We evaluated dietary intake in US Hispanics/Latinos with diabetes, and found that diabetes awareness is associated with reduced carbohydrate and sugar intake, and increased monounsaturated fat intake.
Abstract
Context:
Diet modification is a mainstay of diabetes management. US Hispanics/Latinos are disproportionately affected by diabetes, but few studies have examined dietary intake among US Hispanics/Latinos with diabetes, and little is known regarding the influence of diabetes awareness on dietary intake.
Objective:
We evaluated macronutrient intake and its associations with diabetes awareness and glycemic control among US Hispanics/Latinos with diabetes.
Participants:
This analysis included 3310 diabetic adults aged 18–74 years from the Hispanic Community Health Study/Study of Latinos (2008–2011).
Main Outcome Measures:
Diabetes was defined as diagnosed (based on medical history or antihyperglycemic medication use) or undiagnosed diabetes (based on fasting glucose ≥ 126 mg/dL, glycated hemoglobin [HbA1c] ≥ 6.5%, or 2 h glucose ≥ 200 mg/dL in the absence of a physician diagnosis). Dietary intake was assessed using two 24-hour recalls.
Results:
Among Hispanic/Latino adults with diabetes, 21.2%, 55.7%, and 71.2% met the American Diabetes Association recommendations for fiber (≥14 g per 1000 kcal), saturated fat (<10% of total energy), and cholesterol intake (<300 mg), respectively. Compared with those with undiagnosed diabetes, people with diagnosed diabetes consumed less carbohydrate (50.3 vs 52.4% of total energy; P = .017), total sugar (19.1 vs 21.5% of total energy; P = .002), added sugar (9.8 vs 12.1% of total energy; P < .001), and more total fat (30.7 vs 29.3% of total energy; P = .048) and monounsaturated fat (11.5 vs 10.7% of total energy; P = .021). Association between diabetes awareness and low total and added sugar intake was observed in individuals of Mexican and Puerto Rican background but not in other groups (P for interaction < .05). Among people with diagnosed diabetes, those with HbA1c of 7% or greater consumed more total fat, saturated fat, and cholesterol than those with HbA1c less than 7% (all P < .05).
Conclusions:
Among US Hispanics/Latinos with diabetes, fiber intake is low, and diabetes awareness is associated with reduced carbohydrate and sugar intake and increased monounsaturated fat intake. Sugar intake may require special attention in certain Hispanic/Latino background groups.
Hispanics/Latinos are the largest minority group of the US population (1) and suffer disproportionately from diabetes (2). Baseline examination data from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) estimated that the prevalence of diabetes in Hispanic/Latino adults aged 18–74 years was 16.9%, ranging from 10.2% among Hispanics/Latinos of South American background to 18.3% among those of Mexican descent (3). There is some evidence that US Hispanics/Latinos tend to have poorer diabetes outcomes and suboptimal self-monitoring of lipids, blood glucose, and blood pressure compared with non-Hispanic whites (4–6). Moreover, the US Hispanic/Latino population is a heterogeneous group, with differences in diabetes control and management across diverse backgrounds which may be attributed to differences in socioeconomic status, acculturation, access to health care, income level, lifestyles, and dietary patterns (7, 8).
Nutrition therapy is one of the most vital and effective components of diabetes management (9), and what constitutes a healthy dietary pattern may differ across populations and cultures. Carbohydrate intake is a major factor of glycemic control due to its immediate effect on postprandial glucose levels (10). Several randomized trials have indicated that replacement of saturated fatty acids and carbohydrate with monounsaturated fatty acids improves glycemic control and other metabolic parameters among subjects with diabetes (11–14). The American Diabetes Association (ADA) recommends increased consumption of fiber (≥14 g per 1000 kcal) (9), which has been shown to improve cardiovascular disease risk factor control and other complications among individuals with diabetes (10, 15).
Recent data from the National Health and Nutrition Examination Survey (NHANES) 2005–2010 suggested that awareness of diabetes status may favorably affect some dietary patterns (eg, less sugar and carbohydrate intake) in individuals with diabetes (16). Another study also reported that higher fat and lower carbohydrate intakes were associated with worse glycemic control in American Indians (17). However, no study has examined macronutrient and fiber intake among US Hispanics/Latinos of diverse backgrounds with diabetes. Therefore, in this study, we evaluated macronutrient and fiber intake among US Hispanic/Latino adults with diabetes from the HCHS/SOL and further examined whether dietary intake differed by diabetes awareness and glycemic control.
Materials and Methods
Study population
The HCHS/SOL is a population-based cohort study designed to examine the prevalence and development of chronic diseases among Hispanic/Latino individuals residing in four US communities (Bronx, New York; Chicago, Illinois; Miami, Florida; and San Diego, California). Details of the sample design and cohort selection are provided elsewhere (18, 19). Briefly, between March 2008 and June 2011, the HCHS/SOL examined 16 415 Hispanic/Latino men and women aged 18–74 years. Participants were recruited from randomly selected households in the four field centers through a stratified two-stage area probability sampling approach. The data collection included demographic characteristics, standardized clinical examination components, fasting and postglucose load blood collection, medical history, and assessment of health behaviors and acculturation-related factors. In the current study, 3310 subjects with diabetes were included after exclusion of diabetic individuals with two unreliable 24-hour dietary recalls (n = 72); those with abnormal or missing energy intake (n = 91); and those with missing diagnosis status (n = 56). In addition, we also included 12 548 individuals without diabetes for further comparison between people with diabetes and those without diabetes. All participants gave their written informed consent, and the study was approved by the institutional review boards at each participating institution.
