Our study suggested that the Hippo/YAP-signaling play a critical role in the pathogenesis of endometriosis and may present a novel therapeutic method against endometriosis.
Abstract
Context:
The imbalance in cell proliferation and apoptosis is considered an important role in the pathogenesis of endometriosis, but the exact mechanisms remains unclear. A newly established signaling pathway–Hippo/Yes-associated protein (YAP) pathway plays a critical role in the proliferation and apoptosis processes. However, studies focusing on Hippo/YAP pathway and endometriosis are lacking.
Objective:
The objective was to explore the function of the Hippo/YAP pathway in endometriosis.
Setting and Design:
The expression of YAP was first investigated in endometrium of women with or without endometriosis. The role of YAP in cell proliferation and apoptosis is identified by transfection of endometrial stromal cells (ESCs) in vitro, subsequent Verteporfin treatments in eutopic ESCs in vitro, and endometriosis animal model of nude mice in vivo.
Results:
Our results revealed that increased expression of YAP and decreased expression of p-YAP in ectopic and eutopic endometrium compared with normal endometrium. YAP knockdown in eutopic ESCs decreased cell proliferation and enhanced cell apoptosis companied with decreased expression of TEAD1, CTGF, and B-cell lymphoma/leukemia (BCL)-2; whereas overexpression of YAP resulted in increased proliferation and decreased apoptosis of normal ESCs with increased expression of TEAD1, CTGF, and BCL-2. By chromatin immunoprecipitation qPCR CTGF and BCL-2 were identified as directly downstream target genes of YAP-TEAD1 active complex. Eutopic ESCs treated with Verteporfin revealed decreased proliferation and enhanced apoptosis whereas in endometriosis animal models of nude mice treated with Verteporfin, the size of endometriotic lesions was significantly reduced.
Conclusions:
Our study suggests that the Hippo/YAP-signaling pathway plays a critical role in the pathogenesis of endometriosis and should present a novel therapeutic method against endometriosis.
Endometriosis is a commonly encountered gynecologic disease that affects 10–15% of women of reproductive age. This condition arises when endometrial tissue grows outside of the uterine cavity, which can result in chronic pelvic pain, infertility, and an elevated risk of ovarian cancer (1, 2). Current treatments include using medicine to alleviate chronic pelvic pain and surgical removal of lesions, all of which provide a temporary but not permanent cure (3). Until now, the etiology and pathogenesis of endometriosis are still unclear. Understanding the subtle course of its pathogenesis is important for exploring effective, thorough therapy of disease.
As a kind of disease with a feature of growth, the development of endometriosis includes a course of cell proliferation and apoptosis. Cell proliferation and apoptosis are essential yet opposing cellular processes. The coordination and balance between cell proliferation and apoptosis are crucial for normal development and tissue-size homeostasis (4). The disease will occur once the balance is disrupted. Until now, the most widely accepted theory for pathogenesis of endometriosis is Sampson theory, in which the exfoliated menstrual endometrial cells attach to the peritoneal membrane, and subsequent cell proliferation and invasion into the underlying tissue result in endometriotic lesions (5). The retrograde menstruation is a physiologic process that takes place almost in all menstruation cycles of reproductive-age women. But the morbidity of endometriosis is only 10–15%, the answer to this question lies in the defect of eutopic endometrial cells. The menstrual endometrial cells usually do not survive even expelled inside the peritoneal cavity because of programmed cell death. Accumulated evidence suggests that endometrial cells from both eutopic endometrium and ectopic endometrium of endometriosis exhibit impaired apoptosis and excessive proliferation (6–10); besides, aberrant expression of proliferation-related and apoptosis-related molecules such as c-myc, B-cell lymphoma/leukemia (BCL)-2, and connective tissue growth factor (CTGF) should be responsible for viability of ectopic endometrial cells. Therefore, imbalance of cell proliferation and apoptosis is the foundation of progression of endometriosis (6, 11–13). So far, it has been reported that miRNAs, histone deacetylation, and several signaling pathways play a role in regulation of endometrial cell proliferation and apoptosis in disease (14–17). The mechanisms of endometrial cells imbalance are still unclear and need for further study.
