Vitamin D3 regulates Wnt/β-catenin and mTOR signaling pathways that are associated with cell proliferation and tumorigenicity, and thus vitamin D3 may have therapeutic utility as an effective, safe, long-term treatment option for human UFs.
Abstract
Context:
Somatic mutations in the Med12 gene are known to activate Wnt/β-catenin signaling in human uterine fibroids (UFs).
Objective:
The objective of the study was to examine the role of vitamin D3 in the modulation of Wnt/β-catenin and mammalian target of rapamycin (mTOR) signaling in human UF cells.
Design:
Immortalized human UF cells (HuLM) and human primary UF (PUF) cells were treated with increasing concentrations of vitamin D3 and thereafter analyzed using Western blots and immunocytochemistry.
Main Outcome Measures:
Wnt/β-catenin and mTOR signaling proteins in cultured HuLM and PUF cells were measured.
Results:
UF tumors with Med12 somatic mutations showed an up-regulation of Wnt4 and β-catenin as compared with adjacent myometrium. Vitamin D3 administration reduced the levels of Wnt4 and β-catenin in both HuLM and PUF cells. Vitamin D3 also reduced the expression/activation of mTOR signaling in both cell types. In contrast, vitamin D3 induced the expression of DNA damaged-induced transcription 4 (an inhibitor of mTOR) and tuberous sclerosis genes (TSC1/2) in a concentration-dependent manner in HuLM cells. Furthermore, we observed a concentration-dependent reduction of Wisp1 (Wnt induced signaling protein 1) and flap endonuclease 1 proteins in HuLM cells. Additionally, abrogation of vitamin D receptor expression (by silencing) in normal myometrial cells induces Wnt4/β-catenin as well as prompts a fibrotic process including an increase in cell proliferation and increased extracellular matrix production. Together these results suggest that vitamin D3 functions as an inhibitor of Wnt4/β-catenin and mTOR signaling pathways, which may play major roles in fibroid pathogenesis.
Conclusion:
Vitamin D3 may have utility as a novel long-term therapeutic and/or preventive option for uterine fibroids.
Uterine fibroids (UFs), or leiomyomas, are the leading cause of hysterectomy in reproductive-age women (1–3). UFs afflict up to 70% of reproductive-age women and 80% of all women during their lifetime (4). Clinically, UFs present with a variety of symptoms, including vaginal bleeding, pelvic pain, urinary and bowel symptoms, infertility and abortion, early miscarriage, and preterm labor (5). UFs are 3–4 times more prevalent in African American women, who also suffer from vitamin D deficiency (6, 7). We and others have recently established that women with UFs have low levels of serum vitamin D3 as compared with women who do not have UFs (8–10). In our prior study, we also showed a direct association between low levels of serum vitamin D3 and increased size of UFs (8). Furthermore, in our prior publications, we have established that vitamin D3 in a potent growth inhibitor of human UF cells in vitro and that it inhibits the growth of fibroid tumors in several in vivo animal models (11–16).
Recently we and others have demonstrated that gene mutations in the mediator complex subunit 12 (Med12) play key roles in the pathogenesis of human UFs (17–21). Somatic mutations in the Med12 gene have also been linked with the induction of gene expression of wingless-type mouse mammary tumor virus integration site family, member 4 (Wnt4) and activation of β-catenin signaling (19). UFs with missense mutations in the Med12 gene also showed an overexpression of IGF-2 as compared with UFs that have no mutations (22), indicating the functional role of these mutations in fibroid pathogenesis. A more recent study has demonstrated that the conditional expression of a common Med12 somatic variant in the uterus promotes UF formation and genomic instability in a murine model (23). Moreover, a recent study also showed that the mammalian target of rapamycin (mTOR) pathway is one of the most highly up-regulated pathways in both human and rat tumors, and the growth of UFs is dependent on activation of mTOR signaling (24).
The Mediator is a large complex of 30 subunits that regulate eukaryotic transcription and thereby controls organismal development and homeostasis (25). The Mediator is conserved in all eukaryotic organisms and is required for the transcription of almost all genes (26). The Mediator interacts directly with a numbers of transcription factors to facilitate RNA polymerase II recruitment to target genes (27). Med12 has been linked to general functions of the complex and to specific interactions with transcription factors. Med12 is a subunit of the Cdk8 kinase module that can function as a transducer of Wnt/β-catenin signaling (28). This module interacts transiently with the other components of the Mediator and functions as a context-dependent positive or negative regulator (29–31). Using a gene knockdown approach, it has been shown that Med12 is essential for early mouse embryogenesis and for canonical Wnt and Wnt/PCP signaling pathways (32). Our previous study has shown that β-catenin physically and functionally targets the Med12 subunit to activate transcription and that the Med12 gene is essential for the transactivation of Wnt/β-catenin signaling (28). Med12 is functionally linked to the modulation of hedgehog signaling (33). Moreover, Med12 can regulate TGFβ receptor signaling (34) and estrogen receptor-α signaling in human breast cancer cells (35). Furthermore, it has also been demonstrated that Med12 expression is up-regulated in pancreatic cancer, and silencing Med12 by knockdown inhibits the cell-cycle progression in pancreatic cancer cells (36).
Although studies have demonstrated the association of Med12 with canonical Wnt/β-catenin signaling, cell-cycle progression, and the association of Med12 somatic mutations with UF pathogenesis, nevertheless, it is important to establish the therapeutic utility of vitamin D3 by the suppression of Wnt/β-catenin and mTOR signaling because these pathways play major roles in the pathogenesis of human UFs. Therefore, the main objectives of this study are to understand whether Med12 somatic mutations are associated with the activation of Wnt/β-catenin signaling and, if so, whether vitamin D3 has the potential to suppress Wnt/β-catenin and its downstream mTOR signaling pathways, thereby substantiating vitamin D3 as a novel therapeutic approach for the medical treatment for human UFs.
Materials and Methods
Cell lines and cultures
The immortalized human uterine fibroid cell line (HuLM) and immortalized human uterine myometrial smooth muscle cell line (UtSMC) were a generous gift from Dr Darlene Dixon (National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina) (37). Human primary uterine fibroid (PUF) cells were generated in our laboratory as we have described earlier (14). These cells were grown in SmBm medium (Lonza) with 5% fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2 as previously described (11).
