The role of muscle in development of metabolic conditions is poorly understood. The authors show that, while there was no overall association between muscle mass, area, and strength and incident diabetes in older adults, more muscle at baseline was protective against incident diabetes for normal weight women.
Abstract
Context:
Skeletal muscle plays a key role in glucose regulation, yet the association between muscle quantity or quality and the risk of developing type 2 diabetes has not been explored.
Objective:
The objective of the study was to assess the association between muscle quantity and strength and incident diabetes and to explore whether this association differs by body mass index (BMI) category.
Design and Setting:
Participants were 2166 older adults in the Health, Aging, and Body Composition Study who were free of diabetes at baseline (1997–1998). Computed tomography and dual-energy x-ray absorptiometry were used to measure abdominal and thigh muscle area and total body lean mass, respectively. Strength was quantified by grip and knee extensions.
Main Outcome Measure:
Incident diabetes, defined as fasting glucose of 126 mg/dL or greater, a physician's diagnosis, and/or the use of hypoglycemic medication were measured.
Results:
After a median 11.3 years of follow-up, there were 265 incident diabetes cases (12.2%). In fully adjusted models, no association was found between muscle or strength measures and incident diabetes (for all, P > .05). For women, there was a significant interaction with BMI category for both abdominal and thigh muscle, such that greater muscle predicted lower risk of incident diabetes for normal-weight women (hazard ratio 0.37 [0.17–0.83] and 0.58 [0.27–1.27] per SD, respectively) and a greater risk for overweight and obese women (hazard ratio 1.23 [0.98–1.54] and 1.28 [1.00–1.64], respectively). No significant interactions by BMI category existed for strength measures or any measures for men (for all, P > .05).
Conclusions:
Greater muscle area is associated with a lower risk of incident diabetes for older normal-weight women but not for men or overweight women.
Lean muscle plays a key role in metabolic function, contributing to both glucose consumption and storage (1, 2), which in turn can regulate glucose levels and prevent hyperglycemia (1). Muscle quantity and quality could therefore play an important role in type 2 diabetes (diabetes) etiology. Previous studies on muscle and diabetes, however, have primarily focused on muscle wasting concurrent to or after a diabetes diagnosis rather than examining low muscle mass as a potential risk factor for diabetes onset (3, 4). Recent studies have shown cross-sectional associations between low muscle mass and insulin resistance, insulin secretion, prediabetes, the metabolic syndrome, and prevalent diabetes, independent of central and visceral adiposity (2, 5–7). However, it remains uncertain whether low muscle mass contributes to diabetes risk because previous research has been limited to cross-sectional data.
Similarly, few data are available on associations between muscle function or quality and metabolic health. Previous studies have shown associations between strength and health outcomes, such as mortality, that are distinct from associations with muscle mass (8), and participation in strengthening activities predicted lower risk of incident diabetes in the Nurse's Health Study (9). Wander et al (10) showed that greater grip strength predicted lower risk of incident diabetes in a cohort of Japanese American men and women, yet to date this is the only study investigating any strength measures and risk of diabetes.
Little is known about the risk factors for normal-weight metabolic obesity, and it is possible that, in those without excess adipose tissue, muscle mass could play a stronger role in metabolic health. In women in the Rancho Bernardo Study, we found that the inverse association between abdominal muscle area and diabetes prevalence was modified by body mass index (BMI) category and that the inverse association was strongest for normal-weight individuals (5), although this relationship has not been examined in men. In their analyses of grip strength and incident diabetes, Wander et al (10) found that the inverse relationship was strongest at lower BMI percentiles. Relatedly, several studies have found no association between muscle mass and metabolic indicators in exclusively overweight and obese individuals (11, 12).
The role of muscle in metabolic health may be particularly relevant in older adults. Old age is associated with a loss of peripheral fat and overall body weight, yet despite this, diabetes incidence and its association with mortality remains high (13). Aging is also associated with a loss of skeletal muscle and strength (14, 15); thus, it is possible that in older adults excess adiposity gradually plays less of a role in developing diabetes, whereas low muscle could become more of a risk factor.
In the current study, we examined the associations between muscle mass and strength and incident diabetes in the Health, Aging, and Body Composition Study. Participants were 70 to 79 years old and free of diabetes at baseline and were followed up prospectively over the course of up to 14 years for incident type 2 diabetes. We hypothesized that those with lower muscle mass and strength at baseline would have a higher risk of incident diabetes over the course of the follow-up and that this effect would be strongest for normal-weight individuals.
Materials and Methods
Participants
The Health, Aging, and Body Composition Study is a prospective cohort study of older men and women who were well functioning at baseline. Detailed inclusion and exclusion criteria have been published previously (16). Participants were aged 70–79 years at the time of the first study visit (1997–1998) and were recruited from a random sample of white Medicare beneficiaries and all age-eligible black community residents in designated ZIP codes in the Pittsburgh, Pennsylvania, and Memphis, Tennessee, areas. The final sample comprised 3075 white (58.3%) and black (41.7%) men (48%) and women (52%). The study protocol was approved by the Institutional Review Boards at the Universities of Pittsburgh and Tennessee, and all participants provided informed consent.
