In this large, prospective, observational cohort study of postmenopausal women in the WHI, Cox proportional hazard regression models showed that sodium intake at or near recommended levels is not likely to impact bone metabolism.
Abstract
Background:
The relationship of sodium intake to changes in bone mineral density (BMD) in postmenopausal women has not been established, and no study to date has examined its relationship with fracture risk.
Methods:
This was a prospective observational cohort study including 69 735 postmenopausal women in the Women's Health Initiative during an average of 11.4 years of followup to examine whether sodium intake is associated with changes in BMD at the lumbar spine, total hip, femoral neck, and total body and with incident fractures and whether this relationship is modified by potassium and/or calcium intake.
Results:
In adjusted models, there was no association of calibrated sodium intake with changes in BMD at the hip or lumbar spine from baseline to 3 or 6 years (P ≥ .06). Higher sodium intakes were associated with greater increases in total body BMD from baseline to 3 years (P = .00) with a trend from baseline to 6 years (P = .08) and with reduced hip fractures (hazard ratio, 0.81; 95% confidence interval, 0.67–0.97). In sensitivity analyses that included body mass index as an additional covariate in the models, there was no association of sodium intake with changes in BMD at any skeletal site (P ≥ .32) or with incident fractures (P > .28). There was no association of sodium intake with incident fractures after adjusting for potassium intake (P ≥ .30). Calcium intake did not modify the association between sodium intake and incident fractures (P ≥ .20). Levels of sodium intake above or below currently recommended guidelines for cardiovascular disease (≤ 2300 mg/d) were not associated with changes in BMD at any skeletal site from baseline to 3 (P ≥ .66) or 6 years (P ≥ .74) or with incident fractures (P ≥ .70).
Conclusion:
Current population-based recommendations for sodium intake are unlikely to significantly affect osteoporosis.
Osteoporosis is a significant public health problem, particularly for women. Effective pharmacological treatments for osteoporosis exist; however, there are risks with their long-term use (1, 2), and as such, pharmacological strategies are not suitable as a preventive strategy for osteoporosis on a population basis. In contrast, dietary interventions to reduce fractures are appropriate for the population as a whole.
The World Health Organization recommends that sodium intake be reduced to less than 2 g per day (3). In the United States, federal nutrition policy guidelines (4) recommend that sodium intake be reduced to less than 2300 mg daily and to 1500 mg daily or less for certain populations. However, the role of sodium intake in a number of health problems has recently been the subject of substantial controversy (5, 6).
Sodium increases calcium excretion and higher calcium excretion is associated with lower bone mineral density (BMD) (7), a predictor of osteoporotic fractures. Accordingly, it has been hypothesized that high sodium intake may also be a risk factor for osteoporosis (8–10). However, the regulation of sodium balance within the body is quite complex. Adequate total-body sodium content is necessary for maintenance of central blood volume and renal perfusion and is thus tightly regulated by homeostatic defense mechanisms mediated by the renin-angiotensin-aldosterone system (RAAS). The RAAS is initiated as sodium intakes decrease below 3000 mg per day in an average-weight adult (11, 12). Osteoclasts express the angiotensin receptor type 1, angiotensin receptor type 2, and aldosterone receptors. In experimental studies, activation of RAAS or chronic infusion of angiotensin II increases bone resorption, whereas absence of angiotensin receptor type 1 is associated with greater bone strength (13–15).
Higher potassium intakes in some (16) but not all studies (17) have been reported to increase calcium absorption; thus, one might hypothesize that the relationship of sodium intake to osteoporosis might be dependent on calcium and potassium intakes.
Studies of the relationship of sodium intake to osteoporosis are conflicting (18–22). These studies are limited to outcomes of calcium homeostasis or cross-sectional assessments of BMD. The recent development of a calibration methodology (23) using objective biomarkers to correct for errors in self-reported sodium intake measurements permits a detailed and timely examination of the influence of sodium intake on the change in BMD and risk of incident fractures.
The primary goal of this study was to examine the association of sodium intake with changes in BMD and incident fractures in the WHI study during an average of 11.4 years of followup. The secondary objectives were to determine whether potassium or calcium intake modifies the association of sodium intake on these outcomes and whether currently recommended cut-points for sodium intake are optimal for bone health.
