Abstract
Objectives. To examine the relationship between benzodiazepine (BZD) use and HCV seroconversion in 2 linked prospective cohorts of persons who inject drugs (PWID).
Methods. We examined prospective cohorts of 440 PWID (baseline BZD users: n = 102; 23.2%) from the AIDS Care Cohort to Evaluate Access to Survival Services (ACCESS) and the Vancouver Injection Drug Users Study (VIDUS) cohorts, followed-up from 1996 to 2013 in Vancouver, Canada.
Results. At baseline, the prevalence of HCV was higher among those who used BZD (80.5% vs 61.5%; P < .001). After adjustment, BZD use remained independently associated with increased rates of HCV seroconversion (adjusted rate ratio = 1.67; 95% confidence interval = 1.05, 2.66).
Conclusions. BZD use is independently associated with HCV seroconversion in a population of PWID.
Originally marketed more than 40 years ago as a safer alternative to existing sedatives, benzodiazepines (BZD) are commonly used for a broad range of complaints, including anxiety, agitation, depression, insomnia, seizure disorders, spasticity, and alcohol withdrawal.1 As a result, BZD are among the most frequently prescribed medications in North America, with 5.2% of adult Americans reporting taking BZD in recent surveys.2 Despite this widespread use, there remains a paucity of evidence supporting their effectiveness for many of these indications.3,4
Complicating the debate on BZD efficacy is an increasing appreciation for the harms associated with both short- and long-term use, including drowsiness, ataxia, amnesia, and cognitive impairment.5,6 These well-described side effects can lead to downstream consequences, including an increased risk of falls, fractures, and motor vehicles accidents.7,8 An additional concern is physical dependency, which often requires an extended tapering schedule to safely wean patients.
Although the adverse effects of BZD use are relatively common, their euphoric properties have led to widespread issues of abuse, particularly in subpopulations with a history of alcohol abuse.9,10 Polysubstance users are at particularly high risk for BZD abuse because they frequently use BZDs to enhance the high of other drugs or to treat the adverse effects of drug use and the symptoms of drug withdrawal.11 In these populations, BZD use has been correlated with more extensive illicit drug use, higher risk of accidental overdose, more psychiatric comorbidities, poorer health, and increased overall mortality.12–16 Reduction in BZD use has been associated with a reduction in harm in these patients, suggesting that BZD use itself may be driving the poorer clinical outcomes.13
HCV is a blood-borne pathogen that is estimated to affect 2% to 3% of the world’s population, or between 130 million and 170 million people.17 The virus is commonly contracted through use of contaminated syringes during injection drug use, and approximately 10 million of the 16 million persons who inject drugs (PWID) worldwide are believed to be HCV antibody positive.18 Although no vaccines exist to prevent HCV, novel treatments in the form of direct-acting antiviral agents have recently been developed and have had excellent results compared with the older regimens of ribavirin and pegylated interferon.19 This technology has the potential to transform the landscape of HCV treatment within North America, but these medications are extremely expensive and remain out of reach for most individuals, underscoring the continued importance of HCV prevention efforts.
Several previous studies of PWID have noted that the abuse of BZD has been linked to an increase in risky behaviors such as needle sharing and unprotected sex.20,21 It has additionally been observed that BZD use is associated with a higher prevalence of HCV infection.22–24 Because of this association between BZD use and HCV, we examined the hypothesis that BZD use leads to an increased rate of HCV seroconversion in 2 linked prospective cohorts of HCV-negative PWID in a Canadian setting.
METHODS
We obtained data from 2 ongoing prospective studies: the AIDS Care Cohort to Evaluate Access to Survival Services (ACCESS) and the Vancouver Injection Drug Users Study (VIDUS).25 Briefly, participants of these studies are complementary cohorts of illicit drug users (composed of, respectively, HIV-positive and HIV-negative participants) who have been followed in Vancouver, Canada, since 1996. The cohorts are essentially identical in all other respects, to allow merging of data when appropriate. Criteria for eligibility include being at least 18 years of age, living within the Greater Vancouver Area, and having a history of illicit drug use. For VIDUS criteria also include injecting drugs in the month before enrollment, whereas for ACCESS they include use of illicit drugs other than cannabis in the month before enrollment.
Recruitment occurs through street outreach, word of mouth, and self-referral. At time of entry and on a semiannual basis thereafter, participants complete an interviewer-administered questionnaire designed to elicit information on demographics, drug use patterns, high-risk behaviors, and interactions with the criminal justice system. Blood samples are taken at baseline and semiannually to establish HIV and HCV serostatus and incidence and to assess disease progression among those living with HIV. All patients are offered access to addiction treatment services, and any that test positive for HIV are immediately referred to appropriate health services. In return for their time, participants are compensated with a $30 stipend per visit.
