This prospective non-randomized controlled study showed no increased recurrence risk in breast cancer patients who pursued fertility preservation via controlled ovarian stimulation with letrozole during the 5 years after diagnosis.
Abstract
Context and Objective:
There has been increased attention to the issue of fertility preservation (FP). We aimed to investigate the long-term safety of FP via controlled ovarian stimulation with letrozole supplementation (COSTLES) prior to breast cancer treatment.
Design, Setting, and Participants:
This is a prospective, nonrandomized, controlled study conducted between the years 2002 and 2014. A total of 337 women diagnosed with stage 3 or less invasive breast cancer were enrolled during a FP consultation before chemotherapy. Of those, 120 elected to undergo COSTLES for FP prior to chemotherapy (FP group). The remaining 217 patients did not undergo any FP procedure and served as the controls.
Main Outcome Measure:
The primary end point was cancer recurrence defined as the detection of locoregional tumor (chest wall, regional nodal disease), distant metastases, or contralateral invasive breast cancer.
Results:
The baseline characteristics at enrollment were similar between the FP and control groups except for the less frequent lymph node involvement (P = .02) in the former. The mean follow-up after diagnosis was 5.0 years in the FP group and 6.9 years in the control group. In the FP group, the hazard ratio for recurrence after ovarian stimulation was 0.77 (95% confidence interval 0.28–2.13), and the survival was not compromised compared with controls (P = .61). Neither BRCA gene mutation status (P = .57) nor undergoing FP before or after breast surgery (P = .44) affected survival outcomes in the FP group. Likewise, none of the tumor characteristics including the estrogen receptor status affected the survival rates after the COSTLES.
Conclusion:
COSTLES is unlikely to cause a substantially increased recurrence risk in breast cancer during the 5 years after diagnosis.
As a result of the high survival rates and the growing emphasis on the cancer survivors' quality of life, there has been increased attention to the issue of fertility preservation (FP) (1, 2). Accordingly, a recent American Society of Clinical Oncology clinical guideline on FP stressed that, as part of education and informed consent before cancer therapy, health care providers should address the possibility of infertility with patients treated during their reproductive years and be prepared to discuss FP options and/or refer all potential patients to appropriate reproductive specialists (3).
Embryo and oocyte cryopreservation are the most widely used FP methods. Both approaches require ovarian stimulation, which causes marked increases in serum estradiol (E2) levels (4). Given the large body of data implicating estrogen and its metabolites in breast cancer propagation (5), we previously developed a novel ovarian stimulation protocol using an aromatase inhibitor, letrozole, in women with potentially estrogen-sensitive cancers (6–8).
Although controlled ovarian stimulation with letrozole supplementation (COSTLES) has gained better acceptance among women with breast cancer and FP specialists, its long-term safety has not been demonstrated yet. We previously reported our short-term follow-up on the safety of COSTLES, which showed no difference in breast cancer recurrence rates between the treatment and control groups (9, 10). We conducted the current prospective-controlled study to determine the long-term safety of the same protocol in women with breast cancer by comparing the recurrence rates.
Furthermore, the previous study was not powered to conduct subgroup analyses, especially to determine the safety of performing ovarian stimulation in BRCA gene mutation carriers. Accordingly, our secondary aim was to determine whether performing ovarian stimulation in women with BRCA gene mutations is equally safe as performing it in those without these mutations. In addition, we performed subgroup analyses to determine the impact of several factors, such as the estrogen receptor (ER) status and performing ovarian stimulation before tumor resection on tumor recurrence risk.
Patients and Methods
Study patients
This prospective, nonrandomized, controlled study was conducted between the years 2002 and 2014. The study was approved by the institutional review board and was registered at clinicaltrials.gov (identification number NCT00504699). The study population consisted of women with breast cancer who were referred for FP consultation prior to initiation of chemotherapy. All patients meeting the inclusion criteria for FP were offered ovarian stimulation with COSTLES for embryo or oocyte cryopreservation. Those who desired FP underwent embryo or oocyte cryopreservation prior to cancer treatment (FP group). Those who did not want to undergo FP but who agreed to a follow-up served as controls (control group). Some of the patients in the FP (n = 79) and control groups (n = 136) were reported in a prior publication with significantly shorter duration of follow-up (9).
