Abstract
Inflammation is a common feature of Parkinson Disease and other neurodegenerative disorders. Hypochlorous acid (HOCl) is a reactive oxygen species formed by neutrophils and other myeloperoxidase-containing cells during inflammation. HOCl chlorinates the amine and catechol moieties of dopamine to produce chlorinated derivatives collectively termed chlorodopamine. Here, we report that chlorodopamine is toxic to dopaminergic neurons both in vivo and in vitro. Intrastriatal administration of 90 nmol chlorodopamine to mice resulted in loss of dopaminergic neurons from the substantia nigra and decreased ambulation-results that were comparable to those produced by the same dose of the parkinsonian poison, 1-methyl-4-phenylpyridinium (MPP+). Chlorodopamine was also more toxic to differentiated SH SY5Y cells than HOCl. The basis of this selective toxicity is likely mediated by chlorodopamine uptake through the dopamine transporter, as expression of this transporter in COS-7 cells conferred sensitivity to chlorodopamine toxicity. Pharmacological blockade of the dopamine transporter also mitigated the deleterious effects of chlorodopamine in vivo. The cellular actions of chlorodopamine included inactivation of the α-ketoglutarate dehydrogenase complex, as well as inhibition of mitochondrial respiration. The latter effect is consistent with inhibition of cytochrome c oxidase. Illumination at 670 nm, which stimulates cytochrome c oxidase, reversed the effects of chlorodopamine. The observed changes in mitochondrial biochemistry were also accompanied by the swelling of these organelles. Overall, our findings suggest that chlorination of dopamine by HOCl generates toxins that selectively kill dopaminergic neurons in the substantia nigra in a manner comparable to MPP+.
Keywords: dopamine, brain, hypochlorous acid, neuroinflammation, Parkinson Disease
Most cases of Parkinson Disease (PD) arise idiopathically. Seminal studies by Langston et al. (1983) introduced the notion of xenobiotics as possible causes for the noninheritable forms of PD. Following their discovery of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, 1-methyl-4-phenylpyridinium (MPP+), 6-hydroxydopamine, rotenone, and paraquat were also identified as putative parkinsonian poisons (Greenamyre et al., 2010). Humans, in general, are not exposed to these compounds (Cannon and Greenamyre, 2011). Nonetheless other parkinsonian toxins may exist. One intriguing possibility is the conversion of neurotransmitters into selective neural toxins during cerebral inflammation (Bisaglia et al., 2014; Pham and Waite, 2014). We have been particularly interested in conversion of dopamine into poisonous species by reaction with hypochlorous acid (HOCl) (Jeitner et al., 2015). This powerful oxidant results from the oxidation of chloride ions by myeloperoxidase subsequent to the reduction of hydrogen peroxide to water by this enzyme (Winterbourn and Kettle, 2013). HOCl reacts with tyrosyl residues to generate a stable biomarker of its production: 3-chlorotyrosine (Hazen et al., 1997). Our interest in the reactions of HOCl was spurred by the discoveries of myeloperoxidase and 3-chlorotyrosine in the brains of patients with PD or Alzheimer Disease, and animals that serve as models for these diseases (Choi et al., 2005; Green et al., 2004; Maki et al., 2009).
Myeloperoxidase is primarily found in the microglia of diseased brains (Choi et al., 2005; Green et al., 2004; Maki et al., 2009). The highest density of these cells is in the substantia nigra (Kim et al., 2000), the region of brain most affected by PD. Dopaminergic neurons in this nucleus also produce appreciable amounts of hydrogen peroxide (Spanos et al., 2013). These observations suggest that the inflamed substantia nigra is a likely site of significant HOCl production. HOCl is also generated by direct oxidation of chloride ions by hydroxyl radicals (Saran et al., 1999). These radicals are generated by Fenton chemistry, which requires hydrogen peroxide and a source of reduced transition metals. The relevant metals include ferrous and cuprous ions which are abundant in the diseased tissues of PD patients (Akatsu et al., 2012). Chloride ions are present in the brain at 10−2 to 10−1 M, concentrations that favor the oxidation of these ions to HOCl by hydroxyl radicals. Thus, at least 2 pathways exist for the production of HOCl in the tissues affected by PD.
HOCl and other hypohalous acids are generated by the innate immune system as part of its armamentarium against invading pathogens (Winterbourn and Kettle, 2013). Damage to the surrounding uninfected cells is prevented, in part, by a variety of scavengers. The most effective scavengers are those bearing sulfur and typically have second-order rate constants for the reactions with hypohalous acids in the range of 107 to 108 M−1 s−1 (Storkey et al., 2014). Glutathione and thiocyanate are the major scavengers of hypohalous acids outside the brain (Ashby et al., 2004; Ismael et al., 2015; Skaff et al., 2009). These sulfur compounds, however, are unlikely to effectively scavenge HOCl in the brain. The half-life of glutathione varies from 60 to 80 h in the mammalian brain (Chang et al., 1997) as opposed to 1.5 h in liver (Kramer et al., 1985). This implies that any irreversible losses of glutathione in the brain will take days to replenish. Such losses are likely as HOCl reacts with glutathione to produce glutathione sulfonamide, which represents a permanent loss of the parent compound (Harwood et al., 2008). Thiocyanate is present in the brain but at low amounts that are rapidly cleared (Pappius, 1969). Whiteman et al. (2005a) proposed that hydrogen sulfide might act as a scavenger of HOCl in the brain. The amounts of this scavenger in the brain, however, are diminished in PD brains (Bae et al., 2013). These observations suggest that the brain has poor defenses against HOCl. The generation of HOCl therefore represents an important etiological factor in PD.
Dopamine and tyrosine are structurally similar. Therefore, we hypothesized that dopamine—like tyrosine—would undergo ring chlorination by HOCl. This hypothesis was confirmed and dopamine was shown to undergo both ring and N-amine chlorination (Figure 1C: compounds 4, 6, and 7) (Jeitner et al., 2015). The second-order rate constant for the reaction of HOCl and dopamine is 2.5 × 104 M−1 s−1 (Jeitner et al., 2015) and thus within the range of constants derived for chlorination of amines (Hawkins et al., 2003). Some of these chloramines have been identified in vivo (Hawkins, Pattison and Davies, 2003), which implies that the reaction of HOCl and dopamine is likely to occur in vivo. These discoveries suggest the possibility that HOCl reacts with dopamine in vivo to produce chlorinated forms of dopamine, which we collectively term chlorodopamine. The notion that oxidized forms of dopamine participate in PD has been postulated before, but the notion did not include the chlorinated variants of dopamine (Bisaglia et al., 2014; Pham and Waite, 2014). We hypothesized that these various forms of chlorodopamine function as selective poisons of dopaminergic neurons. In the following report, we demonstrate that chlorodopamine acts as a selective toxin of dopaminergic neurons, and may therefore represent a group of endogenous parkinsonian poisons generated when HOCl is produced in the substantia nigra.
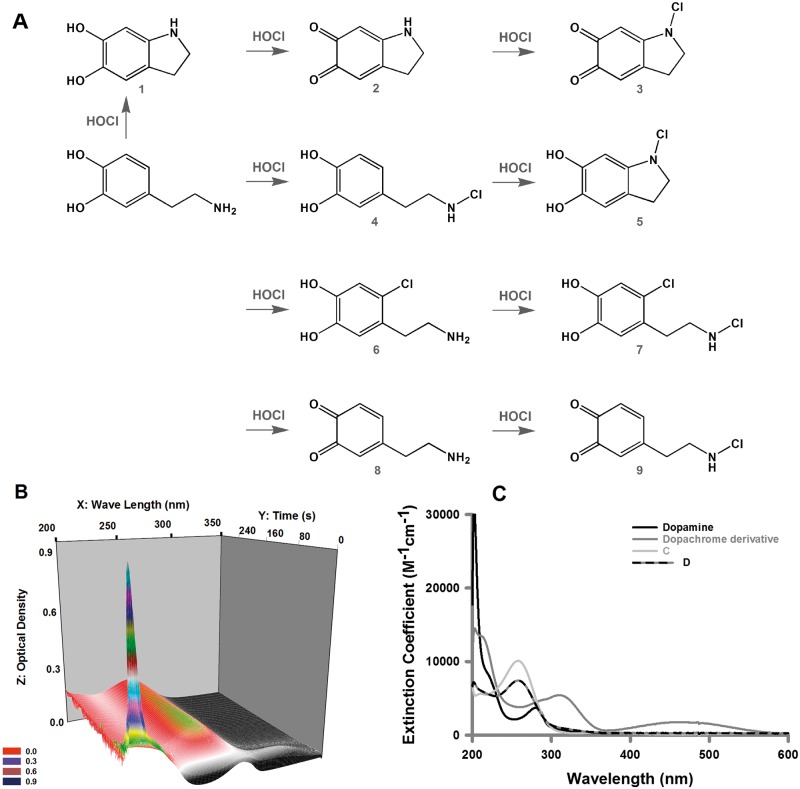
FIG. 1.
The reactions of HOCl and dopamine as observed by UV-Vis spectrometry. The possible individual reactions and products for the reaction of hypochlorous acid (HOCl) and dopamine are given in (A), where 1: indoline-5,6-diol; 2: 2,3-dihydro-1H-indole-5,6-dione; 3: 1-chloro-2,3-dihydro-1H-indole-5,6-dione; 4: 4-(2-(chloroamino)ethyl)benzene-1,2-diol; 5: 1-chloroindoline-5,6-diol; 6: 4-(2-aminoethyl)-5-chlorobenzene-1,2-diol; 7: 4-chloro-5-(2-(chloroamino)ethyl)benzene-1,2-diol; 8: 4-(2-aminoethyl)cyclohexa-3,5-diene-1,2-dione; and 9: 4-(2-(chloroamino)ethyl)cyclohexa-3,5-diene-1,2-dione. Spectra were recorded using a Hewlet-Packard 8453 diode array spectrophotometer. The reaction was initiated by the addition of HOCl (150 µM final concentration) to dopamine (25 µM final concentration) and data were collected every 0.5 s for 300 s. A three-dimensional depiction of the data from 200 to 350 nm is shown (B). The resulting data set was exported and analyzed using global analysis, singular value decomposition via Specfit software (Specsoft Inc.). Data were fitted to a sequential model in which the reactants formed 2 successive transients followed by a final unchanging endpoint specie (C).
Materials and Methods
Primary antibodies were bought from Abcam (Cambridge, Massachusetts), whereas the secondary antibodies were supplied in the kits purchased from ImmunoCruz (Santa Cruz, California) for immunohistochemical staining and the Amersham ECL Western Blotting Analysis System (GE Healthcare). CL-Xposure Film was supplied by Thermo Scientific (Pittsburgh, Pennsylvania). The ELISA kit for tumor necrosis factor-α (TNFα) was obtained from BD Biosciences (San Jose, California). The Halt protease inhibitor cocktail mix was obtained from Thermo Scientific. PlasticsOne (Roanoke, Virginia) supplied the cannulae for microinjection. All other reagents were purchased from Sigma-Aldrich (St. Louis, Missouri).
Animals
Adult Fisher 344X Brown Norway F1 male or female rats (200–250 g) were housed in groups of up to 4 animals with unrestricted access to food and water. Male C57/Bl6 mice aged 8–10 weeks old (20–26 g) were housed individually. Mice were acclimated to the institution’s Animal Care Facility for 1 week prior to surgery and allowed 1 week to recover postsurgery. All protocols used in this study pertaining to the mice were approved by the Institutional Animal Care and Use Committee of Winthrop University, whereas those involving rats were approved by the Institutional Animal Care and Use Committee of the New York Medical College.
