This review details potential mechanisms linking gut dysbiosis to metabolic dysfunction, including lipopolysaccharide, bile acids, short chain fatty acids, gut hormones, and branched-chain amino acids.
Abstract
Context:
Type 2 diabetes mellitus is associated with gastrointestinal dysbiosis involving both compositional and functional changes in the gut microbiome. Changes in diet and supplementation with probiotics and prebiotics (ie, fermentable fibers) can induce favorable changes in gut bacterial species and improve glucose homeostasis.
Objective:
This paper will review the data supporting several potential mechanisms whereby gut dysbiosis contributes to metabolic dysfunction, including microbiota driven increases in systemic lipopolysaccharide concentrations, changes in bile acid metabolism, alterations in short chain fatty acid production, alterations in gut hormone secretion, and changes in circulating branched-chain amino acids.
Methods:
Data for this review were identified by searching English language references from PubMed and relevant articles.
Conclusions:
Understanding the mechanisms linking the gut microbiome to glucose metabolism, and the relevant compositional and functional characteristics of the gut microbiome, will help direct future research to develop more targeted approaches or novel compounds aimed at restoring a more healthy gut microbiome as a new approach to prevent and treat type 2 diabetes mellitus and related metabolic conditions.
The microbiome is an integral part of the human body with the highest density of bacteria in the gut, ranging from 1 × 104 cells/g in the jejunum to 1 × 1014 cells/g in the colon (1). The number of genes in the gut microbiome exceeds that of the human genome by 10- to 100-fold and exert considerable influence on the host (2). Although the composition of the gut microbiome varies between individuals, the underlying functional gene content is similar between individuals, suggesting that the gut microbiome is integral to maintaining aspects of host homeostasis (3). Environmental exposures, such as diet, can induce changes in microbial community composition, metabolism, and functional gene transcription, thereby altering exposure of the host to microbial metabolites and antigens that may influence disease risk (4, 5).
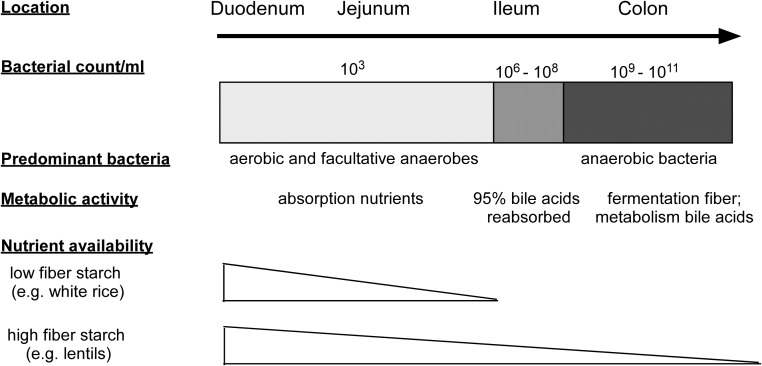
The gut microbiome differs throughout the gastrointestinal (GI) tract (1, 6), and interactions with the host likely vary along this continuum (2, 6–8) (Figure 1). Aerobic and facultative anaerobes dominate the small intestine and rapidly metabolize simple carbohydrates (9), whereas bacteria in the ileum have the ability to degrade more complex fructooligosaccharides (10). In the colon, there is enrichment for strict anaerobic bacteria that ferment otherwise nondigestible dietary fiber (11) and deconjugate bile acids (12). Most studies in humans have relied exclusively on stool samples to characterize the gut microbiome. Stool samples may miss microbial taxa and metabolic pathways among less abundant microbiota of the small intestine relevant to metabolic disorders. Sampling at different sites along the GI tract is needed to obtain a complete picture of the role of the gut microbiome and metabolic disease.
Figure 1.
Diagram detailing the changes in gut bacterial populations, density, and metabolic activity along the gastrointestinal tract (122). The small intestine, which is the main site of nutrient absorption, contains lower populations of bacteria that are mainly aerobic and facultative anaerobes. Bile acids are predominantly reabsorbed in the ileum. The colon contains mainly anaerobic bacteria in large numbers that are able to ferment undigested fiber and metabolize remaining bile acids.
Dysbiosis of the Gut Microbiome in Type 2 Diabetes
Type 2 diabetes (T2DM) is reaching epidemic proportions around the globe (13). In the United States, the prevalence of diabetes (mostly T2DM) in 2012 was estimated at 9.3% (29.1 million individuals), with another 27.5% (86 million) estimated to be prediabetic (14). Development of new strategies to prevent or reverse the underlying pathophysiology of T2DM is critical if we hope to stem the tide of this epidemic.
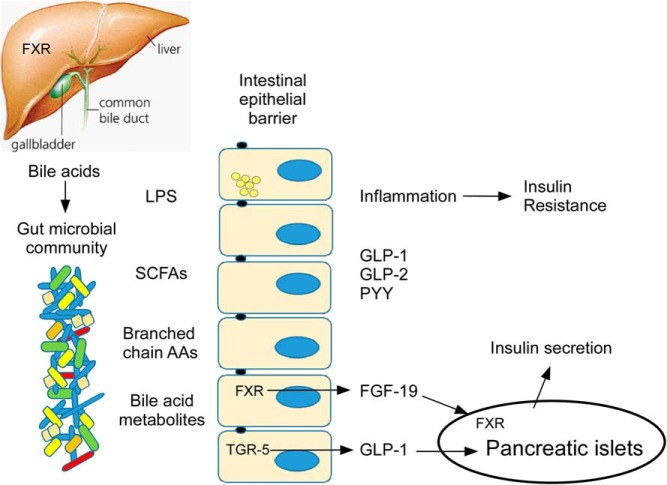
Recent evidence suggests that dysbiosis of the gut microbiome may play a role in the pathogenesis of T2DM (15). Cross-sectional studies in humans demonstrate compositional and functional differences in the gut bacteria of those with T2DM or prediabetes vs normal glucose tolerance (16–18). Fecal transplants from mice with glucose intolerance into healthy germ-free mice induce glucose intolerance (19), demonstrating a causal role at least in rodents. In a human fecal microbiota transplant study, transfer of fecal material from lean donors into individuals with metabolic syndrome resulted in an increase in gut microbial diversity and improved insulin sensitivity (20). These data support the hypothesis that dysbiosis of the gut microbiome contributes to metabolic dysfunction. However, there remains a knowledge deficit regarding which compositional and/or functional characteristics of the gut microbiota are relevant for metabolic health, and through which mechanisms they effect host glucose homeostasis. Proposed mechanisms include increased systemic lipopolysaccharide (LPS), changes in bile acid metabolism, alterations in short chain fatty acid (SCFA) production, alterations in gut hormone secretion, and changes in circulating branched-chain amino acids (Figure 2).
