Abstract
Exposure of rodents to a stimulating environment has beneficial effects on some cognitive functions that are impaired during physiological aging, and especially spatial reference memory. The present study investigated whether environmental enrichment rescues these functions in already declining subjects and/or protects them from subsequent decline. Subgroups of 17-mo-old female rats with unimpaired versus impaired performance in a spatial reference memory task (Morris water maze) were housed until the age of 24 mo in standard or enriched environment. They were then trained in a second reference memory task, conducted in a different room than the first, and recent (1 d) and remote (10 d) memory were assessed. In unimpaired subgroups, spatial memory declined from 17 to 24 mo in rats housed in standard conditions; an enriched environment during this period allowed maintenance of accurate recent and remote spatial memory. At 24 mo, rats impaired at the age of 17 mo housed in enriched environment learned the task and displayed substantial recent memory, but their performance remained lower than that of unimpaired rats, showing that enrichment failed to rescue spatial memory in already cognitively declining rats. Controls indicated carryover effects of the first water maze training, especially in aged rats housed in standard condition, and confirmed the beneficial effect of enrichment on remote memory of aged rats even if they performed poorly than young adults housed for the same duration in standard or enriched condition.
Enriched environment housing provides rodents with enhanced sensory, motor, and cognitive stimulation and more sustained social interaction when compared with standard laboratory housing conditions. Exposure to an enriched environment during adulthood modifies many aspects of rodent behavior and improves learning and memory in a variety of tasks, especially those assessing spatial memory (e.g., Schrijver et al. 2002; Leggio et al. 2005; Birch et al. 2013; Hullinger et al. 2015; Mora-Gallegos et al. 2015). Evaluation of the effects of environmental enrichment on spatial memory is especially relevant in the context of physiological aging, as enrichment was shown to stimulate neuroplasticity in brain areas playing a major role in this cognitive domain (e.g., Faherty et al. 2003; Leggio et al. 2005; Birch et al. 2013; Hullinger et al. 2015), which is particularly sensitive to aging (Gallagher et al. 2006). Several studies using cross-sectional experimental designs showed that spatial memory progressively declines during adulthood. Thus, performances of middle-aged subjects are impaired compared with those of younger adults, but less severely than those of elderly subjects (e.g., Wyss et al. 2000; Bizon et al. 2009; Harati et al. 2013). Conversely, impaired spatial memory is not a systematic hallmark of physiological aging and many experiments have highlighted the heterogeneity of individual performance at a given chronological age (e.g., Gage et al. 1984; Gallagher et al. 1993; Hullinger and Burger 2015; McQuail and Nicolle 2015). Interestingly, several studies showed that a sustained exposure of middle-aged rodents to an enriched environment mitigated spatial memory deficit in old age (e.g., Kobayashi et al. 2002; Harburger et al. 2007; Freret et al. 2012; Kumar et al. 2012; Fuchs et al. 2016; but Bouet et al. 2011). However, whether housing middle-aged rodents in a more stimulating environment rescues spatial memory in already cognitively declining subjects or protects against further decline remains to be explored.
The current study assessed the impact of enriched environment housing on spatial learning and memory in aged rats in which these capabilities were either conserved or already impaired at middle age. For this longitudinal study, middle-aged (17 mo) rats were trained in a first reference memory task in a Morris water maze (i.e., finding an escape platform located in a fixed position in a pool), a place-learning task widely used to distinguish individuals with or without impaired learning and memory during aging (e.g., Gallagher et al. 2006; Cassel et al. 2007). Similar number of rats thereby classified as either unimpaired or impaired were subsequently housed for 6 mo in enriched environment (EE) or standard conditions (SC). At the age of 24 mo, the reference memory capabilities of these now aged rats were again assessed, in a new environment. As a control for the effects of primary training, 18-mo-old naive rats of the same cohort and 2-mo-old rats were randomly housed for 6 mo in either SC or EE and then tested (at the age of 24 and 8 mo, respectively) on the same reference memory task (cross-sectional study). The experimental design is illustrated in Figure 1.
Figure 1.
Experimental design. Middle-aged (17 mo) rats were trained in a first reference memory task (Morris water maze 1). Similar number of rats thereby classified as either unimpaired (MA-UI) or impaired (MA-I) were subsequently housed for 6 mo in standard conditions (SC-UI and SC-I, gray boxes) or enriched environment (EE-UI and EE-I, white boxes). At the age of 24 mo, the reference memory capabilities of these now aged rats were again assessed (Morris water maze 2 for the longitudinal study). As a control for the effects of primary training, 18-mo-old naive rats of the same cohort and 2-mo-old rats were randomly housed for 6 mo in either standard (A-SC and Y-SC) or enriched conditions (A-EE and Y-EE) and then tested (at the age of 24 and 8 mo, respectively) on the same reference memory task (Morris water maze 2 for the cross-sectional study).