Methods
Spectrophotometry
Spectra of the reaction products of dopamine (25 µM) reacted with HOCl (150 µM) were recorded using a Hewlet-Packard 8453 diode array spectrophotometer. Data were collected every 0.5 s for wavelengths from 190 to 1100 nm. The resulting data set was then exported as a single data file and analyzed using global analysis, singular value decomposition via Specfit software (Specsoft Inc.). The data were fit to a sequential model in which the reactants formed 2 successive transients followed by a final unchanging species (A + B→C→D→E).
In vitro toxicity studies
These studies employed SH SY5Y cells differentiated with retinoic acid as described by Jeitner et al. (2015), as well as dopamine transporter (DAT)-expressing and control COS-7 cells that were cultured as described by Ukairo et al. (2007). The toxicity of HOCl, dopamine or chlorodopamine to these cells was assessed by the measuring the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) or the release of lactate dehydrogenase (LDH). These experiments were adapted from those of Whitman et al. (2005a), and began with washing the cells twice with Hank’s Buffered Saline Solution (HBSS) at 37°C. HOCl, dopamine, or combinations thereof, were then added to the cells in Hank's Buffered Salt Solution (HBSS) up to 15 min in a total volume of 0.5 ml per well and incubated under tissue culture conditions. Stocks of HOCl and dopamine were prepared as 100 mM solutions in H2O on ice and diluted into HBSS at 37°C immediately prior to their application to cells. HOCl was quantified in terms of its ability to oxidize 5-thio-2-nitrobenzoic acid as described by Jeitner et al. (2013). The interval between the mixing of the reactants and their addition to the cells was no longer than 60 s. Incubations were terminated by the addition of 1 ml of spent media that served to scavenge any residual HOCl. The mixture of HBSS and spent media was replaced with 0.5 ml fresh media per well and the cells cultured for a further 24 h. In the case of the MTT assay, this incubation was followed by the replacement of the media with 250 µl of 1 mg/ml MTT in HBSS per well and a further 15-min incubation for 15 min under culture conditions. The dye solution was then aspirated from the wells and replaced with 200 μl dimethyl sulfoxide to dissolve the formazan products in the wells. These products were quantified at 510 nm. The LDH assays were carried out as described by Jeitner et al. (2015). This spectrophotometric assay measures the oxidation of NADH by LDH at 340 nm.
Thiol oxidation assay
Chlorodopamine and HOCl solutions were prepared in 1 ml PBS at 22°C. One minute later, 5-μl aliquots were combined with 1 ml 50 μM 5-thio-2-nitrobenzoic acid (TNB) for 2 m, after which absorbance was measured at 412 nm. The residual amounts of TNB were calculated using the Beer-Lambert equation and an extinction coefficient of 14.15 mM cm−1. TNB was prepared as described previously (Jeitner et al., 2013).
α-Ketoglutarate dehydrogenase complex assay
α-Ketoglutarate dehydrogenase (KGDH) complex activity was measured by monitoring the reduction of NAD+ by this complex extracted from SH SY5Y cells cultured on 60-mm-diameter dishes. Cells were exposed to chlorodopamine or HOCl in a volume of 10 ml per dish for 15 min at 37°C. The dishes were then washed with ice-cold HBSS prior to lysis with 20 mM Tris-HCl (pH 7.5), 1 mM dithiothreitol (DTT), 1 mM EDTA, 0.2% Triton X-100, and 1 µl/ml Halt protease inhibitors added at 150 µl per dish. The lysed material was then collected with scraping and assayed for KGDH complex activity as described by Gibson et al. (1988).
Preparation and measurement of isolated rat liver mitochondria
Mitochondria were isolated from 6-month-old male/female Fisher 344X Brown Norway F1 rats (fed ad libitum and with full access to water) by the procedure described previously (Krasnikov et al., 2005). All procedures were carried out on ice in a 4 °C room. Mitochondria isolated using this procedure and kept on ice were stable, as judged by consistent ADP/O and respiratory control ratios values, for at least 12 h after isolation. The mitochondrial protein concentration was determined by the Biuret method. Mitochondrial functioning was assessed by using a multiparameter chamber (Krasnikov et al., 2005), which permits simultaneous measurement of several mitochondrial parameters. Oxygen uptake by mitochondria was measured by a Clark-type closed electrode, and swelling of mitochondria was reflected by changes in transmittance at 660 nm. The experimental incubation buffer consisted of 300 mM sucrose, 5 mM Hepes, 5 mM KH2PO4 supplemented with 5 mM of a respiratory substrate, pH adjusted to 7.4 with Tris-base. The concentrations of the reagents added to the mitochondria in all experiments presented here were such that the volume (1 ml) was altered by < 2%. For these studies, 500 μM chlorodopamine was prepared by mixing 500 μM dopamine and 500 μM HOCl (1:1) immediately prior to the addition of the mixture to mitochondria. All additions listed in the figure legends are final concentrations.
h670 nm Treatments
Retinoic acid-differentiated SH SY5Y cells, grown on 24 multiwell plates, were placed on 25 cm × 10 cm array of 670-nm light emitting diodes (Quantum Devices, Inc., Barnaveld, Wisconsin) and illuminated for 5 min in a darkened room at 22 °C. The power intensity of the illumination was 50 mW/cm2 and yielded a total energy density of 30 J/cm2. Cells were illuminated 24 h, 12 h, and 5 min prior to the application of 50 μM chlorodopamine as described above. Survival was assessed by the MTT assay.
Electron microscopy
For these studies SH SY5Y cells were grown on 10-mm dishes. Cells were exposed to chlorodopamine or HOCl in a volume of 2 ml per dish for 15 min at 37°C. The cells were then fixed in 4% glutaraldehyde buffered in 0.1 M sodium phosphate buffer, pH 7.5, washed with the same buffer, postfixed in buffered 1% osmium tetroxide, en bloc stained with a saturated solution of uranyl acetate in 40% ethanol, dehydrated in a graded series of ethanol solutions, infiltrated with Epon epoxy resin, and embedded in the culture dish. Upon curing, the resin containing the samples was removed from the plastic culture dish, a small piece removed with a jeweler’s saw and glued to an aluminum block for en face thin sectioning. The resulting grids were then poststained with uranyl acetate and lead citrate and visualized on a Zeiss EM 10 transmission electron microscope retro-fitted with an SIA L3C digital camera (SIA, Duluth, Georgia).
Drug treatments and analysis of effects
Each animal received either GBR 12909 (10 mg/kg body weight) or its saline vehicle IP, at 11 am followed by the intrastriatal administration of chlorodopamine or MPP+ 12 noon, as adapted from an earlier protocol (Laloux et al., 2008). Chlorodopamine and MPP+ were administered at a dose of 90 nmol delivered in 2 µl artificial cerebrospinal fluid (124 mM NaCl, 5 mM KCl, 1.2 mM NaH2PO4, 2.7 mM CaCl2, 1.2 mM MgSO4, 26 mM NaHCO3, and 10 mM D-glucose) over a 6-min period as described by Jeitner et al. (2015). The effect of these treatments on the density of dopaminergic neurons was also assessed as described by Jeitner et al. (2015), whereas the effects on locomotor behavior were assessed through the Coulbourn Tru Scan Activity System (Whitehall, Pennsylvania). Mice were placed in this open field system 3 days prior to and 3 days after microinjections for 4 h beginning from an hour prior to dark onset. Total distance travelled was used as the measure for ambulation. After the final day of behavioral monitoring, mice were euthanized by an overdose of Euthasol (150 mg/kg) IP, and the brains were removed and bisected. The cannulated hemisphere was submerged in 4% paraformaldehyde in phosphate buffered saline for 4 h followed by 30% sucrose in phosphate-buffered saline. Brains were sectioned on a cryostat at 40 μm and were processed for tyrosine hydroxylase immunohistochemistry. The other hemisphere was used for protein and cytokine analysis. Free-floating sections were pretreated with peroxidase block and incubated for 1 h in goat serum as supplied by the ImmunoCruz staining system. Sections were then incubated overnight at 4°C with rabbit antityrosine hydroxylase (1:1000). The following day sections were treated with the biotinylated secondary antibody, the horse radish peroxidase-streptavidin complex and visualized with diaminobenzidine for detection. All reagents, except the primary antibody, were supplied by the immunocruz kit. Protocols for cell counts are similar to methods previously published (Laloux et al., 2008) and using published stereotaxic coordinates (Franklin and Paxinos, 2008). Three sections were selected to measure tyrosine hydroxylase-containing neurons in each mouse. The rostral section is rostral to the incipient medial terminal nucleus of the accessory optic tract (MT) (-3.1 bregma); Intermedial segment: between incipient MT and caudal portion of the MT (-3.4 bregma); Caudal segment: between the caudal portion of the MT and the caudal portion of the interfasicular nucleus (-3.7 bregma). Stained sections were visualized by means of a light microscope (Nikon Eclipse TE300, Nikon USA, Melville, New York). Images were taken with the Nikon DS-Ri1 and processed with the NIS-Elements 3.2 (Nikon). The substantia nigra boundaries were established with a medial border as the vertical line passing through the medial tip of the cerebral peduncle, excluding neurons in the ventral tegmental area; the medial/ventral border as the dorsal boundary of the cerebral peduncle. These boundaries include neurons in pars compacta, pars reticulate and pars lateralis and the dorsal boundary that passes above the pars compacta, and below the ventral margin of the medial lemniscus. Microphotographs for dopaminergic neurons were taken at a 40× magnification. Neurons were counted under a light microscope at a magnification of 400×. A neuron was counted if the nucleus was visible and one or more clearly defined processes tapered gradually from the cell body. This cell number was considered to be representative of the number of dopaminergic nigral cells in each animal.
Brain tissue was homogenized in ice-cold lysis buffer (100 mg tissue/ml) consisting of 25 mM Tris, 0.25 M sucrose, 2 mM EDTA, 10 mM EGTA, 1% Triton X-100, and Halt protease inhibitor cocktail mix (200 μl/10 ml). The samples were then centrifuged at 10 000 × g for 20 min and supernatants were for cytokine analysis. Protein concentrations were determined with the Bradford assay. TNFα levels were measured with the mouse TNFα ELISA kit.
Statistics
Data were analyzed using general linear models/ANOVA followed by contrasts to evaluate differences between individual treatments using R (www.r-project.org) or SigmaPlot software (Systat Software). Results are expressed as mean ± SEM and results where P < .05 are considered statistically significant. For analysis of locomotor behaviors, analysis of the model residuals revealed that the variances differed between groups violating a key assumption of ANOVA. Therefore, bootstrapping was applied to estimate standard errors, differences between treatments and P-values.
RESULTS
Alterations in the UV-Visible Spectrum of Dopamine due to HOCl
Structures 4–7 in Figure 1A were identified by 1H-Nuclear Magnetic Resonance (NMR) studies of the reaction of HOCl and dopamine performed in acidified deuterated methanol (Jeitner et al., 2015). This solvent was necessary to prevent the polymerization of chlorodopamine that occurs in aqueous solutions, particularly at the concentrations required for 1H-NMR studies. UV-Vis spectrometry was employed to show rapid formation of chlorinated forms of dopamine in an aqueous environment. Dopamine was mixed with HOCl and allowed to react. The transformation of dopamine to chlorinated species could be observed at all wavelengths between 200 and 600 nm. The data indicate a complex reaction that has 3 primary phases (Figure 1B). The spectra obtained were analyzed using singular value decomposition to deconvolute the data into composite spectra (Figure 1C). Initially, dopamine is converted to a dopachrome derivative as evidenced by relative rapid accumulation of characteristic absorption centered at 460 nm (k1 = 1.4 × 104 M−1 s−1, ε460nm approximately 2000 M−1 cm−1). This specie then decays to a second transient that has a spectrum with 3 maxima (k3 = 0.06 s−1, 207, 240, 291 nm). The decay of this specie forms an endpoint species with a maximal absorption band at 256 nm (k2 = 0.07 s−1, ε256nm approximately 10 000 M−1 cm−1, traces C and D). Spectra of the latter 2 species form in single phases, but have no signature absorption that allows structural assignment (as is available for dopachrome). The possible reaction paths and products resulting from the reaction of dopamine and HOCl are shown in Figure 1A.
