Integrative multiomics analyses of adipose and muscle tissue transcripts, SI, and genotypes revealed novel genetic regulatory mechanisms of insulin resistance in African Americans.
Abstract
Purpose:
Teriparatide (TPTD) improves bone mass and microstructure resulting in reduced risk of vertebral and nonvertebral fractures. However, hip bone mineral density improvements are modest and there are no data confirming that TPTD reduces hip fracture risk. To study the effects of TPTD on the proximal femur, we performed a double-blind trial of TPTD vs placebo (PBO) in patients with osteoarthritis from whom femoral neck (FN) samples were obtained at total hip replacement (THR) surgery.
Methods:
Participants were randomly assigned to receive TPTD or PBO for an average of 40 days before THR. Double tetracycline labeling was initiated 21 days prior to THR to allow histomorphometric assessment of bone formation. During the THR, an intact sample of the FN was procured, fixed, and sectioned transversely. Serum levels of bone turnover markers were measured at baseline and during the THR. Standard histomorphometric parameters were measured and calculated on four bone envelopes (cancellous, endocortical, intracortical, and periosteal). The primary outcome measure was bone formation rate/bone surface (BFR/BS).
Results:
Forty individuals were enrolled (25 women, mean age, 71.5 ± 8.0 y and 15 men, mean age, 68.9 ± 7.7 y). In cancellous and endocortical envelopes, BFR/BS was 100% higher in the TPTD vs PBO group (P < .05). Bone turnover markers measured at the time of THR correlated with BFR/BS.
Conclusion:
TPTD stimulates bone formation rapidly in cancellous and endocortical envelopes of the FN. Our findings provide a mechanistic basis for TPTD-mediated improvement in FN bone mass and ultimately hip strength. This study is the first demonstration of the effect of any osteoporosis medication on osteoblast activity in the human proximal femur.
Hip fracture is the most devastating consequence of osteoporosis, often resulting in profound disability and early mortality (1, 2). The femoral neck (FN) is the site of approximately 50% of all hip fractures (3). The superior/lateral region of the FN is the most vulnerable; it thins the most with advancing age and is most likely to compress and buckle under the force of a sideways fall (4). Several osteoporosis medications have been evaluated specifically for their ability to reduce the risk of hip fracture. Zoledronic acid, denosumab, and alendronate reduce the hip fracture risk by 40–50% (5–7) and risedronate reduces risk in some but not all studies (8–10). Teriparatide (TPTD) is considered to be the most potent of the osteoporosis therapies and in the United States is the only marketed anabolic medication, defined by its ability to stimulate bone formation and increase bone mass (11). TPTD reduces risk of both vertebral and nonvertebral fractures over 18–24 months (12), but the Pivotal Fracture Trial was underpowered to assess the effect of TPTD on hip fracture incidence (there were only eight hip fractures in the three-arm study with a total of only 2450 patient-years of treatment) (12).
Evidence suggesting that TPTD can reduce risk of hip fracture is based on surrogate outcomes (13). TPTD improves hip bone mineral density (BMD) assessed by dual x-ray absorptiometry; however, the effect is modest compared with the TPTD effect on spine BMD (12, 14–16). Studies that use quantitative computed tomography show that TPTD and the full intact PTH peptide produces an increase in volumetric BMD of the hip, but again the effect is modest compared with the effect on volumetric BMD of the spine (17, 18). Importantly, finite element models of quantitative computed tomography data to determine whole-bone strength suggest that TPTD increases hip strength in a simulated sideways fall and would likely protect against hip fracture (19). Furthermore, radionuclide/positron emission tomography studies demonstrate that TPTD stimulates tracer uptake in the proximal femur (20, 21), consistent with an increase in bone formation in the femur. The FN is composed of both cancellous and cortical bone and bone biopsies from the iliac crest confirm that TPTD rapidly stimulates bone formation (within 6 wk) in both cancellous and cortical bone envelopes (22, 23) and ultimately improves cancellous bone volume (BV) and cortical thickness (24, 25). It is unknown, however, whether this finding is generalizable to the proximal femur. In fact, no prior investigations have studied the effects of any osteoporosis medication on osteoblast activity in the human proximal femur. This investigation is most relevant for TPTD, where the salutary action on the femur has not been conclusively demonstrated.
Therefore, the objective of this study was to investigate the effects of TPTD directly on cellular activity in the human proximal femur, using a double-blind trial of TPTD vs placebo (PBO) in patients being scheduled for a total hip replacement (THR) for osteoarthritis. Patients were given two short courses of tetracycline, which binds to the mineralizing front in actively forming bone tissue, to allow assessment of the rate of bone formation. The study design we used is the only ethical way to obtain tetracycline labeled femoral specimens in human beings and demonstrate the direct effect of osteoporosis medication on osteoblast activity in the human proximal femur.
