Results of this trial suggest that the tropic effects of testosterone on the prostate are mediated via its aromatization to estradiol.
Abstract
Context:
T replacement is being increasingly offered to older men with age-related low T; hence, monitoring prostate health is important during T therapy. Data suggest that estrogens have an independent effect on the prostate and some effects of T on the prostate might be mediated via its aromatization to estradiol. Although some studies have assessed the effects of T replacement on prostate volume, the differential effects of T and estradiol have not been delineated.
Objective:
The objective of the study was to investigate the relative effects of T and estradiol on prostate volume in older men with low T.
Participants:
Thirty-one men, 65 years old or older with total T less than 350 ng/dL (measured by mass spectrometry) participated in the study.
Intervention:
The intervention included randomization to 5 g transdermal T gel (TT), 1 mg oral aromatase inhibitor (AI), or placebo daily for 12 months.
Main Outcome Measures:
The primary outcome was prostate volume measured by transrectal ultrasound at baseline and 12 months. Secondary outcomes included prostate-specific antigen levels and lower urinary tract symptoms score.
Results:
Serum T levels increased in both intervention groups; estradiol levels increased in the TT group, whereas it decreased in the AI group. At 12 months, prostate volume significantly increased (4.5 ± 1.76 cc, P < .05) only in the TT group. Increase in prostate-specific antigen levels were seen in both intervention groups at 6 months (P < .01 and P < .001). The lower urinary tract symptoms score increased only in the TT group (P < .05).
Conclusion:
The tropic effects of T on the prostate are mediated via its aromatization to estradiol. Administration of AI for 12 months to older men was not detrimental to the prostate.
Aging in men is associated with a decrease in circulating serum T levels, which has been associated with loss of lean mass, reduced muscle strength, low bone mass, and sexual dysfunction (1). Recently there has been an exponential increase in T prescriptions worldwide; most of these prescriptions written for middle-aged and older men who do not have known pituitary or testicular disease (2, 3). This is also the demographic in which the prevalence of prostate disease is more common (4). Prostate is an androgen-dependent organ and prostate growth, both normal and abnormal, depends on androgens. Indeed, the prostate fails to develop without androgenic stimulation in eunuchs and men with 5α-reductase deficiency (5, 6). Because benign prostatic hyperplasia (BPH) is a frequent feature of male aging (7), concerns have been raised regarding the potential impact of T replacement on the prostate (8). As a result, structured safety monitoring plan has been suggested to assess for potential side effects of androgen replacement on the prostate (9, 10).
In addition to androgen receptors, estrogen receptors (ER) are also expressed in both the stroma and the epithelium of the prostate, and it has been suggested that estrogens play a distinct role in the growth of the prostate (11–13). Indeed, animal studies have shown that administration of estradiol to castrated dogs results in a marked stimulation of prostate growth in a dose-dependent manner (14, 15), and these effects of estradiol could be reversed with concomitant treatment with tamoxifen (16). Population studies have also shown that serum estradiol levels are independent predictors of BPH (17). Immunohistochemical studies have shown that both subtypes of ERs are found in the prostate; ER-α is predominantly localized in the stroma, and its activation leads to cell proliferation (18), whereas ER-β is mainly localized in the epithelium, and its activation results in apoptosis and suppression of prostate growth (13, 19). Indeed, selective estrogen receptor modulators with selectivity for ER-β have shown efficacy in chemoprevention of prostate cancer in both animal studies and small human trials (20). Because T is aromatized to estradiol, it remains unclear whether the tropic effects of T on prostate growth are directly through the androgen receptor or via its aromatization to estradiol. The role of estrogens in prostate growth is supported by the observation that nonaromatizable androgens are unable to induce prostate hypertrophy (21, 22) and that administration of both T and estrogens synergistically induces greater prostate hypertrophy compared to T alone (23). Recently there has been some interest in the use of aromatase inhibitors (AIs) in older men with age-related low T levels because these agents increase endogenous T levels via stimulation of gonadotropins. We recently reported that 12-month intervention with AI in older men with low serum T not only successfully raised and maintained endogenous T levels in the target range but also resulted in an improvement in lean mass, muscle strength, and physical function (24). However, the long-term effects of treatment with AI on prostate volume and other parameters (prostate specific antigen [PSA] levels and lower urinary tract symptoms [LUTS]), remain unknown.