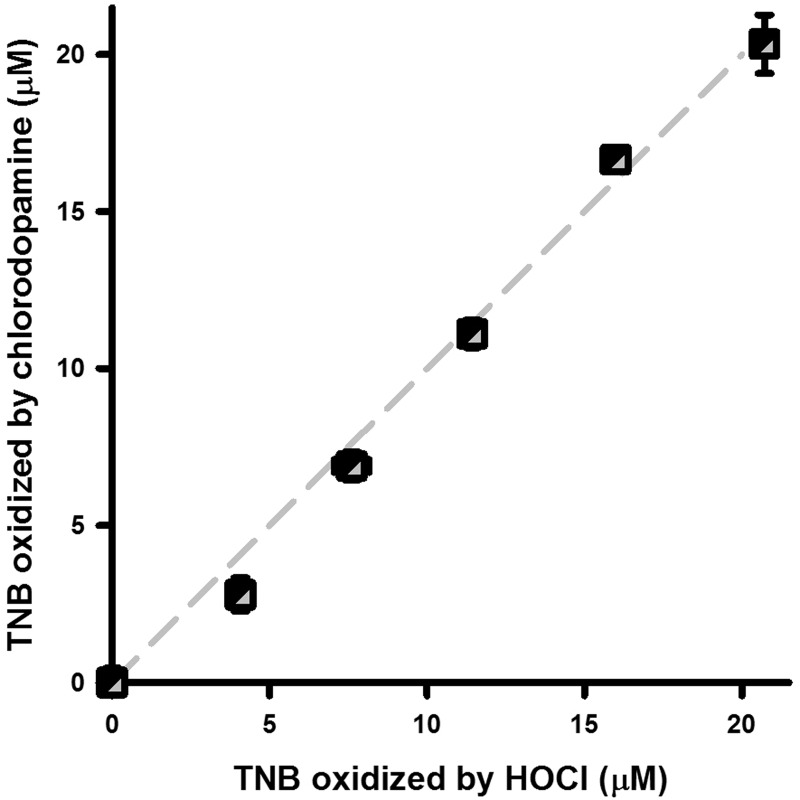
Chlorodopamine Oxidizes TNB
Our earlier 1H-NMR data (Jeitner et al., 2015) and the UV-Vis data presented here indicate that HOCl and dopamine react to produce a variety of chloramines. The presence of the chloramines among the products of the reaction of HOCl and dopamine was supported by the oxidation of TNB (Figure 2). Graded amounts of either HOCl or chlorodopamine were mixed with TNB and allowed to react at 22 °C and the residual thiol content assessed spectrophotometrically. Both HOCl and chlorodopamine oxidized TNB to the same extent, indicating that chlorodopamine solutions retained the same capacity to oxidize TNB as HOCl albeit at a slower rate.
FIG. 2.
Chlorodopamine oxidizes TNB. Oxidation of TNB by HOCl or chlorodopamine is depicted as mean ± SEM of 3 separate determinations. Chlorodopamine and HOCl solutions were prepared in PBS and 1 min later, aliquots of these solutions were combined with TNB. Absorbance was measured at 412 nm for the quantification of residual thiols groups.
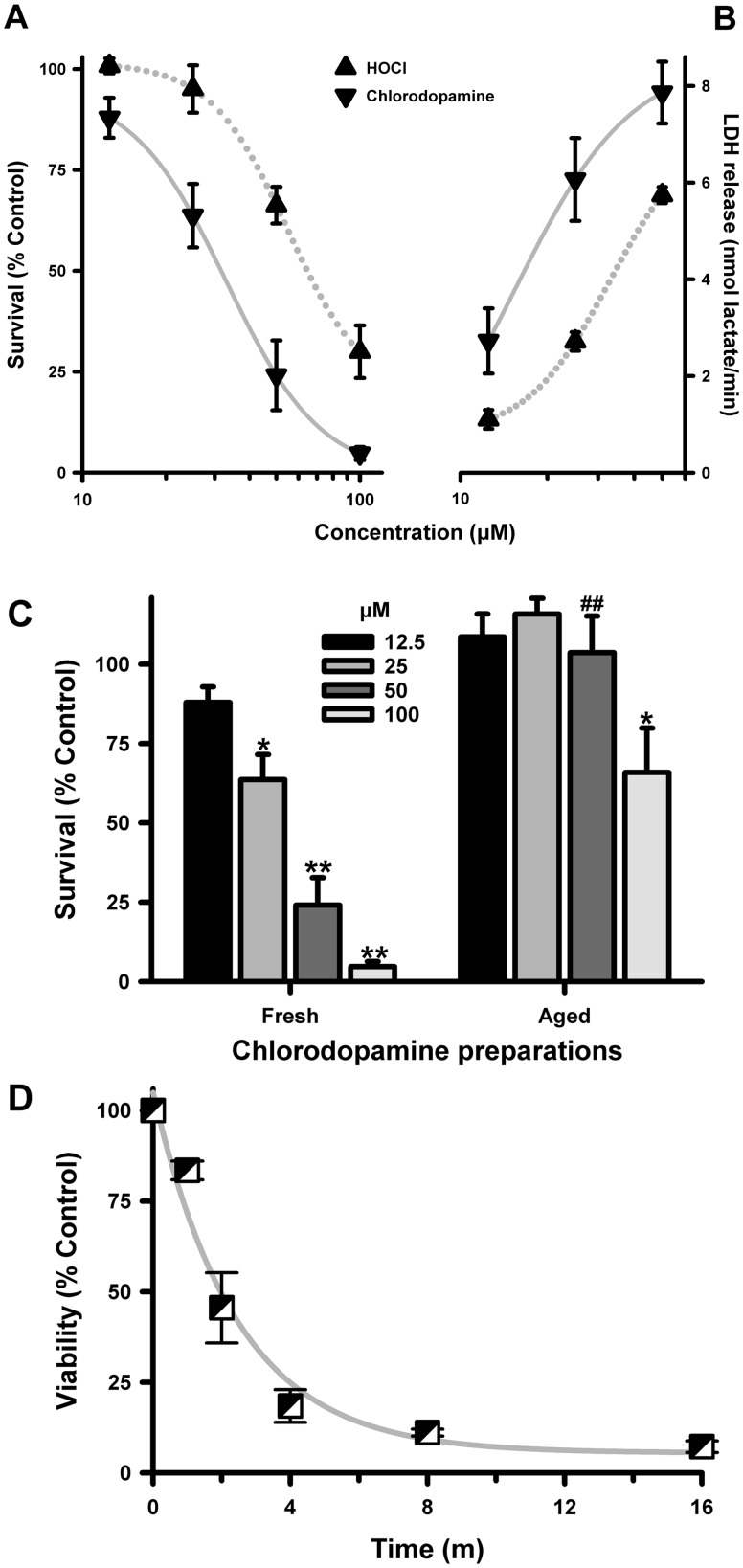
Chlorodopamine Is More Toxic to SH SY5Y Cells Than Is HOCl
The toxicity of chlorodopamine was compared with that of HOCl using retinoic acid-differentiated SH SY5Y cells, which have some characteristics of dopaminergic neurons. Chlorodopamine, dopamine, or HOCl were added to the cells in the absence of scavengers for a period of 15 min and survival assessed 24 h later. Exposure to HOCl significantly reduced the viability of the cells as measured by the reduction of MTT (Figure 3A). The magnitude of these losses was comparable to those reported earlier (Kalogiannis et al. 2016, Whiteman et al., 2005a) and conformed to a 4-parameter logistic curve (R2 = 1.00) with an EC50 = 30.1 μM.
FIG. 3.
Chlorodopamine is more toxic to SH SY5Y cells than is HOCl. The toxicity of HOCl and chlorodopamine to SH SY5Y cells, as measured by MTT staining (A) or the release of LDH (B), is expressed as mean ± SEM of 7 (A) or 5 (B) independent experiments. Significant differences between groups were observed for HOCl (MTT: 50 * and 100 μM **; LDH releases: 50 ** and 100 μM **) and chlorodopamine (MTT: 25 *, 50**, and 100 **; LDH release: 25 *, 50 **, and 100 ** μM), with * and ** denoting P values <.05 and <.01, respectively. Significant differences were also observed between the groups (MTT: 50 * and 100 μM **; LDH release: 25*, 50 **, and 100 μM *). The significant differences were determined by repeated measures ANOVA and Fisher LSD post hoc analysis. Panel (C) depicts the effect of aging the chlorodopamine solution for 60 min at 22 °C, as the mean ± SEM of 10 separate experiments. Significant differences from control in panel (C) were denoted by * and ** for P values <.05 and <.01, respectively. The effect of fresh and aged 50 μM chlorodopamine were also significantly different from each P value <.05 indicated by #. Panel (D) shows the effect of exposure time of the toxicity of 100 μM chlorodopamine, as measured by MTT staining 24 h later is expressed in panel (C) as the mean SEM of between 3 and 5 separate experiments. The changes in viability at all times shown in panel (D) were significantly different at P < .05 as determined by repeated measures ANOVA.
Chlorodopamine was prepared by mixing HOCl and dopamine in prewarmed HBSS 1 min prior the addition of the mixture to SH SY5Y cells. HOCl and dopamine were combined at a ratio of 1:2 to ensure that all of the HOCl was consumed in the reaction (Jeitner et al., 2015). Dopamine at the concentrations used to prepare chlorodopamine was not toxic to SH SY5Y cells. Percent survival following 15-min incubations with 25, 50, 100, and 200 μM dopamine was 110 ± 7, 106 ± 3, 103 ± 4, and 98 ± 4 (mean ± SEM, n = 8), respectively. The indicated chlorodopamine concentrations refer to the initial concentrations of HOCl and allows direct comparison between chlorodopamine and HOCl.
Exposure to chlorodopamine resulted in significantly greater cell losses than that observed with exposure to HOCl alone (Figure 3A). As observed with HOCl, the pattern of cell loss conformed to a 4-parameter logistic curve (R2 = 1.00). The EC50 = 65.3 μM derived from this curve, however, was more than double the EC50 value for the toxicity of HOCl. Similarly, the cell losses due to 100 and 200 μM chlorodopamine were significantly different from those at the corresponding concentrations of HOCl.
The release of cellular LDH was used to confirm the data obtained with MTT staining (Figure 3B). These studies were confined to HOCl and chlorodopamine concentrations up to 50 μM, as higher concentrations of HOCl or chlorodopamine inactivated LDH (not shown). Even so the LDH release studies revealed the same pattern of toxicity observed using vital staining; the toxicities conformed to logistic expressions (R2 = 1 for both curves) and chlorodopamine was more toxic than HOCl to SH SY5Y cells. The LDH release data also correlated linearly with MTT data (R2 = 0.91).
Solutions of chlorodopamine form precipitates, indicative of the formation of polymers and aggregates (Jeitner et al., 2015). The rate of precipitation depends on initial concentrations of the reactants and precipitates were observed in the solutions prepared for the experiments described in Figures 2A and B. This observation indicated that some of the compounds responsible for the toxicity in Figure 3A may polymerize and therefore have short half-lives. This notion was tested by comparing the toxicity of freshly prepared chlorodopamine with toxicity of the same solutions allowed to age for an hour at 22°C (Figure 3C). Aging the chlorodopamine solutions in this manner diminished their toxicity. The diminution was most evident at 50 μM chlorodopamine. In contrast, the effect of aging the 100 μM chlorodopamine solutions was variable: Half of the preparations retained their toxicity whereas the other half became inert.