We used data gathered between May 1996 and November 2013. Participants were eligible for inclusion if they were HCV negative at baseline, had a history of injection drug use, and had at least 1 follow-up visit. Our primary outcome was time to seroconversion to HCV antibody positive (a measure of HCV incidence), and our primary explanatory variable was BZD use as defined by any self-reported use within the previous 6 months (illicit or prescription).
In our analysis, we included the following confounders, which have a known or suspected impact on HCV seroconversion rates: gender (male vs female), age (as a continuous variable, reported in 10-year increments), race/ethnicity (White vs other), homelessness (yes vs no), sex work involvement (yes vs no), unsafe sexual activity (yes vs no), at least daily heroin injection (yes vs no), at least daily cocaine injection (yes vs no), at least daily noninjection crack use (yes vs no), enrollment in methadone maintenance therapy (MMT; yes vs no), and study cohort (as a measure of HIV status). All behavioral variables refer to activities taking place within the previous 6 months, and all variable definitions were identical to those in earlier ACCESS and VIDUS reports.25
First, we compared the baseline characteristics of our sample stratified by BZD use. We used the Pearson χ2 test for categorical variables and the Wilcoxon rank–sum test for continuous variables. We estimated the cumulative probabilities of remaining HCV negative using the Kaplan–Meier product limit method for baseline BZD users and non-BZD users and compared them using the 2-sample log–rank test.
Next, we used Poisson regression to examine bivariable relationships between each of the explanatory variables and time to HCV seroconversion.26 We defined time zero as the time of study entry for each eligible individual. We treated all behavioral variables as time-dependent covariates. Finally, we used multivariable Poisson regression to adjust for possible confounders and establish whether BZD use was independently associated with an increased rate of HCV seroconversion.
As in similar studies, we used a confounding model selection approach.27 We first constructed a full model containing all variables associated with time to HCV seroconversion from our bivariable analyses (at P < .10). Next, we removed secondary explanatory variables stepwise 1 at a time to establish reduced models. Following each step we compared the values of coefficients for BZD status in the full model with those in our reduced models, and we removed secondary explanatory variables corresponding to the smallest relative change. We repeated this process until a minimum change from the full model was greater than 5%, and we reported only these values in the adjusted analysis in our final table. We performed all statistical analyses using SAS version 9.3 (SAS Institute, Cary, NC), and all P values were 2 tailed.
RESULTS
Among all participants in the ACCESS and VIDUS cohorts seen between May 1996 and November 2013, the prevalence of HCV at the time of enrollment was 66.4%. The number was significantly higher among patients who used BZD at baseline than among those who did not (80.5% vs 61.5%; P < .001). A total of 541 baseline HCV-negative participants were recruited; however, follow-up on HCV status was not available for 101 of these participants, and we did not include them in subsequent analyses. The 440 participants available for follow-up assessment of HCV serostatus tended to be older (median age = 29.4 vs 26.0 years; P = .007) and were less likely to practice unsafe sex (40.0% vs 51.5%; odds ratio [OR] = 0.63; 95% confidence interval [CI] = 0.41, 0.97). We saw no other significant differences between the 2 groups, including the prevalence of BZD use. Among the participants, 282 (64.1%) did not report BZD use throughout the entire follow-up period, whereas 158 (35.9%) did report BZD use on at least 1 occasion.
The median age of participants included in our analysis was 29.4 years (interquartile range [IQR] = 22.4–40.0), 68.2% were male, and 57.5% were White. Non-Whites predominantly self-identified as Canadian Aboriginal (20.9%), followed by Hispanic/Latino (7.0%), other or mixed (5.9%), Asian (5.2%), and African American (3.4%). The median number of study visits was 5 (IQR = 3–11), and the median follow-up time per participant was 38.7 months (IQR = 12.7–78.3). Additional baseline demographic data are available in Table 1.