Materials and Methods
Patients
All participants were postmenopausal women and men (age 60–89 y) who had severe hip osteoarthritis requiring an imminent elective THR. Patients having THR for reasons other than osteoarthritis were not included. Participants were selected from two NY hospitals (Helen Hayes Hospital, West Haverstraw, NY; and Hospital for Special Surgery, New York, NY) and inclusion did not require a diagnosis of osteoporosis. Exclusions were tetracycline allergy, diagnosis of rheumatologic disease other than osteoarthritis, severe renal dysfunction (estimated glomerular filtration rate <30 mL/min), uncorrected vitamin D deficiency (≤25 ng/mL), any recent use of glucocorticoids or osteoporosis medication (within 3 mo), use of bisphosphonates within the prior year, and all contraindications to the use of TPTD (Paget's disease of bone, unexplained elevations in alkaline phosphatase, hypercalcemia, hyperparathyroidism, metabolic bone disease other than osteoporosis, history of bone irradiation, or history of bone cancer), any active cancer other than skin, and history of multiple or recent renal calculi.
The study was approved by the institutional review boards of both participant hospitals and all participants provided informed consent. Study progress was monitored by a National Institutes of Health–appointed data safety monitoring board.
Protocol and procedures
After patient consent and inclusion and exclusion criteria were satisfied (including screening laboratory measurements), volunteers were randomly assigned to receive daily sc TPTD (20 mcg) or identically appearing daily sc PBO for approximately 40 days prior to THR. Twenty of the 21 patients who received TPTD were treated for 25–56 days and one patient received TPTD for 84 days due to an unrelated delay in surgery (overall TPTD mean treatment duration, 41 d; median, 40 d). The PBO group was treated for 27–56 days (PBO mean treatment duration, 39 d; median, 40 d). TPTD and PBO were supplied by Eli Lilly. Volunteers were taught to self inject TPTD or PBO and self injection technique was then observed. Double tetracycline labeling was initiated 21 days prior to THR following a standard protocol (tetracycline, 250 mg four times daily for 3 d; 10 d off; demeclocycline, 150 mg four times daily for 3 d). The THR was performed approximately 5 days after the last demeclocycline dose was administered.
During the THR, an intact sample of the mid-FN was procured and the superior and posterior aspects were labeled with ink. The sample consisted of a ring of the FN of 1.0–1.5 cm in thickness, centered on the midpoint of the FN (Figure 1). The specimen was fixed in 10% formalin and embedded without decalcification following standard protocol for undecalcified iliac crest bone biopsy specimens, as previously described in our laboratory (24, 26). The FN was subsequently sectioned transversely using a Reichert Jung Polycut S microtome. Three adjacent sections were cut from two levels 100 microns apart. Within each level, one 20-micron thick section was mounted unstained, and two 7-micron sections were stained with Goldner trichrome and toluidine blue, respectively.
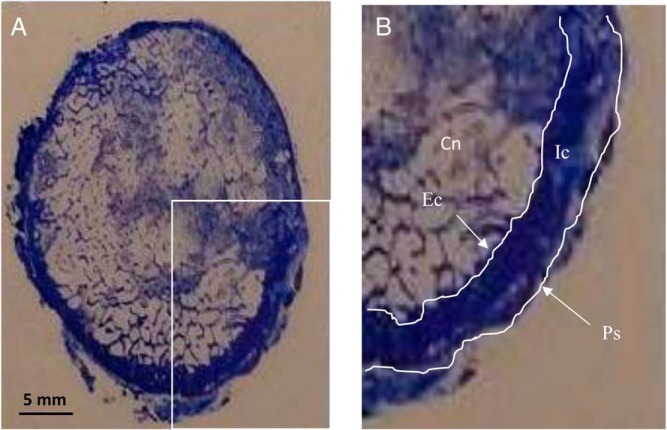
Figure 1.
A, Cross section of the femoral neck, toluidine blue stain. B, Magnified view of lower right quadrant of the FN indicating the four envelopes that were analyzed: Cn, cancellous; Ec, endocortical; Ic, intracortical; Ps, periosteal. The histomorphometric analysis was performed within each envelope for the entire cross section.