In addition to assessing the effects of aging on the chlorodopamine solutions, we sought to determine the minimum period needed to produce maximum toxicity by 100 μM chlorodopamine (Figure 3D). This period was 8 min as the toxicity to this exposure plateaued thereafter. Exposures as little as 1 min also caused a significant 16% cell loss (P < .05, t test). Collectively, these observations indicate that chlorodopamine is more toxic to SH SY5Y cells than is HOCl and kills cells within minutes.
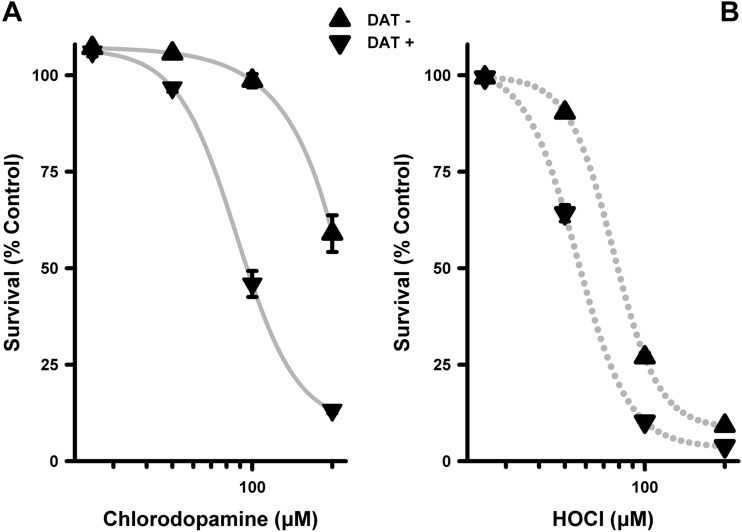
The Toxicity of Chlorodopamine Is Enhanced by the DAT
One possible mechanism to account for the greater toxicity of chlorodopamine to SH SY5Y cells than of HOCl is uptake of the chlorinated forms of dopamine via the DAT. Our initial attempts to investigate this mechanism involved pretreating SH SY5Y cells with 10 μM amounts of the DAT antagonists cocaine, GBR 12909 or GBR 12935 prior to the addition of chlorodopamine. The results of these studies, however, were equivocal in that the higher concentrations of chlorodopamine remained toxic. Given these difficulties, the DAT-expressing cells developed by Surratt and colleagues (Ukairo et al., 2007) were used to assess the aforementioned mechanism. Using these cells, it was found that chlorodopamine was significantly more toxic to cells expressing DAT than the relevant control cells (Figure 4A). In both cases, the relation between cell survival and chlorodopamine concentrations conformed to 4-parameter logistic curves (R2 = 1.00 for both curves). The EC50 value for the death of DAT-expressing cells due to chlorodopamine was 87.6 μM. In contrast, chlorodopamine at 200 μM killed only 40% of control cells. Chlorodopamine was also less toxic to control cells than was HOCl (Figs. 4A and B); the opposite of what was observed using SH SY5Y cells (Figs. 3A and B). HOCl killed both DAT-expressing and control cells in a logistic manner (R2 = 1.00 for both curves) with EC50 values equal to 55.6 and 76.4 μM for the death of DAT-expressing and control cells, respectively. These results indicate that the toxicity of chlorodopamine is enhanced by the activity of DAT. This discovery was corroborated by in vivo studies as described in detail below.
FIG. 4.
The toxicity of chlorodopamine is enhanced by the dopamine transporter (DAT). Shown are the losses of DAT-expressing COS-7 cells (DAT+) or control COS-7 cells (DAT-) due to chlorodopamine (A) or HOCl (B), expressed as the mean ± SEM of 6 independent observations. Cells were exposed to the test compounds for 15 min at 37 °C, and cell survival assessed was 24 h later by the MTT assay. Significant differences between the cell populations were observed for chlorodopamine (DAT+: 50 *, 100 **, and 200 μM **; DAT-: 200 μM **) and HOCl (DAT+/-: 50 **, 100 **, and 200 μM **), with * and ** denoting P values <.05 and <.01, respectively. Significant differences were also observed between the cell populations for DAT+ and DAT- (chlorodopamine and HOCl: 50 **, 100 **, and 200 μM **).
Chlorodopamine Affects Mitochondria
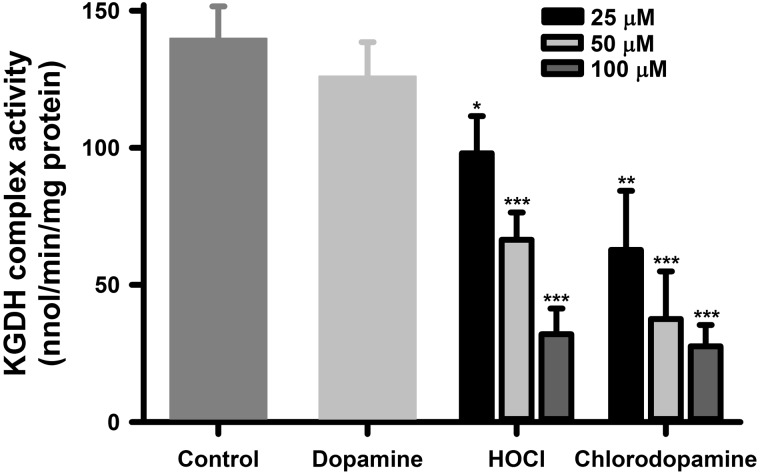
In previous studies, we reported that the KGDH complex was sensitive to inactivation by HOCl and N-chloramine (Jeitner et al., 2005). As the chlorinated compounds include chloramines (Figure 1C) and have a significant ability to oxidize thiol moieties (Figure 4), the inactivation of the cellular KGDH complex was used to further assess the toxic actions of chlorodopamine versus those of HOCl. KGDH complex activity in SH SY5Y cells was assayed following 15-min exposures of the cells to dopamine, chlorodopamine, or HOCl (Figure 5). Exposure to 200 μM dopamine for 15 min had no appreciable effect on KGDH complex activities in SH SY5Y cells consistent with its effect on cell survival (Figure 5). HOCl and chlorodopamine both inactivated cellular KGDH complex activity in a manner that corresponded to their effects on viability of SH SY5Y cells (Figs. 2A and B). The inactivation of the KGDH complex was statistically significant (P < .05) at 25 μM and higher concentrations of the oxidants.
FIG. 5.
Chlorodopamine inactivates the cellular KGDH complex. Shown is the inactivation of KGDH complex activities in SH SY5Y cells by HOCl or chlorodopamine. The cells were exposed to these agents for 15 min at 37 °C. Data are expressed as the mean ± SEM of between 5 and 19 independent experiments. Significant difference between the groups are denoted by * and ** for P values <.05 and <.01, respectively.
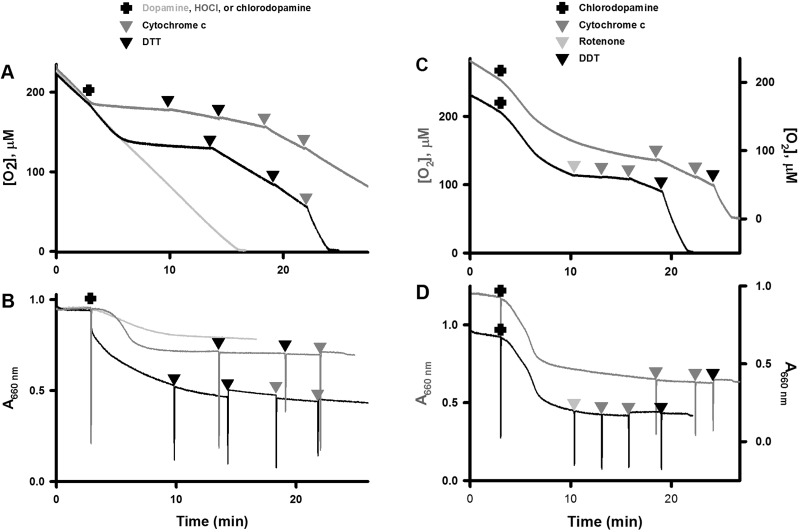
The correspondence between survival and KGDH complex activities suggested that mitochondria might be vulnerable to the actions of chlorodopamine. These organelles are commonly affected in PD. For example, rotenone and MPP+ both inhibit mitochondrial complex I activity (Greenamyre et al., 2010; Kotake and Ohta, 2003). The activity of this complex can be assessed in terms of O2 consumption by isolated mitochondria respiring on glutamate and malate. Oxidation of these substrates by the citrate cycle yields NADH, which in turn is oxidized by complex I. Addition of 500 μM chlorodopamine to mitochondria respiring on glutamate/malate caused an initial increase in O2 consumption followed by slowing of respiration (Figure 6A). The increase in O2 consumption coincided with high-amplitude mitochondrial swelling (Figure 6B).
FIG. 6.
Chlorodopamine affects mitochondrial respiration and dimensions. The effects of 500 μM chlorodopamine on O2 consumption and size of isolated rat liver mitochondria energized with glutamate/malate are depicted in panels (A) and (B), respectively. Two traces are shown in each panel with offset Y axes to aid in the comparison of experiments. The traces differ primarily by the addition of rotenone (1 mM, grey triangle) at 10 min in the second trace to inhibit complex I activity. The effects of chlorodopamine (500 μM, black trace), dopamine (grey trace), and HOCl (500 μM, dark grey trace) on O2 consumption and size of isolated rat liver mitochondria respiring on succinate are depicted in panels (C) and (D), respectively. The effects of cytochrome c (0.2 mg/ml, dark grey triangles) and DTT (500 μM, black triangles) on chlorodopamine-treated mitochondria are also shown. The experiments in these panels were repeated at least 4 times.
Krasnikov et al. (2011) reported that mitochondrial swelling due to positively charged species was associated with dissociation of cytochrome c from the inner membrane. Loss of cytochrome c results in a decreased O2 consumption, which can be restored by the addition of reduced cytochrome c. Addition of reduced cytochrome c partially restored the respiration of chlorodopamine-affected mitochondria (Figure 6A: black trace). The partial nature of the restoration was not due to amount of cytochrome c added (final concentration = 0.2 mg/l), as doubling the concentration of cytochrome c caused no further increase in O2 consumption. These observations suggested that chlorodopamine inactivated cytochrome c oxidase (complex IV) rather than complex I.
Thiol compounds can reduce chlorides from chlorinated compounds; therefore, DTT was tested as a means of reversing the effects of chlorodopamine on mitochondria. Addition of DTT to mitochondria treated sequentially with chlorodopamine and cytochrome c, resulted in O2 consumption comparable to that of uncoupled mitochondria (Figure 6A: black trace). These results suggested that chlorodopamine acts to chlorinate and thereby inactivate complex IV. Electrons for acceleration of respiration due to DTT could have come from the exogenous cytochrome c and the electron transport chain. The latter is a possibility if chlorodopamine inactivated complexes II or III in a manner reversed by DTT. Consequently, the complex I inhibitor rotenone was used to assess the contribution of the electron transport chain to the restoration of O2 consumption by DTT in Figure 6C. Addition of rotenone to these mitochondria led to an almost complete cessation of O2 consumption (Figure 6A: black trace). The subsequent addition of cytochrome c to these mitochondria did not increase respiration, in contrast to mitochondria treated with chlorodopamine and cytochrome c (Figure 6A: black trace). This observation indicates that some the electrons consumed by mitochondria treated by chlorodopamine, cytochrome c, and DTT were derived from the electron transport chain. A second addition cytochrome c to mitochondria treated with chlorodopamine and rotenone resulted in a modest stimulation of respiration, indicative of partial inhibition of complex IV by chlorodopamine (Figure 6C: black trace). This inhibition was reversed by the addition of DTT. Taken together, these observations indicate that chlorodopamine inactivates complex IV and not complex I. The effect of chlorodopamine on mitochondrial dimensions was not altered by the subsequent additions of cytochrome c, DTT, or rotenone, and suggests that chlorodopamine induces a permanent change in mitochondrial size (Figure 6B and D).
