspect. 113, 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]