Four childhood metabolic risk factors were assessed as predictors of future type 2 diabetes; obesity and impaired glucose tolerance were strong predictors, hypertension and dyslipidemia were not.
Abstract
Context:
Data are lacking on how metabolic risk factors during childhood affect the long-term risk of type 2 diabetes.
Objectives:
Assess four metabolic risk factors as predictors of type 2 diabetes and determine whether the risk differs between younger and older children.
Design:
In a prospective cohort study conducted between 1965 and 2007, participants were followed for development of diabetes. Baseline measurements included body mass index (BMI), blood pressure, serum cholesterol, and 2-hour plasma glucose after an oral glucose tolerance test. Additional analyses divided subjects into two groups according to baseline age, 5–11 and 12–19 years.
Setting:
Gila River Indian Community in Arizona.
Participants:
A total of 5532 nondiabetic Pima Indian children 5–19 years old.
Results:
A total of 1281 children developed diabetes (median follow-up, 12.4 years). Diabetes incidence was higher in overweight children (BMI ≥ 85th percentile) than in nonoverweight children. Nonoverweight children had the lowest risk of diabetes (20-year cumulative incidence, 9.5%), whereas overweight children with impaired glucose tolerance (2-hour glucose ≥ 140 mg/dL) had the highest (79.0%). The relative risk for children with metabolic abnormalities compared with their healthy counterparts was higher in younger children than in older children early in follow-up. BMI and 2-hour glucose were related to incident diabetes in multivariable models (predicted 15-year cumulative incidence for the highest vs lowest quartile was 3.9 and 1.8 times as high for BMI and 2-hour glucose, respectively; P < .001), whereas blood pressure and cholesterol were not.
Conclusions:
BMI and impaired glucose tolerance in children are strong predictors of type 2 diabetes. Other components of the “metabolic syndrome” are not.
Children with metabolic risk factors, such as high body mass index (BMI) and impaired glucose tolerance (IGT), are at an increased risk for developing type 2 diabetes (1–5). Combining these metabolic risk factors with other conditions such as hypertension and dyslipidemia into a single “metabolic syndrome” is thought by some to be helpful for assessment of diabetes risk (6–8). Although guidelines have considerable heterogeneity in the measures and cut-points used to define metabolic syndrome, all include central obesity, hyperglycemia and/or insulin resistance, hypertension, and hyperlipidemia; each definition, applied to children, is predictive of diabetes (9).
The International Diabetes Federation has defined metabolic syndrome in children, but only those 10–16 years of age. A study based on similar guidelines from the Adult Treatment Panel III reported that childhood metabolic syndrome predicts the development of diabetes and showed that an increasing number of metabolic syndrome components was associated with higher diabetes risk (10). However, others have suggested that body weight and glucose tolerance are the principal determinants of diabetes risk in children, with little or no additional risk attributable to the other metabolic syndrome components (1–4). Previous studies have had relatively short follow-up and, correspondingly, a small number of incident diabetes cases; thus there is a paucity of data on long-term diabetes risk associated with metabolic risk factors in children. This lack of data is particularly striking for prepubertal children (eg, younger than 10 years old). In the present study, we performed a prospective analysis in a cohort of Pima Indian children, who were followed as long as 40 years, to assess predictive values of four metabolic risk factors for type 2 diabetes. We also determined whether combining these factors improved diabetes prediction, and we examined whether the predictive value of the factors differed between younger and older children.
Subjects and Methods
Study population
From 1965 through 2007, residents of the Gila River Indian Community in Arizona, most of whom were of Pima or Tohono O'odham Indian heritage, participated in a longitudinal study of diabetes and related conditions. In this prospective analysis, we included children and adolescents between the ages of 5 and 19 years who were nondiabetic at a baseline examination and had at least one follow-up examination.
Examinations included measurement of serum cholesterol concentration and a modified 75-g oral glucose tolerance test with plasma glucose concentration measured 2 hours after the glucose load. Diabetes was diagnosed according to 1998 World Health Organization criteria; if the fasting plasma glucose concentration was ≥126 mg/dL, or the 2-hour plasma glucose concentration was ≥200 mg/dL, or a previous diagnosis was documented in the clinical record. Among those without diabetes, IGT was diagnosed if the 2-hour plasma glucose concentration was ≥140 mg/dL but <200 mg/dL. Before 1975, oral glucose tolerance tests were performed without an overnight fast, so a fasting plasma glucose concentration was not available during that period. To allow for the use of data from the entire study period, 2-hour glucose, rather than fasting glucose, was used to define hyperglycemia. Similarly, waist circumference, high-density lipoprotein (HDL) cholesterol, and triglycerides were not measured throughout the study, so to maximize follow-up we used BMI to define adiposity and total serum cholesterol to define lipid status (7, 11). Hypercholesterolemia was diagnosed if the serum cholesterol concentration was ≥ 200 mg/dL.