Diabetes definition
Diagnosed diabetes mellitus was defined as self-reported history of physician-diagnosed diabetes or documented use of antihyperglycemic agents. Undiagnosed diabetes mellitus was defined as not having been diagnosed by a physician and not taking antihyperglycemic medications but meeting laboratory criteria at field examination: fasting plasma glucose of 126 mg/dL or greater, 2-hour postload glucose levels of 200 mg/dL or greater, or a glycated hemoglobin (HbA1c) level of 6.5% or greater. Study participants with diagnosed diabetes were considered to have their blood glycemic levels well controlled if their HbA1c was less than 7.0%; and those with an HbA1c of 7.0% or greater were considered to be uncontrolled.
Dietary intake
Macronutrient intake was assessed using two 24-hour dietary recalls. The first 24-hour dietary recall was conducted in person at the time of the baseline interview, whereas the second was performed primarily via telephone approximately 30 days after the baseline interview. Interviews were conducted in Spanish or English based on the participant's preference using the multiple-pass method. Diet assessments were performed using the Nutrition Data System for Research software (version 11) developed by the Nutrition Coordinating Center at the University of Minnesota. The Nutrition Data System for Research used in the present study included common foods, brand-name products, and Hispanic/Latino foods. Detailed information on dietary data collection has been previously published elsewhere (20).
In this study, dietary intake variables of interest included total energy, carbohydrate, total and added sugar, fiber, protein, total fat, saturated, monounsaturated, and polyunsaturated fatty acids, and total cholesterol. We used the means of two 24-hour diet recalls to quantify usual dietary intake of the aforementioned macronutrients. Recalls with energy below the sequence-sex-specific first percentile or greater than the 99th percentile or those that were considered to be unreliable according to the interviewer were excluded.
Covariates
Height and weight were measured during the in-person examination. Body mass index was calculated as weight in kilograms divided by the square of height in meters and was categorized as normal weight (<25 kg/m2); overweight (≥25 kg/m2 and < 30 kg/m2); and obese (≥30 kg/m2). Self-reported data were used to define Hispanic/Latino background (Dominican, Central American, Cuban, Mexican, Puerto Rican, South American, or mixed/other); nativity (born within 50 US states or not); length of time in the United States (<10 y or ≥ 10 y); annual household income (<$20,000, $20,000-$49,999, or ≥$50,000, not reported); education level (less than high school, high school equivalent, greater than high school); health insurance (yes or no); physical activity (metabolic equivalents in minutes per day); cigarette use (current, former, or never); alcohol use (current, former, or never); diabetes medication use (yes or no); family history of diabetes (yes or no); and medical history of diabetes by using standardized instruments. Physical activity was measured as metabolic equivalents in minutes per day during work and travel and leisure time provided by the Global Physical Activity Questionnaire, which was developed by the World Health Organization (scoring information is available: www.who.int/chp/steps). Participants were instructed to bring in their currently used prescription medications, which were scanned or transcribed into a database and matched to a Medical Therapeutic Classification or the National Drug Code.
Statistical analyses
General linear regression and χ2 tests were used to compare means and proportions of participant characteristics by diabetes diagnosis status. Means and SEs of usual macronutrient intake were estimated using predicted marginals from separate general linear regression models for each macronutrient by diagnosis status and control status. These models were adjusted for age, sex, field center, Hispanic/Latino background, body mass index categories, use of diabetes medication, family history of diabetes, annual household income, education level, nativity, length of time in the United States, health insurance status, cigarette use, alcohol use, and physical activity level. Similarly, separate general linear regression models were used to examine differences in dietary intake between people without diabetes and those with undiagnosed or diagnosed diabetes, respectively. In addition, among participants with diagnosed diabetes separate linear regression models were fit with HbA1c levels as the dependent variable and each macronutrient dietary intake as the independent variable adjusting by previously mentioned covariates. We further conducted stratified analyses by Hispanic/Latino background, sex, and age groups (<45 and ≥ 45 y). All analyses accounted for complex survey design and were performed using SAS version 9.3 software (SAS Institute) or SAS-callable SUDAAN software, release 11 (RTI International, Research Triangle Park, NC).
Results
Table 1 shows the characteristics of the study sample. Among 3310 participants with diabetes, 1820 (55.0%) were females, and 2226 (67.3%) were aware of their diabetes status. Compared with individuals with undiagnosed diabetes, those with diagnosed diabetes were older and were more likely to have been born in the United States (50 US states only), to have resided in the United States for more than 10 years, to have health insurance, to have a family history of diabetes, and to not consume alcohol (all P < .05). Individuals with diagnosed diabetes were more likely to be of Dominican or Puerto Rican background than their undiagnosed counterparts (P < .001).
Table 1.
