We compared effects of teriparatide and denosumab on PTH, bone turnover markers, and bone histomorphometry in osteoporotic postmenopausal women. The findings were inconsistent with an early indirect anabolic effect of denosumab.
Abstract
Context:
Denosumab-induced PTH elevation may stimulate early bone formation.
Objective:
Our objective was to evaluate whether denosumab-induced changes of intact PTH (iPTH) result in early anabolic effects according to histomorphometry and bone turnover markers (BTMs) compared with teriparatide, an established anabolic agent.
Design:
This open-label, randomized study used quadruple labeling to label bone before/after treatment, with a transiliac bone biopsy at 3 months.
Setting:
This study took both in both US and Canadian sites.
Participants:
Sixty-nine postmenopausal women with osteoporosis were included.
Interventions:
Teriparatide (20 μg/day) for 6 months and denosumab (60 mg once) were used in this study.
Main Outcome Measure:
Between-treatment comparison of change from baseline to month 3 in cancellous mineralizing surface/bone surface, histomorphometric indices in four bone envelopes, and BTM and iPTH at baseline, 1, 3, and 6 months was undertaken.
Results:
After denosumab, iPTH peaked at month 1 (P < .001), then declined, remaining above baseline through month 6 (P ≤ .01); after teriparatide, iPTH declined at all time points (P < .001). From baseline to month 3, cancellous mineralizing surface/bone surface increased with teriparatide and decreased with denosumab and at month 3, was higher with teriparatide. Similar results were observed in other bone envelopes. BTMs increased from baseline in teriparatide-treated subjects (procollagen type 1 N-terminal propeptide at month 1 and carboxyterminal cross-linking telopeptide of type 1 collagen at month 3); procollagen type 1 N-terminal propeptide and carboxyterminal cross-linking telopeptide of type 1 collagen decreased from baseline at all time points in denosumab-treated subjects.
Conclusions:
Denosumab treatment increased iPTH but inhibited bone formation indices. In contrast, teriparatide treatment decreased iPTH but stimulated bone formation indices. These findings are not consistent with the hypothesis of early indirect anabolic effect with denosumab.
Osteoporosis drugs can be grouped into either anabolic or anticatabolic (antiresorptive) classes of bone-active agents (1). Teriparatide (recombinant human PTH analog [1–34]), is currently the only US Food and Drug Administration–approved agent in the anabolic class (1, 2). The anticatabolic/antiresorptive drug class consists of bisphosphonates, estrogens, selective estrogen receptor modulators, calcitonin, and denosumab (3–5).
Although denosumab has been characterized as an antiresorptive agent by histomorphometric and bone turnover marker (BTM) assessments (6, 7), some effects of this drug have led to speculation that denosumab may have distinctive properties with respect to its mechanism of action (MOA). Studies have reported that intact PTH (iPTH) levels are increased in the 1–3 months after denosumab administration (8–13). It has been suggested that elevation of PTH may result in indirect stimulation of bone formation during a time of inhibited osteoclast activity (12, 14) and in combination with denosumab-induced reductions in cortical porosity (7, 12, 14, 15), may potentially play a role in continuous long-term bone mineral density (BMD) gains (16) reported with denosumab.
The study objectives were to characterize denosumab-induced changes in iPTH levels in conjunction with bone histomorphometric indices and BTMs and compare these effects with those induced by teriparatide, an anabolic agent. To achieve these goals, this study used quadruple-labeled histomorphometry (17) of transiliac biopsies obtained from postmenopausal women with osteoporosis treated with either denosumab or teriparatide. This is the first study to use quadruple-labeled histomorphometry with an antiresorptive agent and to compare directly the MOAs of an antiresorptive agent and an anabolic agent. Quadruple labeling allowed both baseline and posttreatment information to be obtained from a single biopsy from each subject.
Subjects and Methods
Subjects
Ambulatory postmenopausal women with osteoporosis, aged 55–89 years, were included if their BMD T-score was at least 2.5 at the lumbar spine, femoral neck, or total hip; or less than 1.5 with a prevalent vertebral or nonvertebral fragility fracture. Subjects had laboratory values within reference ranges for serum calcium, PTH, and alkaline phosphatase.
Subjects were excluded if they had any condition specified in the teriparatide boxed warning or any treatment contraindications for teriparatide or denosumab (2, 18). Exclusion criteria included 25-hydroxyvitamin D concentration of less than 10 ng/ml; treatment with drugs or any active or suspected disease, except osteoporosis, known to affect bone metabolism; history of malignancy in the previous 5 years; significantly impaired hepatic or renal function; treatment with systemic glucocorticoids (≥5 mg/day prednisone or equivalent) in the previous 6 months; and prior or current use of teriparatide, PTH, PTH analog, or denosumab or any IV bisphosphonate. Oral bisphosphonate use required a wash-out period before screening (6 months off for use >2 weeks but ≤2 months; 1 year off for use >2 months but ≤1 year; 2 years off for use >1 year).
Study design and treatment
This was an open-label, randomized phase IV study of 6 months' duration comparing teriparatide (20 μg/d) with denosumab (60 mg once) in postmenopausal women with osteoporosis. The primary endpoint was comparison of change from baseline to 3 months in mineralizing surface/bone surface (MS/BS) in the cancellous envelope of transiliac bone biopsies. Biopsies were taken at 3 months because iPTH is known to be elevated at this time point after treatment with denosumab (8–13). The iPTH increase may be maximal with the first dose of denosumab (11–13); therefore, any anabolic properties that are observable by histomorphometric analyses should be apparent by 3 months after the first dose. The effect of both teriparatide and denosumab on remodeling also is apparent by this time based on biochemical marker data (11, 19). Finally, with quadruple-labeled histomorphometry, the possibility was considered that findings in subjects treated with teriparatide might not be reliable beyond 3 months because some of the initial labels could have been lost with remodeling. It was thought that loss of the first set of labels by this mechanism should not be a factor if the biopsy was obtained at 3 months; however, in a study using quadruple labeling, the first set of labels was demonstrated to be intact after 3 months of PTH(1–84) treatment (20). MS/BS was chosen as the primary endpoint because it is a robust variable that readily distinguishes between anabolic and antiresorptive MOAs (19). Other objectives were to characterize changes in: 1) serum iPTH; 2) a panel of histomorphometric indices in 4 bone envelopes (cancellous, endocortical, intracortical, periosteal), and 3) BTMs following denosumab or teriparatide treatment, comparing treatment effects.