The inclusion criteria were age 18–45 years, histologically confirmed invasive breast carcinoma, no prior chemotherapy, and normal basal (menstrual cycle d 2 or 3) FSH (FSH <13 mU/mL) and E2 (<75 pg/mL) prior to cancer treatment. Only women with stage III or lower stage cancers were enrolled in the study. Pathology reports were reviewed to confirm the type of breast surgery, tumor histology, size, grade, lymph node status, estrogen and progesterone receptor, and human epidermal growth factor receptor (HER)-2/neu (overexpression of epidermal growth factor receptors) status.
Follow-up
Follow-up information was collected during return visits, by phone interview, and in some cases by also contacting the patient's referring oncologist to confirm the information. The primary end point was cancer recurrence defined as the detection of locoregional tumor (chest wall, regional nodal disease), distant metastases, or contralateral invasive breast cancer. Time to recurrence was calculated from the time of definitive surgery. Patients were censored when lost to follow-up or at the last time point their disease status was known.
Ovarian stimulation
Ovarian stimulation was performed using letrozole (Femara; Novartis) in combination with gonadotropins as previously described, regardless of the ER status (6). We considered aromatase inhibitors in ER-negative patients because of the possible nonreceptor actions of estrogen. Embryos were cryopreserved by slow freezing at the prezygote (two pronuclei) or cleavage (d 3) stages. If oocyte cryopreservation was to be performed, a slow freezing technique was used until 2008 after which time the technique was switched to vitrification following technological advances.
Statistical analysis
Subjects' demographic characteristics, hormonal data, and ovarian stimulation outcomes were presented as mean ± SD. A t test was used for comparison of population means and χ2 for comparison of proportions; P < .05 (two sided) was considered statistically significant. Whenever possible, 95% confidence intervals (CIs) were calculated. Survival analyses for recurrence were performed using the Kaplan-Meier method and compared using the Mantel-Haenszel (log rank) test. If patients were not available for follow-up, they were considered free of disease in the control group and counted as recurrence in the study group. A Cox proportional hazard regression model was used to compare the effects of multiple prognostic covariates between both groups on survival analysis. A priori power analysis indicated that 80 patients were required in each arm to detect 10% difference in disease-free survival between the groups with a power of 80%. SAS (version 9.3; SAS Institute) was used for conducting statistical analysis.
Results
Overall comparison of recurrence rates and disease-free survival between COSTLES and control groups
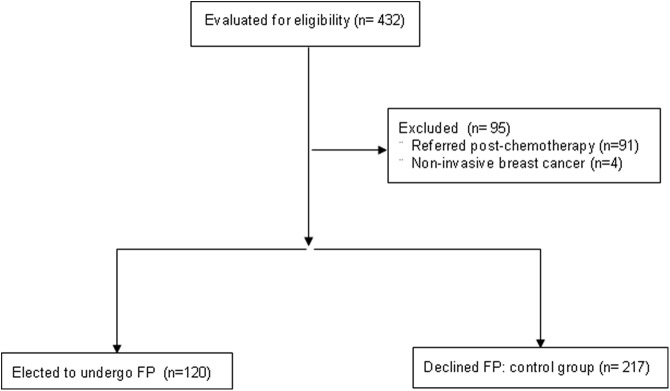
A total of 120 and 217 women were eligible in the FP and control groups, respectively (Figure 1). One hundred nineteen women in the FP group (99%) and 152 in the control group (70%) completed follow-up. There were no differences in demographics and tumor characteristics between those who had follow-up and who did not (Supplemental Table 1).
Figure 1.
Study flow diagram. Of the 432 women evaluated initially, a total of 337 were eligible for the study. Of those, 120 elected to undergo fertility preservation via the COSTLES, whereas 217 declined and served as controls.
The FP and control groups were similar except for the lower frequency of lymph node involvement (34% vs 48%, P = .02) in the FP arm (Table 1). In the FP group, seven women cryopreserved only oocyte(s) and seven cryopreserved both embryos and oocytes. Ovarian stimulation was canceled in four women in the FP group because of the low response; one subsequently pursued ovarian tissue cryopreservation. There were nine women who cryopreserved ovarian tissue in the control group.
Table 1.