Figure 2.
Schematic of potential mechanisms linking the gut microbial community with glucose metabolism. Potential mechanisms include 1) systemic absorption of LPS, either through “leaky” tight junctions or via chylomicron uptake, with subsequent inflammation; 2) bacterial production of SCFAs with signaling effects to stimulate secretion of gut hormones GLP-1 and GLP-2 and PYY as well as nutrient effects; 3) bacterial synthesis and absorption of branched chain amino acids (AAs) that may result in insulin resistance; 4) bacterial metabolism of bile acids with local and organ-specific signaling effects, including stimulation of fibroblast growth factor-19 (FGF-19) and GLP-1. FGF-19 has metabolic effects on the FXR in the pancreatic β-cell and in the liver.
Changes in community composition and microbial metabolism along the digestive track may be particularly relevant to metabolic diseases (16, 21). Indeed, small intestinal bacterial overgrowth is highly prevalent and may play an etiological role in diabetes (22), nonalcoholic steatohepatitis (23), and obesity (24). In small intestinal bacterial overgrowth, complex carbohydrate and bile acid metabolism that is normally reserved for the colon occurs in the upper GI tract leading to early and increased absorption of SCFAs (25) and deconjugated bile acids (26). Small intestinal bacterial overgrowth also contributes to leaky tight junctions, bacterial translocation, and endotoxemia (27).
Effect of Prebiotic, Probiotic, and Synbiotic Supplementation on Glucose Metabolism
Because the gut microbiome is altered rapidly with changes in diet (28), strategies to increase the amount of “beneficial” bacteria in the gut have included use of high-fiber diets or addition of fermentable fibers such as fructooligosaccharides, which are inulin-type prebiotics that favor the growth of the Bifidobacteria species. High-fiber diets improve insulin sensitivity in healthy and overweight/obese men and women (29–31) and reduce fasting glucose and hemoglobin A1c in patients with T2DM (32). Data on prebiotic (33, 34) or probiotic (35) supplementation alone have been mixed. In contrast, supplementation with probiotic cultures combined with a prebiotic to support growth of beneficial bacteria improved glucose levels, insulin sensitivity, and systemic markers of inflammation (36–40).
The “Leaky Gut” Hypothesis and Endotoxemia
LPS or endotoxin is a bacterial cell wall component found predominantly in gram-negative bacteria that stimulates an inflammatory response via activation of toll-like receptor-4 and transforming growth factor-β (TGF-β)-mediated pathways (41, 42). Earlier studies focused on the inflammatory response induced by high concentrations of endotoxin such as those seen in septicemia, but in recent years the focus has shifted to effects of subsepticemic levels (<2 pg/ml) associated with chronic disease. In mice, chronic infusion of LPS mimicked the effects of the high-fat diet on metabolic and inflammatory parameters (43).
In human cross-sectional observational studies, higher systemic LPS or higher LPS binding protein were associated with low-grade, chronic inflammation in obesity (43–46), metabolic syndrome (46) and T2DM (44, 46, 47). Several possible pathways through which the gut microbiome may impact circulating LPS levels are detailed in the following paragraphs (5).
First, changes in the relative amounts or the types of gram-negative vs gram-positive bacteria in the intestinal lumen may influence LPS bioavailability. Dysbiosis in diabetes is characterized by a decrease in many gram-positive butyrate producing Clostridial species that lack LPS and an expansion of many gram-negative opportunistic pathogens including some Bacteroidetes and Proteobacteria species that contain LPS (17). In mice fed a high-fat diet, supplementation with fermentable fiber was associated with an increase in Bifidobacteria and normalization of circulating LPS along with improved glucose tolerance, insulin secretion, and inflammation (43). Both increases in systemic LPS and shifts in LPS subtypes are likely to play a role in the inflammatory process fueled by endotoxemia (48). LPS is composed of a highly variable outer region (O-segment) and a relatively conserved inner region (lipid A). Small changes in the lipid A structure such as differential phosphorylation have been shown to result in dramatic changes in binding affinity to toll-like receptor-4 and downstream host responses (48).
Second, increased intestinal permeability (“leaky gut”) may allow for LPS to translocate through intercellular pathways. The gut microbiome regulates gut permeability by maintaining the health of the intestinal cells, their tight junctions, and a protective mucous layer, which may be due in part by providing nutrients, such as SCFAs, to the epithelial cells (49). Probiotics (Streptococcus thermophilus and Lactobacillus acidophilus) prevented increases in permeability in human intestinal epithelial cells induced by tumor necrosis factor α or gamma interferon in vitro, demonstrating the important role of certain bacteria in maintaining a healthy intestinal barrier (50). In mouse models, high-fat feeding leads to dramatic changes in the gut microbiota, glucose intolerance, insulin resistance, increased plasma LPS, intestinal permeability, inflammation, and oxidative stress, all of which could be partially or completely blocked with antibiotics or by genetically knocking out the LPS receptor. Obese leptin (ob/ob) and leptin-receptor (db/db) deficient mice demonstrate increased intestinal permeability and higher portal LPS levels and circulating inflammatory markers compared to lean, wild-type control mice (51). Analysis of the tight junctions in the small intestine in these animals showed both redistribution and decreases in tight junction proteins (51). These animal data support the concept that dysbiosis of the gut microbiome can increase gut permeability leading to increased LPS entry into the systemic circulation with subsequent inflammation and metabolic dysfunction.