Results
Classification of middle-aged rats before differentiated housing
A group of middle-aged rats (17 mo old, n = 56) was trained on a place-learning task (Fig. 1, Morris water maze task 1; a photograph of the testing room is given in Supplemental Fig. 1A) using a procedure sensitive to age-related spatial learning deficit in SC-housed rats (e.g., Harati et al. 2011). During five training days, a submerged platform was located at an unchanged position in a water maze; the following day, the platform was removed and time spent in each quadrant of the pool was monitored for 30 sec (probe trial). To distinguish subgroups of rats displaying contrasting spatial memory capabilities in the cohort of middle-aged animals, we used a measure of platform search accuracy during this probe trial rather than measures of performance during training, as nonspatial strategies might lead to performance improvement during training (e.g., Cassel et al. 2007; Rutz et al. 2009). This measure was the platform search profile, which was established for each rat as follows: time spent in the quadrant in which the platform had been located during training (target) and, from longest to shortest, times spent in the other quadrants (Q2 to Q4). These profiles were used to divide the population into two subgroups (Fig. 2A, left part) on cluster analysis (K-mean clustering on the time spent in each quadrant). In one subgroup, rats spent more time in the target than in any of the other quadrants (F(3,66) = 73.54, P < 0.0001; P < 0.001 for each comparison). The high specificity of this search profile suggested conserved long-term spatial memory, and this subgroup was named “middle-aged unimpaired” (MA-UI, n = 23). In the other subgroup, rats spent more time in another quadrant than in all others, including the target (F(3,96) = 45.35, P < 0.0001; P < 0.0001 for each comparison), indicating impaired spatial capability; this subgroup was named “middle-aged impaired” (MA-I, n = 33). Swimming speed was similar in these two subgroups (F(1,54) = 0.18, P > 0.05; data not shown). In order to assess whether such an impaired search profile might emerge during aging, data previously obtained in a population of young adult rats (4–5 mo, n = 34; data collected over a 6-yr period from three cohorts of rats of the same strain, sex, and breeder, housed in SC and trained in the water maze with the same material and procedure as those of the current study) were subjected to the same analysis. In this young-adult population (Fig. 2A, right part), the search profile of one subgroup was highly specific (Y-H, n = 15), as rats spent more time in the target than in each of the other quadrants (F(3,42) = 95.70, P < 0.0001; P < 0.001 for each comparison), like the MA-UI subgroup. In the other subgroup, search was less specific (Y-L, n = 19). Indeed, rats of this subgroup spent an equivalent time in the target and one of the other quadrants, but more time in the target than in the other two quadrants (F(3,54) = 68.18, P < 0.0001; P < 0.001 for each comparison), suggesting less accurate memory of platform location without, however, showing the search bias observed in the MA-I subgroup: i.e., specific search in a wrong quadrant. To confirm that this latter subgroup was impaired with respect to younger animals, we compared variables reflecting platform search accuracy during the probe trial (time spent in the target quadrant, and proximity: i.e., mean distance from the previous platform location) obtained in the young adults to those obtained in middle-aged rats of the current study. Statistical analysis indicated that, as a whole, the middle-age group was impaired when compared with young adults (Fig. 2B; time spent in the target quadrant: F(1,88) = 8.44, P < 0.005; proximity: F(1,88) = 6.40, P < 0.05). However, MA-I rats were the only ones to differ from young adults (time spent in the target quadrant: F(2,87) = 53.10, P < 0.001; MA-I versus the two other groups, P < 0.01; proximity: F(2,87) = 33.96, P < 0.001; MA-I versus the two other groups, P < 0.01).
Figure 2.
Classification of middle-aged rats before differentiated housing. (A) Time spent in the four quadrants during the probe trial in middle-aged (MA, left part) and young adult (Y, right part) rats: time spent in the quadrant in which the platform had been located during training (Target) and, from longest to shortest, times spent in the other quadrants (Q2–Q4). MA rats were characterized according to their search profile (see text) as unimpaired (UI) or impaired (I) and Y rats as highly specific (H) or less specific (L). The dashed line indicates chance level. (B) Individual differences in the probe trial in MA (UI and I) and Y groups: time spent in the target quadrant (left part) and proximity (right part). The horizontal line represents the mean of the two groups of age. (C) Performance during the training period in the two subgroups of MA rats: distance swam to the platform (left part) and percentage thigmotaxis (right part). (D) Performance during the training period in the two subgroups of Y rats: distance swam to the platform (left part) and percentage thigmotaxis (right part). Data are mean + SEM. Statistics: *, difference between time spent in the target quadrant and time spent in the other three quadrants; µ, difference between time spent in this quadrant and time spent in all the others including the target quadrant; &, difference between MA-I and the other groups (MA-UI and Y). For detailed statistical description see text.
We then analyzed whether the subgroups of rats identified by K-mean clustering had also displayed differences in performance during the 5 d of training. In the middle-aged population, MA-UI outperformed MA-I (Fig. 2C). ANOVA indicated a main effect of Subgroup for distance swam to reach the platform (F(1,54) = 14.36, P < 0.001), thigmotaxis (F(1,54) = 11.04, P < 0.01), and number of successful trials (data not shown; F(1,54) = 9.88, P < 0.01), but performance improvement during training was similar in the two subgroups for these three variables [no Subgroup × training Day interaction, but a main effect of Day for distance (F(4,216) = 33.04), thigmotaxis (F(4,216) = 84.76), and number of successful trials (F(4,216) = 33.24); P < 0.0001]. In contrast, in the young-adult population, no differences were observed between the Y-H and Y-L subgroups during training, whatever the variable analyzed. As illustrated in Figure 2D, Y-H and Y-L displayed similar overall performance and improvement throughout training (main effect of Day: F(4,128) = 56.20, P < 0.0001 for distance swam to reach the platform and F(4,128) = 141.68, P < 0.0001 for thigmotaxis; no significant effects of Subgroup or Subgroup × Day interaction). Their contrasted platform search profiles during the probe test further underlined the importance of a probe trial to assess spatial memory accuracy.
Late enrichment has beneficial effects on platform search accuracy, but only in rats unimpaired when middle aged
After completion of behavioral testing, half of the middle-aged rats of each subgroup were housed in SC and the other half in EE until they were 24 mo old (surviving rats, n = 41). These four groups were referred to as “SC-I,” “SC-UI,” “EE-I,” and “EE-UI” according to their performance at middle age and their late housing condition. Rats were then trained in a second place learning task (Fig. 1, Morris water maze 2 for the longitudinal study; a photograph of the testing room is given in Supplemental Fig. 1B) to assess the impact of this late enrichment on recent and remote memory of aged rats according to their performance when middle aged. To this aim, we used a more extensive training procedure than the one used at 17 mo, as 5 d of training are not sufficient to allow spatial recent memory in aged rats (e.g., Harati et al. 2011; Fuchs et al. 2016). This training procedure was adapted from previous works (F Fuchs et al. unpublished data for aged rats; e.g., Lopez et al. 2008 for adult rats), and consisted of one session with a visible platform followed by eight sessions with the hidden platform. The improvement of performances across the four trials of the visible platform session (i.e., the decrease of the distance swam to reach the platform; data not shown) was similar in all four groups (effect of Trial: F(3,111) = 23.60, P < 0.001; no effect of Housing: F(1,37) = 0.04, P > 0.05; no effect of Subgroup: F(1,37) = 0.006, P > 0.05; and no interaction between these three factors: F(3,111) = 2.13, P > 0.05). Data for training with the hidden platform are illustrated in Figure 3. Analysis of the distance swam to reach the platform (Fig. 3A) indicated that overall distance was shorter in the aged UI subgroup than in the I subgroup (F(1,37) = 4.30, P < 0.05). No difference according to subgroup was observed for thigmotaxis (Fig. 3B), although analyzing successful trials (Fig. 3C) showed subgroup difference that was nearly significant (F(1,37) = 3.22, P = 0.081). Overall performance improved during training (Day; F(7,259) = 11.89, P < 0.0001; F(7,259) = 3.40, P < 0.01; F(7,259) = 7.21, P < 0.0001 for each variable, respectively) at similar rates in all four groups (no significant interactions between Subgroup and Housing condition). The last analysis conducted on the data collected during training assessed the impact of enrichment on long-term and short-term memory components of daily performance improvement. A short-term memory index (mean of the last two daily trials) and a long-term memory index (mean of the first daily trial) were computed for the distance swam to reach the platform from the second to the last training day (adapted from George et al. 2006). Analysis of these memory indices (Fig. 3D) found no difference between subgroups for short-term memory, whereas UI rats tended to show better long-term memory (F(1,37) = 3.97, P = 0.053). Importantly, these analyses showed that Housing had no significant effect on performance during training.