Comparison of Baseline Characteristics From Women With Breast Cancer Who Did (FP) and Did Not Pursue (Control) COSTLES for Fertility Preservation
| FP, %, or Mean ± SD (n = 120) | Control, %, or Mean ± SD (n = 217) | P Value | |
|---|---|---|---|
| Age at cancer diagnosis, y | 34.8 ± 4.5 | 34.9 ± 4.7 | .88 |
| BMI, kg/m2 | 22.8 ± 3.7 | 23.1 ± 3.9 | .56 |
| Ethnicity | .70 | ||
| White | 49 | 43 | |
| Others | 51 | 57 | |
| Node involvement | 34 | 48 | .02 |
| Tumor size, cm | .59 | ||
| <2 | 67 | 66 | |
| 2–5 | 31 | 30 | |
| >5 | 2 | 3 | |
| Lymphovascular space invasion | 31 | 37 | .44 |
| Histological grade | 1.00 | ||
| 1–2 | 40 | 39 | |
| 3 | 57 | 59 | |
| ER positive | 82 | 77 | .27 |
| HER-2/neu positive | 35 | 33 | .78 |
| BRCA1/2 gene mutationa | 29 | 25 | .61 |
| Adjuvant tamoxifen use | 89 | 87 | 1.00 |
| Length of follow-up, y | 5.0 ± 2.1 | 6.9 ± 3.6 | <.001 |
Abbreviation: BMI, body mass index.
A total of 174 women were tested for BRCA mutations.
Mean gonadotropin dose used for ovarian stimulation was 2052.8 ± 1243.4 IU. Peak E2 levels ranged from 103.0 to 2350.6 pg/mL with a mean of 564.5 ± 436.3 pg/mL in the FP group. An average of 13.3 ± 8.4 oocytes were retrieved, and 6.1 ± 4.7 embryos or 12.6 ± 3.5 oocytes were cryopreserved per woman.
The mean length of follow-up after initial FP consultation was 5.0 ± 2.1 years (range 1–13 y) in the FP group and 6.9 ± 3.6 years (range 1–14 y) in the control group (P < .001). When we analyzed patients who completed follow-up, there were six recurrences (5.0%) or contralateral breast cancers (five distant, one locoregional) in the FP group, and 12 (5.5%) in the control group (10 distant, one locoregional, one contralateral breast) (P = .86).
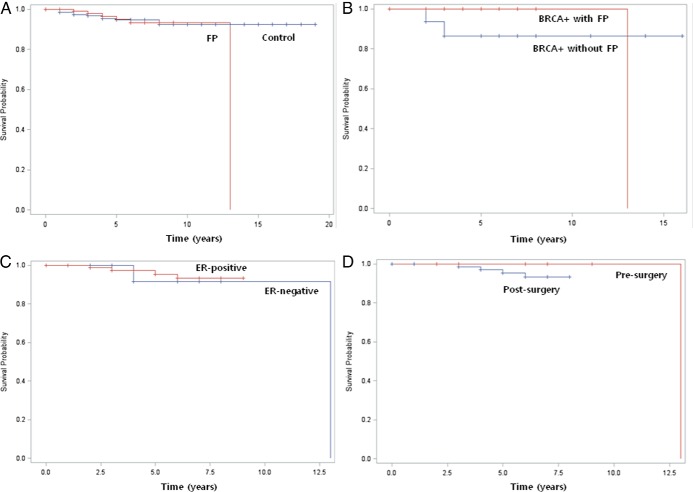
There was no significant difference in the relapse-free survival between the FP and control groups (Kaplan-Meier method, P = .61, hazard ratio 0.77 [95% CI 0.28–2.13]; Figure 2A). A Cox regression analysis was used to study the difference in the effect of tumor size, grade, involvement of lymph node, vascular space invasion, ER and progesterone receptor status, overexpression of HER-2/neu, type of chemotherapy used (anthracycline based, cyclophosphamide + methotrexate + fluorouracil and similar regimens, others), and length of follow-up (together or successively) on the relapse-free survival between the groups. The effect of each of these variables was not significantly different in the FP group compared with the controls.
Figure 2.
