Abstract
Considering the ability of atmospheric-pressure cold plasma (ACP) to disrupt the biofilm matrix and rupture cell structure, it can be an efficient tool against virulent oral biofilms. However, it is fundamental that ACP does not cause damage to oral tissue. So, this study evaluated (1) the antimicrobial effect of ACP on single- and dual-species biofilms of Candida albicans and Staphylococcus aureus as well as (2) the biological safety of ACP on in vitro reconstituted oral epithelium. Standardized cell suspensions of each microorganism were prepared for biofilm culture on acrylic resin discs at 37°C for 48 hours. The biofilms were submitted to ACP treatment at 10 mm of plasma tip-to-sample distance during 60 seconds. Positive controls were penicillin G and fluconazole for S. aureus and C. albicans, respectively. The biofilms were analyzed through counting of viable colonies, confocal laser scanning microscopy, scanning electron microscopy and fluorescence microscopy for detection of reactive oxygen species. The in vitro reconstituted oral epithelium was submitted to similar ACP treatment and analyzed through histology, cytotoxocity test (LDH release), viability test (MTT assay) and imunnohistochemistry (Ki67 expression). All plasma-treated biofilms presented significant log10 CFU/mL reduction, alteration in microorganism/biofilm morphology, and reduced viability in comparison to negative and positive controls. In addition, fluorescence microscopy revealed presence of reactive oxygen species in all plasma-treated biofilms. Low cytotoxicity and high viability were observed in oral epithelium of negative control and plasma group. Histology showed neither sign of necrosis nor significant alteration in plasma-treated epithelium. Ki67-positive cells revealed maintenance of cell proliferation in plasma-treated epithelium. Atmospheric-pressure cold plasma is a promissing approach to eliminate single- and dual-species biofilms of C. albicans and S. aureus without having toxic effects in oral epithelium.
Introduction
Several studies have reported colonization of tooth, prostheses, restorative materials and implants by yeast and bacterial biofilms causing several related diseases [1]. Oral biofilm has also been associated with occurance of aspirative pneumonia, endocarditis and orther systemic disorders [2]. Biofilms represent an aggregation of microbes embedded within a protective carbohydrate matrix, which allows adhesion to a host surface, stabilization of biofilm architecture, maintenance of nutrients, and protection against antimicrobial agents and host immune response [3, 4]. In this sense, Candida albicans is a frequent fungal biofilm-forming pathogen that causes life-threatening infections by colonizing medical and dental devices (i.e. prostheses, implants and catheters) [5, 6]. Dimorphism is an important characteristic experienced by C. albicans in response to adverse environmental conditions, which actually increases its virulence. Furthermore, coaggregation between C. albicans and oral bacteria has been reported as an important factor in microbial colonization and progression of infections in oral cavity [7]. Accordingly, Staphylococcus aureus is an oral bacteria that may cause local infections (e.g. endodontic problem, mucositis, angular cheilits, and parotitis) [8] and systemic diseases (e.g. endocarditis, pneumonia, eczema, abscesses, and septicemia) [9]. Adhesion of S. aureus to C. albicans hyphae has been also demonstrated [10], which increases its virulence and ability to tissue invasion. A previous study showed that dentures are commonly colonised by an association of yeast and bacteria, including C. albicans and S. aureus, even in healthy subjects [11]. Literature has also suggested that these pathogens can be commonly associated as co-infector microorganisms and the clinical outcome results are increased frequency and severity of the disease [10, 12, 13]. Thus, the dual-species biofilm of C. albicans and S. aureus should be further investigated due to its high pathogenicity [14] and resistance to antimicrobial agents [13, 15].
Current treatment of biofilm-related diseases involves mechanical removal of biofilm and the use of antiseptics and antibiotics. Nevertheless, considering that mechanical removal could be ineffective on biofilm inactivation, and that pathogenic microorganisms may develop resistance to antimicrobial agents, alternative therapies are required. In this sense, atmospheric-pressure cold plasma (ACP) is a promising tool against C. albicans [6, 16], S. aureus [17–19], and other microorganisms [20–22] that could be used for clinical treatment of biofilm-related oral diseases. The cold plasma is generated by gas ionization at atmospheric pressure and low temperature; producing reactive oxygen species (ROS), reactive nitrogen species (RNS), UV radiation, ions, electrons, excited molecules, and electromagnetic field [18, 23, 24]. Although plasma effect on microorganism is not fully understood, it has been demonstrated that the reactive species cause cell damage and/or death through dehydration, membrane lysis [19], and damage to DNA, lipids and proteins [25, 26]. It is also noteworthy that plasma plume can reach sites inaccessible by other methods (i.e. deep and interproximal caries and periodontal pockets), so it could be more effective for biofilm decontamination than conventional therapies.
Considering that plasma is usually produced by low-toxicity gases and its activity involves a mixture of products that decay within a few seconds, this approach has been suggested as environmentally friendly with no harmful residues [17]. Thus, production of stable plasma at atmospheric pressure has attracted attention for treating living human cells and tissues without thermal damage [27]. However, studies of the biological safety of plasma are limited [28, 29], particularly on oral mucosa [22]. Given that a novel treatment for biofilm-related oral diseases should not cause irreversible damage to the host cells, further studies are required to evaluate the biocompatibility of plasma on oral tissues.
The aim of this study was to evaluate (1) the antimicrobial efficacy of ACP on single- and dual-species biofilms of C. albicans and S. aureus, and (2) the biological safety of ACP on in vitro reconstituted oral epithelium.
Materials and Methods
Atmospheric-pressure cold plasma (ACP)
The ACP was generated through ionization of argon gas (Ar) at atmospheric pressure using the device Kinpen (INP, Germany) developed by Leibniz Institute for Plasma Science and Technology (INP) [30]. The device consists of a hand-held unit (170 mm in length, 20 mm in diameter and 170 g) for generation of plasma jet, a dc power supply (system power: 8 W at 220 V, 50/60 Hz), and a gas supply unit. The atmospheric-pressure plasma jet was generated from the top of the centered electrode and expanded to the surrounding air outside the nozzle [6]. The treatment of biofilm and oral epithelium samples was conducted at plasma tip-to-sample distance of 10 mm during 60 seconds in continuous working mode [30]. The samples were moved horizontally during plasma application for scanning of the overall surface. The plasma tip-to-sample distance was maintained using an apparatus developed to fix plasma positioning during treatment. The experiment was conducted by a calibrated operator (JAD) that was trained to perform the experiment in a standardized manner in order to minimize bias. The argon gas flow was set to 5 slm and the flow rate was controlled by a flow controller (MKS Instruments, Germany).