The Hippo pathway was originally identified as the signaling that controls organ size in Drosophila, with the core architecture conserved in mammals. In the mammalian Hippo pathway, the central components of this pathway comprise a regulatory serine-threonine kinase module and a transcriptional module. Mammalian Ste20-like kinases (MST1/2) and large tumor suppressor kinases (LATS1/2) as kinase module regulate transcriptional coactivators, Yes-associated protein (YAP) and Transcriptional coactivator with a PDZ-binding motif (TAZ) (14). As a major downstream target of the Hippo pathway, transcription cofactor YAP does not contain its own DNA-binding motifs and initiates transcription by interacting with the DNA-binding transcription factors TEA domain (TEAD) family members; then activate expression of target genes regulating cell proliferation, differentiation, and apoptosis (18). YAP has been found to be dysregulated in a variety of diseases, such as hepatocellular carcinoma, neurological diseases, and malignant mesothelioma (19). These findings highlight that dysregulation by genetic inactivation of core pathway components or amplification of its downstream effector YAP results in increased cell proliferation and decreased cell apoptosis. Verteporfin (trade name, Visudyne by Novartis) is used clinically as a photosensitizer in photodynamic therapy for neovascular macular degeneration. Recently, it has been found that verteporfin repealed liver overgrowth induced by YAP overexpression (OE) in vivo (20). Verteporfin inhibits esophageal cancer development in vivo by down-regulating SOX9 (another YAP-TEAD target gene) in another report (21). These studies predict the therapeutic potential of targeting YAP-TEAD interaction (22).
However, the expression and function of the Hippo/YAP pathway in endometriosis have not been investigated yet. Thus, the goal of this study is to gain further insight of endometriosis pathogenesis by identifying the expression and spatial distribution of YAP in endometriosis and investigate the role of YAP in the regulation of endometrial cell proliferation and apoptosis.
Materials and Methods
Study population
The study was approved by the Institutional Review Board of West China Second University Hospital of Sichuan University, and written informed consent was obtained from each patient. Participants were age 20–40 years and had regular menstrual cycles. None had received steroid hormone treatment for at least 3 months before sampling. All were determined to be in the proliferative phase according to their last menstrual period and further confirmed by histological dating. Normal endometrium was obtained from disease-free women laparoscopically as controls, paired ectopic and eutopic endometrial tissues were obtained from women with revised-American Fertility Society (r-AFS) stage III–IV ovarian endometriosis by laparoscopic and histological diagnosis; the cyst wall of ovarian endometriosis without adjacent ovarian tissue was obtained and the inner side was used for further analysis.
Immunohistochemistry
Human endometrial samples and mice endometriotic lesions were fixed in formalin, embedded in paraffin, cut to 4-μm sections, dried, and kept at 4°C. The hematoxylin-eosin and immunohistochemistry staining were made. The primary antibodies used were 1:400 monoclonal rabbit antihuman YAP (ab52771, Abcam) and 1:100 monoclonal rabbit antihuman Ki67 (ab15580, Abcam). The immunostaining of Ki67 was quantified by normalizing the average number of positively stained cells to the total number of cells from five different fields of each image.
Flow cytometry analysis
Apoptosis was analyzed using cell surface expression of Annexin V. Endometrial stromal cells (ESCs) were separately isolated from normal endometrium of controls or eutopic endometrium of endometriosis as described previously (23). ESCs were transfected for 24 hours and then cultured for 72 hours before determining the extent of apoptosis using the Annexin V-FITC Apoptosis Assay Kit as described by the manufacturer (KeyGEN BioTECH). Before being analyzed by flow cytometry, the cells were trypsinized and incubated for 5 minutes at room temperature with Annexin V-FITC and propidiumiodide.
Cell proliferation assays
The CCK8 proliferation assay was performed according to the manufacturer's instructions (Dojindo Laboratory). Briefly, 96-well plates were seeded with 1000 cells per well. Every 24 hours, cell counting kit-8 reagent was added to the wells of the plate. After 3 hours' incubation, the plate was measured as spectrophotometric absorbance at wave length of 450 nm.