For the current analysis, participants were also excluded if they had type 2 diabetes at baseline, defined as self-reported history of a physician's diagnosis of type 2 diabetes, use of hypoglycemic medication, a fasting glucose measure of 126 mg/dL or greater (7.0 mmol/L) at the baseline visit, and/or a glucose measure of 200 mg/dL or greater (11.1 mmol/L) after a 2-hour glucose tolerance test at baseline. Of the 3075 individuals in the original cohort, 575 (18.7%) had diabetes at baseline, leaving 2500 participants eligible for the current analysis. Of these 2500, complete body composition data were available for 2166, which was the final sample included in our analysis.
Measures
Covariates
Demographics and lifestyle variables were assessed using an interviewer-administered questionnaire. Weight was measured using a calibrated balance-beam scale, and height was measured without shoes using a stadiometer. Participants were classified as normal weight (BMI < 25 kg/m2) or overweight/obese (BMI ≥ 25 kg/m2) (5). Waist circumference was measured at the largest circumference point after expiration between the lower rib and iliac crest while standing. Thigh circumference was measured at the midpoint between the patella and inguinal crease.
Glucose, triglycerides, and high-density lipoprotein were assessed from a fasting (8+ h) blood draw using a Johnson and Johnson Vitros 950 analyzer. Low-density lipoprotein was estimated using the Friedewald equation (17). Participants brought all medications taken in the past 2 weeks to the baseline visit to compile a medication inventory. Blood pressure (millimeters of mercury) was the average of two seated measurements. Participants were classified as hypertensive if they took antihypertensive medications and/or had blood pressure (BP) of 140 mm Hg or greater systolic and/or 90 mm Hg or greater diastolic. An automated glucose oxidase reaction was used to measure fasting glucose and baseline 2-hour postchallenge glucose.
Body composition
Total body fat, percentage body fat, and total lean mass were measured using dual-energy x-ray absorptiometry (DXA) and calculated using Hologic QDR 4500 software (version 8.21). Validity of the DXA measures of fat and muscle mass in this cohort have been published previously (18).
Abdominal body composition was measured using computed tomography (CT) using a 9800 Advantage (GE; Pittsburgh site), Somatom Plus 4 (Siemens; Memphis site), or a Picker PQ 2000S (Marconi Medical Systems; Memphis site). Slices of 10 mm were taken at L4/L5 with participants lying down. Scans were assessed by a single reader at a reading center using a SUN workstation (SPARCstation II; Sun Microsystems). Muscle, fat, and bone were distinguished based on density measured in Hounsfield units (HU) scale. A line was manually drawn around the internal abdominal wall to distinguish visceral (inside) and sc (outside) adipose tissue. The area was determined by the pixel area at a given HU within the specified plane using IDL-based software (RSI Systems). The fat area was defined in the range of −190 to −30 HU, whereas the range for lean muscle was 0–100 HU. The total abdominal muscle area was calculated as the total of the right and left psoas, lateral abdominal muscles, and rectus abdominal muscles.
Muscle and fat in the thigh were measured using CT scans at the midpoint between the greater trochanter and intercondyloid fossa. Fat outside the fascial plane surrounding the thigh muscles was categorized as sc, and fat inside was categorized as intermuscular or im. Total muscle area in the thigh was defined as the total area within the fascial line minus bone (HU > 150) and adipose tissue.
Because measures of abdominal and thigh muscle were derived from single-slice CT scans, these measures will be reported as area (square centimeters). DXA measures of body composition, alternatively, are reported in weight (kilograms) and thus will be referred to as mass.
Strength measures
Grip strength was measured using an isometric dynamometer (Jaymar; JLW Instruments). Participants were excluded if they had severe hand pain or recent surgery. Two trials were performed for each hand; the mean of all four readings was used as the grip strength measure.
Knee extension (quad) strength was measured using an isokinetic dynamometer (125 AP; Kin-Com). A detailed protocol has been described elsewhere (16). Briefly, participants performed three to six extensions at an angular velocity of 60°/sec using the right leg. Strength was defined as the mean maximal torque (in Newton meters) of three trials. Participants were excluded from the strength testing measures if they had a previous diagnosis of stroke, cerebral aneurysms or bleeding, severe hypertension (≥ 200/110 mm Hg), bilateral knee replacement, or severe bilateral knee pain. Because a large number of participants (n = 338) did not have quad strength measures, these analyses were performed separately from the others rather than restricting all analyses to those with complete data (including quad strength).
Incident diabetes
Follow-up assessments were conducted yearly in the clinic and/or by phone through year 15. Fasting glucose measures were available in years 1, 2, 4, 6, 10, and 11. Incident diabetes cases were defined as fasting glucose levels of 126 mg/dL or greater and/or reporting a physician's diagnosis of diabetes and/or use of hypoglycemic medication at the follow-up examination. For years 3, 5, 7, 8, 9, 12, 13, and 14, no fasting glucose was measured. At visits in years 3 and 5, participants were classified as developing incident diabetes if they self-reported a new physician's diagnosis of diabetes and/or used hypoglycemic medication. At years 7, 8, 9, 12, 13, and 14, hypoglycemic medication use was not assessed, and incident diabetes was defined as self-reported a new physician's diagnosis of diabetes.