Children were dressed in light clothing without shoes for height and weight measurements. BMI was converted to a percentile rank based on the Centers for Disease Control and Prevention 2000 age- and sex-specific growth charts (12). A child was considered overweight if the age- and sex-specific BMI was ≥ the 85th percentile, as recommended by the Pediatrics Expert Committee on Obesity (13). For these analyses, we included obese individuals (BMI ≥ 95th percentile) in the overweight category. Blood pressure was measured while the subject was resting in the supine position. Diastolic blood pressure was measured at the fourth Korotkoff sound. Hypertension was defined as blood pressure ≥95th percentile of the age-, sex-, and height-specific value for either systolic or diastolic blood pressure from National Health and Nutrition Examination Survey 1999–2000 (14). For continuous analyses of blood pressure, mean arterial pressure (MAP), calculated as (2 * diastolic blood pressure + systolic blood pressure)/3, was used. To address grouping of the metabolic risk factors, we developed a set of metabolic abnormalities defined by the presence of overweight and at least two of the following conditions: hypertension, hypercholesterolemia, or IGT. These criteria were adapted from the current International Diabetes Federation (IDF) metabolic syndrome recommendations with specific modifications to suit our available data (Supplemental Table 1) (7). Subjects who met these criteria were classified as belonging to the “metabolic set” (MSet).
Study cohorts
The study population was divided into groups according to metabolic profile and baseline age. The groups included nonoverweight, nonoverweight plus hypertension and/or hyperlipidemia, nonoverweight plus IGT, overweight, overweight plus hypertension or hypercholesterolemia (OHH), overweight plus IGT without hypertension or hypercholesterolemia (OIGT), MSet without IGT (MSet-HH), and MSet with IGT (MSet-IGT). To determine whether there were age-related differences in the effect of the metabolic risk factors on diabetes, the cohort was subdivided into two groups according to age at the baseline examination (ages 5–11 and 12–19 years). The same subject could appear in both age cohorts. If a subject had multiple examinations within a given age cohort, the examination nearest the midpoint of the age range was used as the baseline. Thus, we had a total of 7748 baseline examinations in 5532 subjects: 3801 subjects with an examination in the 5- to 11-year-old cohort and 3947 in the 12- to 19-year-old cohort, with 2216 individuals examined in both cohorts. In analyses of the entire sample, if a subject appeared in both age cohorts his/her examination from the 5- to 11-year cohort was considered the baseline examination. If an individual with a 5- to 11-year-old examination developed diabetes before the 12- to 19-year-old examination, he or she was not included in the later age cohort. Due to the small sample size in some groups, the OHH and MSet-HH groups were combined, and the OIGT and MSet-IGT groups were combined for incidence rate calculations. A detailed breakdown of the groups can be found in Supplemental Table 2.
This study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases, and each subject ≥18 years old gave informed consent. Parents of children <18 years old provided informed consent, and the children assented to the study.
Statistical analysis
Subjects were followed from their baseline examination until diabetes was diagnosed or, in those who did not develop diabetes, to their last examination.
Unadjusted cumulative incidence was calculated from the Kaplan-Meier survival function for each metabolic group. Incidence rates were computed for both baseline age cohorts as the number of new cases of diabetes per 1000 person-years at risk. Incidence rate ratios were calculated from the incidence rates using the nonoverweight group without any metabolic abnormalities as the reference. Confidence intervals were determined by calculating the standard error of the logged incidence rate ratio and evaluating the 95% limits of the rate ratio, as described by Rothman et al (15).
Cox regression modeling was used to assess each metabolic risk factor as a continuous variable with adjustment for covariates. Two different models were assessed, one truncated at 5 years of follow-up and one truncated at 15 years. The longer follow-up led to a violation of the proportionality assumption, so we also calculated cumulative incidence rates for each metabolic risk factor adjusted for the other factors from a Poisson regression model. This method allowed the relative risks to vary over the follow-up time.