Figure 3.
Impact of late housing condition on training performance in aged rats according to their performance at middle age. (A) Distance swam to reach the platform. (B) Successful trials as percentage of total trials. (C) Percentage thigmotaxis time. (D) Short-term (left part) and long-term (right part) memory indices. Data are mean + SEM. Statistic: For detailed statistical description see text. SC, aged rats housed in standard condition; EE, aged rats housed in enriched environment; UI and I, their probe performance at 17 mo of age.
Two 30-sec probe trials were conducted to assess recent (1 d after the last training day) then remote memory of platform location (10 d after four additional trials conducted immediately after the first probe test). Data for these probe trials are illustrated in Figure 4, alongside the platform search profile obtained at middle age in the same rats (Fig. 4A). For recent memory, the UI subgroup outperformed the I subgroup for time spent in the target quadrant (Fig. 4B; F(1,37) = 8.18, P < 0.01) and proximity (Fig. 4C; F(1,37) = 8.05, P < 0.01), target area crossings being very low in both subgroups (Fig. 4D). Moreover, post hoc comparison conducted on the nearly significant interaction between Subgroup and Housing condition for time spent in the target quadrant (F(1,37) = 3.2, P = 0.07) showed that UI rats outperformed I rats only in the EE group (P < 0.05). Only EE-UI group displayed a specific platform search profile, as at the age of 17 mo: time spent in the target quadrant was significantly greater than time spent in any other quadrant (P < 0.001 for each comparison), suggesting that recent memory for platform location was conserved. In contrast, SC-UI, SC-I, and EE-I rats showed lower accuracy in platform search, spending more time in both the target and another quadrant than in the two others (P < 0.05). However, rats in these groups, as EE-UI rats, spent significantly longer time than chance in the target quadrant (P < 0.05). In the remote memory test, subgroup differences were no longer observed (Fig. 4E–G). Search profile analysis indicated degraded memory of platform location. In the SC-UI, SC-I, and EE-I groups, time spent in the target quadrant only differed from that spent in the fourth most visited quadrant (P < 0.01). However, platform search was more specific in the EE-UI group and, although time spent in the target quadrant failed to differ significantly from time spent in the second most visited quadrant (P = 0.08), it differed from time spent in the third and fourth most visited quadrant (P < 0.01). Only the EE-UI group spent significantly longer than chance in the target quadrant on the remote probe test (P < 0.05), further confirming the beneficial effects of late enrichment on spatial memory in this group. Swimming speed was similar in the four groups of animals during the two probe trials (data not shown) with no effect of housing (first probe trial: F(1,37) = 2.88, P > 0.05; second probe trial: F(1,37) = 1.43, P > 0.05), no effect of subgroup (first probe trial: F(1,37) = 0.26, P > 0.05; second probe trial: F(1,37) = 0.20, P > 0.05) and no interaction between these two factors (first probe trial: F(1,37) = 0.40, P > 0.05; second probe trial: F(1,37) = 0.18, P > 0.05).
Figure 4.
Platform search profile during the probe trial for recent memory performed at 17 mo in rats that survived until 24 mo (A) and probe trial performances of the same rats at the age of 24 mo during assessment of recent (B–D) and remote (E–G) memory. (A,B,E) Time spent in the four quadrants. The dashed line indicates chance level. (C,F) Proximity (average distance to the platform). (D,G) Number of target area crossings. Data are mean + SEM. Statistics: *, difference between time spent in the target quadrant and time spent in the other three quadrants; µ, difference between time spent in this quadrant and time spent in all the others including the target quadrant; £, significantly above chance level; $, significantly different from EE-I. For detailed statistical description, see text. MA, middle aged; SC, aged rats housed in standard condition; EE, aged rats housed in enriched environment; UI and I, their probe performance at 17 mo of age.