Analysis
Because body composition (particularly muscle) measures are markedly differently distributed between men and women, a priori we decided to conduct all analyses separately for men and women.
Abdominal muscle area was divided into gender-specific tertiles to first assess linear univariate associations of baseline characteristics with abdominal muscle area, using ANOVA or χ2, where appropriate. Next, staged multivariate Cox regression models were used to determine the association between baseline muscle mass and strength as continuous variables and incident diabetes. In model 1, adjustment included age, race, and site (Memphis or Pittsburgh) in addition to total abdominal area (only for the total abdominal muscle analysis) or total thigh area (only for the total thigh muscle analysis). Model 2 included these variables in addition to lifestyle variables (smoking pack-years and physical activity), lipids (high density lipoprotein, low density lipoprotein, and triglycerides), and prevalent hypertension. In a final model, we adjusted for body size and composition by adjusting for BMI, visceral fat area, and total body fat. These variables were entered individually and then all together. All predictor variables were standardized to produce hazard ratios (HRs) per gender-specific SD.
CT scans of the spine and paraspinous muscles were conducted only at the Pittsburgh site. However, using data only from the Pittsburgh site, we found the correlation between abdominal area with and without the paraspinous muscles included was r = 0.96. This suggests quantifying the abdominal muscle area using only the psoas, rectus, and lateral abdominal muscle groups was an acceptable approximation of total abdominal area. Therefore, to maximize the statistical power by including the largest number of participants and the largest number of events, we used data from participants from both the Memphis and Pittsburgh sites and omitted the paraspinal measures from the total abdominal area quantification.
Interactions between muscle and strength measures and BMI category (normal weight vs overweight/obese) were tested on a multiplicative scale in staged Cox regression models with incident diabetes as the outcome. These interaction models adjusted for covariates and potential confounders in stages similar to the models for main effects, although the final model was adjusted only for visceral fat area (because the interaction variable already accounted for BMI category). As a final step, we stratified by BMI category and performed similar Cox regression models separately for normal weight (BMI < 25 kg/m2) and overweight/obese (BMI ≥ 25 kg/m2) participants. These categories were chosen to examine the qualitative difference in those with and without excess adiposity, rather than focusing on the degree of excess adiposity by separating those who are overweight and obese. These two categories were also used in previous research (5), which found no difference between overweight and obese individuals and so combined these categories; thus, this allows us to extend the findings from previous studies. All analyses were conducted using SPSS version 20, and values of P < .05 were considered statistically significant for all analyses including interaction terms.
Results
Table 1 shows unadjusted baseline characteristics of the study sample across tertiles of abdominal muscle area for women and men. Women had markedly lower abdominal muscle area than men; the highest tertile of abdominal muscle in women (>60 cm2) was considerably smaller than the lowest tertile for men (<75 cm2). For both men and women, nearly all demographic and physiological variables differed significantly across tertiles (P < .05). Men and women in the highest tertile of abdominal muscle area had the highest BMI, the largest waist circumference, most visceral fat, total body fat, highest percentage body fat, and the highest fasting glucose. However, these individuals were also slightly younger, had more overall lean mass, and had greater quad strength and grip strength. For both men and women, those in the lowest muscle tertile were also the least physically active.
Table 1.
Baseline Characteristics for Women and Men by Tertiles of Abdominal Muscle Area
| Tertile 1 | Tertile 2 | Tertile 3 | P for Trend | |
|---|---|---|---|---|
| Women | < 51 cm | 51–60 cm | > 60 cm | |
| n | 396 | 366 | 384 | |
| Age, y | 73.9 (2.9) | 73.6 (2.9) | 73.0 (2.7) | <.001 |
| Race, % white | 75% | 60% | 41% | <.001 |
| BMI, kg/m2 | 24.1 (4.0) | 26.8 (4.1) | 30.7 (5.1) | <.001 |