To develop this model, diabetes incidence density data were stratified by sex, age (as a time-dependent variable, changing during follow-up), and baseline metabolic risk factor quartile (16). A Poisson regression model was fit to the incidence density data using the logistic procedure in SAS (17). This model was used to calculate incidence rates of diabetes for each age category during follow-up (<20, 20–29, 30–39, and 40–49 years) and the quartile for a given metabolic risk factor. The model also included sex, the quartile rank of the other three risk factors, and an interaction term between age at follow-up and quartile rank of each risk factor. The age-interaction term allows for changes in the incidence rate ratio for a given metabolic risk factor over time. The adjusted cumulative incidence for a given follow-up time was calculated from the incidence rates using the formula:
where Ij is the incidence rate for the jth age and risk-factor category, tj is the amount of time spent in that category, and the summation is over all relevant categories (15). The summary effect of increasing quartile rank on incidence over all age categories was assessed by adding the corresponding β values of the metabolic risk factor and its age interaction terms. The null hypothesis that this value was zero was analyzed with the Wald χ2 test (one degree of freedom). Model fit was evaluated with the Hosmer-Lemeshow test.
Results
Baseline characteristics
Table 1 shows the demographic, clinical, and biochemical characteristics of the participants at baseline. Over half of the children were overweight or obese. Of the 5532 children, 1281 (23%) developed diabetes during a median follow-up of 12.4 (interquartile range, 6.0–22.9) years. In the 5- to 11-year age cohort, 769 (20%) developed diabetes during a median follow-up period of 12.8 (interquartile range, 6.1–23.3) years; in the 12- to 19-year age cohort, 1114 (28%) participants developed diabetes during a median follow-up period of 12.3 (interquartile range, 6.1–21.3) years. A total of 602 cases were counted in both cohorts because there was overlap between the age groups.
Table 1.
Baseline Characteristics for Both Baseline Age Cohorts
| Full Cohort | Baseline Age Cohort |
||
|---|---|---|---|
| 5–11 y | 12–19 y | ||
| Characteristics | |||
| n | 5532 | 3801 | 3947 |
| Age, y | 11.4 ± 3.6 | 9.2 ± 1.5 | 15.9 ± 1.6 |
| Males, n (%) | 2591 (46.8) | 1836 (48.3) | 1707 (43.3) |
| BMI (percentile) | 87.6 (61.7–97.0) | 86.4 (59.0–97.0) | 91.1 (70.1–97.6) |
| ≥85th percentile | 2972 (53.7%) | 1977 (52.0%) | 2366 (59.9%) |
| 2-hour plasma glucose, mg/dL | 98.3 ± 21.9 | 96.8 ± 20.5 | 101.3 ± 24.6 |
| ≥140 mg/dL | 238 (4.3%) | 119 (3.1%) | 284 (7.2%) |
| MAP, mm Hg | 74.9 ± 11.1 | 71.7 ± 10.1 | 81.2 ± 9.8 |
| ≥95th percentile | 794 (14.4%) | 472 (12.4%) | 656 (16.6%) |
| Serum total cholesterol, mg/dL | 149 ± 28 | 149 ± 25 | 150 ± 30 |
| ≥200 mg/dL | 228 (4.1%) | 114 (3.0%) | 202 (5.1%) |
| Metabolic groups, n (%) | |||
| Nonoverweight | 2226 (40.2) | 1616 (42.5) | 1341 (34.0) |
| Nonoverweight + hypertension and/or hypercholesterolemia | 297 (5.4) | 189 (5.0) | 195 (4.9) |
| Nonoverweight + IGT | 37 (0.7) | 19 (0.5) | 45 (1.1) |
| Overweight | 2170 (39.2) | 1544 (40.6) | 1590 (40.3) |
| OHH | 570 (10.3) | 315 (8.3) | 511 (13.0) |
| OIGT | 132 (2.4) | 64 (1.7) | 163 (4.1) |
| MSet-HH | 31 (0.6) | 18 (0.5) | 26 (0.7) |
| MSet-IGT | 69 (1.2) | 36 (0.9) | 76 (1.9) |
Continuous variables are given as mean ± SD or median (interquartile range). The eight metabolic groupings are shown at the bottom. A total of 5532 children were included; 3801 had an exam between ages 5 and 11 years, 3947 had an exam between ages 12 and 19 years, and 2216 had an exam in both age groups.
Development of diabetes by metabolic group
Figure 1 shows the 20-year cumulative incidence of diabetes for the whole cohort. The two overweight groups with IGT had the highest cumulative incidence (OIGT, 79%; MSet-IGT, 76%), followed by nonoverweight children with IGT and overweight children without IGT (IGT, 34%; overweight, 28%; OHH, 35%; and MSet-HH, 29%). Nonoverweight children without IGT had the lowest cumulative incidence of diabetes (nonoverweight, 10%; HH, 11%). The numbers of subjects in each category per 5-year increments of follow-up time are given in Supplemental Table 3.
Figure 1.