Most of the previous trials of AI in older men have been short term (25, 26) and only one long-term study assessed prostate volume (27). Furthermore, none of these studies compared AI with T replacement in a head-to-head fashion to evaluate the differential effects of T vs estradiol on prostate parameters. Hence, we conducted this long-term, proof-of-concept, mechanistic study to determine the role of estradiol on prostate volume in older men with age-related low T levels by enrolling three groups of men: transdermal T gel (which increases both the T and estradiol level), AI (which increases endogenous T but reduces the estradiol levels), and a placebo group (to observe any changes in prostate volume over a 12 mo time course). In addition to prostate volume, we also assessed PSA levels and LUTS in all three cohorts.
Materials and Methods
Trial participants
Community-dwelling men aged 65 years and older with fasting morning (7:00–10:00 am) total T levels less than 350 ng/dL were enrolled. The trial was conducted at the National Institute on Aging/National Institutes of Health Intramural Research Program and was approved by MedStar Harbor Hospital Institutional Review Board (number NCT00104572). This was a double-blind, randomized, placebo-controlled trial of 12 months' duration. Subjects were randomized to three groups: transdermal T gel 5 g/d and placebo tablet (TT group, n = 11); oral aromatase inhibitor (Anastrozole; AstraZeneca) 1 mg/d, and placebo gel (AI group, n = 11); placebo tablet and placebo gel daily (placebo, n = 9). The primary outcome of the study was prostate volume measured by transrectal ultrasound performed at baseline and at 12 months; secondary outcomes included PSA levels and LUTS assessed at baseline, 6 months, and 12 months. The participants were required to have normal levels of gonadotropins and prolactin and PSA levels of 4.0 ng/dL or less. Men with severe BPH, erythrocytosis, uncontrolled high blood pressure, or recent acute coronary syndrome were excluded. Men were also excluded if they were ever prescribed AI, selective estrogen receptor modulators, 5α-reductase inhibitors or any anabolic agents. Subjects were requested to refrain from drinking more than 30 g of alcohol daily or smoke tobacco or cannabis products for the study duration. All participants provided written informed consent as previously described (24).
Prostate parameters
Participants underwent transrectal ultrasound (Philips HDI 5000) at baseline and at 12 months, which was performed by the same sonographer, and interpretation was performed by a single radiologist (M.G.). Volumetric assessment of the prostate was performed using the ellipsoid formula (volume = height × width × length × 0.523), which requires the measurement of transverse, anteroposterior (in the axial plane), and longitudinal (in the sagittal plane) dimensions (28). Serum PSA was measured using an immunoassay analyzer (Dimension Vista 3000T; Siemens Healthcare). The intra- and interassay coefficient of variation (CVs) for PSA was less than 5%. The severity of LUTS was evaluated using validated International Prostate Symptom Score (IPSS) questionnaire (29).
Gonadal hormones
Serum total T and estradiol levels were measured using liquid chromatography-tandem mass spectrometry (24). Detection limits for T was 2.5 ng/dL; the intra- and interassay CVs were 3.5% and 5.3%, respectively. The detection limit for estradiol was 1 pg/mL; the intra- and interassay CVs were 7.1% and 9.2%, respectively. SHBG was measured using electrochemiluminescence with a detection limit of 10 nmol/L (intra- and interassay CVs of 2.3% and 1.8%, respectively). LH and FSH were measured by an ELISA (Millipore) with a minimum detectable concentration of 0.01 ± 0.02 mIU/mL (intraassay CV < 10%; interassay CV < 15%).
Statistical analysis
Based on previously published data on the effect of T administration on prostate volume (30), we wanted to detect a minimal mean change from baseline of 12% (SD ± 1) in prostate volume between the intervention groups and placebo. The effect size was estimated, based on the sample size of 10 participants per group, to have 90% power at α = .05. Mean change from baseline was calculated from each time point and expressed as mean ± SEM. Baseline characteristics were compared across the three groups using an ANOVA. Any significant difference between the baseline and 12-month measurements were assessed by paired and unpaired t test for group comparisons. A linear mixed-effects model was used for repeated measures with random intercept. Changes from baseline were regressed on the baseline value of the end point, treatment group, time, and treatment group-by-time interactions; linear regression of change scores on treatment groups stratified by time. Values of P < .05 were considered statistically significant (SAS Institute; version 9.3).
Results
Study participants
Baseline characteristics among the three groups were similar (Table 1). The mean age of the participants was 71 years and they were overweight or obese. Serum concentrations of gonadal steroids and gonadotropins were similar between the three groups. There was no difference in prostate volume between the groups; although none of the participants were on treatment for BPH, the average volume in all three groups was consistent with mild BPH. Scores on the IPSS were also similar between the groups. Although serum PSA levels were in the normal range in all the participants, men randomized to the AI group had slightly higher PSA levels at study entry.