To assure a balance in bone turnover between the treatment groups at baseline, subjects were stratified based on their value of nonfasting procollagen type 1 N-terminal propeptide (P1NP) at screening: less than 30 ng/ml, at least 30 ng/ml to less than 50 ng/ml, or more than 50 ng/ml. Using a computer-generated random sequence generated by an interactive voice-response system, subjects were randomly assigned (1:1) to either teriparatide or denosumab treatment groups based on their stratified baseline P1NP value. Subjects received elemental calcium (approximately 1000 mg/d) and vitamin D3 or equivalent (800–1200 IU/day) throughout the study.
The study received ethical review board approval at all sites, and subjects gave informed written consent. The study was conducted in accordance with the Declaration of Helsinki and applicable laws and guidelines.
Assessments
Bone biopsy procedure
Quadruple labeling using the fluorochromes, demeclocycline, and tetracycline was performed (17). Beginning 18–23 days before randomization, subjects took 150 mg demeclocycline 4 times daily following a 3:12:3 day sequence (baseline labeling); (Supplemental Appendix 1). One to 5 days after the last demeclocycline dose, subjects were randomized to either denosumab or teriparatide. Beginning 22–25 days before the bone biopsy, subjects took 250 mg tetracycline (posttreatment labeling) following the same dosing schedule as demeclocycline. The biopsy was performed 5–8 days following the last tetracycline dose.
Transiliac bone biopsies were performed using a Rochester needle or similar large-bore (6–8 mm) manual trephine. Sample preparation and histomorphometric analyses were performed as described (19, 21). The central histomorphometry reader was blinded to treatment assignment and all indices were measured, calculated, and expressed following American Society for Bone and Mineral Research Histomorphometric Nomenclature Committee recommendations (22).
Histomorphometry
Demeclocycline can overestimate the length of fluorochrome labels relative to tetracycline (23, 24). This artifactual difference can potentially affect interpretation of data from indices derived from label length, such as MS/BS, which could be overestimated. To assess treatment effects accurately and minimize label length artifacts, demeclocycline and tetracycline were administered for the first and second labeling periods, respectively (23). The length of the tetracycline labels in both the teriparatide and denosumab treatment groups was multiplied by a correction factor (23) of 1.34, which was empirically determined by the histomorphometry laboratory assessing the biopsies.
For biopsy samples missing labels, the value of MS/BS was zero. Mineral apposition rate (MAR) was reported as missing, and MAR-derived indices, such as bone formation rate, were assigned a value of zero. For biopsies with a single label only, MAR and MAR-derived indices were evaluated by two methods: 1) assigned an imputed value of 0.3 μm/day or 2) counted as missing (25). Results from the imputation method only are reported here as results from the 2 methods were similar.
Baseline and 3-month data were obtained for MS/BS, bone formation rate (BFR)/bone surface (BS), MAR, single-label surface/BS, and double-label surface/BS in all four bone envelopes defined as described (26). Only 3-month data were obtained for BFR/bone volume (BV) (cancellous envelope only), activation frequency, active formation period, adjusted apposition rate, mineralization lag time, osteoid maturation time, and total formation period (cancellous, endocortical, and intracortical envelopes). Data were only available at month 3 because they require static components in their derivation, which cannot be separately assessed at baseline. Static indices measured were osteoid surface/BS, osteoid volume/BV, osteoid thickness, wall thickness (W.Th), and eroded surface (ES)/BS. These indices were assessed in the cancellous, endocortical, and intracortical envelopes with the exception of osteoid volume/BV, which was assessed in only the cancellous envelope.
Biochemical markers of bone turnover and intact PTH
Blood (fasting) was collected at baseline and months 1, 3, and 6 for determination of serum BTM (P1NP and carboxyterminal cross-linking telopeptide of type 1 collagen [CTX]) and iPTH.
Safety
Clinical chemistry and hematology measurements were performed at the screening visit and vital signs were collected at each study visit. Treatment-emergent adverse events (TEAEs) and serious adverse events were recorded at each study visit and at early discontinuation, if applicable.
Sample size
Twenty completers per group with adequate biopsies would provide approximately 80% power to detect a significant difference with a two-sided alpha level of 0.05 using a two-sample t test. Assuming a 40% dropout or unevaluable biopsy rate, the total number of randomized subjects needed was 68.
Statistical analyses
All prespecified efficacy and safety analyses were performed on the full analysis set (FAS) using a modified intent-to-treat principle. The FAS included data from all randomized subjects receiving at least one dose of study drug according to the actual received treatment. Tests of treatment effects were conducted at a two-sided alpha level of 0.05. No adjustment for multiplicity was made for primary and secondary analyses. Demographics and baseline characteristics were summarized by treatment group. Categorical variables were summarized by frequencies and percentages. Continuous variables were summarized by means and standard deviations. Categorical variables were analyzed using Fisher's exact test, and continuous variables were analyzed using t test. Efficacy variables were analyzed using an analysis of covariance model, with treatment group as the main effect and baseline measurement as a covariate. Paired t tests were used to assess changes from baseline within each treatment group. If the normality assumption of efficacy variable was not satisfied at the 0.01 significance level and if visual inspection of the data deemed it necessary, an appropriate nonparametric test, such as the Wilcoxon rank-sum test, was performed to assess treatment differences; the Wilcoxon signed-rank test was used to assess changes from baseline within each treatment group.
Results
Baseline demographics and characteristics
Overall, 109 subjects were included from the United States and Canada; of these, 69 subjects were randomized beginning in February 2013 (33 teriparatide; 36 denosumab) (Supplemental Appendix 2). Thirty teriparatide-treated and 34 denosumab-treated subjects completed the study. The last subject completed the study in June 2014. Reasons for discontinuation in the teriparatide group were adverse event (n = 1) and sponsor decision (n = 2), and in the denosumab group, subject decision (n = 2). Biopsies were collected from 66 subjects (31 teriparatide; 35 denosumab); all biopsies were evaluable for all indices with the exception of one biopsy with a missing cortex.