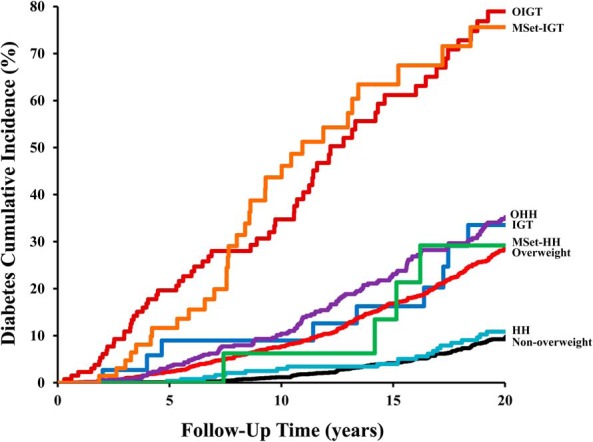
Twenty-year cumulative incidence of type 2 diabetes by baseline metabolic category.
Age-specific incidence of diabetes
Table 2 shows the incidence rates of diabetes for both baseline age cohorts, stratified by baseline metabolic profile and follow-up age. The incidence of diabetes was greater with worse metabolic profile and generally increased with age at follow-up in both cohorts. Follow-up was sparse in the later age categories. Incidence rate ratios by metabolic profile category are shown with the nonoverweight category as the reference. Incidence rates of diabetes in the metabolically abnormal profiles were substantially higher than in the nonoverweight group. The rate ratios were highest in the first 5 years of follow-up and declined over time. In the 5- to 11-year-old cohort, for example, the incidence rate ratio for diabetes in those with OIGT or MSet-IGT over the first 5 years was 188.2 (95% confidence interval [CI], 43.8–808.0), and in the 12- to 19-year-old cohort, the rate ratio was 50.7 (95% CI, 15.3–168.3) relative to the nonoverweight cohort.
Table 2.
Incidence of Type 2 Diabetes Among the Two Baseline Age Cohorts
| Age at follow-Up, y | Category | Baseline Age 5–11 Years |
Baseline Age 12–19 Years |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | Person-Years | Incidence | Rate Ratio (95% CI) | No. of Cases | Person-Years | Incidence | Rate Ratio (95% CI) | ||
| 10–14 | Nonoverweight | 2 | 7001.4 | 0.3 | 1.0 | ||||
| Overweight | 31 | 6402.6 | 4.8 | 16.9 (4.1–70.8) | |||||
| OHH or MSet-HH | 8 | 1357.8 | 5.9 | 20.6 (4.4–97.1) | |||||
| OIGT or MSet-IGT | 19 | 353.4 | 53.8 | 188.2 (43.8–808.0) | |||||
| 15–19 | Nonoverweight | 10 | 5660.0 | 1.8 | 1.0 | 3 | 4948.0 | 0.6 | 1.0 |
| Overweight | 45 | 4851.5 | 9.3 | 5.3 (2.6–10.4) | 25 | 5700.3 | 4.4 | 7.2 (2.2–24.0) | |
| OHH or MSet-HH | 11 | 995.8 | 11.0 | 6.3 (2.7–14.7) | 15 | 1906.8 | 7.9 | 13.0 (3.8–44.8) | |
| OIGT or MSet-IGT | 10 | 221.9 | 45.1 | 25.5 (10.6–61.3) | 24 | 781.1 | 30.7 | 50.7 (15.3–168.3) | |
| 20–24 | Nonoverweight | 21 | 4314.1 | 4.9 | 1.0 | 7 | 5222.1 | 1.3 | 1.0 |
| Overweight | 56 | 3358.7 | 16.7 | 3.4 (2.1–5.7) | 79 | 5638.9 | 14.0 | 10.5 (4.8–22.6) | |
| OHH or MSet-HH | 21 | 749.0 | 28.0 | 5.8 (2.6–4.8) | 33 | 2013.2 | 16.4 | 12.2 (5.4–27.6) | |
| OIGT or MSet-IGT | 4 | 128.3 | 31.2 | 6.4 (2.2–18.7) | 37 | 721.1 | 51.3 | 38.3 (17.1–85.9) | |
| 25–29 | Nonoverweight | 59 | 3370.5 | 17.5 | 1.0 | 45 | 4173.5 | 10.8 | 1.0 |