Reagents and antibodies
1,25-dihydroxyvitamin D3, antifibronectin, and anti-β-actin antibodies were purchased from Sigma Biochemicals. Anticollagen type 1 was purchased from Fitzgerald. Monoclonal anti-β-catenin antibody was purchased from BD Biosciences. Anti-Wnt4, anti-phospho-p70 S6 kinase, and anti-p70 S6 kinase were purchased from Abcam. Anti-DNA damaged-induced transcription 4 (DDIT4), antituberous sclerosis (TSC)-1, anti-TSC2, anti-phospho-mTOR, and anti-mTOR antibodies were purchased from Thermo Scientific. Antiproliferating cell nuclear antigen (PCNA) antibody was purchased from Santa Cruz Biotechnology, and carbocyanine 3-conjugated mouse and rabbit secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc.
Protein extraction from human tissue samples
Human UFs and adjacent normal myometrium tissues were collected from consented individuals who had undergone surgery (hysterectomy or myomectomy) for the removal of UFs as previously described (38). Methodology of protein extraction from collected tissue samples was also previously described (15). Equal amounts of solubilized proteins from the above-mentioned tissue samples were resolved in 10% SDS-PAGE, and then Western blot analyses were performed to determine the expression levels of Wnt4, β-catenin, and Wnt1-inducible-signaling pathway protein 1 (Wisp1) proteins.
Western blot analyses
For analyses of protein expression, 0.7 × 106 HuLM cells were cultured in 60 mm tissue culture dishes, serum starved, and subsequently treated with increasing concentrations of vitamin D3 for 48 hours, as described earlier studies (14, 15, 38). The 0 nM concentration of vitamin D3 in each individual experiment was served as the untreated control. Preparation of protein lysates from vitamin D3-treated and untreated cells and Western blot analyses were performed as previously described (14, 15, 38). In Western blot analyses, the antigen-antibody complex was detected with Pierce enhanced chemiluminescence Western blotting substrate from Thermo Fisher Scientific. Specific protein bands were visualized after exposure to autoradiography films and developed using automatic x-ray developer. The intensity of each protein band was quantified and normalized against corresponding β-actin, as described in the figure legends where applicable.
Immunofluorescence analyses
Immunofluorescence analyses were performed as previously described (15, 38). Both HuLM and PUF cells were seeded onto glass coverslips and cultured overnight. After fixation/permeabilization steps, cells were incubated with rabbit polyclonal anti-DDIT4 and anti-Wnt4 antibodies (1:50 dilution each) or monoclonal anti-β-catenin (1:100 dilution) for 1 hour at room temperature followed by incubation for an additional 1 hour with carbocyanine 3-conjugated mouse or rabbit secondary antibodies (1:150 dilution). Fluorescent images were taken using an Axiovert 100 M inverted microscope. Signal intensities were visually compared between untreated vs vitamin D3-treated cells.
Generation of stable vitamin D receptor (VDR) knockdown cells
To examine the role of VDR in normal myometrium, the VDR gene was knocked down in UtSMC cells using VDR gene-specific short hairpin RNA (shRNA). These UtSMC cells expressed normal levels of endogenous VDR protein as we have shown previously (15), and therefore, UtSMC provides an appropriate model to determine the role of VDR in human myometrium. We used lentivirus plasmid constructs that contain human VDR gene-specific short shRNA sequences and nonfunctional scrambled-control shRNA, which were purchased from Origen Inc. These plasmid constructs express green fluorescence protein and contain a puromycin selection marker gene. These lentivirus constructs were transiently transfected into human embryonic kidney cells (293T) using lipofectamin LTX transfection reagent according to the manufacturer's instruction (Invitrogen). Fifteen hours after transfection, fresh DMEM culture medium was added and incubated for another 48 hours. Supernatant media containing lentiviruses were collected and filtered, and subsequently polybrene solution was added at the concentration of 8 μg/mL. These virus-containing media were used to infect cultured UtSMC cells. Fifteen hours after infection, fresh medium was added and then cultured for another 48 hours. Infected cells were selected with puromycin to generate stable populations, which were used for molecular characterization.
Cell proliferation assay
Cell proliferation was performed by a dimethylthiazoldiphenyltetra-zoliumbromide (MTT) assay. Briefly, cells (3000/well) from either VDR knockdown or scrambled control were seeded onto 96-well tissue culture plates (Becton Dickinson), and then the MTT assay was performed at different time points as described in the figure legends. Averaged cell numbers from triplicate wells were used in preparing the data graph. Each data point is the mean (±SD) from an individual experiment performed in triplicate (n = 3).
Statistical analysis
A Student's t test was also used to assess any significant differences between untreated control (0) vs vitamin D3-treated data points. A Student's t test was also used to assess any significant difference between control and VDR-shRNA data points. Values were considered statistically significant at a 95% confidence level when the value was P < .05. Data were presented as SD of the mean (±SD).
Results
Med12 somatic mutations induce the expression of Wnt4 and β-catenin in human uterine fibroids
To examine whether Med12 somatic mutations correlate with Wnt/β-catenin in human UFs, Western blot analyses were used to comparatively profile human UFs with Med12 somatic mutations and adjacent normal myometrium (no Med12 mutation). Med12 mutation-positive tumors expressed higher levels of β-catenin in comparison with adjacent normal myometrium (Figure 1). Med12 mutation-positive tumors also showed induced expression of Wnt4, and Wisp1 when compared with mutation-negative adjacent normal myometrium (Figure 1). These results indicate a correlation between Med12 somatic mutations and the activation of Wnt4/β-catenin signaling in human UFs.
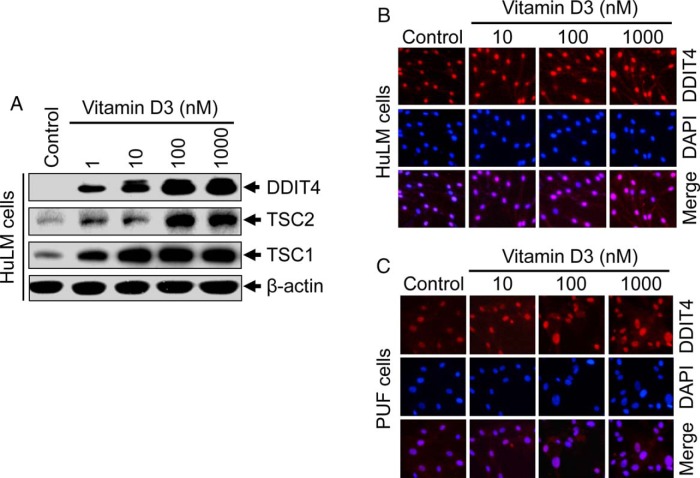
Figure 1.