Cell culture and transfection
Construction and production of Lentiviral vectors were made by Shanghai Genechem Co., Ltd., the YAP1-specific short-hairpin RNA (shRNA)-targeting coding sequence (GGTGATACTATCAACCAAA) was cloned into GV118 vector to produce YAP-knockdown (KD) vector. Human YAP cDNA (BC038235) was amplified by PCR as a template, then cloned into GV358 vector to generate YAP expression vector. The ESCs were suspended in DMEM/F12 (1:1) supplemented with 10% fetal bovine serum (life technologies) and 50 U/mL penicillin/streptomycin at 37°C under a 5% CO2 condition, plated at 1 × 106 cells per six-well plate and cultured to 40% confluency, then transfected with optional virus-containing supernatant supplemented with polybrene. The cells were transfected with YAP expression Lentiviral vector to establish the YAP-overexpressing normal ESCs line; 72 hours after transfection, they were selected with 4 μg/mL puromycinin culture medium for more than 10 days. Transfection efficiency of YAP was measured by RT-qPCR and Western blotting. All experiments were performed in triplicate and repeated three times.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were conducted following the manufacturer's protocol (EMD Millipore). Briefly, to crosslink proteins to DNA, fresh formaldehyde (final concentration, 1%) was added to the culture medium and incubated for 10 minutes at room temperature. Cells were scraped and collected and then lysed successively with cell lysis buffer and nuclear lysis buffer containing protease inhibitors. Aliquots of cell lysates were sonicated to shear DNA into 0.2–1.0-kb fragments, and the cellular debris was removed. Chromatin aliquots were incubated with fully suspended protein A magnetic beads and 2.5 μg specific YAP antibody (ab52771, Abcam), 5 μg TEAD1 antibody (ab133533, Abcam), 5 μg Histone H3 antibody (positive control; ab4729), or 5 μg normal rabbit IgG (negative control; #12–370, EMD Millipore) overnight at 4°C with rotation. Beads were collected with the magnetic separator and then washed. Protein/DNA complexes were decrosslinked with proteinase K for 2 hours at 62°C, 10 minutes at 95°C. After that, DNA was purified using spin columns and resuspended. The purified DNA was then subjected to quantitative PCR (qPCR) with indicated ChIP primers in Supplemental Table 1.
qRT-PCR
Total RNA was extracted from all tissue samples or primary cultured ESCs using TRIzol according to the manufacturer's protocol (Life Technologies) and then followed the protocol (supporting information). Relative gene expression was calculated using the 2−ΔΔCT method, normalizing with glyceraldehyde-3-phosphate dehydrogenase (GADPH) levels.
Western blotting
Total proteins were collected with immunoprecipitation lysis buffer supplemented with protease inhibitors and phosphatase inhibitors. Protein concentration was determined using a bicinchoninicacid assay kit (ThermoFisher Scientific) and then followed the protocol (supporting information). Protein bands was analyzed with Quantity One (Bio-Rad Laboratories). Protein levels were normalized to that of the internal control β-actin.
Verteporfin treatments
Verteporfin (Selleckchem) was dissolved in dimethyl sulfoxide (100 mg/mL), aliquoted, and stored at −80°C. In vitro, ESCs were treated with dimethyl sulfoxide or verteporfin with a dose of 1μM. In vivo, working solution of verteporfin was prepared at 10 mg/mL in PBS freshly before use, and mice were administered ip at a dose of 100 mg/kg every other day for 12 days after day 2 of implantation whereas control mice were injected with equivalent PBS.
Animal experiments
All animal experiments were conducted in accordance with the National Institutes of Health Guide lines for the Care and Use of Laboratory Animals. The protocol was approved by the Ethics Committee of Animal Experiments of Sichuan University. Female BALB/c nude mice age 6–8 weeks were purchased from Chengdu Dashuo Experimental Animals Limited Company. Nude mice were housed in a barrier unit in a controlled pathogen-free environment and regulated light/dark cycles (12 h/12 h). All equipment and food entering the barrier were autoclaved. Mice had free access to food and water.
Explanted eutopic endometrial tissue collected from women with endometriosis was cut into 5-mm fragments under sterile conditions and kept for 1 hour in culture medium (DMEM/F12; 1:1; Life Technologies) supplemented with Pen-Strep (MP Biomedicals) at 37°C prior to transplantation. Fragments of human endometrium were transplanted into nude mice when local anesthesia by ip administration of chloralhydrate (KeLong Chemical) at a dose of 10 mg/kg. All mice were implanted two pieces of implants and fixed on both sides of the lateral abdominal wall with surgical sutures as described before (25). The experimental mice (n = 5) and control mice (n = 5) were treated as previously described. Finally, mice were euthanized by cervical dislocation. Implanted endometrial lesions were dissected, subsequently measured in two perpendicular diameters (d < D) with a caliper, and their volume was calculated with the following formula: V = (3/4) πr2R (r and R are the radii; r < R) (24). Then, the lesions were divided into two parts: one part was frozen immediately in liquid nitrogen for RNA/protein extraction; and another one fixed in 10% formalin for morphological and immunohistochemical analysis.
Bromodeoxyuridine labeling
For bromodeoxyuridine (BrdU) labeling, mice were injected ip with BrdU (50 mg/kg; Sigma-Aldrich) for 24 hours before euthanasia. The endometriotic lesions were collected, fixed, and sectioned as described above, then detected with an anti-BrdU antibody (R&D systems). The percentage of BrdU incorporation was determined by counting BrdU+ nuclei among the total number of cells in distinct fields.
TUNEL assay
Apoptosis was detected by In Situ Cell Death Detection Kit (Roche) following the manufacturer's protocol with some modifications (supporting information).
