Patients with lipodystrophy had elevated apolipoprotein CIII (apoCIII) compared to healthy controls. ApoCIII did not change with leptin treatment despite significant reductions in serum triglyceride.
Abstract
Context:
Apolipoprotein CIII (apoCIII), an inhibitor of lipoprotein lipase, plays an important role in triglyceride metabolism. However, the role of apoCIII in hypertriglyceridemia in lipodystrophy and the effects of leptin replacement on apoCIII levels are unknown.
Objective:
The objective of the study was to test the hypotheses that apoCIII is elevated in hypertriglyceridemic patients with lipodystrophy and that leptin replacement in these patients lowers circulating apoCIII.
Design, Setting, Study Participants, Intervention, and Outcome Measures:
Using a post hoc cross-sectional case-control design, we compared serum apoCIII levels from patients with lipodystrophy not associated with HIV (n = 60) and age-, gender-, race-, and ethnicity-matched controls (n = 54) participating in ongoing studies at the National Institutes of Health. In a prospective, open-label, ongoing study, we studied the effects of 6–12 months of leptin replacement on apoCIII in lipodystrophy patients as an exploratory outcome.
Results:
ApoCIII was higher in lipodystrophy patients (geometric mean [25th and 75th percentiles]) (23.9 mg/dL [14.6, 40.3]) compared with controls (14.9 mg/dL [12.3, 17.7]) (P < .0001). ApoCIII and triglyceride levels were positively correlated in patients with lipodystrophy (R = 0.72, P < .0001) and healthy controls (R = 0.6, P < .0001). Leptin replacement (6–12 mo) did not significantly alter apoCIII (before leptin: 23.4 mg/dL [14.5, 40.1]; after leptin: 21.4 mg/dL [16.7, 28.3]; P = .34).
Conclusions:
Leptin replacement in lipodystrophy did not alter serum apoCIII levels. Elevated apoCIII may play a role in the hypertriglyceridemia of lipodystrophy independent of leptin deficiency and replacement.
Lipodystrophy is a rare disorder of complete or partial loss of sc adipose tissue. Patients with lipodystrophy have deficiency of adipokines such as leptin, resulting in hyperphagia. Because excess calories cannot be stored in adipose tissue, they are deposited as ectopic fat in the liver and muscle, causing severe insulin resistance, diabetes mellitus, and hypergtriglyceridemia. Moderate to severe hypertriglyceridemia is frequently observed in generalized and partial lipodystrophy and is highly responsive to leptin therapy (1, 2).
Hypertriglyceridemia, a consistent feature of the metabolic syndrome, is associated with increased cardiovascular disease risk (3, 4). The plasma triglyceride (TG) level is determined by the production of very low-density lipoproteins (VLDLs) and chylomicrons by the liver and intestine, respectively, and their catabolism to their remnant forms. One of the regulators of the plasma TG level is apolipoprotein CIII (apoCIII). apoCIII is a 79-amino acid glycoprotein synthesized mainly in the liver and to a lesser extent in the small intestine and is a component of VLDL and high-density lipoproteins (HDL) (5–7). apoCIII is a known noncompetitive inhibitor of lipoprotein lipase (LPL), which hydrolyzes TGs to free fatty acids for muscle and adipose tissue uptake (8), and transforms TG-rich lipoproteins to their remnant forms to be cleared by the liver (9). Additional mechanisms by which apoCIII regulates TG metabolism include inhibition of hepatic lipase (10, 11), reduction of apolipoprotein B- and apolipoprotein E-mediated binding of lipoproteins to the low-density lipoproteins (LDL) receptor (12, 13), and promotion of intrahepatic VLDL assembly and secretion (14).
In lipodystrophy, as in other insulin-resistant states, hypertriglyceridemia is thought to occur secondary to an inability to store surplus energy, increased free fatty acid turnover, and VLDL production, and reduced LPL activity. Increased plasma apoCIII concentration and production rate is associated with insulin resistance and is proposed to be involved in the pathogenesis of hypertriglyceridemia in the metabolic syndrome (15, 16). Conversely, lowering apoCIII levels reduces plasma TGs in different forms of hypertriglyceridemia (17, 18). Leptin replacement in patients with lipodystrophy lowers serum TGs (1). However, whether the leptin-induced reduction in TGs is mediated via reduced apoCIII levels in patients with lipodystrophy is unknown. To explore this question, in this study we tested the hypotheses that circulating apoCIII concentrations are elevated in patients with lipodystrophy and that leptin replacement lowers apoCIII.