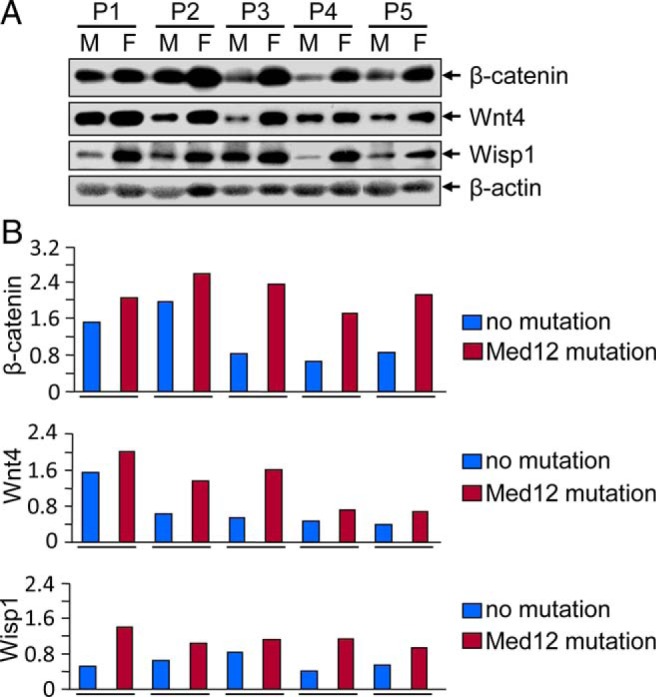
UFs having Med12 somatic mutations showed an up-regulation of β-catenin, Wnt4, and Wisp1 as compared with Med12-negative adjacent normal myometrium. A, Protein lysates were prepared from paired myometrium (M) and uterine fibroid (F; n = 5) from five individual subjects. Equal amounts of each protein lysates (30 μg) were analyzed by Western blots using anti-β-catenin, anti-Wnt4, and anti-Wisp1 antibodies. B, The intensity of each protein band was quantified using image-analyzing software, normalized to corresponding β-actin, and relative values were used to generate data graphs. Each underline shows UFs and the adjacent myometrium from the same patient. P1, P2, P3, P4, and P5 indicate fibroid subjects. Med12 mutation status in fibroids from subjects P1, P2, P3, P4, and P5 are 130G>A, 107T>C, 105A>T, 131 G>A, and 105A>T, respectively.
Vitamin D3 inhibits β-catenin protein expression in cultured HuLM cells
To first examine whether vitamin D3 can affect protein expression of β-catenin in HuLM cells, Western blot analyses were performed using lysates from HuLM cells treated with increasing concentrations of vitamin D3. Vitamin D3 treatment reduced the levels of β-catenin in a concentration-dependent manner when compared with untreated control (Figure 2A). Vitamin D3 at 100 nM concentration significantly reduced the levels of β-catenin, which was further reduced by a higher (1000 nM) concentration (Figure 2A). Immunofluorescence analyses showed β-catenin expression in both the cytoplasm and nuclei of HuLM cells, whereas vitamin D3 effectively reduced β-catenin expression in both compartments in a concentration-dependent manner. These results suggest that vitamin D3 administration reduces the levels of β-catenin in HuLM cells.
Figure 2.
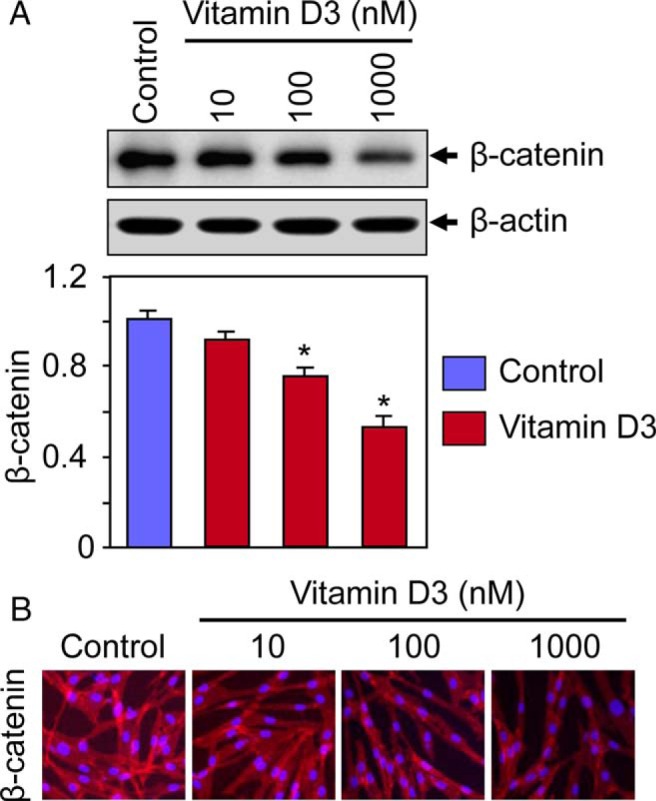
Effect of vitamin D3 on β-catenin protein expression in cultured HuLM cells. A, HuLM cells were serum starved and treated with increasing concentrations of vitamin D3 (0, 10, 100, and 1000 nM) for 48 hours. Equal amounts of each cell lysate were analyzed by Western blots using anti-β-catenin antibody. β-Actin Western blot was used as loading control. The intensity of each protein band was quantified and normalized to corresponding β-actin. *, P < .05 when compared with control. B, Immunofluorescence analyses were performed using HuLM cells cultured on glass coverslips and treated with increasing concentrations of vitamin D3 (0, 10, 100, and 1000 nM) for 48 hours. Cells were fixed, permeabilized, and stained with monoclonal anti-β-catenin antibody followed by incubating with carbocyanine 3-conjugated antimouse secondary antibody. β-Catenin staining (red) was monitored by a fluorescence microscopy. Nuclei of cells were stained with 4′,6-diamino-2-phenylindole. Pictures were taken at ×200 magnification. These data are representative of at least two independent experiments, each performed in duplicate.