Baseline characteristics between the two treatment groups were similar with the exception of mean age, which was higher in the denosumab group than in the teriparatide group (65.2 years vs 61.6 years; P = .041) (Table 1); this difference did not affect any treatment outcomes (data not shown). The groups were balanced with respect to P1NP stratification.
Table 1.
Baseline Characteristics
| Teriparatide n = 33 | Denosumab n = 36 | |
|---|---|---|
| Age, y (mean [sd]) | 61.6 (5.8) | 65.2 (8.3)a |
| Race, n (%) | ||
| Asian | 0 (0) | 2 (5.6) |
| Black or African American | 1 (3.0) | 2 (5.6) |
| Multiple | 1 (3.0) | 1 (2.8) |
| White | 31 (93.9) | 31 (86.1) |
| Body mass index, kg/m2 (mean [sd]) | 24.7 (4.6) | 24.0 (3.5) |
| Previous osteoporosis therapy, n (% yes) | 11 (33.3) | 14 (38.9) |
| Prevalent clinical fracture, n (% yes) | 17 (51.5) | 16 (44.4) |
| Lumbar spine T-score, mean (sd) | −2.58 (0.91) | −2.45 (0.93) |
| Femoral neck T-score, mean (sd) | −2.24 (0.92) | −2.39 (0.63) |
| Total hip T-score, mean (sd) | −1.68 (0.93) | −1.92 (0.76) |
| Serum P1NP strata, n (%) | ||
| <30 ng/ml | 0 (0) | 2 (5.6) |
| ≥30 ng/ml to ≤50 ng/ml | 13 (39.4) | 14 (38.9) |
| >50 ng/ml | 20 (60.6) | 20 (55.6) |
| 25-hydroxyvitamin D (mean [se]), ng/mlb | 35.5 (2.5) (n = 32) | 38.0 (3.1) (n = 35) |
| iPTH (mean [se]), pg/mlc | 34.8 (2.4) (n = 33) | 33.7 (2.4) (n = 34) |
P < .05 using Fisher's exact test for categorical variables and using t test for continuous variables.
Reference range: ≥29 ng/ml for >18 y of age.
Reference range: 14–72 pg/ml for >18 y of age.
Of the five significant protocol violations (one each in three teriparatide-treated subjects; one denosumab-treated subject with two violations), all involving violations of inclusion/exclusion criteria, one subject was removed from the study. Analyses excluding these subjects were performed for the primary endpoint; inclusion of these subjects in the FAS did not significantly change the result or conclusions.
iPTH and BTMs
In the denosumab group, iPTH was significantly increased from baseline (89.6 mean percent change; P < .001) after 1 month of treatment (mean absolute values, baseline: 38.1 pg/ml; month 1: 63.5 pg/ml; reference range: 14–72 pg/ml) (Figure 1A). Thereafter, iPTH levels declined but were still significantly greater than baseline at month 3 (68.0%, 51.5 pg/ml) and at month 6 (31.9%, 47.9 pg/ml). However, despite the elevated iPTH levels, both P1NP and CTX levels were significantly reduced (P < .001 vs baseline at all time points). In contrast, after 1 month of teriparatide treatment, there was a significant reduction from baseline (−27.1 mean percent change; P < .001) in iPTH levels (mean absolute values, baseline: 38.6 pg/ml; month 1: 27.1 pg/ml), continuing through month 6 (month 3: −22.4%, 25.1 pg/ml; month 6: −35.3%, 22.2 pg/ml) (Figure 1B). However, both P1NP and CTX levels increased significantly starting at 1 and 3 months, respectively, and rose through 6 months with P1NP approximately 400% above baseline at 6 months.
Figure 1.
Changes in serum bone turnover markers and intact parathyroid hormone. (A) Denosumab; (B) teriparatide. Mean percent change ± SE is shown. †P < .001 for within treatment group comparison from baseline to each time point. *P = .01 for within treatment group comparison from baseline to each time point using t test.
Bone histomorphometry
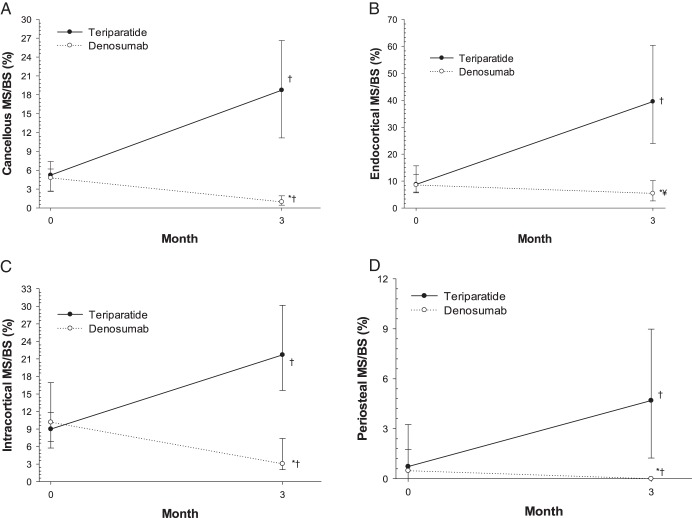
At baseline, in the cancellous envelope, median MS/BS values were not significantly different between teriparatide (5.22%) and denosumab (4.78%) (Figure 2A, Table 2). At month 3, median MS/BS was significantly higher in the teriparatide group (18.73%) than in the denosumab group (0.96%) (P < .001). From baseline to month 3, MS/BS increased with teriparatide treatment (P < .001) and decreased with denosumab treatment (P < .001) (median of change, +12.4% vs −2.5%; P < .001 for between-group comparison), indicating increased extent of new bone formation with teriparatide and decreased extent with denosumab. Similar results for MS/BS were observed in the endocortical, intracortical, and periosteal envelopes (Figure 2, B–D).
Figure 2.