Table 1.
Baseline Characteristics of the Participants (Data Presented as Mean ± SEM)
| Parameters | Placebo Group (n = 9) | TT Group (n = 11) | AI Group (n = 11) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 72 ± 1 | 72 ± 1 | 70 ± 1 | .61 |
| Race (white/African American), n | 7/2 | 9/2 | 11/0 | – |
| Body mass index, kg/m2 | 27.6 ± 1.2 | 30.1 ± 1.1 | 27.8 ± 1.2 | .27 |
| Sex hormones | ||||
| Total T, ng/dL | 303.8 ± 16.6 | 300.1 ± 13.4 | 271.6 ± 12.7 | .40 |
| Total estradiol, pg/mL | 16 ± 2.0 | 20 ± 2.0 | 15 ± 2.0 | .16 |
| LH, mIU/mL | 12.2 ± 3.4 | 11.4 ± 2.3 | 6.4 ± 0.8 | .17 |
| FSH, mIU/mL | 8.2 ± 3.6 | 8.0 ± 1.8 | 6.5 ± 1.6 | .85 |
| SHBG, nmol/L | 58.5 ± 7.1 | 43.3 ± 6.1 | 40 ± 5.6 | .11 |
| Prostate parameters | ||||
| Prostate volume, cca | 34.8 ± 4.7 | 32.2 ± 3.9 | 39.3 ± 4.9 | .69 |
| PSA, ng/mL | 0.7 ± 0.1 | 1.2 ± 0.2 | 1.7 ± 0.3 | .03 |
| IPSS scores | 8.2 ± 1.1 | 8.2 ± 1.7 | 8.3 ± 2.5 | .99 |
Ten men in both the TT group and the AI group underwent prostate ultrasound at baseline and 12 months.
Changes in gonadal hormones
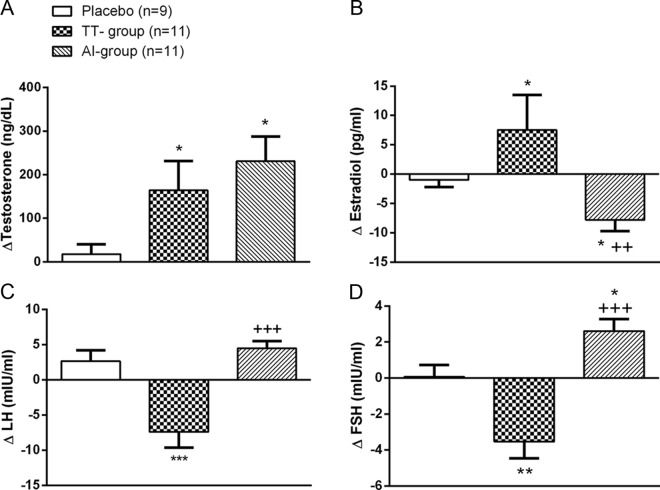
In both the intervention groups, total T levels significantly increased from baseline into the target range, which was determined a priori to be between 500 and 1000 ng/dL. At 12 months, serum T levels significantly increased from baseline in both the treatment groups (TT group = Δ164.2 ± 67.2 ng/dL; AI group = Δ231.00 ± 56.8 ng/dL), whereas there was no change in the placebo group (Figure 1). Serum total estradiol levels significantly increased from baseline in the TT group (Δ8 ± 6 pg/mL), whereas they decreased in the AI group (Δ − 8 ± 2 pg/mL). As expected, gonadotropin levels were suppressed in the TT group, whereas they increased in the AI group. Serum SHBG levels did not change in any of the groups.
Figure 1.
Change in gonadal steroids and gonadotropins after 12 months of intervention. Data are expressed as mean ± SEM. *, P < .05, **, P < .01, ***, P < .001 compared with placebo; ++, P < .01, +++, P < .001 compared with baseline values.