TABLE 1—
Baseline Characteristics of Persons Who Inject Drugs Stratified by Benzodiazepine Use: Vancouver, Canada, 1996–2013
| Characteristic | BZD Users, (n = 102, 23.2%), IQR or No. (%) | Non-BZD Users (n = 338, 76.8%), IQR or No. (%) | OR (95% CI) |
| Median age | 24.6 (20.4–36.1) | 30.7 (23.7–41.3) | . . . |
| Gender | |||
| Male | 65 (63.7) | 235 (69.5) | 0.77 (0.48, 1.23) |
| Female | 37 (36.3) | 103 (30.5) | |
| Race/ethnicity | |||
| White | 65 (63.7) | 188 (55.6) | 1.40 (0.89, 2.21) |
| Non-White | 37 (36.3) | 150 (44.4) | |
| Homelessa | |||
| Yes | 19 (18.6) | 104 (30.8) | 0.52 (0.30, 0.90) |
| No | 82 (80.4) | 232 (68.6) | |
| Engages in methadone maintenancea | |||
| Yes | 8 (7.8) | 44 (13.0) | 0.56 (0.26, 1.24) |
| No | 94 (92.2) | 292 (86.4) | |
| Injects heroin dailya | |||
| Yes | 46 (45.1) | 102 (30.2) | 1.88 (1.20, 2.97) |
| No | 56 (54.9) | 234 (69.2) | |
| Injects cocaine dailya | |||
| Yes | 25 (24.5) | 54 (16.0) | 1.70 (0.99, 2.90) |
| No | 77 (75.5) | 282 (83.4) | |
| Uses noninjection crack dailya | |||
| Yes | 22 (21.6) | 92 (27.2) | 0.73 (0.43, 1.24) |
| No | 80 (78.4) | 245 (72.5) | |
| Engages in unsafe sexa | |||
| Yes | 58 (56.9) | 118 (34.9) | 2.45 (1.56, 3.84) |
| No | 44 (43.1) | 219 (64.8) | |
| Involved in sex worka | |||
| Yes | 26 (25.5) | 74 (21.9) | 1.22 (0.73, 2.03) |
| No | 76 (74.5) | 263 (77.8) | |
| Study cohort | |||
| ACCESS | 3 (2.9) | 82 (24.3) | 0.09 (0.03, 0.31) |
| VIDUS | 99 (97.1) | 256 (75.7) |
Note. ACCESS = AIDS Care Cohort to Evaluate Access to Survival Services; BZD = benzodiazepine; CI = confidence interval; IQR = interquartile range; OR = odds ratio; VIDUS = Vancouver Injection Drug Users Study. The sample size was n = 440.
Activities or situations occurring in the past 6 mo.
BZD users tended to be younger (median age = 24.6 vs 30.7 years; P < .001), were less likely to be homeless (18.6% vs 30.8%; OR = 0.52; 95% CI = 0.30, 0.90), were more likely to inject heroin at least daily (45.1% vs 30.2%; OR = 1.88; 95% CI = 1.20, 2.97), and were more likely to practice unsafe sex (56.9% vs 34.9%; OR = 2.45; 95% CI = 1.56, 3.84). We did not see any significant differences between groups in gender, race/ethnicity, MMT enrollment, at least daily injection cocaine use, at least daily noninjection cocaine use, involvement in sex work, or study cohort.
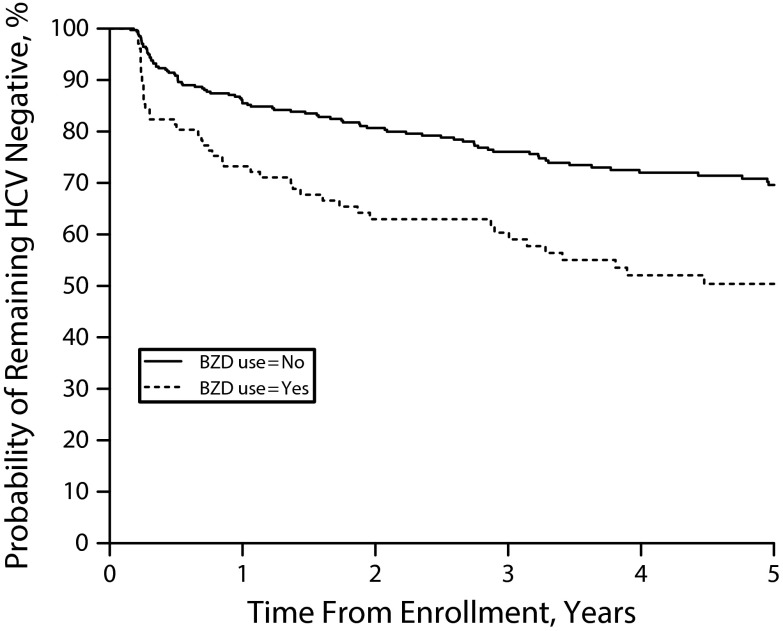
A total of 142 HCV seroconversion events occurred over the duration of the study, for an incidence density of 7.6 (95% CI = 6.5, 9.0) cases per 100 person-years. The median number of months to seroconversion following enrollment was 11.5 (IQR = 3.6–32.9). As shown in Figure 1, participants who reported BZD use at baseline were less likely to remain HCV negative over the course of the study period. At 5 years after recruitment, the cumulative probability of remaining HCV negative was 50.4% among baseline BZD users and 69.6% among baseline non-BZD users (log–rank P < .001).