Six-months’ EE housing in young adult and middle-aged naive rats induces beneficial effects on place learning
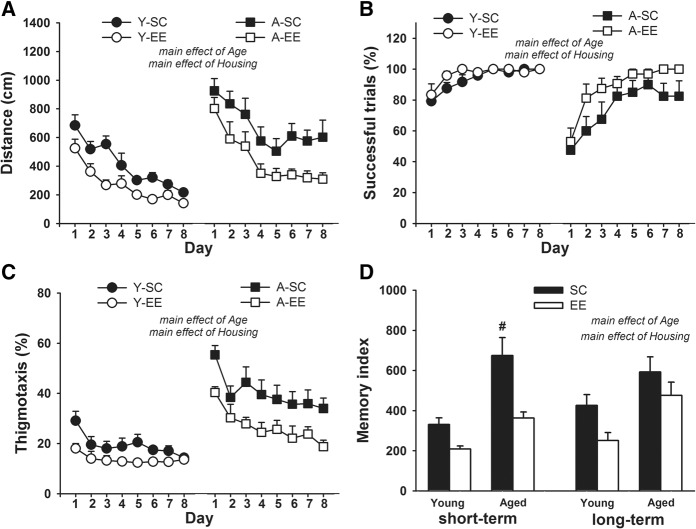
The previous analyses showed that late enrichment did not improve performances during training. As a control for the effects of primary training, naive rats of the same cohort and 2-mo-old rats were randomly housed for 6 mo in either SC or EE and then tested (at the age of 24 and 8 mo, respectively) on the same reference memory task (Fig. 1, Morris water maze 2 for the cross-sectional study). The improvement of performances across the four trials of the visible platform session (data not shown) was similar in all groups (no significant interaction between “Trial” and the other factors) whereas the distance swam was globally higher in aged rats than in young adults (Age, F(1,38) = 5.79, P < 0.05) and in SC than in EE rats (Housing, F(1,38) = 4.95, P < 0.05; no interaction between Age and Housing). The data obtained during the training with the hidden platform are presented in Figure 5. The overall distance swam to reach the platform (Fig. 5A) was shorter in EE than SC groups (Housing, F(1,38) = 18.48, P < 0.001) and in young adult than aged groups (Age, F(1,38) = 26.95, P < 0.0001). The improving effect of enrichment seemed greater in aged groups, but statistical analysis failed to support this impression (no significant Housing × Age interaction). Performances improved at similar rates in all groups (Day, F(7,266) = 30.49, P < 0.0001; no significant interaction between Day and the other factors). Similarly, statistical analysis of successful trials (Fig. 5B) and thigmotaxis (Fig. 5C) indicated significant effects of Day (F(7,266) = 27.73, P < 0.0001 and F(7,266) = 15.85, P < 0.0001, respectively), Housing (F(1,38) = 5.55, P < 0.05 and F(1,38) = 13.52, P < 0.001, respectively), and Age (F(1,38) = 15.95, P < 0.001 and F(1,38) = 41.98, P < 0.0001, respectively), but no significant interaction between Housing and Age. However, a significant interaction between Age and Day for each of these two variables (F(7,266) = 3.85, P < 0.001 and F(7,266) = 2.65, P < 0.05, respectively) indicated that young adult and aged rats improved at different rates; post hoc comparisons showed that successful trials increased only from the first to the second day of training in young adult groups (P < 0.05), but until the third day in aged groups (P < 0.05), indicating that optimal performance levels were more rapidly reached in young adults. There was also no significant decrease in thigmotaxis after the third day in young adult groups (P < 0.05 from Day 1 to Day 3), whereas thigmotaxis continued to decrease from Day 3 to Day 7 in aged groups (P < 0.05), remaining significantly higher than in young adult groups until the last training day (P < 0.05). As illustrated in Figure 5D, enrichment had a major impact on age-related impairment of short-term memory assessed by the index: the SC aged group was greatly impaired when compared with its young adult counterpart, but this age-related deficit was reversed by enrichment (Housing, F(1,38) = 25.56, P < 0.0001; Age, F(1,38) = 19,40, P < 0.0001; Housing × Age, F(1,38) = 3.69, P = 0.06; post hoc P < 0.001 for each comparison). Figure 5D seems to suggest that the impact of enrichment on long-term memory index was more limited in aged group, but also less affected by aging; however, analysis revealed significant effects of Housing (F(1,38) = 6.13, P < 0.05) and Age (F(1,38) = 11.10, P < 0.01), but no interaction between the two. These results clearly show that enrichment had a beneficial effect in naive rats, and suggest that its failure to ameliorate training performance in aged rats exposed to a first reference memory task when middle aged, might be due to a carryover effect of the first training in SC rats. Figures 3 and 5 clearly show that it was mainly SC aged rats that benefited from pretraining; complementary analyses (Supplemental data) indicated that pretraining significantly reduced the distance swam to reach the platform, increased the number of successful trials, reduced thigmotaxis and improved short-term memory index only in SC rats (Supplemental Fig. 2).
Figure 5.
Effect of 6 months’ environmental enrichment on training performance in young adult and aged naive rats. (A) Distance swam to reach the platform. (B) Successful trials as percentage of total trials. (C) Percentage thigmotaxis time. (D) Short-term (left part) and long-term (right part) memory indices. Data are mean + SEM. Statistic: #, significantly different from all other groups. For detailed statistical description see text. Y, young adult rats; A, aged rats; SC, rats housed in standard condition; EE, rats housed in enriched environment.
Results obtained during the probe tests are illustrated in Figure 6. In the recent memory test, platform search accuracy measurements indicated age-related impairment. Thus, aged rats were impaired for time spent in the target quadrant (Fig. 6A; F(1,38) = 12.59, P < 0.01), proximity (Fig. 6B; F(1,38) = 19.73, P < 0.0001), and target area crossings (Fig. 6C; F(1,38) = 38.17, P < 0.0001). Enrichment had no impact on these variables in aged rats, but seemed to increase target area crossings in young adults. Statistical analysis confirmed these observations, with a significant interaction between Housing and Age (F(1,38) = 4.77, P < 0.05). Post hoc comparison showed that EE young adults outperformed all other groups on this variable (P < 0.05). Analysis of platform search profile (Fig. 6A) indicated that the SC and EE groups behaved similarly in each age group, but that search was less specific in aged than young adult rats, the latter spending more time in the target than in any other quadrant (P < 0.001 for each comparison in SC and EE groups) whereas aged rats spent significantly more time in the target and another quadrant than in the other two (P < 0.05 for each comparison in SC and EE groups). Comparison of time spent in the target quadrant at chance level (i.e., 7.5 sec) showed that aged rats, whatever their late housing condition, spent significantly more time in the target quadrant (P < 0.05), confirming that they displayed recent memory for platform location. In the remote memory test, age was the only factor supporting between-group differences in platform search accuracy. Thus, young adult rats outperformed aged ones for time spent in the target quadrant (Fig. 6D; F(1,38) = 11.91, P < 0.01), proximity (Fig. 6E; F(1,38) = 14.24, P < 0.001), and target area crossings (Fig. 6F; F(1,38) = 6.98, P < 0.05). Analysis of search profiles (Fig. 6D) indicated that, like in the recent memory test, young adult rats spent significantly more time in the target than in any other quadrant (P < 0.001 for each comparison in SC and EE groups), indicating that platform search remained very specific even with this longer training-to-test interval. The aged rats still spent more time in a more extended zone of the pool including the target quadrant and another one (P < 0.05 when compared with the two other quadrants in SC and EE groups), suggesting remote memory for platform location, even if less specific than in young adult rats. However, at this longer training-to-test interval, only SC aged rats failed to spend significantly more time than chance level in the target quadrant (P < 0.05 for the other groups), showing that their remote memory of platform location was impaired. Importantly, even if aged rats swim more slowly than younger ones during the two probe trials (data not shown; first probe trial, F(1,38) = 6.43, P < 0.05; second probe trial: F(1,38) = 12.29, P < 0.01), there was no effect of Housing (first probe trial: F(1,38) = 0.87, P > 0.05; second probe trial: F(1,38) = 0.72, P > 0.05), nor interaction between Housing and Age (first probe trial: F(1,38) = 0.69, P > 0.05; second probe trial: F(1,38) = 0.007, P > 0.05).
Figure 6.