There are fewer studies in humans examining the role of gut permeability in circulating LPS concentrations. In human cross-sectional studies, gut permeability is increased in subjects with T2DM (47, 52–54) and obese vs normal weight individuals (55). An intervention study in healthy young volunteers fed inulin-enriched pasta showed decreased gut permeability (56), but lacked data on changes in the gut microbiome, glucose metabolism, or inflammatory markers. In a separate study, 4 weeks of a probiotic yogurt in elderly patients with and without small bowel bacterial overgrowth decreased LPS markers and soluble CD14 but had no impact on measures of intestinal permeability (57). Although the data in animal models are convincing, more data are needed in humans.
Third, LPS can be taken up by gut epithelial cells and incorporated into chylomicrons in the small intestine after a high-fat meal. LPS contains a lipid moiety and can be taken up by enterocytes (58) and transported to the Golgi apparatus (59), where chylomicrons are also formed (60). In mice, the rise in plasma LPS after long-chain dietary fatty acid intake was almost entirely accounted for by LPS associated with the chylomicron remnant fraction. Furthermore, the mechanism of absorption appeared dependent on chylomicron formation as the rise in LPS after an oral fat load was blocked by an inhibitor of chylomicron formation (61). Circulating LPS levels increase after a high-fat meal in healthy men (62) and are higher in obese subjects with postprandial hypertriglyceridemia (63). In the latter study, postprandial chylomicron LPS levels correlated with the rise in plasma triglycerides, but not with insulin resistance (63). Chylomicrons increase the rate of clearance of LPS by hepatocytes, decrease LPS-mediated inflammation, and reduce mortality in rat models of sepsis (64). LPS binding protein associates with chylomicrons and enhances the amount of LPS binding to chylomicrons, thereby allowing clearance of bacterial toxins and preventing cell activation (65). In these experimental settings, chylomicrons appear to serve a protective role. However, they may also facilitate absorption of low levels of LPS into the body, especially in response to diets high in fat, and thus contribute to a low-grade chronic inflammatory state.
Bile Acid Metabolism by Gut Bacteria and Effects of Bile Acid Metabolites on Glucose Homeostasis
The gut microbiome plays a major role in bile acid metabolism (4, 66). Primary bile acids produced in the liver are conjugated with either glycine or taurine to form bile salts before active secretion into the small intestine. Upon reaching the ileum, 95% of bile salts undergo enterohepatic circulation and are transported back to the liver. Roughly 400–600 mg of bile salts enter the large intestine, where they undergo bacterial transformation to secondary bile acids, primarily deoxycholic acid and lithodeoxycholic acid, by a variety of anaerobic bacteria (67–69).
Several lines of evidence suggest that bile acids are involved in the regulation of glucose homeostasis. First, treatment with bile acid sequestrants improves both insulin sensitivity and glycemic control (70). Second, treatment with a synthetic bile acid (tauro-ursodeoxycholic acid) improved hepatic and muscle insulin sensitivity by approximately 30% compared to placebo (71). Third, taurine-conjugated deoxycholic acid and chenodeoxycholic acid were associated with insulin resistance among nondiabetic individuals, and were also significantly higher in patients with T2DM than those without diabetes (72, 73).
The exact mechanism through which bile acids affect glucose homeostasis remains incompletely understood, but may be mediated through activation of the nuclear farnesoid X receptor (FXR), which is expressed in the ileum, liver, and pancreas, and the membrane-bound G-protein coupled receptor, TGR5 (70). Some bile acids are agonists for FXR, whereas others are known or suspected FXR antagonists (72–74). Established FXR agonists include (in the order of ligand activity) CDCA, lithocholic acid, deoxycholic acid, and cholic acid (66). The antidiabetic effects of vertical sleeve gastrectomy, a type of bariatric surgery, were shown to be dependent on signaling through FXR (75). Similarly, treatment with an intestinal FXR agonist improved insulin sensitivity (76). In the ileum, activation of FXR leads to the production of fibroblast growth factor-19, a hormone that affects glucose tolerance through mechanisms that are largely independent of insulin (77, 78). Also in the ileum, activation of TGR5 leads to production of glucagon-like peptide-1 (GLP-1), which has beneficial impacts in both energy and glucose homeostasis (79). In the pancreas, activation of FXR plays a role in insulin transport and secretion (80), and may protect islets against lipotoxicity (81). In the liver, FXR activation likely improves insulin sensitivity, as illustrated by improved insulin sensitivity in patients with T2DM and nonalcoholic fatty liver disease (82) in response to treatment with obeticholic acid, a first-in-class FXR agonist. Taken together, bile acids could have major impacts on all determinants of glucose homeostasis.
The gut microbial composition can alter the amount and type of secondary bile acids formed with the potential for differential metabolic effects via FXR and TGR5 signaling. For example, the bacterial enzymes and genes involved in the deconjugation (bile salt hydrolase), dehydroxylation (7-alpha dehydroxylase), and epimerization (7-α-hydroxysteroid dehydrogenase) of bile acids are distributed across many genera, with reduced abundance of gut microbial bsh genes observed in individuals with T2DM compared to healthy controls (83). Additionally, alteration of the gut microbiome by oral administration of vancomycin to humans with the metabolic syndrome decreased secondary bile acids and worsened insulin sensitivity. In that study, decreases in secondary bile acids correlated with decreases in insulin sensitivity (84).