FIGURE 1—
Probability of Remaining HCV Negative Among Persons Who Inject Drugs Stratified by Benzodiazepine Use: Vancouver, Canada, 1996–2013
Note. Log rank P < .001. The sample size was n = 440.
In bivariable analyses (Table 2), factors associated with increased rate of HCV seroconversion included BZD use (unadjusted rate ratio [RR] = 3.44; 95% CI = 2.20, 5.37), homelessness (RR = 1.67; 95% CI = 1.17, 2.38), at least daily heroin injection (RR = 3.50; 95% CI = 2.51, 4.87), at least daily cocaine injection (RR = 3.97; 95% CI = 2.72, 5.80), unsafe sex (RR = 1.61; 95% CI = 1.14, 2.28), and sex work involvement (RR = 3.41; 95% CI = 2.33, 5.00). In the same unadjusted analysis, factors associated with a decreased rate of HCV seroconversion included age (per 10 years older; RR = 0.62; 95% CI = 0.52, 0.74) and male gender (RR = 0.69; 95% CI = 0.49, 0.96).
TABLE 2—
Factors Associated With HCV Seroconversion Among Persons Who Inject Drugs: Vancouver, Canada, 1996–2013
| Characteristic | HCV Seroconversion,a n = 142, 32.3%, IQR or No. (%) | HCV Nonseroconversion, n = 298, 67.7%, IQR or No. (%) | Unadjusted RR (95% CI) | Adjusted RRb (95% CI) |
| Uses BZDc | 3.44 (2.20, 5.37) | 1.67 (1.05, 2.66) | ||
| Yes | 67 (47.2) | 91 (30.5) | ||
| No | 75 (52.8) | 207 (69.5) | ||
| Median age, by 10-y increments | 27.0 (21.5–36.8) | 30.9 (23.4–41.2) | 0.62 (0.52, 0.74) | 0.87 (0.73, 1.03) |
| Gender | 0.69 (0.49, 0.96) | |||
| Male | 87 (61.3) | 213 (71.5) | ||
| Female | 55 (38.7) | 85 (28.5) | ||
| Race/ethnicity | 1.01 (0.73, 1.41) | |||
| White | 81 (57.0) | 172 (57.7) | ||
| Non-White | 61 (43.0) | 126 (42.3) | ||
| Homelessc | 1.67 (1.17, 2.38) | |||
| Yes | 73 (51.4) | 177 (59.4) | ||
| No | 69 (48.6) | 121 (40.6) | ||
| Engages in methadone maintenancec | 0.97 (0.67, 1.42) | |||
| Yes | 43 (30.3) | 109 (36.6) | ||
| No | 99 (69.7) | 189 (63.4) | ||
| Injects heroin dailyc | 3.50 (2.51, 4.87) | 1.93 (1.34, 2.79) | ||
| Yes | 93 (65.5) | 152 (51.0) | ||
| No | 49 (34.5) | 146 (49.0) | ||
| Injects cocaine dailyc | 3.97 (2.72, 5.80) | 2.27 (1.52, 3.37) | ||
| Yes | 64 (45.1) | 86 (28.9) | ||
| No | 78 (54.9) | 212 (71.1) | ||
| Uses noninjection crack dailyc | 1.07 (0.73, 1.57) | |||
| Yes | 60 (42.3) | 153 (51.3) | ||
| No | 82 (57.7) | 145 (48.7) | ||
| Engages in unsafe sexc | 1.61 (1.14, 2.28) | |||
| Yes | 96 (67.6) | 188 (63.1) | ||
| No | 46 (32.4) | 110 (36.9) | ||
| Involved in sex workc | 3.41 (2.33, 5.00) | |||
| Yes | 51 (35.9) | 80 (26.8) | ||
| No | 91 (64.1) | 218 (73.2) | ||
| Study cohort | 0.42 (0.23, 0.78) | |||
| ACCESS | 131 (92.3) | 224 (75.2) | ||
| VIDUS | 11 (7.7) | 74 (24.8) |
Note. ACCESS = AIDS Care Cohort to Evaluate Access to Survival Services; BZD = benzodiazepine; CI = confidence interval; IQR = interquartile range; RR = rate ratio; VIDUS = Vancouver Injection Drug Users Study. The sample size was n = 440.