The possible inhibition of complex II activity by chlorodopamine was examined using mitochondria respiring succinate. This substrate is oxidized in mitochondria by on succinate dehydrogenase, which is a component of complex II. The effects of chlorodopamine on mitochondria respiring on succinate (Figure 6C) were comparable to those respiring on glutamate/malate (Figure 6A) with some important differences. For example, the period prior to the slowing of O2 consumption was protracted in the presence of glutamate/malate as compared with that in the presence of succinate (6.9 vs 2.8 min). In addition, the rate of the initial acceleration of respiration due to chlorodopamine was greater when mitochondria oxidized glutamate/malate rather than succinate. These changes were accompanied by an approximate doubling of the swelling observed with mitochondria respiring on succinate and treated with chlorodopamine (cf. Figs. 6B and D).
Addition of DTT to mitochondria respiring on succinate and treated with chlorodopamine partly restored O2 consumption (Figure 6A). Doubling the concentration of DTT, however, resulted in no further improvement in respiration. The addition of cytochrome c, however, led to O2 utilization comparable to that of uncoupled mitochondria. These results suggest that DTT reversed the inactivation of succinate-supported respiration by chlorodopamine, and that cytochrome c replenished the pool of this cytochrome lost from the inner membrane. As observed with mitochondria respiring on glutamate/malate, the additions of DTT and cytochrome had not effect on the swelling induced by chlorodopamine (Figure 6D).
The effects of chlorodopamine on mitochondrial respiration and size were also compared with the effects of the parent compounds: dopamine and HOCl. Addition of HOCl to mitochondria oxidizing succinate caused an immediate inhibition of O2 consumption, as well as high-amplitude swelling. The effect on respiration was marginally alleviated by the additions of DTT and cytochrome c. These observations suggest that HOCl caused irreversible damage to mitochondria. Addition of dopamine to mitochondria respiring on succinate resulted in a modest increase in respiration and low-amplitude swelling (Figs. 6C and D).
The initial increases in respiration observed with mitochondria oxidizing either glutamate/malate or succinate indicate that chlorodopamine inhibits neither complex I nor II. Such increases in O2 consumption have been attributed to inhibition at complex III or IV causing electrons to flow back to and leak from complex I. The leaked electrons reduce O2 to superoxide, which manifests as accelerated O2 consumption. In summary, rather than inhibiting complex I, chlorodopamine inhibits complex IV and possibly complex III. Chlorodopamine also causes cytochrome c to dissociate from the inner mitochondrial membrane.
Whiteman et al. (2005b) reported that the swelling of mitochondria induced by HOCl was due to opening of the mitochondrial permeability pore. The likelihood that this pore might be similarly opened by chlorodopamine was examined using cyclosporine A, which prevents its opening. Mitochondria were subjected to Ca2+ load, which resulted in immediate increase in oxygen uptake and high-rate high amplitude swelling that were completely prevented by cyclosporine A (not shown). In the case of chlorodopamine, however, cyclosporine A caused a small reduction of the initial increase in mitochondrial respiration, but largely mitigated the inhibition of respiration by chlorodopamine (not shown). The mitochondrial swelling due to chlorodopamine was also only partially attenuated by cyclosporine A (not shown).
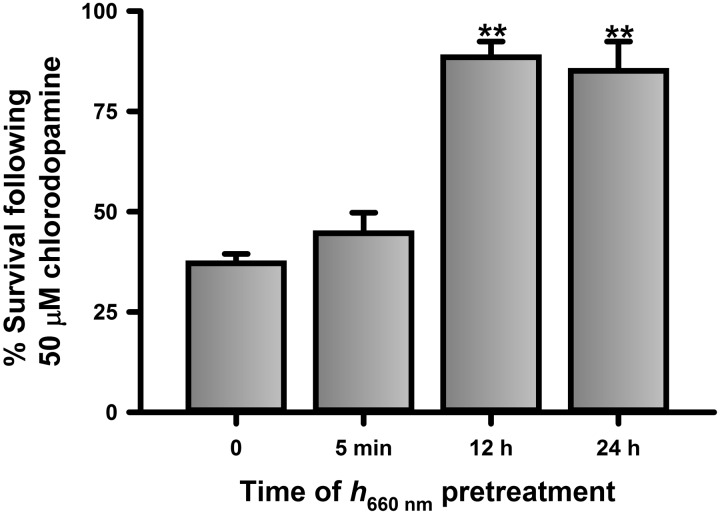
Inhibition of complex IV by cyanide is mitigated by prior illumination of cells with red light (Wong-Riley et al., 2005). Thus, the possibility that chlorodopamine affects complex IV activity was examined further by testing the ability of illumination at 670 nm (h670 nm) to reverse the toxicity of 50 μM chlorodopamine to SH SY5Y cells (Figure 7). Pretreatment of SH SY5Y cells with 5-min illumination at h670 nm at 12 and 24 h prior to the addition of 50 μM chlorodopamine mitigated the toxicity of the chlorinated compounds. In contrast, illumination with h670 nm immediately before the application of chlorodopamine had no effect on toxicity. The mitigation of the toxicity of chlorodopamine by h670 nm is consistent with the effect of this illumination on cyanide, a known cytochrome c oxidase poison.
FIG. 7.
h670 nm pretreatment reverses the toxicity of chlorodopamine to SH SY5Y cells. SH SY5Y cells were pretreated with h670 nm for 5 min at 24 h, 12 h, and immediately prior to the application of 50 μM chlorodopamine, as described for Fig. 2. Shown are the mean ± SEM of 5 independent experiments with significant differences between groups indicated by ** and denotes P values <.01.
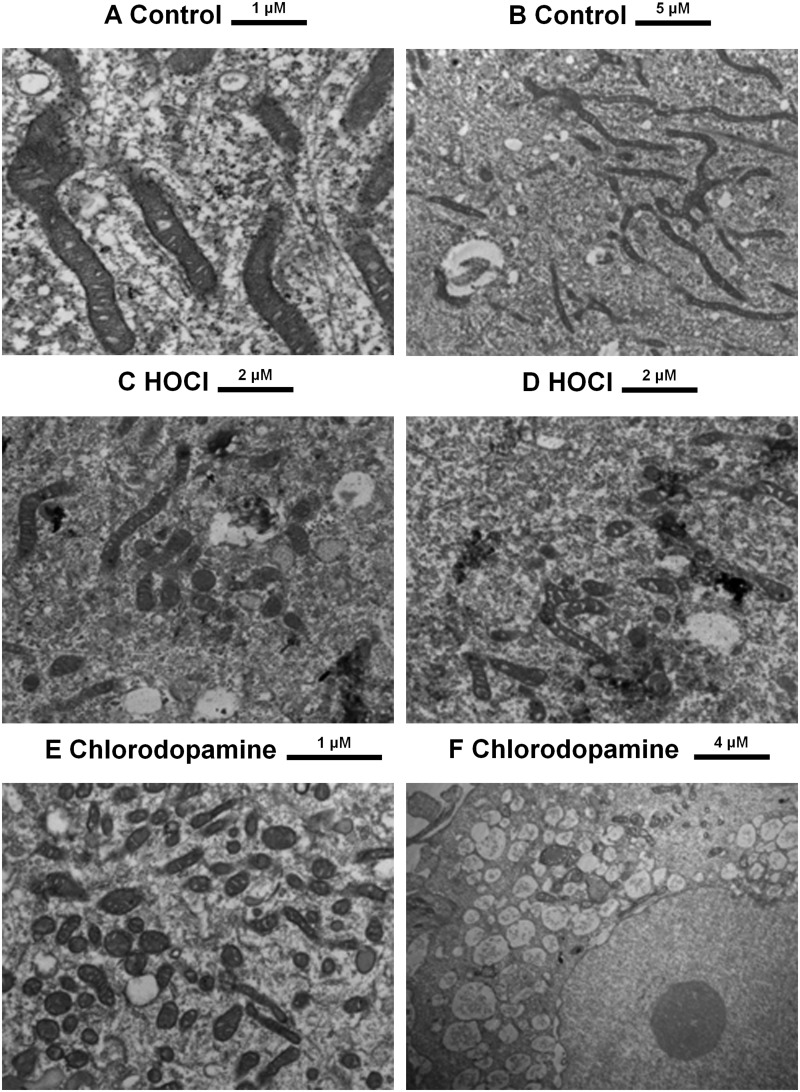
The swelling observed with isolated mitochondria (Figs. 6B and D) also was confirmed using SH SY5Y cells treated with HBSS, HOCl, or chlorodopamine for 15 min (Figure 8). The mitochondria in the control cells exhibited the established morphology of these organelles: long tubular structures with clearly defined cristae (Figs. 8A and B). HOCl caused mitochondrial swelling as indicated by the ovoid structures in Figures 8C and D. The densely pigmented material in these panels is indicative of destroyed mitochondria. Even so, apparently normal mitochondria are also present in the HOCl-treated cells. Exposure of the cells to chlorodopamine, however, caused most of the mitochondria to swell (Figure 8E). In addition, the cells exhibited significant vacuolation (Figure 8F).
FIG. 8.
Ultrastructural changes induced by HOCl and chlorodopamine. SH SY5Y cells were treated with 50 μM HOCl or chlorodopamine for 15 min and then processed for electron microscopy. Size bars are given above each panel.