Four bone envelopes (cancellous, endocortical, intracortical, and periosteal) were analyzed as previously described using OsteoMeasure version 3.0. (OsteoMetrics, Inc.) (24, 26). Within each envelope, all bone within the periosteal perimeter was analyzed (Figure 1). Depending on the size of each specimen, the ranges of areas and perimeters measured were as follows: trabecular area, 61.5–241.2 mm2; trabecular perimeter, 1115–3757 mm; cortical area, 53.3–224.3 mm2; endocortical perimeter, 91.8–168.7 mm; periosteal perimeter, 123.9–227.5 mm. The following variables were measured on the Goldner trichrome–stained sections: cancellous BV/total volume (TV), bone surface (BS)/TV, cortical width and porosity, eroded surface/BS, osteoid surface/BS, osteoid volume/BV, and osteoid surface/BS. The following variables were measured on the toluidine blue–stained sections: wall thickness and osteoclast number/BS. The following variables were measured on the unstained sections; single-labeled surface/BS, double-labeled surface/BS, and mineral apposition rate (MAR). The bone formation rate was calculated as the sum of double-labeled surface plus one half of the single-labeled surface multiplied by MAR. All histomorphometric parameters were defined, calculated, and expressed according to the most recent recommendations of the American Society for Bone and Mineral Research (27).
Serum for biochemical bone turnover markers (BTMs) was collected at baseline and in the operating room on the day of the THR. The markers of bone formation measured were procollagen of type I N- propeptide (PINP) and osteocalcin (OC), and the marker of bone resorption was crosslinked C-terminal telopeptide (CTX), all measured by Elecsys cobas (Roche Diagnostics Corporation). Reagents for these biochemical markers were also provided by Roche Diagnostics Corporation. The ranges of intra- and interassay coefficients of variation, based on the analysis of control samples with high, medium, and low concentrations were for OC, 0.5–1.1% and 2.4–4%; for PINP, 1.6–2.5% and 1.9–3%; and for CTX, 1.0–1.6% and 2.9–4.2%.
Statistical analyses
Gender distribution between groups was compared by χ2 Square analysis. Other demographic variables between groups were compared by Student t tests. Group differences in the BTMs and histomorphometric variables were assessed by Wilcoxon two-sided tests or Student t tests, as appropriate. The primary outcome was BFR/BS. Relationships between BFR/BS and BTMs were evaluated by Pearson correlation coefficients (SAS version 9.3; SAS Institute).
Results
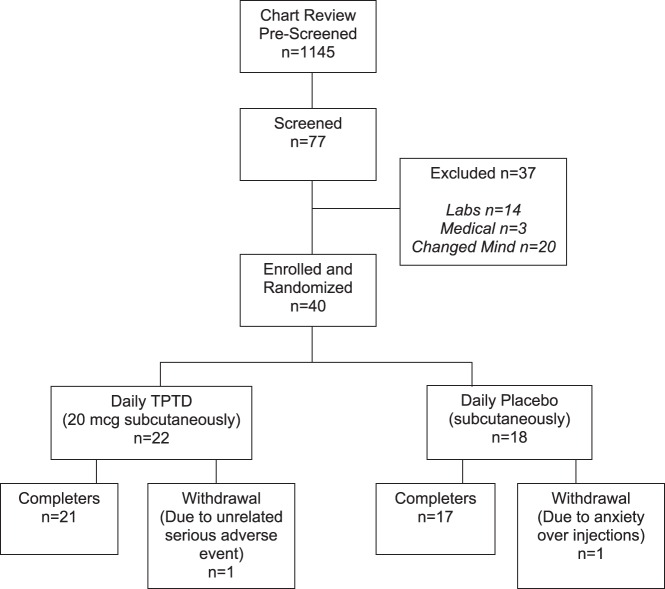
Figure 2 illustrates the derivation of subjects for the trial. Table 1 provides the demographic data for the 40 individuals enrolled (25 postmenopausal women, mean age, 71.5 + 8.0 y; and 15 men, mean age, 68.9 + 7.7 y). The mean body mass index was 27.4 and 30.2 kg/m2 in the TPTD and PBO groups, respectively. There were no significant differences between TPTD- and PBO-treated subjects for any of the demographic variables. Thirty-eight subjects completed the study (Figure 2). One subject dropped out for an unrelated new medical diagnosis of pancreatic cancer (after taking TPTD for 10 d) and the other discontinued due to anxiety from injections (after taking PBO for 9 d).
Figure 2.
CONSORT diagram. Forty subjects were enrolled and randomized, of whom 38 completed.
Table 1.
Baseline Characteristics
| Characteristic | TPTD (n = 22) | PBO (n = 18) |
|---|---|---|
| Female | 59% | 67% |
| Male | 41% | 33% |
| Mean ± sd | Mean ± sd | |
| Age, y | 71.6 ± 9.3 | 69.2 ± 5.8 |
| Height, in | 65.1 ± 4.7 | 65.7 ± 4.0 |
| Weight, lb | 166 ± 42 | 186 ± 44 |
| body mass index, kg/m2 | 27.4 ± 5.5 | 30.2 ± 6.1 |
| Serum Osteocalcin, ng/mL | 20.0 ± 1.7 | 19.8 ± 1.4 |
| Serum P1NP, ng/mL | 47.5 ± 20.8 | 52.6 ± 5.7 |
| Serum C-telopeptide, pg/mL | 378.9 ± 65.1 | 437.2 ± 72.8 |
There were no significant between-group differences.