Materials and Methods
Participants
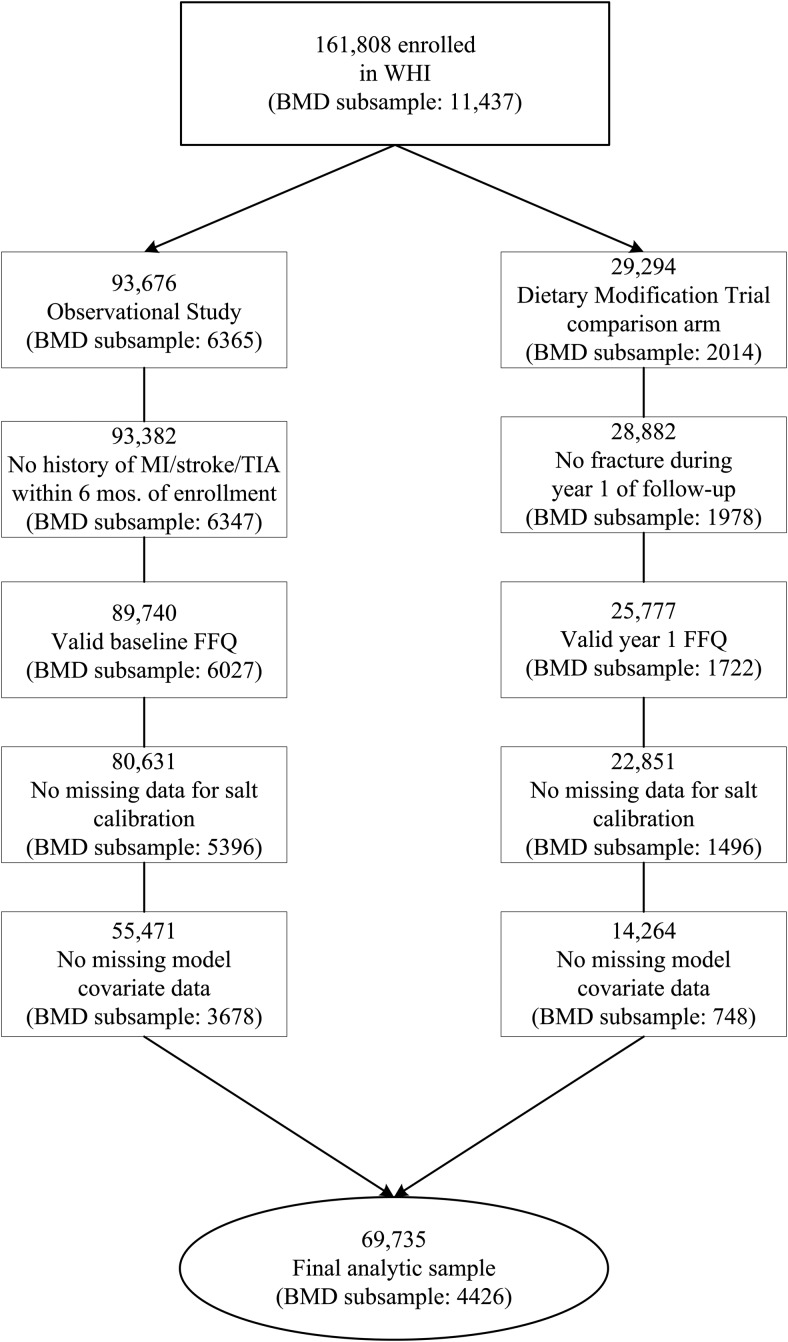
The WHI included postmenopausal women age 50–79 years enrolled between October 1, 1993 and December 31, 1998 at 40 clinical centers across the United States (24). For the purposes of this article, baseline dietary data and follow-up fracture data from the Women's Health Initiative–Observational Study (WHI-OS) participants, WHI Dietary Modification Trial (WHI-DM) participants, and participants in the comparison (nonintervention) arm of the WHI-DM (WHI-DM-C) were used through September 2010 (Figure 1). All protocols were approved by the institutional review board at each participating center. All participants provided written informed consent.
Figure 1.
Derivation of the analytic sample.
Predictors of nutrient intake
Data from the 450 women enrolled onto the Nutrition and Physical Activity Assessment Study (NPAAS), a WHI substudy, were used for the development of calibration equations for sodium/energy, potassium/energy, and for sodium/potassium using 24-hour urine concentrations; and for total energy using a doubly labeled water protocol (23). Details of the NPAAS study have been published (23).
Calibrated intake estimates for sodium/energy, potassium/energy, sodium/potassium, and energy derived from self-reported semiquantitative Block Food Frequency Questionnaires (FFQ) and other data were derived in a manner that adjusts for systematic biases associated with body mass index (BMI) and with other factors (23). Given that the baseline FFQ was used to determine eligibility in the WHI-DM-C, the FFQ from year 1 in DM-C was also used as 'baseline' in these analyses to avoid related assessment biases. Dietary intakes of calcium, magnesium, and vitamin D were assessed by the FFQ without biomarker calibration. Total calcium, magnesium, and vitamin D intakes were defined as the sum of all intakes from medications, supplements, and diet. We excluded those women who reported extremes of caloric intakes (< 600 kcal/d or > 5000 kcal/d). The cutoff of 450 mg per day of calcium or less was used to define “low calcium intake”—approximately ten percent of the WHI study population fell below this cutoff. This value was chosen because it was the amount of calcium consumed in the comparison arm of the Dietary Approaches to Stop Hypertension study on the effects of sodium reduction on bone metabolism (25).
Outcomes
Incident clinical fractures were determined by self-report semiannually through the end of the clinical trial (CT) and annually when the trial finished; in the OS, fractures were self reported annually. All hip fractures were centrally adjudicated in both the CT and OS within WHI. All other fracture outcomes were locally adjudicated in the CT trials and in the dual-energy x-ray absorptiometry (DXA) cohort through September 2005. Self-reported nonhip fractures in the OS participants not part of the DXA cohort were not adjudicated.