| Waist circumference, cm | 91.0 (11.5) | 96.2 (11.8) | 103.8 (13.1) | <.001 |
| Visceral fat, cm2 | 102.1 (46.8) | 119.5 (52.5) | 152.9 (64.3) | <.001 |
| Total body fat, kg | 24.0 (7.3) | 28.1 (7.7) | 33.7 (9.2) | <.001 |
| Percentage body fat | 39.0 (5.8) | 40.4 (5.6) | 42.3 (5.1) | <.001 |
| SBP | 135.4 (20.9) | 135.7 (21.4) | 137.2 (21.0) | .37 |
| DBP | 69.2 (11.8) | 70.6 (11.3) | 71.9 (12.3) | .01 |
| Exercise, kcal/wk | 732.0 (1366) | 776.7 (1353) | 761.9 (1038) | .89 |
| Smoking, pack-years | 13.6 (25.4) | 10.4 (19.0) | 11.8 (23.8) | .14 |
| Lean mass, kg | 36.5 (4.1) | 40.2 (4.3) | 45.4 (5.4) | <.001 |
| Fasting glucose, mg/dL | 89.4 (8.9) | 91.0 (9.2) | 95.1 (9.8) | <.001 |
| Hypertensive medications, % | 52 | 48 | 38 | <.001 |
| Thigh muscle, cm2 | 157.7 (24.6) | 181.5 (25.5) | 208.5 (29.2) | <.001 |
| Grip strength, mean, kg | 21.1 (5.1) | 22.0 (5.4) | 24.2 (5.7) | <.001 |
| Quad strength, mean, Nm | 73.2 (20.2) | 81.4 (19.4) | 89.3 (23.1) | <.001 |
| Men | < 75 cm | 75–87 cm | > 87 cm | |
| n | 365 | 316 | 339 | |
| Age, y | 74.3 (2.9) | 73.8 (2.9) | 73.1 (2.7) | <.001 |
| Race, white % | 67% | 69% | 57% | .04 |
| BMI, kg/m2 | 24.4 (3.0) | 26.4 (3.1) | 29.2 (3.7) | <.001 |
| Waist circumference, cm | 94.5 (8.7) | 99.6 (11.6) | 105.8 (12.1) | <.001 |
| Visceral fat, cm2 | 118.9 (52.7) | 149.5 (63.7) | 176.9 (71.9) | <.001 |
| Total body fat, kg | 20.6 (5.7) | 23.2 (6.5) | 27.2 (7.4) | <.001 |
| Percentage body fat | 28.1 (5.0) | 28.8 (5.0) | 30.1 (4.7) | <.001 |
| SBP | 133.2 (18.9) | 136.1 (20.8) | 134.7 (21.7) | .20 |
| DBP | 72.1 (11.4) | 73.7 (11.2) | 74.2 (11.4) | .03 |
| Exercise, kcal/wk | 1222 (1616) | 1308 (1773) | 1760 (2987) | .003 |
| Smoking, pack-years | 28.1 (31.3) | 26.3 (31.5) | 23.6 (30.3) | .16 |
| Lean mass, kg | 51.5 (5.5) | 56.0 (5.4) | 61.9 (6.4) | <.001 |
| Fasting glucose, mg/dL | 93.6 (10.1) | 95.4 (9.9) | 95.7 (9.6) | .01 |
| Hypertensive meds, % | 55 | 52 | 51 | .53 |
| Thigh muscle, cm2 | 233.1 (35.1) | 258.8 (31.0) | 291.7 (40.3) | <.001 |
| Grip strength, mean kg | 35.0 (7.7) | 37.2 (8.2) | 40.6 (7.7) | <.001 |
| Quad strength, mean Nm | 119.4 (29.2) | 133.4 (29.0) | 147.9 (38.9) | <.001 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
Median follow-up time was 11.3 years, during which incident diabetes occurred in 11.8% of women (n = 135) and 12.7% of men (n = 130). Associations between muscle area and strength variables and incident diabetes are shown in Table 2. In unadjusted models, greater abdominal muscle area was associated with a greater risk of developing diabetes (HR 1.29 and 1.57 per SD greater muscle area for men and women, respectively). This relationship was no longer significant, however, after adjusting for demographic variables and total abdominal area (model 1; HR 0.96 and 1.16 for men and women, respectively).
Table 2.
Associations of Muscle Mass and Strength With Incident Diabetes in Community-Living Older Men and Women
| Men | Incident Diabetes HR (95% CI) | Women | Incident Diabetes HR (95% CI) |
|---|---|---|---|
| Abdominal muscle area | Abdominal muscle area | ||
| Unadjusted | 1.29 (1.09–1.52) | Unadjusted | 1.57 (1.36–1.82) |
| +Model 1a | 0.96 (0.77–1.19) | +Model 1a | 1.16 (0.94–1.42) |
| +Model 2b | 0.99 (0.78–1.24) | +Model 2b | 1.13 (0.91–1.39) |
| +Model 3c | 0.93 (0.73–1.19) | +Model 3c | 1.04 (0.83–1.30) |
| Thigh muscle area | Thigh muscle area | ||
| Unadjusted | 1.10 (0.94–1.30) | Unadjusted | 1.65 (1.42–1.91) |
| +Model 1a | 0.82 (0.63–1.07) | +Model 1a | 1.38 (1.12–1.70) |
| +Model 2b | 0.80 (0.60–1.07) | +Model 2b | 1.31 (1.05–1.63) |
| +Model 3c | 0.92 (0.64–1.34) | +Model 3c | 1.16 (0.92–1.47) |
| Total lean mass | Total lean mass | ||
| Unadjusted | 1.21 (1.03–1.43) | Unadjusted | 1.56 (1.35–1.80) |
| +Model 1a | 1.18 (1.00–1.39) | +Model 1a | 1.43 (1.21–1.67) |
| +Model 2b | 1.12 (0.94–1.35) | +Model 2b | 1.27 (1.08–1.51) |
| +Model 3c | 0.84 (0.66–1.07) | +Model 3c | 0.96 (0.75–1.25) |
| Quad strength | Quad strength | ||
| Unadjusted | 1.05 (0.89–1.24) | Unadjusted | 1.52 (1.29–1.78) |
| +Model 1a | 1.04 (0.87–1.24) | +Model 1a | 1.38 (1.17–1.63) |
| +Model 2b | 1.01 (0.83–1.22) | +Model 2b | 1.28 (1.08–1.52) |
| +Model 3c | 0.97 (0.79–1.18) | +Model 3c | 1.16 (0.98–1.37) |
| Grip strength | Grip strength | ||
| Unadjusted | 0.96 (0.81–1.13) | Unadjusted | 1.24 (1.06–1.45) |
| +Model 1a | 0.88 (0.74–1.05) | +Model 1a | 1.16 (0.99–1.36) |
| +Model 2b | 0.89 (0.75–1.07) | +Model 2b | 1.17 (0.99–1.38) |
| +Model 3c | 0.90 (0.74–1.08) | +Model 3c | 1.12 (0.94–1.33) |
Abbreviation: CI, confidence interval.