BTMs in the PBO and TPTD groups at baseline are shown in Table 1. There were no significant changes in any of these markers in the PBO group whereas all BTMs increased after a mean of 41 days of TPTD with an approximately 100% increase for the bone formation markers and 25% increase for the bone resorption marker (Mean serum PINP increased from 51.8 to 110.4 ng/mL; mean serum OC from 19.6 to 43.5 ng/mL; mean serum CTX increased from 443.6 to 550.3 pg/mL; all P < .01).
Table 2 provides structural, static, and dynamic variables in the cancellous, endocortical, and intracortical envelopes for both groups. There were no group differences for any structural variables. Endocortical and intracortical osteoid thickness measurements were significantly lower in the TPTD compared with PBO groups, with a similar strong trend for cancellous osteoid thickness. Activation frequency was approximately 2-fold higher with TPTD vs PBO treatment in both cancellous and endocortical envelopes. Adjusted apposition rate was somewhat higher in the TPTD compared with PBO group in all three envelopes and the group difference was significant for the intracortical envelope. Wall thickness was slightly higher in the PBO compared with TPTD group in the endocortical envelope only.
Table 2.
Structural, Static, and Dynamic Histomorphometric Data
| Variable | TPTD (n = 21) |
PBO (n = 17) |
P-Value | ||
|---|---|---|---|---|---|
| Mean | sd | Mean | sd | ||
| Structural | |||||
| Cancellous bone volume, % | 17.51 | 3.01 | 17.03 | 4.64 | NS |
| Trabecular thickness, μm | 117.4 | 15.54 | 119.4 | 15.92 | NS |
| Trabecular number, No./mm | 1.49 | 0.18 | 1.41 | 0.30 | NS |
| Trabecular separation, μm | 668.2 | 83.3 | 732.0 | 190.0 | NS |
| Cortical width, μm | 866.2 | 258.3 | 917.9 | 227.0 | NS |
| Cortical porosity area, % | 10.56 | 3.64 | 9.49 | 2.79 | NS |
| Cortical porosity number, No./mm2 | 5.17 | 1.32 | 4.80 | 1.48 | NS |
| Cancellous | |||||
| Osteoclast number/BS | 0.072 | 0.062 | 0.052 | 0.049 | NS |
| Osteoid volume/BV, % | 0.523 | 0.484 | 0.625 | 1.116 | NS |
| Osteoid surface/BS, % | 4.17 | 3.75 | 2.83 | 3.55 | NS |
| Osteoid thickness, μm | 4.98 | 0.72 | 5.73 | 1.39 | .056 |
| Wall thickness, μm | 25.13 | 2.54 | 26.13 | 1.77 | NS |
| Eroded surface/BS, % | 3.36 | 1.57 | 3.12 | 1.21 | NS |
| Adjusted apposition rate, μm/d | 1.59 | 1.03 | 1.17 | 0.70 | .16 |
| Activation frequency, cycle/y | 0.712 | 0.571 | 0.305 | 0.263 | .007 |
| Endocortical | |||||
| Osteoclast number/BS | 0.102 | 0.102 | 0.091 | 0.083 | NS |
| Osteoid surface/BS, % | 8.95 | 7.71 | 5.73 | 4.72 | .12 |
| Osteoid thickness, μm | 5.08 | 1.11 | 5.82 | 0.76 | .02 |
| Wall thickness, μm | 27.70 | 2.86 | 29.43 | 1.85 | .037 |
| Eroded surface/BS, % | 4.49 | 2.19 | 4.89 | 3.01 | NS |
| Adjusted apposition rate, μm/d | 1.67 | 1.02 | 1.39 | 1.07 | NS |
| Activation frequency, cycle/y | 1.41 | 0.90 | 0.73 | 0.48 | .006 |
| Intracortical | |||||
| Osteoclast number/BS | 0.224 | 0.201 | 0.156 | 0.128 | NS |
| Osteoid surface/BS, % | 5.16 | 3.83 | 6.06 | 3.90 | NS |
| Osteoid thickness, μm | 5.12 | 1.04 | 6.09 | 0.81 | .003 |
| Wall thickness, μm | 34.88 | 3.79 | 36.25 | 2.33 | NS |
| Eroded surface/BS, % | 6.43 | 4.16 | 4.81 | 2.44 | .15 |
| Adjusted apposition rate, μm/d | 3.01 | 2.52 | 1.62 | 0.67 | .02 |
| Activation Frequency, cycle/y | 1.08 | 0.60 | 0.98 | 0.66 | NS |
Abbreviation: NS, not significant.