BMD of the total hip, femoral neck, anterior-posterior lumbar spine, and total body was measured at baseline, year 3, and year 6 in participants at three of the forty clinical centers of the WHI (Pittsburgh, Pennsylvania; Birmingham, Alabama; and Phoenix/Tucson, Arizona) by DXA using a Hologic QDR densitometer Model 2000, 2000+ or 4500 Fan-beam technology (Hologic, Inc.). Spine and hip phantoms were used. Calibration phantoms were scanned across instruments and clinical sites with interscanner variability of less than 1.5% for the lumbar spine, less than 4.8% for the hip, and less than 1.7% for linearity.
Covariates
Current medication use at baseline was ascertained by interviewers. Questionnaires obtained at the baseline visit were used to collect demographic and clinical information included as covariates in this report. Weekly recreational physical activity and walking was calculated by multiplying an assigned energy expenditure level for each category of activity by the hours exercised per week to calculate total metabolic equivalents per week. The Short Form Health Survey (SF-36) was used as a measure of physical function construct score, with higher scores indicating better function.
Statistical analysis
Previously published calibration equations for (log-transformed) sodium/energy, potassium/energy, total energy, and the sodium/potassium ratio (23) were updated with additional covariates related to osteoporotic fractures and BMD. Covariates listed above that were significantly associated with the nutrient biomarkers were retained. These calibration equations were then applied to the FFQ data and other study participant measures throughout the entire study sample. Calibrated sodium and potassium values were then derived by multiplying the calibrated sodium/energy and potassium/energy values by the corresponding calibrated energy.
Cox proportional hazard regression models were used to estimate the risk of fracture associated with binary calibrated nutrient intake level categorized as above or below a cutoff value, or with the linear effect of log-transformed calibrated nutrient intake values as the modeled regression variable. Follow-up time started at enrollment for OS participants, at year 1 for DM-C participants, and ended at the fracture event; or if none, the earliest of death, loss-to-followup, or September 2010. All Cox models were stratified by 5-year intervals of age at screening for OS and age at year 1 for DM-C, study component (OS vs DM-C), and randomization arms in the hormone therapy (HT) and calcium and vitamin D trials for participants randomly assigned in these trials. Models were also adjusted for potential confounding factors listed in the tables below, and data from participants missing baseline values for any of the factors were excluded. Geographic study site by region and trial enrollment were also included as covariates.
Hazard ratios (HRs) compare fracture risk for nutrient levels at or above vs below the median for each nutrient intake; for sodium intake, two additional cutoffs were considered: 1500 and 2300 mg per day (26). In addition, HRs were estimated for a 20% increment in nutrient intake.
The association between log-transformed BMD change and calibrated nutrient intake was evaluated using linear regression models, adjusted for the same stratification and confounding variables as used for the Cox models, with an additional covariate for the DXA scanner ID. BMD levels measured at baseline and followup were log transformed, and the change estimated using the following equation: percent change = [exp(change in log-BMD) − 1] × 100. The differences in “percentage change” were estimated comparing above- vs below-median nutrient level for each nutrient intake and the two additional cutoff values for sodium intake, as well as for each 20% higher intake. SEs for calibrated intake were obtained using a resampling procedure, with 1000 bootstrap samples where the resampling was stratified by study component (OS vs DM-C) and DXA cohort. This resampling procedure allows for sampling variation in calibration equation coefficients estimates and in Cox regression and linear risk modeling.
To access interaction between nutrient intake and calcium intake, an interaction term with calcium intake (< 450 vs ≥ 450 mg/d) was entered into the model for evaluation.
The calibration equations include BMI both to correct systematic bias, given that women with high BMI underreport both sodium and energy more than do women having lower BMI (23), and to provide a source of information on longer-term intake, especially for energy. Whether BMI also needs to be included as a covariate in the outcome models is an unresolved issue. Doing so may overcorrect given that the outcome analyses reflect associations with short-term intake having a weak association with the biomarker data, whereas not doing so could incur residual confounding if BMI is associated with the outcome via mechanisms other than the intakes under study. Our primary analyses were based on outcome regression models that do not adjust for BMI. Separate sensitivity analyses were carried out that included BMI as a covariate in the outcome models. Separate sensitivity analyses were also performed excluding participants with diabetes from the models, and excluding users of medications significantly associated with calibrated sodium intake. All analyses were performed using SAS version 9.3 (SAS Institute) and R version 3.1.2 (R Foundation for Statistical Computing). Reported P-values are two sided.
Results
Baseline characteristics of the study population by WHI substudy are shown in Supplemental Table 1, A (entire population) and B (BMD subset). The geometric mean intake of sodium from the FFQ was 2457.8 mg per day (range, 373.1–13 280.2 mg/d), and for the biomarker calibrated sodium was 2986.1 mg per day (range, 1234.5–7574.9 mg/d). Based on the uncalibrated intake, 11% and 42% of the women had sodium intakes at or below 1500 and 2300 mg per day, respectively, compared with 0.1 and 12% using the calibrated intake. The median calibrated sodium intake was 2891.6 mg per day and was 2489.8 mg per day based on uncalibrated intake.
