Abstract
Subtypes of innate lymphoid cells (ILC), defined based on their cytokine secretion profiles and transcription factor expression, are important for host protection from pathogens and maintaining tissue homeostasis. ILCs develop from common lymphoid progenitors (CLP) in the bone marrow. Using the methods described here, we have previously shown that loss of the transcriptional regulator TOX (Thymocyte-selection associated HMG-box protein) leads to specific changes in ILC development and differentiation. Here, we describe how to obtain ILCs from in vivo isolated CLP grown in vitro.
Materials and Reagents
5 ml Syringe (BD biosciences, catalog number: 309646)
25 G × 5/8 Needle (BD biosciences, catalog number: 305122)
15 ml Conical Tubes (Corning, Falcon, catalog number: 352097)
50 ml Conical Tubes (USA Scientific, catalog number: 1500–1811)
60 × 15 mm culture dish (Corning, Falcon, catalog number: 351007)
Sterile 48 well plates, tissue culture treated (Greiner Bio-One GmbH, catalog number: 677180)
5 ml and 10 ml serological pipets
OP9-DL1 cells (not commercially available, must privately request from Dr. J. C. Zuniga-Pflucker, University of Toronto, Sunnybrook Research Institute)
OP9-DL1 stromal cells (OP9-DL1 made by and obtained from Dr. J. C. Zuniga-Pflucker)
- Antibodies (all purchased from eBiosciences, Affymetrix)
- Blocking antibody Anti-CD16/CD32 Functional Grade Purified (clone 93) (eBioscience, Affymetrix, catalog number: 16-0161)
- Anti-mouse lineage antibodies (concentrations as per vendor instructions), Flouorochromes were conjugated to FITC or APC (Lineage markers), APC-Cy7 (IL7Rα⊔, PerCP eFIour® 710 (Flt-3)] and PE-Cy7 (Ly6A/E)
- CD4 (clone 6K1.5)
- CD8α (53-6.7)
- CD3ε (145-2C11)
- CD11b (M1/70)
- CD11c (N418)
- CD19 (1D3)
- B220 (RA3-6B2)
- Gr-1 (RB6-8C5)
- Nk1.1 (PK136)
- Ter-119 (TER-119)
- Thy1.2 (30-H12)
- Common lymphoid progenitor defining cell surface antibodies (concentrations as per vendor instructions)
- Flt3 (A2F10)
- IL7Rα (A7R34)
- Ly6A/E (Sca-1) (D7)
Dulbecco’s Phosphate Buffered Saline (DPBS) (Thermo Fisher Scientific, Gibco™, catalog number: 14190-144)
Dulbecco’s Modified Eagle Medium, High Glucose (DMEM) (Thermo Fisher Scientific, Gibco™, catalog number: 11965-092)
Minimum Essential Media (MEM) alpha, No Nucleosides (Thermo Fisher Scientific, Gibco™, catalog number: 12561-049)
Fetal Bovine Serum (FBS) (Omega Scientific, catalog number: FB-01)
100× Penicillin-Streptomycin (PSA) (Thermo Fisher Scientific, Gibco™, catalog number: 10378-016)
Sodium Azide (Sigma-Aldrich, catalog number: S2002)
2-mercaptoethanol (Sigma-Aldrich, catalog number: M7522)
Sodium pyruvate (Thermo Fisher Scientific, Gibco™, catalog number: 11360-070)
MEM nonessential amino acids (Mediatech, Corning® glutagro™, catalog number: 25-025-CI)
HEPES (Thermo Fisher Scientific, Gibco™, catalog number: 15630)
Trypsin-EDTA (0.05%), phenol red (Thermo Fisher Scientific, Gibco™, catalog number: 25300-054)
Bovine Serum Albumin (BSA) (Sigma-Aldrich, catalog number: A8022)
Recombinant mouse Interleukin 7 (IL-7) carrier free (Biolegend, catalog number: 577802)
Recombinant mouse Stem Cell Factor (SCF) carrier free (Biolegend, catalog number: 579702)
Recombinant mouse IL-33 carrier free (Biolegend, catalog number: 580504)
Mitomycin C (Santa Cruz Biotechnology, catalog number: sc-3514)
Phosphate buffered saline (PBS)
NaN3
FACS Buffer (see Recipes)
Sterile Sorting Buffer (see Recipes)
Innate lymphoid cell media (ILC media) (see Recipes)
OP9-DL1 media (see Recipes)
Equipment
Sterile Biosafety cabinet
Incubator set at 37 °C, 5% CO2
Vortex Genie 2 (Thermo Fisher Scientific, catalog number: 12–812)
Sterile FACS Tubes
Cell sorter (BD Biosciences, model: FACS aria III)
Reichert Bright-Line Hemocytometer (Sigma-Aldrich, catalog number: Z359629)
Dissection scissors (Roboz Surgical Instrument Co., catalog number: RS-5912)
Forceps (Roboz Surgical Instrument Co., catalog number: RS-5137)
Procedure
Part I. Maintaining OP9-DL1 cells
- Thawing OP9-DL1
- Prepare OP9-DL1 media (recipes) and place 10 ml media in a 15 ml conical tube.