Characteristics of Hispanics/Latinos With Diabetes, Hispanic Community Health Study/Study of Latinos, 2008–2011
| All (n = 3310) | Undiagnosed (n = 1084) | Diagnosed (n = 2226) | P Value | |
|---|---|---|---|---|
| Age, y | 53.8 (0.4) | 52.3 (0.6) | 54.6 (0.5) | .003 |
| Body mass index, kg/m2 | 32.1 (0.2) | 32.1 (0.3) | 32.1 (0.2) | .989 |
| Females, % | 55.0 (1.2) | 56.3 (2.1) | 54.4 (1.5) | .437 |
| Self-reported Hispanic background, % | <.001 | |||
| Dominican | 9.9 (1.0) | 8.1 (1.1) | 10.8 (1.2) | |
| Central American | 6.7 (0.6) | 7.8 (1.0) | 6.1 (0.7) | |
| Cuban | 21.7 (2.2) | 25.3 (2.7) | 20.0 (2.3) | |
| Mexican | 36.1 (2.0) | 38.3 (2.7) | 35.0 (2.2) | |
| Puerto Rican | 19.4 (1.2) | 13.9 (1.5) | 22.2 (1.4) | |
| South American | 3.3 (0.4) | 4.4 (0.7) | 2.8 (0.4) | |
| More than one/other heritage | 2.9 (0.7) | 2.4 (0.7) | 3.2 (0.9) | |
| Annual family income, % | .096 | |||
| ≤ $20 000 | 49.3 (1.3) | 45.1 (2.1) | 51.4 (1.7) | |
| $20 001-$50 000 | 33.3 (1.2) | 37.4 (2.0) | 31.3 (1.5) | |
| > $50 000 | 8.2 (0.9) | 8.0 (1.3) | 8.2 (1.0) | |
| Not reported | 9.2 (0.7) | 9.5 (1.2) | 9.1 (0.8) | |
| Education, % | .075 | |||
| Less than high school | 45.4 (1.4) | 42.8 (2.3) | 46.7 (1.6) | |
| High school or equivalent | 22.5 (1.2) | 26.0 (2.0) | 20.7 (1.5) | |
| Greater than high school | 32.2 (1.4) | 31.2 (2.2) | 32.7 (1.7) | |
| Born in United States, % | 11.7 (0.9) | 9.3 (1.2) | 12.9 (1.1) | .022 |
| US residence > 10 y, % | 78.2 (1.5) | 72.1 (2.6) | 81.3 (1.4) | <.001 |
| Health insurance, % | 61.2 (1.3) | 48.5 (2.3) | 67.7 (1.4) | <.001 |
| Family history of diabetes, % | 60.5 (1.4) | 52.6 (2.2) | 64.4 (1.7) | <.001 |
| Current smoking, % | 18.4 (1.1) | 17.3 (1.7) | 18.9 (1.3) | .592 |
| Current drinking, % | 37.1 (1.3) | 41.7 (2.0) | 34.8 (1.7) | .048 |
| Physical activity, MET, min/d | 495.4 (23.4) | 532.7 (38.8) | 476.9 (28.3) | .235 |
Abbreviation: MET, metabolic equivalent. Data are means (SEs) or percentages (SEs). All values were weighted to account for complex survey design.
Macronutrient intake by diagnosis status of diabetes
Table 2 presents macronutrient and fiber intakes among all diabetic participants as well as by diabetes diagnosis status. Overall, on a given day, Hispanic/Latino adults with diabetes consumed 51.1% of their daily total energy from carbohydrate (19.9% and 10.6% from total sugar and added sugar, respectively), 18.1% from protein, 30.2% from total fat (9.9% from saturated fats), and consumed 10.9 g fiber per 1000 kcal and 246.8 mg total cholesterol. Moreover, 21.2% of Hispanic/Latino adults with diabetes met the ADA recommendations for fiber intake (≥14 g per 1000 kcal), ranging from 9.5% among those of Puerto Rican background to 33.6% among those of Mexican background; 55.7% of Hispanic/Latino adults with diabetes met the ADA recommendations for saturated fat intake (<10% of total kilocalories), ranging from 50.8% (Puerto Rican background) to 70.1% (Dominican background); 71.2% of Hispanics/Latino adults with diabetes met the ADA recommendations for total cholesterol intake (<300 mg), ranging from 64.3% (Mexican background) to 86.6% (Dominican background) (Supplemental Table 1).
Table 2.
Macronutrient Intake Among Hispanics/Latinos With Diabetes by Diagnosis Status, Hispanic Community Health Study/Study of Latinos, 2008–2011
| All (n = 3310) | Undiagnosed (n = 1084) | Diagnosed (n = 2226) | P Value | |
|---|---|---|---|---|
| Total energy, kcal | 1706.9 (15.3) | 1688.0 (39.2) | 1716.9 (24.2) | .598 |
| Carbohydrate, g | 219.2 (2.2) | 221.0 (5.5) | 218.2 (3.6) | .728 |
| Total kilocalories, % | 51.1 (0.2) | 52.4 (0.6) | 50.3 (0.4) | .017 |
| Total sugar, g | 83.4 (1.1) | 89.3 (2.9) | 80.3 (1.7) | .022 |
| Total kilocalories, % | 19.9 (0.2) | 21.5 (0.6) | 19.1 (0.3) | .002 |
| Added sugar, g | 46.2 (0.9) | 52.0 (2.4) | 43.1 (1.4) | .008 |
| Total kilocalories, % | 10.6 (0.2) | 12.1 (0.5) | 9.8 (0.3) | <.001 |
| Fiber, g | 17.9 (0.2) | 17.5 (0.5) | 18.1 (0.3) | .454 |
| Fiber, g per 1000 kcal | 10.9 (0.1) | 10.8 (0.3) | 10.9 (0.2) | .624 |
| Protein, g | 74.7 (0.8) | 71.1 (2.0) | 76.6 (1.4) | .069 |
| Total kilocalories, % | 18.1 (0.1) | 17.6 (0.3) | 18.4 (0.2) | .077 |
| Total fat, g | 60.7 (0.7) | 57.0 (1.9) | 62.6 (1.3) | .060 |
| Total kilocalories, % | 30.2 (0.2) | 29.3 (0.5) | 30.7 (0.3) | .048 |
| Saturated fatty acids, g | 19.6 (0.2) | 18.7 (0.7) | 20.0 (0.4) | .163 |
| Total kilocalories, % | 9.9 (0.1) | 9.6 (0.2) | 10.0 (0.1) | .185 |
| Monounsaturated fatty acids, g | 22.6 (0.3) | 21.0 (0.8) | 23.5 (0.6) | .033 |
| Total kilocalories, % | 11.2 (0.1) | 10.7 (0.2) | 11.5 (0.2) | .021 |
| Polyunsaturated fatty acids, g | 13.2 (0.2) | 12.4 (0.6) | 13.7 (0.4) | .143 |
| Total kilocalories, % | 6.5 (0.1) | 6.4 (0.2) | 6.5 (0.1) | .589 |
| Total cholesterol, mg | 246.8 (3.9) | 229.8 (9.0) | 255.7 (6.3) | .047 |
| Total cholesterol, mg per 1000 kcal | 147.0 (2.2) | 136.9 (4.9) | 152.4 (3.6) | .031 |
Data are means (SEs), adjusted for age, sex, field center, Hispanic/Latino background, body mass index categories, use of diabetes medications, family history of diabetes, annual household income, education, born in the United States, US residence more than 10 years, health insurance status, smoking, alcohol consumption, and physical activity. All values were weighted to account for complex survey design.