Characteristics of the population with diabetes are shown in Supplemental Table 2. Supplemental Table 3 illustrates baseline medication use by quartiles of calibrated sodium intake.
There was no significant association of sodium intake with changes in BMD at the lumbar spine or hip (P ≥ .06 for baseline to 3 years and P ≥ .16 for baseline to 6 years [Table 1]). There was evidence of a larger total body BMD increase from baseline to year 3 with higher sodium intakes (P = .00), with a trend from baseline to year 6 (P = .08) (Table 1; Figure 2). There were significant increases by quartiles of sodium intake in BMD at the total hip site, an area enriched in cortical bone, from baseline to 3 years (P < .001), whereas there were no significant changes in BMD from baseline to 3 years in areas of the hip that contain predominantly trabecular bone including the hip trochanter (P = .394) and Ward's triangle (P = .229) (data not shown). However, there were no significant associations of 20% increments in sodium intake with changes in BMD from baseline to 3 or 6 years in the hip including the femoral neck and total hip site (P ≥ .16; Table 1) or in the intertrochanteric, trochanteric, or Ward's triangle (P > .11 for all, data not shown).
Table 1.
Association of Sodium Intake with Changes in BMD by Increments of Sodium Intake (n = 4426)
| By 20% Increment in Intake | Baseline to Year 3 |
Baseline to Year 6 |
||||
|---|---|---|---|---|---|---|
| β | se | P-Value | β | se | P-Value | |
| Total hip, % change | 0.14 | 0.10 | .16 | −0.17 | 0.14 | .24 |
| Femoral neck, % change | 0.09 | 0.13 | .46 | −0.11 | 0.18 | .55 |
| Total spine, % change | 0.28 | 0.15 | .06 | 0.32 | 0.23 | .16 |
| Total body,% change | 0.36 | 0.12 | .00 | 0.26 | 0.15 | .08 |
Models adjusted for age, study component, HT, and calcium/vitamin D (CaD) trial randomization arms, race/ethnicity, education, income, smoking, alcohol intake, history of fracture, family history of hip fracture, physical functioning, self-reported health status, treated diabetes, HT use, medication use (bisphosphonate, oral corticosteroid, thiazide, anticonvulsant, protein pump inhibitor, thyroid), calcium, vitamin D, and magnesium intake from diet and supplements.
All BMD measures included in models were log transformed; β and se are based on the log-transformed values.
Figure 2.
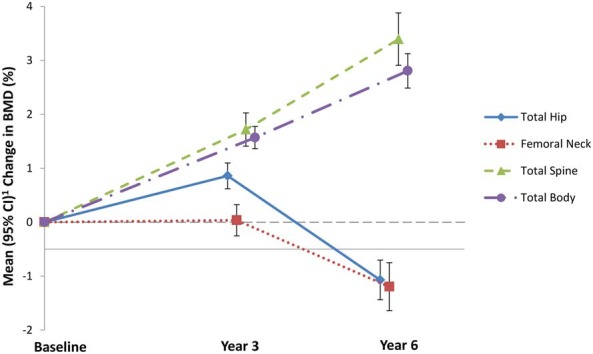
Change in BMD (%) corresponding to a 20% increment in sodium intake (n = 4426), estimated at the mean value of modeled variables. Estimated from linear regression models for log BMD change on log sodium intake, adjusted for age, study component, HT and CaD trial randomization arms, race/ethnicity, income, smoking, alcohol intake, history of fracture, family history of hip fracture, physical functioning, self-reported health status, treated diabetes, hormone therapy use, medication use (bisphosphonate, oral corticosteroid, thiazide, anticonvulsant, protein pump inhibitor, thyroid), calcium, vitamin D and magnesium intake from diet and supplements. % change in BMD is estimated at the mean value of modeled variables .
In sensitivity analyses that 1) excluded participants with treated diabetes and 2) excluded participants on medications significantly associated with calibrated sodium intake there remained no significant association of 20% increments in sodium intake with changes in BMD from baseline to 3 (P ≥ .07) or 6 years (P ≥ .10) at the total hip, femoral neck, and total spine in either analyses. Significant differences in changes in total body BMD from baseline to 3 years (P = .00 excluding persons with diabetes) and (P = .00 excluding medication use) and with a trend from baseline to 6 years (P = .06 excluding persons with diabetes) and (P = .07 excluding medication use) (data not shown) remained. In sensitivity analyses that included BMI in the BMD models, there was no significant association sodium intake with percent changes in BMD from baseline to 3 or 6 years at any skeletal site (data not shown).