- Thaw a prepared cyro-vial from liquid nitrogen (frozen in FBS plus 10% DMSO, 1ml total volume) by placing the tube in a 37 °C water bath until the ice is melted (approximately 3 min).
- Add this to 10 ml of OP9-DL1 media using a 1 ml micropipette and centrifuge 235 rcf for 5 min at room temperature.
- Carefully remove the media, leaving only the cell pellet.
- Add 10 ml fresh OP9-DL1 media to the tube and pipet up and down to dislodge the pellet.
- Add this 10 ml to a T75 flask containing 5 ml OP9-DL1 media for a total of 15B ml.
- Incubate at 37 °C, 5% CO2, split cells 1:3 every 3–4 day.
-
Passaging OP9-DL1
Note: OP9-DL1 cells will need to be split 1:3 every 3–4 d depending on growth kinetics (70%–75% confluence or approximately 0.6–1 × 106 cells in a T75 flask). If OP9-DL1 reaches a high density they must be discarded, as they will turn into adipocytes.- Prepare OP9-DL1 media (recipes) and place 10 ml media in a 15 ml conical tube.
- Remove media from T75 flask and add 7 ml Trypsin.
- Incubate 37 °C, 5% CO2 for approximately 3 min or until OP9-DL1 start to detach.
- Carefully remove Trypsin and immediately add 10 ml OP9-DL1 media.
- Wash walls of flask with 10 ml pipet to dislodge OP9-DL1 cells.
- Centrifuge 260 × g for 5 min at 25 °C.
- Remove supernatant and add 1 ml OP9-DL1 media to pellet.
- Count cells using a hemocytometer and split appropriately (1 T75 flask should have approximately 300,000 cells).
- Plating OP9-DL1 for differentiation of CLP
- Prepare OP9-DL1 media (recipes) and place 10 ml media in a 15 ml conical tube.
- Remove media from T75 flask and add 7 ml Trypsin.
- Incubate 37 °C, 5% CO2 for approximately 3 min or until OP9-DL1 start to detach.
- Carefully remove Trypsin and immediately add 10 ml OP9-DL1 media.
- Wash walls of flask with 10 ml pipet to dislodge OP9-DL1 cells.
- Centrifuge 260 × g for 5 min at 25 °C.
- Remove supernatant and add 1 ml OP9-DL1 media to pellet.
- Count cells using a hemocytometer and plate OP9-DL1 at a density of 30,000 cells/well in 200–300 μl in a 48 well plate.
- Allow at least 6 h for cells to attach to surface.
- Add 1× Mitomycin-C (200–300 μl/well at a 50 μg/ml final concentration) to each well and incubate 37 °C, 5% CO2 for 25 min.
- Gently add 1 ml per well of warmed OP9-DL1 media to each well to wash out Mitomycin-C.
- Aspirate media and repeat step C11, 3 times.
- The OP9-DL1 feeder layer is now ready to plate isolated CLPs.
Part II. Differentiation of CLP to ILCs
- Common Lymphoid Progenitor (CLP) isolation and immunocytochemistry
- Prepare a 60 × 15 mm culture dish containing 3 ml FACS buffer (FACS buffer kept at 4 °C).
- Prepare a 5 ml Syringe with a 25G × 5/8 needle containing 5 ml PBS.
- Remove the mouse hind limb femur and tibia by cutting proximal to the joints as to have both ends of the bone open. Remove the femur completely with both ends cut.
- Holding the bone with a forceps, flush the Bone Marrow (BM) out of the bone by slowly adding the 5 ml PBS in the Syringe.
- Dissociate the BM by aspirating with the 5 ml Syringe/25G needle 2×.