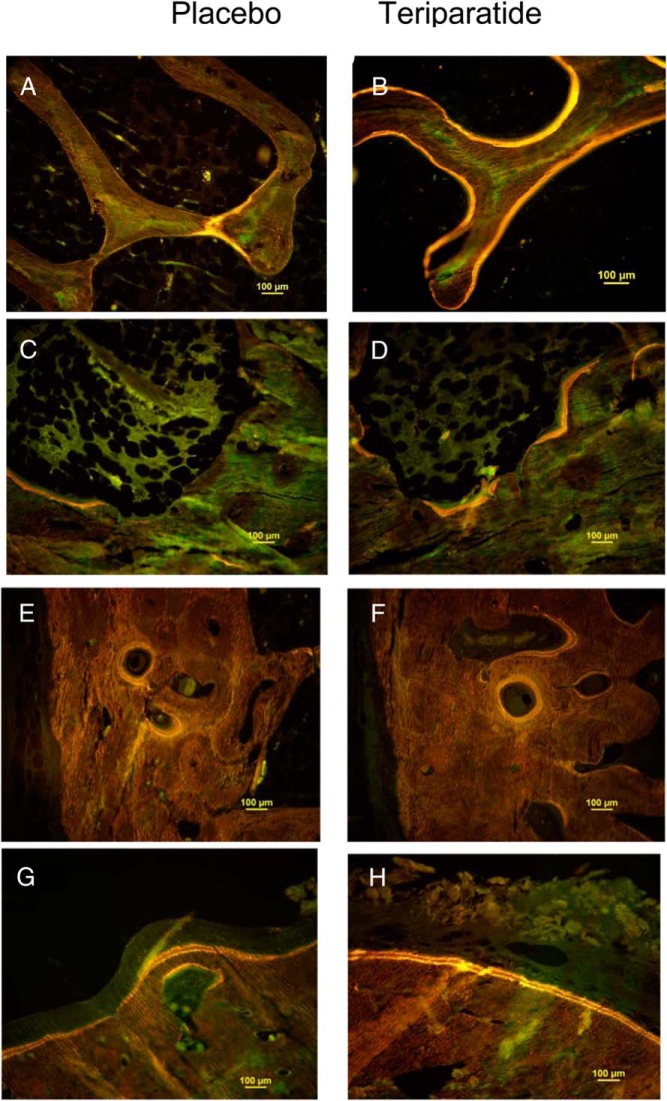
Figure 3 shows representative samples of tetracycline-labeled FN bone from individual patients from the PBO and TPTD groups for each of the four bone envelopes. The cancellous and endocortical envelopes displayed much more tetracycline label in the TPTD patients, indicating more active bone formation. In contrast, in the intracortical envelope, there was no obvious qualitative difference. In the periosteal envelope, both the PBO-treated and TPTD-treated patient had extensive tetracycline label.
Figure 3.
Individual examples of tetracycline labels from representative subjects in the PBO group (left panels) and TPTD group (right panels) from each of the four bone envelopes (A and B, cancellous; C and D, endocortical; E and F, intracortical; and G and H, periosteal).
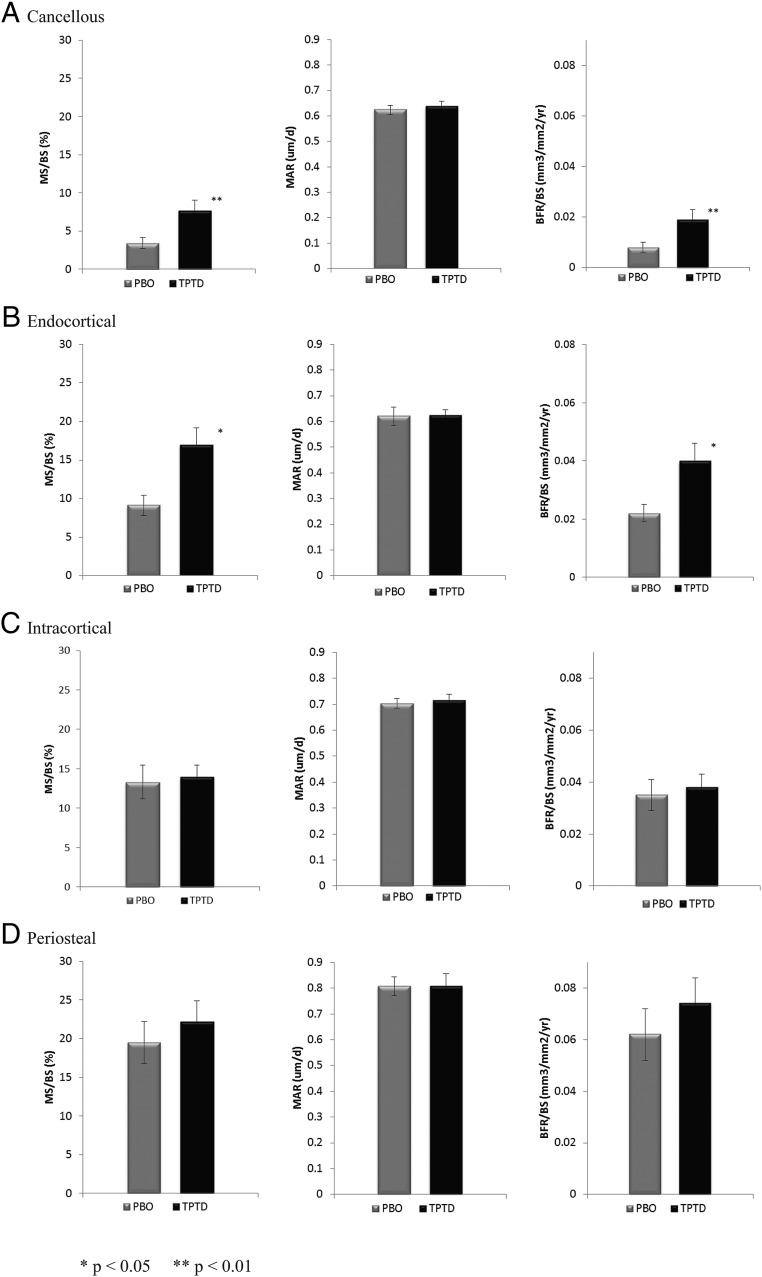
Figure 4 illustrates mean values of mineralizing surface (MS)/BS, MAR, and BFR/BS by group. As suggested by the individual images (Figure 3), on average, there was much more extensive bone surface engaged in active formation (MS/BS) in both cancellous (120% group difference; P < .01) and endocortical bone envelopes (85% group difference; P < .05) in TPTD-treated patients compared with PBO-treated patients. There was no significant difference in MAR between the two groups. Group differences in BFR/BS mirrored those of MS/BS (Figure 4). In contrast, there were no group differences in any of the bone formation variables in the intracortical or periosteal envelopes.
Figure 4.
Dynamic indices (MS/BS), MAR, BFR/BS (in the FN in four bone envelopes: A, cancellous; B, endocortical; C, intracortical; and D, periosteal). Statistical differences were analyzed by Wilcoxon two-sided tests. *, P < .05; **, P < .001 for TPTD vs PBO group.
There were significant correlations between bone formation marker concentrations on the day of THR and BFR/BS in the FN in cancellous and endocortical envelopes, both within the entire population, as well as in the TPTD group alone. For the TPTD group alone (n = 17), serum OC and PINP correlated with cancellous BFR/BS (r = 0.533, P = .013 and r = 0.590, P = .008, respectively) and endocortical BFR/BS (r = 0.537, P = .012 and r = 0.644, P = .003, respectively). For the entire population (n = 38), cancellous BFR/BS correlated with, OC (r = 0.608, P < .0001) and PINP (r = 0.674, P < .0001). Endocortical BFR/BS correlated with OC (r = 0.605, P < .001) and PINP (r = 0.675, P < .001).