The self-reported mean total energy intake was 1706.9 kcal/d in US Hispanics/Latinos with diabetes (Table 2), and there was no significant difference between individuals with diagnosed diabetes and those with undiagnosed diabetes. The relatively low energy intake reflects significant underreporting, which has been confirmed in our prior work when compared with the biomarker doubly labeled water (21). Compared with adults with undiagnosed diabetes, individuals with diagnosed diabetes consumed less carbohydrate (50.3 vs 52.4% of total energy, P = .017), total sugar (80.3 vs 89.3 g/d, P = .022; 19.1 vs 21.5% of total energy, P = .002), added sugar (43.1 vs 52.0 g/d, P = .008; 9.8 vs 12.1% of total energy, P < .001), and more total fat (30.7 vs 29.3% of total energy, P = .048), monounsaturated fatty acids (23.5 vs 21.0 g/d, P = .033; 11.5 vs 10.7% of total energy, P = .021), total cholesterol (255.7 vs 229.8 mg/d, P = .047; 152.4 vs 136.9 mg per 1000 kcal, P = .031). In particular, adults with diagnosed diabetes were less likely to consume sugar-sweetened beverages than their undiagnosed counterparts (38.6 vs 47.4 g/d, P = .010; 8.8 vs 11.0% of total energy, P = .004) (Supplemental Table 2). No significant differences in daily total energy, fiber, or protein intake were observed between individuals with diagnosed and undiagnosed diabetes (Table 2). In addition, we also repeated these analysis by excluding socioeconomic status (indicated by education, annual household income, born in the United States, and US residence more than 10 y), access to medical care (indicated by health insurance status), and family history of diabetes as covariates in the models one at a time. The results did not change materially (data not shown).
To further investigate the influence of diabetes status on dietary intake, we also compared dietary intake among individuals with diabetes and those without diabetes. Individuals with undiagnosed and diagnosed diabetes tended to consume less total sugar and added sugar and more fiber and protein, compared with those without diabetes (Table 3). These differences in dietary intake were more significant in the comparison between individuals with diagnosed diabetes and those without diabetes. In addition, adults with diagnosed diabetes were more likely to consume less carbohydrate (49.3 vs 51.8% of total energy, P = .002) and more total fat (31.7 vs 30.2% of total energy, P = .032) and monounsaturated fat (11.8 vs 11.1% of total energy, P = .018), compared with those without diabetes.
Table 3.
Macronutrient Intake Among Hispanics/Latinos by Diagnosis Status, Hispanic Community Health Study/Study of Latinos, 2008–2011
| Nondiabetics (n = 12 548) | Undiagnosed (n = 1084) | Diagnosed (n = 2226) | P1 | P2 | |
|---|---|---|---|---|---|
| Total energy, kcal | 1914.2 (10.1) | 1871.7 (30.1) | 1891.6 (42.7) | .160 | .636 |
| Carbohydrate, g | 250.0 (1.6) | 242.5 (4.4) | 238.3 (6.5) | .086 | .106 |
| Total kilocalories, % | 51.8 (0.2) | 51.4 (0.4) | 49.3 (0.7) | .332 | .002 |
| Total sugar, g | 105.7 (0.9) | 99.2 (2.4) | 89.8 (3.2) | .007 | <.001 |
| Total kilocalories, % | 22.4 (0.1) | 21.6 (0.5) | 19.4 (0.6) | .077 | <.001 |
| Added sugar, g | 69.3 (0.8) | 62.8 (1.9) | 53.8 (2.7) | <.001 | <.001 |
| Total kilocalories, % | 14.3 (0.1) | 13.3 (0.4) | 11.2 (0.5) | .016 | <.001 |
| Fiber, g | 17.2 (0.1) | 17.6 (0.4) | 17.9 (0.6) | .462 | .266 |
| Fiber, g per 1000 kcal | 9.3 (0.1) | 9.8 (0.2) | 9.9 (0.2) | .033 | .022 |
| Protein, g | 79.0 (0.5) | 79.0 (1.3) | 84.1 (2.5) | .956 | .067 |
| Total kilocalories, % | 17.0 (0.1) | 17.5 (0.2) | 18.2 (0.4) | .016 | .002 |
| Total fat, g | 67.9 (0.6) | 65.7 (1.3) | 71.1 (2.4) | .100 | .219 |
| Total kilocalories, % | 30.2 (0.1) | 30.1 (0.3) | 31.7 (0.6) | .813 | .032 |
| Saturated fatty acids, g | 22.2 (0.2) | 21.3 (0.5) | 22.7 (0.8) | .072 | .584 |
| Total kilocalories, % | 9.9 (0.1) | 9.8 (0.1) | 10.3 (0.2) | .545 | .196 |
| Monounsaturated fatty acids, g | 24.9 (0.2) | 24.1 (0.5) | 26.4 (0.9) | .102 | .142 |
| Total kilocalories, % | 11.1 (0.1) | 11.0 (0.1) | 11.8 (0.3) | .589 | .018 |
| Polyunsaturated fatty acids, g | 14.8 (0.1) | 14.5 (0.4) | 15.9 (0.7) | .501 | .185 |
| Total kilocalories, % | 6.5 (0.04) | 6.6 (0.1) | 6.8 (0.2) | .400 | .257 |
| Total cholesterol, mg | 269.4 (2.7) | 259.8 (6.3) | 283.6 (10.7) | .143 | .232 |
| Total cholesterol, mg per 1000 kcal | 142.6 (1.5) | 141.4 (3.7) | 155.7 (6.1) | .760 | .051 |
P1 indicated differences between individuals without diabetes and those with undiagnosed diabetes. P2 indicated differences between individuals without diabetes and those with diagnosed diabetes. Data are means (SEs), adjusted for age, sex, field center, Hispanic/Latino background, body mass index categories, use of diabetes medications, family history of diabetes, annual household income, education, born in the United States, US residence more than 10 years, health insurance status, smoking, alcohol consumption, and physical activity. All values were weighted to account for complex survey design.