Statistical analysis
Statistical analysis was performed using SPSS version 18.0 (SPSS). All data were expressed as mean ± SD. The Student t test was used for comparisons between the two groups, and one-way ANOVA with a post-hoc test least-significant difference was used for multiple comparisons. P < .05 was considered statistically significant (two tailed).
Results
Increased expression of YAP in eutopic and ectopic endometrium of endometriosis
To investigate the functional relevance of YAP during endometriosis, we performed qRT-PCR to detect mRNA level of YAP in ectopic and eutopic endometrium from women with endometriosis and normal endometrium from disease-free women. The YAP mRNA expression was significantly higher in ectopic endometrium than eutopic and normal endometrium (Figure 1A). Then we detected protein level of YAP and its phosphorylated form phosphoS127-YAP (serine to alanine at residue 127) by Western blotting, The result showed that not only up-regulation of Yap in endometrium of women with endometriosis, but an inhibition of phospho-YAP in this disease (Figure 1, B and C). In immunohistochemistry, YAP immune expression was distributed strongly in the nucleus and cytoplasm of epithelial and stromal cells of ectopic and eutopic endometrium whereas YAP protein expression was weak in the nucleus and cytoplasm of normal endometrium (Figure 1D). The Above results suggest up-regulated YAP in endometriosis.
Figure 1.
YAP expression in normal endometrium and in paired eutopic and ectopic endometrium. A, RT-qPCR-analyzed expression of YAP mRNA levels in normal endometrium (n = 10) and in paired eutopic and ectopic endometrium (n = 15). B, Western-blot analysis of YAP protein levels in normal endometrium and in paired eutopic and ectopic endometrium (n = 6). C, Western-blot analysis of phospho-YAP(S127) protein levels in normal endometrium and in paired eutopic and ectopic endometrium (n = 6). D, Immunohistochemical analysis of YAP was performed on sections of normal endometrium and in paired eutopic and ectopic endometrium. Black arrows indicate positive staining. Scale bars, 200 μm. Data were analyzed by one-way ANOVA with least-significant difference correction.
KD of YAP in eutopic ESCs of endometriosis reduces proliferation and promotes apoptosis
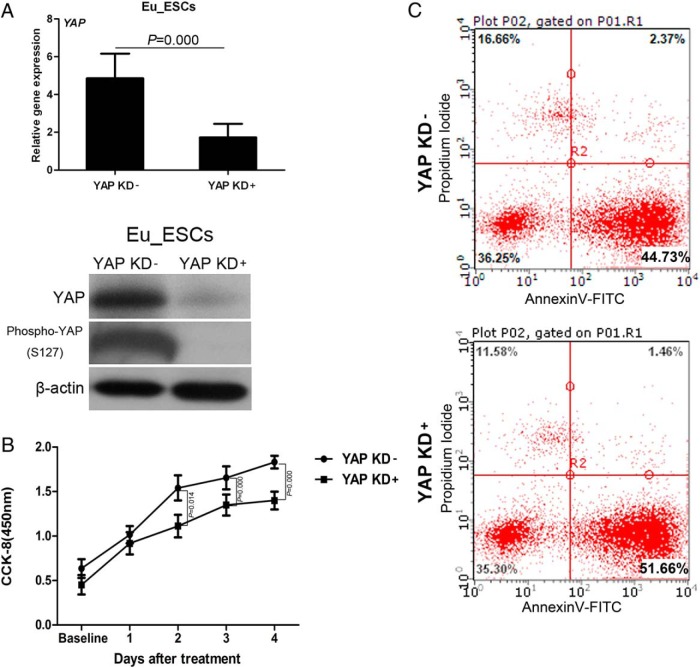
Based on the abundant expression of YAP in endometriosis and its well-established ability to regulate cell proliferation and apoptosis, we hypothesized that YAP may control proliferation and apoptosis of endometrial cells. To test this hypothesis, we performed an shRNA-based KD of YAP in eutopic ESCs of endometriosis. GFP-labeled scrambled shRNA was used as controls. We obtained greater than 70% KD efficiency at protein and mRNA levels (Figure 2A). Then the transfected eutopic ESCs were subjected to CCK8 assays. The data showed that YAP-KD eutopic ESCs have a retarded proliferation rate when compared with the control group (Figure 2B). The flow cytometry revealed a statistically higher percentage of early apoptotic cells in YAP-KD eutopic ESCs (Figure 2C). These data demonstrated that loss of YAP expression resulted in decreased proliferation and enhanced apoptosis of eutopic ESCs.
Figure 2.