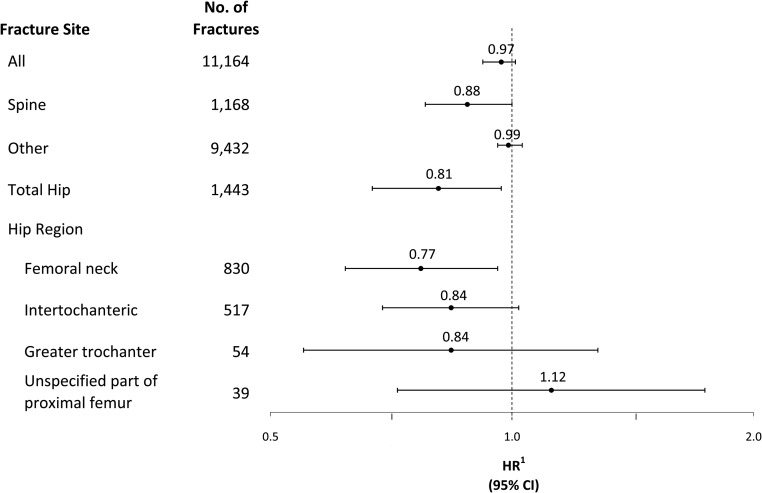
There was no significant association of sodium intake with all fractures (HR, 0.97; 95% confidence interval [CI], 0.92–1.01), spine fractures (HR, 0.88; 95% CI, 0.78–1.00), or other fractures (HR, 0.99; 95% CI, 0.96–1.03). There was evidence of reduced hip fracture incidence with increases in sodium intake (HR, 0.81; 95% CI, 0.67–0.97). Among sites of hip fractures, 20% increments in sodium intake were significantly inversely associated with femoral neck fractures (HR, 0.77; 95% CI, 0.62–0.96) but not intertrochanteric (HR, 0.84; 95% CI, 0.69–1.02), greater trochanteric (HR, 0.84; 95% CI, 0.55–1.28) or unspecified part of proximal femur fractures (HR, 1.12; 95% CI, 0.72–1.74) (Figure 3). However, in sensitivity analyses that included BMI in the fracture models, there was no association of sodium intake with all fractures or any fracture site (data not shown).
Figure 3.
Fracture HRs corresponding to a 20% increment in sodium. Hazard ratios (HR) and corresponding confidence intervals (CI) are estimated from Cox proportional hazard regression models stratified by 5-year age group, study component, HT and CaD trial randomization arms, and adjusted for race/ethnicity, education, income, smoking, alcohol intake, history of fracture, family history of hip fracture, physical functioning, self-reported health status, treated diabetes, hormone therapy use, medication use (bisphosphonate, oral corticosteroid, thiazide, anticonvulsant, protein pump inhibitor, thyroid), calcium, vitamin D and magnesium intake from diet and supplements.
In sensitivity analyses that 1) excluded participants with treated diabetes, 2) excluded participants on medications significantly associated with calibrated sodium intake 20% increments in sodium intake remained significantly inversely associated with hip fractures (HR, 0.81; 95% CI, 0.67–0.97) (excluding persons with diabetes) and 0.79 (95% CI, 0.64–0.97) (excluding medication use).
There was no significant association of above vs below the median value of sodium intakes, with percent changes from baseline to 3 years (P ≥ .24) or baseline to 6 years in BMD at any skeletal site (P ≥ .16) (Table 2). There was evidence of a larger total body BMD increase from baseline to year 3 at sodium intakes above the median (P = .02) (Table 2). There was no significant association of sodium intake above or below median levels of intake with all fractures (HR, 0.95; 95% CI, 0.88–1.10), spine fractures (HR, 0.96; 95% CI, 0.78–1.18), or other fractures (HR, 0.96; 95% CI, 0.90–1.03). There was evidence of reduced hip fracture incidence at sodium intake levels above the median (HR, 0.79; 95% CI, 0.64–0.98): (910 hip fractures in those below the median [annualized rate 0.23%] vs 533 hip fractures [annualized rate 0.13%] in those above the median). In sensitivity analyses that included BMI in the BMD and fracture models, there was no significant association of median sodium intake with percent changes in BMD from baseline to 3 or 6 years at any skeletal site or with incident fractures (data not shown).
Table 2.
Association of Sodium Intake with Changes in BMD by Median Sodium Intakes (n = 4426)
| By Median Intake | Baseline to Year 3 |
Baseline to Year 6 |
||||
|---|---|---|---|---|---|---|
| Mean | (95% CI) | P-Value | Mean | (95% CI) | P-Value | |
| Total hip | ||||||
| % change | .76 | .16 | ||||
| < median | 0.48 | (0.22 to 0.74) | 0.00 | (−0.35 to 0.35) | ||
| ≥ median | 0.55 | (0.31 to 0.78) | −0.38 | (−0.69 to 0.08) | ||
| Femoral neck | ||||||
| % change | .84 | .38 | ||||
| < median | −0.17 | (−0.47 to 0.14) | −0.22 | (−0. 63 to 0.19) | ||
| ≥ median | −0.11 | (−0.38 to 0.16) | −0.51 | ( − 0.88 to − 0.13) | ||
| Total spine | ||||||
| % change | .24 | .69 | ||||
| < median | 1.54 | (1.21 to 1.87) | 3.16 | (2.69 to 3.62) | ||
| ≥ median | 1.87 | (1.56 to 2.18) | 3.30 | (2.90 to 3.71) | ||
| Total body | ||||||
| % change | .02 | .36 | ||||
| < median | 0.72 | (0.48 to 0.96) | 1.74 | (1.39 to 2.10) | ||
| ≥ median | 1.17 | (0.96 to 1.38) | 1.99 | (1.70 to 2.28) | ||
Models adjusted for age, study component (CT or OS), HT and CaD trial randomization arms, race/ethnicity, education, income, smoking, alcohol intake, history of fracture, family history of hip fracture, physical functioning, self-reported health status, treated diabetes, HT use, medication use (bisphosphonate, oral corticosteroid, thiazide, anticonvulsant, protein pump inhibitor, thyroid), calcium, vitamin D, and magnesium intake from diet and supplements.