Effect of 6 months’ environmental enrichment in young adult and aged rats on probe trial performance assessing recent (A–C) and remote (D–F) memory. (A,D) Time spent in the four quadrants. The dashed line indicates chance level. (B,E) Proximity (average distance to the platform). (C,F) Number of target area crossings. Data are mean + SEM. Statistics: *, difference between time spent in the target quadrant and time spent in the other three quadrants; £, significantly above chance level; #, significantly different from all the other groups; §, significantly different from the A-EE group. For detailed statistical description, see text. Y, young adult rats; A, aged rats; SC, rats housed in standard condition; EE, rats housed in enriched environment.
Discussion
In this study, a reference memory task was used to assess the impact of late enriched environmental housing (from the age of 18 mo) on spatial learning and memory in aged (24 mo) rats. The longitudinal study indicated that late enriched environmental housing maintained accurate spatial memory in aged rats that were unimpaired in the same cognitive domain when middle aged (17 mo), but failed to rescue spatial memory in already cognitively declining rats. The cross-sectional study showed that late enrichment facilitated learning and remote memory in a group of naive aged rats, but that aged animals remained impaired when compared with younger adults (8 mo) on all measures of platform-search accuracy.
Some middle-aged rats display accurate spatial memory whereas others are severely impaired
Cross-sectional studies have shown that spatial reference memory in rats declines between the ages of 3–6 mo and 12–18 mo, depending on the strain, sex, and chronological ages under investigation (Aitken and Meaney 1989; Markowska 1999; Wyss et al. 2000; Bizon and Gallagher 2003; Bizon et al. 2009; McQuail and Nicolle 2015). Some of these studies also reported further decline following these ages (e.g., Bizon et al. 2009; McQuail and Nicolle 2015). In Long-Evans female rats, previous cross-sectional studies by our team also showed impaired performance at 15 mo when compared with 6-mo-old subjects (Harati et al. 2013), and further degradation of spatial reference memory between the age of 13–15 mo and 25–27 mo (Harati et al. 2011, 2013). However, several studies highlighted the fact that the age-related decline in spatial memory reported in studies comparing age-groups concerns only some of the subjects (e.g., Gallagher et al. 1993; Hullinger and Burger 2015; McQuail and Nicolle 2015). Coherently with these reports, using a criterion able to differentiate rats according to platform-search accuracy on a probe trial, the present results showed that some rats displayed accurate spatial memory at 17 mo, while others were severely impaired, searching for the platform in another quadrant than the target one. Importantly, this most preferred quadrant was not the same for all animals. Indeed, respectively 36.36% and 24.24% of rats preferred the two quadrants adjacent to the target one and 39.39% of rats preferred the opposite quadrant. Thus, it seems unlikely that one cue could have been more salient than the other ones and have misled the animals. Taking into account the lower performance improvement of MA-I rats during training, this bias seen in the probe trial might indicate that they were using spatial cues from outside the maze (test-room features) to guide their behavior at the end of the training period but that these cues were not relevant enough to allow accurate platform search. This hypothesis suggests that these rats might need more trials to use extra-maze cues to efficiently locate the platform. The fact that this subgroup no longer displayed this bias when tested with a longer training period when they were aged is in agreement with this hypothesis; although this subgroup also performed worse than subgroup of unimpaired rats during training at the age of 24 mo, they displayed recent memory for platform location and a search profile that was correct even if low in accuracy.
Spatial memory is impaired in aged rats housed in standard conditions
The results of our cross-sectional study confirmed that aged rats show impairment in training. They swam longer distances to reach the platform and were less efficient (fewer successful trials) than young adults. Aged rats were also impaired on probe trials, whatever the variable used to assess platform-search accuracy. However, they displayed recent memory for platform location, even if they searched for it in a broader zone of the pool than young adults. These results confirm that, although impaired compared with young adults, aged rats are able to learn a reference memory task in the Morris water maze if they have had extended training (Gallagher et al. 1993; Clayton et al. 2002). The present results also showed that the impaired performance of aged rats during the training phase was due to a pronounced short-term memory deficit (higher short-term memory index), but also to a long-term memory deficit (higher long-term memory index), as previously reported using within- and between-days performance improvement as measures of short- and long-term memory, respectively (Aitken and Meaney 1989; Shukitt-Hale et al. 1998). However, age-related deficits in short-term (Lindner et al. 1992; McQuail and Nicolle 2015) or long-term memory (Foster and Kumar 2007) have also been reported. Variations in study design (massed versus distributed training trials, and the presence or the absence of an interpolated probe during the training phase) might explain these discrepancies. The present data also demonstrated that, while aged rats exhibited recent memory, they did not display remote memory for platform location, a result that might suggest that their less specific memory trace was also more sensitive to degradation. Only a few studies have evaluated the impact of aging on the persistence of memory traces, and all of them report that aging impaired remote memory but left recent memory intact (spatial memory) (Morel et al. 2015); (contextual fear memory that also depends on hippocampus integrity) (Oler and Markus 1998; Houston et al. 1999).
In the present longitudinal study, in agreement with previous studies in which even short training period during middle age improved the performance of aged rats in a spatial task (van Groen et al. 2002; Hansalik et al. 2006), pretraining at the age of 17 mo facilitated spatial learning at the age of 24 mo, especially in rats housed in standard conditions. This beneficial effect was not related, in the present experiment, to memory for spatial cues as, in contrast to the aforementioned studies, aged rats were tested in a different room at 24 mo. As also discussed by others (e.g., van Groen et al. 2002; Hansalik et al. 2006), this beneficial effect of pretraining in the present study might be due to a carryover effect for two main aspects of the task: first, a procedural aspect (i.e., remembering that an escape platform has to be found); and second, memory of a previously learned strategy for solving the task, and especially a strategy enabling the platform to be located using extra-maze spatial cues. The fact that pretrained rats exhibited less thigmotaxis and less frequent failure to find the platform (high rate of successful trials) as early as the first day of training, but also showed a lower short-term index, clearly supports such a carryover effect. Importantly, the present results showed that pretraining had a beneficial effect on neither the accuracy nor the persistence of spatial memory in aged rats. The platform search profiles of pretrained and naive aged rats were similar during both recent and remote memory tests, and neither group displayed remote memory for platform location. Therefore, even when pretrained in a task that clearly allows a carryover effect, aged rats displayed spatial memory deficit. Importantly, during the recent memory probe trial, aged rats with accurate spatial memory at middle age behaved similarly to aged rats showing impairment at middle age, searching for the platform in an extended part of the pool, whereas they had focused specifically on the target quadrant when middle aged. These results indicate that, although the combined effect of pretraining and extended training before recent memory assessment allowed impaired aged rats to display recent memory for platform location, spatial memory capabilities nevertheless declined with age in the subgroup of rats unimpaired at middle age.