Vitamin D3 inhibits protein expression of Wnt4, Wisp1, and flap endonuclease 1 (FEN1) in cultured human uterine fibroid cells
To examine whether vitamin D3 administration affects the expression of Wnt4 as well as Wnt signaling-associated proteins in HuLM cells, Western blot analyses were performed using lysates from HuLM cells treated with increasing concentrations of vitamin D3. Vitamin D3 reduced the levels of Wnt4, Wisp1, and FEN1 in a concentration-dependent manner (Figure 3A). At 10 nM concentration, vitamin D3 markedly reduced the levels of Wisp1, and FEN1, and these levels were further decreased by the higher concentrations of vitamin D3. The reduction of Wnt4 by vitamin D3 was not as robust as Wisp1 and FEN1 (Figure 3A). To further determine the localization of Wnt4 in HuLM cells, immunofluorescence analyses were performed as described above. Wnt4 staining signals (red) were predominantly localized in the nuclei of HuLM cells, whereas vitamin D3 decreased those signals in a concentration-dependent manner (Figure 3B). To substantiate these findings in human UFs, similar immunofluorescence analyses were performed using human PUF cells. Similar to HuLM cells, vitamin D3 was able to decrease nuclear Wnt4 in PUF cells (Figure 3C). These results suggest that vitamin D3 has the potential to suppress the expression of Wnt4, Wisp1, and FEN1 in cultured human uterine fibroid cells.
Figure 3.
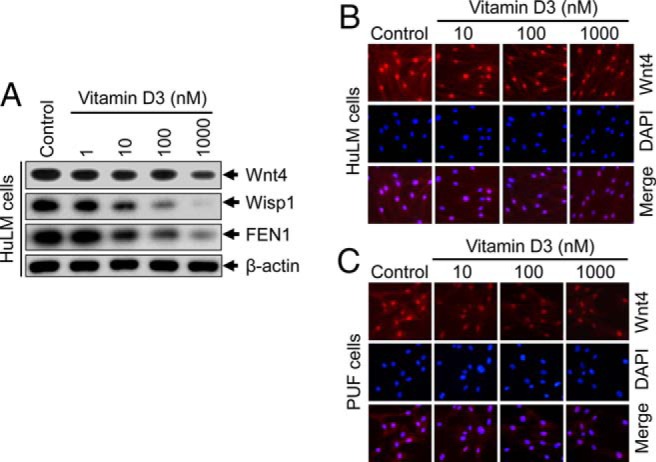
Effect of vitamin D3 on protein expression of Wnt4, Wisp1, and FEN1 in cultured human uterine fibroid cells. A, HuLM cells were serum starved and treated with increasing concentrations of vitamin D3 (0, 1, 10, 100, and 1000 nM) for 48 hours. Equal amounts of each cell lysate were analyzed by Western blots using anti-Wnt4, anti-Wisp1, and anti-FEN1 antibodies. β-Actin Western blot was used as loading control. B and C, Immunofluorescence analyses were performed using both HuLM cells (B) and human PUF cells (C) cultured on glass coverslips and treated with increasing concentrations of vitamin D3 (0, 10, 100, and 1000 nM) for 48 hours. Cells were fixed, permeabilized, and stained with anti-Wnt4 antibody (1:50 dilution) followed by incubating with carbocyanine 3-conjugated antirabbit secondary antibody. Wnt4 staining (red) was monitored by fluorescence microscopy. Nuclei of cells were stained with 4′, 6-diamino-2-phenylindole. Pictures were taken at ×200 magnification.
Vitamin D3 induces protein expression of DDIT4, TSC1, and TSC2 in cultured human uterine fibroid cells
The DDIT4 plays an important role in regulating cell proliferation in DDIT4-knockout mouse (39). Vitamin D3 inhibits osteoblast cell proliferation through the induction of DDIT4 (40). In our previous study, we showed that vitamin D3 inhibits HuLM cell proliferation (11). To test whether vitamin D3 administration affects the expression of DDIT4 in HuLM cells, Western blot analyses were performed using protein lysates from vitamin D3-treated HuLM cells as described above. Vitamin D3 induced the levels of DDIT4 in a concentration-dependent manner in HuLM cells. DDIT4 was induced at a low concentration of vitamin D3 (1 nM), whereas its induction was further stimulated by higher concentrations of vitamin D3 (Figure 4A). Similar to DDIT4, vitamin D3 treatment also induced the levels of tumor suppressors, TSC1 and TSC2, in HuLM cells (Figure 4A). To further determine the localization of DDIT4, immunofluorescence analyses were performed using HuLM cells. DDIT4 was present in the nuclei of HuLM cells, whereas vitamin D3 treatment induced nuclear DDIT4 in a concentration-dependent manner (Figure 4B). To substantiate these findings in human UFs, similar immunofluorescence analyses were performed using human PUF cells. Similarly, vitamin D3 was able to induce nuclear DDIT4 in PUF cells (Figure 4B). These results suggest that DDIT4, TSC1, and TSC2 are key vitamin D3 targets in human uterine fibroid cells.
Figure 4.
Effect of vitamin D3 on protein expression of DDIT4, TSC1, and TSC2 in cultured human uterine fibroid cells. A, HuLM cells were serum starved and treated with increasing concentrations of vitamin D3 (0, 1, 10, 100, and 1000 nM) for 48 hours, as described above. Equal amounts of each cell lysate were analyzed by Western blots using anti-DDIT4, anti-TSC1, and anti-TSC2 antibodies. β-Actin Western blot was used as loading control. B and C, Immunofluorescence analyses were performed using both HuLM cells (B) and human PUF cells (C) cultured on glass coverslips and treated with increasing concentrations of vitamin D3 (0, 10, 100, and 1000 nM) for 48 hours. Cells were fixed, permeabilized, and stained with anti-DDIT4 antibody (1:50 dilution) followed by incubating with carbocyanine 3-conjugated antirabbit secondary antibody. DDIT4 staining (red) was monitored by fluorescence microscopy. Nuclei of cells were stained with 4′,6-diamino-2-phenylindole. Pictures were taken at ×200 magnification.