Prostate volume
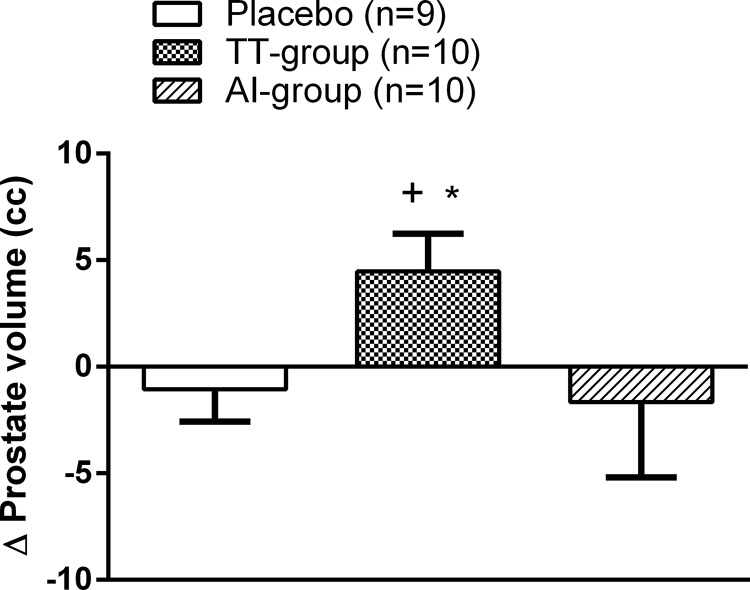
At 12 months, prostate volume significantly increased in the TT group compared with baseline (Δ4.5 ± 1.76 cc, P = .03). Prostate volume did not change significantly from baseline in either the AI group or the placebo group (Figure 2). In the TT group, the change in on-treatment serum T levels were not significantly correlated with the change in prostate volume (data not shown).
Figure 2.
Change in prostate volume after 12 months of intervention. Data are expressed as mean ± SEM. +, P < .05 compared with baseline values; *, P < .05 compared with placebo.
PSA levels
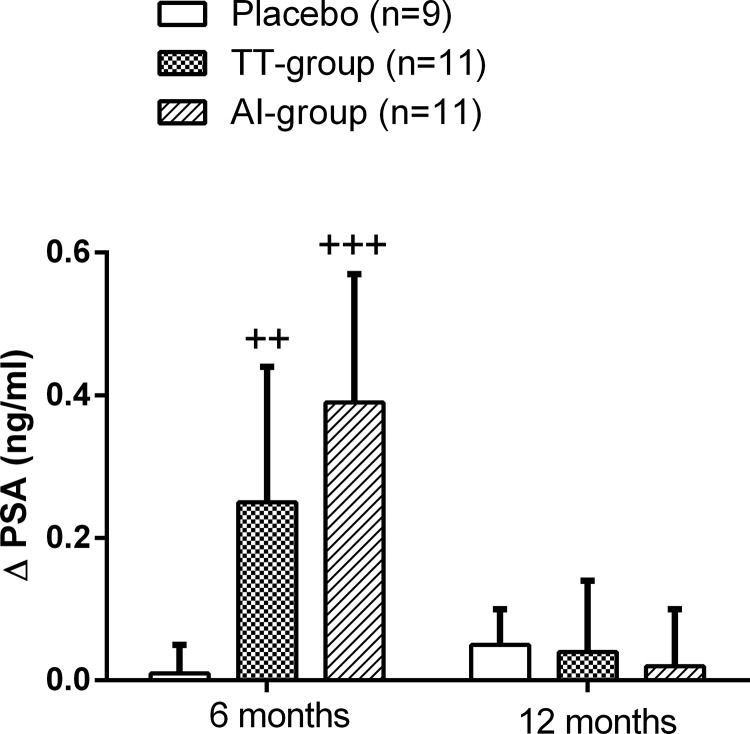
Serum PSA levels significantly increased at 6 months in both the TT group (Δ0.25 ± 0.19 ng/mL, P = .02) and the AI group (Δ0.39 ± 0.18 ng/ml, P = .0006) compared with baseline (Figure 3). However, at 12 months, the levels decreased and remained slightly above baseline levels.
Figure 3.
Change in serum PSA levels after 12 months of intervention. Data are expressed as mean ± SEM. ++, P < .01, +++, P < .001 compared with baseline values.