| Overweight | 73 | 2274.2 | 32.1 | 1.8 (1.3–2.6) | 113 | 3839.0 | 29.4 | 2.7 (1.9–3.9) | |
| OHH or MSet-HH | 15 | 553.6 | 27.1 | 1.6 (0.9–2.7) | 47 | 1362.1 | 34.5 | 3.2 (2.1–4.8) | |
| OIGT or MSet-IGT | 6 | 73.1 | 82.1 | 4.7 (2.0–10.9) | 38 | 344.9 | 110.2 | 10.2 (6.6–15.7) | |
| 30–34 | Nonoverweight | 58 | 2383.6 | 24.3 | 1.0 | 59 | 3209.3 | 18.4 | 1.0 |
| Overweight | 66 | 1428.8 | 46.2 | 1.9 (1.3–2.7) | 91 | 2493.3 | 36.5 | 2.0 (1.4–2.8) | |
| OHH or MSet-HH | 16 | 399.4 | 40.1 | 1.6 (0.9–2.9) | 36 | 947.9 | 38.0 | 2.1 (1.4–3.1) | |
| OIGT or MSet-IGT | 5 | 45.8 | 109.1 | 4.5 (1.8–11.2) | 16 | 150.1 | 106.6 | 5.8 (3.3–10.1) | |
| 35–39 | Nonoverweight | 56 | 1385.9 | 40.4 | 1.0 | 57 | 2177.2 | 26.2 | 1.0 |
| Overweight | 38 | 730.2 | 52.0 | 1.3 (0.9–1.9) | 63 | 1479.4 | 42.6 | 1.6 (1.1–2.3) | |
| OHH or MSet-HH | 9 | 266.1 | 33.8 | 0.8 (0.4–1.7) | 42 | 582.2 | 72.1 | 2.8 (1.8–4.1) | |
| OIGT or MSet-IGT | 2 | 17.8 | 112.4 | 2.8 (0.7–11.4) | 10 | 56.6 | 176.6 | 6.7 (3.4–13.2) | |
| 40–44 | Nonoverweight | 42 | 1162.5 | 36.1 | 1.0 | ||||
| Overweight | 50 | 674.1 | 74.2 | 2.1 (1.4–3.1) | |||||
| OHH or MSet-HH | 20 | 325.1 | 61.5 | 1.7 (1.0–2.9) | |||||
| OIGT or MSet-IGT | 1 | 10.0 | 99.5 | 2.8 (0.4–20.0) | |||||
Incidence is stratified into categories using baseline metabolic groups and age at follow-up and is shown in cases/1000 person-years.
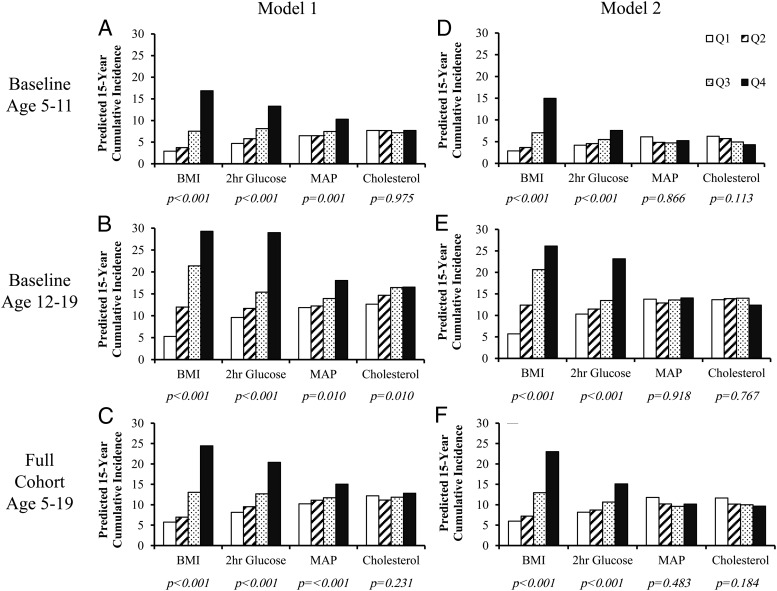
Analysis of multiple metabolic components
Results from the Poisson regression model are shown in Figure 2. With adjustment for age and sex, an increase in quartile rank for BMI, 2-hour glucose, and MAP strongly predicted the incidence of diabetes; in the 12- to 19-year baseline age cohort, cholesterol also predicted diabetes incidence. Further adjustment for the other three metabolic risk factors had little effect on BMI and 2-hour glucose, but the effects of MAP and cholesterol were attenuated and no longer significant. In the fully adjusted model, sex was not a significant predictor of diabetes. These findings were similar to those of the Cox regression analyses (Supplemental Table 4).
Figure 2.
Predicted cumulative incidence of diabetes by quartile rank of risk factor. A–C, Model 1 is adjusted for follow-up age, sex, and the interaction between age and the risk factor of interest. D–F, Model 2 is further adjusted for the other risk factors and their age-interactions. P values are given for the linear trend.