Single- and dual-species biofilms
Strains of C. albicans (ATCC SC5314) and S. aureus (ATCC 25923) were used for in vitro biofilm culture [12]. Stock cultures were maintained at—80°C, streaked onto specific medium (Sabouraud Dextrose Agar with chloramphenicol for C. albicans and Mannitol Salt Agar for S. aureus) and incubated at 37°C for 48 hours. Each microorganism culture was transferred to 10 mL of specific medium (Yeast Nitrogen Base—YNB with 100 mM of glucose for C. albicans and Tryptic Soy Broth—TSB for S. aureus) and incubated at 37°C for 18 hours. Cells of each microorganism culture were harvested, washed twice with 10 mL of phosphate-buffered saline (PBS—10 mM PO43-, 137 mM NaCl, 2.7 mM KCl) at 4000 rpm for 5 minutes, resuspended in 10 mL of RPMI medium (Roswell Park Memorial Institute) (RPMI-1640, Sigma-Aldrich Co., USA), and standardized to 1 X 107 cells/mL. For single-specie biofilm (C. albicans or S. aureus), 1 mL of the standardized cell suspension was added to 1 mL of RPMI medium, while for dual-species biofilm (C. albicans + S. aureus), 1.0 mL of the standardized cell suspension of each microorganism were mixed.
The biofilms were cultured on heat-polymerized acrylic resin discs (Vipi Wave, VIPI Ind. e Com. Exp. e Imp. de Produtos Odontológicos Ltda., Brazil) with 10 mm in diameter and 2 mm in thickness. The surface roughness of the discs was standardized to Ra = 3.0 μm [31] using a specific matrix. The discs were sterilized using microwave energy for 3 minutes at 650W in sterile ultrapure water [32].
Initially, the samples were incubated in the inoculum for 90 minutes [33] at 37°C in an orbital shaker. Then, non-adherent cells were removed by gently washing twice with 2 mL of PBS, and 2 mL of fresh RPMI medium was added to each sample. After 24 hours of incubation at 37°C in an orbital shaker, 1 mL of the RPMI medium was removed and equal volume of fresh medium was added for additional 24 hours of incubation under similar conditions [34]. After 48-hour incubation [12], a mature biofilm was formed so each disc was transferred to a new well and the biofilm was gently washed with 2 mL of PBS.
Experimental samples were submitted to ACP treatment, whereas negative control had no treatment. For positive control, C. albicans and S. aureus strains were exposed to fluconazole (80mg/L) and penicillin G (100mg/L) for 30 minutes at 37°C, respectively.
In vitro reconstituted oral epithelium (ROE)
The in vitro reconstituted oral epithelium EpiOralTM (ORL-200, MatTek Corporation, USA) was used in this study. The EpiOralTM model is based on primary oral keratinocytes cultured on a collagen-based matrix [35].
Upon receipt, the cell culture inserts were maintained in pre-warmed MatTek assay medium into a humidified 37°C, 5% CO2 incubator for 1 hour to recover from stress of shipping. Following 1 hour incubation, medium was aspirated and replaced by pre-warmed fresh assay medium.
Similar to biofilm samples, experimental tissues were submitted to ACP treatment and the negative control had no treatment. Experimental and negative tissues were maintained into a humidified 37°C and 5% CO2 incubator for 24 hours after the experiments. Positive control tissues were incubated in similar conditions, except that 400 μL of 1% Triton X-100 solution (MatTek Corporation, USA) was added atop the tissue samples at 20 hours of incubation.
Biofilm analyses
For counting of viable colony forming units (CFU), the biofilm was removed from the surface of the resin discs using sterilized cotton swab, which was subsequently transferred to a 1.5 mL centrifuge tube with 1 mL of PBS. After vortexing, serially diluted aliquots were plated to determine the number of CFU/mL of each microorganism after 24 hours of incubation at 37°C. C. albicans colonies were cultured in Sabouraud Dextrose Agar supplemented with chloramfenicol (0.1 mg/mL) [12] while S. aureus colonies were cultured in Brain Heart Infusion Agar supplemented with amphotericin B (0.025 mg/mL) [13]. The experiment was conducted in triplicate in three independent occasions. Data were analyzed by ANOVA and Tukey’s test (p<0.05).
The biofilm was also stained using the Live/Dead BacLight Viability kit (Invitrogen-Molecular Probes, USA) to reveal the proportion of live or active cells (fluorescent green) and dead or inactive cells (fluorescent red) [30]. The live/dead stain was prepared by diluting 1.5 μL of staining component A (SYTO 9) and 1.5 μL of staining component B (propidium iodide) in 1.0 mL of sterile 1% phosphate buffered solution (PBS–pH7.4) (Invitrogen, USA). Each sample was covered with 1 mL of the reagent mixture and incubated for 20 minutes at room temperature protected from light exposure. A series of images were obtained in the z section using a Leica TCS SP5 II confocal microscope (Leica, Germany) with the objective Leica HCX APO L 40x/0.8 W U-V-I water dipping lens (Leica, Germany). Five representative optical fields were examined for each specimen. The experiment was conducted in duplicate in two independent occasions.
For scanning electron microscopy (SEM), discs containing biofilm were fixed with 4% paraformaldehyde for 1 hour and then dehydrated in increasing ethanol concentrations (70%, 85%, and 100%) for 5 minutes in each solution. After dehydration, the samples were placed in a desiccator for 24 hours before gold sputter coating. The images were captured using the SEM Hitachi S-3500N (Hitachi High Technologies America, USA) under 8 mm working distance and 10–15 kV. The experiment was conducted in duplicate in three independent occasions.
The presence of intracellular reactive oxygen species (ROS) in plasma-treated biofilms was observed using the fluorescent probe dihydrorhodamine 123 (DHR 123) (Invitrogen-Molecular Probes, USA) [36–38]. This product is an uncharged and nonfluorescent ROS indicator that can passively diffuse across membranes where it is oxidized to cationic rhodamine 123, which exhibits green fluorescence. The biofilms were incubated with 15 μM DHR 30 minutes before ACP treatment. Fluorescent pictures were captured in the stereoscopic microscope Nikon SMZ1500 (Nikon Corporation, Japan) using 1.6 × lens and 5.0 × zoom with the microscope imaging software NIS-Elements Br (Nikon Corporation, Japan).
ROE analyses
The CytoTox-ONETM Homogeneous Integrity Assay kit (Promega, USA) was used as a fluorometric method to estimate cell cytotoxicity based on the release of lactate dehydrogenase (LDH) from cells with damaged membrane [35, 39]. LDH release into the culture medium was measured with this enzymatic assay that results in the conversion of resazurin into resorufin. After 24-hour incubation, the culture medium of each sample was collected, transferred to 96-well plate (100 μL of culture medium per well, totalizing 6 measurements for each sample) and cooled to room temperature. Then, the kit reagent was added to each well (100 μL per well) and incubated for 10 minutes. After addition of stop solution (50 μL per well), fluorescent signal was measured in a microplate reader (SpectraMax M5 Multi-Mode microplate reader, Molecular Devices, USA) with excitation wavelength of 560 nm and emission wavelength of 590 nm. The experiment was conducted in duplicates in three independent occasions. Percent cytotoxicity was determined according to the following calculation:
| (1) |
The MatTek MTT toxicology kit (MTT-100, MatTek Corporation, USA) was used as a colorimetric assay to check tissue viability [40]. According to this method, the NAD(P)H-dependent cellular oxidoreductase enzymes present in viable cells are capable of reducing the tetrazolium dye MTT to its insoluble formazan, which has a purple color. The tissue inserts were gently rinsed twice with PBS and placed in 24-well plate containing 300 μL of MTT solution (MTT concentrate + MTT diluent) per well. After 3 hours of incubation at humidified 37°C and 5% CO2, the tissue inserts were transferred to another 24-well plate and immersed into 2 mL of the extractant solution per well. After 2 hours of extraction under agitation protected from light, the extractant solution was added into a 96-well plate (200 μL per well, totalizing 6 measurements for each sample) to determine the optical density at 570 nm in a microplate reader (SpectraMax M5 Multi-Mode microplate reader, Molecular Devices, USA). The extractant solution was used as a blank. The experiment was conducted in duplicates in three independent occasions. Percent viability was determined according to the following calculation:
| (2) |
Data about cytotoxity and viability were analyzed by ANOVA test with Dunnett’s or Tukey’s post-hoc for multiple comparison. p<0.05 was considered significance.