Discussion
This is the first study to evaluate the direct effects of an osteoporosis medication on cellular activity in the human proximal femur. We found that TPTD stimulates bone formation in the FN, the site of approximately 50% of all hip fractures, after less than 6 weeks of treatment. The findings were most striking in endocortical and cancellous bone envelopes. Based on our prior study of similar TPTD treatment duration in the iliac crest, it is likely that the early mechanism of action is stimulation of ongoing remodeling-based bone formation as well as initiation of modeling-based formation on previously quiescent bone surfaces (23). Our findings provide a mechanistic basis for TPTD-mediated improvement in bone mass at the hip, which has been shown to result in an increase in estimated femoral strength (19). Even a small amount of bone formed in the thinnest, weakest area of the FN (the superior segment) is thought to produce an effect on hip strength (4, 19, 28). Our investigation also illustrates an innovative technique for the study of bone-active therapies in human proximal femoral bone.
Given the short treatment period, there were no group differences expected for any structural variables. The higher values for activation frequency are consistent with the mechanism of action of TPTD. The interesting finding that osteoid thickness was consistently lower in the TPTD group suggests an early increase in the rate of osteoid mineralization with TPTD. Accordingly, we might have expected to see a higher value for MAR in the TPTD group. This was not the case; however, the higher adjusted apposition rate in the TPTD group is consistent with this finding. The observation that endocortical wall thickness was slightly higher in the PBO-treated patients was unexpected and has no plausible explanation; it may represent a spurious finding.