International Prostate Symptom Score
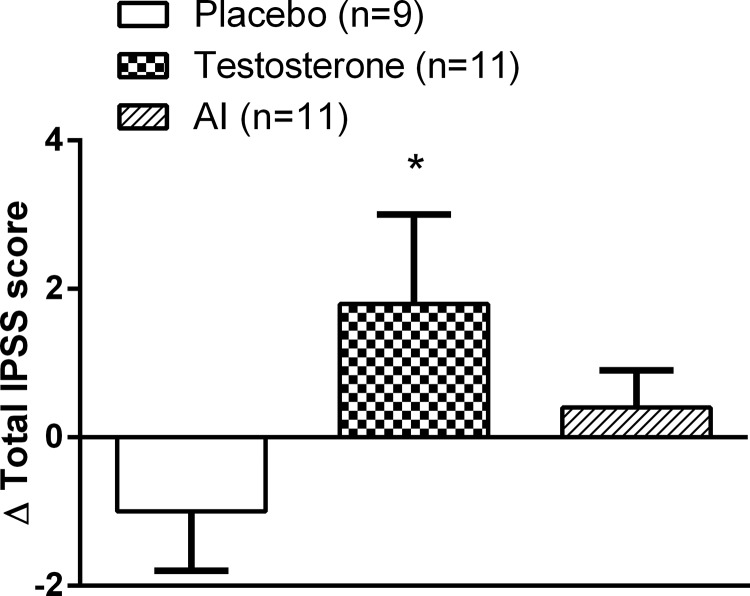
At 12 months, the total IPSS score was significantly higher (Δ1.8 ± 1.2 points, P = .03) only in the TT group, whereas no increase was seen either in the AI group or the placebo group (Figure 4).
Figure 4.
Change in IPSS after 12 months of intervention. Data are expressed as mean ± SEM. *, P < .05 compared with placebo.
Discussion
The past decade has seen an exponential increase in T prescriptions worldwide, which are mainly written for middle-aged and older men who do not have classic androgen deficiency (2). Because some previous studies have shown an increase in prostate volume during T therapy (31, 32) and because the incidence of BPH increases with age, monitoring of prostate safety during T replacement has been recommended (10). Although prostate is an androgen-dependent organ, both animal and human data suggest that estradiol has an independent effect on the prostate. Because T is aromatized to estradiol, it is conceivable that some of the tropic effects of T on the prostate might be mediated via its aromatization to estradiol. Indeed, studies have shown higher concentrations of both estradiol and estrone in prostate tissues of men with BPH compared with androgen levels, suggesting direct tropic effects of estrogens on the prostate (33). Recently there has been a growing interest in the use of AI in the treatment of age-related decline in serum T levels (25). Although a handful of trials using AIs have been performed in older men with low T, the majority have been short term, and none of the studies have measured prostate volume. This proof-of-concept, randomized-controlled trial is not only the first study that evaluated the long-term effects of AI on prostate volume; it also compared AI with transdermal T gel to evaluate the differential effects of T and estradiol on prostate volume. Additionally, a placebo group was also enrolled to evaluate any changes in the prostate parameters over a 12-month time period. We demonstrate that prostate volume significantly increased only in the TT group (even though on-treatment serum T levels were similar in the AI group), suggesting that the tropic effects of T on prostate volume are mediated via its aromatization to estradiol. To the contrary, serum PSA increased significantly (although within the normal range) in both the intervention groups, suggesting that the increase in PSA is primarily an androgen-driven process.
The findings of this proof-of-concept study are made all the more convincing by the strength of its design, including blinding, placebo and TT groups, concealed randomization, and the parallel-group design. Randomization effectively generated three groups that were similar in their baseline hormonal and prostate parameters. Both baseline and on-treatment serum T and estradiol levels were measured using liquid chromatography-mass spectrometry, the current gold standard method for the measurement of gonadal steroids. At baseline, mean total T levels were well below the lower limits of established norms in community-based samples (34) and interventions with both TT and AI effectively raised serum T levels into the target range. This trial also brings novelty because it is the first trial of AI that has evaluated prostate volume. Lastly, prostate ultrasonography, both at baseline and at 12 months, was performed by a single experienced sonographer and read by a single investigator (M.G.) on all participants, removing the possibility of measurement error due to interobserver variability. Because this long-term proof-of-concept trial did not show any adverse effect of AI treatment on prostate parameters, this study should provide an impetus for larger trials with AI (to evaluate both efficacy and safety) in older men with low T. The limitation of this trial is the small sample size (with potential impact on some of the analyses using multiple comparisons); however, this was a proof-of-concept trial that was designed to answer mechanistic questions. Nonetheless, we were able to find statistically and clinically significant changes in the prostate volume.