Subjects and Methods
Study design and study subjects
Using a post hoc cross-sectional, case-control design, we compared serum apoCIII levels from adult patients with lipodystrophy and healthy volunteers with overweight or obesity. Lipodystrophy patients were enrolled in a study evaluating the natural history of insulin resistance (https://clinicaltrials.gov/ct2/show/NCT00001987) and/or the effects of recombinant human methionyl leptin (metreleptin) therapy in patients with lipodystrophy (https://clinicaltrials.gov/ct2/show/NCT00025883). Healthy volunteers were recruited for a study of the phenotype of overweight and obese adults (https://clinicaltrials.gov/ct2/show/NCT00428987). All studies were conducted at the Clinical Center of the National Institutes of Health and were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Written informed consent was obtained from all participants. The inclusion criteria of the lipodystrophy group were as follows: a clinical diagnosis of lipodystrophy; in patients on metreleptin low serum leptin level (<8 ng/mL in males, < 12 ng/mL in females); and one or more metabolic abnormalities including diabetes mellitus defined per the 2007 American Diabetes Association criteria (19), insulin resistance, or hypertriglyceridemia (fasting TGs > 200 ng/mL).
The cohort included in this study comprised 60 patients with either generalized or partial lipodystrophy not associated with HIV, who were selected based on the lack of prior treatment with metreleptin age 18 years or older, and availability of baseline metabolic data and a stored, frozen serum sample. Healthy control subjects were in good general health, over the age of 18 years, and had a body mass index (BMI) greater than 25 kg/m2. The 54 subjects in the healthy volunteer group were selected from a large database by matching to lipodystrophy patients based on age, gender, race, and ethnicity. In six cases, an appropriate match to a lipodystrophy patient could not be identified. In a prospective, open-label, ongoing study, we studied the effects of 6–12 months of leptin replacement on apoCIII in lipodystrophy patients as an exploratory outcome. To test this objective, we included 54 subjects from our cohort of 60 lipodystrophy patients, in whom we could compare data before and after 6–12 months of treatment with metreleptin. Four subjects of the larger cohort of 60 were never started on metreleptin replacement, and two subjects did not yet have follow-up data on metreleptin. These 54 lipodystrophy patients received self-administered sc metreleptin injections in doses ranging from 0.04 to 0.16 mg/kg body weight per day.
Baseline and follow-up measurements
All subjects had baseline anthropometric measurements (height, weight, and BMI) and routine laboratory assays including fasting lipid panel, insulin, leptin, glycated hemoglobin (HbA1c), and general chemistry. The lipodystrophy group also had measurement of body fat percentage (measured using whole body dual energy x-ray absorptiometry [Hologic QDR 4500; Hologic]) and a standard 75-g oral glucose tolerance test (OGTT) with measurement of glucose and insulin at −10, 0, 30, 60, 90, 120, and 180 minutes. The results of the OGTT are expressed as area under the curve (AUC) calculated using the trapezoidal method. To assess insulin sensitivity/resistance, the Quantitative Insulin Sensitivity Check Index (QUICKI) was calculated from fasting insulin and fasting glucose values (20): 1/(log [fasting insulin in microunits per milliliter) + log( [fasting glucose in milligrams per deciliter]). In lipodystrophy patients the severity of nonalcoholic fatty liver disease was determined by a liver biopsy and was quantified using the nonalcoholic steatohepatitis Clinical Research Network (NASH-CRN) scoring system (21). All 54 lipodystrophy subjects on metreleptin therapy had anthropometric measurements and routine laboratory assays at their follow-up visit; however, there were limited data on fasting insulin, OGTT, body fat percentage, and liver biopsy.
apoCIII was measured in serum samples that were stored at −70°C using an ELISA from Abcam. Serum samples were diluted 1:4000 and the lower limit of detection was 0.0004 μg/mL. The intraassay and interassay coefficients of variation were 3.4% and 8.2%, respectively. Leptin was measured by a RIA using a commercial kit (Linco Research).
Leptin treatment of ob/ob mice and gene expression analysis (Supplemental Methods)
Statistical analysis
Statistical analyses were performed using Microsoft Excel, GraphPad Prism, version 6.01 (GraphPad) and SAS Enterprise Guide, version 5.1 (SAS Institute). Baseline characteristics of lipodystrophy patients vs overweight/obese controls were compared using an unpaired Student's t test for continuous variables and a χ2 test or Fisher's exact test for categorical variables. Nonnormally distributed data were log transformed for analyses. Comparisons among generalized lipodystrophy, partial lipodystrophy, and overweight/obese control groups were performed by a one-way ANOVA. Correlations between apoCIII and other parameters were evaluated using linear regression, and multivariate analyses to test the influence of lipid medications and insulin therapy on apoCIII levels were done using an analysis of covariance. Data on lipodystrophy patients before and after metreleptin therapy were compared using a paired Student's t test for parameters with normal distribution, Wilcoxon test for those with nonnormal distribution, and a χ2 test for medications. Results are presented as mean ± SD except for apoCIII and TGs, which are expressed as geometric mean (25th and 75th percentiles) of the untransformed data. P < .05 was considered statistically significant.