Histological analysis was conducted in order to study the microscopic anatomy of the cells and tissue architecture. For histological analysis [35], the samples were immersed in 10% formalin, washed with PBS and dehydrated with 50% and 70% ethanol. Samples were then prepared for paraffin embedding, cutting and hematoxylin and eosin staining. The slides were scanned using the digital slide scanner ScanScope CS2 (Aperio, USA) at 20X. The experiment was conducted in duplicate in two independent occasions.
To identify actively proliferating cells, immunohistochemistry was performed on 4 μm formalin fixed, paraffin embedded samples using a cell proliferation marker (rabbit anti-human Ki67 clone SP6) (Thermo Scientific, USA) [35]. The sections were deparaffinized in xylene, rehydrated through graded alcohols (100% and 95% ethanol) and rinsed in distilled water. Heat induced epitope retrieval was performed in a 1200-Watt microwave oven at 100% power in 10 mM sodium citrate buffer, pH 6 for 20 and 10 minutes, respectively. Sections were allowed to cool for 30 minutes and then rinsed in distilled water. Antibody incubation and detection were carried out at 40°C on a Discovery instrument (Ventana Medical Systems, USA) using Ventana’s reagent buffer and detection kits. Endogenous peroxidase activity was blocked with hydrogen peroxide. Antibody was diluted (1:400) in Dulbecco’s Phosphate Buffered Saline, (Life Technologies, USA). Samples were incubated overnight at room temperature. Antibody was detected with biotinylated goat anti-rabbit diluted 1:200 (Vector Laboratories, USA) for 30 minutes. This was followed by application of streptavidin-horseradish-peroxidase conjugate. The complex was visualized with 3,3 diaminobenzidene and enhanced with copper sulfate. Slides were washed in distilled water, counterstained with hematoxylin, dehydrated and mounted with permanent media. The experiment was conducted in duplicate in two independent occasions.
Results
Biofilms
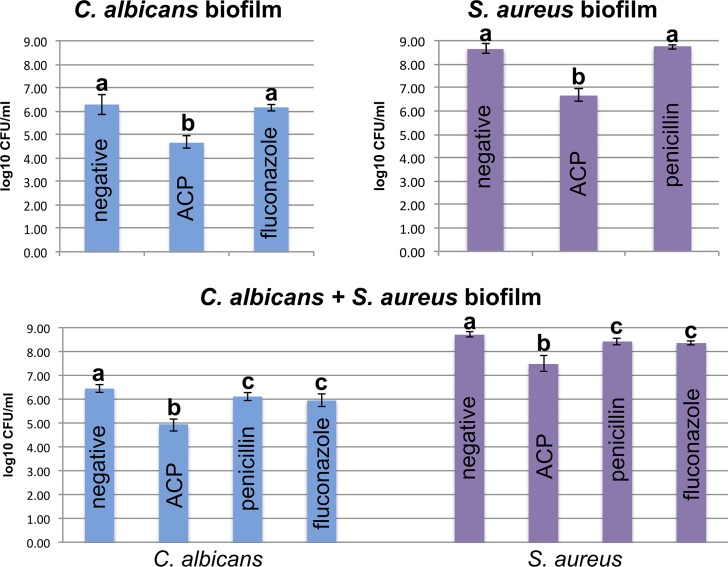
Fig 1 shows the results of log10 CFU/mL of C. albicans and S. aureus for single and dual-species biofilms. It was observed a significant log10 CFU/mL reduction in all plasma-treated samples in comparison to the negative and positive controls. For single-specie biofilms, plasma group showed lower log10 CFU/mL (C. albicans: 4.68 ± 0.25 / S. aureus: 6.68 ± 0.29) than negative (C. albicans: 6.27 ± 0.42 / S. aureus: 8.67 ± 0.21) (p<0.05) and positive (C. albicans: 6.14 ± 0.12 / S. aureus: 8.74 ± 0.10) (p<0.05) controls. However, there was no significant difference in log10 CFU/mL between the negative and positive control samples (p>0.05). In dual-species biofilm, for both C. albicans and S. aureus strains, plasma group showed lower log10 CFU/mL (C. albicans: 4.92 ± 0.25 / S. aureus: 7.48 ± 0.33) than negative (C. albicans: 6.44 ± 0.16 / S. aureus: 8.71 ± 0.12) (p<0.05) and positive (C. albicans—fluconazole: 5.94 ± 0.25 / C. albicans—penicillin: 6.10 ± 0.18 / S. aureus—fluconazole: 8.35 ± 0.08 / S. aureus—penicillin: 8.42 ± 0.14) (p<0.05) controls. Positive controls also showed lower log10 CFU/mL than negative control (p<0.05). However, there was no significant difference (p>0.05) between positive controls with fluconazole and penicillin (Fig 1).
Fig 1. Log10 CFU/mL for both C. albicans and S. aureus strains in single- and dual-species biofilms.
Individual statistical analyses were conducted only for comparison between the negative control group, ACP group and positive control group (i.e. penicillin for S. aureus and fluconazole for C. albicans) of the same strain in each biofilm. * different letters denote statistically significant difference (p<0.05) only between groups of the same strain in each graph.