In this trial, after 12 months of intervention, an increase in prostate size of 4.5 cc was seen in the TT group. This increase is in agreement with some previous studies that have measured prostate volume during T replacement (31, 35). Although prostate is an androgen-dependent organ, data from laboratory studies in animals and population studies suggest that estrogens have an independent effect on the prostate. Indeed, aromatase activity has been demonstrated in the prostate stroma, and laboratory studies have shown that estradiol stimulates proliferation of human prostate stromal cells via activation of the ERK pathway and by increasing intracellular cAMP (36, 37). Estrogens also stimulate prostate hypertrophy by inducing growth factors such as insulin-like growth factor and epidermal growth factor (38). Animal studies in castrated dogs and monkeys have shown that administration of exogenous estradiol increases prostate size in a dose-dependent manner (15), whereas administration of aromatase inhibitors prevent the induction of BPH in animals (39), suggesting a direct tropic effect of estradiol on the prostate.
Both ER-α and ER-β are expressed in the prostate; ER-α is mainly expressed in the stroma, whereas ER-β is expressed in the epithelial cells (40, 41). These receptor subtypes play variable roles in the prostate; stimulation of ER-α leads to prostate hypertrophy, whereas activation of ER-β initiates apoptosis (13, 19). Indeed, selective estrogen receptor modulators have been used in the management of BPH and in chemoprevention of prostate cancer, both in animal studies and in men with high-grade prostatic intraepithelial neoplasia (20, 42, 43). Cohort studies have also shown that serum estradiol levels are an independent risk factor for BPH (17), and this risk increases with increasing serum estradiol levels (44). Despite these data, clinical trials have not attempted to elucidate the distinct effects of estrogens on prostate volume. Previous trials of AI in older men mainly focused on skeletal effects (27, 45) and did not evaluate prostate size. Furthermore, none of the previous studies directly compared AI with exogenous T replacement to disentangle the effects of estradiol vs T on various outcomes. This is the first trial that evaluated the effects of AI on prostate volume and directly compared the use of AI with exogenous T replacement in a head-to-head fashion. We found that treatment with AI did not increase prostate volume despite achieving serum T levels that were not only in the target range but also similar to the TT group, suggesting that the tropic effects of T on the prostate are mediated via its aromatization to estradiol.
In contrast to the prostate volume, the increases in serum PSA levels were seen early in the trial (6 mo) in both intervention groups. Although statistically significant, these increments were modest and consistent with those seen in previous trials of T administration (9). These findings suggest that the increase in PSA levels is predominantly an androgen-dependent process. Indeed, prostate biopsies in men undergoing T replacement have shown an increase in PSA gene expression (46). Nevertheless, it was reassuring that even the early increase in PSA levels in the AI group was within the normal range, findings consistent with another trial (27). At 12 months, although no statistical difference was observed in IPSS scores between the TT and AI groups, there was a statistically significant increase in the total IPPS score within the TT group. However, this increase was modest and the total score at the end of intervention was consistent with mild LUTS. The effect of T administration on LUTS remains unclear; in fact, some studies have shown an improvement in LUTS scores in older men on T therapy (47, 48). Hence, it is reassuring that 12 months of AI treatment did not worsen PSA or LUTS in a clinically meaningful way.
In conclusion, this proof-of-concept study demonstrated that the tropic effects of T on the prostate are mediated via its aromatization to estradiol. Based on the observation that long-term intervention with AI was not detrimental to prostate health and that AI has shown efficacy in older men with age-related decline in serum T (24), a larger trial to assess both the efficacy and safety of AI should be planned. Because the use of AI might impact bone mass negatively, the safety parameters of such a trial should include evaluation of bone mineral density and assessment of fracture risk. Without ensuring safety of the male skeleton, the clinical use of AI cannot be advocated even in the absence of any harm to the prostate.
Acknowledgments
This study had a clinical trial registration number of NCT00104572.
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Disclosure Summary: S.B. has previously received research grants from AbbVie and consulting fees from Eli Lilly and Takeda. The other authors have nothing to disclose.
Footnotes
- AI
- aromatase inhibitor
- BPH
- benign prostatic hyperplasia
- CV
- coefficient of variation
- ER
- estrogen receptor
- IPSS
- International Prostate Symptom Score
- LUTS
- lower urinary tract symptoms
- PSA
- prostate specific antigen.