Systemic bile acid concentrations follow a diurnal pattern with levels of conjugated bile acids increasing following food intake (85). Considering that, it may be a major limitation that all existing studies have focused on fasting bile acid concentrations only. An additional limitation to the existing literature is that many investigations have measured only total bile acids or have not differentiated between taurine- and glycine-conjugated bile acids. Thus, studies that are longitudinal, measure bile acid concentrations with a good, comprehensive assay, and ideally include diurnal or at least postprandial samples, are needed.
Role of Short Chain Fatty Acids (SCFAs)
The bacteria in the colon ferment nondigestible carbohydrates into SCFAs, with acetate, butyrate, and propionate predominating. There is an interplay between dietary fiber content, microbiota, and SCFAs as oligosaccharide-containing diets alter the microbial composition, increase SCFA production and lower the luminal pH (86, 87). In addition, SCFAs are also produced during amino acid catabolism by gut bacteria. Protein fermentation accounts for 17–38% of the SCFA produced in the cecum and sigmoid/rectum (88). The SCFA products vary depending upon the amino acid substrate and the species of gut bacteria (89, 90). For example, branched-chain fatty acids isobutyrate, 2-methylbutyrate, and isovalerate are produced from branched-chain amino acids; propionate and butyrate are produced from threonine; and acetate and butyrate are produced from glutamate, histidine, lysine, arginine, and alanine. Although isobutyrate, 2-methylbutyrate, and isovalerate have not been associated with host health outcomes, acetate, propionate, and butyrate are used as energy substrates by the host. Butyrate serves as an important energy source for colonocytes whereas acetate and propionate are primarily absorbed and extracted by the liver where they are used as substrates for lipogenesis and gluconeogenesis (91).
In addition to supplying additional nutrients, SCFAs function as signaling molecules by activating AMP kinase and free fatty acid receptors 2 and 3 (FFAR2 and 3) also known as G-protein coupled receptors 43 and 41 (92). SCFAs stimulate fatty acid oxidation and inhibit de novo lipogenesis and lipolysis (91) and therefore may protect against the development of nonalcoholic fatty liver disease. Butyrate has received increased focus as a potential beneficial intermediary. Butyrate producing bacteria are less abundant in subjects with T2DM (17, 18) and butyrate supplementation in rodents improves insulin sensitivity (93, 94). Unfortunately, because of absorption and rapid uptake by the liver and/or local metabolism of SCFAs, measurement of SCFAs in stool or peripheral blood provides little information (91). Some insights into the influence of butyrate on diabetes may be gained by analyzing the microbiome for functional genes (metagenomics) with specific focus on metabolic pathways associated with SCFA production (95, 96).
The Microbiome and Gut Hormone Secretion
SCFAs have also been implicated in the regulation of secretion of several gut hormones, including GLP-1 and the anorectic hormone peptide YY (PYY), that regulate energy homeostasis and glucose metabolism (reviewed in Hullar and Lampe (5)). GLP-1 arises from tissue-specific posttranslational processing of proglucagon in response to nutrient intake. GLP-1 augments glucose-mediated insulin secretion from the pancreatic β-cell and both PYY and GLP-1 act in the hypothalamus to decrease food intake (97). GLP-1 and PYY are both made and secreted from intestinal L cells, which are concentrated in the terminal ileum and colon where fermentation of resistant starches occurs. The L cells contain the SCFA receptors FFAR2 and FFAR3 and respond to SCFAs with an increase in intra-cellular calcium (98). Based on in vitro and in vivo experiments in knockout models, FFAR2 but not FFAR3 appears to be important in GLP-1 secretion in response to SCFAs (98). Additional research suggests that GLP-1 secretion can also be modulated by indole, a bacterial metabolite of tryptophan, (99) and by bile acids via TGR5 (100).
In vitro studies using cultured human colonic cells demonstrated significant increases in PYY and GLP-1 in response to propionate (101). The same investigators also used a novel propionate-inulin ester to deliver the SCFA directly to the colon. They found that the propionate-inulin ester acutely increased postprandial PYY and GLP-1 concentrations. If given over 24 weeks, the supplement prevented weight gain and increases in intra-abdominal fat (101). In a rodent model, the prebiotic oligofructose decreased glucose levels during an oral glucose tolerance test and increased both GLP-1 and PYY concentrations (102). Studies in humans on the effect of prebiotics on GLP-1 secretion have been mixed, with a meta-analysis showing conflicting studies with no overall significant changes in GLP-1 or PYY concentrations (103).
Studies using probiotics to modulate the intestinal microbiome have also shown effects on GLP-1 secretion. Mice fed the probiotic VSL#3 (σ-Tau Pharmaceuticals), which contains 8 different strains of “beneficial” bacteria, for 8 weeks demonstrated increased GLP-1 concentrations, reduced food intake, decreased weight gain, and improved glucose tolerance (104). These effects were associated with increased fecal butyrate levels and changes in the abundance of bacterial species, including a decrease in Firmicutes and an increase in Bacteroidetes and Bifidobacteria (104). In a human study in obese children with nonalcoholic steatohepatitis, treatment with VSL#3 resulted in decreased liver fat and increased fasting GLP-1 levels (105). In a separate prospective, double-blind, randomized trial in healthy, glucose-tolerant adults, daily supplementation with Lactobacillus reuteri for 4 weeks increased GLP-1, insulin, and c-peptide in response to an oral glucose load, but did not alter insulin sensitivity (106). However, treatment with oral vancomycin in humans with metabolic syndrome did not change GLP-1 levels despite significant changes in fecal microbial species and decreased insulin sensitivity (84). Taken together, these data support the concept that the composition of the gut microbiome affects the regulation of GLP-1 secretion and thereby may impact glucose metabolism, but need further confirmation.