Model 1 included age, race, clinical site (+ total abdominal area for abdominal muscle area analyses + total thigh area for thigh muscle area analyses).
Model 2 included model 1 + physical activity, smoking, lipids, and hypertension.
Model 3: included model 2 + BMI, visceral fat, and total body fat by DXA.
For men, greater thigh muscle area and total lean mass were also significantly associated with greater diabetes risk in unadjusted models, although these were no longer significant after adjusting for other potential confounders. Quad strength and grip strength were not associated with diabetes in any models for men. In unadjusted models, thigh muscle, total lean mass, quad strength, and grip strength were all significantly associated with higher risk of incident diabetes in women (for all, P < .05). However, none of these associations remained significant after adjusting for body size and composition (BMI, visceral fat area, and total fat mass; model 3). Height, rather than BMI, was also adjusted for as a measure of overall body size, but this did not change the results (data not shown). Entering these measures of body size and composition individually did not markedly change the results for any variables. Sensitivity analyses performed only on participants from the Pittsburgh site including the paraspinous muscles were not materially different (data not shown).
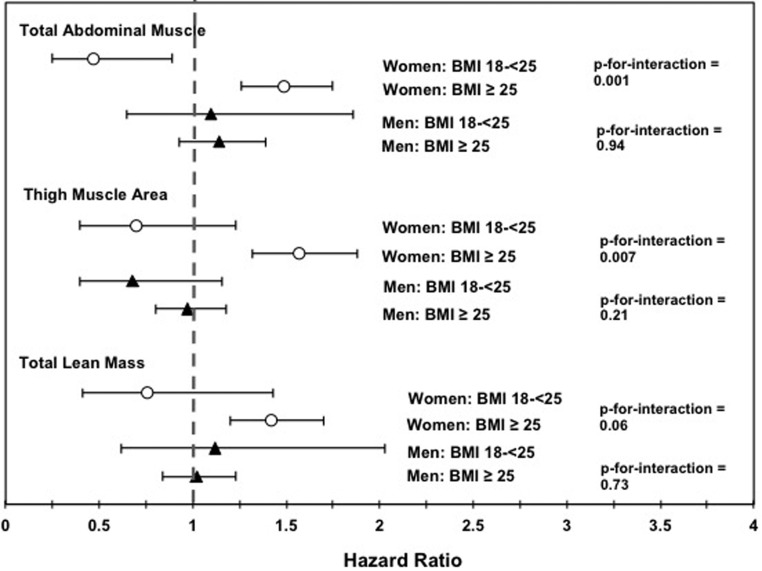
Figure 1 shows the unadjusted associations between muscle measures and incident diabetes stratified by BMI category and sex. For women, there was a significant interaction between BMI category (normal weight vs overweight/obese) and both abdominal muscle area (P = .001) and thigh muscle area (P = .007), and the interaction with total lean mass approached statistical significance (P = .06). For all three measures, greater muscle area was associated with a lower risk of incident diabetes for normal-weight women (HR 0.47, 0.70, and 0.76 per SD greater muscle area, respectively), whereas greater muscle area was associated with a higher risk of incident diabetes for overweight/obese women (HR 1.49, 1.57, and 1.42 per SD, respectively). As shown in Table 3, interactions between BMI category and abdominal and thigh muscle remained significant after further adjustment for covariates, including visceral fat area. In fully adjusted models, there was a 37% lower risk of incident diabetes per SD of abdominal muscle area for normal-weight women, compared with a 23% increased risk of incident diabetes for overweight women (P for interaction = .004). For thigh muscle, normal-weight women experienced a 58% lower risk of incident diabetes in fully adjusted models, compared with a 28% increased risk in overweight/obese women (P for interaction = .01). Trends were similar, but not significant, in fully adjusted models for total lean mass (P for interaction = .18) (Table 3). Number of participants with and without diabetes in each weight category are shown in Table 4.
Figure 1.
Unadjusted hazard ratios for incident diabetes by gender and BMI category. White circles, women; black triangles, men.
Table 3.