KD YAP in eutopic ESCs (Eu_ESCs) reduced cell proliferation and promotes apoptosis. A, mRNA and protein levels of YAP/phosphor-YAP(S127) following YAP KD as measured by RT-qPCR and Western blotting (n = 3). B, CCK8 analysis of proliferation of YAP KD eutopic ESCs (n = 3). C, Annexin V-FITC/PI staining of YAP KD eutopic ESCs as analyzed by flow cytometry (n = 3). Right lower quadrant indicates the percentage of early apoptosis cells. Data were analyzed by Student t test.
OE of YAP in ESCs of normal endometrium promotes proliferation and decreases apoptosis
To further explore the role of YAP in cell proliferation and apoptosis of ESCs, we established a normal ESC line, which expresses the wild type (WT) YAP protein (YAP OE). The empty vector (GV358) was also transfected into ESCs and served as controls. Results showed that normal ESCs expressed more YAP mRNA and protein than controls (Figure 3A). By CCK8 assays, YAP OE ESCs revealed a higher proliferation rate compared with controls (Figure 3B). In addition, the percentage of early apoptotic cells significantly decreased after OE of YAP in ESCs (Figure 3C).
Figure 3.
OE of YAP in normal ESCs (No_ESCs) promotes cell proliferation and cell antiapoptosis. A, mRNA and protein levels of YAP/phosphor-YAP (S127) following YAP OE as measured by RT-qPCR and Western blotting (n = 3). B, CCK8 analysis of the proliferation of YAP OE ESCs (n = 3). C, Annexin V-FITC/PI staining of YAP OE normal ESCs as analyzed by flow cytometry (n = 3). Right lower quadrant indicates the percentage of early apoptosis cells. Data were analyzed by Student t test.
BCL-2 and CTGF participate in cell proliferation and apoptosis as downstream targets of YAP-TEAD1 complex
The TEAD/TEF family of transcription factors is necessary for YAP's biological activity through a stable, transcriptionally active complex. In our experiments, we found that KD YAP decreased TEAD1 mRNA and protein expression in eutopic ESCs whereas OE YAP increased TEAD1 mRNA and protein levels in normal ESCs (Figure 4, A and B). Furthermore, YAP KD in eutopic ESCs or OE in normal ESCs significantly altered CTGF and BCL-2 mRNA and protein expression, both had been previously characterized for their important roles in regulating cell proliferation and cell apoptosis. Therefore, we considered that YAP-TEAD1 complex regulated ESCs through mediation of CTGF and BCL-2. Two TEAD1-binding sites (GGGATTCCTGCG and GACATTTCTGTG) in the promoter region of human BCL-2 gene were identified according to prediction of Jaspar database (Figure 4C). The target of YAP-TEAD1 on CTGF had already been identified (26). Our ChIP experiments confirmed YAP-TEAD1 complex binding to the promoter region of CTGF and BCL-2 (Figure 4D). Together, the results suggestd that YAP involve in ESC cell proliferation and apoptosis through transcriptionally regulated CTGF and BCL-2 expression.
Figure 4.
BCL-2 and CTGF are the direct targets of YAP-TEAD1 transcriptional active complex. A, mRNA and protein levels of TEAD1, BCL-2, and CTGF following YAP KD in eutopic ESCs (Eu_ESCs) (n = 3). B, mRNA and protein levels of TEAD1, BCL-2, and CTGF following YAP OE in normal ESCs (No_ESCs) (n = 3). C, The human BCL-2 promoter region contains two possible TEAD1-binding sites. The possible TEAD1-binding sites are shown in red. D, Cross-linked chromatin was prepared from ESCs immunoprecipitated with anti-YAP antibody, anti-TEAD1 antibody, antihistone H3 antibody, or IgG alone followed by qPCR using primers specific to CTGF and BCL-2 promoter. Data were analyzed by Student t test.