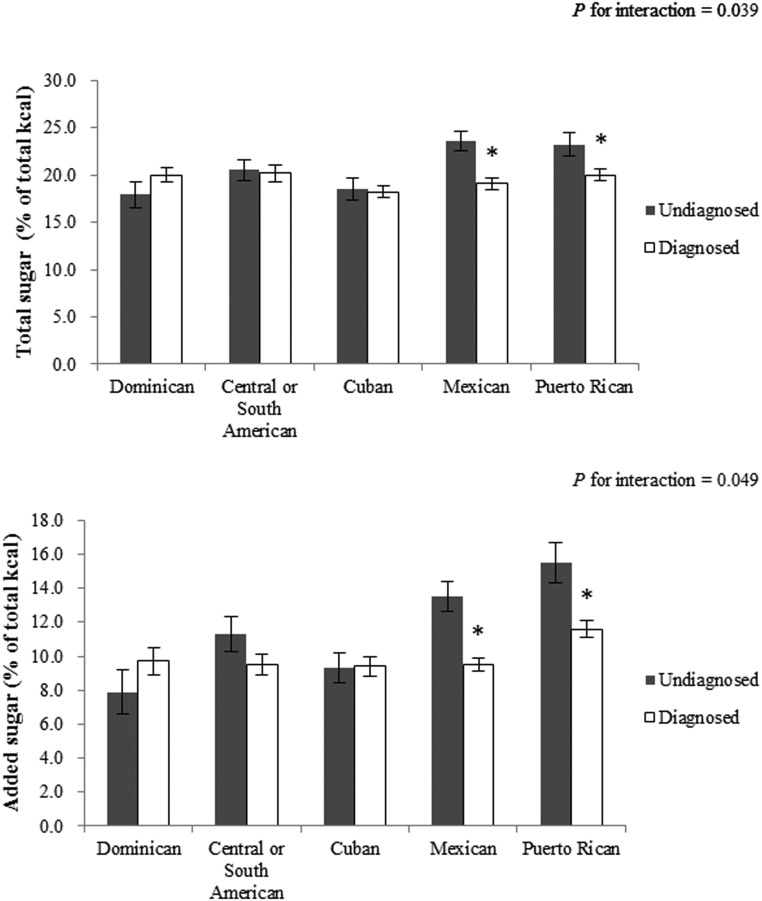
We then examined total and added sugar intake (as percentage of total energy) between diagnosed and undiagnosed adults with diabetes across Hispanic/Latino backgrounds. As shown in Figure 1, total and added sugar intakes were lower in participants with diagnosed diabetes than their undiagnosed counterparts among those of Mexican (total sugar: 19.1 vs 23.6% of total energy, P < .001; added sugar: 9.5 vs 13.5% of total energy; P < .001) and Puerto Rican backgrounds (total sugar: 20.0 vs 23.2% of total energy; P = .043; added sugar: 11.6 vs 15.5% of total energy; P = .009). No significant difference was found in other Hispanic/Latino background groups.
Figure 1.
Total and added sugar intake (% from energy) among Hispanics/Latinos with diabetes by diagnosis status of diabetes, stratified by Hispanic background, Hispanic Community Health Study/Study of Latinos, 2008–2011. *, P < .05 comparing diagnosed vs undiagnosed groups within Hispanic background. Data are means (SEs), adjusted for age, sex, field center, body mass index categories, use of diabetes medications, family history of diabetes, annual household income, education, born in the United States, US residence more than 10 years, health insurance status, smoking, alcohol consumption, and physical activity, stratified by Hispanic/Latino background. All values were weighted to account for complex survey design.
We also performed stratified analyses by Hispanic/Latino background (Supplemental Table 3), sex (Supplemental Table 4), and age groups (Supplemental Table 5). Results were generally consistent pertaining to differences in macronutrient intake between adults who had diagnosed vs undiagnosed diabetes across subgroups.
Macronutrient intake by glycemic control
We examined associations between dietary intake and glycemic control among individuals with diagnosed diabetes. As shown in Table 4, among Hispanic/Latino adults with diabetes, individuals with HbA1c less than 7% consumed less total fat (30.1 vs 31.0% of total energy, P = .039), saturated fat (9.7 vs 10.2% of total energy, P = .001), and total cholesterol (144.0 vs 156.4 mg per 1000 kcal, P = .017) compared with those with HbA1c of 7% or greater. We also found a borderline significantly greater intake of carbohydrate (51.1 vs 50.1% of total energy, P = .069) in diabetic individuals with HbA1c less than 7% than those with HbA1c of 7% or greater. In addition, we also examined the associations between macronutrient dietary intake and HbA1c levels as continuous variables. There were no statistically significant associations between the macronutrient dietary intake and HbA1c levels (Supplemental Table 6), but the directions of the associations were generally consistent with the results in Table 4 by comparing individuals with HbA1c less than 7% and those with HbA1c of 7% or greater.