Discussion
Of the four metabolic risk factors analyzed in this study, BMI and 2-hour glucose predicted diabetes. Overweight or overweight plus IGT during childhood or adolescence predicted an increased incidence of diabetes up to 25 years after the baseline examination. Even in children 5–11 years old, a BMI ≥85th percentile and/or IGT were risk factors for diabetes in adulthood, suggesting that interventions should be considered in young children presenting with metabolic abnormalities as well as in older children. An MSet classification provided little, if any, information to enhance prediction of diabetes beyond overweight and IGT alone. After adjustment for the other three metabolic risk factors, higher MAP and cholesterol were not associated with increased diabetes incidence.
Previous studies suggest that a childhood metabolic syndrome diagnosis predicts diabetes development; however, it is not superior to BMI and glucose tolerance alone (3–5). In a joint analysis of the Bogalusa Heart Study and the Cardiovascular Risk in Young Finns Study, metabolic syndrome was no better than a BMI in the upper quartile of the study group for predicting subclinical atherosclerosis, adult metabolic syndrome, or diabetes (4). The Princeton Lipid Research Clinics Follow-Up Study found that fasting glucose (but not BMI, triglycerides, HDL cholesterol, or blood pressure) predicted either IGT or diabetes (5).
To our knowledge, the present analysis represents the largest longitudinal study of the incidence of diabetes into adulthood based on early childhood measurements. We characterized the effect of metabolic abnormalities on diabetes risk in 5-year windows of follow-up time, allowing for the visualization of short-, medium-, and long-term risk of diabetes attributable to early risk factors. Previous analyses of the Pima cohort identified obesity and elevated glucose in children as risk factors for subsequent type 2 diabetes, but were limited in sample size and follow-up and thus did not characterize long-term risk (2, 3). In the present study, the incidence of diabetes was remarkably elevated in subjects who were overweight or had IGT (Figure 1), and the increased risk persisted over a span of 25 years (Table 2). The incidence rate ratios for overweight children with or without IGT relative to the nonoverweight group declined over time, even as the incidence rate of diabetes increased for all groups, suggesting that the risk associated with these early childhood abnormalities diminished relative to other risk factors as the cohorts aged.
The significance of metabolic risk factors in children below age 10 has not been well characterized. Current IDF criteria do not provide for a metabolic syndrome diagnosis before 10 years of age, although they recommend that abdominal obesity prompt a weight loss intervention (7). Morrison et al (18) showed that hyperinsulinemia and metabolic syndrome at age 10 predicted impaired glucose regulation in young adulthood. By contrast, in a previous analysis of a subset of the present cohort with normal glucose tolerance, BMI, 2-hour glucose, and fasting insulin measured in 5–9 year olds were not significantly associated with the 10-year cumulative incidence of diabetes. However, these variables were predictive in 10–19 year olds (2). Franks et al (3) studied another subset 10 years later and found that only waist circumference predicted diabetes in 5–9 year olds. The present findings indicate that even in early childhood, poor glucose regulation and excess weight independently increase diabetes risk. Moreover, the earlier presence of these conditions was associated with a larger relative increase in diabetes risk. Incidence rate ratios for children with overweight and impaired glucose or MSet with impaired glucose tolerance were higher in 5–11 year olds than 12–19 year olds during the first 5 years of follow-up; after 5 years, the ratios became comparable between the two groups. This suggests that metabolic abnormalities are of great importance in young children. The present study has more subjects and longer follow-up time than our previous reports in this population. Previous cross-sectional studies have identified metabolic abnormalities including IGT, hypertension, dyslipidemia, and hepatic steatosis in overweight or obese children younger than 10 years of age (19–21). Taken together with prior research, our findings illustrate the importance of early metabolic abnormalities as risk factors for diabetes later in life.
Although our population has a higher risk of type 2 diabetes than other racial and ethnic groups, the pathophysiology of diabetes development is similar. We reported previously in Pima adults that diabetes is preceded by obesity, insulin resistance, and reduced insulin secretion, and these findings correspond closely to those of studies in adolescents of other population groups (22, 23). We also recently tested a number of established genetic variants associated with diabetes in other populations and found that they are similarly associated in Pimas, although they do not account for the higher prevalence of diabetes (24). Thus, we believe our findings can be generalized for other populations.
Strengths of the present study include its detailed characterization of the study population from an early age, the long duration of follow-up, the large number of children with IGT, and the statistical precision provided by the large number of diabetes cases that developed during follow-up. Limitations include our inability to distinguish type 1 diabetes, maturity onset diabetes of the young (MODY), and type 2 diabetes, and the lack of data on waist circumference, insulin, fasting glucose, triglycerides, and HDL cholesterol during the early years of the study. We have previously shown that the overwhelming majority, if not all, diabetes in Pima Indians is type 2. Very few Pimas present with islet cell or glutamic acid decarboxylase antibodies at the time of diabetes diagnosis, and virtually none display insulin dependence (25, 26). Additionally, we have found no evidence that mutations in known MODY or MODY candidate genes are associated with diabetes risk in Pimas (27).