Although we have studied the cellular effects of TPTD extensively through iliac crest bone biopsies (22, 24, 25), the femur is a weight-bearing site, in contrast with the nonweight bearing iliac crest. Looking across different populations, our study of the proximal femur suggests that levels of MS/BS in cancellous, endocortical, and intracortical envelopes are in fact quite similar to those seen in iliac crest biopsy specimens (22–24, 29). The periosteal envelope is extremely different, however, with much greater MS/BS and BFR/BS in the femur compared with the ilium. This could be related to differences between weight-bearing and nonweight-bearing bone, but also could be due to increased remodeling activity associated with the underlying disease of osteoarthritis.
Our study provides the first demonstration that serum BTMs correlate strongly with histomorphometrically determined bone formation rate in the human FN. The biochemical marker level after approximately 6 weeks of treatment reflects the level of bone formation at the most clinically important site in patients with osteoporosis.
As TPTD can positively influence bone formation in the endocortical envelope of the FN, it is conceivable that it could benefit the outcome of THR. Harris Hip Scores, a standard quality-of-life indicator for THR patients (30) did not differ between groups at 3 months or 1 year, but all patients had marked improvement after THR. The sample may have been too small or treatment exposure might have been too brief to see an effect. However, a prolonged residual improvement (at 1 y) of a similar short course of TPTD vs PBO (6 wk) was seen on jaw bone in a similarly small group of patients (n = 40) with advanced periodontal disease. (31) Any possible beneficial effects of TPTD to improve osseointegration or reduce the likelihood of prosthesis loosening, suggested in some animal studies, might not be appreciated until a later time point (5–10 y) (32, 33).
This study has several limitations. All subjects had severe osteoarthritis of the hip, which might affect remodeling characteristics in the FN (34). This might be the explanation for why the bone formation rate was so high in the periosteal envelope in both PBO and TPTD-treated individuals. If this is so, the reactive bone formation might have obscured a more subtle stimulation of BFR/BS by TPTD in the periosteal envelope. The duration of exposure to TPTD was brief, so TPTD-mediated changes in structural parameters (such as cancellous BV and cortical width) would not be expected and were not seen. The brief TPTD treatment period was chosen for ethical and practical reasons; it was not possible to delay the THR surgery once the necessity for THR was demonstrated. In addition, pre-THR treatment duration was determined by surgical scheduling and therefore ranged from 4–8 weeks, with one patient receiving 12 weeks of TPTD. There did not seem to be any relationship between BFR/BS and duration of TPTD treatment. Moreover, the average treatment duration was the same between the TPTD and PBO groups.
Important strengths of the study include that it was placebo controlled, and histomorphometric analysis was performed on four bone envelopes. This is the first investigation of the effect of any osteoporosis medication on osteoblast activity (assessed by tetracycline labeling) in the human proximal femur. This study helps close important gaps in our knowledge about the effects of TPTD on the hip.
We conclude that TPTD stimulates bone formation rapidly in the FN, with prominent effects in cancellous and endocortical envelopes. Our findings provide a mechanistic basis for TPTD-mediated improvement in bone mass of the FN and, ultimately, bone strength of the hip.
Acknowledgments
Teriparatide and placebo were supplied by Eli Lilly and Company. Eli Lilly and Company played no role in the design or conduct of the trial, nor in the data interpretation or manuscript preparation. We thank Roche Diagnostics for providing the PINP assay reagents free of charge for the purpose of conducting this research study. These reagents are not currently commercially available in the United States.
This study was registered in ClinicalTrials.gov as trial number NCT01309399.
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number ARO59204.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Summary: F.C. consults for Eli Lilly, Amgen, Radius, and Merck; received lecture fees from Eli Lilly and Amgen; and received grant support from Eli Lilly and Amgen. D.W.D. consults for Eli Lilly, Amgen, Radius, and Merck; received lecture fees from Eli Lilly and Amgen; and received grant support from Eli Lilly and Amgen. R.L. consults for Eli Lilly and received grant support from Eli Lilly. J.W.N., H.Z., M.Z., C.R., Y.H., and M.B. have nothing to declare.
Footnotes
- BFR
- bone formation rate
- BMD
- bone mineral density
- BS
- bone surface
- BTM
- bone turnover marker
- BV
- bone volume
- CTX
- C-terminal telopeptide
- FN
- femoral neck
- MAR
- mineral apposition rate
- MS
- mineralizing surface
- OC
- osteocalcin
- PBO
- placebo
- PINP
- procollagen of type I N propeptide
- THR
- total hip replacement
- TPTD
- teriparatide
- TV
- total volume.