Number of individuals ever reporting each characteristic during follow-up.
A confounder selection approach was used to select values.
Activities or situations occurring in the past 6 months.
In an adjusted model, BZD use remained independently associated with an increased rate of HCV seroconversion (adjusted RR = 1.67; 95% CI = 1.05, 2.66; Table 2). To assess whether the effect of BZD use differs between HIV-seropositive and HIV-seronegative participants, we built a multivariable model that included an interaction term between BZD use and study cohort indicator. The interaction term was not statistically significant (P = .387), indicating that there was no difference in the effect of BZD use between HIV-seropositive and HIV-seronegative participants.
DISCUSSION
We found a higher prevalence of HCV among BZD users at baseline and an elevated rate of HCV seroconversion associated with BZD use, even after adjusting for demographic factors, drug use patterns, and high-risk behaviors. These data build on numerous previous cross-sectional studies linking BZD use with an increased prevalence of HCV among polysubstance abusers.
A correlation between BZD and HCV infection was first reported in a retrospective cohort of PWID in Amsterdam, where an association was noted between BZD use and HCV seropositivity.24 A similar relationship was later identified in a retrospective cohort of middle-class polysubstance abusers in the United States, in which BZD use was again linked to increased HCV prevalence.22 In a population of patients enrolled in MMT in Israel, BZD use at the time of entry into treatment was not associated with increased HCV rates; however, in a multivariable analysis of patient characteristics 1-year into treatment, baseline HCV seropositivity was associated with the ongoing misuse of BZD.23 Because BZD users may be at higher risk for other comorbidities and are more likely to engage in high-intensity polysubstance use, it was not clear according to these studies whether BZD use was independently associated with HCV seroconversion. Our results help fill this gap and demonstrate for the first time, to our knowledge, that BZD users have a significantly higher risk of HCV seroconversion even after other risk factors are considered in multivariable analyses.
The negative effects of BZD use in polysubstance users are pervasive and are likely underappreciated because measurements of BZD use are not routinely incorporated into research that examines patterns of illicit drug use. Previous studies have focused largely on patients enrolled in MMT programs and clearly describe the deleterious effects these medications have on treatment. In general, patients enrolled in MMT with a history of BZD use tend to have lengthier and more extensive drug use histories, have more psychiatric comorbidities, and engage in more high-risk behaviors.15,20,21,28,29 Ongoing BZD use by patients engaged in MMT has been associated with poorer retention, increased illicit drug use, and worse outcomes.12,30–33 An increased risk of overdose has also been described in opiate users on BZD both in and outside of MMT programs.14,34–37 More encouraging is the finding that successful discontinuation of BZD use in patients enrolled in MMT predicts improved retention, and equivalent outcomes have been seen in BZD-abstinent patients compared with those who were BZD nonusers at baseline.13,38
Examining our results in the context of what is known about BZD abuse, multiple potential explanations may contribute to the increased risk of HCV infection. It has been noted that BZD use in PWID is correlated with an increase in risky behaviors that may increase transmission rates of infections such as HIV and HCV.20,21 Because BZD are known to decrease inhibitions and impair judgment and can result in long-term cognitive impairment, these effects may directly contribute to higher risk injecting habits and may increase the likelihood of HCV transmission.5,6
BZD use has also been associated with more aggressive and problematic drug use; thus, although we attempted to control for drug use patterns, it is possible that this subpopulation may still have an increased overall frequency of injections.12 Finally, patients with severe mental illnesses are at increased risk for contracting HCV.39 Because this population is also more likely to abuse BZD, teasing out whether the increased susceptibility is secondary to BZD use versus mental illness remains a challenge.15,20,21,28,29 Nevertheless, because of the limited proven use for BZD and the existence of safer alternatives for common clinical ailments in which BZD may be considered, physician education to promote greater recognition of the safety concerns related to BZD use in this population is needed.