To maximize follow-up time, we used metabolic risk factors that were available throughout the study and combined them into a single set, based on IDF metabolic syndrome criteria, to assess the effects of combining risk factors. A prior study in Pima Indian children and adolescents found a strong correlation (r = 0.96–0.98) between BMI and fat mass obtained using dual energy x-ray absorptiometry, indicating that BMI is a reliable measure of adiposity in our population (28). BMI is generally preferred for assessing obesity in children and adolescents because it is easy to obtain and identifies the most overweight/obese subjects with reasonable accuracy (29, 30). Two-hour plasma glucose concentration continues to be used for the diagnosis of IGT and diabetes, and a total serum cholesterol concentration of >200 mg/dL can be used to diagnose dyslipidemia (31). In the IDF metabolic syndrome guidelines, HDL cholesterol and triglycerides are used in lieu of total cholesterol, presumably because the syndrome was initially described as reflecting a clustering of risk factors for cardiovascular disease and insulin resistance, including low HDL cholesterol and hypertriglyceridemia (32, 33). In addition, childhood obesity is associated with a dyslipidemic pattern that includes elevated triglycerides and low HDL cholesterol, and this pattern is related to the initiation and progression of atherosclerotic lesions (34). In regard to diabetes risk, a previous analysis of a smaller subset of the Pima cohort found that low HDL cholesterol was associated with diabetes, but its predictive power was weaker than that of BMI and 2-hour glucose (3).
The presence of metabolic risk factors is common in Pima Indian children and predicts type 2 diabetes. Nevertheless, most, if not all, of the predictive power is attributable to BMI and glucose tolerance. Blood pressure and cholesterol did not predict diabetes risk after adjustment for BMI and glucose tolerance. Under current IDF guidelines, the definition of pediatric metabolic syndrome does not account for the presence of IGT, and thus it may under- or overestimate diabetes risk depending on IGT status. Additionally, we found that higher BMI and 2-hour glucose in children at least as young as 5 years old—the youngest age enrolled in this study—increases their risk of diabetes. The heightened diabetes risk associated with a poor metabolic profile in childhood and adolescence can persist for up to 25 years, although the added risk is attenuated as the cohort ages.
Acknowledgments
The authors thank members of the Gila River Indian Community for participation in these studies and the staff of the National Institute of Diabetes and Digestive and Kidney Diseases Phoenix Branch for assistance.
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. Part of this work was presented at the American Diabetes Association 75th Scientific Sessions, June 5–9, 2015, Boston, MA.
Disclosure Summary: The authors have indicated no financial relationships relevant to this article to disclose.
Footnotes
- BMI
- body mass index
- CI
- confidence interval
- HDL
- high-density lipoprotein
- IGT
- impaired glucose tolerance
- MAP
- mean arterial pressure
- MODY
- maturity onset diabetes of the young
- MSet
- metabolic set
- MSet-HH
- MSet without IGT
- MSet-IGT
- MSet with IGT
- OHH
- overweight plus hypertension and/or hyperlipidemia
- OIGT
- overweight plus IGT.
References
- 1. Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–206. [DOI] [PubMed] [Google Scholar]
- 2. McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Bennett PH, Knowler WC. Glucose, insulin concentrations and obesity in childhood and adolescence as predictors of NIDDM. Diabetologia. 1994;37:617–623. [DOI] [PubMed] [Google Scholar]
- 3. Franks PW, Hanson RL, Knowler WC, et al. Childhood predictors of young-onset type 2 diabetes. Diabetes. 2007;56:2964–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magnussen CG, Koskinen J, Chen W, et al. Pediatric metabolic syndrome predicts adulthood metabolic syndrome, subclinical atherosclerosis, and type 2 diabetes mellitus but is no better than body mass index alone: the Bogalusa Heart Study and the Cardiovascular Risk in Young Finns Study. Circulation. 2010;122:1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morrison JA, Glueck CJ, Wang P. Childhood risk factors predict cardiovascular disease, impaired fasting glucose plus type 2 diabetes mellitus, and high blood pressure 26 years later at a mean age of 38 years: the Princeton-lipid research clinics follow-up study. Metabolism. 2012;61:531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 7. Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–2061. [DOI] [PubMed] [Google Scholar]
- 8. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 9. Morandi A, Maffeis C. Predictors of metabolic risk in childhood obesity. Horm Res Paediatr. 2014;82:3–11. [DOI] [PubMed] [Google Scholar]
- 10. Schubert CM, Sun SS, Burns TL, Morrison JA, Huang TT. Predictive ability of childhood metabolic components for adult metabolic syndrome and type 2 diabetes. J Pediatr. 2009;155:S6.e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Viner RM, Segal TY, Lichtarowicz-Krynska E, Hindmarsh P. Prevalence of the insulin resistance syndrome in obesity. Arch Dis Child. 2005;90:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years). http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm Accessed December 12, 2014.