Survival analysis by Kaplan-Meier. A, Relapse-free survival in FP and control groups (log rank, P = .61). B, Relapse-free survival in BRCA mutation-positive patients pursuing and not pursuing COSTLES (log rank, P = .57). C, Relapse-free survival in women with ER-positive and ER-negative breast cancer (log rank, P = .75). D, Relapse-free survival in pre- and postsurgery groups (log rank, P = .44).
Impact of BRCA mutation status on overall recurrence risks
Among the study participants, 188 underwent BRCA mutation screening test (47 BRCA positive, 127 BRCA negative, and 14 unverified results). Among the 47 subjects with BRCA mutations (28 BRCA1, 18 BRCA2, and 1 both BRCA1 and BRCA2), 26 pursued FP treatment using COSTLES and 21 did not. The FP group was more likely to have tumor size <2 cm compared with controls (P = .02) (Table 2). In the FP group, the mean gonadotropin dose used for ovarian stimulation was 2511.0 ± 1557.0 IU. There was one recurrence in the FP group and two recurrences in the control group. There was no significant difference in the relapse-free survival between the FP and control groups (Kaplan-Meier method, P = .57) (Figure 2B). Likewise, we did not find any difference in survival when the BRCA mutation status was compared among women who underwent FP (one in BRCA positive vs four in BRCA negative, Kaplan-Meier method, P = .18).
Table 2.
Comparison of Tumor and Cycle Characteristics of Women With BRCA Gene Mutations
| BRCA-Positive COSTLES FP, %, or Mean ± SD (n = 26) | BRCA-Positive Control, %, or Mean ± SD (n = 21) | P Value | |
|---|---|---|---|
| Age at cancer diagnosis, y | 31.8 ± 3.8 | 33.8 ± 3.3 | .08 |
| BMI, kg/m2 | 22.0 ± 2.5 | 20.5 ± 2.1 | .23 |
| Tumor size, cm | .02 | ||
| <2 | 65 | 58 | |
| ≥2 | 35 | 42 | |
| Lymphovascular space invasion | 38 | 43 | .85 |
| ER positive | 43 | 53 | .55 |
| Progesterone receptor positive | 38 | 50 | .49 |
| HER-2/neu positive | 15 | 30 | .33 |
| Adjuvant tamoxifen use | 75 | 57 | .46 |
| Length of follow-up, y | 4.7 ± 2.7 | 6.9 ± 4.3 | .06 |
Abbreviation: BMI, body mass index.
Other subgroup analyses
Impact of ER status on overall recurrence risks
Among the 120 women in the FP group, 98 had ER-positive and 22 had ER-negative breast cancer. The ER-positive and ER-negative groups were similar except for the significantly younger age at cancer diagnosis for the latter (P = .01). The peak E2 levels after ovarian stimulation were also similar between the ER-positive (547.8 ± 398.0 pg/mL) and ER-negative (522.9 ± 470.2 pg/mL) groups (P = .82). There were four recurrences or contralateral breast cancers in the ER-positive group (4%), and two in the ER-negative group (9%) (P = .30). There was no significant difference in the relapse-free survival between the ER-positive and ER-negative groups (Kaplan-Meier method, P = .75; hazard ratio 0.63 [95% CI 0.06–5.31)]) (Figure 2C). A Cox regression analysis was used to account for the differences in the age at cancer diagnosis and FP treatment and showed to have no effect on the results.
Pre- vs postsurgery groups
Of the 120 subjects in the FP group, 14 underwent ovarian stimulation before (presurgery group) and 106 did after surgery (postsurgery group). The presurgery group was more likely to have lymph node involvement (P = .05) and ER-negative breast cancer (P = .04). There was one recurrence in the presurgery group (7%), and five in the postsurgery group (4%) (P = .47). The relapse-free survival rates were not statistically significantly different between the pre- and postsurgery groups (Kaplan-Meier method, P = .44; Figure 2D).
Discussion
As the acceptability and the usage rates of FP strategies increase, the safety of such treatments has become the center of interest among patients and health care providers. In this study with the only safety data about FP using COSTLES in women with breast cancer, we demonstrated that when ovarian stimulation was performed with concurrent use of letrozole, the relapse-free survival rate in breast cancer patients was unlikely to be affected, after a mean follow-up of 5.5 years. In the subgroup analysis, BRCA mutation status, having undergone tumor resection before COSTLES, and ER status of the tumor were not related to any change in the probability of relapse-free survival.