Changes in mineralizing surface. Median and interquartile range (Q1, Q3) are shown for the four bone envelopes at baseline and 3 months: (A) cancellous; (B) endocortical; (C) intracortical; and (D) periosteal. For samples with no label, MS/BS was assigned a value of zero. *P < .001 for between treatment group comparison at baseline or 3 months in each envelope using Wilcoxon rank-sum test. †P < .001 for within treatment group comparison from baseline to 3 months in each envelope using Wilcoxon signed-rank test. ¥P < .01 for within treatment group comparison from baseline to 3 months in each envelope using Wilcoxon signed-rank test.
Table 2.
| Indices, Median (Q1, Q3) | Cancellous |
Endocorticalc |
Intracorticalc |
Periostealc |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Month 3 |
Baseline |
Month 3 |
Baseline |
Month 3 |
Baseline |
Month 3 |
|||||||||
| TPTD | DMAb | TPTD | DMAb | TPTD | DMAb | TPTD | DMAb | TPTD | DMAb | TPTD | DMAb | TPTD | DMAb | TPTD | DMAb | |
| MS/BS, %d | 5.22 | 4.78 | 18.73e | 0.96e,f | 8.73 | 8.54 | 39.50e | 5.42f,g | 8.99 | 10.16 | 21.69e | 3.05e,f | 0.73 | 0.48 | 4.69e | 0.00e,f |
| (2.63, 7.39) | (2.68, 6.19) | (11.13, 26.64) | (0.44, 1.93) | (5.90, 15.64) | (5.62, 12.44) | (23.92, 60.39) | (2.63, 10.15) | (5.74, 11.84) | (6.85, 16.95) | (15.61, 30.17) | (2.03, 7.38) | (0.00, 3.25) | (0.00, 1.76) | (1.24, 8.98) | (0.00, 0.00) | |
| n = 31 | n = 35 | n = 31 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | |
| BFR/BS, mm3/mm2/yh | 0.0096 | 0.0091 | 0.0366e | 0.0014e,f | 0.0201 | 0.0169 | 0.0889e | 0.0096e,f | 0.0216 | 0.0229 | 0.0668e | 0.0048e,f | 0.0008 | 0.0005 | 0.0051e | 0.0000e,f |
| (0.0051, 0.0148) | (0.0048, 0.0115) | (0.0226, 0.0543) | (0.0005, 0.0033) | (0.0096, 0.0353) | (0.0092, 0.0241) | (0.0445, 0.1501) | (0.0029, 0.0193) | (0.0127, 0.0295) | (0.0144, 0.0376) | (0.0465, 0.0950) | (0.0025, 0.0099) | (0.0000, 0.0033) | (0.0000, 0.0023) | (0.0014, 0.0111) | (0.0000, 0.0000) | |
| n = 31 | n = 35 | n = 31 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | |
| MAR, μm/dayh | 0.55 | 0.52i | 0.57 | 0.41e,f | 0.56 | 0.50i | 0.60j | 0.45e,f | 0.63 | 0.63 | 0.84e | 0.40e,f | 0.30 | 0.30 | 0.30 | 0.30 |
| (0.48, 0.58) | (0.45, 0.54) | (0.49, 0.59) | (0.30, 0.48) | (0.52, 0.61) | (0.46, 0.55) | (0.53, 0.63) | (0.32, 0.50) | (0.54, 0.70) | (0.54, 0.70) | (0.78, 0.90) | (0.30, 0.45) | (0.30, 0.43) | (0.30, 0.37) | (0.30, 0.33) | (0.30, 0.30) | |
| n = 31 | n = 35 | n = 31 | n = 33 | n = 29 | n = 34 | n = 30 | n = 31 | n = 30 | n = 35 | n = 30 | n = 34 | n = 20 | n = 25 | n = 29 | n = 5 | |
| sLS/BS, % | 4.68 | 4.13 | 15.44e | 1.28e,f | 7.80 | 7.90 | 24.24e | 7.18f | 8.34 | 11.69 | 15.28e | 4.66e,f | 1.45 | 0.77 | 9.37e | 0.00e,f |
| (2.71, 7.19) | (2.90, 6.29) | (10.16, 18.53) | (0.66, 2.62) | (5.93, 12.59) | (5.69, 9.93) | (14.65, 30.32) | (4.13, 10.58) | (5.75, 11.24) | (7.09, 13.89) | (11.17, 17.26) | (2.54, 9.57) | (0.00, 5.50) | (0.00, 3.50) | (2.47, 13.92) | (0.00, 0.00) | |
| n = 31 | n = 35 | n = 31 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | |
| dLS/BS, % | 2.47 | 2.43 | 10.44e | 0.22e,f | 5.11 | 4.72 | 28.84e | 1.68e,f | 4.72 | 5.82 | 14.96e | 0.83e,f | 0.00 | 0.00 | 0.00 | 0.00i,g |
| (1.54, 3.16) | (1.19, 3.31) | (6.00, 15.41) | (0.00, 0.79) | (2.21, 8.57) | (3.33, 7.47) | (12.83, 43.96) | (0.00, 5.08) | (2.61, 6.36) | (2.80, 9.73) | (10.55, 19.69) | (0.00, 1.87) | (0.00, 0.39) | (0.00, 0.29) | (0.00, 0.62) | (0.00, 0.00) | |
| n = 31 | n = 35 | n = 31 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | |
Abbreviations: dLS/BS, double labels per bone surface; DMAb, denosumab; sLS/BS, single labels per bone surface; TPTD, teriparatide. Median values are in bold.
Baseline values were derived from demeclocycline labels.
Month 3 values were derived from tetracycline labels.
One sample (teriparatide group) had a missing cortex and was not included in analysis of cortical envelopes.
For samples with no label, MS/BS was assigned a value of zero.
P < .001 for within treatment group comparison from baseline to 3 months in each envelope using Wilcoxon signed-rank test.
P < .001 for between treatment group comparison at baseline or 3 months in each envelope using Wilcoxon rank-sum test.
P < .01 for within treatment group comparison from baseline to 3 months in each envelope using Wilcoxon signed-rank test.