Chlorodopamine Causes Neuronal Loss, Behavioral Deficits, and Inflammation In Vivo
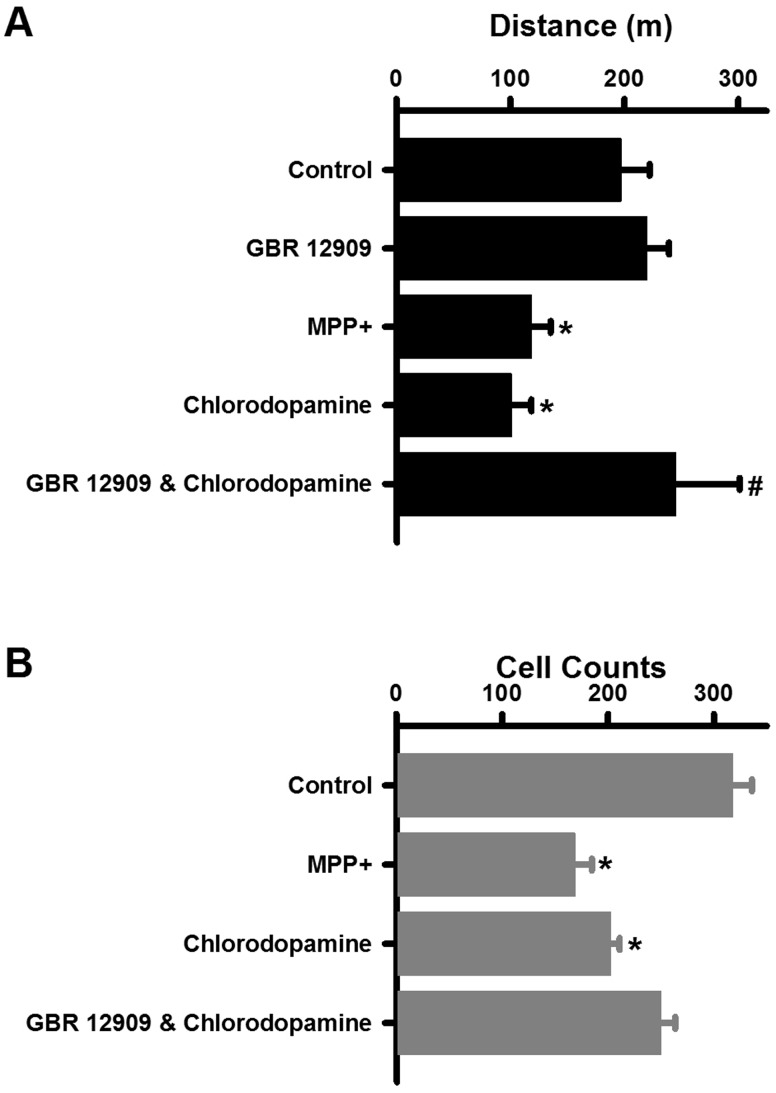
The properties of chlorodopamine described thus far are consistent with those of a parkinsonian drug, in that, this collection of chlorinated compounds selectively kills dopaminergic cells by disrupting mitochondrial function. This possibility was tested by injecting chlorodopamine into striatum of mice and comparing the acute effects of this treatment with those of the known parkinsonsian drug MPP+ (Giovanni et al., 1994). Chlorodopamine or MPP+ was injected into the striata of mice at a dose of 90 nmol per animal. These injections target the dopaminergic projections from the substantia nigra to striatum that are the first to exhibit degeneration in PD (Burke and O’Malley, 2013). Previous studies have demonstrated that losses of dopaminergic neurons from the striatum reduce ambulatory behavior (Giovanni et al., 1994; Laloux et al., 2008). Both MPP+ and chlorodopamine caused significant reductions in ambulation 1 day following their administration (Figure 9A). The reduction due to chlorodopamine was attenuated by pretreatment with GBR 12909. These changes in behavior were reflected in the densities of dopaminergic neurons in the substantia nigra (Figure 9B). MPP+ reduced the number of dopaminergic neurons from 318 ± 18 in the control striata to 169 ± 16 in the striata of mice treated with MPP+, a 47% reduction comparable to that reported in similar studies of MPP+ (Giovanni et al., 1994). Similarly, 203 ±8 cells remained 3 days following the intrastriatal injection of a 90 nmol dose of chlorodopamine: a significant 36% reduction (Figure 9A). Pretreatment with GBR 12909 mitigated the cell losses due to chlorodopamine, and thereby confirmed the importance of DAT to the toxicity of chlorinated dopamine (Figure 9A).
FIG. 9.
Chlorodopamine causes neuronal loss and behavioral deficits in vivo. Behavioral changes due to these treatments are indicated by the distance traveled by the mice 3 days after the intrastriatal injections (A). Significant difference from control is denoted by * for P values <.05. Similarly, the significant difference between the chlorodopamine and GBR 12909 + chlorodopamine groups at P <.05 is indicated by #. Changes in the density of dopaminergic neurons in substantia nigra (mean ± SEM) are indicated by the tyrosine hydroxylase staining of tissues taken from animals 3 days following 2 μl intrastriatal injections consisting of artificial cerebrospinal fluid (control, n = 13), 90 nmol MPP + (n = 12), 90 nmol chlorodopamine (n = 11), or chlorodopamine to mice pretreated with 10 μg/kg GBR12909 (n = 10) is depicted in panel (B). Significant difference from control is denoted by * for P values <.05. These differences were determined as described in the Methods section.
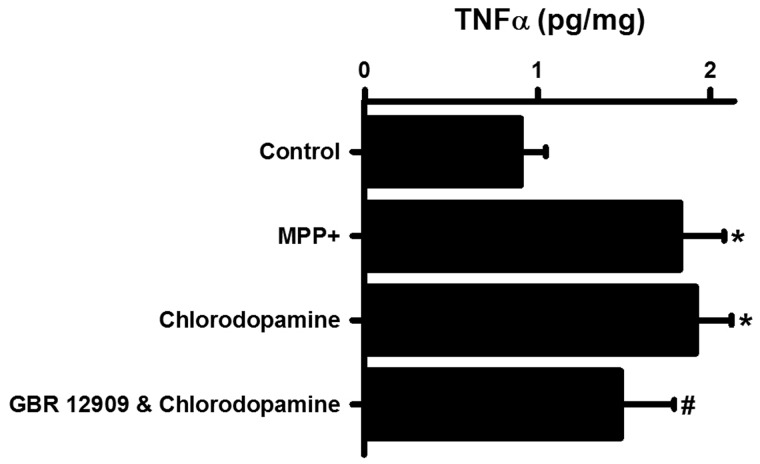
Inflammation is a common consequence of the administration of parkinsonian drugs (Wilms et al., 2007). The possibility that inflammation accompanied the intrastriatal administration of chlorodopamine was investigated by measuring the changes in the cerebral content of TNFα (Imai and Kohsaka, 2002) (Figure 10). Chlorodopamine increased the TNFα content of the brain as did MPP+ (Figure 10). The increase due to chlorodopamine was partially offset by prior treatment with GBR 12909.
FIG. 10.
Chlorodopamine induces cerebral inflammation in vivo. Chlorodopamine increases the brain contents of TNFα. The groups in panel (A) are the same as for Fig. 9. Significant differences from control are denoted by * for P values <.05. The significant difference between the chlorodopamine and GBR 12909 + chlorodopamine groups is indicated by # for P < .05. These differences were assessed by ANOVA followed by Fisher's least significant difference post hoc analysis.
DISCUSSION
HOCl is one of the most powerful oxidants produced during inflammation of extracerebral tissues. However, evidence that this oxidant is also produced in the brain is accumulating (Choi et al., 2005; Green et al., 2004; Maki et al., 2009). Here, we report that HOCl reacts with dopamine to produce toxins capable of selectively destroying dopaminergic neurons. The major products of the reaction of HOCl and dopamine were identified as 4-(2-(chloroamino)ethyl)benzene-1,2-diol; 4-(2-aminoethyl)-5-chlorobenzene-1,2-diol; and 4-chloro-5-(2-(chloroamino)ethyl)benzene-1,2-diol (Figure 1: compounds 4, 6, and 7) in 1H-NMR studies of the reaction in acidified deuterated methanol (Jeitner et al., 2015). UV-Vis spectrophotometric investigation of the reaction of HOCl and dopamine provided evidence for the formation of these compounds in aqueous media. The chloramines in the mixtures are also capable of oxidizing the thiol compound TNB. KGDH complex, which is sensitive to inactivation by N-chloramine (Jeitner et al., 2005), is also inactivated by chlorodopamine. The likely cause of this inactivation is oxidation of the cysteinyl thiols by chlorodopamine, based on the proclivity of chloramines to react with thiols (Peskin and Winterbourn, 2001) and the sensitivity of the KGDH complex to inhibition by agents that alkylate its thiol groups (Angelides and Hammes, 1979).
Chlorination of dopamine by HOCl in situ will depend on the pools of this catecholamine and the extent to which these are accessed by HOCl. Dopamine is present in the cytosol, synaptic vesicles, and synaptic cleft at concentrations of 50 μM (Schmidt et al., 2013; Spanos et al., 2013), 0.3 M (Omiatek et al., 2013), and 50 nM (Mosharov et al., 2003; Woods et al., 2005), respectively. Given the proximity of microglia to the termini of dopaminergic neurons, the most probable site for the reaction of HOCl and dopamine is at synapses (Nakadate and Tanaka-Nakadate, 2015). DAT—while not present within the synapse—are concentrated nearby and could facilitate uptake of chlorodopamine into the termini, which are richly endowed with mitochondria. Chlorodopamine would then damage the mitochondria and thus compromise synaptic transmission and cellular viability. As reported here, the toxicity of chlorodopamine depends on both its cellular uptake via DAT and its actions on the mitochondria. These properties are shared by MPP+ (Giovanni et al., 1994), 6-hydroxydopamine (Lehmensiek et al., 2006), and paraquat (Rappold et al., 2011), but not by rotenone (Hirata et al., 2008).
In addition to the synapse, HOCl could also access cytosol and vesicular pools of dopamine. The pKa of HOCl is 7.54 at 25°C. Thus, under physiological conditions approximately half of the HOCl is present in the protonated form and therefore able to diffuse through membranes. The remaining hypochlorite anion (−OCl) may transit though chloride channels, which allow the diffusion of other similarly sized anions such as −SCN and NO3− (Suzuki et al., 2006). Cells treated with HOCl swell prior to the loss of ATP (Ochoa et al., 1997), suggesting that the intracellular accumulation of −OCl is due to influx via chloride channels or to the cytosolic ionization of HOCl. A variety of cytosolic and intraorganellar moieties are also oxidized in cells exposed to HOCl at concentrations that preserve cellular integrity (Yap et al., 2010). In short, HOCl accesses both the cytosol and organelles and is therefore likely to chlorinate dopamine in these pools. Synaptic vesicles contain 0.3 M dopamine (Omiatek et al., 2013) a concentration that favors chlorination by HOCl (Jeitner et al., 2015). The formation of aggregates, however, would be retarded by acidic milieu of these vesicles (Roz and Rehavi, 2003). Release of chlorinated dopamine into the synapse—where the pH is near neutral—is likely to promote the formation of melanic polymers and aggregates. Such structures could then be phagocytized by microglia and stimulate inflammation (Jeitner et al., 2015).
Chlorodopamine affects at least 2 mitochondrial pathways: the inner mitochondrial membrane electron transport and the citrate cycle. The earliest effects of chlorodopamine on mitochondria are to increase O2 consumption and to cause swelling. These effects are likely due to the loss of cytochrome c from the inner mitochondrial membrane and a partial inhibition of complex III. The dissociation of cytochrome c was evident from the increase in O2 consumption following the addition of exogenous cytochrome c. Inhibition of complex III was inferred from the initial increase in respiration due to chlorodopamine acting on mitochondria respiring on either glutamate/malate or succinate. Electrons can be induced to flow in a reverse direction (back flow) within the mitochondrial electron transport chain by certain inhibitors (Zoccarato et al., 2009). These electrons exit the chain through complex I to reduce O2 to superoxide, which in turn, undergoes dismutation to hydrogen peroxide. The latter specie is one of 3 possible candidates as causes for the swelling of mitochondria by chlorodopamine (Kakkar et al., 1998). In addition to the generation of hydrogen peroxide, chlorodopamine could damage the outer membrane by chlorination of its lipidaceous constituents (Nusshold et al., 2010). Finally, the chlorinated forms of dopamine might activate opening of the mitochondrial permeability pore, as has been reported for HOCl (Whiteman et al., 2005b). The swelling of isolated mitochondria by chlorodopamine and HOCl was also observed in cells treated with these compounds.
Chlorodopamine inhibited complex IV as determined by the restoration of O2 utilization by the additions of both DTT and cytochrome c. Illumination of SH SY5Y cells with h670 nm also mitigated the toxicity of chlorodopamine to these cells, consistent with the inhibition of complex IV (Wong-Riley et al., 2005). The restoration of respiration following treatment of chlorodopamine-affected mitochondria with DTT and cytochrome c was comparable to O2 consumption by uncoupled mitochondria. One explanation for this phenomenon is damage to complex V that uncouples proton conductance through the complex with the phosphorylation of ADP. KGDH complex activity was also inhibited in accordance with our earlier observations that HOCl and N-chloramines inhibit the activity of the purified and cellular forms of this complex (Jeitner et al., 2005). The effects of chlorodopamine on mitochondria, while widespread, were selective relative to the actions of HOCl on these organelles, as reported here and elsewhere (Whiteman et al., 2005b). Given the multiplicity of mitochondrial entities affected by chlorodopamine, we elected to investigate these in later studies using the individual forms of chlorinated dopamine. If some entities (eg, complexes III and IV) are uniquely damaged by these chlorinated molecules, they may serve as indices of the generation of chlorodopamine in vivo and thus account for some cases of idiopathic PD.