Enrichment favors spatial learning and allows remote memory, but only in aged rats unimpaired when middle aged
The cross-sectional study showed that 6 mo of enriched environment housing facilitated spatial learning, whether initiated in young adults or middle-aged rats. EE groups swam shorter distances to reach the platform than SC groups and were more efficient in solving the task (more successful trials). Although enrichment failed to protect learning against age-related deficit, it alleviated the deficit in short-term memory exhibited by SC aged rats. Thus, late enriched environment exposure mitigates age-related deficit, in agreement with previous studies (e.g., Kobayashi et al. 2002; Harburger et al. 2007; Freret et al. 2012; Kumar et al. 2012; Morse et al. 2015; Fuchs et al. 2016; but Bouet et al. 2011). The present results also showed that, although EE young adults outperformed SC young adults on a measure of platform-search accuracy (annulus crossing), enrichment had no beneficial effects on the recent memory probe trial in aged subjects. In particular, both SC and EE aged rats displayed a less accurate platform-search than young adults. However, in the remote memory test, although impaired compared with young adults, only EE aged rats had any significant memory of platform location. Only a few studies have assessed whether enrichment has an impact on remote memory. Enrichment was shown to enhance long-term memory for object recognition (i.e., 48 h after exposure) in both young (Bruel-Jungerman et al. 2005; Leger et al. 2015) and aged rats (Leal-Galicia et al. 2008), and our team has previously shown that long-life environmental enrichment protects young and middle-aged rats against spontaneous forgetting (Harati et al. 2013). The results of the present study extend these findings by showing that late enrichment also enables aged rats to form memory traces that are less sensitive to degradation.
The results of the longitudinal study shed new light by demonstrating that late enrichment only benefits aged rats displaying accurate spatial memory at middle age. The subgroup of unimpaired rats housed in enriched environment was the only one to display accurate recent spatial memory, but also the only one with remote memory for platform location. These results suggest that late enrichment may protect against subsequent decline, but is not able to restore already impaired capacity. This might explain why some authors failed to find beneficial effects of EE exposure when initiated too late (Bouet et al. 2011), as the proportion of impaired subjects increases with age (e.g., McQuail and Nicolle 2015). However, the hypothesis that an age-related decline in exploratory behavior elicited by EE might also contribute to the lower efficiency of late enrichment cannot be ruled out.
One of the last issues raised by the current study concerns the similarity of the effects of pretraining and enrichment on performance improvement during the training stage. The performances of pretrained SC aged rats were indistinguishable from those of naive EE rats, and the impact of pretraining was very slight in EE aged rats. The first result suggests that enrichment favors the same processes as those involved in the carryover effects found in pretrained SC rats: i.e., it accelerates learning of the procedural aspect of the task but also promotes the use of a spatial strategy. The second result may reflect a ceiling effect on the performance of aged animals in training.
Conclusions
The main result of this study is that late enrichment allows maintenance of accurate recent spatial memory and enables the formation of a memory trace that is less sensitive to degradation, in a subgroup of rats displaying unimpaired spatial memory when middle aged. However, enrichment failed to rescue these capabilities in a subgroup of rats showing already declining spatial memory. Taken together, these results highlight the beneficial effects of exposure to a stimulating environment for the maintenance of some cognitive functions throughout the late part of life, and also its genuine ability to facilitate learning throughout life.
Materials and Methods
Subjects and housing conditions
One hundred female Long-Evans rats purchased at the age of 4–5 wk from Janvier Labs (Le Genest-St-Isle, France) were housed in large transparent cages (60 × 38 × 20 cm) in groups of eight. At the age of 2 mo, they were rehoused in pairs in smaller cages [46 × 26 × 15 cm; standard condition (SC)]. From 18 to 24 mo, rats were assigned to either SC or enriched environment (EE). In EE, rats were housed in groups of 10–12 in wire-mesh cages (112 × 40 × 40 cm) with various objects changed five times a week as previously described (e.g., Harati et al. 2011). A cohort of 24 rats (4–5 wk old) purchased later was housed at the age of 2 mo in either SC or EE, at the same time as the 18-mo-old rats and for the same duration (6 mo). All rats were housed in the same animal facility, with controlled temperature (22 ± 1°C) and humidity (55 ± 5%) under a 12 h–12 h light–dark cycle (lights on at 7:00 a.m.), with ad libitum access to food and water. Commercially available food pellets for adult rats (Muceloda, Italy: RF 21) were changed (RF 18) when rats were 6 mo old, as recommended by the breeder. Experimental protocols and animal care were in compliance with European Community Council Directive 2010/63/UE and the current project was approved by the local ethics committee (CREMEAS, authorization no. AL/36/43/02/13). Rats were observed daily to detect health problems. During aging of the cohorts, some rats died from natural courses or were euthanized (often for tumor onset, but occasionally for other health problems such as hindlimb paralysis). Percentage survivors was 86% at the age of 17 mo. At the end of behavioral testing, percentage survivors was 56% in the 24-mo-old cohort and 100% in 8 mo olds. To avoid isolated housing in SC aging rats, the survivor was housed with another congener from our sentinel group.
Apparatus
The mazes were circular pools (diameter 160 cm, height 60 cm) filled with water to half the height and virtually divided into four equal quadrants. The water (20 ± 1°C) was opacified with powdered milk. A circular platform, 11 cm in diameter, could be placed in the pools, either 1 cm underneath (hidden platform) or above (visible platform) the water surface. The pools were located in two experimental rooms (Rooms 1 and 2, see Supplemental Fig. 1), both with many extra-maze cues (e.g., chair, desk, pictures hanging on the wall, etc.), and were each equipped with a video-tracking system (Noldus, Wageningen in Room 1; SMART, SD Instruments in Room 2) to collect various aspects of the rat's behavior.