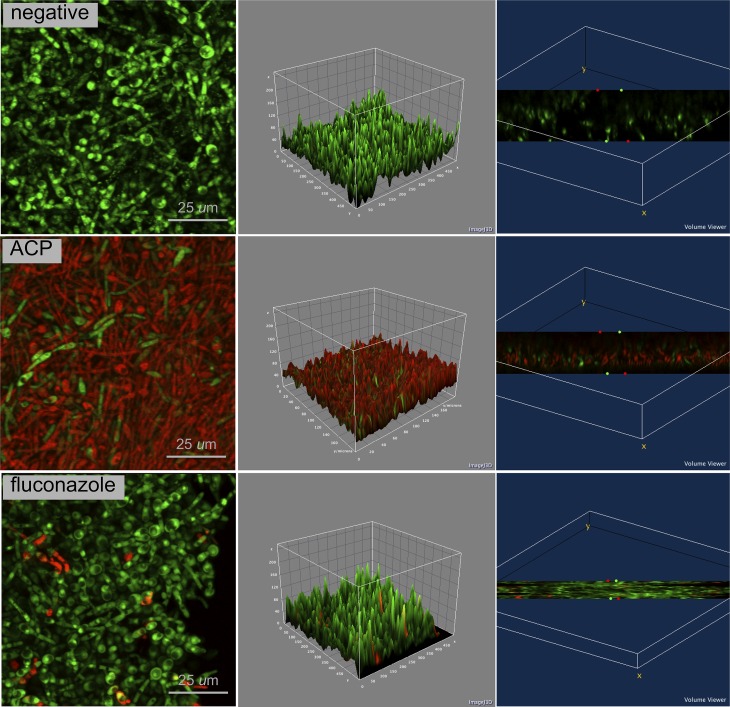
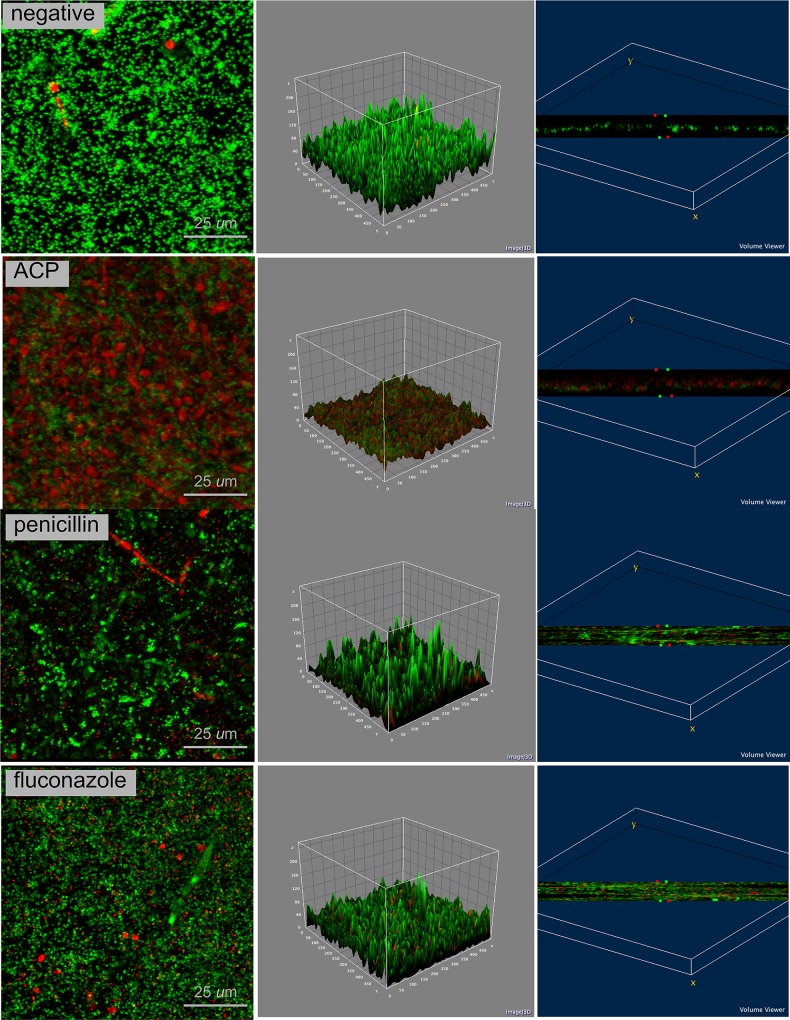
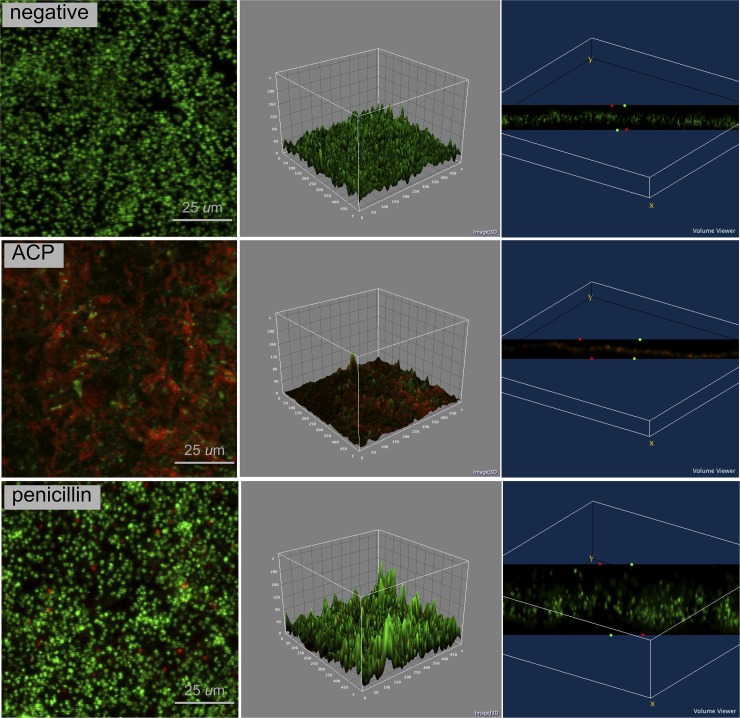
Confocal analysis (Figs 2–4) clearly demonstrates that plasma-treated samples showed higher amount of dead biofilm than the negative and positive control groups. As displayed by the 3D volume images, dead cells were seen even at bottom layer of samples treated with plasma. After plasma treatment, images suggest higher survival of S. aureus when this microorganism was grown in a dual-species biofilm model compared with the single-specie biofim.
Fig 2. Confocal laser scanning microscopy of C. albicans biofilm in negative control group, ACP group and positive control group (fluconazole); 4 × zoom.
Biofilm stained with Live/Dead BacLight Viability kit. Live cells in fluorescent green and dead cells in fluorescent red. 2D view (left side image), surface plot of 3D volume image (center image), and cross-section of 3D volume image (right side image) show the distribution of live and dead cells throughout biofilm layers.
Fig 4. Confocal laser scanning microscopy of C. albicans + S. aureus biofilm in negative control group, ACP group and positive control groups (fluconazole and penicillin); 4 × zoom.
Biofilm stained with Live/Dead BacLight Viability kit. Live cells in fluorescent green and dead cells in fluorescent red. 2D view (left side image), surface plot of 3D volume image (center image), and cross-section of 3D volume image (right side image) show the distribution of live and dead cells throughout biofilm layers.
Fig 3. Confocal laser scanning microscopy of S. aureus biofilm in negative control group, ACP group and positive control group (penicillin); 4 × zoom.
Biofilm stained with Live/Dead BacLight Viability kit. Live cells in fluorescent green and dead cells in fluorescent red. 2D view (left side image), surface plot of 3D volume image (center image), and cross-section of 3D volume image (right side image) show the distribution of live and dead cells throughout biofilm layers.