Vitamin D3 inhibits activation of mTOR signaling in cultured human uterine fibroid cells
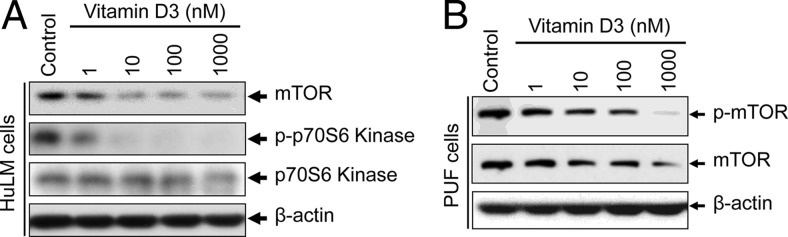
mTOR functions downstream of Wnt/β-catenin signaling, which is known to be a master regulator of cell growth and proliferation (41). Vitamin D3 has been shown to inhibit osteoblast cell proliferation by inducing the levels of DDIT4 and by suppressing mTOR signaling (40). Vitamin D3 also inhibits HuLM cell proliferation (11). Next, the effect of vitamin D3 was verified on the status of mTOR signaling in both HuLM and PUF cells. These cells were treated with vitamin D3, and cell lysates were analyzed by Western blotting as described above. Vitamin D3 reduced the protein expression of mTOR in both cell types (Figure 5, A and B). The levels of phospho-mTOR was also reduced after vitamin D3 treatment in PUF cells (Figure 5B). The p70S6 kinase activity is regulated by mTOR. Accordingly, vitamin D3 reduced the expression of phospho-p70S6 kinase in HuLM cells, whereas the total levels of p70S6 kinase was unchanged. These results suggest that vitamin D3 has the potential to reduce the activation of mTOR signaling and downstream p70S6 kinase activity, which may play important roles in the regulation of human uterine fibroid cell proliferation.
Figure 5.
Effect of vitamin D3 on activation of mTOR signaling in cultured HuLM and human PUF cells. HuLM cells (A) and human PUF cells (B) were serum starved and treated with increasing concentrations of vitamin D3 (0, 1, 10, 100, and 1000 nM) for 48 hours. Equal amounts of each cell lysate were analyzed by Western blots using anti-mTOR, anti-p-mTOR, anti-p-p70S6 kinase, and anti-p70S6 kinase antibodies, as indicated. β-Actin Western blot was used as loading control.
Silencing VDR gene induces protein expression of Wnt4/β-catenin and mTOR signaling in cultured UtSMC cells
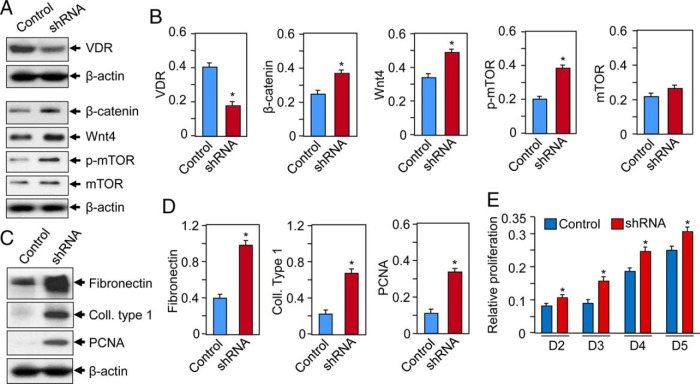
To examine the direct role of VDR in uterine myometrial cells, the VDR was depleted in UtSMC cells using lentiviral-based RNA interference. Cultured UtSMC cells were infected with lentiviruses-expressing, VDR-specific shRNA or nonfunctional scramble control (Origen Inc). Infected cells were selected with puromycin to generate stable cell populations. Western blot analyses showed that VDR-knockdown cells expressed markedly reduced levels of VDR (∼60% reduction) as compared with scrambled control cells (Figure 6, A and B). VDR-knockdown cells concurrently expressed higher levels of Wnt4 and β-catenin as compared with scrambled-control cells (Figure 6, A and B). Furthermore, VDR-knockdown cells showed higher levels of phosphorylated mTOR as compared with scrambled control, whereas the levels of mTOR was unchanged (Figure 6, A and B). These results indicate an important role for VDR in the regulation of Wnt4/β-catenin and mTOR signaling in UtSMC cells.
Figure 6.
Knockdown VDR induces Wnt4/β-catenin and mTOR signaling and induces proliferation of UtSMC cells. A, VDR was targeted for knockdown in UtSMC cells by infecting with lentiviruses expressing VDR-specific shRNA or scrambled control. Cell lysates were analyzed by Western blotting using anti-VDR, anti-β-catenin, anti-Wnt4, anti-p-mTOR, and anti-mTOR antibodies. β-Actin was used as loading control. B, Quantification of protein expression of above proteins were normalized to β-actin and shown. *, P < .05 as compared with control. C, Cell lysates were also analyzed by Western blotting using antifibronectin, anticollagen type 1, and anti-PCNA antibodies. D, Normalized protein levels are shown. *, P < .05 as compared with control. E, Both scrambled control and VDR knockdown cells were seeded into 12-well plates and cultured in phenol-free DMEM/F12 medium containing 10% charcoal stripped fetal bovine serum. Cultures were replenished every other day with fresh conditioned media. Cell proliferation MTT assay was performed at days 2, 3, 4, and 5. Each data point is the mean SD of triplicate wells (n = 3). *, P < .05 as compared with the corresponding control.
Silencing VDR gene affects extracellular matrix (ECM)-associated pathways in cultured UtSMC cells
To further test whether the disruption of VDR gene expression affects ECM-associated protein expression in human uterine myometrial smooth muscle cells, we performed Western blot analyses using cell lysates from VDR-knockdown cells and scrambled control cells as described above. Silencing of VDR gene expression was associated with increased ECM production such as induction of fibronectin and collagen type 1 expression and cell proliferation-associated PCNA (Figure 6, C and D). VDR-knockdown cells also showed induced proliferation when compared with scrambled control (Figure 6E). These results suggest that the disruption of the normal VDR gene expression in the UtSMC cells induces ECM deposition and cell proliferation.
Discussion
The purpose of this study was to evaluate the association of Med12 somatic mutations with activation of Wnt/β-catenin signaling in human UFs. More importantly, we verified the effect of vitamin D3 on the regulation of Wnt/β-catenin and its downstream mTOR signaling in human UF cells.
The Mediator is a large multiprotein complex involved in global and gene-specific transcriptional regulation. The Mediator can activate and repress transcription by virtue of its ability to interface directly with transcription factors and the RNA polymerase II initiation machinery (42). Within the Mediator, Med12 is an essential regulator of the kinase activity of cyclin-dependent kinase-8 submodule, and the protein directly interacts with various transcription factors (42, 43). Med12 is known to participate in various oncogenic signaling pathways, including p53 and Wnt/β-catenin, that have central roles in tumor development (28, 44).