Results
Baseline characteristics
Baseline characteristics of lipodystrophy patients and overweight/obese controls are shown in Table 1. Because subjects were matched based on their age, gender, race and ethnicity, there was no significant difference in these variables. BMI was significantly higher in overweight and obese controls vs lipodystrophy patients. TG levels were significantly higher in lipodystrophy patients vs controls (485.4 mg/dL [252.3, 940.0] vs 133.9 mg/dL [106.8, 193.3]; P < .001). Use of lipid and glucose-lowering medications was higher in lipodystrophy patients. Baseline characteristics of generalized lipodystrophy (n = 21) vs partial lipodystrophy (n = 39) patients are shown in Supplemental Table 1. There was a female predominance in both lipodystrophy groups. BMI and percentage body fat were significantly lower in generalized lipodystrophy. There was no difference in TGs or HbA1c. Insulin use was greater in generalized lipodystrophy, and thiazolidinedione (TZD) use was greater in partial lipodystrophy.
Table 1.
Baseline Characteristics of Lipodystrophy Patients Versus Overweight/Obese Controls
| Lipodystrophy (n = 60) | Overweight/obese (n = 54) | P Value | ||
|---|---|---|---|---|
| Gender, n, % | Females | 54 (90%) | 48 (89%) | 1.0 |
| Males | 6 (10%) | 6 (11%) | ||
| Age, y, mean ± SD | 35.1 ± 13.7 | 37.1 ± 13.6 | .45 | |
| Ethnicity/race, n, % | Caucasian | 40 (67%) | 38 (70%) | .41 |
| Hispanic | 9 (15%) | 10 (19%) | ||
| African-American | 4 (6.6%) | 4 (7.4%) | ||
| Asian | 3 (5%) | 2 (3.7%) | ||
| Other | 4 (6.7%) | 0 | ||
| BMI, kg/m2, mean ± SD | 24.5 ± 4.1 | 34.9 ± 10.1 | <.001 | |
| Plasma leptin, ng/mL | 3.03 (1.22, 7.02) | 35.31 (18.34, 65.24) | <.001 | |
| TGs, mg/dL, geometric mean (25th, 75th percentiles) | 485.4 (252.3, 940.0) | 133.9 (106.8, 193.3) | <.001 | |
| HbA1c, %, mean ± SD | 8.2 ± 2.1 | 5.6 ± 0.7 | <.001 | |
| Fasting glucose, mg/dL | 175 ± 79 | 97 ± 20 | <.001 | |
| Fasting insulin, μU/mL | 35.9 (14.0, 66.2) | 10.4 (6.6, 23.2) | <.001 | |
| Medications, n, % | Statin | 21 (35%) | 8 (14.8%) | .017 |
| Fibrate | 32 (53.3%) | 0 (0%) | <.001 | |
| Fish oil | 19 (31.7%) | 3 (5.5%) | <.001 | |
| Metformin | 36 (60%) | 0 (0%) | <.001 | |
| TZD | 14 (23.3%) | 0 (0%) | <.001 | |
| Insulin | 37 (61.7%) | 0 (0%) | <.001 |
apoCIII levels were higher in lipodystrophy patients vs overweight and obese controls
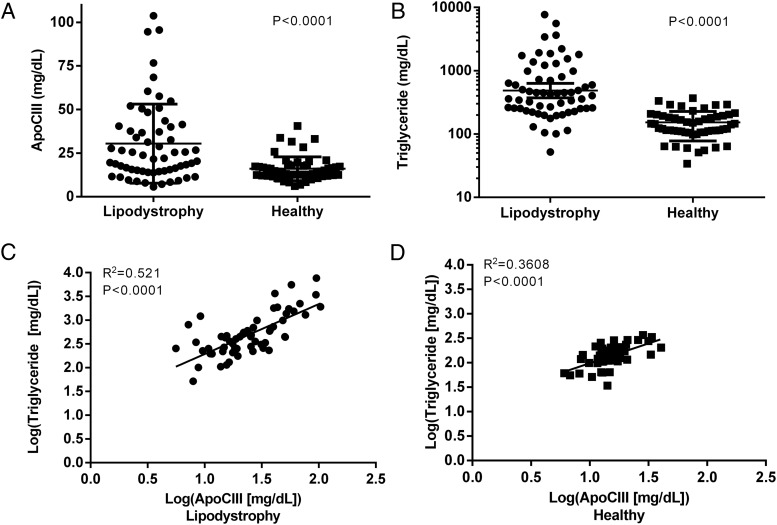
apoCIII levels were significantly elevated in the lipodystrophy group (23.9 mg/dL [14.6, 40.3]) compared with overweight/obese controls (14.9 mg/dL [12.3, 17.7]; P < .0001) (Figure 1). This difference remained significant after adjustment for use of individual medications (statins, fibrates, fish oil, TZDs, and total daily dose of insulin) and was borderline significant when all medication use was considered in a single model (P = .05). There was no difference in apoCIII between generalized (24.3 mg/dL [14.2, 40.9]) vs partial lipodystrophy patients (23.7 mg/dL [15.2, 37.6]; P = .9), and both were elevated compared with controls.