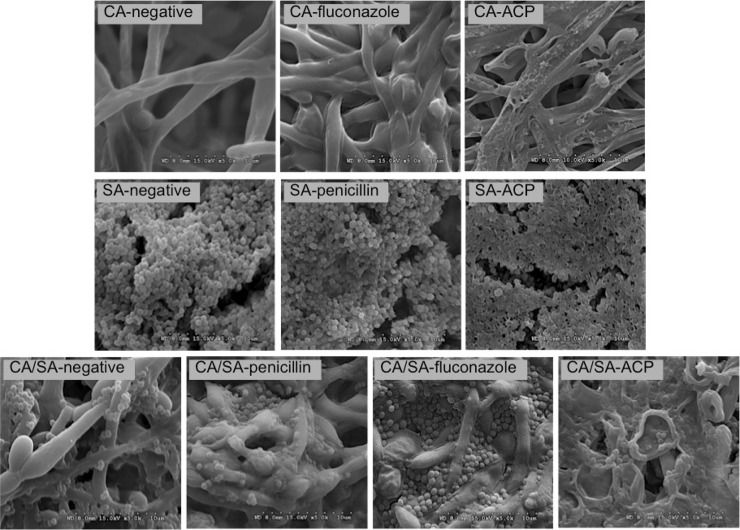
All plasma-treated biofilms showed alteration in the morphological structure of the microorganisms/biofilm in comparison to the negative and positive controls. SEM micrographs (Fig 5) revealed massive perforation of C. albicans and S. aureus cell wall and membrane after plasma treatment. As a consequence, plasma-treated biofilms seemed flatten, representing damage to biofilm architecture. In contrast, control samples looked as undamaged. In this sense, it was also observed adhesion of S. aureus to C. albicans hyphae in dual-specie biofilm, indicating some collaborative process between both microorganisms for host tissue invasion. Interestingly, it was found that some S. aureus cells remained preserved within C. albicans scaffold in the dual-species biofilm.
Fig 5. SEM micrographs of single and dual-species biofilms in negative control group, ACP group and positive control groups (fluconazole and penicillin); 5000 × zoom.
C. albicans (CA), S. aureus (SA) and C. albicans + S. aureus (CA/SA) biofilms. Note rupture of cells architecture in all biofilms after ACP treatment (groups CA-ACP, SA-ACP and CA/SA-ACP).
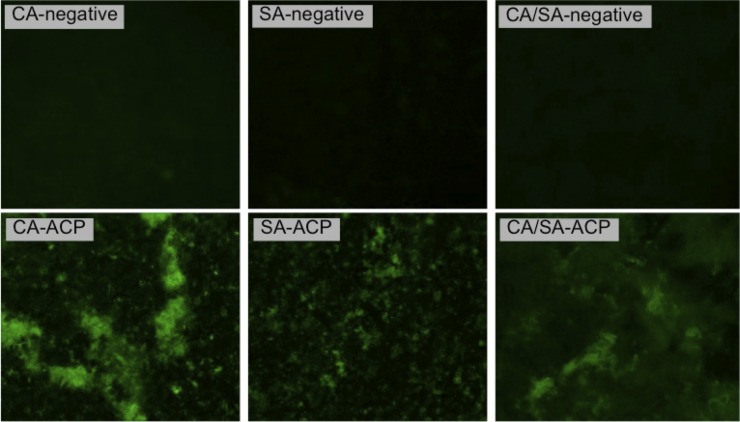
Fig 6 demonstrates presence of ROS exclusively in plasma-treated biofilms, as shown by the fluorescent green (oxidized rhodamine 123). The detection of ROS in plasma-treated biofilms suggested some antimicrobial pathway of the atmospheric-pressure cold plasma used in this study.
Fig 6. Fluorescence microscopy images of single and dual-species biofilms in negative control group and ACP group; 1.6 × lens and 5.0 × zoom.
Fluorescent green (oxidized rhodamine 123) represents presence of intracellular ROS. CA—C. albicans biofilm, SA—S. aureus biofilm, and CA/SA—C. albicans + S. aureus biofilm.
In vitro reconstituted oral epithelium (ROE)
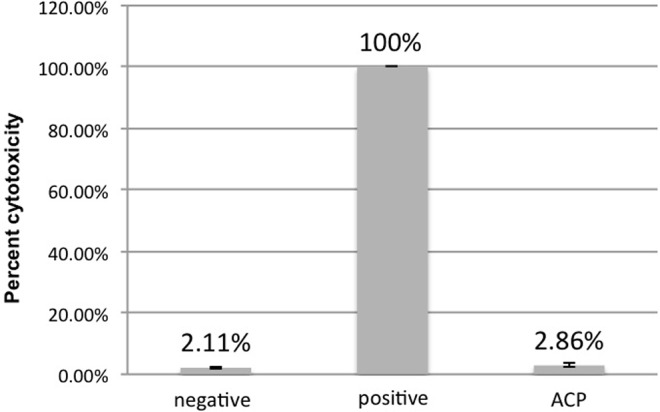
The measurement of LDH release demonstrated lower percent cytotoxicity for negative control (2.11% ± 0.26) and plasma group (2.86% ± 0.56) in comparison to the positive control (100% ± 0.00). Considering the positive control as a reference for cytotoxicity calculation, the post-hoc Dunnett’s test showed significant difference between positive control and plasma group (p<0.05) as well as between positive and negative controls (p<0.05). In addition, Tukey’s test showed no significant difference between negative control and plasma group (p = 0.094) (Fig 7). The similarity between the results of the negative control and plasma samples indicates a non-cytotoxic effect of plasma treatment on oral epithelium within the dosage applied in this study.
Fig 7. Mean (± SD) percent cytotoxicity values of ROE for negative control group, positive control group and ACP group.
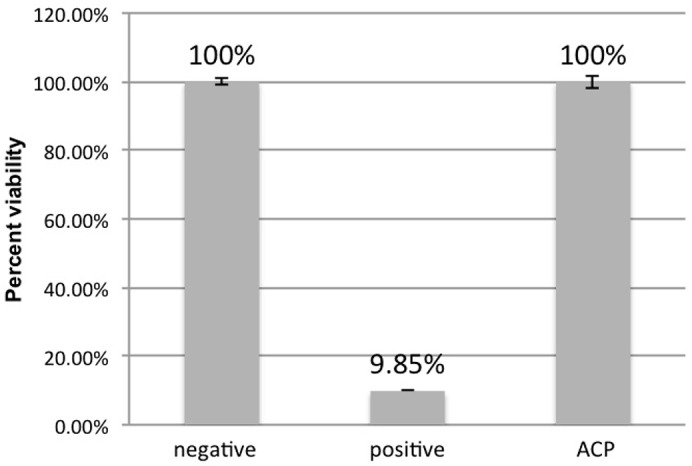
The MTT assay revealed high percent viability for both negative control (100% ± 0.00) and plasma group (100% ± 1.85) in comparison to the positive control (9.85% ± 0.84). Considering the negative control as a reference for viability calculation, the post-hoc Dunnett’s test showed no significant difference between negative control and plasma group (p = 0.596). However it was found a statistically significant difference (p<0.05) between positive and negative controls (Fig 8). The similarity between the results of the negative control and plasma samples corroborates with the cytotoxicity results.
Fig 8. Mean (± SD) percent viability values of ROE for negative control group, positive control group and ACP group.