GLP-2 is another gut hormone resulting from posttranslational processing of proglucagon in the intestinal L cells. GLP-2 displays intestinotrophic protective properties and a recently approved GLP-2 analog, teduglutide, is indicated for treatment of patients with short bowel syndrome. GLP-2 receptor expression is mainly confined to the intestinal tract, but has also been reported in the central nervous system, mesenteric fat, lymph nodes, pancreas, liver, bladder, and spleen (107).
There is evidence in both animals and humans that GLP-2 secretion is impacted by the gut microbiota. In a high-fat diet mouse model that results in endotoxemia, inflammation, and glucose intolerance, treatment with the prebiotic oligofructose increased GLP-2 production and Bifidobacterium numbers, decreased intestinal permeability, improved tight junction integrity, lowered plasma LPS and cytokines, and reduced hepatic inflammation and oxidative stress. Most of the beneficial effects of the prebiotic were abolished with treatment with a GLP-2 antagonist, and treatment with pharmacologic doses of GLP-2 mimicked the effects of treatment with the prebiotic (108). A separate study found that treatment of diet-induced obese rats with the probiotic Bifidobacterium animalis but not the prebiotic oligofructose increased portal GLP-2 concentrations; however, there were no significant changes in intestinal permeability or circulating LPS and cytokine levels (109).
In one human cross-sectional study of 24 obese adults, GLP-2 secretion in response to a meal test was inversely related to insulin sensitivity (110), but there was no evaluation of the gut microbiome. In an interventional study in glucose tolerant adults, 4 weeks ingestion of Lactobacillus reuteri increased both GLP-1 and GLP-2 secretion in response to an oral glucose load compared to placebo. Insulin secretion was also increased, although there was no change in insulin sensitivity, glucose tolerance, liver fat, or cytokines (106). In a separate study, healthy young volunteers were fed pasta enriched with the prebiotic inulin for five weeks in a crossover study design. Fasting GLP-2 levels were increased in response to the inulin, although there was no significant change in GLP-2 secretion in response to a standardized meal. Measures of intestinal permeability decreased in the inulin treatment group suggesting improved intestinal barrier function potentially modulated by increased GLP-2 levels (56). Although the data are limited, they suggest that GLP-2 secretion can be altered by supplementation with probiotics or prebiotics and may be an important factor in maintaining a healthy and intact gut epithelial lining.
Microbial Synthesis of Amino Acids
Circulating amino acids contribute to glucose homeostasis with amino acids stimulating both insulin and glucagon secretion. Branched-chain amino acids appear to play a particular role in glucose homeostasis and may contribute to diabetes risk. In a prospective, nested case-control study, plasma concentrations of five branched-chain and aromatic amino acids (isoleucine, leucine, valine, tyrosine, and phenylalanine) were identified as predictors of the development of diabetes, independent of traditional risk factors (111). Similar findings were observed in the Metabolic Syndrome in Men study in which alanine, leucine, isoleucine, tyrosine, and glutamine predicted incident T2DM, an association that was largely mediated by insulin resistance (112). In a pediatric population, elevated branched-chain amino acids were associated with obesity and were predictive of the development of insulin resistance (113). Additionally, an acute infusion of a mixture of amino acids that included three branched-chain amino acids into healthy humans decreased insulin sensitivity at the level of both liver and muscle (114). Deprivation of dietary branched-chain amino acids in animal models improves insulin sensitivity (115, 116), lending additional support to the hypothesis that branched-chain amino acids contribute to insulin resistance.
The human microbial community contributes to the synthesis of amino acids and circulating amino acid levels (117). Bacteria may provide a source of branched-chain amino acids since their proportions are higher in bacterial cells than eukaryotic cells. Bacteria can synthesize de novo all of the 20 amino acids required for protein synthesis. There are several lines of evidence that suggest the gut bacteria contribute to and influence both the composition and levels of amino acids absorbed into the body. Early studies in germ-free rats showed an altered distribution of amino acids along the gut compared to conventionalized rats (118). Use of 15NH4Cl tracers demonstrated that bacterial de novo biosynthesis is a significant source of host amino acids in pigs (119) and humans (120). Additionally, recent metagenomic analysis showed that genes associated with amino acid biosynthesis were enriched in the human distal gut (121).
Concluding Remarks
Although limited, the existing data strongly suggest that the gut microbiota affect glucose homeostasis. Possible mechanisms linking the gut microbiota to glucose homeostasis may include increased intestinal permeability, low-grade endotoxemia, changes in the production of SCFAs or branched-chain amino acids, alterations in bile acid metabolism, and/or effects on the secretion of gut hormones. A better understanding of the mechanisms linking the gut microbiome to glucose metabolism, and the relevant compositional and functional characteristics of the gut microbiome, will help direct future research to develop more targeted dietary approaches, probiotic and/or prebiotic supplements, or novel compounds aimed at restoring a more healthy gut microbiome as a new approach to prevent and treat T2DM.
Acknowledgments
This work was supported in part by the Veteran Affairs Administration (to K.U.), the Diabetes Research Center P30DK017047 (to K.U.), the Fred Hutchinson Cancer Research Center (to M.H., M.K.), and CA168530 from the National Cancer Institute of the National Institutes of Health (to M.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- FFAR
- free fatty acid receptor
- FXR
- farnesoid X receptor
- GI
- gastrointestinal
- GLP
- glucagon-like peptide-1
- LPS
- lipopolysaccharide
- PYY
- peptide YY
- SCFA
- short chain fatty acid
- T2DM
- type 2 diabetes.