UFs are benign smooth muscle tumors in the uterus that occur in approximately 70% of women by the age of 50 years (6). Recently several studies have demonstrated that Med12 gene exon 2 somatic mutations are frequently associated (up to 85% cases) in human UFs (19, 20, 45, 46). Despite the high prevalence of Med12 somatic mutations, little is known about the mechanisms of how these mutations are involved in the tumorigenicity of this disease. Although studies have linked Med12 somatic mutations with the activation of Wnt/β-catenin signaling (19, 28), the function of these mutations is quite unknown. Moreover, it is also not well characterized whether Med12 somatic mutations have association with the activation of Wnt/β-catenin signaling in human UFs. To establish whether Med12 somatic mutations are associated with Wnt/β-catenin signaling, we performed protein expression analyses. In Figure 1, our Western blot analyses showed that human UFs having Med12 somatic mutations showed higher levels of protein expression of Wnt4 and β-catenin as compared with adjacent normal myometrium that do not have Med12 somatic mutations. These findings suggest that Med12 somatic mutations can activate Wnt4/β-catenin signaling, which may play roles in the development and progression of human UFs.
We and others have recently demonstrated that vitamin D3 deficiency is a risk factor for the occurrence of human UFs (8–10). Our recent findings also showed reduced levels of VDR in human UFs as compared with the adjacent myometrium (15). Moreover, our published studies demonstrated the potentials of vitamin D3 in the inhibition of HuLM cell proliferation in vitro and fibroid tumor growth in vivo animal models (11–16). These findings suggest that both vitamin D3 deficiency and reduced expression of VDR and thus cumulative attenuation of vitamin D signaling may be important contributing factors in the pathogenesis of human UFs. Figure 2 shows that vitamin D3 has the potential to reduce the expression of β-catenin in HuLM cells. Moreover, β-catenin is localized in both the cytosol and nucleus of HuLM cells, whereas vitamin D3 treatment considerably reduced its expression at physiological concentrations, suggesting that vitamin D3 possess the ability to reduce β-catenin expression in UF cells. In contrast, vitamin D3 treatment was unable to modulate the expression of Med12 protein in PUF cells (Supplemental Figure 1), indicating that vitamin D3 targets Med12-associated downstream signaling targets. Wnt signaling-associated proteins such as Wnt4 and Wisp1 as well as FEN1 was further verified using Western blot analyses. Vitamin D3 can reduce Wnt4 in a concentration-dependent manner in HuLM cells (Figure 3), which indicates the inhibitory role of vitamin D3 on the activation of Wnt4/β-catenin signaling and that the inhibitory function of vitamin D3 may ultimately reduce the pathogenesis of human UFs.
β-Catenin functions through translocation to the nucleus and binds to the T-cell factor/lymphocyte enhancer factor family of transcription factors, resulting in the expression of specific target genes (47). Several β-catenin target genes such as c-Myc, Wisp1, and cyclin D1 are involved in cell proliferation, and β-catenin signaling plays an important role in the development and neoplasia (47). The study also demonstrated that overexpression of constitutively activated β-catenin in the uterine mesenchyme during embryonic development and in adults gives rise to leiomyoma-like tumors in the uterus in female mice (48), suggesting the involvement of Wnt/β-catenin signaling in the development of UFs. The Wisp1 gene is overexpressed in human breast cancer as well as other cancers. Wisp1 is known to be involved in tumorigenesis and the progression of many types of cancers such as breast (49), prostate (50), lung (51), and oral squamous cell carcinoma (52), indicating the vital role that Wisp1 plays in cancer development and progression. The effect of vitamin D3 on expression of Wisp1 in UF cells was verified, and our results indicate concentration-dependent inhibition of Wisp1 by vitamin D3 (Figure 3), suggesting the role of vitamin D3 is the inhibition of fibroid cell proliferation through the reduction of Wisp1.
The FEN1 genes are multifunctional proteins involved in DNA replication and damage repair. The products of FEN1 and PCNA genes are highly conserved when compared with other DNA repair genes. An earlier study has shown the up-regulation of FEN1 in human breast and other cancers, and its levels inversely correlated with the survival of breast cancer patients (53). Genomic and protein analyses revealed that FEN1 is a biomarker in breast and ovarian cancer (54). We tested the possibility that vitamin D3 has the potential to reduce FEN1 protein expression in human UF cells because this gene play a role in the tumorigenesis of several cancers. Figure 3 shows that vitamin D3 effectively suppressed FEN1 in a concentration-dependent manner in HuLM cells, indicating the therapeutic utility of vitamin D3 for fibroid treatment.
mTOR is a member of the phosphoinositol kinase-related kinase family, which is localized downstream of Wnt/β-catenin signaling, and plays an important role in the regulation of cell growth and proliferation (41, 55). The role of atypical activation of mTOR signaling in human UFs has been suggested in several studies. It has also been demonstrated that mTOR signaling was the most induced in human UFs as compared with adjacent myometrium (24). In addition, the activation of mTOR signaling and the development of UFs in the Eker rat animal model has been reported previously (56). Recent studies demonstrated a central role of dysregulated phosphoenositide 3-kinase-protein kinase B/AKT pathway leading to the activation of mTOR in the tumorigenicity of UFs (24, 56). Furthermore, the activation of AKT/mTOR and the ensuing phosphorylation of downstream target p70S6K and 4E-binding protein 1 can promote cell growth and proliferation (57). Herein we verified the effect of vitamin D3 on the regulation of mTOR signaling because this signaling pathway is known to be involved in the pathogenesis of human UFs. Our results showed that vitamin D3 has the potential to suppress mTOR signaling by reducing the protein expression of mTOR and by reducing phosphorylated p70S6 kinase activity in HuLM and PUF cells (Figure 5, A and B), and thus, these findings indicate the therapeutic potential of vitamin D3 for nonsurgical treatment of human UFs. Moreover, we observed that vitamin D3 administration induced DDIT4 and inhibits mTOR signaling in human UF cells (Figures 4 and 5). This result is consistent with the previous finding that established the growth-inhibitory role of vitamin D3 on osteoblast cell proliferation through the induction of DDIT4 and the suppression of mTOR (40). In addition, our findings in Figure 4 shows that vitamin D3 can induce tumor suppressor genes, TSC1/2, in human UF cells, which play important roles in the inhibition of downstream mTOR signaling. The mTOR signaling has been shown to be activated in UF tumors that developed in the Eker rat animal model due to the inactivation of the TSC2 gene (56).