In addition to demonstrating a relationship between BZD use and HCV infection, our results highlight the high overall prevalence of BZD use in Canada. At baseline, 35.9% of our participants reported BZD use, a rate that is in keeping with similar cohorts and that reflects the high prevalence of BZD use in Canada.28,32,37,40,41 The 2012 Canadian Alcohol and Drug Use Monitoring Survey reported that, although opioids remained the most frequent prescription drug of abuse, 1.5% of the Canadian population (approximately 410 000 citizens) had used a nonopioid psychoactive pharmaceutical within the past year for purposes other than prescribed.42 BZD are likely the majority of these psychoactive compounds, particularly considering that Canadian consumption rates of BZD as anxiolytics were among the highest in the world in 2012.43 Such a high volume of prescribing creates a huge potential for drug diversion, and it is believed that the majority of illicit BZD use originates from physician prescriptions. We could not distinguish between prescribed versus illicitly obtained BZD because these data were not collected. Future studies should examine these issues further. Overall, our results underscore the importance of limiting the unnecessary prescription of these addictive medications.
Our results highlight the immense burden of HCV in a population of PWID. Among all our cohort members, the prevalence of HCV at baseline was 66.4%, consistent with other estimates from this region.44 An incidence density of 7.6 cases per 100 person-years is in keeping with similarly described cohorts of PWID around the world.45,46 Our results suggest that developing strategies to address BZD abuse may provide a new opportunity to reduce the transmission of this virus and its associated morbidity and mortality.
Limitations
This study has several limitations. First, as our participants were not randomly recruited, there remain questions regarding the generalizability of our results, although we believe that this is a representative population of local PWID. Second, some participants were not available for any follow-up visits after initial enrollment, although we did not see any differences in BZD use in this population versus those included in the study.
Third, our analysis included data on behaviors such as drug use patterns and high-risk activities that may be of a sensitive nature. Because this information was obtained through self-reporting, there is a possibility that response bias may have affected our survey results, although self-reports of drug users have previously been shown to be valid.47 Fourth, our study would have benefited from detailed measures of mental health comorbidities. Unfortunately, these data were not collected, and we could not adjust for them. Lastly, as this is an observational study, there remains the possibility that this association is not a causal one, although randomized studies focused on these issues would not be ethical, and our results are in keeping with the literature on this subject.
Conclusions
We found a high rate of BZD use in a population of PWID and an independent association between BZD use and an increased rate of HCV infection. These data illustrate yet another negative consequence of BZD use and add to a growing body of literature describing the detrimental effects of these medications for PWID. Our results highlight the need to increase awareness regarding the limited evidence-based indications for BZD prescription and draw attention to the importance of identifying and treating BZD abuse in patients with polysubstance use disorders.
ACKNOWLEDGMENTS
The study was supported by the US National Institutes of Health (grants R01DA021525 and R01DA011591), the Canadian Institutes of Health Research (new investigator award 201412MSH-340060-196922 to K. H.), the Canadian Institutes of Health Research (fellowship to M-J M.), the Michael Smith Foundation for Health Research (fellowship to M-J M.), and the Canada Research Chairs program through a Tier 1 Canada Research Chair in Inner City Medicine (to E. W.).
The authors thank the study participants and current and past researchers and staff for their contribution to this research.
HUMAN PARTICIPANT PROTECTION
The cohort studies received annual ethics approval from the University of British Columbia/Providence Healthcare research ethics board.
REFERENCES
- 1.Bostwick J, Casher M, Yasugi S. Benzodiazepines: a versatile clinical tool. Curr Psychiatr. 2012;11(4):55–63. [Google Scholar]
- 2.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–142. doi: 10.1001/jamapsychiatry.2014.1763. [DOI] [PubMed] [Google Scholar]