References
- 1. Basaria S. Male hypogonadism. Lancet. 2014;383:1250–1263. [DOI] [PubMed] [Google Scholar]
- 2. Spitzer M, Huang G, Basaria S, Travison TG, Bhasin S. Risks and benefits of testosterone therapy in older men. Nat Rev Endocrinol. 2013;9:414–424. [DOI] [PubMed] [Google Scholar]
- 3. Handelsman DJ. Global trends in testosterone prescribing, 2000–2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199:548–551. [DOI] [PubMed] [Google Scholar]
- 4. Liverman C, Blazer D, eds. Testosterone and Aging. Clinical Research Directions. Washington, DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- 5. Imperato-McGinley J, Guerrero L, Gautier T, Peterson RE. Steroid 5α-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974;186:1213–1215. [DOI] [PubMed] [Google Scholar]
- 6. Wilson JD, Roehrborn C. Long-term consequences of castration in men: lessons from the Skoptzy and the eunuchs of the Chinese and Ottoman courts. J Clin Endocrinol Metab. 1999;84:4324–4331. [DOI] [PubMed] [Google Scholar]
- 7. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. [DOI] [PubMed] [Google Scholar]
- 8. Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011;82:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhasin S, Singh AB, Mac RP, Carter B, Lee MI, Cunningham GR. Managing the risks of prostate disease during testosterone replacement therapy in older men: recommendations for a standardized monitoring plan. J Androl. 2003;24:299–311. [DOI] [PubMed] [Google Scholar]
- 10. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. [DOI] [PubMed] [Google Scholar]
- 11. Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Am J Pathol. 1999;155:641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cunha GR, Wang YZ, Hayward SW, Risbridger GP. Estrogenic effects on prostatic differentiation and carcinogenesis. Reprod Fertil Dev. 2001;13:285–296. [DOI] [PubMed] [Google Scholar]
- 13. Cheung CP, Yu S, Wong KB, et al. Expression and functional study of estrogen receptor-related receptors in human prostatic cells and tissues. J Clin Endocrinol Metab. 2005;90:1830–1844. [DOI] [PubMed] [Google Scholar]
- 14. Coffey DS, Walsh PC. Clinical and experimental studies of benign prostatic hyperplasia. Urol Clin North Am. 1990;17:461–475. [PubMed] [Google Scholar]
- 15. Rhodes L, Ding VD, Kemp RK, et al. Estradiol causes a dose-dependent stimulation of prostate growth in castrated beagle dogs. Prostate. 2000;44:8–18. [DOI] [PubMed] [Google Scholar]
- 16. Funke PJ, Tunn UW, Senge T, Neumann F. Effects of the antioestrogen tamoxifen on steroid induced morphological and biochemical changes in the castrated dog prostate. Acta Endocrinol (Copenh). 1982;100:462–472. [DOI] [PubMed] [Google Scholar]
- 17. Hammarsten J, Damber JE, Karlsson M, et al. Insulin and free oestradiol are independent risk factors for benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2009;12:160–165. [DOI] [PubMed] [Google Scholar]
- 18. Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8:29–41. [DOI] [PubMed] [Google Scholar]
- 19. Omoto Y, Iwase H. Clinical significance of estrogen receptor β in breast and prostate cancer from biological aspects. Cancer Sci. 2015;106:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Price D, Stein B, Sieber P, et al. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: results of a double-blind, placebo controlled, phase IIB clinical trial. J Urol. 2006;176:965–970. [DOI] [PubMed] [Google Scholar]
- 21. Pollard M, Snyder DL, Luckert PH. Dihydrotestosterone does not induce prostate adenocarcinoma in L-W rats. Prostate. 1987;10:325–331. [DOI] [PubMed] [Google Scholar]
- 22. Idan A, Griffiths KA, Harwood DT, et al. Long-term effects of dihydrotestosterone treatment on prostate growth in healthy, middle-aged men without prostate disease: a randomized, placebo-controlled trial. Ann Intern Med. 2010;153:621–632. [DOI] [PubMed] [Google Scholar]
- 23. Suzuki K, Takezawa Y, Suzuki T, Honma S, Yamanaka H. Synergistic effects of estrogen with androgen on the prostate—effects of estrogen on the prostate of androgen-administered rats and 5α-reductase activity. Prostate. 1994;25:169–176. [DOI] [PubMed] [Google Scholar]
- 24. Dias JP, Melvin D, Simonsick EM, et al. Effects of aromatase inhibition vs. testosterone in older men with low testosterone: randomized-controlled trial. Andrology. 2015;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leder BZ, Rohrer JL, Rubin SD, Gallo J, Longcope C. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels. J Clin Endocrinol Metab. 2004;89(3):1174–1180. [DOI] [PubMed] [Google Scholar]