Because vitamin D3 functions through the induction/activation of VDR signaling in UF cells, the direct role of vitamin D3/VDR was verified in uterine myometrial smooth muscle cells by silencing VDR gene expression in UtSMC cells using VDR-specific shRNA. UtSMC cells were selected in this study because these cells were generated from human uterine myometrium, and they express normal levels of VDR and maintained the normal myometrial cell phenotype. Our findings in Figure 6 indicate that VDR-knockdown UtSMC cells showed induced levels of Wnt4/β-catenin and mTOR signaling as well as higher levels of ECM deposition, indicating that VDR plays a critical role in the homeostasis and normalcy of human UtSMC cells and that abrogation of VDR expression (by silencing) elicits a fibrotic process including an increase in myometrial cell proliferation as well as increased ECM production.
In summary, we observed an association of Med12 somatic mutations with the activation of Wnt/β-catenin signaling in human UFs. Vitamin D3 administration reduced the protein expression of Wnt4 and β-catenin in human uterine fibroid cells. Additionally, vitamin D3 reduced the protein expression of Wisp1 and FEN1 in HuLM cells. In contrast, vitamin D3 induced the levels of DDIT4 and TSC1/2 while reducing the activation of mTOR signaling in HuLM cells. In parallel, vitamin D3 also reduced the activation of mTOR signaling in human PUF cells. By the silencing of VDR gene expression in human myometrial smooth muscle cells, we showed an induction of Wnt4/β-catenin and mTOR signaling and that abrogation of VDR expression elicits a fibrotic process including an increase in myometrial cell proliferation and ECM production, which are the hallmarks of the fibroid phenotype. Together our results demonstrate the critical roles that vitamin D3 plays in the suppression of tumor-promoting Wnt4/β-catenin and further downstream mTOR signaling in human UF cells. Our findings suggest that vitamin D3 may have utility as a novel therapeutic approach for the medical treatment of human UFs.
Acknowledgments
This work was supported by the Georgia Regents University Start-up package and by the Research Centers in Minority Institutions (RCMI) pilot grant 2G12RR003032-26 (to SKH), and National Institutes of Health R01 Grant 2R01HD046228-11 (to A.A.-H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DDIT4
- damaged-induced transcription 4
- ECM
- extracellular matrix
- FEN1
- flap endonuclease 1
- Med12
- mediator complex subunit 12
- mTOR
- mammalian target of rapamycin
- MTT
- dimethylthiazoldiphenyltetra-zoliumbromide
- PCNA
- proliferating cell nuclear antigen
- shRNA
- short hairpin RNA
- TSC
- tuberous sclerosis
- UF
- uterine fibroid
- VDR
- vitamin D receptor
- Wisp1
- Wnt1-inducible-signaling pathway protein 1
- Wnt4
- wingless-type mouse mammary tumor virus integration site family, member 4.
References
- 1. Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308(5728):1589–1592. [DOI] [PubMed] [Google Scholar]
- 2. Marsh EE, Bulun SE. Steroid hormones and leiomyomas. Obstet Gynecol Clin North Am. 2006;33(1):59–67. [DOI] [PubMed] [Google Scholar]
- 3. Payson M, Leppert P, Segars J. Epidemiology of myomas. Obstet Gynecol Clin North Am. 2006;33(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laughlin SK, Schroeder JC, Baird DD. New directions in the epidemiology of uterine fibroids. Semin Reprod Med. 2010;28(3):204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta S, Jose J, Manyonda I. Clinical presentation of fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):615–626. [DOI] [PubMed] [Google Scholar]
- 6. Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology. 2003;14(2):247–250. [DOI] [PubMed] [Google Scholar]
- 7. Nesby-O'Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–192. [DOI] [PubMed] [Google Scholar]
- 8. Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int J Womens Health. 2013;5:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin d and the risk of uterine fibroids. Epidemiology. 2013;24(3):447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paffoni A, Somigliana E, Vigano P, et al. Vitamin D status in women with uterine leiomyomas. J Clin Endocrinol Metab. 2013;98(8):E1374–E1378. [DOI] [PubMed] [Google Scholar]
- 11. Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al-Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril. 2011;95(1):247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-β3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2011;96(4):E754–E762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halder SK, Sharan C, Al-Hendy A. 1,25-Dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod. 2012;86(4):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halder SK, Osteen KG, Al-Hendy A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum Reprod. 2013;28(9):2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halder SK, Osteen KG, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol Reprod. 2013;89(6):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Halder SK, Sharan C, Al-Hendy O, Al-Hendy A. Paricalcitol, a vitamin D receptor activator, inhibits tumor formation in a murine model of uterine fibroids. Reprod Sci. 2014;21(9):1108–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Makinen N, Mehine M, Tolvanen J, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334(6053):252–255. [DOI] [PubMed] [Google Scholar]
- 18. Makinen N, Heinonen HR, Moore S, Tomlinson IP, van der Spuy ZM, Aaltonen LA. MED12 exon 2 mutations are common in uterine leiomyomas from South African patients. Oncotarget. 2011;2(12):966–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Markowski DN, Bartnitzke S, Loning T, Drieschner N, Helmke BM, Bullerdiek J. MED12 mutations in uterine fibroids—their relationship to cytogenetic subgroups. Int J Cancer. 2012;131(7):1528–1536. [DOI] [PubMed] [Google Scholar]
- 20. McGuire MM, Yatsenko A, Hoffner L, Jones M, Surti U, Rajkovic A. Whole exome sequencing in a random sample of North American women with leiomyomas identifies MED12 mutations in majority of uterine leiomyomas. PLoS One. 2012;7(3):e33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Halder SK, Laknaur A, Miller J, Layman LC, Diamond M, Al-Hendy A. Novel MED12 gene somatic mutations in women from the southern United States with symptomatic uterine fibroids. Mol Genet Genomics. 2015;290(2):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Tommaso S, Tinelli A, Malvasi A, Massari S. Missense mutations in exon 2 of the MED12 gene are involved in IGF-2 overexpression in uterine leiomyoma. Mol Hum Reprod. 2014;20(10):1009–1015. [DOI] [PubMed] [Google Scholar]