Cerebral inflammation plays a crucial role in propagating parkinsonism and PD (Wilms et al., 2007). This process is mediated, to a large extent, by TNFα. One of the major actions of TNFα in PD is to activate microglia. Intrastriatal administration of chlorodopamine stimulated the production of TNFα. This index of inflammation was measured in brain homogenates and is likely to underestimate elevations in TNFα within specific nuclei. The intrastriatal injection of chlorinated melanin also stimulated the production of TNFα in mice (Jeitner et al., 2015).
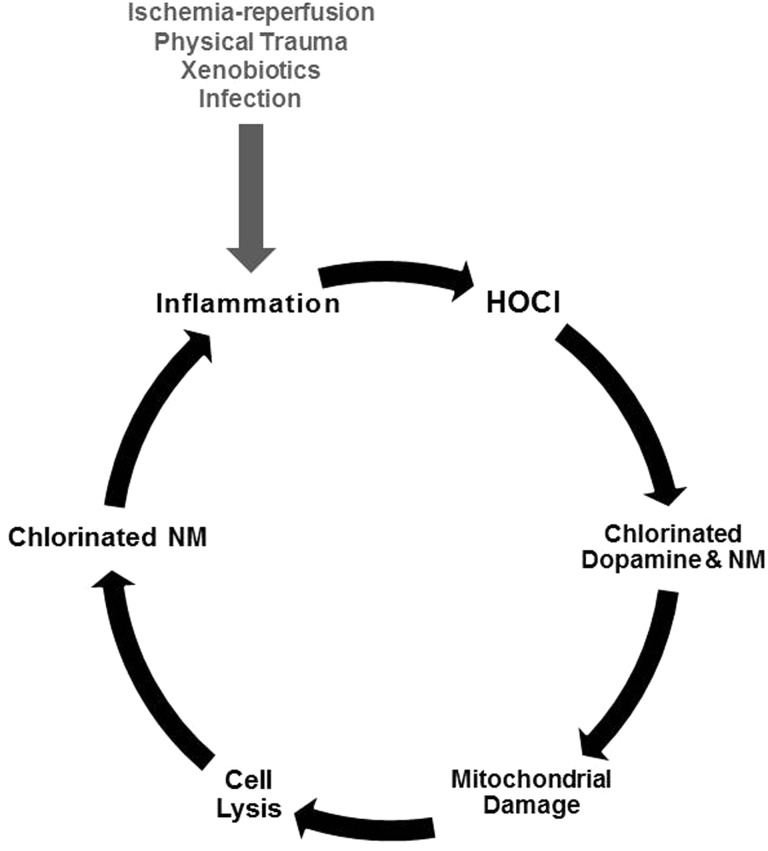
The above observations support our hypothesis that chlorination of dopamine by HOCl result in the formation of selective poisons of dopaminergic neurons. Given the densities of microglia and dopaminergic neurons in the substantia nigra, the generation of chlorinated forms of dopamine is more likely in this nucleus of the brain and could lead to PD. The studies present here and elsewhere (Jeitner et al., 2015) indicate that the chlorodopamine acts both directly and indirectly to kill neurons according to the model depicted in Figure 11. This model postulates that the activation of glial cells in substantia nigra by microstrokes, physical trauma, infections, or xenobiotics results in the aberrant expression of myeloperoxidase. Hydrogen peroxide is readily available in this region of the brain (Spanos et al., 2013), and thus, a portion of the peroxide will be reduced by myeloperoxidase leading to the production of HOCl. As described earlier, the brain has relatively poor cellular defenses against HOCl. Some of the HOCl will therefore escape to the interstitial spaces and react with dopamine within the synapses bordered by dopaminergic neurons. Chlorodopamine could then enter dopaminergic cells through the DAT and poison the mitochondria. Another important cellular target for HOCl is neuromelanin (NM) present in the neurons vulnerable to PD. Loss of mitochondrial energy would then lead to cell lysis and the release of chlorinated NM, which as we have shown may promote inflammation (Jeitner et al., 2015). This model provides a mechanism for the propagation of PD that relies on the continual stimulation of inflammation by chlorinated NM, released from neurons poisoned by chlorinated forms of dopamine. As noted above, chlorinated melanins could also arise from the exocytosis of synaptic chlorodopamine and its subsequent polymerization within the synapse.
FIG. 11.
Model for the etiology of some cases of idiopathic PD. The model postulates that microglia in the substantia nigra become aberrantly activated by a variety of events implicated in PD etiology: physical trauma, inflammation, exposure to xenobiotics, strokes, and others. This activation leads to the anomalous expression of myeloperoxidase. Reduction of ambient H2O2 by this enzyme results in the production of HOCl, which chlorinates both dopamine and MN. Extracellular chlorodopamine is then enters the cells via passage through the DAT, and together with cytoplasmic chlorodopamine, damages mitochondria. The consequent loss of ATP leads to cell lysis and the release of chlorinated neuromelanin (NM). Another form of chlorinated NM arises from the release of synaptic chlorodopamine into the synapse. This release involves the transition of chlorodopamine from and acidic to neutral environment, which promotes the polymerization and aggregation of chlorodopamine. The chlorinated NMs then activate local inflammatory cells, and thereby, initiate new cycles of inflammation and cell death.
One of the important implications of these studies is that idiopathic PD could arise from certain inflammatory events in the brain. This implies that it may be possible to diagnose the nonheritable forms of PD by imaging inflammation in the brain, and studies are currently in progress (Jucaite et al., 2015). The chlorinated forms of dopamine may also be useful biomarkers and pharmacological targets of PD. Unlike most reactive oxygen species, the reactions of hypohalous acids can be elucidated by following the destinations of the halide (eg, 3-chlorotyrosine (Hazen et al., 1997) or chlorodopamine (Jeitner et al., 2015). Therefore, it may be possible to identify specific biomarkers for the reactions of chlorodopamine or methods for imaging this species. Hypohalous acids are readily scavenged by sulfur- and selenium-based antioxidants (Ashby et al., 2004; Ismael et al., 2015; Skaff et al., 2009), and may therefore be used to treat PD with known myeloperoxidase involvement. Thus, the chlorination of dopamine suggests exciting opportunities for the diagnosis and ready treatment of some forms of idiopathic PD.
FUNDING
National Institute of Neurological Disorders and Stroke (RO3-NS074286) and Theresa Pantnode Santmann Foundation Award to T.M.J.
ACKNOWLEDGMENTS
The authors are grateful to Dr Jim Delikatny, Dr Louis Ragolia, Dr Arthur Cooper, Dr Patricia Patrick, and Dr Kevin Battaile for their insightful comments on these studies and ensuring manuscript, as well as Jim Mathew, B.Sc.; Thomas Palaia, M.Sc; Ahmed Ibrahim, B.Sc.; and Shihir Sihna, M.D. for their assistance with the experiments. Professor C.K Surratt of the Mylan School of Pharmacy, Duquesne University provided the DAT-expressing COS-7 cells and the corresponding control cells.
REFERENCES
- Akatsu H., Hori A., Yamamoto T., Yoshida M., Mimuro M., Hashizume Y., Tooyama I., Yezdimer E. M. (2012). Transition metal abnormalities in progressive dementias. Biometals 25, 337–350. 10.1007/s10534-011-9504-8. [DOI] [PubMed] [Google Scholar]
- Angelides K. J., Hammes G. G. (1979). Structural and mechanistic studies of the alpha-ketoglutarate dehydrogenase multienzyme complex from Escherichia coli. Biochemistry 18, 5531–5537. [DOI] [PubMed] [Google Scholar]
- Ashby M. T., Carlson A. C., Scott M. J. (2004). Redox buffering of hypochlorous acid by thiocyanate in physiologic fluids. J. Am. Chem. Soc. 126, 15976–15977. 10.1021/ja0438361. [DOI] [PubMed] [Google Scholar]
- Bae S. K., Heo C. H., Choi D. J., Sen D., Joe E. H., Cho B. R., Kim H. M. (2013). A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in Parkinson's disease gene knockout astrocytes. J. Am. Chem. Soc. 135, 9915–9923. 10.1021/ja404004v. [DOI] [PubMed] [Google Scholar]
- Bisaglia M., Filograna R., Beltramini M., Bubacco L. (2014). Are dopamine derivatives implicated in the pathogenesis of Parkinson's disease? Ageing Res. Rev. 13, 107-14. 10.1016/j.arr.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Burke R. E., O’Malley K. (2013). Axon degeneration in Parkinson's disease. Exp. Neurol. 246, 72–83. 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. R., Greenamyre J. T. (2011). The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol. Sci. 124, 225–250. 10.1093/toxsci/kfr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. L., Klaidman L. K., Adams J. D. (1997). The effects of oxidative stress on in vivo brain GSH turnover in young and mature mice. Mol. Chem. Neuropathol. 30, 187–197. [DOI] [PubMed] [Google Scholar]
- Choi D. K., Pennathur S., Perier C., Tieu K., Teismann P., Wu D. C., Jackson-Lewis V., Vila M., Vonsattel J. P., Heinecke J. W., et al. (2005). Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson's disease in mice. J. Neurosci. 25, 6594–6600. 10.1523/JNEUROSCI.0970-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K. B, Paxinos G. (2008). The Mouse Brain in Stereotaxic Coordinates. Elsevier, New York. [Google Scholar]
- Gibson G. E., Sheu K. F., Blass J. P., Baker A., Carlson K. C., Harding B., Perrino P. (1988). Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer's disease. Arch. Neurol. 45, 836–840. [DOI] [PubMed] [Google Scholar]
- Giovanni A., Sonsalla P. K., Heikkila R. E. (1994). Studies on species sensitivity to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Part 2: Central administration of 1 dinium. J. Pharmacol. Exp. Ther. 270, 1008–1014. [PubMed] [Google Scholar]
- Green P. S., Mendez A. J., Jacob J. S., Crowley J. R., Growdon W., Hyman B. T., Heinecke J. W. (2004). Neuronal expression of myeloperoxidase is increased in Alzheimer's disease. J. Neurochem. 90, 724–733. 10.1111/j.1471-4159.2004.02527. x. [DOI] [PubMed] [Google Scholar]
- Greenamyre J. T., Cannon J. R., Drolet R., Mastroberardino P. G. (2010). Lessons from the rotenone model of Parkinson's disease. Trends Pharmacol. Sci. 31, 141–142. Author reply 142–143, 10.1016/j.tips.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood D. T., Nimmo S. L., Kettle A. J., Winterbourn C. C., Ashby M. T. (2008). Molecular structure and dynamic properties of a sulfonamide derivative of glutathione that is produced under conditions of oxidative stress by hypochlorous acid. Chem. Res. Toxicol. 21, 1011–1016. 10.1021/tx800050n. [DOI] [PubMed] [Google Scholar]
- Hawkins C. L., Pattison D. I., Davies M. J. (2003). Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids 25, 259–274. 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- Hazen S. L., Crowley J. R., Mueller D. M., Heinecke J. W. (1997). Mass spectrometric quantification of 3-chlorotyrosine in human tissues with attomole sensitivity: A sensitive and specific marker for myeloperoxidase-catalyzed chlorination at sites of inflammation. Free Radic. Biol. Med. 23, 909–916. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Suzuno H., Tsuruta T., Oh-hashi K., Kiuchi K. (2008). The role of dopamine transporter in selective toxicity of manganese and rotenone. Toxicology 244, 249–256. 10.1016/j.tox.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kohsaka S. (2002). Intracellular signaling in M-CSF-induced microglia activation: Role of Iba1. Glia 40, 164–174. 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- Ismael F. O., Proudfoot J. M., Brown B. E., van Reyk D. M., Croft K. D., Davies M. J., Hawkins C. L. (2015). Comparative reactivity of the myeloperoxidase-derived oxidants HOCl and HOSCN with low-density lipoprotein (LDL): Implications for foam cell formation in atherosclerosis. Arch. Biochem. Biophys. 573, 40–51. 10.1016/j.abb.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Jeitner T. M., Kalogiannis M., Mathew J. (2013). Preparation of 2-nitro-5-thiobenzoate for the routine determination of reagent hypochlorous acid concentrations. Anal. Biochem. 441, 180–181. 10.1016/j.ab.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeitner T. M., Kalogiannis M., Patrick P. A., Gomolin I., Palaia T., Ragolia L., Brand D., Delikatny E. J. (2015). Inflaming the diseased brain: A role for tainted melanins. Biochim. Biophys. Acta 1852, 937–950. 10.1016/j.bbadis.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeitner T. M., Xu H., Gibson G. E. (2005). Inhibition of the alpha-ketoglutarate dehydrogenase complex by the myeloperoxidase products, hypochlorous acid and mono-N-chloramine. J. Neurochem. 92, 302–310. 10.1111/j.1471-4159.2004.02868.x. [DOI] [PubMed] [Google Scholar]