- Add cells (8 ml) to a 15 ml conical tube.
- Centrifuge the cells for 5 min at 260 × g, 25 °C.
- Resuspend in 10 ml sterile sort buffer at 4 °C and count the cells by using a hemocytometer. Concentration of cells depends on manufacturer instructions for antibody staining. Here, a test was defined as 1 × 106 cells/test and was performed in a volume of 200 μl/test.
- Block by pre-incubating cells (1 × 106 cells/test) with CD16/CD32 on ice for 10 min.
- Stain for cell surface molecules using the specified antibodies listed under “antibodies” as per manufacturer instruction.
- Incubate at 4 °C for 30 min.
- Wash cells by adding 3 ml sterile sorting buffer and centrifuge at 235 rcf for 5 min.
- Resuspend in appropriate amount of sterile FACS buffer (see manufacturer instructions for cell numbers per test) and proceed to sorting.
- Gate and sort CLP as lineage negative, CD127+ Flt3+ Sca-1int (see Figure 1 for gating example) on an ARIA III or ARIA II using a 70 μm nozzle. Sort CLPs directly into a 1.5 ml eppendorf tube containing 300 μl ILC media supplemented with 20 ng/ml IL-7.
- Following sort, plate 7,500 CLPs/well in a 48 well plate in 300 μl cDMEM/IL-7 per well (sort should net approximately 15,000 CLPs/animal) on mitomycin-c treated OP9-DL1 (see part I, section C above).
- Feed with 300 μl fresh cDMEM media supplemented with 20 ng/ml IL-7 and 20 ng/ml SCF every three days. Replate on fresh OP9-DL1 at a concentration of 7,500 cells/well every six days. Add cytokines according to what ILC subtype is under investigation (i.e. for ILC-2 add 20 ng/ml IL-33).
- Cells can be analyzed for ILC using previously reported cell surface markers and transcription factors (see reference) as early as six days following plate-down of CLPs. If one wishes to polarize populations specifically to an ILC2 fate, IL-33 is added to the media at a concentration of 20 ng/ml (i.e. CLPs are sorted directly into media containing cytokine) (Figure 1).
Figure 1. Gating strategy for the isolation of common lymphoid progenitor cells (CLPs) from bone marrow of wild-type mice.
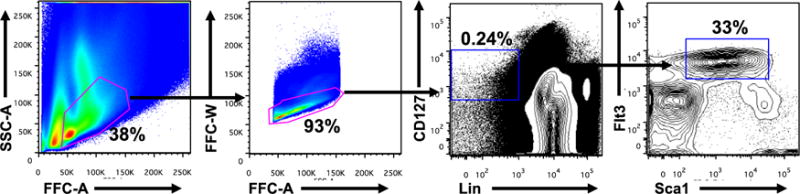
Numbers adjacent to outlined areas indicate percent cells in each gate. CLPs are specifically defined as Lin−CD127+Flt3+Sca-1+. Data are representative of 10 experiments with cells pooled from two mice in each.
Recipes
- FACS buffer (for 500 ml)
- 480 ml phosphate buffered saline (PBS)
- 15 ml FBS (3% final)
- 5 ml of a 10% sodium azide solution (10 g NaN3 in 100 ml deionized water)
- Stored at 4 °C
- Sterile sorting buffer (for 500 ml)
- 2.5 g BSA (0.5% final)
- 12.5 HEPES (25 mM final)
- 485 ml PBS
- ILC media
- DMEM high glucose
- 10% FBS
- 50 μM 2-mercaptoethanol
- 1% penicillin-streptomycin
- 1 mM sodium pyruvate
- 1× non-essential amino acids
- 20 mM HEPES (pH 7.4)
- IL-7 (20 ng/ml)
- SCF (50 ng/ml)
- OP9-DL1 media
- Alpha-MEM
- 20% FBS
- 1% penicillin-streptomycin
Acknowledgments
This work was supported by the U. S National Institutes of Health. This protocol was modified from reference (Seehus et al., 2015).
References
- 1.Seehus CR, Aliahmad P, de la Torre B, Iliev ID, Spurka L, Funari VA, Kaye J. The development of innate lymphoid cells requires TOX-dependent generation of a common innate lymphoid cell progenitor. Nat Immunol. 2015;16(6):599–608. doi: 10.1038/ni.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, Radtke F, Hardman CS, Hwang YY, Fallon PG, McKenzie AN. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13(3):229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]