In 2008, we reported that the ovarian stimulation with the concurrent use of letrozole did not affect the relapse-free survival (9). However, because of the short median follow-up (median follow-up after chemotherapy was 2.0 y in the FP group and 2.5 y in the control group) and relatively small number of patients in that report (79 patients in the FP group and 136 patients in the control group), it was not yet possible to draw a population-based inference. In this follow-up report, we were able to validate our previous findings with a larger number of patients and longer follow-up (5.5 y). This long-term follow-up is especially meaningful because most recurrences occur during the first 5 years after breast cancer treatment (11, 12).
In theory, there may be several important factors that can modify the risk of relapse-free survival in breast cancer patients after COSTLES. Because BRCA mutation incidence is higher among premenopausal breast cancer and, in turn, the women undergoing FP with breast cancer and because these women have higher risks of recurrence and occurrence of other estrogen-sensitive cancers, it is important to determine the specific risk of ovarian stimulation in this group of patients. In this study, we did not find a detrimental effect of BRCA mutations on survival after ovarian stimulation. However, this observation is based on limited power, raising the possibility of a type I error. Given that young breast cancer patients with BRCA mutations tend to have recurrences 5–7 years after the first diagnosis, our report cannot provide conclusive evidence of safety of ovarian stimulation in this specific population (13). Larger studies with longer follow up periods are required to confirm our findings.
In addition, we could not consider the impact of risk-reducing salpingo-oophorectomy on cancer recurrence after COSTLES in BRCA mutation carriers because this information was not collected. However, even when we performed a sensitivity analysis, assuming a 35% risk-reducing salpingo-oophorectomy rate among BRCA mutation carriers and 4% among noncarriers based on previously published data (14), we did not find any increase of recurrence risk related to BRCA mutations.
Estrogen exposure is a well-known risk factor for ER-positive breast cancer (15, 16), but the role of estrogen in carcinogenesis of ER-negative breast cancer is still not well delineated. Biologically, estrogens are expected to have little effect on breast carcinogenesis in ER-negative breast cancer (17). Nevertheless, several reports suggested a possible association between estrogen-dependent reproductive characteristics such as high parity and the incidence of ER-negative breast cancer (18, 19). In our study, we chose to use COSTLES in all patients, regardless of the ER status, with the aim to prevent any possible nonreceptor-dependent impact of estrogens in cancer propagation. In our study, the two populations were similar except for expected younger age in women with ER-negative breast tumors (20). Notwithstanding the relatively small subgroup sample size, we did not find a difference in relapse-free survival between the ER+ and ER− women, even after controlling for the difference in age at diagnosis.
Although the impact of estrogen metabolites on the progression of preexisting breast cancer has been well validated (5), the effect of short-term exposure to supraphysiological estrogen levels on breast cancer progression and recurrence has not been studied. A prospective randomized study of standard ovarian stimulation vs COSTLES protocol may not be feasible or ethical. However, given that iatrogenic estrogen exposure remains contraindicated in women with breast cancer, the COSTLES protocol offers a more acceptable alternative for oncologists and patients alike.
Pursuing ovarian stimulation for FP with the tumor in situ may create higher risk of relapse-free survival compared with pursuing the same treatment after tumor resection. Current American Society of Clinical Oncology guidelines on FP recommend referral of patients who are eligible and interested in FP treatments to a fertility specialist as soon as possible. As the scope of neoadjuvant chemotherapy has been expanded in breast cancer treatment (21), a growing number of patients seek FP prior to undergoing breast surgery to complete FP before the initiation of chemotherapy. We have shown that referral before breast surgery reduces the delay to adjuvant chemotherapy and enables women to preserve larger number of eggs or embryos for FP (22). As part of that understanding, some of our patients underwent COSTLES before breast surgery (1). Because neoadjuvant chemotherapy is preferentially used for locally advanced breast cancer (22), these women who pursued COSTLES before tumor resection tended to bear more aggressive tumors. They were more likely to have lymph node involvement and present with ER-negative tumors. Both of these characteristics are related to worse prognosis (23) and less operability (24). Despite this poor prognostic profile, the disease-free survival was not compromised in patients who underwent COSTLES before tumor resection. This finding, however, should be interpreted with caution because the number of women analyzed in the preresection group was relatively small. As the treatment strategies and modalities for locally advanced breast cancer change, we expect to have larger number of patients in this category to draw inferences from in future studies.