References
- 1. Hayashi H, Takahashi R, Nishi T, Sakamoto M, Benno Y. Molecular analysis of jejunal, ileal, caecal and recto-sigmoidal human colonic microbiota using 16S rRNA gene libraries and terminal restriction fragment length polymorphism. J Med Microbiol. 2005;54:1093–1101. [DOI] [PubMed] [Google Scholar]
- 2. Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. [DOI] [PubMed] [Google Scholar]
- 3. Human Microbiome Project C. A framework for human microbiome research. Nature. 2012;486:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hullar MA, Burnett-Hartman AN, Lampe JW. Gut microbes, diet, and cancer. Cancer Treat Res. 2014;159:377–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hullar MA, Lampe JW. The gut microbiome and obesity. Nestle Nutr Instit Workshop Series. 2012;73:67–79. [DOI] [PubMed] [Google Scholar]
- 6. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Larsson E, Tremaroli V, Lee YS, et al. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut. 2012;61:1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol. 2002;68:3401–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zoetendal EG, Raes J, van den Bogert B, et al. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012;6:1415–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cecchini DA, Laville E, Laguerre S, et al. Functional metagenomics reveals novel pathways of prebiotic breakdown by human gut bacteria. PloS One. 2013;8:e72766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. [DOI] [PubMed] [Google Scholar]
- 12. Shindo K, Machida M, Koide K, Fukumura M, Yamazaki R. Deconjugation ability of bacteria isolated from the jejunal fluid of patients with progressive systemic sclerosis and its gastric pH. Hepato-gastroenterology. 1998;45:1643–1650. [PubMed] [Google Scholar]
- 13. WHO. Global status report on noncommunicable diseases 2014. 2014. [Google Scholar]
- 14. Association AD. Statistics about diabetes. 2015; http://www.diabetes.org/diabetes-basics/statistics/ Accessed May 12, 2015.
- 15. Vrieze A, Holleman F, Zoetendal EG, de Vos WM, Hoekstra JB, Nieuwdorp M. The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia. 2010;53:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. [DOI] [PubMed] [Google Scholar]
- 17. Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. [DOI] [PubMed] [Google Scholar]
- 18. Zhang X, Shen D, Fang Z, et al. Human gut microbiota changes reveal the progression of glucose intolerance. PloS One. 2013;8:e71108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. [DOI] [PubMed] [Google Scholar]
- 20. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916 e917. [DOI] [PubMed] [Google Scholar]
- 21. Karlsson F, Tremaroli V, Nielsen J, Backhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62:3341–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Virally-Monod M, Tielmans D, Kevorkian JP, et al. Chronic diarrhoea and diabetes mellitus: prevalence of small intestinal bacterial overgrowth. Diabetes Metab. 1998;24:530–536. [PubMed] [Google Scholar]
- 23. Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sabate JM, Jouet P, Harnois F, et al. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obesity Surg. 2008;18:371–377. [DOI] [PubMed] [Google Scholar]
- 25. Hoverstad T, Bjorneklett A, Fausa O, Midtvedt T. Short-chain fatty acids in the small-bowel bacterial overgrowth syndrome. Scand J Gastroenterol. 1985;20:492–499. [DOI] [PubMed] [Google Scholar]
- 26. van Dijk J, Hartog J, Boschman TA. A new class of diuretics with the 1,4-dioxino(2,3-g)quinolone structure. J Med Chem. 1976;19:982–983. [DOI] [PubMed] [Google Scholar]
- 27. Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. [DOI] [PubMed] [Google Scholar]
- 28. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Priebe MG, Wang H, Weening D, Schepers M, Preston T, Vonk RJ. Factors related to colonic fermentation of nondigestible carbohydrates of a previous evening meal increase tissue glucose uptake and moderate glucose-associated inflammation. Am J Clin Nutr. 2010;91:90–97. [DOI] [PubMed] [Google Scholar]
- 30. Weickert MO, Mohlig M, Schofl C, et al. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care. 2006;29:775–780. [DOI] [PubMed] [Google Scholar]
- 31. Pereira MA, Jacobs DR, Jr., Pins JJ, et al. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;75:848–855. [DOI] [PubMed] [Google Scholar]
- 32. Post RE, Mainous AG, 3rd, King DE, Simpson KN. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Bd Family Med. 2012;25:16–23. [DOI] [PubMed] [Google Scholar]
- 33. Delzenne NM, Neyrinck AM, Cani PD. Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome. Microb Cell Factories. 2011;10(Suppl 1):S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kelly G. Inulin-type prebiotics: a review (Part 2). Altern Med Re. 2009;14:36–55. [PubMed] [Google Scholar]
- 35. Gomes AC, Bueno AA, de Souza RG, Mota JF. Gut microbiota, probiotics and diabetes. Nutr J. 2014;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535–542. [DOI] [PubMed] [Google Scholar]
- 37. Eslamparast T, Zamani F, Hekmatdoost A, et al. Effects of synbiotic supplementation on insulin resistance in subjects with the metabolic syndrome: a randomised, double-blind, placebo-controlled pilot study. Br J Nutr. 2014;112:438–445. [DOI] [PubMed] [Google Scholar]
- 38. Malaguarnera M, Vacante M, Antic T, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545–553. [DOI] [PubMed] [Google Scholar]
- 39. Tajadadi-Ebrahimi M, Bahmani F, Shakeri H, et al. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. AnnNutr Metab. 2014;65:34–41. [DOI] [PubMed] [Google Scholar]
- 40. Taghizadeh M, Asemi Z. Effects of synbiotic food consumption on glycemic status and serum hs-CRP in pregnant women: a randomized controlled clinical trial. Hormones. 2014;13:398–406. [DOI] [PubMed] [Google Scholar]
- 41. Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. [DOI] [PubMed] [Google Scholar]
- 42. Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31:817–844. [DOI] [PubMed] [Google Scholar]
- 43. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. [DOI] [PubMed] [Google Scholar]