Median intake based on calibrated sodium is 2891.6 mg/d. All BMD measures included in the models were log transformed, and back-transformed means and 95% CI are reported. Percent change was calculated as [exp(change in log BMD from baseline) − 1] × 100.
Sodium intakes above or below United States federal guidelines for sodium intake (≤ 2300 mg/d) were not significantly associated with changes in BMD at any skeletal site from baseline to 3 (P ≥ .49) or 6 years (P ≥ .74 [Table 3]). There was no significant association of sodium intakes at or below 2300 mg per day with all fractures (HR, 0.94; 95% CI, 0.79–1.12), spine fracture (HR, 0.86; 95% CI, 0.55–1.34), other fractures (HR, 0.99; 95% CI, 0.83–1.18) or hip fractures, (HR, 0.70; 95% CI, 0.20–2.42). In sensitivity analysis that included BMI in the models, there was no significant association above or below guidelines for sodium intake with percent changes in BMD from baseline to 3 or 6 years at any skeletal site or with incident fractures (data not shown).
Table 3.
Association of Sodium Intake with Changes in BMD by Recommended Sodium Intakes (n = 4426)
| By Intake > vs ≤ 2300 mg | Baseline to Year 3 |
Baseline to Year 6 |
||||
|---|---|---|---|---|---|---|
| Mean1 | (95%CI) | P-Value | Mean2 | (95%CI) | P-Value | |
| Total hip | ||||||
| % change | .83 | .80 | ||||
| ≤ 2300 mg | 0.39 | (−0.89 to 1.66) | 0.00 | (−1.82 to 1.83) | ||
| > 2300 mg | 0.53 | (0.40 to 0.66) | −0.23 | ( − 0.43 to − 0.04) | ||
| Femoral neck | ||||||
| % change | .98 | .79 | ||||
| ≤ 2300 mg | −0.15 | (−1.62 to 1.33) | −0.05 | (−2.65 to 2.55) | ||
| > 2300 mg | −0.14 | (−0.30 to 0.03) | −0.41 | ( − 0.65 to − 0.18) | ||
| Total spine | ||||||
| % change | .78 | .88 | ||||
| ≤ 2300 mg | 1.51 | (−0.07 to 3.10) | 3.44 | (0.67 to 6.20) | ||
| > 2300 mg | 1.74 | (1.57 to 1.91) | 3.22 | (2.97 to 3.47) | ||
| Total body | ||||||
| % change | .49 | .74 | ||||
| ≤ 2300 mg | 0.52 | (−0.86 to 1.91) | 1.55 | (−0.57 to 3.67) | ||
| > 2300 mg | 1.01 | (0.89 to 1.14) | 1.91 | (1.71 to 2.11) | ||
Models adjusted for age, study component, HT and CaD trial randomization arms, race/ethnicity, education, income, smoking, alcohol intake, history of fracture, family history of hip fracture, physical functioning, self-reported health status, treated diabetes, HT use, medication use (bisphosphonate, oral corticosteroid, thiazide, anticonvulsant, protein pump inhibitor, thyroid), calcium, vitamin D, and magnesium intake from diet and supplements.
All BMD measures included in the models were log transformed, and back-transformed means and 95% CI are reported. Percent change was calculated as [exp(change in log BMD from baseline) − 1] × 100.
Higher sodium/potassium intake ratios were not significantly associated with incident fractures including all fractures (P = .60), clinical spine fractures (P = .60), other osteoporotic fractures (P = .41), or hip fractures (P = .54). There was no significant association between above vs below the median for sodium/potassium intake ratios with incident fractures including all fractures HR 1.01 (95% CI, 0.94–1.08) clinical spine fractures (HR, 0.87; 95% CI, 00.71–1.08) other osteoporotic fractures (HR, 0.98; 95% CI, 0.91–1.05) or hip fractures HR, 0.93 (95% CI, 0.75–1.16). Associations between sodium intake and incident fractures did not vary by calcium intake level (P ≥ .20) (data not shown).
Discussion
In more than 4000 postmenopausal women in WHI, there was no significant association between calibrated sodium intake and changes in BMD at the lumbar spine, hip or total body, or incident fractures including all, other, and clinical spine sites. Higher levels of sodium intake and intakes above the WHI population median were associated with significantly fewer hip fractures. Sodium intakes at or below currently recommended guidelines for cardiovascular disease prevention in the United States (26) were not associated with changes in BMD or incident fractures. There was no association of sodium intake with changes in BMD and incident fractures after consideration for sodium/potassium ratios or when including calcium intake as an interaction term.