References
- 1. Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: A systematic epidemiological review. Osteoporos Int. 2009;20:1633–1650. [DOI] [PubMed] [Google Scholar]
- 2. Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta-analysis: Excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152:380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karagas MR, Lu-Yao GL, Barrett JA, Beach ML, Baron JA. Heterogeneity of hip fracture: Age, race, sex, and geographic patterns of femoral neck and trochanteric fractures among the US elderly. Am J Epidemiol. 1996;143:677–682. [DOI] [PubMed] [Google Scholar]
- 4. Reeve J, Loveridge N. The fragile elderly hip: Mechanisms associated with age-related loss of strength and toughness. Bone. 2014;61:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. [DOI] [PubMed] [Google Scholar]
- 6. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. [DOI] [PubMed] [Google Scholar]
- 7. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. [DOI] [PubMed] [Google Scholar]
- 8. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–1352. [DOI] [PubMed] [Google Scholar]
- 9. McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340. [DOI] [PubMed] [Google Scholar]
- 10. Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) study group. Osteoporos Int. 2000;11:83–91. [DOI] [PubMed] [Google Scholar]
- 11. Recker RR, Kimmel DB, Dempster D, Weinstein RS, Wronski TJ, Burr DB. Issues in modern bone histomorphometry. Bone. 2011;49:955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. [DOI] [PubMed] [Google Scholar]
- 13. Eriksen EF, Keaveny TM, Gallagher ER, Krege JH. Literature review: The effects of teriparatide therapy at the hip in patients with osteoporosis. Bone. 2014;67:246–256. [DOI] [PubMed] [Google Scholar]
- 14. McClung MR, San Martin J, Miller PD, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165:1762–1768. [DOI] [PubMed] [Google Scholar]
- 15. Obermayer-Pietsch BM, Marin F, McCloskey EV, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res. 2008;23:1591–1600. [DOI] [PubMed] [Google Scholar]
- 16. Leder BZ, Tsai JN, Uihlein AV, et al. Two years of denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): A randomized controlled trial. J Clin Endocrinol Metab. 2014;99:1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95:1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. [DOI] [PubMed] [Google Scholar]
- 19. Keaveny TM, McClung MR, Wan X, Kopperdahl DL, Mitlak BH, Krohn K. Femoral strength in osteoporotic women treated with teriparatide or alendronate. Bone. 2012;50:165–170. [DOI] [PubMed] [Google Scholar]
- 20. Moore AE, Blake GM, Taylor KA, et al. Assessment of regional changes in skeletal metabolism following 3 and 18 months of teriparatide treatment. J Bone Miner Res. 2010;25:960–967. [DOI] [PubMed] [Google Scholar]
- 21. Frost ML, Siddique M, Blake GM, et al. Differential effects of teriparatide on regional bone formation using (18)F-fluoride positron emission tomography. J Bone Miner Res. 2011;26:1002–1011. [DOI] [PubMed] [Google Scholar]
- 22. Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1–34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2007;22:495–502. [DOI] [PubMed] [Google Scholar]
- 23. Lindsay R, Cosman F, Zhou H, et al. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: Early actions of teriparatide. J Bone Miner Res. 2006;21:366–373. [DOI] [PubMed] [Google Scholar]
- 24. Dempster DW, Cosman F, Kurland ES, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: A paired biopsy study. J Bone Miner Res. 2001;16:1846–1853. [DOI] [PubMed] [Google Scholar]
- 25. Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18:1932–1941. [DOI] [PubMed] [Google Scholar]
- 26. Dempster D, Shane E. Bone quantification and dynamics of bone turnover. Principles and practice of endocrinology and metabolism Lippincott, Philadelphia: 2002:475–479. [Google Scholar]
- 27. Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poole KE, Treece GM, Gee AH, et al. Denosumab rapidly increases cortical bone in key locations of the femur: A 3D bone mapping study in women with osteoporosis. J Bone Miner Res. 2015;30:46–54. [DOI] [PubMed] [Google Scholar]
- 29. Dempster DW, Zhou H, Recker RR, et al. Skeletal histomorphometry in subjects on teriparatide or zoledronic acid therapy (SHOTZ) study: A randomized controlled trial. J Clin Endocrinol Metab. 2012;97:2799–2808. [DOI] [PubMed] [Google Scholar]
- 30. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: A systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bashutski JD, Eber RM, Kinney JS, et al. Teriparatide and osseous regeneration in the oral cavity. N Engl J Med. 2010;363:2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fitch DA, Ancarani C, Bordini B. Long-term survivorship and complication rate comparison of a cementless modular stem and cementless fixed neck stems for primary total hip replacement. Int Orthop. 2015;39(9):1827–1832. [DOI] [PubMed] [Google Scholar]
- 33. Yang X, Ricciardi BF, Dvorzhinskiy A, et al. Intermittent parathyroid hormone enhances cancellous osseointegration of a novel murine tibial implant. J Bone Joint Surg Am. 2015;97:1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jordan GR, Loveridge N, Power J, Clarke MT, Reeve J. Increased cancellous bone in the femoral neck of patients with coxarthrosis (hip osteoarthritis): A positive remodeling imbalance favoring bone formation. Osteoporos Int. 2003;14:160–165. [DOI] [PubMed] [Google Scholar]