Table 4.
Macronutrient Intake Among Hispanics/Latinos With Diagnosed Diabetes by Glycemic Control, Hispanic Community Health Study/Study of Latinos, 2008–2011
| HbA1C < 7.0% (n = 967) | HbA1C ≥ 7.0% (n = 1242) | P Value | |
|---|---|---|---|
| Total energy, kcal | 1693.2 (29.9) | 1657.8 (24.6) | .364 |
| Carbohydrates, g | 218.3 (4.3) | 208.0 (3.40) | .054 |
| Total kilocalories, % | 51.1 (0.4) | 50.1 (0.4) | .069 |
| Total sugar, g | 81.1 (2.0) | 76.1 (1.8) | .066 |
| Total kilocalories, % | 19.5 (0.4) | 18.8 (0.4) | .149 |
| Added sugar, g | 43.2 (1.5) | 39.7 (1.5) | .118 |
| Total kilocalories, % | 9.8 (0.3) | 9.3 (0.3) | .252 |
| Fiber, g | 18.4 (0.4) | 17.5 (0.4) | .105 |
| Fiber, g per 1000 kcal | 11.2 (0.2) | 11.0 (0.2) | .364 |
| Protein, g | 74.7 (1.4) | 74.8 (1.4) | .971 |
| Total kilocalories, % | 18.3 (0.2) | 18.5 (0.2) | .553 |
| Total fat, g | 60.2 (1.4) | 60.8 (1.3) | .752 |
| Total kilocalories, % | 30.1 (0.3) | 31.0 (0.3) | .039 |
| Saturated fatty acids, g | 19.1 (0.5) | 19.8 (0.4) | .279 |
| Total kilocalories, % | 9.7 (0.1) | 10.2 (0.1) | .001 |
| Monounsaturated fatty acids, g | 22.5 (0.6) | 22.8 (0.6) | .766 |
| Total kilocalories, % | 11.2 (0.1) | 11.5 (0.2) | .141 |
| Polyunsaturated fatty acids, g | 13.4 (0.4) | 13.0 (0.3) | .486 |
| Total kilocalories, % | 6.6 (0.1) | 6.5 (0.1) | 0.635 |
| Total cholesterol, mg | 238.7 (6.9) | 257.2 (6.7) | 0.044 |
| Total cholesterol, mg per 1000 kcal | 144.0 (3.9) | 156.4 (3.7) | 0.017 |
Data are means (SEs), adjusted for age, sex, field center, Hispanic/Latino background, body mass index categories, use of diabetes medications, family history of diabetes, annual household income, education, born in the United States, US residence more than 10 years, health insurance status, smoking, alcohol consumption, and physical activity. All values were weighted to account for complex survey design.
Discussion
The ADA dietary guidelines for patients with diabetes recommend increasing fiber intake to 14g per 1000 kcal and reducing saturated fat to less than 10% of total energy intake, dietary cholesterol less than 300 mg/d (9). Our study found that only 21.2% of US Hispanic/Latino adults with diabetes met the ADA recommendations for fiber intake. This is consistent with data from NHANES 2005–2010 (predominantly non-Hispanic white population) (16). Although it would be impracticable to consume the required amount of fiber (>50 g/d) for glycemic control improvement, individuals with diabetes are still encouraged to increase fiber intake due to its potential benefits on cardiovascular disease risk factors such as decreases in total and low-density lipoprotein cholesterol (9, 10). Whereas NHANES data showed that US diabetic patients consumed on average slightly more saturated fat than is recommended (16), the average saturated fat intake in our Hispanic/Latino diabetic population (9.9% of calories) was aligned with the ADA recommendations, regardless of diagnosis status of diabetes. In addition, the majority of individuals in the HCHS/SOL with diabetes (71.2%) met the ADA recommendations for total cholesterol intake.
A major contribution of this study is that we extend previous findings (16) to US Hispanics/Latinos of diverse backgrounds. Both the HCHS/SOL and the NHANES 2005–2010 (16) demonstrated that individuals who were aware of having diabetes consumed less carbohydrates and sugars than their undiagnosed counterparts. People who are aware of their diabetes might have more knowledge about healthful diets and modify their diet/lifestyle to control their glucose levels. This is particularly pertinent for consumption of sugar-containing foods/drinks, especially sugar-sweetened beverages, which should be limited or avoided in people with diabetes according to the ADA recommendations (9). Consistent with our results, a previous study has reported that adults with diagnosed diabetes had a lower consumption of added sugar from sugar-sweetened beverages than those with undiagnosed diabetes (22). Also, our study found that individuals with diagnosed diabetes tended to consume more monounsaturated fat than their undiagnosed counterparts. Numerous studies have indicated that high monounsaturated fat intake is associated with glycemic control and improved insulin responsiveness, blood lipid profiles, and cardiovascular risk factors (9, 14, 23–25).