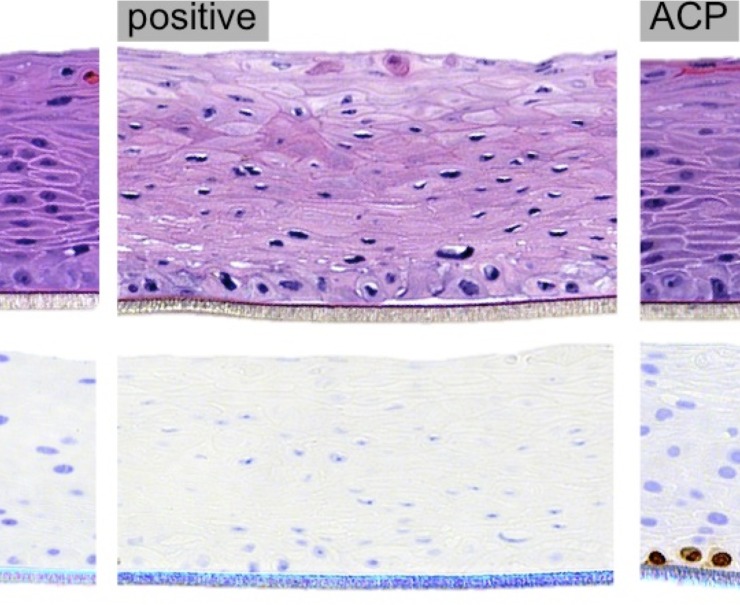
Histological analysis showed minor epithelium alteration in plasma samples with slight keratinization at the top layer in comparison to the negative control. In contrast, positive control exhibited significant tissue damage with cell vacuolization and nuclear shrinkage (i.e. pyknosis). Furthermore, Ki67-positive cells were found in the basal layer of plasma samples, representing actively proliferating cells, analogous to the normal epithelium of the negative control group. In contrast, positive control showed no Ki67 expression (Fig 9).
Fig 9. Oral epithelium sections of negative control group, positive control group and ACP group; 20 × zoom.
Sections stained by hematoxylin and eosin for histology (top) and immunohistochemistry for Ki67 (bottom).
Discussion
Cold plasma has emerged as a physical treatment with microbicidal effectiveness on bacteria, parasites, fungi, spores, and viruses [41]. In the present study, log10 CFU data (Fig 1) and confocal laser scanning microscopy (Figs 2–4) confirmed the antimicrobial effect of ACP since all plasma-treated samples exhibited significant lower viability than positive and negative controls. In addition, reactive oxygen species were found in all plasma-treated samples (Fig 6).
It has been suggested that the reactive oxygen species (i.e. ozone, atomic oxygen, superoxide, peroxide, hydroxyl radical, and nitric oxide) produced by plasma generate oxidative effects in cellular biomacromolecules including DNA, lipids and proteins [17]. As a consequence, oxidative stress causes lipid peroxidation and oxidation of several amino acids of proteins, which compromises the function and integrity of membrane and cell wall [6, 20]. In addition, membrane desestabilization affects the ability to maintain proper intracellular pH [4] and releases cellular contents in the surrounding medium [28]. It was also suggested that plasma species break down hydrogen, sulphide and peptide bonds of the proteins; leading to changes in protein structure and dramatically decrease of enzyme activity [42]. On the other hand, low doses of oxidative stress have been demonstrated to favor biofilm formation of some strains so that treatment time and dose must be carefully controlled [42].
The assumption about membrane damage caused by plasma was confirmed by SEM images (Fig 5), revealing cell rupture. Membrane perforation was previously reported since plasma reactive particles produce a general mechanical effect on the surface of living organisms called ‘etching’ [25]. Etching results from the reaction of highly reactive radicals with organic materials, generating by-products that are desorbed from the surface, causing membrane perforation [18]. In addition, charged particles generated by plasma may lead to membrane lysis when electrostatic force overcomes the tensile strength of the membrane [23].
The inactivation of biological agents promoted by ACP may also result from deconstruction of the microorganism genetic material (DNA) by UV radiation produced with plasma and erosion of the microorganisms through intrinsic photodesorption. The photon-induced desorption results from damage of chemical bonds in the microorganism after being exposed to UV radiation, allowing its atoms to form volatile compounds [25, 43]. However, the role of UV radiation in atmospheric-pressure plasma sterilization remains controversial [23]. According to some authors [43], even when no significant UV emission is present with low-temperature atmospheric-pressure plasma, the synergy of other species such as radicals and charged particles still plays a dominant role for sterilization.
In contrast to conventional therapy, literature suggests that a great benefit on using ACP is that antimicrobial resistance is less likely to occur because of its multiple modes of action and diversity of active agents [17, 42]. While strongly charged or chemically reactive agents in conventional therapy fail to reach the biofilm due to the negatively charged extracellular polymeric matrix, which acts as an ion-exchange barrier, some small molecules of plasma are able to travel into the biofilm by channels with varying diameters [19]. Interestingly, it was shown that pre-treatment with attenuated gas plasma restored antibiotic sensitivity of methicillin-resistant S. aureus (MRSA) [44]. In contrast, the results of positive control groups for both species showed that the drugs were not strongly effective to reduce biofilm viability probably because only a single dose was applied and not enough to inactivate the pathogens.
The results about reconstituted oral epithelium showed no sign of significant damage after ACP application, as demonstrated by the low cytotoxicity (Fig 7) and high viability (Fig 8) levels in plasma-treated samples. Only few studies have been conducted on the effect of ACP on eukaryotic cells [28]. In this sense, some authors [22] evaluated the effect of ACP treatment on buccal and tongue mucosa of rabbits. Even though the treatment was conducted for a much longer period (10 minutes) than that from the present study (one minute), the histological analysis showed no hyperemia, swelling, ulcer or anabrosis. Furthermore, the authors concluded that ACP might offer such advantages over the traditional methods as high bacterial killing efficiency, time saving and less toxic by-products produced. Similarly, the histological analysis in our study showed no sign of necrosis or significant alteration in oral tissue after ACP application (Fig 9). A previous study also reported no morphological changes or signs of necrosis or apoptosis while treating bacterial loads on the pig skin with cold plasma [29]. In another investigation [27], a 2- to 5-minute cold plasma treatment substantially inactivated ocular pathogens without causing significant tissue and DNA damage. Together with our results, these findings support future application of ACP for disinfection of living tissue.
Considering that both C. albicans and ROE cells are eukaryotic, a question remains about plasma mechanism that inactivated C. albicans without causing tissue damage at the same dosage. It was previously suggested that killing of C. albicans by neutrophils is driven synergistically by combinatorial effects of oxidative plus cationic stress. In this sense, although C. albicans activates signaling pathways for detoxification of the oxidative stress and repair of damage caused by ROS, cations inhibit catalase enzyme activity; which leads to the hyper accumulation of intracellular ROS [45]. Thus, it can be indicated that plasma effect against C. albicans is driven by a combination of oxidative stress and cation fluxes as a result of reactive species and charged particles, similarly to neutrophils pathway. However, it has been indicated that the effect of ROS in mammalian cells is dose-dependent, ranging from cell proliferation to cytotoxic damage with apoptosis [46]. Furthermore, at least 40 genes products are involved in the adaptive response of mammalian cells to oxidative stress through antioxidant defense, damage removal or repair enzymes [47]. As an early defensive response, mitotic mammalian cells also enter into a transient growth-arrested state to protect DNA from injuries caused by oxidative stress [47].
The absence of harmful effect observed on ROE can be also explained by the following mechanisms: 1- the lifetime of the atoms is very short (only several milliseconds) and fail to cause injury to the normal tissue, 2—the role of the UV is weak and it cannot damage mucosa in such a short period of exposure time, 3—mammalian cells are protected from ROS by their different cell metabolism and better resistance mechanisms to external stress, and 4—prokaryotic cells have a much higher surface to volume ratio so that a lower plasma dose is required for inactivation [22, 48].