- Jucaite A., Svenningsson P., Rinne J. O., Cselényi Z., Varnäs K., Johnström P., Amini N., Kirjavainen A., Helin S., Minkwitz M., et al. (2015). Effect of the myeloperoxidase inhibitor AZD3241 on microglia: A PET study in Parkinson's disease. Brain 138, 2687–2700. 10.1093/brain/awv184. [DOI] [PubMed] [Google Scholar]
- Kalogiannis M., Delikatny E. J., Jeitner T. M. (2016). Serotonin as a scavenger of hypochlorous acid in the brain. Biochim. Biophys. Acta. 1862, 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar P., Mehrotra S., Viswanathan P. N. (1998). Influence of antioxidants on the peroxidative swelling of mitochondria in vitro. Cell Biol. Toxicol. 14, 313–321. [DOI] [PubMed] [Google Scholar]
- Kim W. G., Mohney R. P., Wilson B., Jeohn G. H., Liu B., Hong J. S. (2000). Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: Role of microglia. J. Neurosci. 20, 6309–6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake Y., Ohta S. (2003). MPP+ analogs acting on mitochondria and inducing neuro-degeneration. Curr. Med. Chem. 10, 2507–2516. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Schuller H. M., Smith A. C., Boyd M. R. (1985). Effects of buthionine sulfoximine on the nephrotoxicity of 1-(2-chloroethyl)-3-(trans-4-methylcyclohexyl)-1-nitrosourea (MeCCNU). J. Pharmacol. Exp. Ther. 234, 498–506. [PubMed] [Google Scholar]
- Krasnikov B. F., Melik-Nubarov N. S., Zorova L. D., Kuzminova A. E., Isaev N. K., Cooper A. J., Zorov D. B. (2011). Synthetic and natural polyanions induce cytochrome c release from mitochondria in vitro and in situ. Am. J. Physiol. Cell Physiol. 300, C1193–C1203. 10.1152/ajpcell.00519.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnikov B. F., Zorov D. B., Antonenko Y. N., Zaspa A. A., Kulikov I. V., Kristal B. S., Cooper A. J., Brown A. M. (2005). Comparative kinetic analysis reveals that inducer-specific ion release precedes the mitochondrial permeability transition. Biochim. Biophys. Acta 1708, 375–392. 10.1016/j.bbabio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Laloux C., Derambure P., Kreisler A., Houdayer E., Bruezière S., Bordet R., Destée A., Monaca C. (2008). MPTP-treated mice: Long-lasting loss of nigral TH-ir neurons but not paradoxical sleep alterations. Exp. Brain Res. 186, 635–642. 10.1007/s00221-008-1268-1. [DOI] [PubMed] [Google Scholar]
- Langston J. W., Ballard P., Tetrud J. W., Irwin I. (1983). Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980. [DOI] [PubMed] [Google Scholar]
- Lehmensiek V., Tan E. M., Liebau S., Lenk T., Zettlmeisl H., Schwarz J., Storch A. (2006). Dopamine transporter-mediated cytotoxicity of 6-hydroxydopamine in vitro depends on expression of mutant alpha-synucleins related to Parkinson's disease. Neurochem. Int. 48, 329–340. 10.1016/j.neuint.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Maki R. A., Tyurin V. A., Lyon R. C., Hamilton R. L., DeKosky S. T., Kagan V. E., Reynolds W. F. (2009). Aberrant expression of myeloperoxidase in astrocytes promotes phospholipid oxidation and memory deficits in a mouse model of Alzheimer disease. J. Biol. Chem. 284, 3158–3169. 10.1074/jbc.M807731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov E. V., Gong L. W., Khanna B., Sulzer D., Lindau M. (2003). Intracellular patch electrochemistry: Regulation of cytosolic catecholamines in chromaffin cells. J. Neurosci. 23, 5835–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakadate K., Tanaka-Nakadate S. (2015). Three-dimensional electron microscopy reconstruction of degenerative dopaminergic neurons surrounded by activated microglia in substantia nigra. Ultrastruct. Pathol. 39, 1–9. 10.3109/01913123.2015.1042609. [DOI] [PubMed] [Google Scholar]
- Nusshold C., Kollroser M., Köfeler H., Rechberger G., Reicher H., Ullen A., Bernhart E., Waltl S., Kratzer I., Hermetter A., et al. (2010). Hypochlorite modification of sphingomyelin generates chlorinated lipid species that induce apoptosis and proteome alterations in dopaminergic PC12 neurons in vitro. Free Radic. Biol. Med. 48, 1588–1600. 10.1016/j.freeradbiomed.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa L., Waypa G., Mahoney J. R., Rodriguez L., Minnear F. L. (1997). Contrasting effects of hypochlorous acid and hydrogen peroxide on endothelial permeability: Prevention with cAMP drugs. Am. J. Respir. Crit. Care Med. 156(4 Pt 1), 1247–1255. 10.1164/ajrccm.156.4.96-10115. [DOI] [PubMed] [Google Scholar]
- Omiatek D. M., Bressler A. J., Cans A. S., Andrews A. M., Heien M. L., Ewing A. G. (2013). The real catecholamine content of secretory vesicles in the CNS revealed by electrochemical cytometry. Sci. Rep. 3, 1447. 10.1038/srep01447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappius H. M. (1969). Water spaces In Structural Neurochemistry (Abel Lajtha N. S., Ed.), Vol. II, pp. 5 Springer, New York. [Google Scholar]
- Peskin A. V., Winterbourn C. C. (2001). Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radic. Biol. Med. 30, 572–579. [DOI] [PubMed] [Google Scholar]
- Pham A. N., Waite T. D. (2014). Cu(II)-catalyzed oxidation of dopamine in aqueous solutions: Mechanism and kinetics. J. Inorg. Biochem. 137, 74–84. 10.1016/j.jinorgbio.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Rappold P. M., Cui M., Chesser A. S., Tibbett J., Grima J. C., Duan L., Sen N., Javitch J. A., Tieu K. (2011). Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc. Natl Acad. Sci. U. S. A. 108, 20766–20771. 10.1073/pnas.1115141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roz N., Rehavi M. (2003). Hyperforin inhibits vesicular uptake of monoamines by dissipating pH gradient across synaptic vesicle membrane. Life Sci. 73, 461–470. [DOI] [PubMed] [Google Scholar]
- Saran M., Beck-Speier I., Fellerhoff B., Bauer G. (1999). Phagocytic killing of microorganisms by radical processes: Consequences of the reaction of hydroxyl radicals with chloride yielding chlorine atoms. Free Radic. Biol. Med. 26, 482–490. [DOI] [PubMed] [Google Scholar]
- Schmidt A. C., Wang X., Zhu Y., Sombers L. A. (2013). Carbon nanotube yarn electrodes for enhanced detection of neurotransmitter dynamics in live brain tissue. ACS Nano 7, 7864–7873. 10.1021/nn402857u. [DOI] [PubMed] [Google Scholar]
- Skaff O., Pattison D. I., Davies M. J. (2009). Hypothiocyanous acid reactivity with low-molecular-mass and protein thiols: Absolute rate constants and assessment of biological relevance. Biochem. J. 422, 111–117. 10.1042/BJ20090276. [DOI] [PubMed] [Google Scholar]
- Spanos M., Gras-Najjar J., Letchworth J. M., Sanford A. L., Toups J. V., Sombers L. A. (2013). Quantitation of hydrogen peroxide fluctuations and their modulation of dopamine dynamics in the rat dorsal striatum using fast-scan cyclic voltammetry. ACS Chem. Neurosci. 4, 782–789. 10.1021/cn4000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkey C., Davies M. J., Pattison D. I. (2014). Reevaluation of the rate constants for the reaction of hypochlorous acid (HOCl) with cysteine, methionine, and peptide derivatives using a new competition kinetic approach. Free Radic. Biol. Med. 73, 60–66. 10.1016/j.freeradbiomed.2014.04.024. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Morita T., Iwamoto T. (2006). Diversity of Cl(-) channels. Cell Mol. Life Sci. 63, 12–24. 10.1007/s00018-005-5336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukairo O. T., Ramanujapuram S., Surratt C. K. (2007). Fluctuation of the dopamine uptake inhibition potency of cocaine, but not amphetamine, at mammalian cells expressing the dopamine transporter. Brain Res. 1131(1), 68–76. 10.1016/j.brainres.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman M., Cheung N. S., Zhu Y. Z., Chu S. H., Siau J. L., Wong B. S., Armstrong J. S., Moore P. K. (2005a). Hydrogen sulphide: A novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem. Biophys. Res. Commun. 326, 794–798. 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- Whiteman M., Rose P., Siau J. L., Cheung N. S., Tan G. S., Halliwell B., Armstrong J. S. (2005b). Hypochlorous acid-mediated mitochondrial dysfunction and apoptosis in human hepatoma HepG2 and human fetal liver cells: Role of mitochondrial permeability transition. Free Radic. Biol. Med. 38, 1571–1584. 10.1016/j.freeradbiomed.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Wilms H., Zecca L., Rosenstiel P., Sievers J., Deuschl G., Lucius R. (2007). Inflammation in Parkinson's diseases and other neurodegenerative diseases: Cause and therapeutic implications. Curr. Pharm. Des. 13, 1925–1928. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Kettle A. J. (2013). Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal 18, 642–660. 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. T., Liang H. L., Eells J. T., Chance B., Henry M. M., Buchmann E., Kane M., Whelan H. T. (2005). Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J. Biol. Chem. 280, 4761–4771. 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- Woods L. A., Powell P. R., Paxon T. L., Ewing A. G. (2005). Analysis of mammalian cell cytoplasm with electrophoresis in nanometer inner diameter capillaries. Electroanalysis 17, 1192–1197. 10.1002/elan.200403240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap Y. W., Chen M. J., Choy M. S., Peng Z. F., Whiteman M., Manikandan J., Melendez A. J., Cheung N. S. (2010). Temporal transcriptomic profiling reveals cellular targets that govern survival in HOCl-mediated neuronal apoptosis. Life Sci. 87, 457–467, 10.1016/j. lfs.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Zoccarato F., Cavallini L., Alexandre A. (2009). Succinate is the controller of O2-/H2O2 release at mitochondrial complex I: Negative modulation by malate, positive by cyanide. J. Bioenerg. Biomembr. 41, 387–393. 10.1007/s10863-009-9238-2. tpd. [DOI] [PubMed] [Google Scholar]