Procedures
Classification of middle-aged rats before differential housing
At the age of 17 mo, rats (n = 56) were singly housed in transparent cages (46 × 26 × 15 cm) and transferred to another animal facility. They were weighed, and handled 1 min per day for five consecutive days. These middle-aged (MA) rats were then trained in the water maze (Room 1) (Supplemental Fig. 1A) using a procedure sensitive to age-related spatial deficit in SC-housed rats (e.g., Harati et al. 2011). Briefly, rats were trained for five consecutive days to find the hidden platform located at a fixed position in one quadrant of the pool. Each day, four trials were performed, for which the rat was placed in the pool, facing the wall, at one of seven fixed starting points in a pseudorandom order. Twenty-four hours after training, long-term recent memory of the platform location was assessed on a single 30-sec trial without platform (probe trial). For this test, all animals were released from the same point. Rats were classified according to their search profile during this probe (i.e., time spent in the target quadrant and from longest to shortest time spent in the other three quadrants). A nonhierarchical cluster-analysis method (K-mean clustering) based on the double principle of maximizing intersubgroup and minimizing intrasubgroup variability, was used to subdivide the population into two clusters (called “subgroups” hereafter). Three days after the probe trial, each rat was implanted with a subcutaneous microchip (biolog-id) in order to be later identified. At the age of 18 mo, half of the rats of each subgroup were housed in SC and the other half in EE, in a dedicated room, until they were 24 mo old.
Water maze for 24- and 8-mo-old rats
After 6 mo differential housing, pretrained aged (remaining n = 41; EE I, n = 12; EE UI, n = 7; SC I, n = 14; SC UI, n = 8), naive aged (remaining n = 18; EE, n = 8; SC, n = 10), and young adult rats (n = 24; EE n = 12; SC, n = 12) were housed singly in transparent cages (46 × 26 × 15 cm) and transferred in another animal facility. They were weighed, handled (1 min per day for 5 consecutive days), then trained in the water maze. The task differed from that described for MA rats only on the following: (1) the task was performed in another room (Room 2) Supplemental Fig. 1B); (2) a visible platform test (four consecutive trials with a visible platform located in one quadrant of the pool) was conducted the day before training with the hidden platform; (3) rats were trained for 8 consecutive days (with the hidden platform located in another quadrant of the pool); and (4) both recent and remote memory for platform location were assessed. For recent memory, 24 h after the last training day, rats were given a 30-sec probe trial. It was followed immediately by four additional training trials with the platform replaced at the same location as in training. For remote memory, a second 30-sec probe trial was conducted 10 d later. Given the large number of animals to be tested, training of experimentally naive young adult and aged rats began after completion of training for pretrained aged rats (naive rats were housed singly 1 wk after pretrained rats, to maintain a constant time between the end of differential housing and the testing period).
Data collection and analysis
For training, performance (mean of the daily trials with the hidden platform) was assessed by computing the distance swam to reach the platform corrected according to the method described by Lindner (1997). This method consists of assigning to all trials in which a rat did not locate the platform the median swam distance calculated with all of those trials. This maximum distance was also assigned to trials in which the distance swam to reach the platform was higher than this distance. The percentage thigmotaxis (percentage time spent in the 10 cm peripheral annulus) and the number of successful trials (i.e., trials in which the rat located the platform) (Ruediger et al. 2012) were also computed. For the water maze in Room 2, a short-term (mean of the last two daily trials) and a long-term memory index (mean of the first daily trials) was computed for distance swam to reach the platform from the second to the last training day (adapted from George et al. 2006). For probe trials, several variables reflecting platform-search accuracy were collected: time spent in each quadrant of the pool, mean distance to the platform, and target area (platform enlarged by a 10-cm annulus) crossings. Swimming speeds during training and probe tests were also analyzed. Data were subjected to analysis of variance (ANOVA) with either Subgroup, or Age and Housing condition, or Subgroup and Housing condition as between-subject factors. For visible platform session, performance improvement was assessed using Trial as within-subject factor. For training, performance improvement was assessed using Day as within-subject factor. For probe trials, the platform-search profile of each group was analyzed using Quadrant as within-subject factor. The ANOVAs were completed by post hoc comparison using the Tukey test (for unequal samples for between group comparisons). The threshold for rejecting the null hypothesis was 0.05 throughout.
Supplementary Material
Acknowledgments
This work was supported by the University of Strasbourg and the CNRS. The authors would like to express their gratitude to O. Bildstein, G. Edomwony, and O. Egesi for their valuable and constant investment in the care provided to the rats.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.041236.115.
References
- Aitken DH, Meaney MJ. 1989. Temporally graded, age-related impairments in spatial memory in the rat. Neurobiol Aging 10: 273–276. [DOI] [PubMed] [Google Scholar]
- Birch AM, McGarry NB, Kelly AM. 2013. Short-term environmental enrichment, in the absence of exercise, improves memory, and increases NGF concentration, early neuronal survival, and synaptogenesis in the dentate gyrus in a time-dependent manner. Hippocampus 23: 437–450. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. 2003. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci 18: 215–219. [DOI] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. 2009. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol Aging 30: 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouet V, Freret T, Dutar P, Billard JM, Boulouard M. 2011. Continuous enriched environment improves learning and memory in adult NMRI mice through θ burst-related-LTP independent mechanisms but is not efficient in advanced aged animals. Mech Ageing Dev 132: 240–248. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. 2005. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci 21: 513–521. [DOI] [PubMed] [Google Scholar]
- Cassel JC, Lazaris A, Birthelmer A, Jackisch R. 2007. Spatial reference- (not working- or procedural-) memory performance of aged rats in the water maze predicts the magnitude of sulpiride-induced facilitation of acetylcholine release by striatal slices. Neurobiol Aging 28: 1270–1285. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. 2002. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J Neurosci 22: 3628–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faherty CJ, Kerley D, Smeyne RJ. 2003. A Golgi-Cox morphological analysis of neuronal changes induced by environmental enrichment. Brain Res Dev Brain Res 141: 55–61. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. 2007. Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiol Learn Mem 87: 522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freret T, Billard JM, Schumann-Bard P, Dutar P, Dauphin F, Boulouard M, Bouet V. 2012. Rescue of cognitive aging by long-lasting environmental enrichment exposure initiated before median lifespan. Neurobiol Aging 33: 1005.e1–1005.e10. [DOI] [PubMed] [Google Scholar]