The immunohistochemistry revealed Ki67-positive cells in the plasma-treated samples (Fig 9), which indicates maintenance of actively cell proliferation in oral epithelium after ACP application. Considering that Ki67-positive cells were present at basal layer, it can be suggested that plasma was not able to achieve deeper tissue structure. Keratinized epithelium may also have functioned a defense barrier against plasma reactive species. In addition, cold plasma can produce different effects in mammalian cells that range from cell proliferation to apoptosis, depending on plasma dose [49]. In this sense, sub-lethal doses of plasma have been used to induce apoptosis in cancer cells but not necrosis [50]. A previous study also demonstrated that ACP was effectively employed in the treatment of chronic skin and wound infections by simultaneously killing bacteria and promoting wound healing [42].
It is also noteworthy that the relatively low temperature is a critical feature of ACP system for in vivo applications since the majority of the electrical energy used to electrify the gaseous environment goes into the production of energetic electrons, instead of heating the surrounding gas [4]. So, ACP can be used on sensitive surfaces without causing heating, electric shock or pain [42]. However, although the literature confirms that ACP has several beneficial applications in medicine, there have not been sufficient studies to comprehensively assess the potential side effects of the treatment.
As a limitation, even in well-defined plasma-generating conditions it is difficult to characterize plasma and resulting active species for each individual reaction. So, further understanding and standardization are required to control microbial response and avoid possible cytotoxic effects [42]. In addition, although no visibly damage was observed in oral epithelium, direct plasma effects on animal tissues and immune cells should be further investigated [18].
Acknowledgments
The authors would like to acknowledge the Experimental Pathology Immunohistochemistry Core Laboratory at Langone Medical Center—NYU.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grant 2012/20699-6—Sao Paulo Research Foundation (http://www.fapesp.br/en/) (JAD), grant 2013/21388-7—Sao Paulo Research Foundation (http://www.fapesp.br/en/) (JAD), and grant 2012 GSK/IADR Innovation in Oral Care Award (http://www.iadr.com/i4a/pages/index.cfm?pageid=3426#.Vfcgnc6hsfo) (SD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm formation on dental restorative and implant materials. J Dent Res. 2010;89(7): 657–665. 10.1177/0022034510368644 [DOI] [PubMed] [Google Scholar]
- 2.Parahitiyawa NB, Jin LJ, Leung WK, Yam WC, Samaranayake LP. Microbiology of odontogenic bacteremia: beyond endocarditis. Clin Microbiol Rev. 2009;22(1): 46–64. 10.1128/CMR.00028-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black CE, Costerton JW. Current concepts regarding the effect of wound microbial ecology and biofilms on wound healing. Surg Clin North Am. 2010;90(6): 1147–1160. 10.1016/j.suc.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 4.Ferrell JR, Shen F, Grey SF, Woolverton CJ. Pulse-based non-thermal plasma (NTP) disrupts the structural characteristics of bacterial biofilms. Biofouling. 2013;29(5): 585–599. 10.1080/08927014.2013.795554 [DOI] [PubMed] [Google Scholar]
- 5.Fricke K, Koban I, Tresp H, Jablonowski L, Schroder K, Kramer A, et al. Atmospheric pressure plasma: a high-performance tool for the efficient removal of biofilms. PLoS One. 2012;7(8): e42539 10.1371/journal.pone.0042539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koban I, Matthes R, Hubner NO, Welk A, Meisel P, Holtfreter B, et al. Treatment of Candida albicans biofilms with low-temperature plasma induced by dielectric barrier discharge and atmospheric pressure plasma jet. New J Phys. 2010;12: 073039. [Google Scholar]
- 7.Pereira-Cenci T, Del Bel Cury AA, Crielaard W, Ten Cate JM. Development of Candida-associated denture stomatitis: new insights. J Appl Oral Sci. 2008;16(2): 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith AJ, Jackson MS, Bagg J. The ecology of Staphylococcus species in the oral cavity. J Med Microbiol. 2001;50(11): 940–946. [DOI] [PubMed] [Google Scholar]
- 9.Glass RT, Bullard JW, Hadley CS, Mix EW, Conrad RS. Partial spectrum of microorganisms found in dentures and possible disease implications. J Am Osteopath Assoc. 2001;101(2): 92–94. [PubMed] [Google Scholar]
- 10.Peters BM, Jabra-Rizk MA, Scheper MA, Leid JG, Costerton JW, Shirtliff ME. Microbial interactions and differential protein expression in Staphylococcus aureus -Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol. 2010;59(3): 493–503. 10.1111/j.1574-695X.2010.00710.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro DG, Pavarina AC, Dovigo LN, Machado AL, Giampaolo ET, Vergani CE. Prevalence of Candida spp. associated with bacteria species on complete dentures. Gerodontology. 2012;29(3): 203–208. 10.1111/j.1741-2358.2011.00578.x [DOI] [PubMed] [Google Scholar]
- 12.Zago CE, Silva S, Sanita PV, Barbugli PA, Dias CM, Lordello VB, et al. Dynamics of Biofilm Formation and the Interaction between Candida albicans and Methicillin-Susceptible (MSSA) and -Resistant Staphylococcus aureus (MRSA). PLoS One. 2015;10(4): e0123206 10.1371/journal.pone.0123206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harriott MM, Noverr MC. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob Agents Chemother. 2009;53(9): 3914–3922. 10.1128/AAC.00657-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pulimood S, Ganesan L, Alangaden G, Chandrasekar P. Polymicrobial candidemia. Diagn Microbiol Infect Dis. 2002;44(4): 353–357. [DOI] [PubMed] [Google Scholar]
- 15.Shirtliff ME, Peters BM, Jabra-Rizk MA. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett. 2009;299(1): 1–8. 10.1111/j.1574-6968.2009.01668.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki H, Ohshima T, Tsubota Y, Yamaguchi H, Jayawardena JA, Nishimura Y. Microbicidal activities of low frequency atmospheric pressure plasma jets on oral pathogens. Dent Mater J. 2011;30(3): 384–391. [DOI] [PubMed] [Google Scholar]
- 17.Alkawareek MY, Algwari QT, Gorman SP, Graham WG, O'Connell D, Gilmore BF. Application of atmospheric pressure nonthermal plasma for the in vitro eradication of bacterial biofilms. FEMS Immunol Med Microbiol. 2012;65(2): 381–384. 10.1111/j.1574-695X.2012.00942.x [DOI] [PubMed] [Google Scholar]
- 18.Ermolaeva SA, Varfolomeev AF, Chernukha MY, Yurov DS, Vasiliev MM, Kaminskaya AA, et al. Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J Med Microbiol. 2011;60: 75–83. 10.1099/jmm.0.020263-0 [DOI] [PubMed] [Google Scholar]
- 19.Traba C, Liang JF. Susceptibility of Staphylococcus aureus biofilms to reactive discharge gases. Biofouling. 2011;27(7): 763–772. 10.1080/08927014.2011.602188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joaquin JC, Kwan C, Abramzon N, Vandervoort K, Brelles-Marino G. Is gas-discharge plasma a new solution to the old problem of biofilm inactivation? Microbiology. 2009;155: 724–732. 10.1099/mic.0.021501-0 [DOI] [PubMed] [Google Scholar]