- 3.Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106(12):2086–2109. doi: 10.1111/j.1360-0443.2011.03563.x. [DOI] [PubMed] [Google Scholar]
- 4.Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol. 2014;77(2):295–301. doi: 10.1111/j.1365-2125.2012.04418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25(3):158–165. [Google Scholar]
- 6.Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs. 2004;18(1):37–48. doi: 10.2165/00023210-200418010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Cumming RG, Le Couteur DG. Benzodiazepines and risk of hip fractures in older people: a review of the evidence. CNS Drugs. 2003;17(11):825–837. doi: 10.2165/00023210-200317110-00004. [DOI] [PubMed] [Google Scholar]
- 8.Rapoport MJ, Lanctot KL, Streiner DL et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663–673. doi: 10.4088/JCP.08m04325. [DOI] [PubMed] [Google Scholar]
- 9.Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74–89. doi: 10.1016/j.pbb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919–934. doi: 10.1517/14740338.2014.925444. [DOI] [PubMed] [Google Scholar]
- 11.O’brien CP. Benzodiazepine use, abuse, and dependence. J Clin Psychiatry. 2005;66(suppl 2):28–33. [PubMed] [Google Scholar]
- 12.Bleich A, Gelkopf M, Schmidt V, Hayward R, Bodner G, Adelson M. Correlates of benzodiazepine abuse in methadone maintenance treatment. A 1 year prospective study in an Israeli clinic. Addiction. 1999;94(10):1533–1540. doi: 10.1046/j.1360-0443.1999.941015339.x. [DOI] [PubMed] [Google Scholar]
- 13.Darke S, Ross J, Mills K, Teesson M, Williamson A, Havard A. Benzodiazepine use among heroin users: baseline use, current use and clinical outcome. Drug Alcohol Rev. 2010;29(3):250–255. doi: 10.1111/j.1465-3362.2009.00101.x. [DOI] [PubMed] [Google Scholar]
- 14.Kerr T, Fairbairn N, Tyndall M et al. Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend. 2007;87(1):39–45. doi: 10.1016/j.drugalcdep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Ross J, Darke S. The nature of benzodiazepine dependence among heroin users in Sydney, Australia. Addiction. 2000;95(12):1785–1793. doi: 10.1046/j.1360-0443.2000.951217858.x. [DOI] [PubMed] [Google Scholar]
- 16.Caplehorn JR. Risk factors for non-HIV-related death among methadone maintenance patients. Eur Addict Res. 1996;2(1):49–52. [Google Scholar]
- 17.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17(2):107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 18.Nelson PK, Mathers BM, Cowie B et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015;385(9973):1124–1135. doi: 10.1016/S0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drake S, Swift W, Hall W, Ross M. Drug use, HIV risk-taking and psychosocial correlates of benzodiazepine use among methadone maintenance clients. Drug Alcohol Depend. 1993;34(1):67–70. doi: 10.1016/0376-8716(93)90047-t. [DOI] [PubMed] [Google Scholar]
- 21.Metzger D, Woody G, De Philippis D, McLellan AT, O’Brien CP, Platt JJ. Risk factors for needle sharing among methadone-treated patients. Am J Psychiatry. 1991;148(5):636–640. doi: 10.1176/ajp.148.5.636. [DOI] [PubMed] [Google Scholar]
- 22.Abraham HD, Degli-Esposti S, Marino L. Seroprevalence of hepatitis C in a sample of middle class substance abusers. J Addict Dis. 1999;18(4):77–87. doi: 10.1300/J069v18n04_07. [DOI] [PubMed] [Google Scholar]
- 23.Peles E, Rados V, Adelson M. Characterization of former heroin addict patients with Hepatitis C virus antibodies in a methadone maintenance treatment (MMT) clinic in Israel. Subst Use Misuse. 2007;42(9):1477–1484. doi: 10.1080/10826080701212550. [DOI] [PubMed] [Google Scholar]
- 24.van den Hoek JA, van Haastrecht HJ, Goudsmit J, de Wolf F, Coutinho RA. Prevalence, incidence, and risk factors of hepatitis C virus infection among drug users in Amsterdam. J Infect Dis. 1990;162(4):823–826. doi: 10.1093/infdis/162.4.823. [DOI] [PubMed] [Google Scholar]
- 25.Strathdee SA, Patrick DM, Currie SL et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS. 1997;11(8):F59–F65. doi: 10.1097/00002030-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Kleinbaum DG, Klein M. Survival Analysis: A Self-Learning Text. New York, NY: Springer-Verlag; 1996. [Google Scholar]
- 27.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 28.Chen KW, Berger CC, Forde DP, D’Adamo C, Weintraub E, Gandhi D. Benzodiazepine use and misuse among patients in a methadone program. BMC Psychiatry. 2011;11:90. doi: 10.1186/1471-244X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rooney S, Kelly G, Bamford L, Sloan D, O’Connor JJ. Co-abuse of opiates and benzodiazepines. Ir J Med Sci. 1999;168(1):36–41. doi: 10.1007/BF02939579. [DOI] [PubMed] [Google Scholar]