- Fuchs F, Cosquer B, Penazzi L, Mathis C, Kelche C, Majchrzak M, Barbelivien A. 2016. Exposure to an enriched environment up to middle age allows preservation of spatial memory capabilities in old age. Behav Brain Res. 299: 1–5. [DOI] [PubMed] [Google Scholar]
- Gage FH, Kelly PA, Björklund A. 1984. Regional changes in brain glucose metabolism reflect cognitive impairments in aged rats. J Neurosci 4: 2856–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. 1993. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci 107: 618–626. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Colantuoni C, Eichenbaum H, Haberman RP, Rapp PR, Tanila H, Wilson IA. 2006. Individual differences in neurocognitive aging of the medial temporal lobe. Age (Dordr) 28: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Parducz A, Dupret D, Kharouby M, Le Moal M, Piazza PV, Mayo W. 2006. Smad-dependent alterations of PPT cholinergic neurons as a pathophysiological mechanism of age-related sleep-dependent memory impairments. Neurobiol Aging 27: 1848–1858. [DOI] [PubMed] [Google Scholar]
- Hansalik M, Skalicky M, Viidik A. 2006. Impairment of water maze behaviour with ageing is counteracted by maze learning earlier in life but not by physical exercise, food restriction or housing conditions. Exp Gerontol 41: 169–174. [DOI] [PubMed] [Google Scholar]
- Harati H, Majchrzak M, Cosquer B, Galani R, Kelche C, Cassel JC, Barbelivien A. 2011. Attention and memory in aged rats: Impact of lifelong environmental enrichment. Neurobiol Aging 32: 718–736. [DOI] [PubMed] [Google Scholar]
- Harati H, Barbelivien A, Herbeaux K, Muller MA, Engeln M, Kelche C, Cassel JC, Majchrzak M. 2013. Lifelong environmental enrichment in rats: impact on emotional behavior, spatial memory vividness, and cholinergic neurons over the lifespan. Age (Dordr) 35: 1027–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger LL, Lambert TJ, Frick KM. 2007. Age-dependent effects of environmental enrichment on spatial reference memory in male mice. Behav Brain Res 185: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston FP, Stevenson GD, McNaughton BL, Barnes CA. 1999. Effects of age on the generalization and incubation of memory in the F344 rat. Learn Mem 6: 111–119. [PMC free article] [PubMed] [Google Scholar]
- Hullinger R, Burger C. 2015. Learning impairment identified early in life are predictive of future impairments associated with aging. Behav Brain Res 294: 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullinger R, O'Riordan K, Burger C. 2015. Environmental enrichment improves learning and memory and long-term potentiation in young adult rats through a mechanism requiring mGluR5 signaling and sustained activation of p70s6k. Neurobiol Learn Mem 125: 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Ohashi Y, Ando S. 2002. Effects of enriched environments with different durations and starting times on learning capacity during aging in rats assessed by a refined procedure of the Hebb-Williams maze task. J Neurosci Res 70: 340–346. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rani A, Tchigranova O, Lee WH, Foster TC. 2012. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol Aging 33: 828.e1–1828.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Galicia P, Castañeda-Bueno M, Quiroz-Baez R, Arias C. 2008. Long-term exposure to environmental enrichment since youth prevents recognition memory decline and increases synaptic plasticity markers in aging. Neurobiol Learn Mem 90: 511–518. [DOI] [PubMed] [Google Scholar]
- Leger M, Paizanis E, Dzahini K, Quiedeville A, Bouet V, Cassel JC, Freret T, Schumann-Bard P, Boulouard M. 2015. Environmental enrichment duration differentially affects behavior and neuroplasticity in adult mice. Cereb Cortex 25: 4048–4061. [DOI] [PubMed] [Google Scholar]
- Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L. 2005. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res 163: 78–90. [DOI] [PubMed] [Google Scholar]
- Lindner MD. 1997. Reliability, distribution, and validity of age-related cognitive deficits in the Morris water maze. Neurobiol Learn Mem 68: 203–220. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Balch AH, VanderMaelen CP. 1992. Short forms of the “reference-” and “working-memory” Morris water maze for assessing age-related deficits. Behav Neural Biol 58: 94–102. [DOI] [PubMed] [Google Scholar]
- Lopez J, de Vasconcelos AP, Cassel JC. 2008. Environmental cue saliency influences the vividness of a remote spatial memory in rats. Neurobiol Learn Mem 90: 285–289. [DOI] [PubMed] [Google Scholar]
- Markowska AL. 1999. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci 19: 8122–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Nicolle MM. 2015. Spatial reference memory in normal aging Fischer 344 × Brown Norway F1 hybrid rats. Neurobiol Aging 36: 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Gallegos A, Rojas-Carvajal M, Salas S, Saborio-Arce A, Fornaguera-Trias J, Brenes JC. 2015. Age-dependent effects of environmental enrichment on spatial memory and neurochemistry. Neurobiol Learn Mem 118: 96–104. [DOI] [PubMed] [Google Scholar]
- Morel GR, Andersen T, Pardo J, Zuccolilli GO, Cambiaggi VL, Hereñú CN, Goya RG. 2015. Cognitive impairment and morphological changes in the dorsal hippocampus of very old female rats. Neuroscience 303: 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse AJ, Butler AA, Davis RL, Soller IJ, Lubin FD. 2015. Environmental enrichment reverses histone methylation changes in the aged hippocampus and restores age-related memory deficits. Biology 4: 298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Markus EJ. 1998. Age-related deficits on the radial maze and in fear conditioning: hippocampal processing and consolidation. Hippocampus 8: 402–415. [DOI] [PubMed] [Google Scholar]
- Ruediger S, Spirig D, Donato F, Caroni P. 2012. Goal-oriented searching mediated by ventral hippocampus early in trial-and-error learning. Nat Neurosci 15: 1563–1571. [DOI] [PubMed] [Google Scholar]
- Rutz S, Majchrzak M, Siedschlag V, Barbelivien A, Harati H, Rothmaier AK, Feuerstein TJ, Jackisch R, Cassel JC. 2009. The modulation of striatal dopamine release correlates with water-maze performance in aged rats. Neurobiol Aging 30: 957–972. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Bahr NI, Weiss IC, Wurbel H. 2002. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol Biochem Behav 73: 209–224. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Mouzakis G, Joseph JA. 1998. Psychomotor and spatial memory performance in aging male Fischer 344 rats. Exp Gerontol 33: 615–624. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. 2002. Old rats remember old tricks; memories of the water maze persist for 12 months. Behav Brain Res 136: 247–255. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Chambless BD, Kadish I, van Groen T. 2000. Age-related decline in water maze learning and memory in rats: strain differences. Neurobiol Aging 21: 671–681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.