A unique contribution of this study is that we evaluated dietary intake in adults with diabetes of diverse Hispanic/Latino backgrounds. Interestingly, we found that people of Mexican and Puerto Rican backgrounds with diagnosed diabetes consumed less total and added sugar than their undiagnosed counterparts, but there was no obvious such difference among other Hispanic/Latino background groups. This may suggest that, after diagnosis of diabetes, individuals of Mexican and Puerto Rican Hispanic backgrounds might make a greater effort to limit total and added sugar intake or be less likely to report higher levels of intake compared with those of other Hispanic/Latino backgrounds, although the underlying reasons are unclear. At the same time, it should be noted that total and added sugar intake was much higher in people with undiagnosed diabetes of Mexican (total sugar: 23.6% of total energy; added sugar: 13.5% of total energy) and Puerto Rican (total sugar: 23.2% of total energy; added sugar: 15.5% of total energy) backgrounds than in those of other Hispanic/Latino backgrounds (total sugar: 17.9%–20.5% of total energy; added sugar: 7.9%–11.3% of total energy). This may imply that total and added sugar intake is high among Mexican and Puerto Rican background individuals, and therefore reducing its consumption after diabetes diagnosis is more feasible compared with those of other Hispanic/Latino backgrounds. Nevertheless, our findings should be interpreted with caution because of reporting bias in self-reported dietary data. It is possible that some individuals might be more familiar with macronutrient composition of traditional meals or food commonly consumed by their group of background.
Another major finding of our study is that low total and saturated fat intake and low total cholesterol intake were associated with good glycemic control among US Hispanic/Latino adults with diagnosed diabetes. Our results are consistent with those observed in US diabetic men from the NHANES 2005–2010 (16), American Indians with diabetes (17), and African American women with diabetes (26). An adverse effect of high saturated fat consumption on HbA1c levels has been also observed in individuals without diabetes (27). Interestingly, we also found that carbohydrate intake was marginally significantly higher in diabetic people with good glycemic control than those with poor glycemic control, which is consistent with previous studies (16, 17). However, it should be noted that quality and/or resources of carbohydrates were not examined, and these data were based on cross-sectional observational studies. In addition, underreporting of sugar-sweetened beverage intake was observed in individuals with overweight and obesity, which might influence the positive association between sugar-sweetened beverage intake and risk of obesity (28). Thus, we speculate that carbohydrate intake including sugar intake might be underreported in those diabetic individuals with poor glycemic control, but further research is needed to clarify this.
This study may have important public health implications because our findings further emphasize the importance of diabetes awareness, which may help people to improve dietary eating behaviors. This might be of particular importance to US Hispanic/Latinos who have a high prevalence of diabetes mellitus. Moreover, a large proportion of diabetes cases (∼40%) have been found to be undiagnosed in our US Hispanic/Latino study (3), which was similar to that found in non-Hispanic whites (29). Many diet recommendations have been developed for the management of individuals with diabetes. For example, carbohydrate intake from vegetables, fruits, whole grains, legumes, and dairy products should be advised over intake from other carbohydrate sources, and total fiber intake, especially from natural food sources (vs supplements) may have a beneficial effect on major cardiovascular disease risk factor control (9).
To the best of our knowledge, this is the first study evaluating macronutrient intake by awareness of diabetes in a relatively large representative population sample of US Hispanics/Latinos of diverse national backgrounds. Several limitations of this study should be noted. Due to the cross-sectional nature of this study, we were unable to test causality of the observed differences in dietary intake by diabetes awareness and glycemic control. In addition, bias and measurement error in self-reported dietary intake data are inevitable. Participants might have inaccurately described their dietary intake and have underreported foods detrimental for glycemic control or overreported healthy foods beneficial to glycemic control (20). Notably, underestimation of total energy intake, a prevalent finding in most of dietary self-report surveys (30–32), was also observed in this study of Hispanics/Latinos with diabetes. Indeed, our previous analysis has indicated similar mean energy intake in the general US Hispanics/Latinos (20), and this systematic underreporting of energy intake has been also confirmed using objective recovery biomarkers (21). However, it should be noted that our analyses were controlled for total energy intake by using a percentage of kilocalories or a ratio per 1000 kcal for nutrient intake, but the results could be biased if underestimation of total energy intake was associated with diabetes diagnosed status. Although we have adjusted for multiple covariates in the analyses, there might be other unknown and unmeasured confounding factors. Because of the complexity of the biological and cultural diversity within the US Hispanics/Latinos, information on internal or detailed disparities in acculturation and custom might not be fully captured in our study. Finally, we defined undiagnosed diabetes mellitus based on one-time laboratory measurements. Therefore, some individuals who were classified as having undiagnosed diabetes may have not actually met clinical criteria for diabetes on repeat tests.
In conclusion, our data suggested that Hispanic/Latino adults with diabetes, in general, adhere to the ADA recommendations for restricting saturated fat and total cholesterol intake but should need to increase fiber intake to benefit their diabetes management. Diabetes awareness is favorably associated with some aspects of dietary intake (less carbohydrate and sugar intake and greater monounsaturated fat intake) among US Hispanics/Latinos with diabetes, further emphasizing the importance of screening for diabetes in this population. Additionally, there is significant heterogeneity in sugar intake by diabetes awareness among Hispanic/Latino backgrounds, but this needs to be validated in further studies. These findings may provide useful information for the development of effective diet strategies for diabetes management and control among US Hispanics/Latinos.
Acknowledgments
We thank the staff of Hispanic Community Health Study/Study of Latinos for their important contributions. A complete list of staff and investigators has been provided by P. Sorlie et al (Ann Epidemiol. 2010;20:642–649) and is also available on the study web site (http://www.cscc.unc.edu/hchs/).
The opinions shared by the authors of this manuscript do not represent the opinions of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the federal government.
This work was supported by contracts from the National Heart, Lung, and Blood Institute to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following institutes/centers/offices contributed to the Hispanic Community Health Study/Study of Latinos through a transfer of funds to the National Heart, Lung, and Blood Institute: National Center on Minority Health and Health Disparities, the National Institute of Deadness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements. X.W. was supported by the China Scholarship Council.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADA
- American Diabetes Association
- HbA1c
- glycated hemoglobin
- HCHS/SOL
- Hispanic Community Health Study/Study of Latinos
- NHANES
- National Health and Nutrition Examination Survey.