Figure 1.
apoCIII and TG levels and their correlation in lipodystrophy and overweight/obese controls. A, ApoCIII was significantly elevated in the lipodystrophy group compared with overweight/obese controls. I bars indicate SD. B, TGs were also significantly elevated in the lipodystrophy group vs overweight/obese controls. The horizontal bar is the geometric mean and I bars indicate 95% CI. C and D, There was a strong positive correlation between apoCIII and TGs in both lipodystrophy (panel C) and overweight/obese controls (panel D). Solid circles indicate lipodystrophy patients, and solid squares indicate overweight/obese controls.
Correlation analysis between apoCIII and metabolic parameters
There was a strong positive correlation between apoCIII and TG levels in both lipodystrophy patients (R2 = 0.52; P < .0001; 95% confidence interval [CI] 0.79–1.32) and overweight/obese controls (R2 = 0.36; P < .0001; 95% CI 0.51–1.1) (Figure 1). This correlation was equivalent in the two lipodystrophy subgroups: generalized lipodystrophy (R2 = 0.40; P = .0023; 95% CI 0.33–1.30) and partial lipodystrophy (R2 = 0.60; P < .0001; 95% CI 0.85–1.5). There were positive correlations between non-HDL cholesterol and apoCIII in both overweight/obese controls and lipodystrophy patients (Table 2). There was no correlation between LDL and apoCIII in any of the groups. There was no correlation between HDL and apoCIII except in the partial lipodystrophy group (R2 = 0.13, P = .036). There was no correlation between the fat content of the liver or percentage body fat and apoCIII in lipodystrophy patients.
Table 2.
Results of Univariate Correlation Analysis Between apoCIII and Age, Body Fat Content, Lipid Metabolism Parameters, Liver Fat Content, and Glucose Metabolism Parameters
| Log (apoCIII) |
||||
|---|---|---|---|---|
| Lipodystrophy |
Overweight/Obese |
|||
| R2 | P Value | R2 | P Value | |
| Age, y | 0.040 | .127 | 0.060 | .072 |
| BMI, kg/m2 | 0.040 | .136 | 0.002 | .734 |
| Body fat: total percentage fat | 0.008 | .563 | N/A | N/A |
| Log (TGs) | 0.521 | <.0001 | 0.361 | <.0001 |
| Non-HDL cholesterol, mg/dL | 0.312 | <.0001 | 0.106 | .017 |
| LDL, mg/dL | 0.0004 | .902 | 0.029 | .219 |
| HDL, mg/dL | 0.027 | .224 | 0.001 | .826 |
| NASH-CRN fat score | 0.032 | .297 | N/A | N/A |
| Log (HbA1c) | 0.047 | .095 | N/A | N/A |
| Fasting glucose, mg/dL | 0.022 | .255 | 0.102 | .019 |
| Fasting insulin, μU/mLa | 0.003 | .710 | 0.04 | .14 |
| Fasting C-peptide, ng/mL | 0.004 | .654 | N/A | N/A |
| OGTT glucose AUC | 0.003 | .711 | N/A | N/A |
| OGTT insulin AUC | 0.007 | .555 | N/A | N/A |
| Plasma leptin, ng/mL | 0.001 | .87 | 0.004 | .65 |
Abbreviation: N/A, not available. Statistically significant results are marked in bold.
Fasting insulin levels in both insulin users and nonusers.
There was a positive correlation between apoCIII and fasting glucose in the overweight/obese controls but not in lipodystrophy patients. HbA1c positively correlated with apoCIII only in the partial lipodystrophy group (R2 = 0.17, P = .014). There was no correlation between apoCIII and fasting insulin level, fasting C-peptide level, OGTT glucose AUC, OGTT insulin AUC, or QUICKI in any of the groups. We examined the association between leptin and TGs and/or apoCIII. In the combined cohort (both lipodystrophy and control), plasma apoCIII (R2 = 0.098, P < .001) and TGs (R2 = 0.28, P < .001) were negatively associated with leptin levels. However, in the individual cohorts, there were no significant relationships between plasma leptin and TG or apoCIII levels (Table 2).
Metabolic effects of leptin replacement therapy
The changes seen after 6–12 months of leptin replacement therapy with metreleptin in anthropometric and metabolic parameters are shown in Table 3. Consistent with prior analyses of this data set (1), metreleptin significantly decreased BMI, percentage body fat, TGs, non-HDL cholesterol, LDL, HbA1c, fasting glucose, and glucose AUC during OGTT. Likewise, metreleptin improved insulin sensitivity as assessed by QUICKI and liver fat content assessed by NASH-CRN Fat Score on liver biopsy. However, there was no significant change in the fasting insulin and C-peptide levels and insulin secretion during an OGTT.
Table 3.