Inhibition of YAP pharmacologically attenuates cell proliferation and induces cell apoptosis of eutopic ESCs in vitro and ectopic endometrial cells in vivo
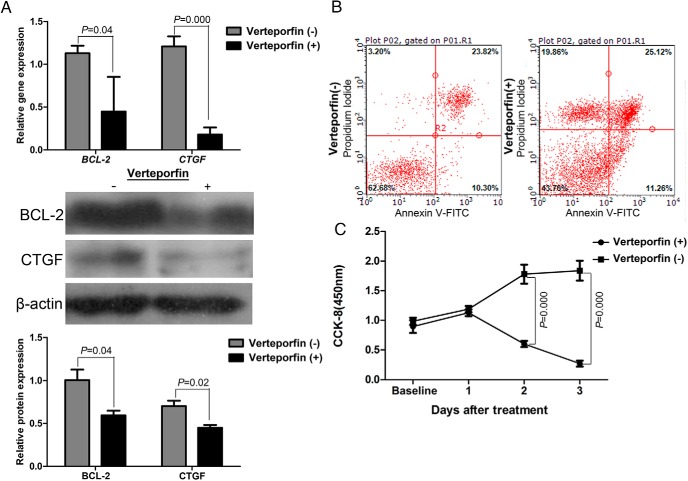
After treatment with verteporfin (1μM) for 18 hours, we found that a reduction in the expression of antiapoptosis marker BCL-2 and proliferation marker CTGF (Figure 5A). The data also showed that eutopic ESCs treated by verteporfin have a significantly lower proliferation rate after 1 day when compared with control group (Figure 5B). Moreover, the flow cytometry revealed a significantly higher percentage of dying cells in verteporfin-treated eutopic ESCs (Figure 5C). These data demonstrated that inhibition of YAP-TEAD complex resulted in decreased proliferation and enhanced apoptosis of eutopic ESCs.
Figure 5.
Inhibition of YAP pharmacologically attenuates proliferation and induces apoptosis of eutopic ESCs in vitro. A, mRNA and protein levels of BCL-2 and CTGF in eutopic ESCs treated with verteporfin for 18 h (n = 3). B, CCK8 analysis of the proliferation of eutopic ESCs treated with verteporfin (n = 3). C, Annexin V-FITC/PI staining of YAP eutopic ESCs treated with verteporfin as analyzed by flow cytometry (n = 3). Left upper quadrant indicates the percentage of dying cells. Data were analyzed by Student t test.
In the nude mouse model of endometriosis, the implantation of eutopic endometrial fragments collected from women with endometriosis successfully led to the development of endometrial-like lesions in gross and histological findings (Figure 6A). After treated ip verteporfin (100 mg/kg), the size of the ectopic lesion in mice experienced a marked reduction compared with those treated with PBS (Figure 6B). In addition, these lesions shrank at least 50% and even some lesions disappeared (Figure 6B). However, the endometrial-like tissue and inflammatory adhesions could be observed inside the peritoneal cavity of control mice treated by PBS (Figure 6B). The cell proliferation of the endometriotic lesions was severely affected by verteporfin treatment, as suggested by Ki67 staining and BrdU staining (Figure 6C). In addition, verteporfin led to higher cells apoptosis ratio in the endometriotic lesions (Figure 6C). This effect was concomitant with a reduction in the expression of antiapoptosis marker BCL-2 and proliferation marker CTGF (Figure 6D).
Figure 6.
Inhibition of YAP pharmacologically attenuates proliferation and induces apoptosis of ectopic endometrial cells in the nude mice of endometriosis. A, H&E staining of endometriotic lesions from a control mouse. Scale bars, 0.5 mm. Magnified view of the boxed region is shown in the panel below (S, stroma; Epi, epithelium.). Black arrow is blood vessels. Scale bars, 200 μm. B, The visible lesions within the peritoneal cavity of a control mouse and a mouse treated with verteporfin for 12 d. C, Proliferative cells were assessed by BrdU and ki67 staing on endometriotic lesions sections from mice treated with verteporfin and control mice. Red arrows indicate positive staining cells. Scale bars, 100 μm; apoptotic cells were assessed by TUNEL assay on endometriotic lesions sections from mice treated with verteporfin and control mice. Red arrows indicate TUNEL+ cells, Scale bars, 100 μm. D, mRNA and protein levels of BCL-2 and CTGF in endometriotic lesions from mice treated with verteporfin and control mice. n = 5 mice per group; Data were analyzed by Student t test.
Discussion
In the present study, we found for the first time that YAP mRNA and protein up-regulating and decreased phosphoS127-YAP in ectopic and eutopic endometrium. Immune staining intensity of YAP was higher in epithelial and stromal cells in both eutopic and ectopic endometrium compared with normal endometrium. This observation suggested that YAP and related Hippo signals may play a role in the development and progression of endometriosis. The complexity of YAP regulation has expanded considerably in recent years. Until now, it has been well established that YAP is a primary downstream effector of the Hippo pathway crucial for organ size control, regeneration, and tumorigenesis by controlling proliferation, apoptosis, and stemness in mammals (27). In 2014, Fu et al (28) found that YAP KD not only resulted in reduction in cell proliferation, but also decreased aromatase (CYP19A1) protein expression and estrogen synthesis in human ovarian granulosa cell tumors cell line. In our study, we knocked down YAP in eutopic ESCs to investigate whether YAP regulates ESCs viability in endometriosis; the ESCs exhibited a lower proliferation and higher apoptosis after the process. Meantime, YAP OE in normal ESCs showed an increased proliferation and antiapoptosis of ESCs. These data showed that YAP involve in the viability of ESCs. Previous studies had suggested that eutopic endometrium of endometriosis possesses many different characteristics when compared with normal endometrium of disease-free women, and most part of lies in ESCs viability (6–10). In our study, up-regulated YAP adjusted the viability of ESCs in endometriosis. It seems as though activated Hippo/YAP signal pathway gives increase to eutopic ESCs viability. These dynamic endometrial cells attach to the peritoneal membrane through retrograde menstruation, forward to continuous growth.