For MAR and BFR/BS derived from MAR, MAR measured on samples with double label and on samples with single label only that were imputed to 0.3 μm/day (samples with no label reported as missing for MAR and assigned a value of zero for BFR/BS).
P < .05 for between treatment group comparison at baseline or 3 months in each envelope using Wilcoxon rank-sum test.
P < .05 for within treatment group comparison from baseline to 3 months in each envelope using Wilcoxon signed-rank test.
Results for supporting histomorphometric indices for cancellous and other envelopes are shown in Tables 2 and 3. In all bone envelopes, the median month 3 values for most dynamic and static indices of bone formation were significantly different between treatment groups and were greater for teriparatide vs denosumab. The static index of bone resorption, ES/BS, was significantly lower in the denosumab vs teriparatide group (P < .001) at month 3 in all envelopes.
Table 3.
Histomorphometric Indices (Month 3)a
| Indices, Median (Q1, Q3)c | Cancellous |
Endocorticalb |
Intracorticalb |
|||
|---|---|---|---|---|---|---|
| TPTD | DMAb | TPTD | DMAb | TPTD | DMAb | |
| Dynamic | ||||||
| BFR/BV, % per y | 0.66 | 0.03d | ||||
| (0.45, 0.88) | (0.01, 0.07) | NM | NM | NM | NM | |
| n = 31 | n = 35 | |||||
| Ac.f, cycle/y | 1.41 | 0.06d | 2.77 | 0.34d | 1.88 | 0.12d |
| (0.88, 2.02) | (0.02, 0.14) | (1.48, 4.31) | (0.09, 0.59) | (1.13, 2.48) | (0.06, 0.27) | |
| n = 31 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | |
| a.FP, y | 0.13 | 0.16d | 0.15 | 0.19d | 0.13 | 0.26d |
| (0.12, 0.14) | (0.14, 0.20) | (0.14, 0.16) | (0.16, 0.22) | (0.11, 0.14) | (0.22, 0.35) | |
| n = 31 | n = 33 | n = 30 | n = 31 | n = 30 | n = 34 | |
| Aj.AR, μm/day | 0.58 | 0.14d | 0.76 | 0.58e | 1.03 | 0.35d |
| (0.46, 0.67) | (0.08, 0.33) | (0.56, 0.88) | (0.22, 0.81) | (0.88, 1.18) | (0.19, 0.73) | |
| n = 31 | n = 33 | n = 30 | n = 30 | n = 30 | n = 33 | |
| Mlt, day | 10.93 | 21.68f | 9.28 | 9.15 | 7.21 | 12.04f |
| (9.66, 13.78) | (12.20, 48.83) | (7.37, 11.46) | (5.50, 18.00) | (5.92, 8.52) | (7.08, 24.94) | |
| n = 31 | n = 33 | n = 30 | n = 31 | n = 30 | n = 34 | |
| Omt, day | 10.88 | 9.86e | 11.43 | 10.76 | 8.40 | 11.45d |
| (10.11, 11.97) | (8.28, 11.80) | (9.94, 12.87) | (7.89, 12.92) | (7.45, 9.48) | (9.45, 13.73) | |
| n = 31 | n = 33 | n = 30 | n = 31 | n = 30 | n = 34 | |
| Tt.FP, y | 0.12 | 0.47d | 0.12 | 0.15 | 0.10 | 0.30d |
| (0.11, 0.15) | (0.21, 0.91) | (0.10, 0.15) | (0.11, 0.38) | (0.09, 0.12) | (0.16, 0.52) | |
| n = 31 | n = 33 | n = 30 | n = 31 | n = 30 | n = 33 | |
| Static | ||||||
| OV/BV, % | 2.58 | 0.39d | ||||
| (1.67, 3.51) | (0.17, 0.65) | NM | NM | NM | NM | |
| n = 31 | n = 35 | |||||
| OS/BS, % | 20.51 | 3.46d | 30.85 | 6.95d | 17.81 | 4.34d |
| (12.50, 24.13) | (2.05, 5.40) | (21.47, 48.02) | (2.82, 10.88) | (11.89, 22.50) | (2.07, 6.17) | |
| n = 31 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | |
| O.Th, μm | 6.12 | 3.78d | 6.99 | 4.33d | 7.08 | 4.68d |
| (5.52, 6.71) | (3.49, 4.18) | (5.61, 7.99) | (3.49, 4.78) | (6.15, 7.92) | (3.68, 5.13) | |
| n = 31 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | |
| W.Th, μm | 26.29 | 24.03d | 31.88 | 30.08e | 38.25 | 37.80 |
| (25.58, 27.84) | (22.79, 25.23) | (29.75, 32.99) | (27.61, 32.03) | (33.87, 39.84) | (34.61, 40.15) | |
| n = 31 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | |
| ES/BS, % | 3.73 | 1.52d | 3.79 | 1.65d | 11.19 | 1.27d |
| (2.95, 6.05) | (0.92, 2.87) | (2.79, 6.25) | (0.78, 2.47) | (7.37, 13.71) | (0.90, 3.82) | |
| n = 31 | n = 35 | n = 30 | n = 35 | n = 30 | n = 35 | |
Abbreviations: a.FP, active formation period; Ac.f, activation frequency; Aj.AR, adjusted apposition rate; DMAb, denosumab; Mlt, mineralization lag time; NM, not measured; O.Th, osteoid thickness; Omt, osteoid maturation time; OS, osteoid surface; OV, osteoid volume; Tt.FP, total formation period; TPTD, teriparatide. Median values are in bold.
Month 3 values were derived from tetracycline labels.
One sample (teriparatide group) had a missing cortex and was not included in the analysis of cortical envelopes.
For dynamic parameters derived from MAR, MAR measured on samples with double label and on samples with single label only imputed to 0.3 μm/day (samples with no label reported as missing with the exception of BFR/BV and Ac.f, which were assigned a value of zero).
P < .001 for between treatment group comparison using Wilcoxon rank-sum test.
P < .05 for between treatment group comparison using Wilcoxon rank-sum test.
P < .01 for between treatment group comparison using Wilcoxon rank-sum test.