- 44. Creely SJ, McTernan PG, Kusminski CM, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–E747. [DOI] [PubMed] [Google Scholar]
- 45. Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. [DOI] [PubMed] [Google Scholar]
- 46. Sun L, Yu Z, Ye X, et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care. 2010;33:1925–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jayashree B, Bibin YS, Prabhu D, et al. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mole Cell Biochem. 2014;388:203–210. [DOI] [PubMed] [Google Scholar]
- 48. Dixon DR, Darveau RP. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J Dental Res. 2005;84:584–595. [DOI] [PubMed] [Google Scholar]
- 49. Delzenne NM, Cani PD. Interaction between obesity and the gut microbiota: relevance in nutrition. Annu Rev Nutr. 2011;31:15–31. [DOI] [PubMed] [Google Scholar]
- 50. Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130:731–746. [DOI] [PubMed] [Google Scholar]
- 51. Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastro Liver Physiol. 2007;292:G518–G525. [DOI] [PubMed] [Google Scholar]
- 52. Valentini L, Ramminger S, Haas V, et al. Small intestinal permeability in older adults. Physiol Rep. 2014;2:e00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang D, Zhang L, Zheng Y, Yue F, Russell RD, Zeng Y. Circulating zonulin levels in newly diagnosed Chinese type 2 diabetes patients. Diabetes Res Clin Pract. 2014;106:312–318. [DOI] [PubMed] [Google Scholar]
- 54. Horton F, Wright J, Smith L, Hinton PJ, Robertson MD. Increased intestinal permeability to oral chromium (51 Cr) -EDTA in human type 2 diabetes. Diabetic Med. 2014;31:559–563. [DOI] [PubMed] [Google Scholar]
- 55. Zak-Golab A, Kocelak P, Aptekorz M, et al. Gut microbiota, microinflammation, metabolic profile, and zonulin concentration in obese and normal weight subjects. Inte J Endocrinol. 2013;2013:674106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Russo F, Linsalata M, Clemente C, et al. Inulin-enriched pasta improves intestinal permeability and modifies the circulating levels of zonulin and glucagon-like peptide 2 in healthy young volunteers. Nutr Res. 2012;32:940–946. [DOI] [PubMed] [Google Scholar]
- 57. Schiffrin EJ, Parlesak A, Bode C, Bode JC, van't Hof MA, Grathwohl D, Guigoz Y. Probiotic yogurt in the elderly with intestinal bacterial overgrowth: endotoxaemia and innate immune functions. Br J Nutr. 2009;101:961–966. [DOI] [PubMed] [Google Scholar]
- 58. Neal MD, Leaphart C, Levy R, Prince J, et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176:3070–3079. [DOI] [PubMed] [Google Scholar]
- 59. Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sabesin SM, Frase S. Electron microscopic studies of the assembly, intracellular transport, and secretion of chylomicrons by rat intestine. J Lipid Res. 1977;18:496–511. [PubMed] [Google Scholar]
- 61. Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–97. [DOI] [PubMed] [Google Scholar]
- 62. Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–1292. [DOI] [PubMed] [Google Scholar]
- 63. Clemente-Postigo M, Queipo-Ortuno MI, Murri M, et al. Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J Lipid Res. 2012;53:973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Harris HW, Grunfeld C, Feingold KR, et al. Chylomicrons alter the fate of endotoxin, decreasing tumor necrosis factor release and preventing death. J Clin Invest. 1993;91:1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vreugdenhil AC, Rousseau CH, Hartung T, Greve JW, van 't Veer C, Buurman WA. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J Immunol. 2003;170:1399–1405. [DOI] [PubMed] [Google Scholar]
- 66. Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Doerner KC, Takamine F, LaVoie CP, Mallonee DH, Hylemon PB. Assessment of fecal bacteria with bile acid 7 alpha-dehydroxylating activity for the presence of bai-like genes. Appl Environ Microbiol. 1997;63:1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ye HQ, Mallonee DH, Wells JE, Bjorkhem I, Hylemon PB. The bile acid-inducible baiF gene from Eubacterium sp. strain VPI 12708 encodes a bile acid-coenzyme A hydrolase. J Lipid Res. 1999;40:17–23. [PubMed] [Google Scholar]
- 69. Wells JE, Hylemon PB. Identification and characterization of a bile acid 7alpha-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl Environ Microbiol. 2000;66:1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap–bile acids in metabolic control. Nat Revs Endocrinol. 2014;10:488–498. [DOI] [PubMed] [Google Scholar]
- 71. Kars M, Yang L, Gregor MF, et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62:4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wewalka M, Patti ME, Barbato C, Houten SM, Goldfine AB. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J Clin Endocrinol Metab. 2014;99:1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metabolism. 2013;17:225–235. [DOI] [PubMed] [Google Scholar]
- 75. Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schaap FG. Role of fibroblast growth factor 19 in the control of glucose homeostasis. Curr Opin Clin Nutr Metabol Care. 2012;15:386–391. [DOI] [PubMed] [Google Scholar]
- 78. Morton GJ, Matsen ME, Bracy DP, et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest. 2013;123:4799–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sandoval DA, D'Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015;95:513–548. [DOI] [PubMed] [Google Scholar]
- 80. Renga B, Mencarelli A, Vavassori P, Brancaleone V, Fiorucci S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta. 2010;1802:363–372. [DOI] [PubMed] [Google Scholar]
- 81. Popescu IR, Helleboid-Chapman A, Lucas A, et al. The nuclear receptor FXR is expressed in pancreatic beta-cells and protects human islets from lipotoxicity. FEBS Lett. 2010;584:2845–2851. [DOI] [PubMed] [Google Scholar]
- 82. Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582. [DOI] [PubMed] [Google Scholar]
- 83. Labbe A, Ganopolsky JG, Martoni CJ, Prakash S, Jones ML. Bacterial bile metabolising gene abundance in Crohn's, ulcerative colitis and type 2 diabetes metagenomes. PloS One. 2014;9:e115175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vrieze A, Out C, Fuentes S, et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60:824–831. [DOI] [PubMed] [Google Scholar]