We have recently reported the pregnancy outcomes with COSTLES, and the pregnancy rate was comparable with those expected in a noncancer population undergoing in vitro fertilization (25). Although the sample size was relatively small and a definite conclusion should not be rendered yet, our interim results showed no recurrences among women who conceived with frozen-thawed embryos. This finding is in keeping with previous studies that did not demonstrate a detrimental effect of pregnancy on disease-free survival (26).
There may be alternatives to the COSTLES protocol. We reported an alternative ovarian costimulation protocol with tamoxifen in earlier prospective studies (7, 27). Others have also reported on the feasibility of this protocol in women with breast cancer undergoing fertility preservation (28). Because our original data indicated that letrozole costimulation (COSTLES) may provide a larger number oocytes compared with tamoxifen, we chose COSTLES as the main stimulation protocol for women with breast cancer. Although the safety data are limited with tamoxifen costimulation (7, 28), we consider this protocol for those patients who cannot tolerate or who have contraindications to letrozole.
Our study has several strengths and limitations. It is an extension of the only prospective controlled study that provided safety information for FP by ovarian stimulation in women with breast cancer. All subjects were evaluated and treated for FP by the senior author (K.O.), providing uniformity to patient counseling and recruitment. We performed a respectable mean follow-up of 5.5 years on the participants. The study also provided novel information on the safety of performing embryo or oocyte cryopreservation in BRCA mutation carriers.
The peak hazard of recurrence in breast cancer occurred in the interval of 1–2 years and decreased consistently in the interval of 2–5 years, and beyond 5 years, it decreased very slowly through year 12 (29). However, considering that especially women with ER-positive cancers can have late recurrences (29), a longer follow-up to a 10-year period will be our final future aim. In the meantime, it is highly important to have the safety information on the COSTLES protocol available so that practitioners and patients can have further assurance on the feasibility of FP with this approach.
Whereas participation among the FP patients was nearly 100%, follow-up information was available for only 70% of controls. This difference is understandable because the performance of a FP procedure with the storage of gametes or embryos in a center establishes an ongoing relationship with patients, which does not exist with those who chose not to undergo FP. Given that we assumed the nonresponders in the control group as disease free for analysis of recurrence and survival, the lower response rate in the control group would have overestimated the recurrence risk in the FP group, strengthening our conclusion that ovarian stimulation with letrozole supplementation for the purposes of fertility preservation is safe to perform in women with breast cancer. In addition, the follow-up duration in the FP group was shorter than that of the control group. This likely reflects the increased acceptance of fertility preservation in women with breast cancer in more recent years.
In conclusion, this study presents greater than 5-year safety data regarding the use of COSTLES for FP via embryo or oocyte cryopreservation in women with breast cancer. Ovarian stimulation with concurrent use of aromatase inhibitors seems to provide a safe FP option to young women with breast cancer who are at risk for losing their fertility secondary to chemotherapy.
Acknowledgments
This work was supported in part by National Institutes of Health Grant RO1 HD053112 (to K.O.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- COSTLES
- controlled ovarian stimulation with letrozole supplementation
- E2
- estradiol
- ER
- estrogen receptor
- FP
- fertility preservation
- HER
- human epidermal growth factor receptor.