Some (18, 27), albeit not all (19, 20, 28), reports are in agreement with the findings in WHI of no association of sodium intake with BMD. Our study differs from prior reports in the inclusion of a multiethnic cohort, adjustment for a number of important potential confounders that these studies could not, including diet, family history of osteoporosis, prior fracture, and medication use, and most importantly, inclusion of longitudinal and not simply cross-sectional BMD. Our findings that total-body BMD was higher with higher sodium intakes is, to our knowledge, the first report of the association between changes in total-body BMD and sodium intake.
In WHI, that sodium intake was not associated with incident fractures including all fractures, clinical spine fractures, and other osteoporotic sites is novel and important information. That hip fractures were associated with higher and not lower sodium intakes is an interesting finding. Mechanistically, it is biologically plausible that lower sodium intakes might stimulate the RAAS, which might increase osteoclastogenesis and bone resorption (13–15), leading to greater fracture risk. However, as previously discussed, it is uncertain whether BMI needs to be included in the outcome models to control confounding, and when BMI was included in the models, this association disappeared.
Theoretically, sodium intake might be a determinant of fractures at low calcium intakes (7, 21). We did not observe this in the WHI, given that at calcium intakes of less than or equal to 450 mg per day, there was no clear evidence of a relationship between sodium intake and incident fractures.
There are a number of important strengths to this study. This is the largest cohort of postmenopausal women studied to examine changes in BMD as they relate to sodium intake and the only study to date to include prospective data on incident fractures. It is the first study to evaluate whether potassium or calcium intake modify the relationship of sodium intake to osteoporosis. It is novel in using data from a biomarker substudy to correct sodium and potassium self-reported intake values.
A number of limitations to this study deserve consideration. The methodology used to ascertain sodium intake has been substantiated (23); however, the putative biomarker for sodium ascertainment remains the collection of multiple 24-hour urine specimens (29). The possibility of residual confounding from unmeasured or poorly measured variables exists. In particular, the multiple roles that BMI may play in these analyses have potential to complicate the interpretation of the association analyses. It is uncertain whether BMI needs to be included in the outcome models to control confounding, and when BMI was included in the models, the association of hip fracture with sodium intake was attenuated. Thus, this analysis may involve some residual confounding after controlling for the other listed covariates. The analyses that include BMI in the outcome models may be lacking in power, given that the “signal” from self-reported (FFQ) sodium is weak. Some covariates, for example, weight, diet, and physical activity levels were only captured at baseline and may not reflect past levels and/or may have changed during the study. Dietary magnesium, obtained from the FFQ, was included as a covariate in the models; however, erythrocyte magnesium levels were not measured. The estimated inverse association between sodium intake and hip fracture could also be attributable to multiple testing. There were too few women (n = 37; 0.1%) to examine whether sodium intakes of less than 1.5 g of sodium intake, which would be the recommended sodium intake for women in the WHI population (age 51 y and older) would have yielded different findings. There may be a differential association of sodium intake with trabecular compared with cortical bone that this study could not fully address that deserves further consideration.
In conclusion, sodium intake in postmenopausal women within current guidelines is unlikely to significantly affect osteoporosis.
Acknowledgments
Author Contributions: L.C. formulated the idea for the study, participated in data interpretation, and drafting and revision of manuscript; R.P. and M.P. performed the data analyses; and K.C.J., Y.H., F.T., J.C., C.C., L.T., M.S.L., J.W.-W., M.B., and W.L. participated in data interpretation, and drafting and revision of manuscript.
A list of Women's Health Initiative investigators can be found at: http://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf.
The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
The Women's Health Initiative program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 95% CI
- 95% confidence interval
- BMD
- bone mineral density
- BMI
- body mass index
- CaD
- calcium/vitamin D
- CT
- clinical trial
- DXA
- dual-energy x-ray absorptiometry
- FFQ
- Block Food Frequency Questionnaires
- HR
- hazard ratio
- NPAAS
- Nutrition and Physical Activity Assessment Study
- RAAS
- renin-angiotensin-aldosterone system
- SF-36
- Short Form Health Survey
- WHI-DM
- Women's Health Initiative–Dietary Modification Trial
- WHI-DM-C
- participants in the comparison (nonintervention) arm of the WHI-DM
- WHI-OS
- Women's Health–Observational Study.