- 26. Lapauw B, T'Sjoen G, Mahmoud A, Kaufman JM, Ruige JB. Short-term aromatase inhibition: effects on glucose metabolism and serum leptin levels in young and elderly men. Eur J Endocrinol. 2009;160:397–402. [DOI] [PubMed] [Google Scholar]
- 27. Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab. 2009;94:4785–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Myschetzky PS, Suburu RE, Kelly BS, Jr, Wilson ML, Chen SC, Lee F. Determination of prostate gland volume by transrectal ultrasound: correlation with radical prostatectomy specimens. Scand J Urol Nephrol Suppl. 1991;137:107–111. [PubMed] [Google Scholar]
- 29. Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. [DOI] [PubMed] [Google Scholar]
- 30. Holmang S, Marin P, Lindstedt G, Hedelin H. Effect of long-term oral testosterone undecanoate treatment on prostate volume and serum prostate-specific antigen concentration in eugonadal middle-aged men. Prostate. 1993;23:99–106. [DOI] [PubMed] [Google Scholar]
- 31. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–2677. [DOI] [PubMed] [Google Scholar]
- 32. Behre HM, Bohmeyer J, Nieschlag E. Prostate volume in testosterone-treated and untreated hypogonadal men in comparison to age-matched normal controls. Clin Endocrinol (Oxf). 1994;40:341–349. [DOI] [PubMed] [Google Scholar]
- 33. Krieg M, Nass R, Tunn S. Effect of aging on endogenous level of 5α-dihydrotestosterone, testosterone, estradiol, and estrone in epithelium and stroma of normal and hyperplastic human prostate. J Clin Endocrinol Metab. 1993;77:375–381. [DOI] [PubMed] [Google Scholar]
- 34. Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96:2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E. Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic study. J Clin Endocrinol Metab. 2003;88:2049–2054. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Z, Duan L, Du X, et al. The proliferative effect of estradiol on human prostate stromal cells is mediated through activation of ERK. Prostate. 2008;68:508–516. [DOI] [PubMed] [Google Scholar]
- 37. Nakhla AM, Ding VD, Khan MS, et al. 5α-Androstan-3α,17β-diol is a hormone: stimulation of cAMP accumulation in human and dog prostate. J Clin Endocrinol Metab. 1995;80:2259–2262. [DOI] [PubMed] [Google Scholar]
- 38. Steiner MS. Review of peptide growth factors in benign prostatic hyperplasia and urological malignancy. J Urol. 1995;153:1085–1096. [PubMed] [Google Scholar]
- 39. Ito K, Fukabori Y, Shibata Y, et al. Effects of a new steroidal aromatase inhibitor, TZA-2237, and/or chlormadinone acetate on hormone-induced and spontaneous canine benign prostatic hyperplasia. Eur J Endocrinol. 2000;143:543–554. [DOI] [PubMed] [Google Scholar]
- 40. Leav I, Lau KM, Adams JY, et al. Comparative studies of the estrogen receptors β and α and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garcia-Florez M, Oliveira CA, Carvalho HF. Early effects of estrogen on the rat ventral prostate. Braz J Med Biol Res. 2005;38:487–497. [DOI] [PubMed] [Google Scholar]
- 42. Steiner MS, Raghow S, Neubauer BL. Selective estrogen receptor modulators for the chemoprevention of prostate cancer. Urology. 2001;57:68–72. [DOI] [PubMed] [Google Scholar]
- 43. Raghow S, Hooshdaran MZ, Katiyar S, Steiner MS. Toremifene prevents prostate cancer in the transgenic adenocarcinoma of mouse prostate model. Cancer Res. 2002;62:1370–1376. [PubMed] [Google Scholar]
- 44. Gann PH, Hennekens CH, Longcope C, Verhoek-Oftedahl W, Grodstein F, Stampfer MJ. A prospective study of plasma hormone levels, nonhormonal factors, and development of benign prostatic hyperplasia. Prostate. 1995;26:40–49. [DOI] [PubMed] [Google Scholar]
- 45. Leder BZ, Finkelstein JS. Effect of aromatase inhibition on bone metabolism in elderly hypogonadal men. Osteoporos Int. 2005;16:1487–1494. [DOI] [PubMed] [Google Scholar]
- 46. Marks LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296:2351–2361. [DOI] [PubMed] [Google Scholar]
- 47. Pearl JA, Berhanu D, Francois N, et al. Testosterone supplementation does not worsen lower urinary tract symptoms. J Urol. 2013;190:1828–1833. [DOI] [PubMed] [Google Scholar]
- 48. Yassin DJ, El Douaihy Y, Yassin AA, Kashanian J, Shabsigh R, Hammerer PG. Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year prospective, observational and longitudinal registry study. World J Urol. 2014;32:1049–1054. [DOI] [PubMed] [Google Scholar]