References
- 1. Kim J, Oktay K, Gracia C, Lee S, Morse C, Mersereau JE. Which patients pursue fertility preservation treatments? A multicenter analysis of the predictors of fertility preservation in women with breast cancer. Fertil Steril. 2012;97:671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodriguez-Wallberg KA, Oktay K. Fertility preservation and pregnancy in women with and without BRCA mutation-positive breast cancer. Oncologist. 2012;17(11):1409–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mitwally MF, Bhakoo HS, Crickard K, Sullivan MW, Batt RE, Yeh J. Estradiol production during controlled ovarian hyperstimulation correlates with treatment outcome in women undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2006;86:588–596. [DOI] [PubMed] [Google Scholar]
- 5. Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. [DOI] [PubMed] [Google Scholar]
- 6. Oktay K, Hourvitz A, Sahin G, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. [DOI] [PubMed] [Google Scholar]
- 7. Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. [DOI] [PubMed] [Google Scholar]
- 8. Pfister CU, Martoni A, Zamagni C, et al. Effect of age and single versus multiple dose pharmacokinetics of letrozole (Femara) in breast cancer patients. Biopharm Drug Dispos. 2001;22:191–197. [DOI] [PubMed] [Google Scholar]
- 9. Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–2635. [DOI] [PubMed] [Google Scholar]
- 10. Oktay K. Further evidence on the safety and success of ovarian stimulation with letrozole and tamoxifen in breast cancer patients undergoing in vitro fertilization to cryopreserve their embryos for fertility preservation. J Clin Oncol. 2005;23:3858–3859. [DOI] [PubMed] [Google Scholar]
- 11. Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100:1179–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bosco JL, Lash TL, Prout MN, et al. Breast cancer recurrence in older women five to ten years after diagnosis. Cancer Epidemiol Biomarkers Prev. 2009;18:2979–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drooger JC, Akdeniz D, Pignol JP, et al. Adjuvant radiotherapy for primary breast cancer in BRCA1 and BRCA2 mutation carriers and risk of contralateral breast cancer with special attention to patients irradiated at younger age. Breast Cancer Res Treat. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pierce LJ, Levin AM, Rebbeck TR, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol. 2006;24:2437–2443. [DOI] [PubMed] [Google Scholar]
- 15. Advani P, Moreno-Aspitia A. Current strategies for the prevention of breast cancer. Breast Cancer. 2014;6:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou W, Slingerland JM. Links between oestrogen receptor activation and proteolysis: relevance to hormone-regulated cancer therapy. Nat Rev Cancer. 2014;14:26–38. [DOI] [PubMed] [Google Scholar]
- 17. Nilsson S, Gustafsson JA. Estrogen receptor action. Crit Rev Eukaryot Gene Expr. 2002;12:237–257. [DOI] [PubMed] [Google Scholar]
- 18. Huang C, Wang X, Sun B, et al. Study on mouse model of triple-negative breast cancer: association between higher parity and triple-negative breast cancer. Target Oncol. 2015;10(1):85–97. [DOI] [PubMed] [Google Scholar]
- 19. Palmer JR, Boggs DA, Wise LA, Ambrosone CB, Adams-Campbell LL, Rosenberg L. Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol Biomarkers Prev. 2011;20:1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–1728. [DOI] [PubMed] [Google Scholar]
- 21. von Minckwitz G, Fontanella C. Selecting the neoadjuvant treatment by molecular subtype: how to maximize the benefit? Breast. 2013;22:S149–S151. [DOI] [PubMed] [Google Scholar]
- 22. Lee S, Ozkavukcu S, Heytens E, Moy F, Oktay K. Value of early referral to fertility preservation in young women with breast cancer. J Clin Oncol. 2010;28:4683–4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu KD, Wu J, Shen ZZ, Shao ZM. Hazard of breast cancer-specific mortality among women with estrogen receptor-positive breast cancer after five years from diagnosis: implication for extended endocrine therapy. J Clin Endocrinol Metab. 2012;97:E2201–E2209. [DOI] [PubMed] [Google Scholar]
- 24. Bentzon N, During M, Rasmussen BB, Mouridsen H, Kroman N. Prognostic effect of estrogen receptor status across age in primary breast cancer. Int J Cancer. 2008;122:1089–1094. [DOI] [PubMed] [Google Scholar]
- 25. Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol. 2015;33(22):2424–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gelber S, Coates AS, Goldhirsch A, et al. Effect of pregnancy on overall survival after the diagnosis of early-stage breast cancer. J Clin Oncol. 2001;19:1671–1675. [DOI] [PubMed] [Google Scholar]
- 27. Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod. 2003;18:90–95. [DOI] [PubMed] [Google Scholar]
- 28. Meirow D, Raanani H, Maman E, et al. Tamoxifen co-administration during controlled ovarian hyperstimulation for in vitro fertilization in breast cancer patients increases the safety of fertility-preservation treatment strategies. Fertil Steril. 2014;102(2):488–495. [DOI] [PubMed] [Google Scholar]
- 29. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. [DOI] [PubMed] [Google Scholar]