For indices with baseline and month 3 data, the directional change with treatment was generally consistent across envelopes (increased with teriparatide; decreased with denosumab), with the exception of MAR. In the cancellous envelope, there was no significant longitudinal change in median MAR for the teriparatide group (P = .311), but a significant decrease was observed for the denosumab group (P < .001). Consequently, MAR was higher with teriparatide than with denosumab at 3 months (P < .001). In the endocortical and intracortical envelopes, MAR increased with teriparatide and decreased with denosumab. There was no change in MAR with either treatment in the periosteal envelope, perhaps because of a lower number of samples with labels in this envelope than in the other envelopes.
Figure 3 shows representative images from two subjects each in the teriparatide and denosumab groups. These subjects were chosen because their MS/BS values were similar to the median value for their assigned treatment group. There are many more posttreatment labels (red arrows) in the teriparatide subject (Figure 3A) than in the denosumab subject (Figure 3B), and the labels are longer in the teriparatide subject. Additionally, with teriparatide treatment, the posttreatment label is shifted downward with respect to the baseline label (Figure 3C, see asterisk), confirming that the mineralization front was moving laterally during the bone formation phase.
Figure 3.
Quadruple labeling in teriparatide- and denosumab-treated subjects in cancellous bone. Low- (top) and high-power (bottom) images of samples, each from a different subject, representative of the month 3 median values for MS/BS for each treatment group (18.73% teriparatide vs 0.96% denosumab). The white arrows indicate first labeling period (demeclocycline) at baseline; red arrows indicate second labeling period (tetracycline) at 3 months. A, Teriparatide, low power; B, Denosumab low power; C, Teriparatide, high power; D, Denosumab, high power.
Safety
There were no deaths in this trial. Two subjects in each group reported serious adverse events (teriparatide: chest pain and lung adenocarcinoma; denosumab: appendicitis and deep vein thrombosis); none of these events were considered related to teriparatide or denosumab treatment, and none led to study discontinuation. One teriparatide-treated subject discontinued because of an adverse event of abdominal pain, which was considered by the investigator to be unrelated to teriparatide.
At least one TEAE was experienced by 27 (81.8%) teriparatide-treated and 23 (63.9%) denosumab-treated subjects. The most common TEAEs experienced by teriparatide-treated subjects were contusion related to the biopsy procedure (30.3%); arthralgia (15.2%); and nausea, fatigue, and headache (12.1% each). The most common TEAEs experienced by denosumab-treated subjects were constipation, contusion, and postprocedural discomfort (11.1% each). TEAEs were generally mild or moderate in severity with the exception of one subject in the teriparatide group (lung adenocarcinoma) and two subjects in the denosumab group (appendicitis and carpal tunnel syndrome), whose events were considered severe.
Discussion
Our results confirm previous observations that iPTH levels are elevated after a single administration of denosumab. The response profile is consistent with these studies, with maximal iPTH elevations occurring at 1 month and then declining toward baseline through 6 months (10–13). Although we did not assess the kinetics of endogenous PTH in this study, it is unlikely that they would be similar to the pulsatile increments that occur with daily subcutaneous injection of PTH peptides and have been associated with bone anabolism (27). The increase in PTH that we observed in our study is not unique to denosumab and may be an attribute of the antiresorptive drug class because iPTH also increases in response to alendronate and zoledronic acid treatment (11, 12, 28, 29). It is likely that PTH increases are compensatory responses to maintain serum calcium levels, which decrease with antiresorptive treatment (8–11, 13, 28, 29). In this study, we could not address this hypothesis because serum calcium was assessed at screening only. Likewise, our finding that iPTH levels decrease during teriparatide treatment has been reported previously (28, 30, 31). This effect is expected and is likely because of teriparatide-mediated suppression of endogenous PTH release from the parathyroid glands.
Theoretically, to be defined as a bone anabolic agent, treatment must result in a significant increase in bone mass because of an increase in bone remodeling with a positive basic multicellular unit balance in which bone formation is greater than resorption (1, 25). In the presence of an anabolic agent, indices of bone formation, including MS/BS, BFR/BS, osteoid surface/BS, and W.Th, are expected to increase relative to placebo or baseline because they reflect an increase in osteoblast number and activity. Despite the significant elevation in serum iPTH levels during 6 months of denosumab treatment, these four indices (except W.Th in the intracortical envelope), along with others, including MAR (except in the periosteal envelope), were significantly lower with denosumab compared to teriparatide at 3 months. In addition, with quadruple labeling, we demonstrated that MS/BS and BFR/BS decreased from baseline to 3 months with denosumab treatment, whereas these indices increased with teriparatide treatment.
The finding that teriparatide treatment resulted in significant increases in MS/BS and BFR/BS in the periosteal envelope is interesting and adds to the current body of evidence for this effect (32, 33). Using quadruple labeling, these results provide for the first time direct evidence in a single biopsy for stimulation of bone formation in the periosteal envelope with teriparatide. These same indices, however, decreased with denosumab treatment.
The median baseline value for MAR was significantly different between treatment groups in the cancellous and endocortical envelopes, being higher with teriparatide treatment. This difference does not appear to be the result of differences in baseline bone remodeling rate, because there were no differences in the distribution of subjects in the two treatment groups among the P1NP strata at randomization. After adjusting for baseline MAR, results for primary and secondary endpoints were similar to the original findings and conclusions remained the same. MAR increased with teriparatide in the endocortical and intracortical envelopes, whereas it decreased with denosumab in the cancellous, endocortical, and intracortical envelopes. The current finding that teriparatide is able to stimulate MAR as previously reported (17, 32) and that denosumab reduced MAR is of interest. This is the first study to use quadruple labeling to assess the effects of an antiresorptive agent, and it is the first prospective demonstration of an inhibitory effect of an antiresorptive agent on MAR. It is important to note that this decrease in MAR with denosumab remains significant when only the data from subjects with double label were used (data not shown). The mechanism for such an effect is unclear, but there are possible explanations. One could be because of denosumab's action to reduce osteoclast number and activity, resulting in a decrease in osteoclast-derived osteoblast stimulating factors or “clastokines” (34). A second possibility relates to the decline of MAR over time during the bone formation phase (35, 36). Early in antiresorptive treatment, there is a rapid reduction of initiation of new remodeling, and sites that were active before treatment complete their formation phase (1). Consequently, a greater proportion of the formation sites with denosumab treatment will be at a later stage in the bone formation phase, and, therefore, MAR would be expected to be lower. By contrast, more remodeling units will be initiated with teriparatide with a greater proportion of formation sites at an earlier stage in the bone formation phase, and MAR would be expected to be higher. Treatment effects on osteoblasts are not needed to explain these findings; this could be explained by different effects of denosumab and teriparatide on activation frequency.