Changes in BMI, Body Fat Percentage, and Parameters of Glucose and Lipid Metabolism After Leptin Replacement in Lipodystrophy Patients
| Before leptin | After leptin | P Value | |
|---|---|---|---|
| BMI, kg/m2 | 24.49 ± 4.12 | 23.62 ± 4.35 | <.001 |
| Body fat: total percentage fat | 18.85 ± 9.06 | 14.44 ± 9.90 | .05 |
| apoCIII, mg/dLa | 23.4 (14.5, 40.1) | 21.37 (16.7, 28.3) | .34 |
| TGs, mg/dLa | 483.47 (252.8, 745.8) | 253.07 (154.5, 357) | <.001 |
| Non-HDL cholesterol, mg/dL | 185.9 ± 84.35 | 145.2 ± 66.05 | <.001 |
| LDL, mg/dL | 98.97 ± 43.76 | 89.33 ± 37.18 | .03 |
| HDL, mg/dL | 30.52 ± 9.02 | 31.83 ± 9.04 | .42 |
| NASH-CRN fat score | 1.75 ± 0.97 | 0.67 ± 0.58 | <.001 |
| HbA1c | 8.38 ± 2.06 | 6.91 ± 1.5 | <.001 |
| Fasting glucose, mg/dL | 178 ± 78.5 | 128.7 ± 48.49 | <.001 |
| Fasting insulin, μU/mLb | 44.48 ± 40.74 | 36.64 ± 32.47 | .24 |
| Fasting C-peptide, ng/mL | 3.68 ± 2.08 | 3.55 ± 1.95 | .92 |
| QUICKI | 0.27 ± 0.03 | 0.29 ± 0.04 | .01 |
| OGTT glucose AUC | 51 078 ± 14 720 | 44 113 ± 14 823 | .008 |
| OGTT insulin AUC | 21 986 ± 33 490 | 25 981 ± 56 566 | .62 |
Data are expressed as mean ± SD. Statistically significant results are marked in bold.
Expressed in geometric mean (25th and 75th percentiles).
Fasting insulin levels in both insulin users and nonusers.
Leptin replacement with metreleptin did not affect apoCIII level
Although apoCIII decreased after metreleptin, this change did not reach significance (before metreleptin: 23.4 mg/dL [14.5, 40.1], n = 54; after metreleptin: 21.4 mg/dL [16.7, 28.3]; P = .34, Table 3). The decrease in apoCIII with metreleptin was driven by changes in the generalized lipodystrophy group, although this change was still not significant (generalized lipodystrophy before metreleptin: 24.9 [14.1, 41.1]; after metreleptin: 18.7 [14.0, 21.9], P = .1; partial lipodystrophy before metreleptin: 22.6 [15.1, 37.3]; after metreleptin: 23.1 [17.7, 30.2]; P = .8). In a post hoc subgroup analysis of individuals with lower post-metreleptin treatment TG levels (n = 40), apoCIII levels were significantly lower: 25.1 (15.3, 42.9); after metreleptin: 20.7 (14.3, 27.6) (P = .01).
Correlation analysis between the change in apoCIII level and changes in metabolic parameters with metreleptin
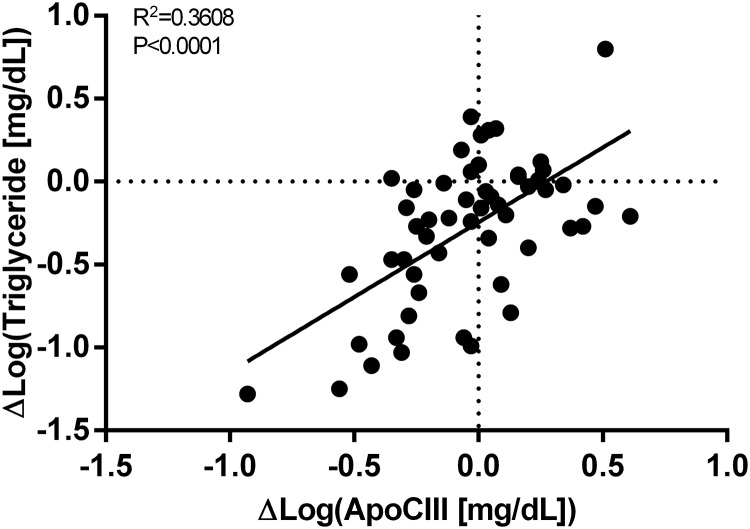
To understand the role of apoCIII during metabolic improvements induced by leptin, we investigated how changes in anthropometric, lipid, and glucose metabolism parameters due to metreleptin related to changes in apoCIII. There was a strong positive correlation between changes in apoCIII and changes in TG and non-HDL cholesterol (Figure 2). Although the overall change in apoCIII levels after metreleptin therapy was not significantly different, in a subset of patients (n = 40), apoCIII levels were lower after leptin replacement. This explains the positive correlation observed in Figure 2. There was no correlation between changes in apoCIII and changes in weight, BMI, percentage body fat, liver fat, or LDL cholesterol. There was a strong positive correlation between changes in apoCIII and changes in fasting glucose and glucose AUC during an OGTT but no correlation with changes in HbA1c, fasting insulin, or insulin AUC during an OGTT (Table 4).