References
- 1. Ennis S, Rios-Vargas M, Albert N. 2010 Census briefs: the Hispanic population: 2010. US Census Bureau. http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf Accessed May 1, 2015
- 2. Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312:1218–1226. [DOI] [PubMed] [Google Scholar]
- 3. Schneiderman N, Llabre M, Cowie CC, et al. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care. 2014;37:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Axon RN, Gebregziabher M, Echols C, Msph GG, Egede LE. Racial and ethnic differences in longitudinal blood pressure control in veterans with type 2 diabetes mellitus. J Gen Intern Med. 2011;26:1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Egede LE, Gebregziabher M, Lynch CP, Gilbert GE, Echols C. Longitudinal ethnic differences in multiple cardiovascular risk factor control in a cohort of US adults with diabetes. Diabetes Res Clin Pract 2011;94:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sequist TD, Adams A, Zhang F, Ross-Degnan D, Ayanian JZ. Effect of quality improvement on racial disparities in diabetes care. Arch Intern Med. 2006;166:675–681. [DOI] [PubMed] [Google Scholar]
- 7. Mainous AG, 3rd, Majeed A, Koopman RJ, et al. Acculturation and diabetes among Hispanics: evidence from the 1999–2002 National Health and Nutrition Examination Survey. Public Health Rep (Washington, DC: 1974). 2006;121:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vega WA, Rodriguez MA, Gruskin E. Health disparities in the Latino population. Epidemiol Rev. 2009;31:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37(suppl 1):S120–S143. [DOI] [PubMed] [Google Scholar]
- 10. Franz MJ, Powers MA, Leontos C, et al. E The evidence for medical nutrition therapy for type 1 and type 2 diabetes in adults. J Am Diet Assoc. 2010;110:1852–1889. [DOI] [PubMed] [Google Scholar]
- 11. Brunerova L, Smejkalova V, Potockova J, Andel M. A comparison of the influence of a high-fat diet enriched in monounsaturated fatty acids and conventional diet on weight loss and metabolic parameters in obese non-diabetic and type 2 diabetic patients. Diabet Med. 2007;24:533–540. [DOI] [PubMed] [Google Scholar]
- 12. Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. [DOI] [PubMed] [Google Scholar]
- 13. Esposito K, Maiorino MI, Ciotola M, et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med. 2009;151:306–314. [DOI] [PubMed] [Google Scholar]
- 14. Brehm BJ, Lattin BL, Summer SS, et al. One-year comparison of a high-monounsaturated fat diet with a high-carbohydrate diet in type 2 diabetes. Diabetes Care. 2009;32:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wheeler ML, Dunbar SA, Jaacks LM, et al. Macronutrients, food groups, and eating patterns in the management of diabetes: a systematic review of the literature, 2010. Diabetes Care. 2012;35:434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bardenheier BH, Cogswell ME, Gregg EW, Williams DE, Zhang Z, Geiss LS. Does knowing one's elevated glycemic status make a difference in macronutrient intake? Diabetes Care. 2014;37:3143–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu J, Eilat-Adar S, Loria CM, et al. Macronutrient intake and glycemic control in a population-based sample of American Indians with diabetes: the Strong Heart Study. Am J Clin Nutr. 2007;86:480–487. [DOI] [PubMed] [Google Scholar]
- 18. Lavange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siega-Riz AM, Sotres-Alvarez D, Ayala GX, et al. Food-group and nutrient-density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr. 2014;99:1487–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mossavar-Rahmani Y, Shaw PA, Wong WW, et al. Applying recovery biomarkers to calibrate self-report measures of energy and protein in the Hispanic Community Health Study/Study of Latinos. Am J Epidemiol. 2015;181:996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bleich SN, Wang YC. Consumption of sugar-sweetened beverages among adults with type 2 diabetes. Diabetes Care. 2011;34:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on glycaemic control in patients with abnormal glucose metabolism: a systematic review and meta-analysis. Ann Nutr Metab. 2011;58:290–296. [DOI] [PubMed] [Google Scholar]
- 24. Itsiopoulos C, Brazionis L, Kaimakamis M, et al. Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr Metab Cardiovasc Dis. 2011;21:740–747. [DOI] [PubMed] [Google Scholar]
- 25. Tanasescu M, Cho E, Manson JE, Hu FB. Dietary fat and cholesterol and the risk of cardiovascular disease among women with type 2 diabetes. Am J Clin Nutr. 2004;79:999–1005. [DOI] [PubMed] [Google Scholar]
- 26. Bell RA, Summerson JH, Konen JC. Dietary intakes by levels of glycemic control for black and white adults with non-insulin dependent diabetes mellitus (NIDDM). J Am Coll Nutr. 1995;14:144–151. [DOI] [PubMed] [Google Scholar]
- 27. Harding AH, Sargeant LA, Welch A, et al. Fat consumption and HbA(1c) levels: the EPIC-Norfolk study. Diabetes Care. 2001;24:1911–1916. [DOI] [PubMed] [Google Scholar]
- 28. Emond JA, Patterson RE, Jardack PM, Arab L. Using doubly labeled water to validate associations between sugar-sweetened beverage intake and body mass among white and African-American adults. Int J Obes (2005). 2014;38:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the US population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Briefel RR, Sempos CT, McDowell MA, Chien S, Alaimo K. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr. 1997;65:1203s–1209s. [DOI] [PubMed] [Google Scholar]
- 31. Poslusna K, Ruprich J, de Vries JH, Jakubikova M, van't Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr. 2009;101(suppl 2):S73–S85. [DOI] [PubMed] [Google Scholar]
- 32. Olendzki BC, Ma Y, Hebert JR, et al. Underreporting of energy intake and associated factors in a Latino population at risk of developing type 2 diabetes. J Am Diet Assoc. 2008;108:1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]