This study has several strengths. The ability to measure baseline histomorphometric data as a result of using quadruple-labeling allowed for a head-to-head comparison of directional changes, providing a more accurate and robust picture of drug effects on bone metabolism and MOA. Additionally, although this study was powered for MS/BS, a full panel of supporting histomorphometric indices was assessed across four bone envelopes providing a comprehensive assessment of the effects of these drugs on bone at an early time point. Moreover, based on preclinical findings that suggest a role for modeling-based bone formation in the MOA of denosumab (37), we have the ability to assess both modeling- and remodeling-based formation in all four envelopes in these human biopsy samples. This work is ongoing. Finally, changes in iPTH, histomorphometric indices, and BTMs were assessed during the same time frame in the same subjects. This study was, however, limited by a short duration with a bone biopsy taken after 3 months of treatment.
In conclusion, denosumab-induced increases in iPTH, when examined in parallel with changes in BTMs and a full panel of histomorphometric indices across four bone envelopes, are not consistent with and do not support early bone-forming activity of this drug. Our data suggest that the sustained and substantial increases in BMD seen with denosumab treatment are not explained by indirect anabolic action induced by increases in endogenous PTH and must be caused by other mechanism(s).
Acknowledgments
We thank the study investigators, site personnel, and patients and their families. Medical writing support was provided by Lori Kornberg (INC Research, Raleigh, NC).
This work was sponsored by Eli Lilly and Company.
Trial Registration: ClinicalTrials.gov: NCT01753856.
Disclosure Summary: D.W.D. has received research grants from and serves as a consultant and speaker for Eli Lilly and Company and Amgen, and is a consultant for Merck, Tarsa Pharmaceuticals, and Radius Health. R.R.R. receives research grants from and is a consultant for Eli Lilly and Company and Merck. J.P.B. receives research grants from Amgen, Eli Lilly and Company, and Novartis; serves as a consultant for Amgen, Eli Lilly and Company, and Radius; and is a speaker for Amgen and Eli Lilly and Company. C.P.R. has received grant/research support from Amgen, Merck, and Eli Lilly and Company and Novartis, and has served on scientific advisory boards for Amgen, Merck, Eli Lilly and Company, NPS, and Novartis and receives honorarium and travel expenses for advisory boards. E.M.L. has received institutional grant/research support from Amgen, Merck, and Eli Lilly and Company; has served on scientific advisory boards for Amgen, Merck, Eli Lilly and Company, Radius Health, AgNovos Healthcare, Alexion, NPS, and AbbVie; and receives honorarium and travel expenses for advisory boards. P.D.M. serves on scientific advisory boards for Alexion, Amgen, AgNovos, Lilly, Merck, Radius Pharma, and Roche; receives research grants from Amgen, Boehringer Ingelheim, Immunodiagnostics, Lilly, Merck, Merck Serrano, Novartis, Novo Nordisk, Radius Pharma, Roche Diagnostics, and Takeda; and is a speaker for Alexion Pharmaceuticals and Radius Health. S.D.R. receives research grants from Eli Lilly and Company. D.L.K. receives research grants, consultancies, and/or honoraria from Amgen, Eli Lilly and Company, Pfizer, GSK, Astra Zeneca, and Astalis. R.L. is a speaker for Eli Lilly and Company. J.H.K., J.A., K.A.T., B.J., and V.A.R. are employees of Eli Lilly and Company and own stock. H.Z. has nothing to declare.
Footnotes
- BFR
- bone formation rate
- BMD
- bone mineral density
- BS
- bone surface
- BTM
- bone turnover marker
- BV
- bone volume
- CTX
- carboxyterminal cross-linking telopeptide of type 1 collagen
- ES
- eroded surface
- FAS
- full analysis set
- iPTH
- intact PTH
- MAR
- mineral apposition rate
- MOA
- mechanism of action
- MS
- mineralizing surface
- P1NP
- procollagen type 1 N-terminal propeptide
- TEAE
- treatment-emergent adverse event
- W.Th
- wall thickness.