The mechanism by which YAP regulated ESC proliferation and antiapoptosis needs to be evaluated further. The expression of TEAD1, one of YAP DNA-binding transcription factors, is inhibited by YAP KD in eutopic ESCs, whereas elevated TEAD1 expression is caused by OE YAP in normal ESCs. Our data suggest that YAP-TEAD1 complex may play a critical role in ESC proliferation and apoptosis. Zhao et al (26) reported that YAP up-regulation increased cell growth in MCF10A cell lines in a CTGF-dependent manner. In line with this study, we also found that the YAP-TEAD1-signaling pathway regulating ESCs proliferation to be dependent of regulator CTGF. BCL-2 is a novel downstream target of the YAP-TEAD1 transcriptional active complex to regulate cell apoptosis. In a previous study, a BCL-2 family gene MCL1 was found to be up-regulated in liver from an ApoE/rtTA-YAP transgenic mouse (of which YAP was induced in the liver) and suggests that YAP could partner with some unknown DNA-binding protein(s) to regulate target genes transcription, including MCL1 (29). Our finding demonstrated that TEAD1 was the cofactor of YAP to regulate BCL-2. In addition, variation of CTGF and BCL-2 expression was consistent with TEAD1. Taken together, our study confirmed that both of CTGF and BCL-2 were required for YAP-controlled ESC proliferation and apoptosis.
In vitro, after treatment with verteporfin, eutopic ESCs revealed decreased proliferation and enhanced apoptosis. In vivo, we established a nude mouse model of endometriosis and then treated ip injections of verteporfin. We found that verteporfin could significantly shrink the endometriotic lesion size, and even lead to disappearance of some of them. The experiments in vitro and in vivo both suggested that verteporfin or other specific compounds can disrupt or weaken the YAP-TEAD complex, which could be considered a latent choice in the pharmacological study of endometriosis.
Our study has some limitations. The first is the nude mouse model used. Endometriosis is widely considered inflammatory disease, yet the use of immunocompromised mice precludes assessment of the host immune response in the disease model and to the treatment. The other limitation is that only ESCs were used in in vitro studies. Primary endometrial epithelial cells are very difficult to grow and passage, which limit our study on this kind cell. But the potential role of Hippo/YAP pathway in endometrial epithelial cells is still necessary to be explored in the following study.
In conclusion, up-regulation of YAP in endometriosis has been presented. Elevated expression of YAP promoted ESCs proliferation and antiapoptosis in vitro and in vivo. As direct and functional targets of YAP-TEAD1 complexes, CTGF and BCL-2 involved in regulation of ESC's viability in endometriosis. Hippo/YAP-signaling pathway may be involved in the pathogenesis of endometriosis and should represent as a novel hypothesis for pathogenesis of endometriosis.
Acknowledgments
We thank Dr Wenming Xu, Huaqin Sun, and Ke Wang for their help in the study; and Mr Ruibo Zhang for his help for proofreading the manuscript.
This work was supported by a grant from the Science and Technology Bureau of Sichuan (2012SZ0030).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BCL
- B-cell lymphoma/leukemia
- BrdU
- bromodeoxyuridine
- ChIP
- chromatin immunoprecipitation
- CTGF
- connective tissue growth factor
- ESC
- endometrial stromal cell
- KD
- knockdown
- shRNA
- short hairpin RNA
- TEAD
- TEA domain
- YAP
- Yes-associated protein.