- 30.Eiroa-Orosa FJ, Haasen C, Verthein U, Dilg C, Schafer I, Reimer J. Benzodiazepine use among patients in heroin-assisted vs. methadone maintenance treatment: findings of the German randomized controlled trial. Drug Alcohol Depend. 2010;112(3):226–233. doi: 10.1016/j.drugalcdep.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Peles E, Schreiber S, Adelson M. 15-Year survival and retention of patients in a general hospital-affiliated methadone maintenance treatment (MMT) center in Israel. Drug Alcohol Depend. 2010;107(2–3):141–148. doi: 10.1016/j.drugalcdep.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Raffa JD, Grebely J, Tossonian H et al. The impact of ongoing illicit drug use on methadone adherence in illicit drug users receiving treatment for HIV in a directly observed therapy program. Drug Alcohol Depend. 2007;89(2–3):306–309. doi: 10.1016/j.drugalcdep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Brands B, Blake J, Marsh DC, Sproule B, Jeyapalan R, Li S. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J Addict Dis. 2008;27(3):37–48. doi: 10.1080/10550880802122620. [DOI] [PubMed] [Google Scholar]
- 34.Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances, and the role of benzodiazepines. Aust N Z J Public Health. 2002;26(4):358–362. doi: 10.1111/j.1467-842x.2002.tb00185.x. discussion 62–63. [DOI] [PubMed] [Google Scholar]
- 35.Darke S, Topp L, Ross J. The injection of methadone and benzodiazepines among Sydney injecting drug users 1996–2000: 5-year monitoring of trends from the Illicit Drug Reporting System. Drug Alcohol Rev. 2002;21(1):27–32. doi: 10.1080/09595230220119318. [DOI] [PubMed] [Google Scholar]
- 36.Man LH, Best D, Gossop M, Stillwell G, Strang J. Relationship between prescribing and risk of opiate overdose among drug users in and out of maintenance treatment. Eur Addict Res. 2004;10(1):35–40. doi: 10.1159/000073724. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen S, Dietze P, Lee N, Dunlop A, Taylor D. Concurrent buprenorphine and benzodiazepines use and self-reported opioid toxicity in opioid substitution treatment. Addiction. 2007;102(4):616–622. doi: 10.1111/j.1360-0443.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 38.Peles E, Linzy S, Kreek M, Adelson M. One-year and cumulative retention as predictors of success in methadone maintenance treatment: a comparison of two clinics in the United States and Israel. J Addict Dis. 2008;27(4):11–25. doi: 10.1080/10550880802324382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg SD, Goodman LA, Osher FC et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31–37. doi: 10.2105/ajph.91.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelkopf M, Bleich A, Hayward R, Bodner G, Adelson M. Characteristics of benzodiazepine abuse in methadone maintenance treatment patients: a 1 year prospective study in an Israeli clinic. Drug Alcohol Depend. 1999;55(1–2):63–68. doi: 10.1016/s0376-8716(98)00175-6. [DOI] [PubMed] [Google Scholar]
- 41.Peles E, Schreiber S, Rados V, Adelson M. Low risk for hepatitis C seroconversion in methadone maintenance treatment. J Addict Med. 2011;5(3):214–220. doi: 10.1097/ADM.0b013e31820e13dd. [DOI] [PubMed] [Google Scholar]
- 42.Health Canada. Canadian Alcohol and Drug Use Monitoring Survey: summary of results for 2012. Available at: http://www.hc-sc.gc.ca/hc-ps/drugs-drogues/stat/_2012/summary-sommaire-eng.php. Accessed February 25, 2016.
- 43.International Narcotics Control Board. Psychotropic Substances: Statistics for 2011, Assessments of Annual Medical and Scientific Requirements for Substances in Schedules II, III and IV of the Convention on Psychotropic Substances of 1971. New York, NY: United Nations; 2013. [Google Scholar]
- 44.Vila-Rodriguez F, Panenka WJ, Lang DJ et al. The Hotel Study: multimorbidity in a community sample living in marginal housing. Am J Psychiatry. 2013;170(12):1413–1422. doi: 10.1176/appi.ajp.2013.12111439. [DOI] [PubMed] [Google Scholar]
- 45.Jordan AE, Des Jarlais DC, Arasteh K, McKnight C, Nash D, Perlman DC. Incidence and prevalence of hepatitis C virus infection among persons who inject drugs in New York City: 2006–2013. Drug Alcohol Depend. 2015;152:194–200. doi: 10.1016/j.drugalcdep.2015.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White B, Dore GJ, Lloyd AR, Rawlinson WD, Maher L. Opioid substitution therapy protects against hepatitis C virus acquisition in people who inject drugs: the HITS-c study. Med J Aust. 2014;201(6):326–329. doi: 10.5694/mja13.00153. [DOI] [PubMed] [Google Scholar]
- 47.Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend. 1998;51(3):253–263. doi: 10.1016/s0376-8716(98)00028-3. discussion 67–68. [DOI] [PubMed] [Google Scholar]