- 13. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192. [DOI] [PubMed] [Google Scholar]
- 14. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 15. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Philadelphia, PA: Lippincott Williams, Wilkins; 2008. [Google Scholar]
- 16. Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol. 1978;108:497–505. [DOI] [PubMed] [Google Scholar]
- 17. Frome EL, Checkoway H. Epidemiologic programs for computers and calculators. Use of Poisson regression models in estimating incidence rates and ratios. Am J Epidemiol. 1985;121:309–323. [DOI] [PubMed] [Google Scholar]
- 18. Morrison JA, Glueck CJ, Umar M, Daniels S, Dolan LM, Wang P. Hyperinsulinemia and metabolic syndrome at mean age of 10 years in black and white schoolgirls and development of impaired fasting glucose and type 2 diabetes mellitus by mean age of 24 years. Metabolism. 2011;60:24–31. [DOI] [PubMed] [Google Scholar]
- 19. Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346:802–810. [DOI] [PubMed] [Google Scholar]
- 20. D'Adamo E, Impicciatore M, Capanna R, et al. Liver steatosis in obese prepubertal children: a possible role of insulin resistance. Obesity (Silver Spring). 2008;16:677–683. [DOI] [PubMed] [Google Scholar]
- 21. D'Adamo E, Marcovecchio ML, Giannini C, et al. The possible role of liver steatosis in defining metabolic syndrome in prepubertal children. Metabolism. 2010;59:671–676. [DOI] [PubMed] [Google Scholar]
- 22. Bogardus C, Tataranni PA. Reduced early insulin secretion in the etiology of type 2 diabetes mellitus in Pima Indians. Diabetes. 2002;51(suppl 1):S262–S264. [DOI] [PubMed] [Google Scholar]
- 23. Giannini C, Caprio S. Progression of β-cell dysfunction in obese youth. Curr Diab Rep. 2013;13:89–95. [DOI] [PubMed] [Google Scholar]
- 24. Hanson RL, Rong R, Kobes S, et al. Role of established type 2 diabetes-susceptibility genetic variants in a high prevalence American Indian population. Diabetes. 2015;64:2646–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dabelea D, Palmer JP, Bennett PH, Pettitt DJ, Knowler WC. Absence of glutamic acid decarboxylase antibodies in Pima Indian children with diabetes mellitus. Diabetologia. 1999;42:1265–1266. [DOI] [PubMed] [Google Scholar]
- 26. Knowler WC, Bennett PH, Bottazzo GF, Doniach D. Islet cell antibodies and diabetes mellitus in Pima Indians. Diabetologia. 1979;17:161–164. [DOI] [PubMed] [Google Scholar]
- 27. Baier LJ, Permana PA, Traurig M, et al. Mutations in the genes for hepatocyte nuclear factor (HNF)-1α, -4α, -1β, and -3β; the dimerization cofactor of HNF-1; and insulin promoter factor 1 are not common causes of early-onset type 2 diabetes in Pima Indians. Diabetes Care. 2000;23:302–304. [DOI] [PubMed] [Google Scholar]
- 28. Lindsay RS, Hanson RL, Roumain J, Ravussin E, Knowler WC, Tataranni PA. Body mass index as a measure of adiposity in children and adolescents: relationship to adiposity by dual energy x-ray absorptiometry and to cardiovascular risk factors. J Clin Endocrinol Metab. 2001;86:4061–4067. [DOI] [PubMed] [Google Scholar]
- 29. US Preventive Services Task Force, Barton M. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2010;125:361–367. [DOI] [PubMed] [Google Scholar]
- 30. Krebs NF, Jacobson MS. Prevention of pediatric overweight and obesity. Pediatrics. 2003;112:424–430. [DOI] [PubMed] [Google Scholar]
- 31. Preventing Childhood Obesity: Health in the Balance. In: Koplan JP, Liverman CT, Kraak VI, eds. Washington DC: Institute of Medicine National Academies Press; 2005. [PubMed] [Google Scholar]
- 32. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. [DOI] [PubMed] [Google Scholar]
- 33. Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34:416–422. [DOI] [PubMed] [Google Scholar]
- 34. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]