Figure 2.
Correlation between changes in apoCIII and TGs in lipodystrophy patients after metreleptin. There was a positive correlation between changes in apoCIII and changes in TGs in lipodystrophy patients after leptin replacement for 6–12 months. Zero is marked with a dotted line on both axes.
Table 4.
Results of Correlation Analysis Between Change in apoCIII Level and Changes in Various Anthropometric, Lipid, and Glucose Metabolism Parameters
| Δ Log (apoCIII) |
||
|---|---|---|
| R2 | P Value | |
| Δ Weight, kg | 0.0002 | .92 |
| Δ BMI, kg/m2 | 0.003 | .69 |
| Δ Body fat: total % fat | 0.11 | .25 |
| Δ TGs | 0.36 | <.001 |
| Δ non-HDL cholesterol | 0.09 | .02 |
| Δ LDL | 0.04 | .32 |
| Δ HDL | 0.03 | .25 |
| Δ NASH-CRN fat score | 0.10 | .15 |
| Δ HbA1c | 0.07 | .06 |
| Δ Fasting glucose | 0.17 | .002 |
| Δ Fasting insulina | 0.00 005 | .96 |
| Δ QUICKI | 0.06 | .10 |
| Δ OGTT glucose AUC | 0.11 | .02 |
| Δ OGTT insulin AUC | 0.01 | .49 |
Statistically significant results are marked in bold.
Fasting insulin levels in both insulin users and nonusers.
Leptin treatment improved metabolic parameters and reduced hepatic apoCIII expression in ob/ob mice
To complement our studies in lipodystrophic patients, we treated leptin deficient ob/ob mice with leptin (24 μg/d recombinant leptin) or vehicle (PBS) sc, through an osmotic pump, for 4 days. As expected, leptin treatment caused significant weight loss (−6.3 g) (50 ± 1 g vs 56 ± 2, P = .01), decreased food intake by 60% compared with the vehicle-treated group (1.75 ± 0.17 vs 4.61 ± 0.54 g/d per mouse, P = .0005), and reduced blood glucose levels (136 ± 15 vs 239 ± 42 mg/dL, P = .04). Leptin treatment reduced hepatic apoCIII mRNA levels by more than 40% (P = .04) and reduced expression of a leptin-responsive gene, stearoyl CoA desaturase 1 (Scd1) by 90% (Supplemental Figure 1).
Discussion
In this study, we have shown that lipodystrophy patients have significantly elevated apoCIII levels compared with overweight/obese controls and that leptin replacement did not decrease the apoCIII levels in lipodystrophy. Previous studies in other patient populations have reported positive associations between TG and apoCIII concentrations (18, 22). Consequently, we hypothesized that hypertriglyceridemia in lipodystrophy may be associated with elevated apoCIII levels. We observed a strong positive correlation between in both lipodystrophy patients and overweight/obese controls, supporting the concept that apoCIII is a key regulator of plasma TG levels in both healthy individuals and in extreme insulin-resistant states such as lipodystrophy.
Many lipid-lowering medications have been shown to affect apoCIII expression. Fibrates, statins, and TZDs reduce apoCIII expression by activating peroxisome-proliferator-activated receptor-α (23–28). The mechanism underlying the apoCIII-lowering effects of fish oils is unclear but may also relate to activation of peroxisome-proliferator-activated receptor-α (29). The use of lipid-lowering medications and TZDs was significantly higher in lipodystrophy patients compared with overweight/obese controls. When we adjusted for TZD, insulin use, total daily dose of insulin, statin, fibrate, and fish oil, the difference in apoCIII levels between lipodystrophy patients and overweight/obese controls remained significant, suggesting that lipodystrophy patients have higher apoCIII despite more frequent use of medications that lower apoCIII levels.
Leptin replacement with metreleptin is the only specific approved therapy for lipodystrophy patients. Metreleptin therapy decreases TG levels, lowers glucose, and improves insulin sensitivity. In humans, circulating apoCIII levels positively correlate with plasma fasting glucose and glucose excursion but not fasting insulin or insulin excursion after a glucose bolus in overweight patients (30), suggesting that apoCIII levels are predominantly under the control of glucose and not insulin (30). In fact, insulin causes a dose-dependent down-regulation of the apoCIII gene in both cultured cells and mice (31), and high glucose concentrations induce apoCIII expression in rat and human hepatocytes (30). Caron et al speculated that, in insulin-resistant states, insulin fails to suppress apoCIII expression, whereas chronic hyperglycemia leads to elevated apoCIII production (30). There are no prior data on the effects of leptin on apoCIII expression in humans or rodents. Because metreleptin in lipodystrophy patients lowers TGs and TGs are regulated by apoCIII, we hypothesized that metreleptin might lower TGs by lowering the elevated apoCIII levels in lipodystrophy. In addition, because glucose levels positively modulate apoCIII expression, we believed that metreleptin-induced improvement in glycemic control in patients with lipodystrophy could lead to lower apoCIII levels. Surprisingly, metreleptin did not significantly decrease the apoCIII level in humans.