- 21.Koban I, Holtfreter B, Hubner NO, Matthes R, Sietmann R, Kindel E, et al. Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro—proof of principle experiment. J Clin Periodontol. 2011;38(10): 956–965. 10.1111/j.1600-051X.2011.01740.x [DOI] [PubMed] [Google Scholar]
- 22.Liu D, Xiong Z, Du T, Zhou X, Cao Y, Lu X. Bacterial-killing effect of atmospheric pressure non-equilibrium plasma jet and oral mucosa response. J Huazhong Unive Sci Technol Med Sci, 2011;31(6): 852–856. [DOI] [PubMed] [Google Scholar]
- 23.De Geyter N, Morent R. Nonthermal plasma sterilization of living and nonliving surfaces. Annu Rev Biomed Eng. 2012;14: 255–274. 10.1146/annurev-bioeng-071811-150110 [DOI] [PubMed] [Google Scholar]
- 24.Joshi SG, Paff M, Friedman G, Fridman G, Fridman A, Brooks AD. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: a biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am J Infect Control. 2010;38(4): 293–301. 10.1016/j.ajic.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 25.Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia LH. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm. 2001;226(1–2): 1–21. [DOI] [PubMed] [Google Scholar]
- 26.Moreau M, Orange N, Feuilloley MG. Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv. 2008;26(6): 610–7. 10.1016/j.biotechadv.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 27.Brun P, Brun P, Vono M, Venier P, Tarricone E, Deligianni V, et al. Disinfection of ocular cells and tissues by atmospheric-pressure cold plasma. PLoS One. 2012;7(3): e33245 10.1371/journal.pone.0033245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann C, Berganza C, Zhang J. Cold Atmospheric Plasma: methods of production and application in dentistry and oncology. Med Gas Res. 2013;3(1): 21 10.1186/2045-9912-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maisch T, Shimizu T, Li YF, Heinlin J, Karrer S, Morfill G, et al. Decolonisation of MRSA, S. aureus and E. coli by cold-atmospheric plasma using a porcine skin model in vitro. PLoS One. 2012;7(4): e34610 10.1371/journal.pone.0034610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delben JA, Murata RM, Wei X, Castro ML, Assunção WG, da Silva NRFA, et al. Low-temperature plasma: an effective approach against Candida albicans biofilm. Plasma Med. 2014;4: 231–244. [Google Scholar]
- 31.Zissis AJ, Polyzois GL, Yannikakis SA, Harrison A. Roughness of denture materials: a comparative study. Int J Prosthodont. 2000;13(2): 136–140. [PubMed] [Google Scholar]
- 32.Ribeiro DG, Pavarina AC, Dovigo LN, Palomari Spolidorio DM, Giampaolo ET, Vergani CE. Denture disinfection by microwave irradiation: a randomized clinical study. J Dent. 2009;37(9): 666–672. 10.1016/j.jdent.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 33.Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, et al. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res. 2001;80(3): 903–908. [DOI] [PubMed] [Google Scholar]
- 34.Thein ZM, Samaranayake YH, Samaranayake LP. Effect of oral bacteria on growth and survival of Candida albicans biofilms. Arch Oral Biol. 2006;51(8): 672–680. [DOI] [PubMed] [Google Scholar]
- 35.Yadev NP, Murdoch C, Saville SP, Thornhill MH. Evaluation of tissue engineered models of the oral mucosa to investigate oral candidiasis. Microb Pathog. 2011;50(6): 278–285. 10.1016/j.micpath.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 36.Barraud N, Hassett DJ, Hwang SH, Rice SA, Kjelleberg S, Webb JS. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J Bacteriol. 2006;188(21): 7344–7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price M, Terlecky SR, Kessel D. A role for hydrogen peroxide in the pro-apoptotic effects of photodynamic therapy. Photochem Photobiol. 2009;85(6): 1491–1496. 10.1111/j.1751-1097.2009.00589.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ristic BZ, Milenkovic MM, Dakic IR, Todorovic-Markovic BM, Milosavljevic MS, Budimir MD, et al. Photodynamic antibacterial effect of graphene quantum dots. Biomaterials. 2014;35(15): 4428–4435. 10.1016/j.biomaterials.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 39.Silva S, Henriques M, Hayes A, Oliveira R, Azeredo J, Williams DW. Candida glabrata and Candida albicans co-infection of an in vitro oral epithelium. J Oral Pathol Med. 2011;40(5): 421–427. 10.1111/j.1600-0714.2010.00981.x [DOI] [PubMed] [Google Scholar]
- 40.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2): 55–63. [DOI] [PubMed] [Google Scholar]
- 41.Dobrynin D, Fridman G, Friedman G, Fridman A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J Phys. 2009;11: 115020. [Google Scholar]
- 42.Mai-Prochnow A, Murphy AB, McLean KM, Kong MG, Ostrikov KK. Atmospheric pressure plasmas: infection control and bacterial responses. Int J Antimicrob Agents. 2014;43(6): 508–517. 10.1016/j.ijantimicag.2014.01.025 [DOI] [PubMed] [Google Scholar]
- 43.Pappas D. Status and potential of atmospheric plasma processing of materials. J Vac Sci Technol A. 2011;29(2): 020801. [Google Scholar]
- 44.Bayliss DL, Shama G, Kong MG. Restoration of antibiotic sensitivity in meticillin-resistant Staphylococcus aureus following treatment with a non-thermal atmospheric gas plasma. Int J Antimicrob Agents. 2013;41(4): 398–399. 10.1016/j.ijantimicag.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 45.Kaloriti D, Jacobsen M, Yin Z, Patterson M, Tillmann A, Smith DA, et al. Mechanisms underlying the exquisite sensitivity of Candida albicans to combinatorial cationic and oxidative stress that enhances the potent fungicidal activity of phagocytes. mBio. 2014;5(4): e01334–14. 10.1128/mBio.01334-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandamme M, Robert E, Lerondel S, Sarron V, Ries D, Dozias S, et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int J Cancer. 2012;130(9): 2185–2194. 10.1002/ijc.26252 [DOI] [PubMed] [Google Scholar]
- 47.Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB life. 2000;50(4–5): 279–289. [DOI] [PubMed] [Google Scholar]
- 48.Heinlin J, Isbary G, Stolz W, Morfill G, Landthaler M, Shimizu T, et al. Plasma applications in medicine with a special focus on dermatology. J Eur Acad Dermatol Venereol. 2011;25(1): 1–11. 10.1111/j.1468-3083.2010.03702.x [DOI] [PubMed] [Google Scholar]
- 49.Kalghatgi S, Kelly CM, Cerchar E, Torabi B, Alekseev O, Fridman A, et al. Effects of non-thermal plasma on mammalian cells. PLoS One. 2011;6(1): e16270 10.1371/journal.pone.0016270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sensenig R, Kalghatgi S, Cerchar E, Fridman G, Shereshevsky A, Torabi B, et al. Non-thermal plasma induces apoptosis in melanoma cells via production of intracellular reactive oxygen species. Ann Biomed Eng. 2011;39(2): 674–687. 10.1007/s10439-010-0197-x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.