Risk of Incident Diabetes in Women and Men by Muscle Variables and Overweight Status
| Normal Weight HR (95% CI) | P Value | Overweight/Obese HR (95% CI) | P Value | P for Interaction | ||
|---|---|---|---|---|---|---|
| Women | BMI 18–24.9 kg/m2 | BMI ≥ 25 kg/m2 | ||||
| Abdominal muscle area | ||||||
| Unadjusted | 0.47 (0.25–0.89) | .02 | 1.49 (1.26–1.76) | <.001 | .001 | |
| +Model 1a | 0.38 (0.18–0.81) | .01 | 1.28 (1.03–1.58) | .03 | .003 | |
| +Model 2b | 0.37 (0.17–0.82) | .02 | 1.26 (1.01–1.58) | .04 | .004 | |
| +Model 3c | 0.37 (0.17–0.83) | .02 | 1.23 (0.98–1.54) | .08 | .004 | |
| Thigh muscle area | ||||||
| Unadjusted | 0.70 (0.40–1.23) | .22 | 1.57 (1.32–1.88) | <.001 | .007 | |
| +Model 1a | 0.79 (0.40–1.56) | .49 | 1.44 (1.15–1.81) | .002 | .009 | |
| +Model 2b | 0.73 (0.35–1.52) | .40 | 1.41 (1.11–1.79) | .005 | .01 | |
| +Model 3c | 0.58 (0.27–1.27) | .18 | 1.28 (1.00–1.64) | .05 | .01 | |
| Total lean mass | ||||||
| Unadjusted | 0.76 (0.41–1.43) | .40 | 1.42 (1.20–1.70) | <.001 | .06 | |
| +Model 1a | 0.71 (0.38–1.34) | .29 | 1.32 (1.09–1.60) | .004 | .08 | |
| +Model 2b | 0.73 (0.37–1.44) | .37 | 1.21 (1.00–1.48) | .055 | .12 | |
| +Model 3c | 0.66 (0.32–1.34) | .24 | 1.10 (0.89–1.36) | .40 | .18 | |
| Men | BMI 18–24.9 kg/m2 | BMI ≥ 25 kg/m2 | ||||
| Abdominal muscle area | ||||||
| Unadjusted | 1.10 (0.65–1.86) | .71 | 1.14 (0.93–1.39) | .21 | .94 | |
| +Model 1a | 0.94 (0.52–1.71) | .84 | 0.94 (0.74–1.19) | .58 | .50 | |
| +Model 2b | 1.14 (0.61–2.13) | .69 | 0.95 (0.73–1.23) | .69 | .44 | |
| +Model 3c | 1.13 (0.60–2.12) | .71 | 0.94 (0.73–1.22) | .64 | .42 | |
| Thigh muscle area | ||||||
| Unadjusted | 0.68 (0.40–1.16) | .16 | 0.97 (0.80–1.18) | .79 | .21 | |
| +Model 1a | 0.64 (0.28–1.44) | .28 | 0.84 (0.63–1.11) | .22 | .27 | |
| +Model 2b | 0.70 (0.29–1.73) | .44 | 0.82 (0.60–1.11) | .20 | .28 | |
| +Model 3c | 0.81 (0.32–2.08) | .66 | 0.87 (0.63–1.18) | .36 | .31 | |
| Total lean mass | ||||||
| Unadjusted | 1.12 (0.62–2.03) | .71 | 1.02 (0.84–1.23) | .88 | .73 | |
| +Model 1a | 1.11 (0.62–2.00) | .73 | 0.95 (0.77–1.17) | .62 | .66 | |
| +Model 2b | 1.23 (0.65–2.30) | .53 | 0.93 (0.75–1.16) | .53 | .65 | |
| +Model 3c | 1.19 (0.63–2.26) | 0.59 | 0.87 (0.69–1.09) | .22 | .54 | |
Abbreviation: CI, confidence interval.
Model 1 included age, race, and clinical site (+ total abdominal area for abdominal muscle area analyses + total thigh area for thigh muscle area analyses).
Model 2 included model 1 + physical activity, smoking, lipids, and hypertension.
Model 3 included model 2 + visceral fat area.
Table 4.
Number of Participants in Each BMI Category, by Gender and Incident Diabetes Status
| No Incident Diabetes | Incident Diabetes | |
|---|---|---|
| Men | ||
| BMI < 25 kg/m2 | 339/958 (35%) | 26/149 (18%) |
| BMI ≥ 25 kg/m2 | 619/958 (65%) | 123/149 (82%) |
| Women | ||
| BMI < 25 kg/m2 | 438/1118 (39%) | 24/153 (16%) |
| BMI ≥ 25 kg/m2 | 680/1118 (61%) | 129/153 (84%) |
For men, there were no significant interactions between muscle area measures and weight status in any models (for all, P > .2; Table 3). There were also no significant interactions between weight status and strength measures (grip or quad) for men or women (Supplemental Table 1).