The reasons for the lack of effect of metreleptin on apoCIII in humans are unclear. We found a positive correlation between apoCIII and fasting glucose in our overweight/obese controls and HbA1c in the partial lipodystrophy group, but apoCIII was unrelated to fasting or glucose-stimulated insulin. This supports previous observations suggesting a positive role for glucose in apoCIII regulation. As expected, there was a significant improvement in glycemic control in lipodystrophy patients on metreleptin therapy with lower fasting glucose and glucose excursion during OGTT. Moreover, we found a positive correlation between changes in apoCIII and changes in fasting glucose and glucose response during an OGTT (AUC) on metreleptin but we did not find any correlation with changes in fasting insulin, insulin response during an OGTT, or insulin sensitivity. However, the changes in glucose (AUC) during an OGTT accounted for only 11% in the change in apoCIII after leptin therapy. These results suggest that glucose may not be a major regulator of apoCIII levels in this patient population.
Concurrently we also examined the effects of leptin on apoCIII expression in leptin-deficient mice. In contrast with our findings in humans, in leptin deficient ob/ob mice, leptin administration acutely suppressed hepatic apoCIII expression by greater than 40%. As in humans, acute leptin treatment in ob/ob mice significantly reduced blood glucose levels (239 ± 42 vs 136 ± 15 mg/dL). Thus, it is possible that the inhibitory effect of leptin on hepatic apoCIII expression may be mediated by the reduction in glucose levels. Whether leptin has direct effects on apoCIII gene expression is unknown and needs to be examined in future investigations. These data together suggest that although apoCIII regulates TGs in patients with lipodystrophy both in the leptin-deficient and leptin-replete states, the mechanisms by which metreleptin decreases TGs are independent of apoCIII.
Recently promising results have been published on apoCIII inhibition using antisense oligonucleotide technology, in which a dose-dependent and prolonged inhibition of apoCIII led to a 71% decrease in the TG level in various hypertriglyceridemic patients and to a 56%–86% decrease in patients with familial chylomicronemia syndrome, a disease of deficient LPL activity (17, 18). In our study, lipodystrophy patients had elevated apoCIII levels. Because a subgroup of lipodystrophy patients fail to achieve good TG control with metreleptin (1), these patients may benefit from treatment with an apoCIII inhibitor.
In conclusion, lipodystrophy patients have elevated apoCIII levels that are unaffected by leptin replacement. Leptin replacement is not always sufficient to treat the metabolic derangements of lipodystrophy, especially hypertriglyceridemia, and apoCIII inhibitors may represent a possible future therapeutic option.
Acknowledgments/grant support
We acknowledge the patients with lipodystrophy and obesity; the clinical fellows, Michelle Ashmus, Elaine Cochran, Megan Mattingly; the National Institute of Diabetes and Digestive and Kidney Diseases Clinical Core (Anula Bhusry, Yuhai Dai, and Joy Guo) and the nursing stuff at the Clinical Center involved in the care of these patients. We also acknowledge Bristol Meyers Squibb and Astra Zeneca for the metreleptin used in the study.
Clinical studies from which data were used are registered at www.clinicaltrials.gov as identifiers NCT00001987, NCT00025883, and NCT00428987.
This work was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases, and by the Inter-Institute Endocrinology Fellowship Program at the National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosure Summary: S.B.B. has received consulting fees from Novo Nordisk. S.I.T. is a former employee of Bristol-Myers Squibb and a consultant for Isis Pharmaceuticals, Aegerion Pharmaceuticals, Calibrium LLC, and Yabao Pharmaceutical Group. The other authors have nothing to disclose.
Footnotes
- apoCIII
- apolipoprotein CIII
- AUC
- area under the curve
- BMI
- body mass index
- CI
- confidence interval
- HbA1c
- glycated hemoglobin
- HDL
- high-density lipoprotein
- LDL
- low-density lipoprotein
- LPL
- lipoprotein lipase
- OGTT
- oral glucose tolerance test
- NAFLD
- nonalcoholic fatty liver disease
- NASH-CRN
- nonalcoholic steatohepatitis Clinical Research Network
- QUICKI
- Quantitative Insulin Sensitivity Check Index
- TG
- triglyceride
- TZD
- thiazolidinedione
- VLDL
- very low-density lipoprotein.