References
- 1. Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005;20:177–184. [DOI] [PubMed] [Google Scholar]
- 2. Forteo Prescribing Information. Eli Lilly and Company. Indianapolis, IN, 2012. http://pi.lilly.com/us/forteo-pi.pdf Accessed November 30, 2015. [Google Scholar]
- 3. Recker RR, Armas L. The effect of antiresorptives on bone quality. Clin Orthop Relat Res. 2011;469:2207–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reginster JY, Neuprez A, Dardenne N, Beaudart C, Emonts P, Bruyere O. Efficacy and safety of currently marketed anti-osteoporosis medications. Best Pract Res Clin Endocrinol Metab. 2014;28:809–834. [DOI] [PubMed] [Google Scholar]
- 5. Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cummings SR, San Martin J, McClung MR, et al. FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. [DOI] [PubMed] [Google Scholar]
- 7. Reid IR, Miller PD, Brown JP, et al. Denosumab Phase 3 Bone Histology Study Group. Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res. 2010;25:2256–2265. [DOI] [PubMed] [Google Scholar]
- 8. Anastasilakis AD, Polyzos SA, Gkiomisi A, Bisbinas I, Gerou S, Makras P. Comparative effect of zoledronic acid versus denosumab on serum sclerostin and dickkopf-1 levels of naive postmenopausal women with low bone mass: a randomized, head-to-head clinical trial. J Clin Endocrinol Metab. 2013;98:3206–3212. [DOI] [PubMed] [Google Scholar]
- 9. Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–1066. [DOI] [PubMed] [Google Scholar]
- 10. Makras P, Polyzos SA, Papatheodorou A, Kokkoris P, Chatzifotiadis D, Anastasilakis AD. Parathyroid hormone changes following denosumab treatment in postmenopausal osteoporosis. Clin Endocrinol (Oxf). 2013;79:499–503. [DOI] [PubMed] [Google Scholar]
- 11. McClung MR, Lewiecki EM, Cohen SB, et al. AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–831. [DOI] [PubMed] [Google Scholar]
- 12. Seeman E, Libanati C, Austin M, et al. The transitory increase in PTH following denosumab administration is associated with reduced intracortical porosity: a distinctive attribute of denosumab therapy. J Bone Miner Res. 2011; 26(Suppl 1) (presentation 1064). [Google Scholar]
- 13. Sugimoto T, Matsumoto T, Hosoi T, et al. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). Osteoporos Int. 2015;26:765–774. [DOI] [PubMed] [Google Scholar]
- 14. Seeman E, Libanati C, Austin M, et al. Association between transitory increase in PTH following denosumab administration and reduced intracortical porosity is a distinctive attribute of denosumab therapy. Bone. 2012;50:S162. [Google Scholar]
- 15. Zebaze RM, Libanati C, Austin M, et al. Differing effects of denosumab and alendronate on cortical and trabecular bone. Bone. 2014;59:173–179. [DOI] [PubMed] [Google Scholar]
- 16. Papapoulos S, Lippuner K, Roux C, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int. 2015;26:2773–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindsay R, Cosman F, Zhou H, et al. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res. 2006;21:366–373. [DOI] [PubMed] [Google Scholar]
- 18. Prolia Prescribing Information. Amgen. Thousand Oaks, CA, USA, 2015. http://pi.amgen.com/united_states/prolia/prolia_pi.pdf Accessed November 30, 2015. [Google Scholar]
- 19. Dempster DW, Zhou H, Recker RR, et al. Skeletal histomorphometry in subjects on teriparatide or zoledronic acid therapy (SHOTZ) study: a randomized controlled trial. J Clin Endocrinol Metab. 2012;97:2799–2808. [DOI] [PubMed] [Google Scholar]
- 20. Rubin MR, Dempster DW, Sliney J, Jr., Zhou H, et al. PTH(1–84) administration reverses abnormal bone-remodeling dynamics and structure in hypoparathyroidism. J Bone Miner Res. 2011;26:2727–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dempster DW. Bone quantification and dynamics of bone turnover by histomorphometric analysis. In: Becker KL, ed. Principles and Practice of Endocrinology and Metabolism. 2nd ed Philadelphia: J. B. Lippincott Co.; 1995:491–498. [Google Scholar]
- 22. Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lindsay R, Zhou H, Cosman F, Nieves J, Dempster D. Double and quadruple tetracycline labeling of bone: impact of the label itself. J Bone Miner Res. 2013;28:222–223. [DOI] [PubMed] [Google Scholar]
- 24. Parfitt AM, Foldes J, Villanueva AR, Shih MS. Difference in label length between demethylchlortetracycline and oxytetracycline: implications for the interpretation of bone histomorphometric data. Calcif Tissue Int. 1991;48:74–77. [DOI] [PubMed] [Google Scholar]
- 25. Recker RR, Kimmel DB, Dempster D, Weinstein RS, Wronski TJ, Burr DB. Issues in modern bone histomorphometry. Bone. 2011;49:955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dempster DW, Zhou H, Recker RR, et al. A longitudinal study of skeletal histomorphometry at 6 and 24 months across four bone envelopes in postmenopausal women with osteoporosis receiving teriparatide or zoledronic acid in the SHOTZ trial [published online February 3, 2016]. J Bone Miner Res. doi:10.1002/jbmr.2804. [DOI] [PubMed] [Google Scholar]
- 27. Dobnig H, Turner RT. The effects of programmed administration of human parathyroid fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology. 1997;138:4607–4612. [DOI] [PubMed] [Google Scholar]
- 28. Anastasilakis AD, Polyzos SA, Makras P, et al. Circulating semaphorin-4D and plexin-B1 levels in postmenopausal women with low bone mass: the 3-month effect of zoledronic acid, denosumab or teriparatide treatment. Expert Opin Ther Targets. 2015;19:299–306. [DOI] [PubMed] [Google Scholar]
- 29. Polyzos SA, Anastasilakis AD, Terpos E. Transient secondary hyperparathyroidism following intravenous infusion of zoledronic acid. Support Care Cancer. 2009;17:1329–1330. [DOI] [PubMed] [Google Scholar]
- 30. Anastasilakis AD, Polyzos SA, Goulis DG, et al. Endogenous intact PTH is suppressed during Teriparatide (rhPTH 1–34) administration in postmenopausal women with established osteoporosis. Endocr J. 2008;55:613–616. [DOI] [PubMed] [Google Scholar]
- 31. Anastasilakis AD, Polyzos SA, Makras P, et al. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos Int. 2014;25:1633–1642. [DOI] [PubMed] [Google Scholar]
- 32. Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1–34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2007;22:495–502. [DOI] [PubMed] [Google Scholar]
- 33. Ma YL, Zeng Q, Donley DW, et al. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21:855–864. [DOI] [PubMed] [Google Scholar]
- 34. Shiwaku Y, Neff L, Nagano K, et al. The crosstalk between osteoclasts and osteoblasts is dependent upon the composition and structure of biphasic calcium phosphates. PloS One. 2015;10:e0132903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eriksen EF. Normal and pathological remodeling of human trabecular bone: three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr Rev. 1986;7:379–408. [DOI] [PubMed] [Google Scholar]
- 36. Parfitt AM. The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in the cell biology of bone. Calcif Tissue Int. 1984;36:S37–S45. [DOI] [PubMed] [Google Scholar]
- 37. Ominsky MS, Libanati C, Niu QT, et al. Sustained modeling-based bone formation during adulthood in cynomolgus monkeys may contribute to continuous BMD gains with denosumab. J Bone Miner Res. 2015;30:1280–1289. [DOI] [PubMed] [Google Scholar]