- 23. Mittal P, Shin YH, Yatsenko SA, Castro CA, Surti U, Rajkovic A. Med12 gain-of-function mutation causes leiomyomas and genomic instability. J Clin Invest. 2015;125(8):3280–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crabtree JS, Jelinsky SA, Harris HA, et al. Comparison of human and rat uterine leiomyomata: identification of a dysregulated mammalian target of rapamycin pathway. Cancer Res. 2009;69(15):6171–6178. [DOI] [PubMed] [Google Scholar]
- 25. Bourbon HM, Aguilera A, Ansari AZ, et al. A unified nomenclature for protein subunits of mediator complexes linking transcriptional regulators to RNA polymerase II. Mol Cell. 2004;14(5):553–557. [DOI] [PubMed] [Google Scholar]
- 26. Takagi Y, Kornberg RD. Mediator as a general transcription factor. J Biol Chem. 2006;281(1):80–89. [DOI] [PubMed] [Google Scholar]
- 27. Malik S, Roeder RG. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat Rev Genet. 2010;11(11):761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim S, Xu X, Hecht A, Boyer TG. Mediator is a transducer of Wnt/β-catenin signaling. J Biol Chem. 2006;281(20):14066–14075. [DOI] [PubMed] [Google Scholar]
- 29. Clark AD, Oldenbroek M, Boyer TG. Mediator kinase module and human tumorigenesis. Crit Rev Biochem Mol Biol. 2015;50(5):393–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akoulitchev S, Chuikov S, Reinberg D. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature. 2000;407(6800):102–106. [DOI] [PubMed] [Google Scholar]
- 31. Elmlund H, Baraznenok V, Lindahl M, et al. The cyclin-dependent kinase 8 module sterically blocks Mediator interactions with RNA polymerase II. Proc Natl Acad Sci USA. 2006;103(43):15788–15793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rocha PP, Scholze M, Bleiss W, Schrewe H. Med12 is essential for early mouse development and for canonical Wnt and Wnt/PCP signaling. Development. 2010;137(16):2723–2731. [DOI] [PubMed] [Google Scholar]
- 33. Zhou H, Kim S, Ishii S, Boyer TG. Mediator modulates Gli3-dependent Sonic hedgehog signaling. Mol Cell Biol. 2006;26(23):8667–8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang S, Holzel M, Knijnenburg T, et al. MED12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signaling. Cell. 2012;151(5):937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prenzel T, Kramer F, Bedi U, Nagarajan S, Beissbarth T, Johnsen SA. Cohesin is required for expression of the estrogen receptor-α (ESR1) gene. Epigenet Chromatin. 2012;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shaikhibrahim Z, Offermann A, Braun M, et al. MED12 overexpression is a frequent event in castration-resistant prostate cancer. Endocr Relat Cancer. 2014;21(4):663–675. [DOI] [PubMed] [Google Scholar]
- 37. Carney SA, Tahara H, Swartz CD, et al. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest. 2002;82(6):719–728. [DOI] [PubMed] [Google Scholar]
- 38. Al-Hendy A, Diamond MP, El-Sohemy A, Halder SK. 1,25-Dihydroxyvitamin D3 regulates expression of sex steroid receptors in human uterine fibroid cells. J Clin Endocrinol Metab. 2015;100(4):E572–E582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brafman A, Mett I, Shafir M, et al. Inhibition of oxygen-induced retinopathy in RTP801-deficient mice. Invest Ophthalmol Vis Sci. 2004;45(10):3796–3805. [DOI] [PubMed] [Google Scholar]
- 40. Lisse TS, Hewison M. Vitamin D: a new player in the world of mTOR signaling. Cell Cycle. 2011;10(12):1888–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729–734. [DOI] [PubMed] [Google Scholar]
- 42. Taatjes DJ, Marr MT, Tjian R. Regulatory diversity among metazoan co-activator complexes. Nat Rev Mol Cell Biol. 2004;5(5):403–410. [DOI] [PubMed] [Google Scholar]
- 43. Knuesel MT, Meyer KD, Donner AJ, Espinosa JM, Taatjes DJ. The human CDK8 subcomplex is a histone kinase that requires Med12 for activity and can function independently of mediator. Mol Cell Biol. 2009;29(3):650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Galbraith MD, Donner AJ, Espinosa JM. CDK8: a positive regulator of transcription. Transcription. 2010;1(1):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Markowski DN, Helmke BM, Bartnitzke S, Loning T, Bullerdiek J. Uterine fibroids: do we deal with more than one disease? Int J Gynecol Pathol. 2014;33(6):568–572. [DOI] [PubMed] [Google Scholar]
- 46. Heinonen HR, Sarvilinna NS, Sjoberg J, et al. MED12 mutation frequency in unselected sporadic uterine leiomyomas. Fertil Steril. 2014;102(4):1137–1142. [DOI] [PubMed] [Google Scholar]
- 47. Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127(3):469–480. [DOI] [PubMed] [Google Scholar]
- 48. Tanwar PS, Lee HJ, Zhang L, et al. Constitutive activation of β-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81(3):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61(24):8917–8923. [PubMed] [Google Scholar]
- 50. Tai HC, Chang AC, Yu HJ, et al. Osteoblast-derived WNT-induced secreted protein 1 increases VCAM-1 expression and enhances prostate cancer metastasis by down-regulating miR-126. Oncotarget. 2014;5(17):7589–7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen PP, Li WJ, Wang Y, et al. Expression of Cyr61, CTGF, and WISP-1 correlates with clinical features of lung cancer. PLoS One. 2007;2(6):e534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chuang JY, Chang AC, Chiang IP, Tsai MH, Tang CH. Apoptosis signal-regulating kinase 1 is involved in WISP-1-promoted cell motility in human oral squamous cell carcinoma cells. PLoS One. 2013;8(10):e78022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singh P, Yang M, Dai H, et al. Overexpression and hypomethylation of flap endonuclease 1 gene in breast and other cancers. Mol Cancer Res. 2008;6(11):1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abdel-Fatah TM, Russell R, Albarakati N, et al. Genomic and protein expression analysis reveals flap endonuclease 1 (FEN1) as a key biomarker in breast and ovarian cancer. Mol Oncol. 2014;8(7):1326–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nojima H, Tokunaga C, Eguchi S, et al. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem. 2003;278(18):15461–15464. [DOI] [PubMed] [Google Scholar]
- 56. Cook JD, Walker CL. The Eker rat: establishing a genetic paradigm linking renal cell carcinoma and uterine leiomyoma. Curr Mol Med. 2004;4(8):813–824. [DOI] [PubMed] [Google Scholar]
- 57. Distefano G, Boca M, Rowe I, et al. Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol Cell Biol. 2009;29(9):2359–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]