References
- 1. Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100:1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joseph J, Shamburek RD, Cochran EK, Gorden P, Brown RJ. Lipid regulation in lipodystrophy versus the obesity-associated metabolic syndrome: the dissociation of HDL-C and triglycerides. J Clin Endocrinol Metab. 2015;99:E1676–E1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. [DOI] [PubMed] [Google Scholar]
- 4. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. [DOI] [PubMed] [Google Scholar]
- 5. Zannis VI, Cole FS, Jackson CL, Kurnit DM, Karathanasis SK. Distribution of apolipoprotein A-I, C-II, C-III, and E mRNA in fetal human tissues. Time-dependent induction of apolipoprotein E mRNA by cultures of human monocyte-macrophages. Biochemistry. 1985;24:4450–4455. [DOI] [PubMed] [Google Scholar]
- 6. Brown WV, Levy RI, Fredrickson DS. Studies of the proteins in human plasma very low density lipoproteins. J Biol Chem. 1969;244:5687–5694. [PubMed] [Google Scholar]
- 7. Ooi EM, Barrett PH, Chan DC, Watts GF. Apolipoprotein C-III: understanding an emerging cardiovascular risk factor. Clin Sci (Lond). 2008;114:611–624. [DOI] [PubMed] [Google Scholar]
- 8. Jong MC, Hofker MH, Havekes LM. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler Thromb Vasc Biol. 1999;19:472–484. [DOI] [PubMed] [Google Scholar]
- 9. Wang CS, McConathy WJ, Kloer HU, Alaupovic P. Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C-III. J Clin Invest. 1985;75:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jong MC, Rensen PC, Dahlmans VE, van der Boom H, van Berkel TJ, Havekes LM. Apolipoprotein C-III deficiency accelerates triglyceride hydrolysis by lipoprotein lipase in wild-type and apoE knockout mice. J Lipid Res. 2001;42:1578–1585. [PubMed] [Google Scholar]
- 11. Landis BA, Rotolo FS, Meyers WC, Clark AB, Quarfordt SH. Influence of apolipoprotein E on soluble and heparin-immobilized hepatic lipase. Am J Physiol. 1987;252:G805–G810. [DOI] [PubMed] [Google Scholar]
- 12. Clavey V, Lestavel-Delattre S, Copin C, Bard JM, Fruchart JC. Modulation of lipoprotein B binding to the LDL receptor by exogenous lipids and apolipoproteins CI, CII, CIII, and E. Arterioscler Thromb Vasc Biol. 1995;15:963–971. [DOI] [PubMed] [Google Scholar]
- 13. Windler E, Havel RJ. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J Lipid Res. 1985;26:556–565. [PubMed] [Google Scholar]
- 14. Yao Z, Wang Y. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr Opin Lipidol. 2012;23:206–212. [DOI] [PubMed] [Google Scholar]
- 15. Chan DC, Nguyen MN, Watts GF, Barrett PH. Plasma apolipoprotein C-III transport in centrally obese men: associations with very low-density lipoprotein apolipoprotein B and high-density lipoprotein apolipoprotein A-I metabolism. J Clin Endocrinol Metab. 2008;93:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Onat A, Hergenc G, Sansoy V, et al. Apolipoprotein C-III, a strong discriminant of coronary risk in men and a determinant of the metabolic syndrome in both genders. Atherosclerosis. 2003;168:81–89. [DOI] [PubMed] [Google Scholar]
- 17. Gaudet D, Alexander VJ, Baker BF, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373:438–447. [DOI] [PubMed] [Google Scholar]
- 18. Gaudet D, Brisson D, Tremblay K, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2015;371:2200–2206. [DOI] [PubMed] [Google Scholar]
- 19. 2007 Standards of medical care in diabetes.. Diabetes Care. 2007;30(suppl 1):S4–S41. [DOI] [PubMed] [Google Scholar]
- 20. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. [DOI] [PubMed] [Google Scholar]
- 21. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 22. Pollin TI, Damcott CM, Shen H, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Altomonte J, Cong L, Harbaran S, et al. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest. 2004;114:1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hertz R, Bishara-Shieban J, Bar-Tana J. Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. J Biol Chem. 1995;270:13470–13475. [DOI] [PubMed] [Google Scholar]
- 25. Martin G, Duez H, Blanquart C, et al. Statin-induced inhibition of the Rho-signaling pathway activates PPARα and induces HDL apoA-I. J Clin Invest. 2001;107:1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagashima K, Lopez C, Donovan D, et al. Effects of the PPARγ agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. J Clin Invest. 2005;115:1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakamoto J, Kimura H, Moriyama S, et al. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun. 2000;278:704–711. [DOI] [PubMed] [Google Scholar]
- 28. Staels B, Vu-Dac N, Kosykh VA, et al. Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. J Clin Invest. 1995;95:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dallongeville J, Bauge E, Tailleux A, et al. Peroxisome proliferator-activated receptor α is not rate-limiting for the lipoprotein-lowering action of fish oil. J Biol Chem. 2001;276:4634–4639. [DOI] [PubMed] [Google Scholar]
- 30. Caron S, Verrijken A, Mertens I, et al. Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arterioscler Thromb Vasc Biol. 2011;31:513–519. [DOI] [PubMed] [Google Scholar]
- 31. Chen M, Breslow JL, Li W, Leff T. Transcriptional regulation of the apoC-III gene by insulin in diabetic mice: correlation with changes in plasma triglyceride levels. J Lipid Res. 1994;35:1918–1924. [PubMed] [Google Scholar]