- 85. Steiner C, Othman A, Saely CH, et al. Bile acid metabolites in serum: intraindividual variation and associations with coronary heart disease, metabolic syndrome and diabetes mellitus. PloS One. 2011;6:e25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Campbell JM, Fahey GC, Jr., Wolf BW. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr. 1997;127:130–136. [DOI] [PubMed] [Google Scholar]
- 87. Smiricky-Tjardes MR, Grieshop CM, Flickinger EA, Bauer LL, Fahey GC., Jr. Dietary galactooligosaccharides affect ileal and total-tract nutrient digestibility, ileal and fecal bacterial concentrations, and ileal fermentative characteristics of growing pigs. J Animal Sci. 2003;81:2535–2545. [DOI] [PubMed] [Google Scholar]
- 88. Macfarlane G, Gibson G, Beatty E, Cummings J. Estimation of short-chain fatty-acid production from protein by human intestinal bacteria based on branched-chain fatty-acid measurements. FEMS Microbiol Ecol. 1992;101:81–88. [Google Scholar]
- 89. Dai ZL, Zhang J, Wu G, Zhu WY. Utilization of amino acids by bacteria from the pig small intestine. Amino Acids. 2010;39:1201–1215. [DOI] [PubMed] [Google Scholar]
- 90. Smith EA, Macfarlane GT. Dissimilatory amino Acid metabolism in human colonic bacteria. Anaerobe. 1997;3:327–337. [DOI] [PubMed] [Google Scholar]
- 91. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. [DOI] [PubMed] [Google Scholar]
- 93. Beauvieux MC, Roumes H, Robert N, Gin H, Rigalleau V, Gallis JL. Butyrate ingestion improves hepatic glycogen storage in the re-fed rat. BMC Physiol. 2008;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio. 2014;5:e00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vital M, Penton CR, Wang Q, et al. A gene-targeted approach to investigate the intestinal butyrate-producing bacterial community. Microbiome. 2013;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. De Silva A, Bloom SR. Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver. 2012;6:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014;9:1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bala V, Rajagopal S, Kumar DP, et al. Release of GLP-1 and PYY in response to the activation of G protein-coupled bile acid receptor TGR5 is mediated by Epac/PLC-epsilon pathway and modulated by endogenous H2S. Front Physiol. 2014;5:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chambers ES, Viardot A, Psichas A, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pyra KA, Saha DC, Reimer RA. Prebiotic fiber increases hepatic acetyl CoA carboxylase phosphorylation and suppresses glucose-dependent insulinotropic polypeptide secretion more effectively when used with metformin in obese rats. J Nutr. 2012;142:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kellow NJ, Coughlan MT, Reid CM. Metabolic benefits of dietary prebiotics in human subjects: a systematic review of randomised controlled trials. Br J Nutr. 2014;111:1147–1161. [DOI] [PubMed] [Google Scholar]
- 104. Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288:25088–25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Alisi A, Bedogni G, Baviera G, et al. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Simon MC, Strassburger K, Nowotny B, et al. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose tolerant humans: a proof of concept. Diabetes Care. 2015;38:1827–1834. [DOI] [PubMed] [Google Scholar]
- 107. El-Jamal N, Erdual E, Neunlist M, et al. Glucagon-like peptide-2: broad receptor expression, limited therapeutic effect on intestinal inflammation and novel role in liver regeneration. Am J Physiol Gastro Liver Physiol. 2014;307:G274–G285. [DOI] [PubMed] [Google Scholar]
- 108. Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bomhof MR, Saha DC, Reid DT, Paul HA, Reimer RA. Combined effects of oligofructose and Bifidobacterium animalis on gut microbiota and glycemia in obese rats. Obesity. 2014;22:763–771. [DOI] [PubMed] [Google Scholar]
- 110. Geloneze B, Lima MM, Pareja JC, Barreto MR, Magro DO. Association of insulin resistance and GLP-2 secretion in obesity: a pilot study. Arq Bras Endocrinol Metabol. 2013;57:632–635. [DOI] [PubMed] [Google Scholar]
- 111. Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Stancakova A, Civelek M, Saleem NK, et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes. 2012;61:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. McCormack SE, Shaham O, McCarthy MA, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013;8:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tremblay F, Krebs M, Dombrowski L, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54:2674–2684. [DOI] [PubMed] [Google Scholar]
- 115. Xiao F, Huang Z, Li H, et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xiao F, Yu J, Guo Y, et al. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metab Clin Exp. 2014;63:841–850. [DOI] [PubMed] [Google Scholar]
- 117. Metges CC. Contribution of microbial amino acids to amino acid homeostasis of the host. J Nutr. 2000;130:1857S–1864S. [DOI] [PubMed] [Google Scholar]
- 118. Torrallardona D, Harris CI, Coates ME, Fuller MF. Microbial amino acid synthesis and utilization in rats: incorporation of 15N from 15NH4Cl into lysine in the tissues of germ-free and conventional rats. Br J Nutr. 1996;76:689–700. [DOI] [PubMed] [Google Scholar]
- 119. Torrallardona D, Harris CI, Fuller MF. Pigs' gastrointestinal microflora provide them with essential amino acids. J Nutr. 2003;133:1127–1131. [DOI] [PubMed] [Google Scholar]
- 120. Metges CC, Petzke KJ, El-Khoury AE, et al. Incorporation of urea and ammonia nitrogen into ileal and fecal microbial proteins and plasma free amino acids in normal men and ileostomates. Am J Clin Nutr. 1999;70:1046–1058. [DOI] [PubMed] [Google Scholar]
- 121. Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Davis CP. Normal flora. In: Baron S, ed. Medical Microbiology. 4th ed Galveston, TX: University of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]