Discussion
This is the first study to investigate the association between muscle quantity and strength and risk of incident diabetes. In well-functioning, community-living older adults without diabetes at baseline, the association between muscle area and incident diabetes was strongly modified by BMI category for women. Greater abdominal muscle area, thigh muscle, and total lean mass were all associated with markedly lower risk of incident diabetes for normal-weight women. Conversely, higher levels of all three of these muscle measures were associated with greater risk of incident diabetes for overweight and obese women. Surprisingly, BMI had no effect on associations between muscle area and incident diabetes for men. No interactions between BMI category and strength measures were found in either men or women. The current findings extend our previous findings in the Rancho Bernardo Study (5) and are significant in light of these prior research findings.
Greater muscle area was not associated with overall reduced risk of incident diabetes over the 14-year follow-up period. In fact, in unadjusted main effect models with all participants, greater abdominal and thigh muscle areas and total lean mass were generally associated with a higher risk of diabetes. This association appeared to be largely explained by those with more muscle also generally having more body fat because none of these associations remained significant after adjusting for body size and composition. Similar results were found for strength measures, which showed greater strength was associated with higher risk of incident diabetes for women until adjusting for body size and composition.
In a prior study in the Rancho Bernardo Study, we similarly demonstrated a stronger inverse association between abdominal muscle area and diabetes prevalence for normal-weight women, which was markedly attenuated in overweight and obese women (5). However, we were limited to evaluating diabetes prevalence and women only. Thus, the current study extends prior findings in several important ways. First, we extend these findings in women with an independent sample. Second, we extend them to incident diabetes, demonstrating that muscle mass prior to diabetes is relevant to development of disease, and that changes leading to low muscle mass after the onset of disease are unlikely to explain these observations. Third, we found that the effect modification by body composition was limited to women.
These findings suggest that low skeletal muscle could play a role in the development of normal-weight metabolic obesity, a possibility upon which researchers have speculated but which has not been more broadly and generally shown until now (19). These findings could have important public health implications. Behavioral interventions for preventing diabetes have generally focused on weight loss through aerobic exercise and diet change (20). These findings suggest that such approaches may indeed be the most appropriate course for overweight/obese individuals, who would likely benefit more from losing excess adiposity than building muscle. For normal-weight women, however, particularly older women, a focus on muscle maintenance or building may be more beneficial.
The differences in results for men and women may partly be explained by marked differences in body composition. Women had considerably lower total lean mass, abdominal muscle area, thigh muscle, grip strength, and quad strength and had much higher levels of percentage body fat than men. It is possible that low muscle area is a metabolic risk factor only under a certain threshold, one that men generally exceeded. Relatedly, the effects of muscle on metabolic health may be visible only in the absence of excess adipose tissue; that is, it could be that muscle was not protective for overweight or obese women because the harmful effects of large amounts of excess fat are stronger than the protective effects of muscle.
It is unclear, however, why greater muscle at baseline was associated with a suggestion of an increased risk of diabetes for overweight and obese women. It is unlikely that muscle is not at all beneficial, or is even harmful, for overweight women. Given the current data, all we can say is that greater muscle did not show an inverse association with incident type 2 diabetes for overweight women. It could be that muscle is metabolically beneficial for all individuals but that for overweight women, the harmful effects of excess adiposity overpowered the benefits of muscle. It is also possible, given the large range of BMI in this category, that muscle area acted as a proxy measure for body size in this case, with more obese women (at higher risk for diabetes) also having more muscle. This explanation is partly tempered by the fact that associations with abdominal and thigh muscle were still significant after adjusting for total abdominal and thigh size, respectively, and visceral fat area. It is possible that abdominal muscle in obese women contained larger amounts of intermuscular or im fat, which was not measured and has been associated with increased metabolic dysfunction (21, 22).
We found no association between strength measures and incident diabetes in men or women. Other studies in the Health, Aging, and Body Composition cohort also show discrepant results between muscle mass and strength (8, 14). Wander et al (10) found that higher grip strength was associated with lower odds of incident diabetes but, similar to our results for muscle mass, only among normal-weight individuals. We did not observe a similar association with strength in the current study. Because this was only the second study to examine the association between strength measures and incident diabetes, clearly more research is needed to better understand how muscle strength, including changes in strength, may be associated with metabolic health.
This study has a number of strengths, including its prospective design, the large diverse sample, and thorough measures, including both CT and DXA for body composition and repeated fasting glucose measures and medication inventories. The study also has important limitations. Based on inclusion criteria, participants were age older than 70 years at baseline; thus, whether results generalize to younger persons is uncertain. Additional limitations include the reliance on self-reported diabetes status for a number of the follow-up years and no oral glucose tolerance test measures after baseline.
These findings should be replicated with a middle-aged cohort to capture incident cases of diabetes when they typically occur, between age 45 and 65 years (23). More research is also needed to determine whether interventions to increase muscle mass could prevent diabetes and whether such interventions may be particularly effective in normal-weight women relative to heavier women and men. Some evidence exists that increasing muscle mass and/or strength can improve insulin sensitivity and prevent insulin resistance (24, 25), and a program of resistance training reduced the need for diabetes medication in older adults (26), but whether these effects may be differential based on gender and body weight is, to our knowledge, untested. If so, this finding could help direct public health resources toward programs that target improvement in muscle to subgroups of the population who might benefit most.