References
- 1. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. [DOI] [PubMed] [Google Scholar]
- 2. Ness RB. Endometriosis and ovarian cancer: Thoughts on shared pathophysiology. Am J Obstet Gynecol. 2003;189:280–294. [DOI] [PubMed] [Google Scholar]
- 3. Sugihara K, Kobayashi Y, Suzuki A, et al. Development of pro-apoptotic peptides as potential therapy for peritoneal endometriosis. Nat Commun. 2014;5:4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo M, Hay B. Cell proliferation and apoptosis. Curr Opin Cell Biol. 1999;11:745–752. [DOI] [PubMed] [Google Scholar]
- 5. Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- 6. Meresman GF, Vighi S, Buquet RA, Contreras-Ortiz O, Tesone M, Rumi LS. Apoptosis and expression of BCL-2 and Bax in eutopic endometrium from women with endometriosis. Fertil Steril. 2000;74:760–766. [DOI] [PubMed] [Google Scholar]
- 7. Béliard A, Noël A, Foidart JM. Reduction of apoptosis and proliferation in endometriosis. Fertil Steril. 2004;82:80–85. [DOI] [PubMed] [Google Scholar]
- 8. Szymanowski K. Apoptosis pattern in human endometrium in women with pelvic endometriosis. Eur J Obstet Gynecol Reprod Biol. 2007;132:107–110. [DOI] [PubMed] [Google Scholar]
- 9. Klemmt PA, Carver JG, Koninckx P, McVeigh EJ, Mardon HJ. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: towards a mechanistic model for endometriosis progression. Hum Reprod. 2007;22:3139–3147. [DOI] [PubMed] [Google Scholar]
- 10. Park JS, Lee JH, Kim M, Chang HJ, Hwang KJ, Chang KH. Endometrium from women with endometriosis shows increased proliferation activity. Fertil Steril. 2009;92:1246–1249. [DOI] [PubMed] [Google Scholar]
- 11. Pellegrini C, Gori I, Achtari C, et al. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil Steril. 2012;98:1200–1208. [DOI] [PubMed] [Google Scholar]
- 12. Wang C, Jin A, Huang W, et al. Up-regulation of BCL-2 by CD147 Through ERK Activation Results in Abnormal Cell Survival in Human Endometriosis. J Clin Endocrinol Metab. 2015;100:E955–E963. [DOI] [PubMed] [Google Scholar]
- 13. Korkmaz D, Bastu E, Dural O, Yasa C, Yavuz E, Buyru F. Apoptosis through regulation of BCL-2, Bax and Mcl-1 expressions in endometriotic cyst lesions and the endometrium of women with moderate to severe endometriosis. J Obstet Gynaecol. 2013;33:725–728. [DOI] [PubMed] [Google Scholar]
- 14. Yotova IY, Quan P, Leditznig N, Beer U, Wenzl R, Tschugguel W. Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Hum Reprod. 2011;26:885–897. [DOI] [PubMed] [Google Scholar]
- 15. Mei J, Li MQ, Ding D, et al. Indoleamine 2,3-dioxygenase-1 (IDO1) enhances survival and invasiveness of endometrial stromal cells via the activation of JNK signaling pathway. Int J Clin Exp Pathol. 2013;6:431–444. [PMC free article] [PubMed] [Google Scholar]
- 16. Abe W, Nasu K, Nakada C, Kawano Y, Moriyama M, Narahara H. miR-196b targets c-myc and Bcl-2 expression, inhibits proliferation and induces apoptosis in endometriotic stromal cells. Hum Reprod. 2013;28:750–761. [DOI] [PubMed] [Google Scholar]
- 17. Kawano Y, Nasu K, Hijiya N, et al. CCAAT/enhancer-binding protein α is epigenetically silenced by histone deacetylation in endometriosis and promotes the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2013;98:E1474–E1482. [DOI] [PubMed] [Google Scholar]
- 18. Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010;24:862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plouffe SW, Hong AW, Guan KL. Disease implications of the Hippo/YAP pathway. Trends Mol Med. 2015;21:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Song S, Ajani JA, Honjo S, et al. Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res. 2014;74:4170–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perra A, Kowalik MA, Ghiso E, et al. YAP activation is an early event and a potential therapeutic target in liver cancer development. J Hepatol. 2014;61:1088–1096. [DOI] [PubMed] [Google Scholar]
- 23. Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78:642–649. [DOI] [PubMed] [Google Scholar]
- 24. Bastón JI, Barañao RI, Ricci AG, et al. Targeting galectin-1-induced angiogenesis mitigates the severity of endometriosis. J Pathol. 2014;234:329–337. [DOI] [PubMed] [Google Scholar]
- 25. Fechner S, Husen B, Thole H, et al. Expression and regulation of estrogen-converting enzymes in ectopic human endometrial tissue. Fertil Steril. 2007;88:1029–1038. [DOI] [PubMed] [Google Scholar]
- 26. Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu C, Li L, Zhao B. The regulation and function of YAP transcription co-activator. Acta Biochim Biophys Sin. 2015;47:16–28. [DOI] [PubMed] [Google Scholar]
- 28. Fu D, Lv X, Hua G, et al. YAP regulates cell proliferation, migration, and steroidogenesis in adult granulosa cell tumors. Endocr Relat Cancer. 2014;21:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]