References
- 1. Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–2294. [DOI] [PubMed] [Google Scholar]
- 2. Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–1491. [DOI] [PubMed] [Google Scholar]
- 3. Prevention of cardiovascular disease. Guidelines for assessment and management of cardiovascular risk. Geneva WHOW, 2007. Available from: http://whqlibdoc.who.int/publications/2007/9789241547178_eng.pdf. [Google Scholar]
- 4. McGuire S. U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv Nutr. 2011;2(3):293–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371(7):612–623. [DOI] [PubMed] [Google Scholar]
- 6. Mente A, O'Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371(7):601–611. [DOI] [PubMed] [Google Scholar]
- 7. Nordin BE, Need AG, Morris HA, Horowitz M. The nature and significance of the relationship between urinary sodium and urinary calcium in women. J Nutr. 1993;123(9):1615–1622. [DOI] [PubMed] [Google Scholar]
- 8. Jones G, Beard T, Parameswaran V, Greenaway T, von Witt R. A population-based study of the relationship between salt intake, bone resorption and bone mass. Eur J Clin Nutr. 1997;51(8):561–565. [DOI] [PubMed] [Google Scholar]
- 9. Need AG, Morris HA, Cleghorn DB, De Nichilo D, Horowitz M, Nordin BE. Effect of salt restriction on urine hydroxyproline excretion in postmenopausal women. Arch Intern Med. 1991;151(4):757–759. [PubMed] [Google Scholar]
- 10. Heaney RP. Role of dietary sodium in osteoporosis. J Am Coll Nutr. 2006;25(3 Suppl):271S–276S. [DOI] [PubMed] [Google Scholar]
- 11. Brunner HR, Laragh JH, Baer L, et al. Essential hypertension: Renin and aldosterone, heart attack and stroke. N Engl J Med. 1972;286(9):441–449. [DOI] [PubMed] [Google Scholar]
- 12. Luft FC, Rankin LI, Bloch R, et al. Cardiovascular and humoral responses to extremes of sodium intake in normal black and white men. Circulation. 1979;60(3):697–706. [DOI] [PubMed] [Google Scholar]
- 13. Asaba Y, Ito M, Fumoto T, et al. Activation of renin-angiotensin system induces osteoporosis independently of hypertension. J Bone Miner Res. 2009;24(2):241–250. [DOI] [PubMed] [Google Scholar]
- 14. Kaneko K, Ito M, Fumoto T, et al. Physiological function of the angiotensin AT1a receptor in bone remodeling. J Bone Miner Res. 2011;26(12):2959–2966. [DOI] [PubMed] [Google Scholar]
- 15. Shimizu H, Nakagami H, Osako MK, et al. Angiotensin II accelerates osteoporosis by activating osteoclasts. FASEB J. 2008;22(7):2465–2475. [DOI] [PubMed] [Google Scholar]
- 16. Ramsubeik K, Keuler NS, Davis LA, Hansen KE. Factors associated with calcium absorption in postmenopausal women: A post hoc analysis of dual-isotope studies. J Acad Nutr Diet. 2014;114(5):761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moseley KF, Weaver CM, Appel L, Sebastian A, Sellmeyer DE. Potassium citrate supplementation results in sustained improvement in calcium balance in older men and women. J Bone Miner Res. 2013;28(3):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carbone LD, Bush AJ, Barrow KD, Kang AH. The relationship of sodium intake to calcium and sodium excretion and bone mineral density of the hip in postmenopausal African-American and Caucasian women. J Bone Miner Metab. 2003;21(6):415–420. [DOI] [PubMed] [Google Scholar]
- 19. Nordin BE, Polley KJ. Metabolic consequences of the menopause. A cross-sectional, longitudinal, and intervention study on 557 normal postmenopausal women. Calcif Tissue Int. 1987;41 Suppl 1:S1–S59. [PubMed] [Google Scholar]
- 20. Kim SW, Jeon JH, Choi YK, et al. Association of urinary sodium/creatinine ratio with bone mineral density in postmenopausal women: KNHANES 2008–2011. Endocrine. 2015;49(3):791–799. [DOI] [PubMed] [Google Scholar]
- 21. Teucher B, Dainty JR, Spinks CA, et al. Sodium and bone health: Impact of moderately high and low salt intakes on calcium metabolism in postmenopausal women. J Bone Miner Res. 2008;23(9):1477–1485. [DOI] [PubMed] [Google Scholar]
- 22. Ilich JZ, Brownbill RA, Coster DC. Higher habitual sodium intake is not detrimental for bones in older women with adequate calcium intake. Eur J Appl Physiol. 2010;109(4):745–755. [DOI] [PubMed] [Google Scholar]
- 23. Huang Y, Van Horn L, Tinker LF, et al. Measurement error corrected sodium and potassium intake estimation using 24-hour urinary excretion. Hypertension. 2014;63(2):238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–S17. [DOI] [PubMed] [Google Scholar]
- 25. Lin PH, Ginty F, Appel LJ, et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003;133(10):3130–3136. [DOI] [PubMed] [Google Scholar]
- 26. Dietary Guidelines for Americans. 2010. (USDA and HHS). http://health.gov/dietaryguidelines/dga2010/dietaryguidelines2010.pdf.
- 27. Greendale GA, Barrett-Connor E, Edelstein S, Ingles S, Haile R. Dietary sodium and bone mineral density: Results of a 16-year follow-up study. J Am Geriatr Soc. 1994;42(10):1050–1055. [DOI] [PubMed] [Google Scholar]
- 28. Devine A, Criddle RA, Dick IM, Kerr DA, Prince RL. A longitudinal study of the effect of sodium and calcium intakes on regional bone density in postmenopausal women. Am J Clin Nutr. 1995;62(4):740–745. [DOI] [PubMed] [Google Scholar]
- 29. McLean RM. Measuring population sodium intake: A review of methods. Nutrients. 2014;6(11):4651–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]