Acknowledgments
This work was supported Contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106 from the National Institute on Aging, National Institute on Aging Grant R01-AG028050 and National Institute of Nursing Research Grant R01-NR012459. This research was also supported in part by the Intramural Research Program of the National Institute on Aging. B.L. was supported by Career Development Grant K01 DK101650 from the National Institute of Diabetes and Digestive and Kidney Diseases. J.I. was supported by an Established Investigator Award from the American Heart Association (14EIA18560026).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CT
- computed tomography
- DXA
- dual-energy x-ray absorptiometry
- HR
- hazard ratio
- HU
- Hounsfield units.
References
- 1. Moore MC, Cherrington AD, Wasserman DH. Regulation of hepatic and peripheral glucose disposal. Best Pract Res Clin Endocrinol Metab. 2003;17(3):343–364. [DOI] [PubMed] [Google Scholar]
- 2. Mizgier ML, Casas M, Contreras-Ferrat A, Llanos P, Galgani JE. Potential role of skeletal muscle glucose metabolism on the regulation of insulin secretion. Obes Rev. 2014;15(7):587–597. [DOI] [PubMed] [Google Scholar]
- 3. Leenders M, Verdijk LB, van der Hoeven L, et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013;14(8):585–592. [DOI] [PubMed] [Google Scholar]
- 4. Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32(11):1993–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larsen BA, Allison MA, Laughlin GA, et al. The association between abdominal muscle and type II diabetes across weight categories in diverse post-menopausal women. J Clin Endocrinol Metab. 2015;100(1):E105–E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the Third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96(9):2898–2903. [DOI] [PubMed] [Google Scholar]
- 7. Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA, members of the Florey Adelaide Male Ageing Study. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism. 2009;58(7):1013–1022. [DOI] [PubMed] [Google Scholar]
- 8. Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–77. [DOI] [PubMed] [Google Scholar]
- 9. Grontved A, Pan A, Mekary RA, et al. Muscle-strengthening and conditioning activities and risk of type 2 diabetes: a prospective study in two cohorts of US women. PLoS Med. 2014;11(1):e1001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wander PL, Boyko EJ, Leonetti DL, McNeely MJ, Kahn SE, Fujimoto WY. Greater hand-grip strength predicts a lower risk of developing type 2 diabetes over 10 years in leaner Japanese Americans. Diabetes Res Clin Pract. 92(2):261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuk JL, Kilpatrick K, Davidson LE, Hudson R, Ross R. Whole-body skeletal muscle mass is not related to glucose tolerance or insulin sensitivity in overweight and obese men and women. Appl Physiol Nutr Metab. 2008;33(4):769–774. [DOI] [PubMed] [Google Scholar]
- 12. Lee S, Kim Y, White DA, Kuk JL, Arslanian S. Relationships between insulin sensitivity, skeletal muscle mass and muscle quality in obese adolescent boys. Eur J Clin Nutr. 2012;66(12):1366–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith NL, Barzilay JI, Kronmal R, Lumley T, Enquobahrie D, Psaty BM. New-onset diabetes and risk of all-cause and cardiovascular mortality: the Cardiovascular Health Study. Diabetes Care. 2006;29(9):2012–2017. [DOI] [PubMed] [Google Scholar]
- 14. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. [DOI] [PubMed] [Google Scholar]
- 15. Seidell JC, Visscher TL. Body weight and weight change and their health implications for the elderly. Eur J Clin Nutr. 2000;54(suppl 3):S33–S39. [DOI] [PubMed] [Google Scholar]
- 16. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol (1985). 2001;90(6):2157–2165. [DOI] [PubMed] [Google Scholar]
- 17. Friedewald W, Levy R, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18. Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study–Dual-Energy X-Ray Absorptiometry and Body Composition Working Group. J Appl Physiol (1985). 1999;87(4):1513–1520. [DOI] [PubMed] [Google Scholar]
- 19. Florez H, Castillo-Florez S. Beyond the obesity paradox in diabetes: fitness, fatness, and mortality. JAMA. 2012;308(6):619–620. [DOI] [PubMed] [Google Scholar]
- 20. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yim JE, Heshka S, Albu J, et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes (Lond). 2007;31(9):1400–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26(2):372–379. [DOI] [PubMed] [Google Scholar]
- 23. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: National Health Interview Survey, 2012. Vital Health Stat. 10;2014(260):1–161. [PubMed] [Google Scholar]
- 24. Ryan AS, Hurlbut DE, Lott ME, et al. Insulin action after resistive training in insulin resistant older men and women. J Am Geriatr Soc. 2001;49(3):247–253. [DOI] [PubMed] [Google Scholar]
- 25. Shaibi GQ, Cruz ML, Ball GD, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc. 2006;38(7):1208–1215. [DOI] [PubMed] [Google Scholar]
- 26. Castaneda C, Layne JE, Munoz-Orians L, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25(12):2335–2341. [DOI] [PubMed] [Google Scholar]